- 1Shandong Provincial Hospital, Shandong University, Jinan, Shandong, China
- 2The First Clinical Medical College, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 3Department of Endocrinology and Metabology, Shandong Provincial Qianfoshan Hospital, Jining Medical University, Shandong Key Laboratory of Rheumatic Disease and Translational Medicine, Shandong Institute of Nephrology, Jinan, Shandong, China
- 4Department of Endocrinology and Metabology, The First Affiliated Hospital of Shandong First Medical University and Shandong Provincial Qianfoshan Hospital, Shandong Key Laboratory of Rheumatic Disease and Translational Medicine, Shandong Institute of Nephrology, Jinan, Shandong, China
- 5Department of Endocrinology and Metabology, Shandong Provincial Qianfoshan Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
Objective: The aim of the study was to evaluate the effect of dipeptidyl peptidase-4 inhibitors (DPP4i) on cardiac structure and function by cardiac magnetic resonance (CMR). Research Methods & Procedures: Database including PubMed, Cochrane library, Embase and SinoMed for clinical studies of DPP4i on cardiac structure and function by CMR were searched. Two authors extracted the data and evaluated study quality independently. Mean difference (MD) or standardized MD and 95% confidence intervals (CI) were used for continuous variables. Review Manager 5.3 was used to performed the analysis.
Results: Ten references (nine studies) were included in this meta-analysis. Most of the studies were assessed as well quality by the assessment of methodological quality. For clinical control studies, the merged MD values of △LVEF by fixed-effect model and the pooled effect size in favor of DPP4i was 1.55 (95% CI 0.35 to 2.74, P=0.01). Compared with positive control drugs, DPP4i can significantly improve the LVEF (MD=4.69, 95%CI=2.70 to 6.69), but no such change compared to placebo (MD=-0.20, 95%CI=-1.69 to 1.29). For single-arm studies and partial clinical control studies that reported LVEF values before and after DPP4i treatment, random-effect model was used to combine effect size due to a large heterogeneity (Chi2 = 11.26, P=0.02, I2 = 64%), and the pooled effect size in favor of DPP4i was 2.31 (95% CI 0.01 to 4.62, P=0.05). DPP4i significantly increased the Peak filling rate (PFR) without heterogeneity when the effect sizes of two single-arm studies were combined (MD=31.98, 95% CI 13.69 to 50.27, P=0.0006; heterogeneity test: Chi2 = 0.56, P=0.46, I2 = 0%).
Conclusions: In summary, a possible benefit of DPP4i in cardiac function (as measured by CMR) was found, both including ventricular systolic function and diastolic function.
1 Introduction
Patients with type 2 diabetes mellitus (T2DM) have a more than doubled risk of developing cardiovascular disease (CVD) than those without (1). More than half of the deaths in DM patients are caused by CVD (2). In addition to CVD, the pathogenesis of cardiac dysfunction caused by diabetes is quite complex, involving cellular, molecular and structural abnormalities. Diabetic cardiomyopathy is also a very important mechanism and is a type of systolic and diastolic dysfunction different from diabetic microangiopathy. Early active and effective hypoglycemic therapy can reduce the risk of complications (including microvascular and macrovascular events) or death in newly diagnosed diabetic patients (3–7). Since 2008, none of the hypoglycemic drugs have shown any cardiovascular safety concerns compared to placebo. Even some hypoglycemic drugs have shown unique cardiovascular benefits (8). Numerous clinical studies have shown evidence of cardiovascular benefits of glucagon-like peptide 1 (GLP-1) receptor agonist compared to placebo (9–15). Dipeptidyl peptidase-4 inhibitors (DPP4i), one of the commonly used hypoglycemic drugs, can improve blood glucose control in T2DM patients by inhibiting the degradation of glucopeptide-1 and glucopeptide-dependent insulin polypeptides, prolongating the action time of endogenous hormones, inhibiting glucagon levels and increasing endogenous insulin secretion (16). However, the cardiovascular effects of DPP4i in patients with diabetes remain unclear. Some studies have shown that DPP4i reduce the risk of adverse cardiovascular events, while others have been neutral about this effect (17–19).
For clinical trials, endocardial biopsy is the main method to evaluate the changes of myocardial pathological in patients. However, due to such shortcomings as the invasiveness, sampling errors, serious complications and poor consistency between observers, evidence of myocardial pathological in heart disease in human is rare (20, 21). For patients with heart diseases or high risk of heart diseases, it is important to evaluate myocardial tissue or cardiocytes by a non-invasive technique. In recent years, cardiac magnetic resonance (CMR) has been widely used in the diagnosis and prognosis assessment of various heart diseases, and is the most commonly used imaging method to evaluate myocardial damage such as myocardial edema and fibrosis (22, 23). Previous studies have found myocarditis (24, 25), dilated cardiomyopathy (26), myocardial infarction (27, 28), heart failure (29, 30) and other heart diseases that have potential myocardial damage by CMR technology, and they were well correlated with myocardial histopathological changes. Early monitoring of global or local myocardial systolic function abnormalities provides important information for early diagnosis and prognosis evaluation of cardiovascular diseases and cardiomyopathy (31). Appling CMR, a sensitive and non-invasive technique, to evaluate the benefit of hypoglycemic drugs, especially the controversial DPP4i, in heart disease may be a good suggestion.
Therefore, this study searched and summarized the previous application of CMR technology to verify the effects of DPP4i on cardiac structure and function, so as to bring better evidence-based medical evidence for the cardiac benefits of DPP4i.
2 Materials and methods
This meta-analysis was conducted under the guidance of the Preferred Reporting Items Statement for Systematic Evaluation and Meta-Analysis (PRISMA).
2.1 Searching progress
We searched the following databases for clinical studies of DPP4i on cardiac structure and function by CMR: PubMed, Cochrane library, Embase and SinoMed, for clinical studies. A list of references to all eligible articles and related review articles was also manually searched. A literature search of this meta-analysis was limited to published results. Databases were searched from the earliest data to 29 January 2024 with the following search terms: (“Dipeptidyl-Peptidase IV Inhibitors” OR “DPP-4 Inhibitor*” OR “DPP 4 inhibitor*” OR “DPP4 inhibitor*” OR “Dipeptidyl peptidase-4 inhibitor*” OR “DPP-IV Inhibitor*” OR “DPP IV Inhibitor*” OR “saxagliptin” OR “sitagliptin” OR “vildagliptin “ OR “linagliptin” OR “alogliptin” OR “anagliptin” OR “gemigliptin” OR “teneligliptin”) AND (“Cardiac Imaging Techniques” OR “CMR” OR “cardiac magnetic resonance” OR “cardiac MRI imaging” OR “cardiac MR imaging”). Eligible studies were screened and selected based on the following criteria (1): published in English or Chinese (2); evaluated the effect of DPP4i on cardiac structure and function by CMR (3); clinical study (either clinical control study or single-arm study) (4); reported at least one outcome of cardiac structure and function by CMR.
2.2 Study selection and data extraction
The studies were screened independently by two authors, and any differences were resolved by consensus. If there is still doubt, a third experienced author was invited to join the consultation and reach a consensus finally. The following data were extracted from the eligible studies (1): characteristic of populations, interventions, number of participants (2); follow-up time (3); MR system (4); outcome index.
