- Plastic Surgery, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, China
Background: The relationship between sex steroid hormones and high-sensitivity C-reactive protein(hs-CRP) levels in American children and adolescents is understudied. This research will examine this association.
Methods: The study conducted a data analysis from the National Health and Nutrition Examination Survey (NHANES) 2015-2016, adjusting multiple linear regression models with R 4.2.2 and EmpowerStats. A total of 1,768 children and adolescents were surveyed. Data collection involved measurements of serum levels of testosterone, estradiol, sex hormone-binding globulin (SHBG) and hs-CRP.
Results: With the increase in testosterone, a brief rise (β=0.082, P=0.047) followed by an overall decline (β=-0.028, P=0.023) in hs-CRP was observed in the Male Prepubertal population, while a continuous decline (β=-0.002, P<0.05) was seen in the Male Pubertal group. A positive correlation (β=0.047, P<0.05) was found between testosterone and hs-CRP in the Female Prepubertal population, whereas no significant association (β=0.002, P>0.05) was detected in the Female Pubertal group. A significant inverse correlation was observed between estradiol and hs-CRP solely in the Female Pubertal group (β=-0.002, P<0.05), while no association was found in other populations. An inverse relationship between SHBG and hs-CRP was consistently noted across all groups: Male Prepubertal, Male Pubertal, Female Prepubertal, and Female Pubertal.
Conclusions: The association between sex steroid hormones and high-sensitivity C-reactive protein (hs-CRP) levels among American children and adolescents is conditional and influenced by multiple factors.
Introduction
C-reactive protein (CRP), predominantly produced by hepatocytes in the liver, is a homopentameric protein involved in the acute-phase inflammatory response, and its expression is noticeably heightened during inflammation, such as that caused by rheumatoid arthritis, specific cardiovascular disorders, and infections (1). Estrogens are generally observed to exert an anti-inflammatory influence, potentially enhancing outcomes in severe infections and during wound healing processes (2). However, the literature also indicates that the effects of estrogens on inflammation can vary, being pro-inflammatory, anti-inflammatory, or both, depending on specific cytokines, cell types, and variations in estrogen receptor expression (3). Conversely, testosterone levels are consistently found to have anti-inflammatory effects, as they inhibit adipocyte expansion, differentiation, and function, reduce the production of various cytokines, and promote adiponectin secretion (4). Furthermore, estrogen is speculated to alter CRP concentrations, with evidence indicating a notable impact of hormone replacement therapy (HRT) on CRP levels in the elderly population (1).
Prior research has revealed a complicated and varied relationship between sex steroid hormones and CRP levels in adults. In men, there is generally an inverse association between CRP and key hormonal markers like total testosterone, free testosterone, and SHBG, while the relationship with estradiol is often insignificant (5). Interestingly, estrogen treatment in certain male groups could potentially elevate CRP levels (6). In women, the relationship varies greatly with hormonal and menopausal status, presenting an intricate pattern of positive, negative, or even non-significant correlations between different sex hormones and CRP levels (7–9).
To date, only a single study targeting the adolescent population (aged 12-16 years) has examined the relationship between sex hormones and C-reactive protein. It found a negative correlation between testosterone and high-sensitivity C-reactive protein(hs-CRP) in adolescent boys, but no correlation in girls. In both sexes, a negative association was found between SHBG and hs-CRP (10).
The study of sex steroid hormones in children and adolescents has been attracting increasing scholarly attention (11, 12). Concurrently, existing findings regarding the relationship between these hormones and CRP remain contentious. It is noteworthy that there is a significant scarcity of research, particularly in the age group of 6-11 years. This oversight is notable given the critical developmental changes that occur during these years, which could potentially modulate the influence of sex hormones on inflammation differently than in adults. Understanding these relationships is crucial, as it could enable earlier and more effective interventions for diseases related to inflammation and hormonal imbalances, and transform preventative health strategies. To bridge this research gap, our study proposes to harness the 2015-2016 NHANES data. The objective is to elucidate the potential associations between sex steroid hormones and hs-CRP levels within the population of American children and adolescents. Given the cross-sectional nature of NHANES, our study focuses on identifying patterns and associations rather than causative links, which is a pertinent approach when dealing with observational datasets. Building on this framework, our hypothesis posits that the relationships between sex hormone levels and high-sensitivity C-reactive protein (hs-CRP) vary across different stages of puberty in children and adolescents. We anticipate unique patterns in the correlation between sex hormones and hs-CRP across different genders of children and adolescents before puberty. During puberty, this relationship is expected to mirror the complex interactions observed in adult studies. These findings will provide new insights into the correlations between sex hormones and inflammatory markers during different developmental stages.
Participants and methods
Study design and participants
The National Health and Nutrition Examination Survey (NHANES) is a sequence of cross-sectional studies carried out in the United States, assessing the health and nutritional conditions of both adults and children. In contrast to other research, NHANES integrates physical exams and interviews for comprehensive data gathering. The ethical aspects of each part of the study were reviewed and approved by the National Center for Health Statistics Ethics Review Board, and every participant gave their written informed consent (13). For the purpose of our research, we extracted data from 1,768 children(6-11 years) and adolescents(12-19 years) who had hs-CRP, serum total testosterone (TT), estradiol (E2), and sex hormone binding globulin (SHBG) records in the 2015-2016 NHANES dataset. The process of our selection is depicted in Figure 1.
