- 1Department of Endocrinology, Zhongda Hospital, Institute of Diabetes, School of Medicine, Southeast University, Nanjing, China
- 2Institute of Diabetes, School of Medicine, Southeast University, Nanjing, China
- 3Department of Endocrinology, The Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, China
- 4Department of Endocrinology, Nanjing First Hospital, Nanjing Medical University, Nanjing, Jiangsu, China
Background: To investigate the association between oxidative balance score (OBS), cardiovascular mortality (CVM), and all-cause mortality (ACM) in type 2 diabetes mellitus (T2DM) patients.
Methods: We included 6,119 participants with T2DM from the 2005-2020 National Health and Nutrition Examination Surveys (NHANES). The status of CVM and ACM of participants was followed through December 31, 2019. Multivariable Cox regression models, Kaplan-Meier curves, log-rank test, restricted cubic spline regression, and subgroup analysis, were used to evaluate the relationship between OBS, CVM, and ACM.
Results: During a median of 100.9 months follow-up, 1,790 ACM cases had occurred, 508 of which were due to cardiovascular disease. The T2DM participants were divided into four groups based on the quartiles of OBS. Participants with Q4 tended to be younger, financially better-off, married, highly educated, had lower alcohol consumption rates, were non-smokers, and exhibited a lower likelihood of ACM and CVM. In multivariate Cox regression models, compared with the patients with Q4, those with Q1 had a 30% increased risk for ACM (Q1, reference; Q4, HR: 0.70, 95%CI: 0.58-0.86) and a 43% increased risk for CVM (Q1, reference; Q4, HR: 0.57, 95%CI: 0.36-0.88). The restricted cubic spline regression models have no nonlinear relationship between OBS, CVM, and ACM. Kaplan-Meier survival curves showed that patients with Q4 had a lower risk of ACM and CVM (log-rank P < 0.05).
Conclusions: We find that ACM and CVM increase with higher OBS in T2DM patients. Moreover, there are linear relationships between OBS, ACM, and CVM.
1 Introduction
Type 2 diabetes mellitus (T2DM), a chronic metabolic disorder, has exhibited a significant growth trend globally due to changes in lifestyle, population aging, and accelerated urbanization. The number of adults with diabetes has increased from 108 million in 1980 to 463 million in 2019, and 90-95% of these cases are T2DM patients (1). It is predicted that the prevalence of T2DM among adults in the United States will reach 25-28% by 2050 (2). T2DM not only has a profound impact on patients’ quality of life but also can trigger a series of severe complications, posing a significant threat to patients’ health and life. This has become one of the significant challenges in the current public health landscape. Diabetes is a cause of one in nine deaths among adults aged 20-79, emphasizing the crucial clinical significance of identifying an appropriate predictor for T2DM mortality (3).
Cardiovascular disease is the principal cause of death and morbidity among T2DM patients (4). According to statistics, the risk of cardiovascular disease in T2DM patients is twice that of non-diabetic patients. Atherosclerosis and heart failure are the most common complications in T2DM patients, and they are significant contributors to morbidity and mortality (5). Current research indicates that circulating metabolites such as hexanoylcarnitine, tryptophan, and kynurenine have been confirmed to be associated with and improve the prediction of all-cause mortality (ACM) in T2DM patients (6). Furthermore, high uric acid levels in T2DM are linked to cardiovascular mortality (CVM). However, while these biomarkers have improved prediction accuracy to a certain extent, their popularity and clinical application value still require further validation.
The oxidative balance score (OBS) is a composite index based on quantiles or categories related to dietary/lifestyle exposures (7). The OBS measures the balance between oxidant and antioxidant exposures, with a higher OBS indicating a dominance of antioxidant exposure, studies have shown a correlation between OBS and the occurrence of T2DM and cardiovascular diseases (8, 9). OBS has been found to have a significant correlation with the incidence of non-alcoholic fatty liver disease (NAFLD) and metabolic dysfunction-associated fatty liver disease (MASLD) among American adults, as well as with ACM associated with MASLD (10, 11). In addition, OBS has been associated with depression, sleep quality, periodontitis, and kidney disease (12–15). Another study has demonstrated that OBS is inversely associated with ACM and CVM (16). By measuring OBS, we can gain a deeper understanding of the pathogenesis of related diseases, providing novel insights into their treatment and prevention.
OBS possesses a unique advantage in assessing the prevalent oxidative stress state in T2DM. However, the current understanding of the relationship between OBS and ACM or CVM among T2DM is still unclear. Therefore, conducting in-depth studies to explore the association between OBS and the prognosis of T2DM is of significant importance and value to the research.
2 Method
2.1 Study population
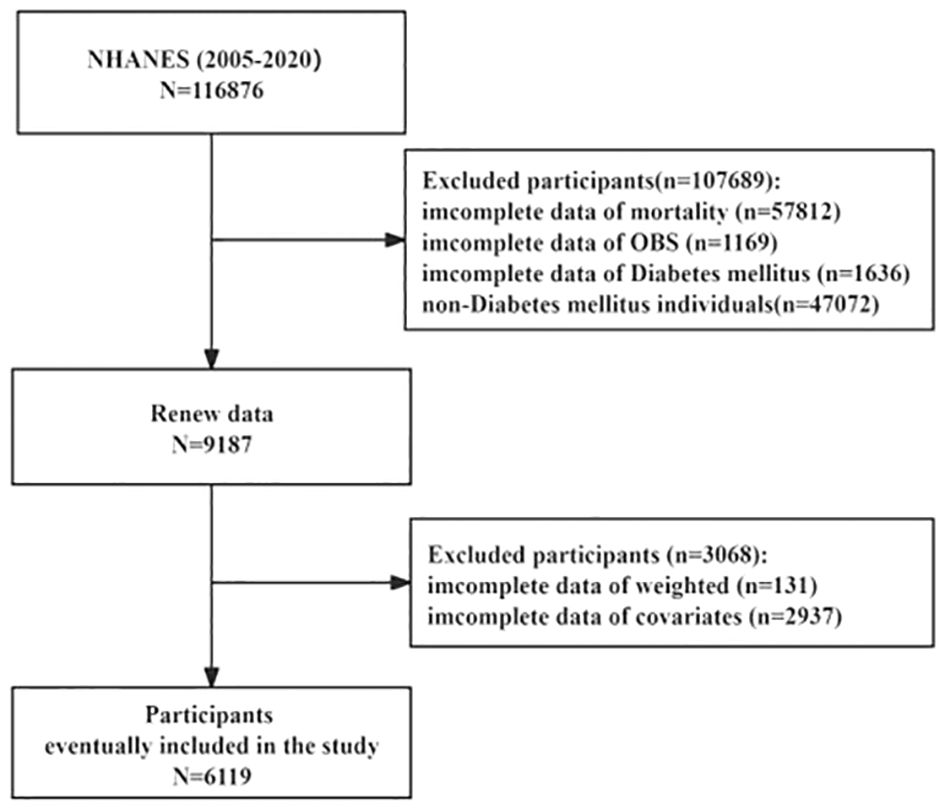
The National Health and Nutrition Examination Surveys (NHANES), a program sponsored by the Centers for Disease Control and Prevention (CDC), aims to evaluate the American populace’s health status comprehensively. The data utilized for our analyses were drawn from the NHANES database spanning 2005 to 2020, encompassing 116,876 participants. The Institutional Review Board of the Centers for Disease Control and Prevention approved the NHANES study protocol, ensuring rigorous ethical standards were upheld. In this study, non-T2DM participants without complete information on mortality, OBS, T2DM, weighted, and covariates were excluded; we enrolled 6,119 participants for the final analysis of this research (Figure 1). The survival status of participants was followed up to December 31, 2019. Additionally, all participants gave informed consent, indicating their voluntary participation and understanding of the study’s objectives and procedures.