2.3 Methodological quality assessment
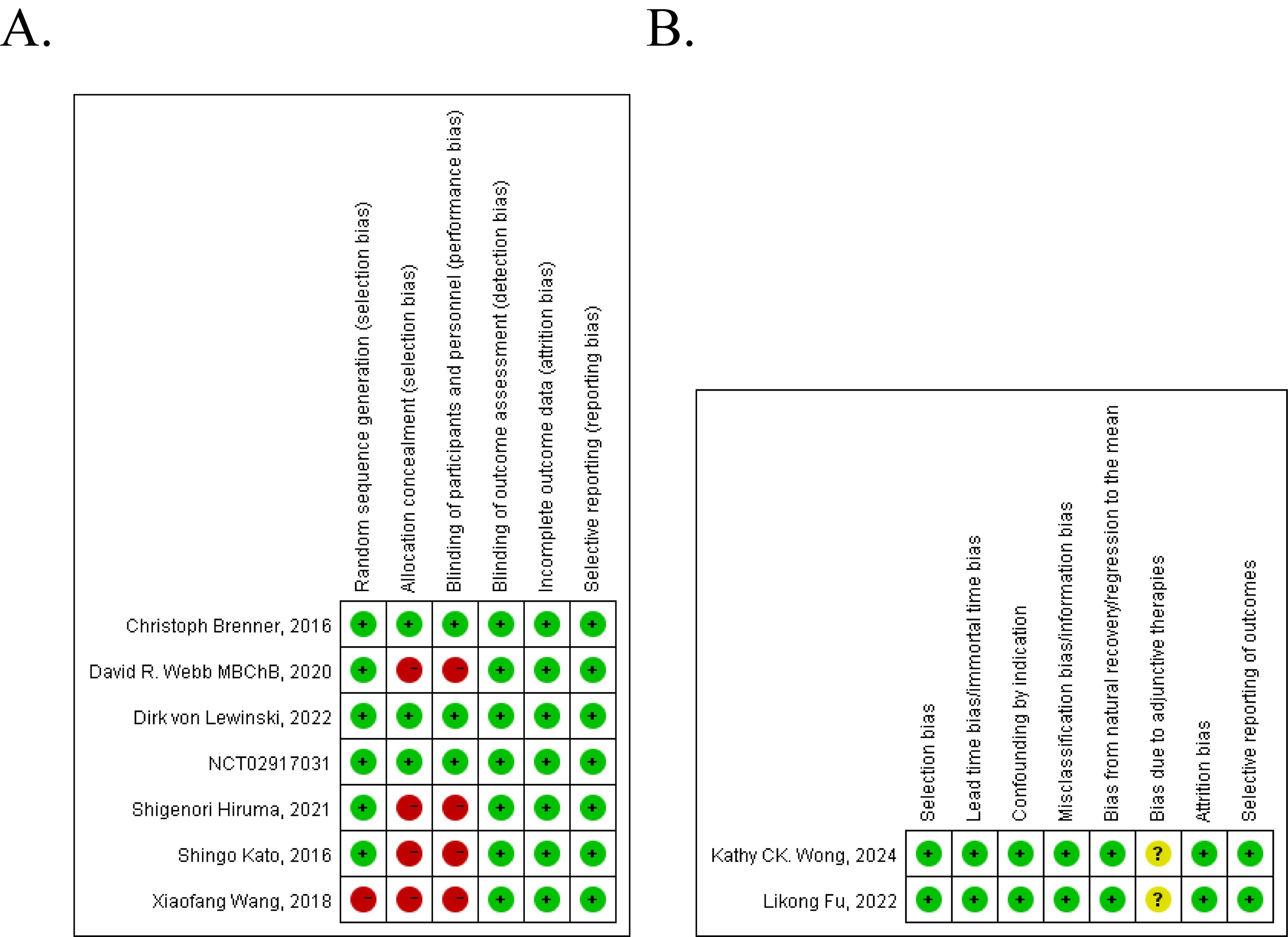
Risk of bias is used for randomized/clinical control studies to assess the methodological quality. A designed tool for assessing risk of bias in single-arm studies considered the following items: selection bias, lead time bias/immortal time bias, confounding by indication, misclassification bias/information bias, bias from natural recovery/regression to the mean, bias due to adjunctive therapies, attrition bias, selective reporting of outcomes. For risk of bias table, a point of 4 or less is considered “poor methodological quality”, while a point above 4 is defined as “good methodological quality”. Two authors scored these items independently.
2.4 Statistical analysis
The main outcome was the change of LVEF over the treatment duration. We also analyzed the change of other index that reflect the cardiac structure and function (including left ventricular function parameters, right ventricular function parameters, heart fat content and myocardial strain parameter) by CMR technique. For continuous variables, mean difference (MD), standardized MD and 95% confidence intervals (CI) were used. Fixed-effect model was used for data analysis. The I2 was calculated as an indicator of the inter-study heterogeneity. If the heterogeneity test showed a large heterogeneity, the random-effects model was replaced. All data analysis was performed by Review Manager 5.3 (Cochrane Collaboration, United Kingdom, http://www.cochrane.org).
3 Results
3.1 Search results and characteristics of included studies
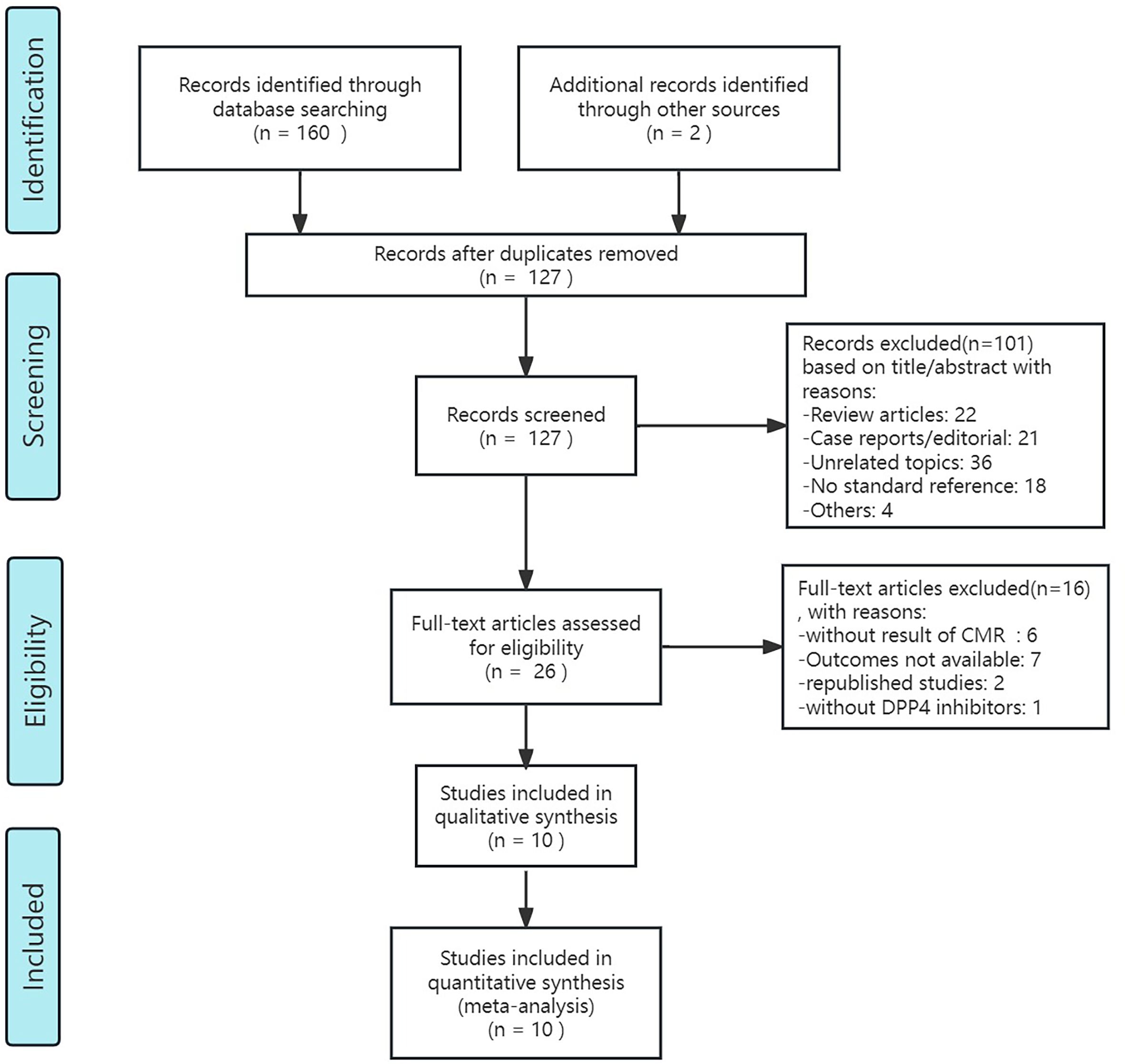
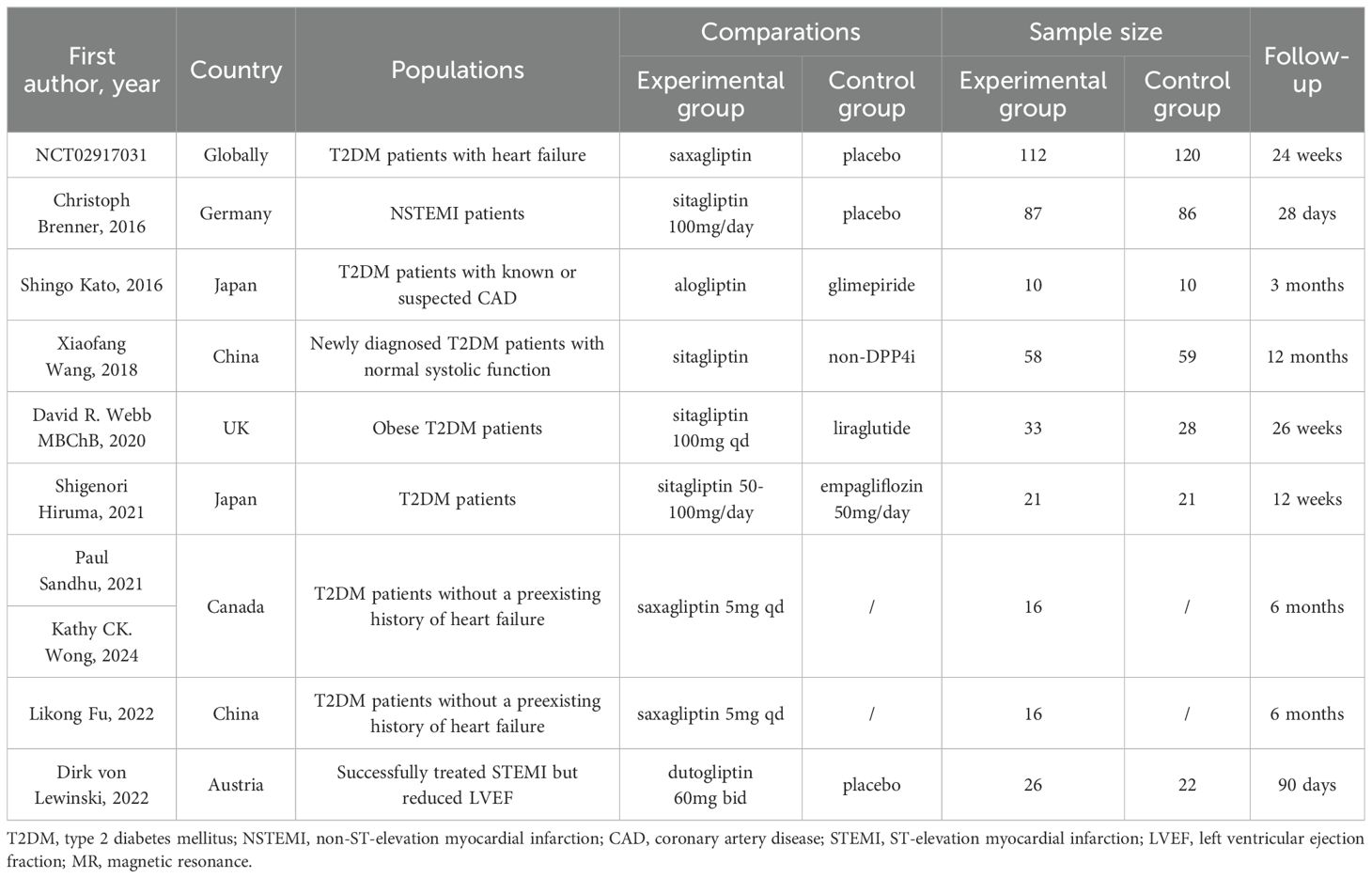