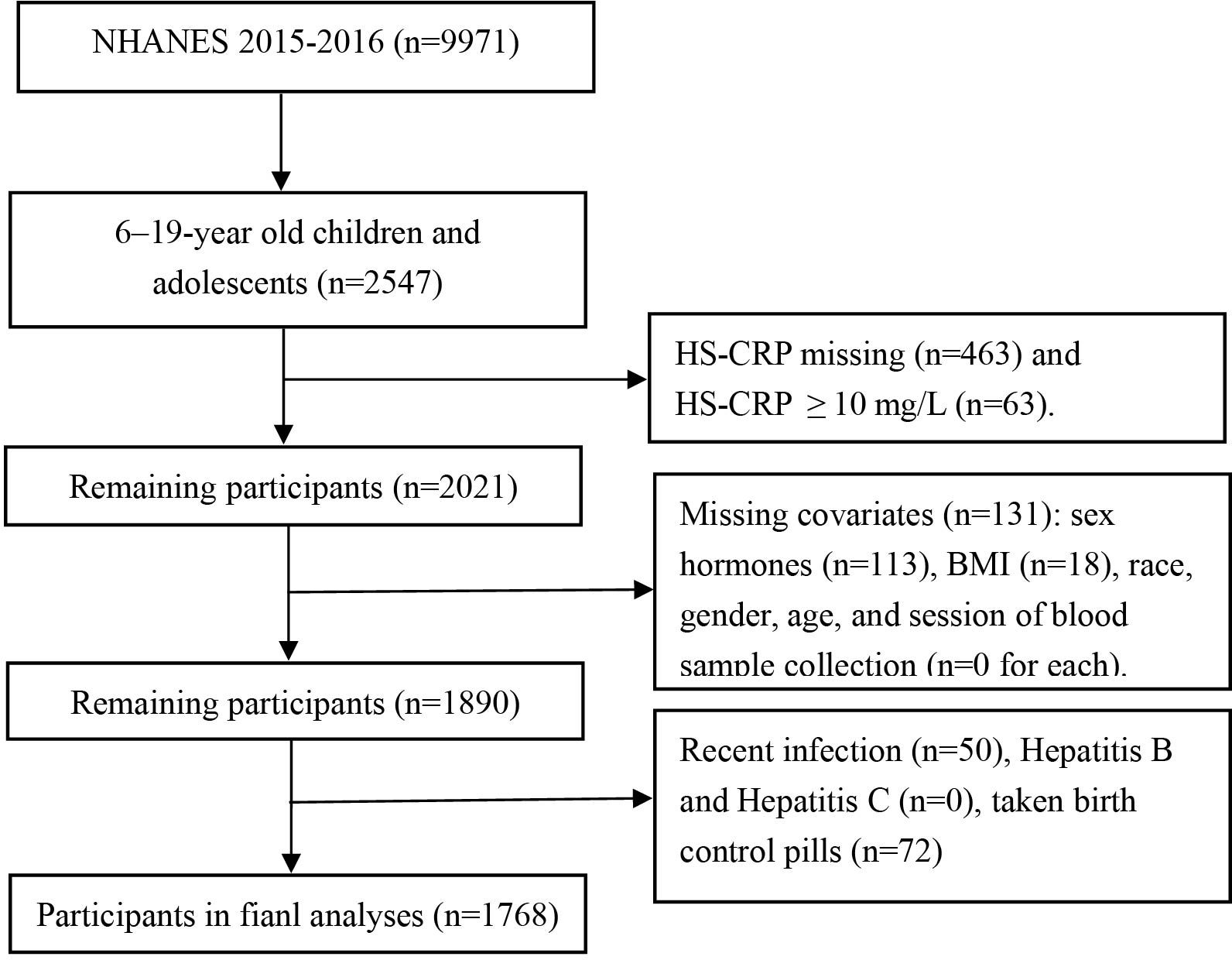
Figure 1. Flowchart for selecting the study population. NHANES, The National Health and Nutrition Examination Survey.
Sex hormone indicators as measured in NHANES
The measurement of testosterone, estradiol, and SHBG in NHANES were conducted by NHANES researchers, following standardized procedures.
The acquisition of testosterone and estradiol involves four principal steps: Dissociation of the analytes from binding proteins, extraction of the analytes from the sample matrix, removal of potentially interfering compounds, and quantitation of the analytes by isotope dilution high performance liquid chromatography tandem mass spectrometry (ID-LC-MS/MS) using stable isotope labeled internal standards and external calibrators. Isolation of the analytes is achieved using liquid-liquid extraction. ID-LC-MS/MS is performed with a triple quadrupole mass spectrometer using electrospray ionization in positive ion mode for testosterone, and negative ion mode for estradiol. Estradiol and testosterone are identified based on chromatographic retention time and on specific mass to charge ratio transitions using selected reaction monitoring (SRM). A 13C isotope-labeled testosterone and a 13C isotope-labeled estradiol are used as internal standards.
The method for measuring Sex Hormone Binding Globulin (SHBG) is based on the reaction of SHBG with immuno-antibodies and chemo-luminescence measurements of the reaction products. It consists of 2 incubation steps and a chemiluminescent measurement via photomultiplier tube that spans 18 minutes.The first incubation period begins by sandwiching the sample of SHBG containing serum between a biotinylated monoclonal SHBG-specific antibody and a monoclonal SHBG-specific antibody that is labeled with ruthenium. The second incubation entails the addition of streptavidin-coated microparticles to the sample mixture. The microparticles bind to the solid phase via biotin and streptavidin interactions. The resulting sample mixture is then aspirated into a measuring cell that is subjected to a magnetic field. This captures the microparticles on an electrode. The remains of the sample mixture are subsequently washed out of the measuring cell. A voltage is applied to the electrode causing a chemiluminescent reaction that is measured by a photomultiplier tube. The readings are compared to an instrument- and lot-specific calibration curve.