2.2 Exposure definitions
The calculation of OBS is based on early research (13, 17, 18). OBS was divided into 16 dietary OBS and four lifestyle OBS, including two prooxidants and 14 antioxidants (19).
The dietary factors were classified into prooxidants (total fat and iron) and antioxidants (β-carotene, dietary fiber, copper, vitamin B6, vitamin B12, vitamin C, niacin, vitamin E, total folate, vitamin B2, magnesium, calcium, zinc, and selenium) according to the effect on oxidative stress. Lifestyle factors were classified as prooxidants (alcohol intake, BMI, and cotinine) and antioxidants (physical activity). Dietary OBS components were assessed in the NHANES using 24-hour food recalls. Physical activity, expressed as weekly metabolic equivalents (MET), was calculated using data on leisure time activities over the past 30 days acquired from household interviews. The primary exposure was OBS and the primary outcomes were ACM and CVM. We used multivariate Cox regression models to assess these relationships, with stepwise adjustments to control for potential confounders.
2.3 Data collection
Questionnaires were collected at baseline to obtain demographic information, including age, personal income ratio (PIR), body mass index (BMI), sex, race, marital status, and education level. Additionally, personal medical history was assessed for T2DM, hypertension, and cardiovascular disease (CVD). Smoking status was categorized as former, current, or never while drinking status was classified as no, moderate, or heavy.
Physical examinations included height, weight, systolic blood pressure (SBP), and diastolic blood pressure (DBP). Blood samples were collected after an 8-hour fast to measure fasting blood glucose (FBG) and conduct other laboratory tests.
2.4 Clinical outcome
ACM refers to deaths occurring for any reason, including cardiovascular disease or cerebrovascular disease, prior to December 31, 2019. Mortality data were obtained from the NHANES-linked mortality files covering 1999-2019. We censored the time from enrollment (date of interview) to death. CVM was defined using the International Classification of Diseases, Tenth Revision codes (I00-I09, I11, I13, I20-I51). Participants who did not have any recorded deaths during the follow-up period were considered alive.
2.5 Statistical analysis
The data were processed by NHANES analytical guidelines (20–22). Continuous variables were expressed as mean ± standard deviation for normally distributed variables or median (interquartile range) if the data were not normally distributed. Categorical variables were presented as numbers (n) and percentages (%). The one-way ANOVA (continuous variables with Gaussian distribution), Kruskal-Wallis H-test (continuous variables with