After retrieval form the database showed above and re-checking, 127 articles of potentially relevant studies need further classified. After screening the abstract, 26 articles were required to be read in full. Of these, 10 articles (9 studies) were eligible (32–41). Figure 1 shows the searching progress. Seven studies are published in English (34–41), the rest two were Chinese (32, 33). Two countries, China and Japan, each had two studies included. Canada, the UK, Germany and Australia each had one study included. And a clinical study conducted simultaneously in several countries around the world was also included in the article. Two of the studies included patients with myocardial infarction (36, 41), while the remaining studies included patients with type 2 diabetes. These studies included diabetic patients with heart failure (37, 40) or without history of heart failure (32–34, 39), or with known or suspected cardiovascular disease (38), or obese T2DM patients (35). In the nine included studies, different DPP4i were applied, including saxagliptin, sitagliptin, alogliptin and dutogliptin, and the doses vary. Totally, for clinical control studies, there are 347 patients received DPP4i treatment and 346 patients assigned to the control group. And the sample size ranges from 10 to 112 in DPP4i treatment group while 10 to 120 in control group (32, 35, 36, 38–41). For single-arm studies, 32 patients were included totally (33, 34, 37). The follow-up ranged from 28 days to 26 weeks. Table 1 summarizes the detailed characteristics of these nine included studies.
3.2 Quality assessment of included studies
The quality assessment of these nine studies is shown in Figure 2. Of the seven controlled clinical studies, four studies got a point of 4 or less and were considered to be of poor methodological quality. The rest three studies were considered as good (Figure 2A). For two single-arm studies, 7 points were obtained, which we can consider to be of high methodological quality (Figure 2B).