High-sensitivity C-reactive protein as measured in NHANES
High-sensitivity C-reactive protein (hs-CRP) levels were obtained from the NHANES 2015-2016 dataset, where the hs-CRP measurements were carried out by NHANES staff using a dual-agent immunoturbidimetric system at designated laboratories. Initially, a sample is amalgamated with a Tris buffer followed by an incubation period. Then, the second reagent, comprising latex particles that are coated with mouse anti-human CRP antibodies, is introduced. Upon exposure to circulating CRP, the latex particles tend to clump together, leading to the formation of immune complexes. These formed complexes induce an increase in the scattering of light, which is directly proportional to the CRP concentration. The resulting light absorption from the scattered light is then measured against a pre-established CRP standard curve to determine the concentration of CRP (14).
Covariates
The selection of covariates in this study was based on previous research on sex hormones (5, 8, 9), and included age (continuous), race (Mexican American, other Hispanic, Non-Hispanic white, Non-Hispanic black, Non-Hispanic Asian, or other race), education level (6th and below 6th grade, above 6th grade), poverty income ratio (PIR, continuous), body mass index (BMI, continuous), diabetes (categorical), session of blood sample collection (morning, afternoon, or evening), total cholesterol(TC, continuous), and physical activity(non-activity, 0.1-0.9 hour/week, 0.1-0.9 hour/week, 1.0-3.4 hour/week, 3.5-5.9 hour/week, and ≥6 hour/week). To identify participants with diabetes, any of the following characteristics were used: (a) hemoglobin A1C concentration ≥ 6.5% or a fasting plasma glucose level ≥126 mg/dL; (b) for those who answered “yes” to the following questions: ‘Take diabetic pills to lower blood sugar?’ or ‘Doctor told you have diabetes?’ or ‘Taking insulin now?’ (15).
Adjustment for pubertal status
It is noteworthy that segmenting the participants aged 6-19 years into categories of children and adolescents based purely on age could result in blending prepubescent and pubescent individuals within each group. Consequently, this could give rise to unusually high or low concentrations of sex hormones within each category, which could skew the relationships between sex hormones and hs-CRP during regression analysis. In addition, the influence of sex hormones on hs-CRP may vary with puberty status. In an effort to mitigate this concern, we subdivided the participants into two categories, namely pubertal and prepubertal groups, based on their serum sex hormone levels and menarcheal status. Participants who displayed levels of TT ≥ 50 ng/dL (in the case of males) or E2 ≥ 20 pg/mL (in the case of females), or had initiated menstrual cycles (applicable to females), were classified under the ‘pubertal group’. The remaining participants, who did not meet these criteria, were assigned to the ‘prepubertal group’ (16).
Statistical analyses