non-Gaussian distribution), or chi-square tests (Differences between groups for categorical variables) were used to assess differences according to OBS quartiles (Q1 ≤ 13, 13<Q2 ≤ 18, 18<Q3 ≤ 24, Q4>24) in groups. Multivariable Cox regression analysis was used to estimate the adjusted hazard ratio (HR) and 95% confidence interval (95% CI) for ACM and CVM according to OBS, lifestyle OBS, and dietary OBS. We used a multivariate Cox regression model to estimate adjusted HRs and 95% CIs because Cox proportional risk models are suitable for analyzing survival data and can handle multiple covariates. Model 1 was constructed without adjusted covariates. Model 2 adjusts for age, sex, and race. Model 3 further adjusts PIR, marital status, education level, smoking status, and hypertension. We performed survival analysis using standardized Kaplan-Meier curves and the log-rank test. The restricted cubic spline (RCS) regression model then tested the association between OBS, ACM, and CVM. Finally, we conducted a subgroup analysis, including age (<60 or ≥60 years), sex (male or female), BMI (<30 or ≥30 kg/m2), smoking (former, now or never), and hypertension (yes or no).
Statistical significance was defined as a two-sided P-value < 0.05. R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analysis.
3 Results
3.1 Baseline characteristics
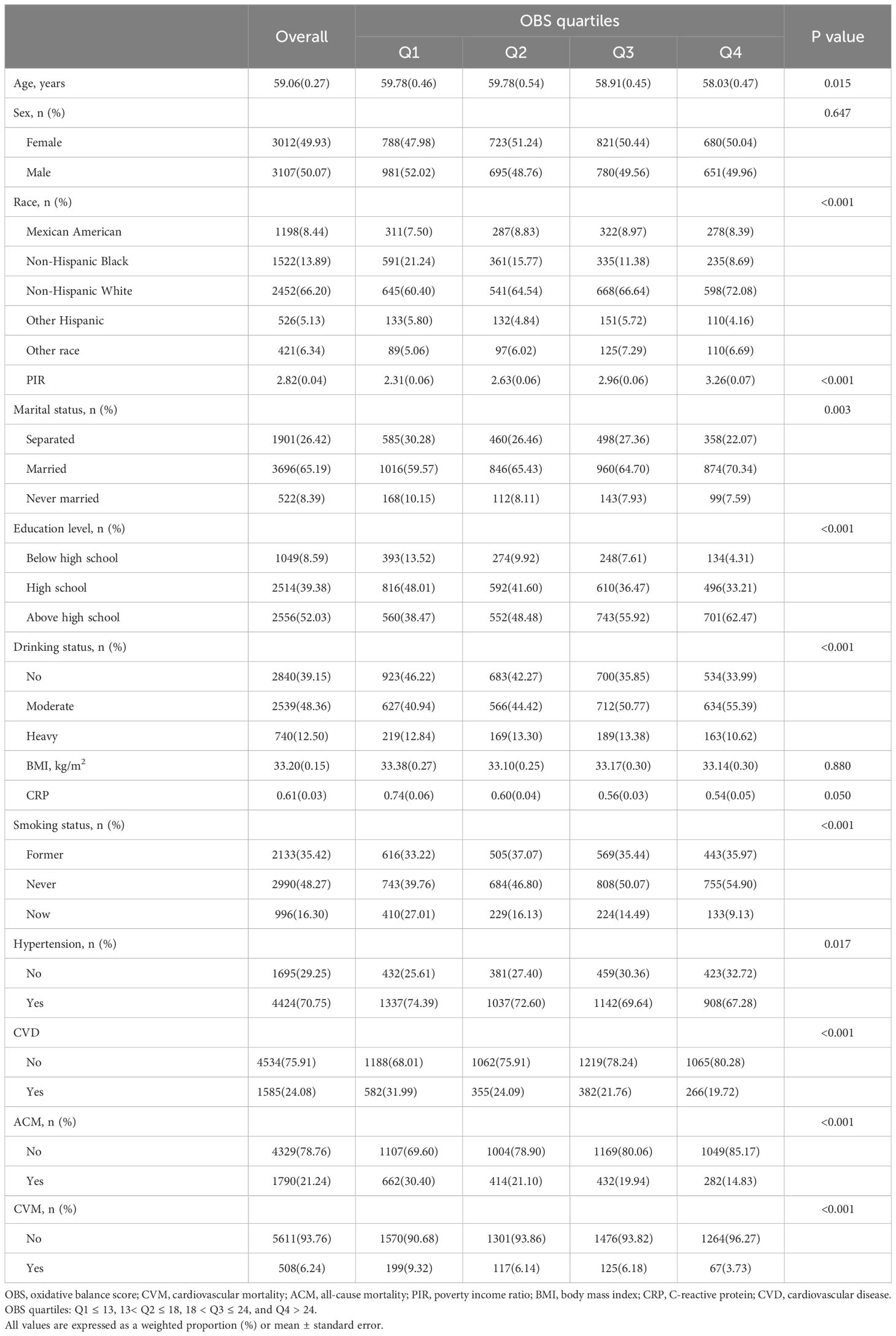
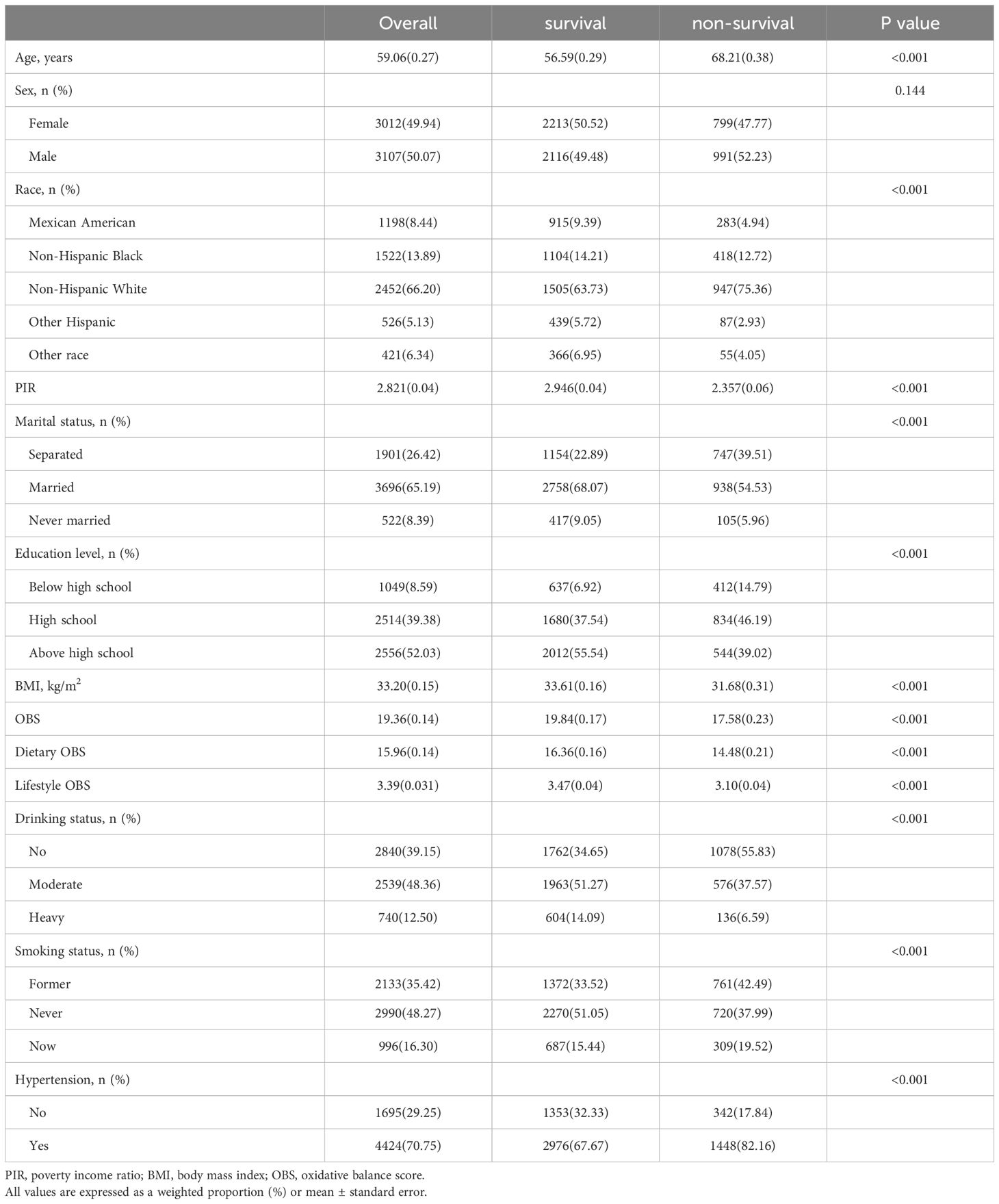
During the continuous NHANES cycles from 2005 to 2020, a total of 6,119 participants were included. Table 1 presents the baseline characteristics of the study participants, categorized by quartiles of OBS. The average age of the included participants was 59.06 ± 0.27 years, with 3,012 females (49.43%). Among the participants, there were 1,198 Mexican Americans (8.44%), 1,522 Non-Hispanic Black individuals (13.89%), 2,452 Non-Hispanic White individuals (66.20%), 526 Other Hispanic individuals (5.13%), and 421 other race individuals (6.34%). While no significant differences were observed in sex, BMI and C-reactive protein (CRP) across the quartiles of OBS, significant variations were found among participants in different quartiles concerning age, PIR, race, marital status, education level, alcohol consumption, smoking, hypertension, and CVD. Specifically, the prevalence of CVD from the lowest to the highest quartile of the OBS was 31.99%, 24.09%, 21.76%, and 19.72%, respectively. In addition, participants with Q4 tended to be younger, financially better-off, married, highly educated, have lower alcohol consumption rates, be non-smokers, and exhibit a lower likelihood of ACM and CVM. Besides, compared to deceased patients, survivors show higher OBS, dietary OBS, and lifestyle OBS. More information can be found in Table 2.