3.3 Effect on global cardiac function
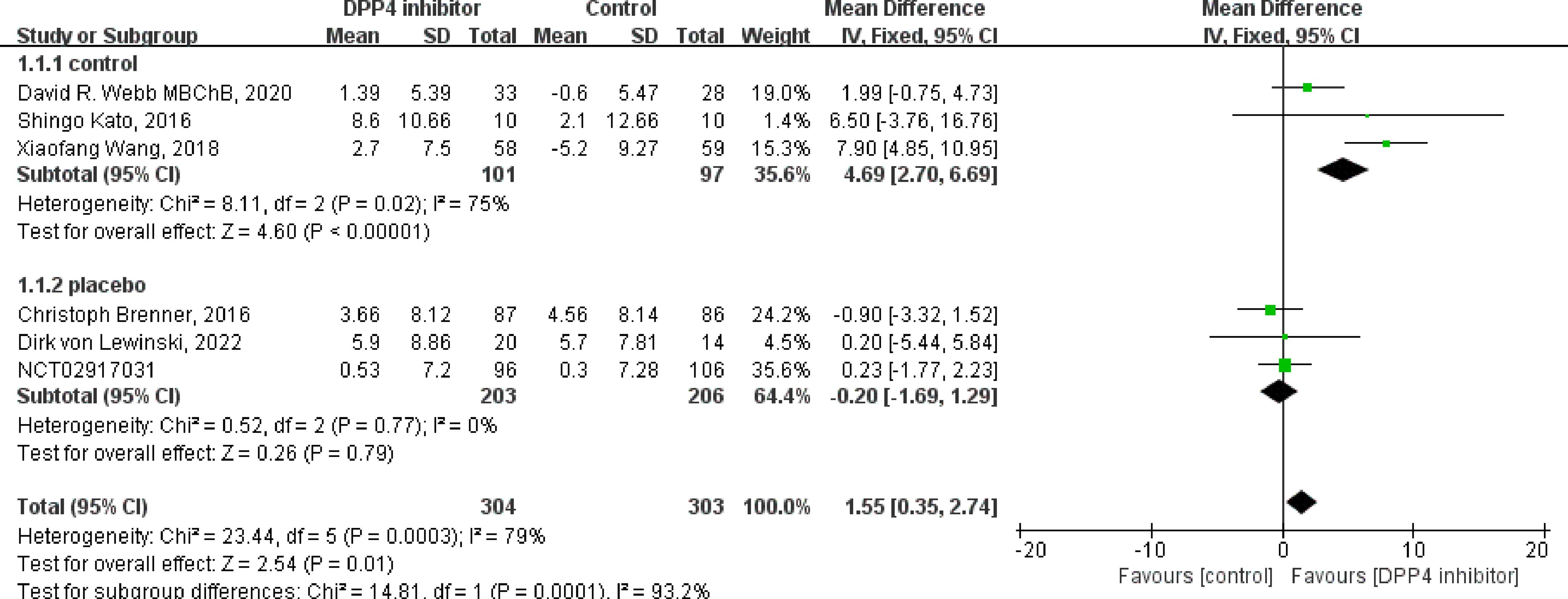
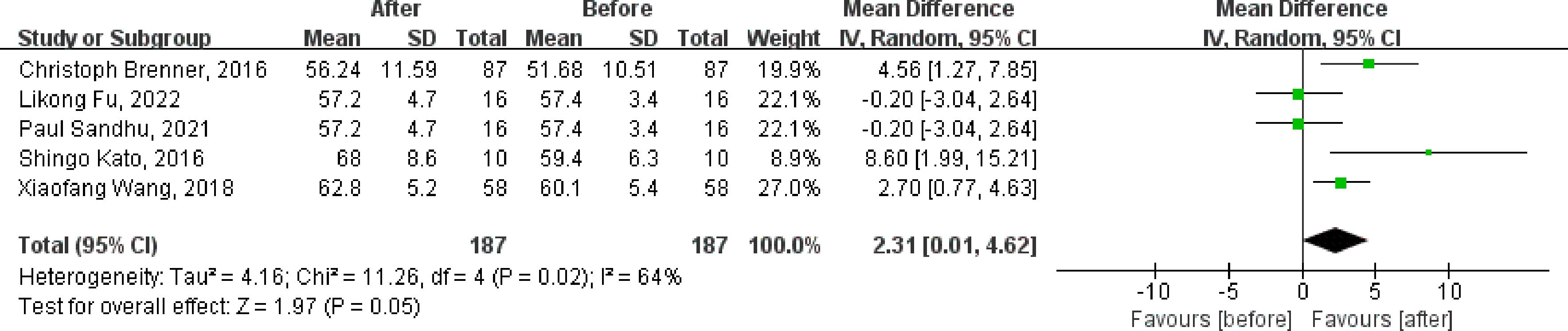
Of the nine included studies, eight studies reported the LVEF as an outcome, and six were clinical control studies (32, 35, 36, 38, 40, 41), five studies reported LVEF both before and after DPP4i treatment, respectively (32, 33, 37, 38, 41). Totally, for clinical control studies, the merged MD values of ΔLVEF by fixed-effect model and the pooled effect size in favor of DPP4i was 1.55 (95% CI 0.35 to 2.74, P=0.01). Heterogeneity analysis showed a huge heterogeneity (Chi2 = 23.44, P=0.0003, I2 = 79%) (Figure 3). Subgroup analysis was performed according to whether the control group was a placebo or not. Compared with positive control drugs, DPP4i can significantly improve the LVEF (MD=4.69, 95%CI=2.70 to 6.69), whereas, a big heterogeneity existed (Chi2 = 8.11, P<0.00001, I2 = 75%). However, there was no such change compared to placebo (MD=-0.20, 95%CI=-1.69 to 1.29). For single-arm studies and partial clinical control studies that reported LVEF values before and after DPP4i treatment, random-effect model was used to combine effect size due to a large heterogeneity (Chi2 = 11.26, P=0.02, I2 = 64%), and the pooled effect size in favor of DPP4i was 2.31 (95% CI 0.01 to 4.62, P=0.05), which indicated that DPP4i could improve LVEF (Figure 4). Overall, from the above results, we can still see the trend of DPP4i’s effect on improving LVEF.
Four studies reported the RVEF value (33, 36, 37, 41), two were clinical control studies (36, 41) and three analyzed the outcome both before and after DPP4i treatment (33, 37, 41). For clinical control studies, the merged MD values of △RVEF by fixed-effect model and the pooled effect size was 0.99 (95% CI -1.64 to 1.83, P=0.92). No heterogeneity was found in the heterogeneity test (Chi2 = 0.76, P=0.38, I2 = 0%). For three single-arm studies, no changes in RVEF were found before and after treatment (MD=-0.06, 95% CI -1.67 to 1.55, P=0.94), and no heterogeneity existed (Chi2 = 0.03, P=0.98, I2 = 0%). In total, DPP4i has no effect on RVEF.