We used sampling weights to adjust for selection probabilities, oversampling, non-response, and differences between the sample and the entire US population. Given the right-skewed distribution of hs-CRP, we performed a natural logarithmic transformation on the hs-CRP values.Weighted univariate linear regression was employed to ascertain the correlations between sex steroid hormones and hs-CRP. We used weighted multivariable linear regression models to examine the relationship between sex hormones and hs-CRP, adjusting for various confounding factors. We performed trend tests to see if the effects of different ranges of sex hormones on hs-CRP were consistent. We adopted the Generalized Additive Model(GAM) to detect the non-linear association. If the relationship between sex hormones and hs-CRP was non-linear, a two-piecewise linear regression model was implemented to estimate the threshold effect of sex steroid hormones on hs-CRP. Subgroup analyses were conducted via stratified linear regression models, with the modification and interaction within subgroups assessed through the likelihood ratio test. These models were designed to explore associations between sex hormones and hs-CRP levels, rather than to infer causal relationships. Given the cross-sectional nature of the NHANES data, the causal direction of any observed associations cannot be determined. Therefore, we selected an analytical approach ideal for identifying correlations in cross-sectional data. We analyzed the data using the R software (version 4.2.2) and EmpowerStats (http://www.empowerstats.com). We considered a P-value < 0.05 as statistically significant.
Results
Baseline characteristics
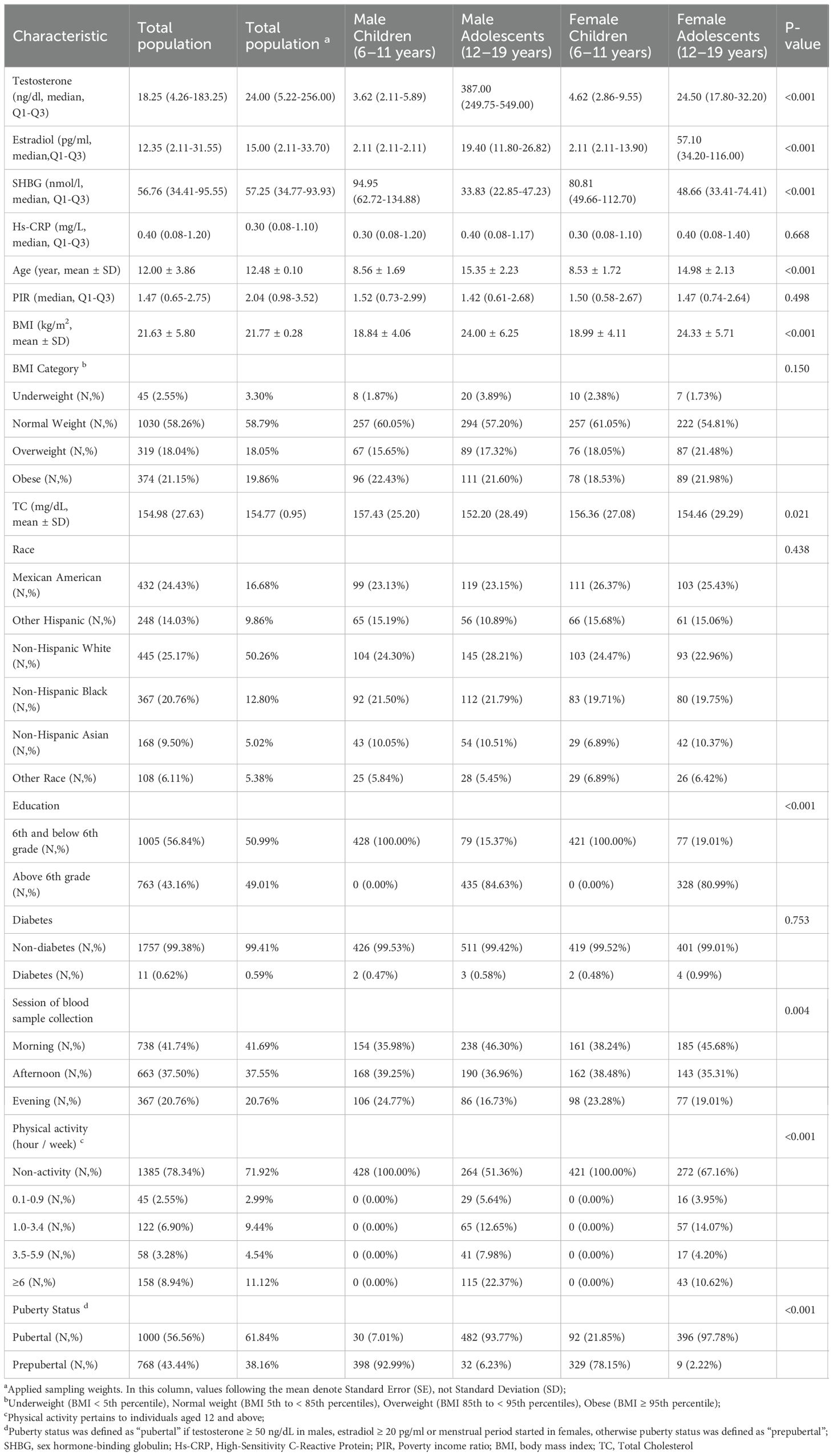
The study population (Table 1), stratified by gender and age groups, exhibited significant disparities in levels of testosterone, estradiol, SHBG, age, and BMI (all P<0.001). Testosterone and estradiol levels were notably higher in adolescents, while SHBG was highest in children. BMI differed significantly between children and adolescents. Most participants were of normal weight (58.26%) and non-diabetic (99.38%).Importantly, pubertal status significantly differed between children and adolescents (P<0.001), with a noteworthy proportion of female children (21.85%) already in puberty. This finding prompted us to mainly categorize our population based on pubertal status for subsequent analysis.
Weighted univariate analysis
The Supplementary Table 1 analysis revealed that testosterone, estradiol, SHBG, age, race, BMI, diabetes status, and examination time were significantly associated with hs-CRP levels across prepubertal and pubertal males and females. Notably, the association strength did not significantly differ between groups, barring estradiol, race, examination time, and BMI.
Association between testosterone and high-sensitivity C-reactive protein
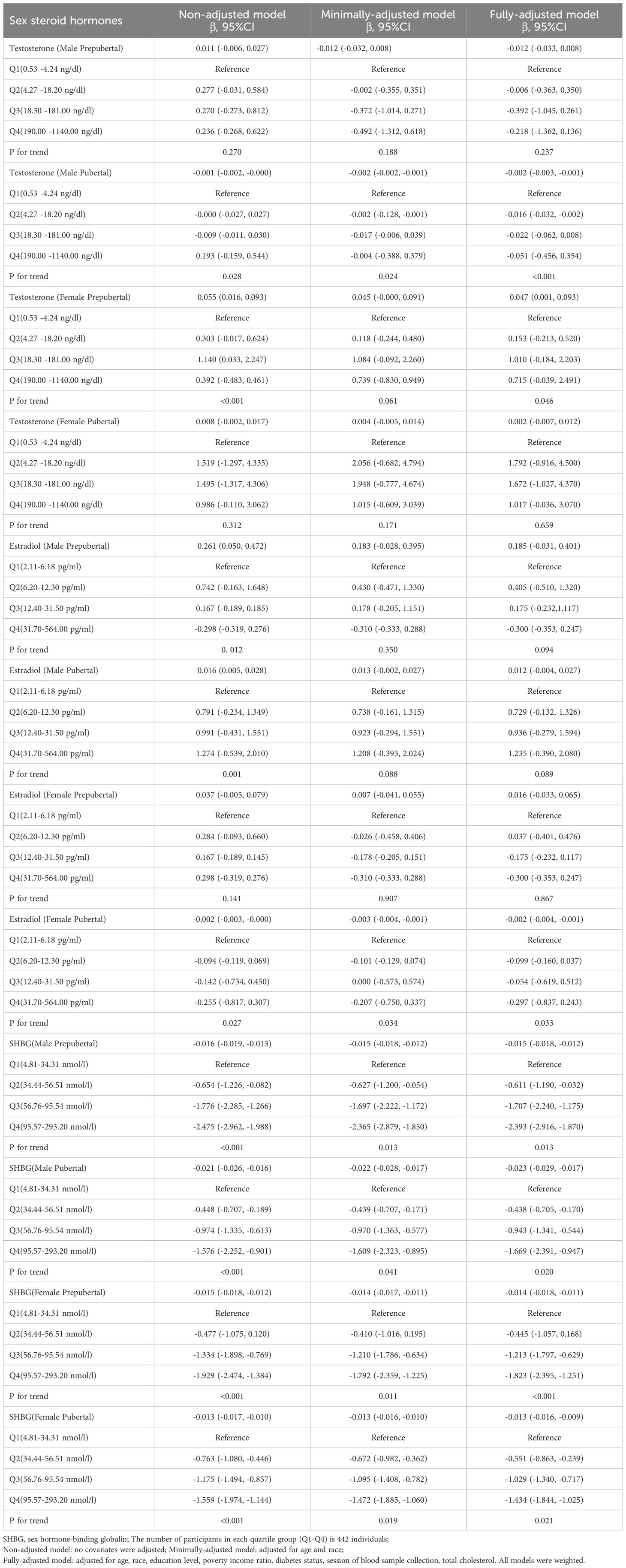
In Male Prepubertal subjects, testosterone exhibited no significant link with hs-CRP (Table 2). A threshold effect was evident at a testosterone level of 8.90 ng/dl (Supplementary Table 2), associating positively below(β=0.082, P=0.047) and negatively above(β=-0.028, P=0.023) this threshold (Figure 2). Stratified analysis (Supplementary Table 3) revealed significant negative correlations in Mexican American(β=-0.027, P<0.05) and Other Hispanic groups(β=-0.044, P<0.05), while the Other Race group showed a positive correlation(β=0.073, P<0.05).