3.2 Relationships between OBS, ACM and CVM
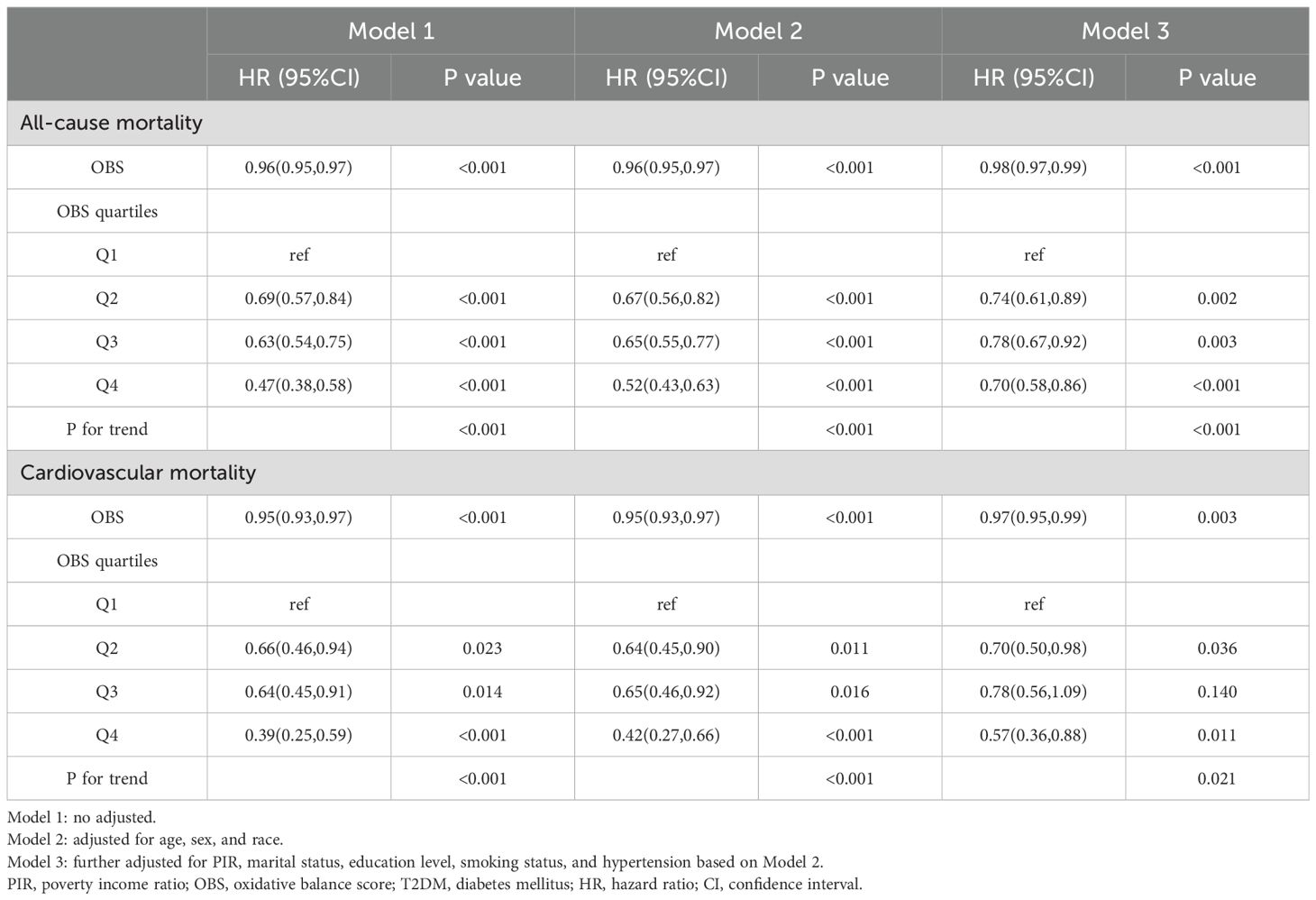
During an average follow-up period of 100.9 months, there were 1,790 cases of ACM and 508 cases of CVM. In multivariate Cox regression models (Table 3), patients with T2DM in Q4 showed significantly lower risk of ACM and CVM compared with patients in Q1. Specifically, in model 1, patients in Q4 had an HR of 0.47 (95% CI: 0.38-0.58) for ACM and 0.39 (95% CI: 0.25-0.59) for CVM. Even after adjusting for confounders such as age, sex, race, PIR, marital status, education level, smoking status, and hypertension, patients in Q4 had an HR of 0.70 (95% CI: 0.58-0.86) for ACM and 0.57 (95% CI: 0.36-0.88) for CVM.
The Kaplan-Meier curves and the log-rank test revealed significant differences among the four groups, with the Q4 group demonstrating a higher survival probability (Figure 2). RCS analysis indicated a linear relationship between OBS, ACM and CVM, with ACM and CVM increasing with higher OBS (Figure 3).
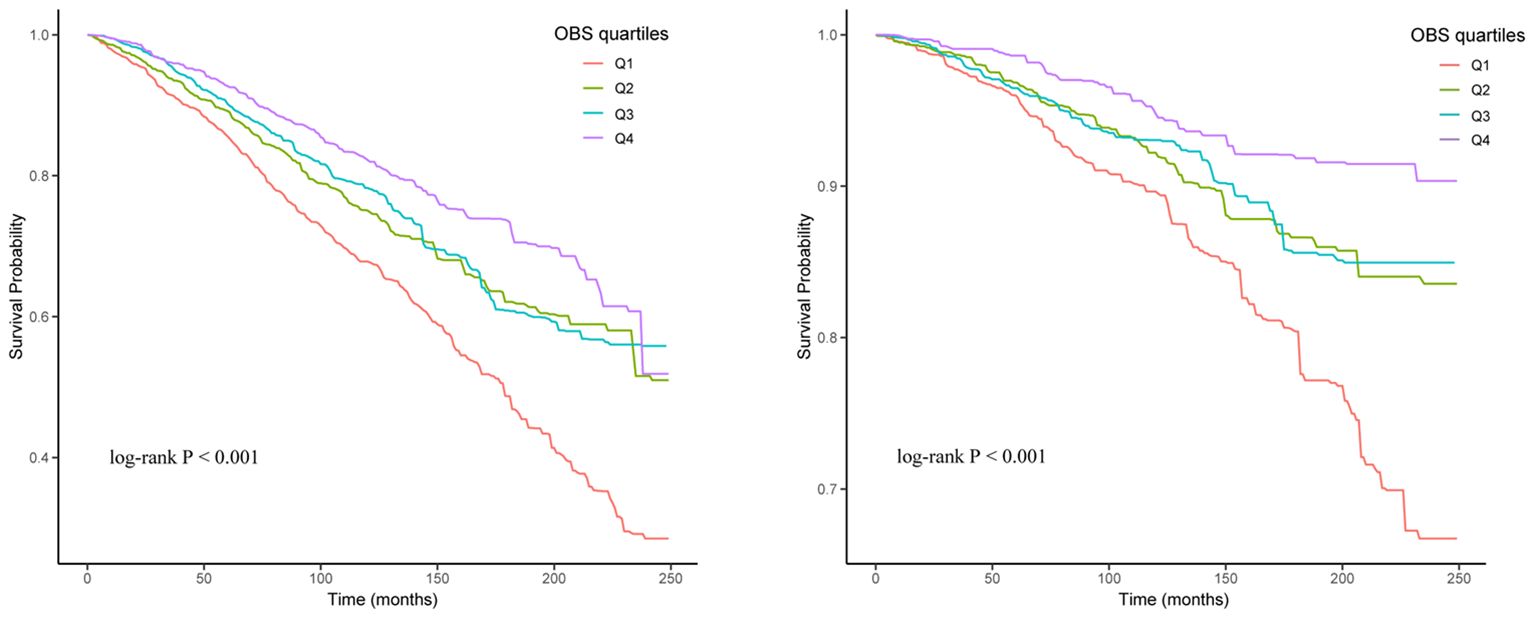
Figure 2 Kaplan-Meier curve of the OBS group for all-cause mortality (Left) and cardiovascular mortality (Right).