3.4 Impact on further left ventricular structure and function parameters
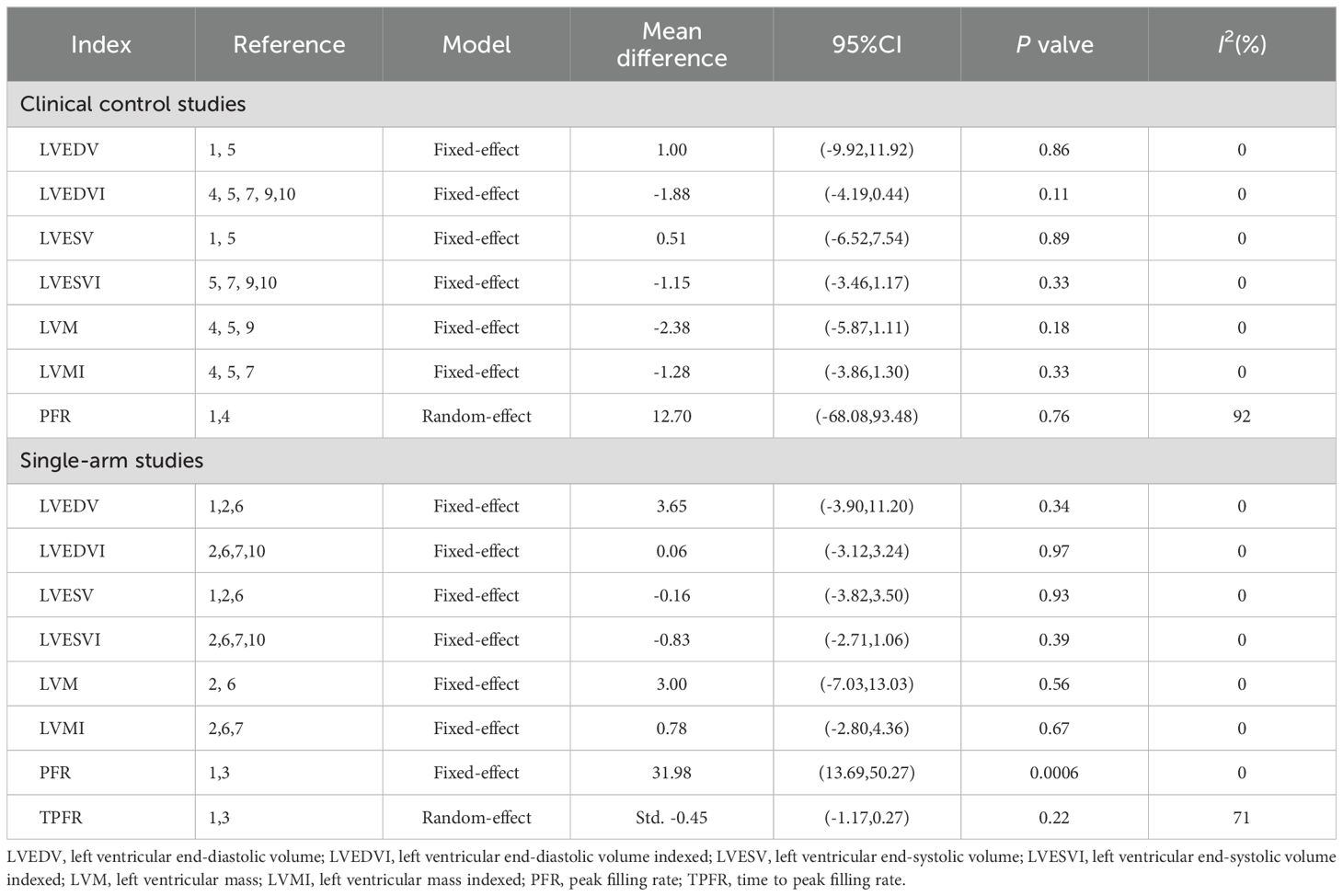
Other outcome representing left ventricular structure and function parameters, such as left ventricular end-diastolic volume (LVEDV), left ventricular end-diastolic volume indexed (LVEDVI), left ventricular end-systolic volume (LVESV), left ventricular end-systolic volume indexed (LVESVI), left ventricular mass (LVM), left ventricular mass indexed (LVMI), peak filling rate (PFR) and time to peak filling rate (TPFR) have also been reported and been further analyzed here. Both in two types of studies, we have not observed the effect of DPP4i on the LVEDV, LVEDVI, LVESVI, LVESV, LVM and LVMI, namely DPP4i don’t change these left ventricular structure and function parameters (Table 2). It was found that DPP4i significantly increased the PFR without heterogeneity when the effect sizes of two single-arm studies were combined (MD=31.98, 95% CI 13.69 to 50.27, P=0.0006; heterogeneity test: Chi2 = 0.56, P=0.46, I2 = 0%) (32, 34). But this result has not been confirmed when combined the results from two clinical control studies (P=0.76) (32, 35).
3.5 Impact on further right ventricular structure and function parameters
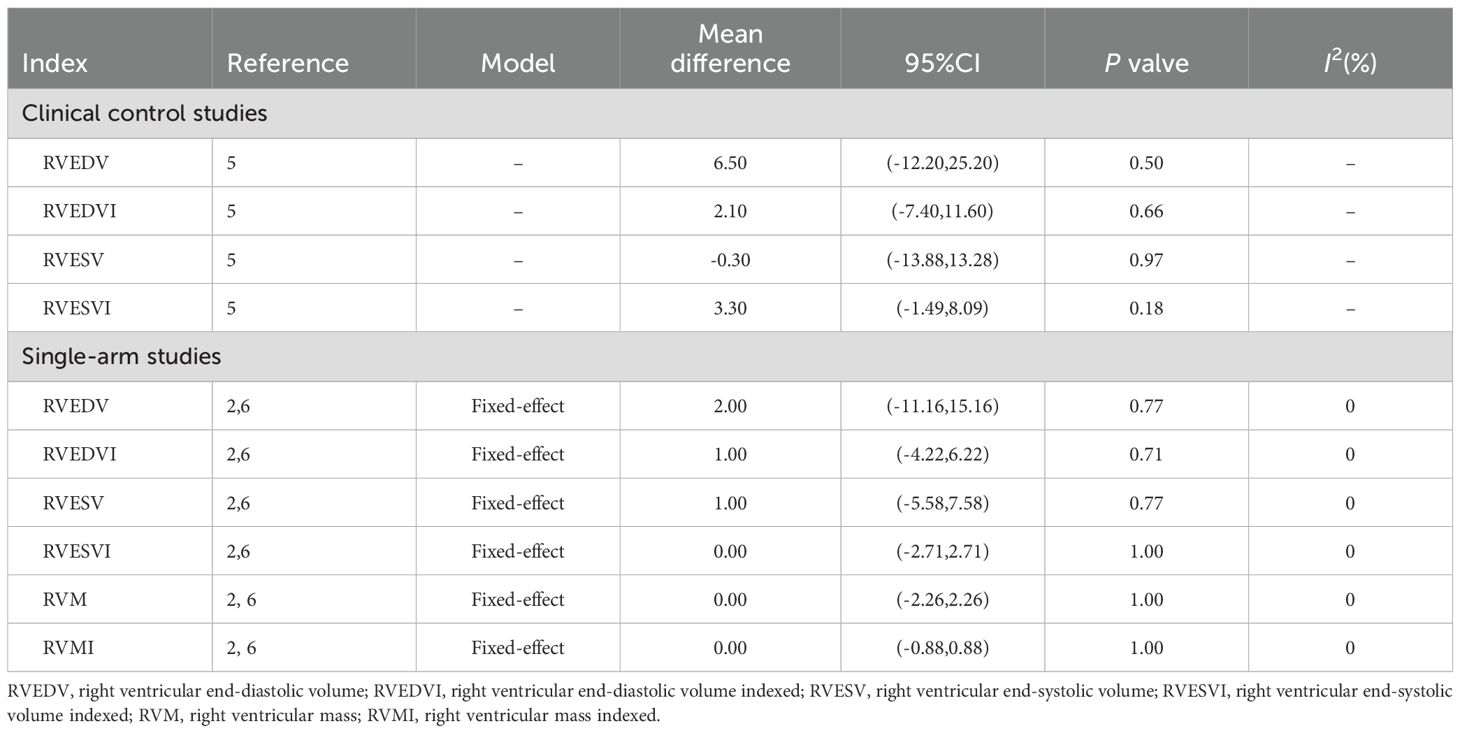
Only one clinical control study reported the change of right ventricular end-diastolic volume (RVEDV), right ventricular end-diastolic volume indexed (RVEDVI), right ventricular end-systolic volume (RVESV), right ventricular end-systolic volume indexed (RVESVI) compare to control group (36). At the same time, two single-arm studies reported the above indicators both before and after treatment (33, 37). Both two study types, DPP4i did not significantly change the above right ventricular structure and functional parameters and DPP4i also did not alter the RVM and RVMI in self-controlled single-arm trials. The details were shown in Table 3.
3.6 Other parameters
Shigenori Hiruma et al. reported the changes in accumulation of pericardial fat and myocardial triglyceride content between sitagliptin and empagliflozin groups (39). DPP4i did not significantly change the heart fat content when the statistics were pooled (accumulation of pericardial fat: MD=-79.8, 95%CI -190.13 to 30.53, P=0.16; myocardial triglyceride content: MD=0.80, 95%CI -2.49 to 4.09, P=0.63). Results of myocardial strain parameters (including global radial strain (GRS), global circumferential strain (GCS), and global longitudinal strain (GLS)) were reported in two single-arm studies (33, 37), but no changes were found.