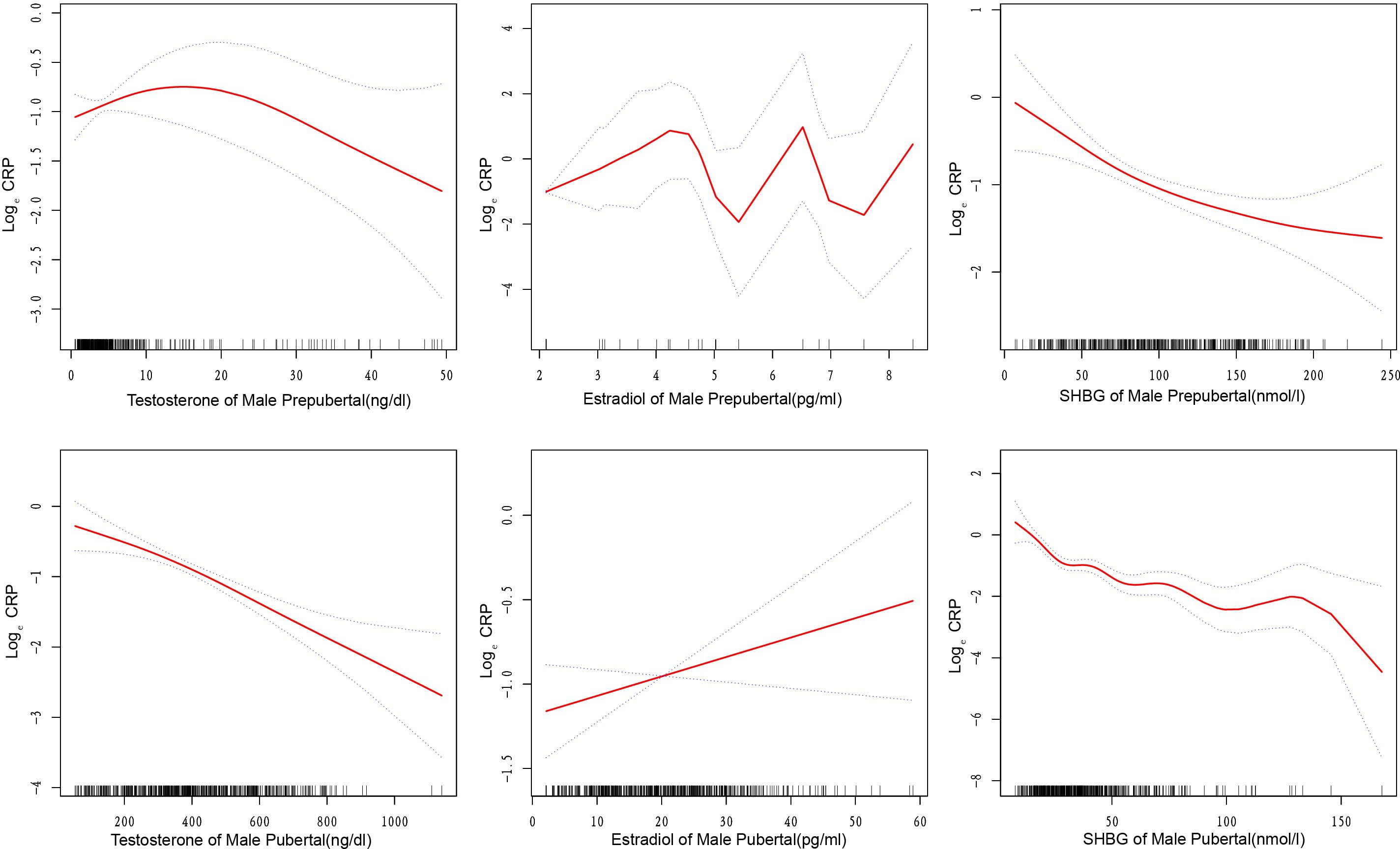
Figure 2. Curve fitting relationships between sex steroid hormones and high-sensitivity C-reactive protein in male children and adolescents. The solid and dashed lines in the graph represent the estimated values and corresponding 95% confidence intervals of high-sensitivity C-reactive protein, respectively. SHBG, sex hormone-binding globulin; Curve adjusted for age, race, education level, poverty income ratio, diabetes status, sample collection session, and total cholesterol.
In Male Pubertal subjects, a consistent negative association was evident between testosterone and hs-CRP levels (β=-0.002, P<0.05, Table 2). Supplementary Table 2 revealed a threshold effect at 224.00 ng/dl, where above this level, the association was more pronounced(β=-0.003, P<0.001) (Figure 2). The results of the stratified analysis (Supplementary Table 3) also support the negative correlation between testosterone and hs-CRP.
For Female Prepubertal subjects, a significant positive association was found between testosterone and hs-CRP in the fully-adjusted model in Table 2 (β=0.047, P<0.05). However, the piecewise linear regression model in Supplementary Table 2 failed to detect any significant threshold effect (Supplementary Figure 1). In the stratified analysis for the Female Prepubertal group (Supplementary Table 3), a significant negative relationship is demonstrated in overweight children (β=-0.077, P<0.05).