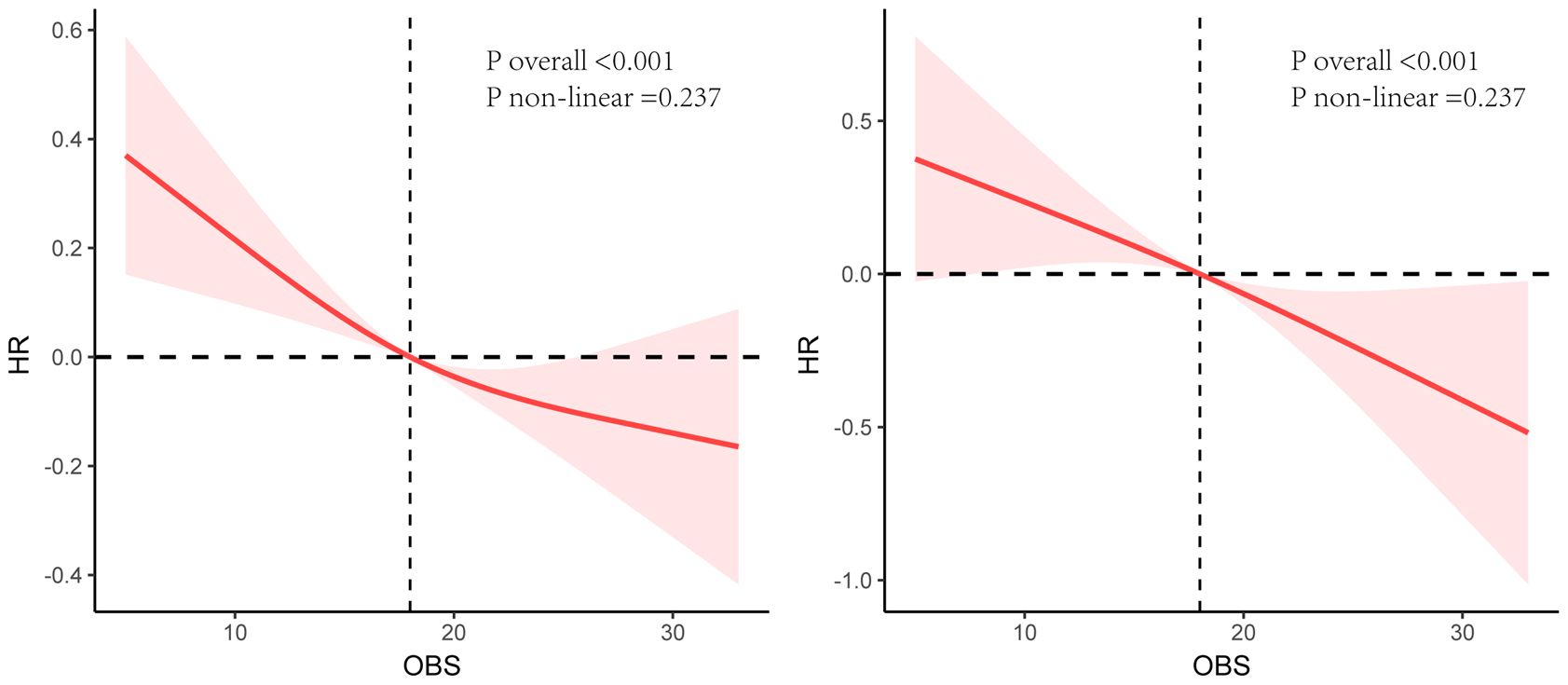
Figure 3 Restricted cubic splines analysis between OBS, all-cause mortality (Left), and cardiovascular mortality (Right).
3.3 Relationships between lifestyle, dietary OBS, ACM, and CVM
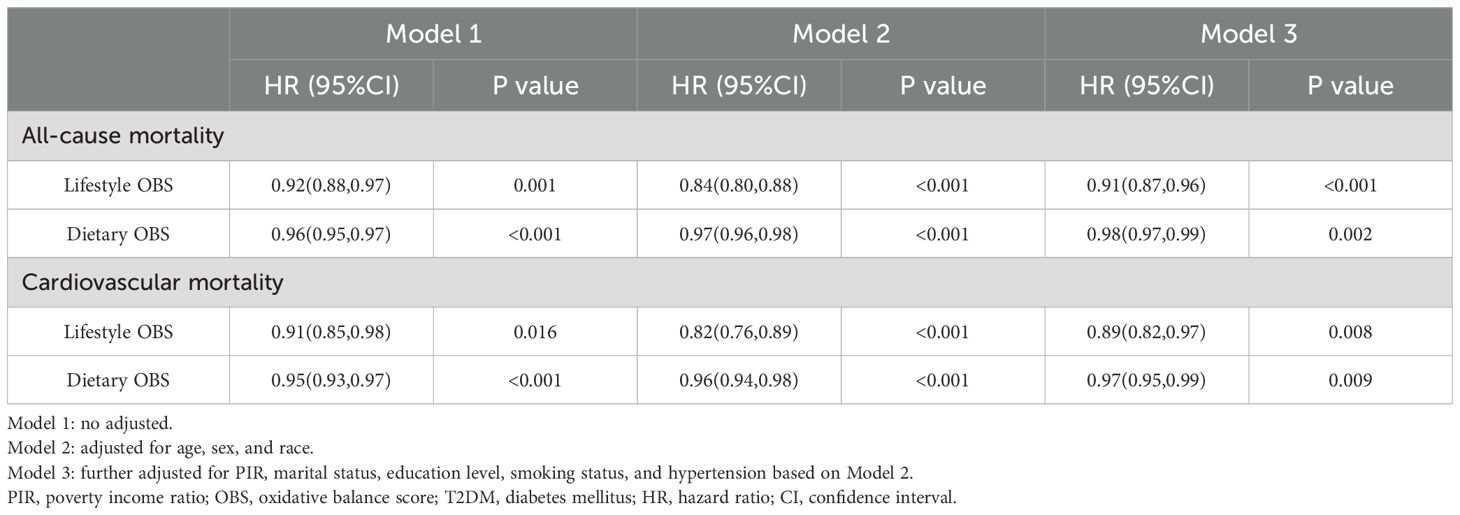
Table 4 illustrates the relationship between lifestyle, dietary OBS, and both ACM and CVM in T2DM. As continuous variables, both dietary OBS and lifestyle OBS were significantly associated with a decreased risk of ACM in T2DM (lifestyle OBS: HR = 0.91, 95% CI 0.87-0.96, P < 0.001; dietary OBS: HR = 0.98, 95% CI 0.97-0.99, P < 0.05) and CVM (lifestyle OBS: HR = 0.89, 95% CI 0.82-0.97, P < 0.05; dietary OBS: HR = 0.97, 95% CI 0.95-0.99, P < 0.05) in fully adjusted Model 3.
3.4 Subgroup analysis
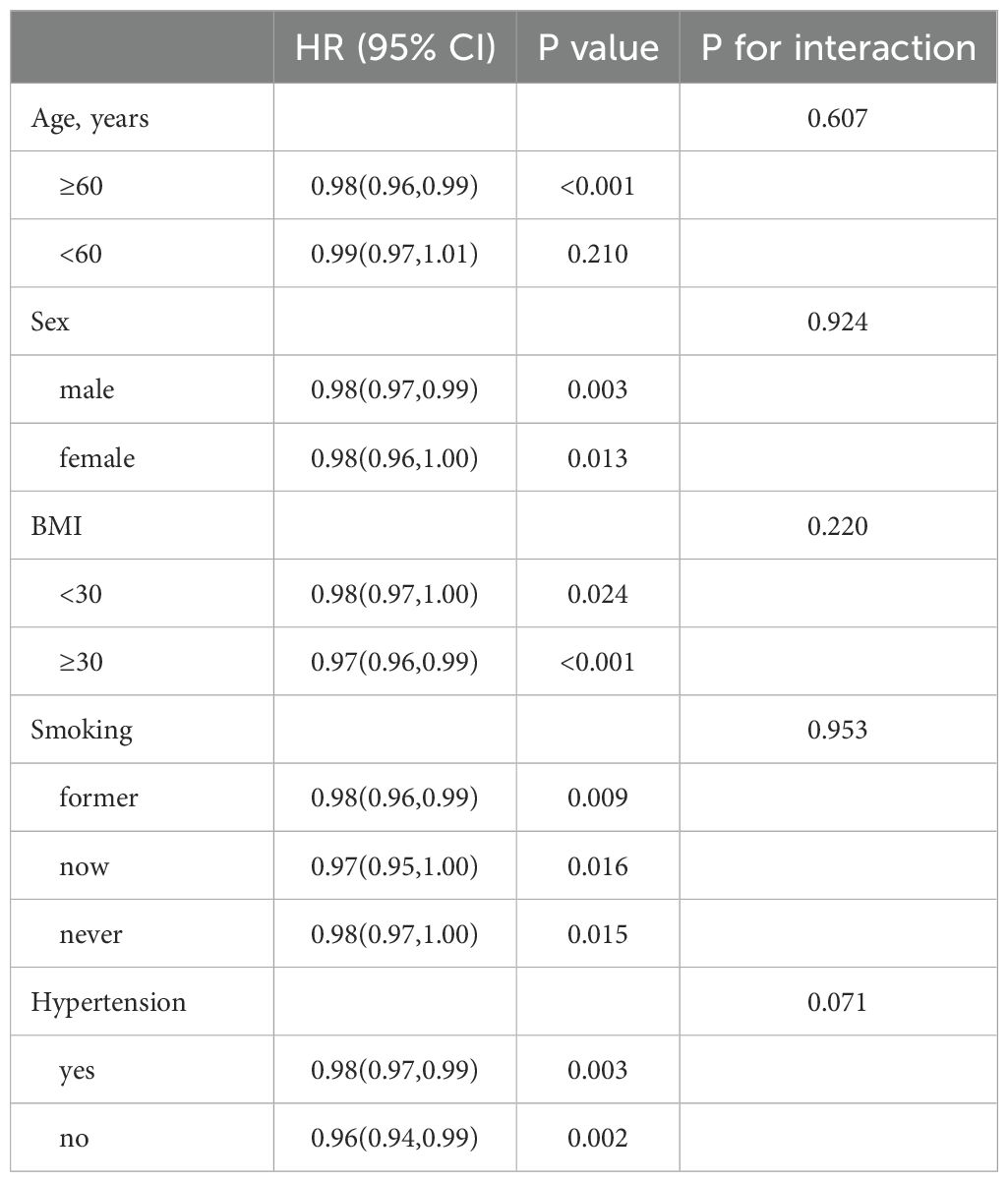
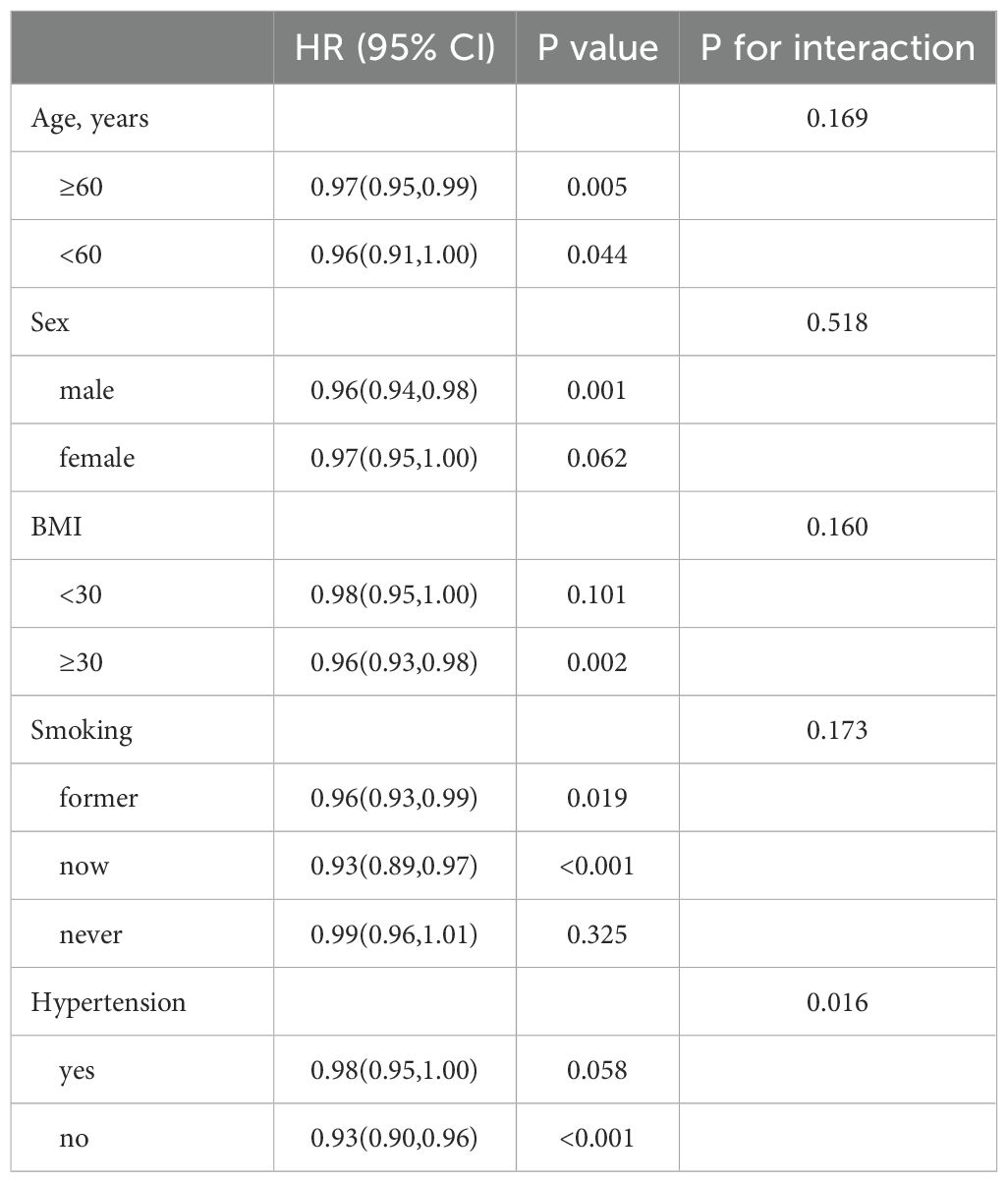
In patients with T2DM, those with high OBS consistently demonstrate lower risks of ACM and CVM across different subgroups based on age, sex, BMI, smoking, and hypertension, as shown in Tables 5, 6. The interaction showed that the presence of hypertensive disorders influenced the negative association between OBS and CVM.
4 Discussion
In this large-scale retrospective study, we identified a significant negative correlation between OBS and both ACM and CVM among T2DM patients, even after adjusting for confounding factors such as age, sex, BMI, smoking, and hypertension. Our findings suggest that higher OBS is associated with a reduced risk of ACM and CVM. These results underscore the importance of promoting health-conscious behaviors, particularly in dietary and lifestyle OBS adjustment, among individuals with T2DM, potentially decreasing ACM and CVM.