3.7 Publication bias
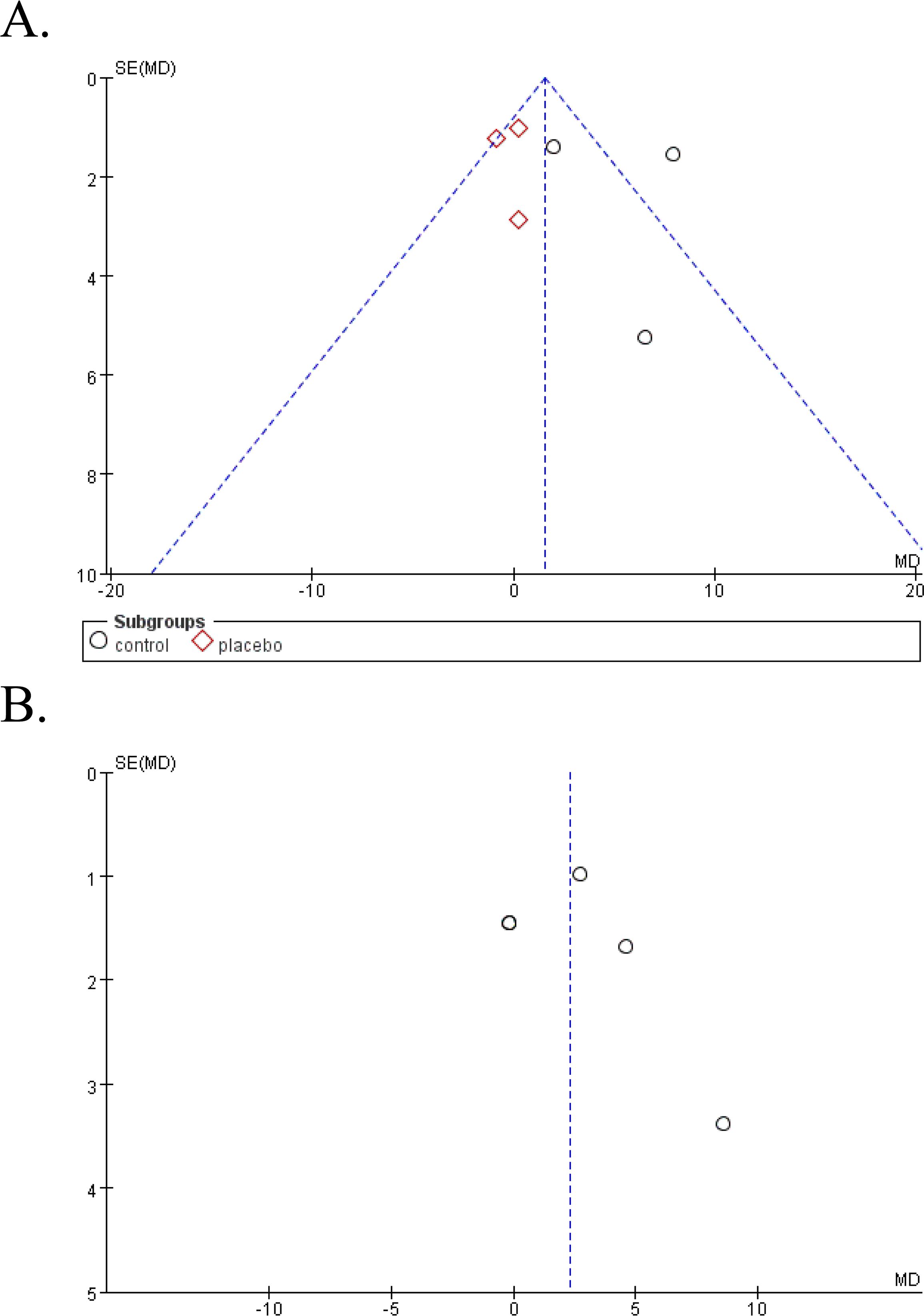
Funnel plot was done to show the publication bias and results were shown in Figure 5. Due to the limited numbers of included studies, selection bias is significant but inevitable.
4 Discussion
Previous clinical studies have suggested the cardiac safety and even cardiac benefits of a variety of hypoglycemic drugs, including DPP4i. Through this study, we found that DPP4i can indeed improve the level of LVEF, and the results from single-arm studies suggest the potential role of DPP4i in improving PFR.
LVEF is an indicator of left ventricular systolic dysfunction, which is of great significance for the prognosis of heart disease. Studies conducted by Read et al. have shown that DPP4i can increase the LVEF assessed by dobutamine stress in patients with ischemic heart disease (42). In addition, echocardiography is a clinical first-line imaging examination of cardiovascular diseases, mainly used to evaluate the heart structure, function and hemodynamics, is currently the highest time resolution of non-invasive imaging technology, little affected by heart rate and rhythm. Compared with other imaging methods, echocardiography has the advantages of real-time, dynamic, convenient and economical, but low spatial resolution, low signal-to-noise ratio, small scanning field of view and large operator dependence are its main shortcomings. Obviously, the use of echocardiography to evaluate the effect of DPP4i on LVEF has also been adopted by some scholars, and the effect of DPP4i on improving LVEF has also been reported (32, 43). However, some studies have come to the opposite conclusion (44). Could this be due to the limitations of echocardiography? Compared with echocardiography, CMR has higher spatial resolution, can accurately delineate the endocardium and the epicardium, and then obtain more accurate cardiac function parameters, and it has become the recognized gold standard for non-invasive evaluation of cardiac structure and function (45, 46). Therefore, CMR was used to evaluate left ventricular systolic function in this study, which has a high sensitivity and makes the study results more accurate. However, the mechanism by which DPP4i increase the level of LVEF is not fully understand. According to Frank-Starling’s law, an increase in LVEDV leads to an increase in ejection fraction. However, our study found that despite an increase in LVEF, there was no increase in LVEDV. It has also been suggested that anti-inflammatory effect may be one of the mechanisms, but it has not been widely verified and recognized (38, 47). Another more accepted theory may be that because DPP4i can increase endogenous GLP-1 and glucose-dependent insulin stimulating polypeptide concentrations by inhibiting DPP4 enzyme activity, and enhance their effects. GLP-1 agonists have been shown to increase the level of LVEF (44). This may due to its role in regulating PI3K/Akt1 and AMPKα signaling that to inhibit angiotensin II and pressure overloading inducing cardiac remodeling (48). Wang et al. (49) also found that the cardioprotective effect of GLP-1 agonists may depend on the inhibition of oxidative stress through the mammalian target rapamycin complex 1/p70 ribosomal protein S6 kinase pathway. In experimental animal models of heart failure, GLP-1 agonists protect the heart during acute ischemia and improve mitochondrial function, microvascular function and myocardial glucose uptake (50, 51). GLP-1 agonists were found in left ventricular cardiomyocytes, thus it may have a direct effect on the ventricle (52). In addition, GLP-1 agonists can reduce inflammation, reduce ischemic injury, increase heart rate, promote plaque stabilization, and reduce smooth muscle proliferation (53). Studies have also shown that the positive effects of GLP-1 agonists on cardiovascular diseases may be the result of direct action on the arteriosclerosis process (54). Therefore, the principal mechanisms underlying this cardioprotection likely involve the suppression of cardiac oxidative stress, apoptosis, ferroptosis, necroptosis, and pyroptosis, which may also directly contribute to enhanced cardiac resistance to ischemia/reperfusion (I/R) injury (55). Furthermore, DPP4i mitigate the progression of diabetic microvascular and cardiovascular complications by reversing alterations associated with hyperglycemic memory. This process is linked to epigenetic modifications, which represent a significant area of current research within the context of metabolic memory phenomena related to diabetic complications (56).