For Female Pubertal subjects, no significant association between testosterone and CRP was found in all models in Table 2, and likewise, no significant threshold effect was detected in Supplementary Table 2 and Supplementary Figure 1. In the stratified analysis of female pubertal development, a significant negative association was observed among Mexican American children(β=-0.016, P<0.05) and those engaging in over 6 hours of physical activity per week(β=-0.048, P<0.05). Conversely, a significant positive relationship was found in Non-Hispanic Asian children (β=0.023, P<0.05).
Association between estradiol and high-sensitivity C-reactive protein
In conducting multivariate regression analysis (Table 2) and threshold effect analysis (Supplementary Table 2), we did not identify significant associations between estradiol and hs-CRP in Male Prepubertal, Male Pubertal, and Female Prepubertal cohorts (Figure 2; Supplementary Figure 1). However, upon conducting stratified analyses within these three populations, we identified several subgroups where the relationship between estradiol and hs-CRP exhibited statistically significant associations. These included both positive and negative correlations (Supplementary Table 4).
In the Female Pubertal group, a significant negative correlation was observed between estradiol and hs-CRP(β=-0.002, P<0.05), and this inverse relationship was more pronounced when estradiol was less than or equal to 183 pg/ml(β=-0.004, P=0.001). In the Female Pubertal group, a significant positive correlation was observed between estradiol and hs-CRP when physical activity was between 3.5-5.9 hours per week(β=0.017, P<0.05, Supplementary Table 4).
Association between SHBG and high-sensitivity C-reactive protein
From Table 2, for each subgroup, there exists a significant negative association between SHBG and hs-CRP, with the relationship holding across various models adjusting for different sets of covariates(all P<0.05). For the Male Prepubertal, Male Pubertal, Female Prepubertal, and Female Pubertal groups, the β coefficients suggest a stronger negative correlation below the respective inflection points of 72.09, 25.74, 149.10, and 56.48 nmol/l (Supplementary Table 2, Figure 2, and Supplementary Figure 1).
The results from stratified analysis also support a negative correlation between SHBG levels and hs-CRP (Supplementary Table 5). However, a notable exception is observed in the ‘Above 6th grade’ group within the Female Prepubertal population, where a positive correlation is identified(β=0.035, P<0.05).
Discussion
Our research uncovers a distinct gender and age-related difference in the relationship between testosterone and hs-CRP. In Male Prepubertal group, an initial increase in testosterone levels leads to a transient surge in hs-CRP (β=0.082, P=0.047), subsequently resulting in an overall decline (β=-0.028, P=0.023). Contrarily, in Male Pubertal, hs-CRP levels consistently decrease under the influence of testosterone (β=-0.002, P<0.05). In Female Prepubertal, a significant positive correlation between testosterone and hs-CRP is observed (β=0.047, P<0.05). However, no significant correlation is found in Female Pubertal (β=0.002, P>0.05). Previous studies commonly support the negative correlation between testosterone and CRP in both adolescent (10) and adult males (5, 17), to some extent, validating the hypothesis that testosterone has anti-inflammatory properties in males. In females, the relationship between testosterone and CRP appears diverse, even contrary. The research conducted by de Dios O and colleagues did not identify any correlation between testosterone and hs-CRP in female adolescents aged between 12 and 16 years (10). In postmenopausal women, some studies have reported a positive correlation between serum testosterone levels and CRP (8). However, there are also studies concluding an inverse relationship between serum testosterone levels and CRP in postmenopausal women (9, 18). The level of testosterone plays a crucial role in modulating inflammatory processes, which is achieved by suppressing the expansion, differentiation, and function of adipocytes, curtailing the formation of cytokines (leptin, IL-6, TNF-α, MCP-1, resistin), and concurrently promoting the secretion of adiponectin (4). Future research is necessary to explore the interaction mechanisms between testosterone and hs-CRP.
Our study found a negative correlation between estradiol and hs-CRP (β=-0.002, P<0.05) solely within the Female Pubertal population. No such correlation was observed in the Male Prepubertal, Male Pubertal, and Female Prepubertal groups. Previous studies on adult females have indicated that the relationship between estradiol and CRP is negative pre-menopause (7, 19), but turns positive post-menopause (8, 18). This suggests a potentially complex and dynamic relationship between these variables across different stages of life. Throughout the inflammatory reaction, pro-inflammatory cytokines prompt the synthesis of NO in cells like monocytes, macrophages, and neutrophils. During the process of phagocytosis, NO, when discharged by tissue macrophages, operates as a positive feedback entity, catalyzing the attraction of additional phagocytes. Physiological levels of estrogen maintained the nCRP-facilitated decrease in NO production in LPS-stimulated monocytes, concurrently, estrogen countered the mCRP-induced increase of NO production in the same cells (20). This aligns with earlier research demonstrating that estrogen attenuates pro-inflammatory responses, which includes the production of NO and inflammatory cytokines.
Our study reveals a consistent negative correlation between SHBG and hs-CRP in both children and adolescents. This finding is not isolated, as it aligns with the results of prior research. Such negative correlation between SHBG and C-reactive protein has been confirmed in various populations, including adolescents (10), adult males (5), premenopausal women (19), and postmenopausal women (9). SHBG, secreted by the liver into the bloodstream, avidly binds to both androgens and estrogens, thereby regulating their bioavailability. BMI has been traditionally considered a primary determinant of circulating SHBG concentration, with a reported consistent negative correlation between BMI and plasma SHBG levels (21). Lower serum SHBG concentrations in overweight individuals serve as a biomarker of metabolic syndrome (22) and indicate an increased risk of type 2 diabetes(T2D) (23) and cardiovascular diseases(CVD) (24). Obesity-related endocrine mechanisms and chronic inflammation are associated with the reduction of SHBG prior to puberty, suggesting that lower SHBG levels could indicate an earlier onset of puberty (25). Reviews have proposed that the subtle inflammation and changes in pro-inflammatory/anti-inflammatory cytokines (i.e., TNFα, IL-1β, and adiponectin) occurring in obesity and T2D may be the primary cause of decreased SHBG levels, rather than hyperinsulinemia (24). It remains to be elucidated whether the decrease in SHBG is merely a biomarker, or if it actively participates in the inflammatory response process related to CRP, and subsequently contributes to the pathogenesis of obesity, T2D, fatty liver, and cardiovascular diseases.