The two main features of T2DM are insulin resistance in target tissues and a relative deficiency in insulin production by pancreatic β-cells, and the production of reactive oxygen species (ROS) is closely related to insulin resistance (23). The ROS production and the antioxidant defense system imbalance lead to oxidative stress (OS). The antioxidant defense system can also reduce ROS accumulation, alleviating oxidative stress (24). The interplay between pro-oxidant and antioxidant factors determines an individual’s oxidative balance. Van Hoydonck et al. first introduced the concept of the OBS, which comprehensively assesses this state by considering dietary intake of vitamin C, beta-carotene, and iron (25).
OBS is now widely used in epidemiological research to assess the association between OS and the risk of chronic diseases (26). Studies have found that OBS is variably associated with reduced risks of T2DM, cardiovascular diseases, chronic kidney disease, periodontitis, sleep disorders, colorectal adenomas, and colorectal cancer (8, 9, 13, 14, 27–29). OBS is also helpful in predicting clinical outcomes and is significantly negatively correlated with all-cause, cardiovascular, and cancer mortality (16, 26). Moreover, current research indicates that OBS can serve as a valuable predictor of prognosis. Early identification of high-risk T2DM patients is crucial for improving prognosis. Existing studies suggest a U-shaped association between the triglyceride-glucose (TyG) index and all-cause as well as CVM in US individuals with diabetes or prediabetes (30). Another study indicates that the TyG-BMI index in US elderly diabetic patients is U-shapedly associated with ACM and linearly associated with CVM (31). Physiologically, OBS may influence health outcomes by modulating the state of OS in the body. OS is thought to be one of the key factors in the development of diabetes and its complications because it can damage cellular components, including lipids, proteins, and nucleic acids (32).