PFR is an important indicator of diastolic function, which decreases when this function is impaired. Diabetic cardiomyopathy is strongly linked to diastolic dysfunction (57). Our research indicates that DPP4i can enhance PFR, highlighting its potential as a treatment for diastolic dysfunction. Earlier studies found that sitagliptin delayed left ventricular diastolic dysfunction in diabetic mice (58). In essence, DPP4i effectively enhances cardiac function.
However, this meta-analysis has some limitations. Only nine studies were included, potentially introducing biases and methodological errors due to their varying designs and poor methodological quality. The small number of studies in subgroup analyses could impact the findings’ reliability. Additionally, DPP4i directly affects ventricular function, but changes in body weight, blood pressure, and waist size could indirectly influence cardiometabolic parameters. While DPP4i’s cardiac benefits seem consistent in patients with or without CVD or HF history, but more research is needed to assess their advantages based on different HF and CVD risk levels due to limited clinical data.
5 Conclusions
In conclusion, the results of our meta-analysis show that DPP4i ameliorates PFR and LVEF levels in patients, as measured by CMR technology.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
HW: Data curation, Formal analysis, Funding acquisition, Writing – original draft. SYG: Data curation, Formal analysis, Writing – original draft. SG: Data curation, Writing – original draft. CL: Data curation, Writing – original draft. FW: Data curation, Writing – original draft. JZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Shandong Provincial Natural Science Foundation of China Grants (ZR2020QH266), the National Natural Science Foundation of China (grant number 82303031), Bethune Public Welfare Foundation (grant number Z04JKM2022E036), and the Imaging Research Fund of LunQin (SD-202008-011).
Acknowledgments
The authors express deep gratitude to the committed researchers and respected institutions contributing to the invaluable databases.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. (2010) 375:2215–22. doi: 10.1016/s0140-6736(10)60484-9
2. Atlas IDFD. Individual, social and economic impact (2019). Available online at: https://diabetesatlas.org/en/sections/individual-social-and-economic-impact.html (Accessed October 26, 2020).
3. Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the diabetes & Aging study). Diabetes Care. (2019) 42:416–26. doi: 10.2337/dc17-1144
4. Turner RC, Holman RR, Cull CA, Stratton IM, Matthews DR, Frighi V, et al. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (Ukpds 33). Lancet. (1998) 352:837–53. doi: 10.1016/s0140-6736(98)07019-6
5. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. (1993) 329:977–86. doi: 10.1056/nejm199309303291401
6. de Boer IH, Sun WJ, Gao XY, Cleary PA, Lachin JM, Molitch ME, et al. Effect of Intensive Diabetes Treatment on Albuminuria in Type 1 Diabetes: Long-Term Follow-up of the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications Study. Lancet Diabetes Endocrinol. (2014) 2:793–800. doi: 10.1016/s2213-8587(14)70155-x
7. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. (2008) 358:2560–72. doi: 10.1056/NEJMoa0802987
8. Thethi TK, Bilal A, Pratley RE. Cardiovascular outcome trials with glucose-lowering drugs. Curr Cardiol Rep. (2021) 23:75. doi: 10.1007/s11886-021-01505-3
9. Marso SP, Poulter NR, Nissen SE, Nauck MA, Zinman B, Daniels GH, et al. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (Leader) trial. Am Heart J. (2013) 166:823–30.e5. doi: 10.1016/j.ahj.2013.07.012
10. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2016) 375:311–22. doi: 10.1056/NEJMoa1603827
11. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2016) 375:1834–44. doi: 10.1056/NEJMoa1607141
12. Green JB, Hernandez AF, D’Agostino RB, Granger CB, Janmohamed S, Jones NP, et al. Harmony outcomes: A randomized, double-blind, placebo-controlled trial of the effect of albiglutide on major cardiovascular events in patients with type 2 diabetes mellitus—Rationale, design, and baseline characteristics. Am Heart J. (2018) 203:30–8. doi: 10.1016/j.ahj.2018.03.030
13. Hernandez AF, Green JB, Janmohamed S, D'Agostino RB, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony outcomes): A double-blind, randomised placebo-controlled trial. Lancet. (2018) 392:1519–29. doi: 10.1016/s0140-6736(18)32261-x
14. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Design and baseline characteristics of participants in the researching cardiovascular events with a weekly incretin in diabetes (Rewind) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab. (2017) 20:42–9. doi: 10.1111/dom.13028
15. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (Rewind): A double-blind, randomised placebo-controlled trial. Lancet. (2019) 394:121–30. doi: 10.1016/s0140-6736(19)31149-3
16. Demuth H-U, McIntosh CHS, Pederson RA. Type 2 diabetes—Therapy with dipeptidyl peptidase iv inhibitors. Biochim Biophys Acta (BBA) - Proteins Proteomics. (2005) 1751:33–44. doi: 10.1016/j.bbapap.2005.05.010
17. Patil HR, Al Badarin FJ, Al Shami HA, Bhatti SK, Lavie CJ, Bell DSH, et al. Meta-analysis of effect of dipeptidyl peptidase-4 inhibitors on cardiovascular risk in type 2 diabetes mellitus. Am J Cardiol. (2012) 110:826–33. doi: 10.1016/j.amjcard.2012.04.061
18. Scheller NM, Mogensen UM, Andersson C, Vaag A, Torp-Pedersen C. All-cause mortality and cardiovascular effects associated with the dpp-iv inhibitor sitagliptin compared with metformin, a retrospective cohort study on the danish population. Diabetes Obes Metab. (2014) 16:231–6. doi: 10.1111/dom.12197
19. Eurich DT, Simpson S, Senthilselvan A, Asche CV, Sandhu-Minhas JK, McAlister FA. Comparative safety and effectiveness of sitagliptin in patients with type 2 diabetes: retrospective population based cohort study. Bmj. (2013) 346:f2267–f. doi: 10.1136/bmj.f2267
20. Chow LH, Radio SJ, Sears TD, McManus BM. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. (1989) 14:915–20. doi: 10.1016/0735-1097(89)90465-8
21. Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clinic Proc. (1989) 64:1235–45. doi: 10.1016/s0025-6196(12)61286-5
22. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: A jacc white paper. J Am Coll Cardiol. (2009) 53:1475–87. doi: 10.1016/j.jacc.2009.02.007
23. Hamlin SA, Henry TS, Little BP, Lerakis S, Stillman AE. Mapping the future of cardiac mr imaging: case-based review of T1 and T2 mapping techniques. Radiographics. (2014) 34:1594–611. doi: 10.1148/rg.346140030
24. Hinojar R, Foote L, Arroyo Ucar E, Jackson T, Jabbour A, Yu CY, et al. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: A proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging. (2015) 8:37–46. doi: 10.1016/j.jcmg.2014.07.016
25. Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G, et al. Cmr in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging. (2014) 7:667–75. doi: 10.1016/j.jcmg.2014.02.005
26. Hong YJ, Park CH, Kim YJ, Hur J, Lee H-J, Hong SR, et al. Extracellular volume fraction in dilated cardiomyopathy patients without obvious late gadolinium enhancement: comparison with healthy control subjects. Int J Cardiovasc Imaging. (2015) 31:115–22. doi: 10.1007/s10554-015-0595-0