Our study possesses several strengths. First, we have filled a research gap in exploring the relationship between sex steroid hormones and hs-CRP in children populations(6-11 years). Additionally, we categorized children and adolescents into prepubertal and pubertal groups based on hormone levels, offering a more reliable demographic foundation for researching the association between sex steroid hormones and hs-CRP. Furthermore, the data utilized in our study was obtained from the NHANES database, ensuring the accuracy of serum hormone and hs-CRP measurements. We also weighted our data to ensure that our final results would be representative of the overall health status of children and adolescents in the United States.
Nevertheless, there are some limitations in our study. Although we conducted detailed stratified analyses for various covariates, the unequal distribution of sample sizes across certain groups may have resulted in insufficient statistical power for some analyses. For instance, in the stratified analysis of testosterone and high-sensitivity C-reactive protein (Supplementary Table 3), the Normal Weight group included 1,030 participants, while the Underweight group comprised only 45 participants. Therefore, the interpretation of these results must take into account the limitations posed by the small sample sizes. Even though we adjusted for potential confounders, there might still be unadjusted factors that could influence our results. The lack of data on gonadotropin-releasing hormone, gonadotropins, and crucial enzymes involved in hormone responses restricted us from further delving into the underlying biological mechanisms. Importantly, it should be noted that our study is cross-sectional, thereby preventing us from establishing a causal relationship between sex steroid hormones and hs-CRP. This design captures data at a single point in time, limiting our ability to track changes in variables over time or to ascertain causal sequences. Future research should employ longitudinal designs to overcome these limitations and validate our findings.
Conclusions
Our findings indicate that the association between sex steroid hormones and high-sensitivity C-reactive protein (hs-CRP) levels among American children and adolescents is conditional and influenced by multiple factors. Specifically, this association is influenced by several factors including age, body mass index (BMI), pubertal status, hormone levels, among others. For instance, in prepubertal males, testosterone levels below 8.90 ng/dL positively correlate with hs-CRP levels, while levels above this threshold show a negative correlation. This indicates that the association between sex steroid hormones and inflammatory markers involves a multifaceted interplay, potentially including other biomarkers and environmental factors not assessed in this study. Further research is needed to explore these dimensions to better elucidate the correlations between sex hormones and inflammatory markers.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by NCHS Ethics Review Board (ERB) Approval. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZSZ: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. XGL: Conceptualization, Resources, Writing – review & editing, Methodology, Project administration. CYW: Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing – review & editing. SZZ: Data curation, Funding acquisition, Investigation, Validation, Visualization, Writing – review & editing. XYZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to express our sincere gratitude to the investigators who conducted the original NHANES study, as well as the USA National Center for Health Statistics for sharing the data. We would like to acknowledge that an earlier version of this manuscript was deposited as a preprint on Research Square (https://www.researchsquare.com/article/rs-3935965/v1) (26), which has not undergone peer review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1431984/full#supplementary-material
Supplementary Figure 1 | Curve fitting relationships between sex steroid hormones and high-sensitivity C-reactive protein in female children and adolescents. The solid and dashed lines in the graph represent the estimated values and corresponding 95% confidence intervals of high-sensitivity C-reactive protein, respectively. SHBG: sex hormone-binding globulin; Curve adjusted for age, race, education level, poverty income ratio, diabetes status, sample collection session, and total cholesterol.
References
1. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. (2018) 9:754. doi: 10.3389/fimmu.2018.00754
2. Harding AT, Heaton NS. The impact of estrogens and their receptors on immunity and inflammation during infection. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14040909
3. Dragin N, Nancy P, Villegas J, Roussin R, Le Panse R, Berrih-Aknin S. Balance between estrogens and proinflammatory cytokines regulates chemokine production involved in thymic germinal center formation. Sci Rep. (2017) 7:7970. doi: 10.1038/s41598-017-08631-5
4. Bianchi VE. The anti-inflammatory effects of testosterone. J Endocr Soc. (2019) 3:91–107. doi: 10.1210/js.2018-00186
5. Kupelian V, Chiu GR, Araujo AB, Williams RE, Clark RV, McKinlay JB. Association of sex hormones and C-reactive protein levels in men. Clin Endocrinol (Oxf). (2010) 72:527–33. doi: 10.1111/j.1365-2265.2009.03713.x
6. Kovacs A, Henriksson P, Hamsten A, Wallén H, Björkegren J, Tornvall P. Hormonal regulation of circulating C-reactive protein in men. Clin Chem. (2005) 51:911–3. doi: 10.1373/clinchem.2004.046169