Kwon et al. conducted a study involving 7,369 participants aged 40-69 years enrolled in the Korean Genome and Epidemiology Study (33). They found that during a mean follow-up period of 13.6 years, 908 men and 880 women developed T2DM. The conclusion drawn was that individual with high OBS had a lower risk of developing T2DM. Previous studies have suggested the potential role of OBS in the risk of ACM and CVM. Rodriguez et al. conducted a prospective investigation of participants in the Seguimiento Universidad de Navarra Study and found a negative correlation between OBS, ACM and CVM (16). However, this study only focused on university graduates aged 20 and above. The study by Hoydonck et al. targeted male smokers; Kong et al. focused on individuals at high risk of cardiovascular disease; and Mao et al. conducted a similar study among older women in Iowa (25, 34, 35). However, these studies did not consider the relationship between OS and T2DM, nor did they account for the impact of OBS on this specific population. Utilizing the NHANES database, our study benefits from a large amount of observational data and long-term follow-up, taking full advantage of its inclusion of diverse racial backgrounds, educational levels, etc., thus contributing to the current body of research evidence.
In our results, the increase in PIR and education level from Q1 to Q4 in participants is consistent with previous studies, where individuals who are better off or have a higher level of education are more likely to adopt health-conscious behaviors, including a higher intake of antioxidants, which can help to improve their oxidative homeostasis and thus reduce the risk of ACM and CVM (36, 37). In addition, the interaction showed that the presence or absence of comorbid hypertensive disease in patients with T2DM affected the negative association between OBS and CVD. Previous studies have identified that hypertensive disease increases the body’s OS, inflammatory response, and vasoconstriction, and leads to structural changes in the heart and blood vessels and that these changes lead to an increased risk of cardiovascular mortality in patients with T2DM, thereby interfering with OBS (38, 39).
Our study possesses several strengths. Firstly, utilizing the nationally representative NHANES population constitutes the primary and most significant advantage. Secondly, subgroup analysis enhances the robustness of the observed relationship between OBS, ACM, and CVM in T2DM patients, underscoring the reliability of the findings regardless of other cardiovascular risk factors such as age, sex, BMI, smoking, and hypertension. Furthermore, we conducted independent investigations into the influence of lifestyle OBS and dietary OBS on the association between ACM and CVM in T2DM patients.
Our study also exhibits several limitations. Firstly, OBS was only measured at baseline, thereby precluding consideration of potential changes or fluctuations in OBS over time. Consequently, our analysis cannot address longitudinal variations in OBS. To overcome the limitation of measuring OBS only at baseline, future studies may consider prospective cohort studies in which OBS is measured at regular intervals to assess its changes over time and its long-term impact on health outcomes. Secondly, our findings cannot establish causal relationships due to the inherent nature of observational study designs. Moreover, given the intricate interplay of factors influencing the association between T2DM and all-cause and CVM, we endeavored to incorporate numerous covariates, encompassing age, sex, race, PIR, marital status, education level, smoking status, and hypertension. Nevertheless, despite adjusting for these variables, residual confounding factors may persist. Finally, this is a population-based study conducted among T2DM patients in the US. Although it includes 1,198 Mexican Americans, 1,522 Non-Hispanic Black individuals, 2,452 Non-Hispanic White individuals, 526 Other Hispanic individuals, and 421 individuals of other races, our study results may not be generalizable to other populations.
5 Conclusion
For the first time, we revealed an association between OBS, ACM, and CVM among patients with T2DM. We demonstrated that there was a linear association between OBS, ACM, and CVM.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (NSFC-82270848).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ali MK, Pearson-Stuttard J, Selvin E, Gregg EW. Interpreting global trends in type 2 diabetes complications and mortality. Diabetologia. (2022) 65:3–13. doi: 10.1007/s00125-021-05585-2
2. Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. (2010) 8:29. doi: 10.1186/1478-7954-8-29
3. Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, et al. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2020) 162:108086. doi: 10.1016/j.diabres.2020.108086
4. Bei Y, Yang T, Wang L, Holvoet P, Das S, Sluijter JPG, et al. Circular RNAs as potential theranostics in the cardiovascular system. Mol Ther Nucleic Acids. (2018) 13:407–18. doi: 10.1016/j.omtn.2018.09.022
5. Dabravolski SA, Zhuravlev AD, Kartuesov AG, Borisov EE, Sukhorukov VN, Orekhov AN. Mitochondria-mediated cardiovascular benefits of sodium-glucose co-transporter 2 inhibitors. Int J Mol Sci. (2022) 23:5371. doi: 10.3390/ijms23105371
6. Scarale MG, Mastroianno M, Prehn C, Copetti M, Salvemini L, Adamski J, et al. Circulating metabolites associate with and improve the prediction of all-cause mortality in type 2 diabetes. Diabetes. (2022) 71:1363–70. doi: 10.2337/db22-0095
7. Xu Z, Lei X, Chu W, Weng L, Chen C, Ye R. Oxidative balance score was negatively associated with the risk of metabolic syndrome, metabolic syndrome severity, and all-cause mortality of patients with metabolic syndrome. Front Endocrinol. (2023) 14:1233145. doi: 10.3389/fendo.2023.1233145
8. Golmohammadi M, Ayremlou P, Zarrin R. Higher oxidative balance score is associated with better glycemic control among Iranian adults with type-2 diabetes. Int J Vitam Nutr Res Int Z Vitam- Ernahrungsforschung J Int Vitaminol Nutr. (2021) 91:31–9. doi: 10.1024/0300-9831/a000596
9. Ilori TO, Wang X, Huang M, Gutierrez OM, Narayan KMV, Goodman M, et al. Oxidative balance score and the risk of end-stage renal disease and cardiovascular disease. Am J Nephrol. (2017) 45:338–45. doi: 10.1159/000464257
10. Peng L, Li L, Liu J, Li Y. New insights into metabolic dysfunction-associated steatotic liver disease and oxidative balance score. Front Nutr. (2023) 10:1320238. doi: 10.3389/fnut.2023.1320238
11. Li Y, Liu Y. Adherence to an antioxidant diet and lifestyle is associated with reduced risk of cardiovascular disease and mortality among adults with nonalcoholic fatty liver disease: evidence from NHANES 1999-2018. Front Nutr. (2024) 11:1361567. doi: 10.3389/fnut.2024.1361567
12. Liu X, Liu X, Wang Y, Zeng B, Zhu B, Dai F. Association between depression and oxidative balance score: National Health and Nutrition Examination Survey (NHANES) 2005-2018. J Affect Disord. (2023) 337:57–65. doi: 10.1016/j.jad.2023.05.071
13. Lei X, Xu Z, Chen W. Association of oxidative balance score with sleep quality: NHANES 2007-2014. J Affect Disord. (2023) 339:435–42. doi: 10.1016/j.jad.2023.07.040
14. Qu H. The association between oxidative balance score and periodontitis in adults: a population-based study. Front Nutr. (2023) 10:1138488. doi: 10.3389/fnut.2023.1138488
15. Ke R, He Y, Chen C. Association between oxidative balance score and kidney stone in United States adults: analysis from NHANES 2007-2018. Front Physiol. (2023) 14:1275750. doi: 10.3389/fphys.2023.1275750
16. Talavera-Rodriguez I, Fernandez-Lazaro CI, Hernández-Ruiz Á, Hershey MS, Galarregui C, Sotos-Prieto M, et al. Association between an oxidative balance score and mortality: a prospective analysis in the SUN cohort. Eur J Nutr. (2023) 62:1667–80. doi: 10.1007/s00394-023-03099-8
17. Zhang W, Peng S-F, Chen L, Chen H-M, Cheng X-E, Tang Y-H. Association between the oxidative balance score and telomere length from the national health and nutrition examination survey 1999-2002. Oxid Med Cell Longev. (2022) 2022:1345071. doi: 10.1155/2022/1345071
18. Lu Y, Wang M, Bao J, Chen D, Jiang H. Association between oxidative balance score and metabolic syndrome and its components in US adults: a cross-sectional study from NHANES 2011-2018. Front Nutr. (2024) 11:1375060. doi: 10.3389/fnut.2024.1375060
19. Liu Y, Chen M. Dietary and lifestyle oxidative balance scores are independently and jointly associated with nonalcoholic fatty liver disease: a 20 years nationally representative cross-sectional study. Front Nutr. (2023) 10:1276940. doi: 10.3389/fnut.2023.1276940
20. Zhou D, Liu X-C, Kenneth L, Huang Y-Q, Feng Y-Q. A non-linear association of triglyceride glycemic index with cardiovascular and all-cause mortality among patients with hypertension. Front Cardiovasc Med. (2021) 8:778038. doi: 10.3389/fcvm.2021.778038
21. Shen G, Liu Z, Wang L, Li J. Inter-leg systolic blood pressure difference has been associated with all-cause and cardiovascular mortality: analysis of NHANES 1999-2004. BMC Public Health. (2024) 24:1071. doi: 10.1186/s12889-024-18508-8
22. Jia S, Huo X, Liu L, Sun L, Chen X. The predictive value of the prognostic nutritional index for all-cause mortality and cardiovascular mortality in frail population: insights from NHANES 2007-2018. J Nutr Health Aging. (2024) 28:100216. doi: 10.1016/j.jnha.2024.100216
23. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. (2020) 21:6275. doi: 10.3390/ijms21176275
24. Hernández-Ruiz Á, García-Villanova B, Guerra-Hernández EJ, Carrión-García CJ, Amiano P, Sánchez M-J, et al. Oxidative balance scores (OBSs) integrating nutrient, food and lifestyle dimensions: development of the nutrientL-OBS and foodL-OBS. Antioxid Basel Switz. (2022) 11:300. doi: 10.3390/antiox11020300
25. Van Hoydonck PGA, Temme EHM, Schouten EG. A dietary oxidative balance score of vitamin C, beta-carotene and iron intakes and mortality risk in male smoking Belgians. J Nutr. (2002) 132:756–61. doi: 10.1093/jn/132.4.756
26. Hernández-Ruiz Á, García-Villanova B, Guerra-Hernández E, Amiano P, Ruiz-Canela M, Molina-Montes E. A review of A priori defined oxidative balance scores relative to their components and impact on health outcomes. Nutrients. (2019) 11:774. doi: 10.3390/nu11040774
27. Son D-H, Lee HS, Seol S-Y, Lee Y-J, Lee J-H. Association between the oxidative balance score and incident chronic kidney disease in adults. Antioxidants. (2023) 12:335. doi: 10.3390/antiox12020335
28. Kong SYJ, Bostick RM, Flanders WD, McClellan WM, Thyagarajan B, Gross MD, et al. Oxidative balance score, colorectal adenoma, and markers of oxidative stress and inflammation. Cancer Epidemiol biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. (2014) 23:545–54. doi: 10.1158/1055-9965.EPI-13-0619
29. Slattery ML, Lundgreen A, Welbourn B, Wolff RK, Corcoran C. Oxidative Balance and Colon and Rectal Cancer: interaction of lifestyle factors and genes. Mutat Res. (2012) 734:30–40. doi: 10.1016/j.mrfmmm.2012.04.002
30. Zhang Q, Xiao S, Jiao X, Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. (2023) 22:279. doi: 10.1186/s12933-023-02030-z
31. Ding L, Fu B, Zhang H, Dai C, Zhang A, Yu F, et al. The impact of triglyceride glucose-body mass index on all-cause and cardiovascular mortality in elderly patients with diabetes mellitus: evidence from NHANES 2007-2016. BMC Geriatr. (2024) 24:356. doi: 10.1186/s12877-024-04992-5
32. Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. (2015) 4:180–3. doi: 10.1016/j.redox.2015.01.002
33. Kwon Y-J, Park H-M, Lee J-H. Inverse association between oxidative balance score and incident type 2 diabetes mellitus. Nutrients. (2023) 15:2497. doi: 10.3390/nu15112497
34. Kong SY, Goodman M, Judd S, Bostick RM, Flanders WD, McClellan W. Oxidative balance score as predictor of all-cause, cancer, and noncancer mortality in a biracial US cohort. Ann Epidemiol. (2015) 25:256–262.e1. doi: 10.1016/j.annepidem.2015.01.004
35. Mao Z, Prizment AE, Lazovich D, Bostick RM. Associations of dietary and lifestyle oxidative balance scores with mortality risk among older women: the Iowa Women’s Health Study. Eur J Nutr. (2021) 60:3873–86. doi: 10.1007/s00394-021-02557-5
36. James WP, Nelson M, Ralph A, Leather S. Socioeconomic determinants of health. The contribution of nutrition to inequalities in health. BMJ. (1997) 314:1545–9. doi: 10.1136/bmj.314.7093.1545
37. Kilander L, Berglund L, Boberg M, Vessby B, Lithell H. Education, lifestyle factors and mortality from cardiovascular disease and cancer. A 25-year follow-up of Swedish 50-year-old men. Int J Epidemiol. (2001) 30:1119–26. doi: 10.1093/ije/30.5.1119
38. Franco C, Sciatti E, Favero G, Bonomini F, Vizzardi E, Rezzani R. Essential hypertension and oxidative stress: novel future perspectives. Int J Mol Sci. (2022) 23:14489. doi: 10.3390/ijms232214489
Keywords: oxidative balance score, cardiovascular, all-cause mortality, type 2 diabetes mellitus, dietary
Citation: Ni C, Wang X, Zhou Y, Wang Q, Cai Z, Wang H, Chen Y, Liu Y and Sun Z (2024) Association of oxidative balance score, cardiovascular, and all-cause mortality among patients with type 2 diabetes mellitus. Front. Endocrinol. 15:1429662. doi: 10.3389/fendo.2024.1429662
Received: 08 May 2024; Accepted: 06 August 2024;
Published: 20 August 2024.
Edited by:
Lu Cai, University of Louisville, United StatesReviewed by:
Dae Young Cheon, Hallym University Dongtan Sacred Heart Hospital, Republic of KoreaSimina Crisan, Victor Babes University of Medicine and Pharmacy, Romania
Ying Zhang, Guangdong Provincial People’s Hospital, China
Copyright © 2024 Ni, Wang, Zhou, Wang, Cai, Wang, Chen, Liu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zilin Sun, c3VuemlsaW4xOTYzQHNldS5lZHUuY24=; Xiaohang Wang, d2FuZ3hpYW9oYW5nMTIzNEBmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Chengming Ni
Chengming Ni Xiaohang Wang
Xiaohang Wang Yunting Zhou4
Yunting Zhou4 Zhensheng Cai
Zhensheng Cai Zilin Sun
Zilin Sun