7. Park JM, Lee YJ. Serum oestradiol levels are inversely associated with C-reactive protein levels in premenopausal women, but not postmenopausal women. J Int Med Res. (2020) 48:300060520961228. doi: 10.1177/0300060520961228
8. Störk S, Bots ML, Grobbee DE, van der Schouw YT. Endogenous sex hormones and C-reactive protein in healthy postmenopausal women. J Intern Med. (2008) 264:245–53. doi: 10.1111/j.1365-2796.2008.01946.x
9. Joffe HV, Ridker PM, Manson JE, Cook NR, Buring JE, Rexrode KM. Sex hormone-binding globulin and serum testosterone are inversely associated with C-reactive protein levels in postmenopausal women at high risk for cardiovascular disease. Ann Epidemiol. (2006) 16:105–12. doi: 10.1016/j.annepidem.2005.07.055
10. de Dios O, Herrero L, Vales-Villamarín C, Mahíllo-Fernández I, Soriano-Guillén L, Garcés C. Sex steroid hormones, leptin, and high-sensitivity C-reactive protein levels in adolescents. Andrology. (2021) 9:829–36. doi: 10.1111/andr.12962
11. Wang ZY, Li BC, Xing JJ, Liu SY, Zhang TT, Xu AM, et al. Associations of waist circumference with sex steroid hormones among 4031 USA children and adolescents. Asian J Androl. (2023) 25:505–11. doi: 10.4103/aja202284
12. Zhang Y, Xing H, Hu Z, Xu W, Tang Y, Zhang J, et al. Independent and combined associations of urinary arsenic exposure and serum sex steroid hormones among 6-19-year old children and adolescents in NHANES 2013-2016. Sci Total Environ. (2023) 863:160883. doi: 10.1016/j.scitotenv.2022.160883
13. Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. (2013) 19:221–31. doi: 10.1093/humupd/dms050
14. Ansley SD, Howard JT. Dietary intake and elevated C-reactive protein levels in US military veterans. Int J Environ Res Public Health. (2021) 18. doi: 10.3390/ijerph18020403
15. Kramer H, Boucher RE, Leehey D, Fried L, Wei G, Greene T, et al. Increasing mortality in adults with diabetes and low estimated glomerular filtration rate in the absence of albuminuria. Diabetes Care. (2018) 41:775–81. doi: 10.2337/dc17-1954
16. Luo K, Liu J, Wang Y, Aimuzi R, Luo F, Ao J, et al. Associations between organophosphate esters and sex hormones among 6-19-year old children and adolescents in NHANES 2013-2014. Environ Int. (2020) 136:105461. doi: 10.1016/j.envint.2020.105461
17. Tsilidis KK, Rohrmann S, McGlynn KA, Nyante SJ, Lopez DS, Bradwin G, et al. Association between endogenous sex steroid hormones and inflammatory biomarkers in US men. Andrology. (2013) 1:919–28. doi: 10.1111/j.2047-2927.2013.00129.x
18. Conroy SM, Neilson HK, O'Reilly R, Woolcott CG, Stanczyk FZ, Courneya KS, et al. Associations between postmenopausal endogenous sex hormones and C-reactive protein: a clearer picture with regional adiposity adjustment? Menopause. (2017) 24:1040–8. doi: 10.1097/gme.0000000000000883
19. Blum CA, Müller B, Huber P, Kraenzlin M, Schindler C, De Geyter C, et al. Low-grade inflammation and estimates of insulin resistance during the menstrual cycle in lean and overweight women. J Clin Endocrinol Metab. (2005) 90:3230–5. doi: 10.1210/jc.2005-0231
20. Sproston NR, El Mohtadi M, Slevin M, Gilmore W, Ashworth JJ. The effect of C-reactive protein isoforms on nitric oxide production by U937 monocytes/macrophages. Front Immunol. (2018) 9:1500. doi: 10.3389/fimmu.2018.01500
21. Glass AR, Swerdloff RS, Bray GA, Dahms WT, Atkinson RL. Low serum testosterone and sex-hormone-binding-globulin in massively obese men. J Clin Endocrinol Metab. (1977) 45:1211–9. doi: 10.1210/jcem-45-6-1211
22. Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. (2004) 27:1036–41. doi: 10.2337/diacare.27.5.1036
23. Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. (2009) 361:1152–63. doi: 10.1056/NEJMoa0804381
24. Simó R, Sáez-López C, Barbosa-Desongles A, Hernández C, Selva DM. Novel insights in SHBG regulation and clinical implications[J. Trends Endocrinol Metab. (2015) 26:376–83. doi: 10.1016/j.tem.2015.05.001
25. Pinkney J, Streeter A, Hosking J, Mostazir M, Jeffery A, Wilkin T. Adiposity, chronic inflammation, and the prepubertal decline of sex hormone binding globulin in children: evidence for associations with the timing of puberty (Earlybird 58). J Clin Endocrinol Metab. (2014) 99:3224–32. doi: 10.1210/jc.2013-3902
26. Zhu Z, Lin X, Wang C, Zhu S, Zhou X. Associations of sex steroid hormones with C-reactive protein levels in American children and adolescents: evidence from NHANES 2015-2016. Res Square [Preprint]. (2024). Available online at: https://www.researchsquare.com/article/rs-3935965/v1 (accessed September 11, 2024).
Keywords: children, adolescents, NHANES, sex steroid hormones, C-reactive protein
Citation: Zhu Z, Lin X, Wang C, Zhu S and Zhou X (2024) Conditional associations of sex steroid hormones with C-reactive protein levels in American children and adolescents: evidence from NHANES 2015-2016. Front. Endocrinol. 15:1431984. doi: 10.3389/fendo.2024.1431984
Received: 13 May 2024; Accepted: 26 August 2024;
Published: 24 September 2024.
Edited by:
Sally Radovick, Rutgers, The State University of New Jersey, United StatesReviewed by:
Guoqiang Wang, Rutgers, The State University of New Jersey, United StatesNancy Reichman, Rutgers, The State University of New Jersey, United States
Copyright © 2024 Zhu, Lin, Wang, Zhu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianying Zhou, emhvdXhpYW55aW5nQGZqbXUuZWR1LmNu
 Zhisheng Zhu
Zhisheng Zhu Xingong Lin
Xingong Lin Xianying Zhou
Xianying Zhou