27. Ugander M, Oki AJ, Hsu L-Y, Kellman P, Greiser A, Aletras AH, et al. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. (2012) 33:1268–78. doi: 10.1093/eurheartj/ehr481
28. Chan W, Duffy SJ, White DA, Gao XM, Du XJ, Ellims AH, et al. Acute left ventricular remodeling following myocardial infarction: coupling of regional healing with remote extracellular matrix expansion. JACC Cardiovasc Imaging. (2012) 5:884–93. doi: 10.1016/j.jcmg.2012.03.015
29. Mascherbauer J, Marzluf BA, Tufaro C, Pfaffenberger S, Graf A, Wexberg P, et al. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circulation: Cardiovasc Imaging. (2013) 6:1056–65. doi: 10.1161/circimaging.113.000633
30. Iles LM, Ellims AH, Llewellyn H, Hare JL, Kaye DM, McLean CA, et al. Histological validation of cardiac magnetic resonance analysis of regional and diffuse interstitial myocardial fibrosis. Eur Heart J - Cardiovasc Imaging. (2014) 16:14–22. doi: 10.1093/ehjci/jeu182
31. Maceira AM, Tuset-Sanchis L, López-Garrido M, San Andres M, López-Lereu MP, Monmeneu JV, et al. Feasibility and reproducibility of feature-tracking-based strain and strain rate measures of the left ventricle in different diseases and genders. J Magnetic Resonance Imaging. (2017) 47:1415–25. doi: 10.1002/jmri.25894
32. Wang XF, Yang LL, Li WS, Zhao XY, Zhang JY. Effect of sitagliptin on cardiac function and myocardial fibrosis in patients with diabetes mellitus. J Clin Cardiol (China). (2018) 34:376–80. doi: 10.13201/j.issn.1001-1439.2018.04.016
33. Fu LK, Meng S, Lu JY, Zhou C, Liu HZ, Li ZW. A prospective study of the effects of salbutamol on cardiac structure and function by cardiac magnetic resonance imaging. J Mol Imaging. (2022) 45:223–8. doi: 10.12122/j.issn.1674-4500.2022.02.12
34. Wong KCK, Ismail HS, Connelly KA, Verma S, Ng M-Y, Deva DP, et al. Relationship between saxagliptin use and left ventricular diastolic function assessed by cardiac mri. Acta Diabetologica. (2023) 61:91–7. doi: 10.1007/s00592-023-02177-x
35. Webb DR, Htike ZZ, Swarbrick DJ, Brady EM, Gray LJ, Biglands J, et al. A randomized, open-label, active comparator trial assessing the effects of 26 Weeks of liraglutide or sitagliptin on cardiovascular function in young obese adults with type 2 diabetes. Diabetes Obes Metab. (2020) 22:1187–96. doi: 10.1111/dom.14023
36. von Lewinski D, Benedikt M, Alber H, Debrauwere J, Smits PC, Édes I, et al. Dutogliptin in combination with filgrastim in early recovery post-myocardial infarction—the rec-dut-002 trial. J Clin Med. (2022) 11:5728. doi: 10.3390/jcm11195728
37. Sandhu P, Ong JP, Garg V, Altaha M, Bello O, Singal SR, et al. The effects of saxagliptin on cardiac structure and function using cardiac mri (Scarf). Acta Diabetologica. (2021) 58:633–41. doi: 10.1007/s00592-020-01661-y
38. Kato S, Fukui K, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, et al. Inhibition of dpp-4 by alogliptin improves coronary flow reserve and left ventricular systolic function evaluated by phase contrast cine magnetic resonance imaging in patients with type 2 diabetes and coronary artery disease. Int J Cardiol. (2016) 223:770–5. doi: 10.1016/j.ijcard.2016.08.306
39. Hiruma S, Shigiyama F, Hisatake S, Mizumura S, Shiraga N, Hori M, et al. A prospective randomized study comparing effects of empagliflozin to sitagliptin on cardiac fat accumulation, cardiac function, and cardiac metabolism in patients with early-stage type 2 diabetes: the asset study. Cardiovasc Diabetol. (2021) 20:32. doi: 10.1186/s12933-021-01228-3
40. World Health Organization. A 24-Week study to investigate the effects of saxagliptin,saxagliptin combined with dapagliflozin, sitagliptin and placebo in patients with type 2 diabetes mellitus and heart failure (2018). Available online at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2015-004825-14/results (accessed August 19, 2021).
41. Brenner C, Adrion C, Grabmaier U, Theisen D, von Ziegler F, Leber A, et al. Sitagliptin plus granulocyte colony-stimulating factor in patients suffering from acute myocardial infarction: A double-blind, randomized placebo-controlled trial of efficacy and safety (Sitagrami trial). Int J Cardiol. (2016) 205:23–30. doi: 10.1016/j.ijcard.2015.11.180
42. Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. Dpp-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circulation: Cardiovasc Imaging. (2010) 3:195–201. doi: 10.1161/circimaging.109.899377
43. Lee SJ, Lee KH, Oh HG, Seo HJ, Jeong SJ, Kim CH. Effect of sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase 4 inhibitors on cardiovascular function in patients with type 2 diabetes mellitus and coronary artery disease. J Obes Metab Syndrome. (2019) 28:254–61. doi: 10.7570/jomes.2019.28.4.254
44. Zhang DP, Xu L, Wang LF, Wang HJ, Jiang F. Effects of antidiabetic drugs on left ventricular function/dysfunction: A systematic review and network meta-analysis. Cardiovasc Diabetol. (2020) 19:10. doi: 10.1186/s12933-020-0987-x
45. Leiner T, Bogaert J, Friedrich MG, Mohiaddin R, Muthurangu V, Myerson S, et al. Scmr position paper (2020) on clinical indications for cardiovascular magnetic resonance. J Cardiovasc Magnetic Resonance. (2020) 22:76. doi: 10.1186/s12968-020-00682-4
46. Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, et al. Accf/acr/aha/nasci/scmr 2010 expert consensus document on cardiovascular magnetic resonance. J Am Coll Cardiol. (2010) 55:2614–62. doi: 10.1016/j.jacc.2009.11.011
47. Satoh-Asahara N, Sasaki Y, Wada H, Tochiya M, Iguchi A, Nakagawachi R, et al. A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism. (2013) 62:347–51. doi: 10.1016/j.metabol.2012.09.004
48. Li R, Shan Y, Gao L, Wang X, Wang X, Wang F. The glp-1 analog liraglutide protects against angiotensin ii and pressure overload-induced cardiac hypertrophy via pi3k/akt1 and ampka signaling. Front Pharmacol. (2019) 10:537. doi: 10.3389/fphar.2019.00537
49. Wang D, Jiang L, Feng B, He N, Zhang Y, Ye H. Protective Effects of Glucagon-Like Peptide-1 on Cardiac Remodeling by Inhibiting Oxidative Stress through Mammalian Target of Rapamycin Complex 1/P70 Ribosomal Protein s6 Kinase Pathway in Diabetes Mellitus. J Diabetes Invest. (2019) 11:39–51. doi: 10.1111/jdi.13098
50. Nielsen R, Jorsal A, Iversen P, Tolbod LP, Bouchelouche K, Sørensen J, et al. Effect of liraglutide on myocardial glucose uptake and blood flow in stable chronic heart failure patients: A double-blind, randomized, placebo-controlled live sub-study. J Nucl Cardiol. (2019) 26:585–97. doi: 10.1007/s12350-017-1000-2
51. Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen Y-T, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. (2006) 317:1106–13. doi: 10.1124/jpet.106.100982
52. Giblett JP, Axell RG, White PA, Aetesam-Ur-Rahman M, Clarke SJ, Figg N, et al. Glucagon-Like Peptide-1–Mediated Cardioprotection Does Not Reduce Right ventricular Stunning and Cumulative Ischemic Dysfunction after Coronary Balloon Occlusion. JACC: Basic to Trans Sci. (2019) 4:222–33. doi: 10.1016/j.jacbts.2018.12.002
53. Bizino MB, Jazet IM, Westenberg JJM, van Eyk HJ, Paiman EHM, Smit JWA, et al. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol. (2019) 18:55. doi: 10.1186/s12933-019-0857-6
54. Kim GS, Park JH, Won JC. The role of glucagon-like peptide 1 receptor agonists and sodium-glucose cotransporter 2 inhibitors in reducing cardiovascular events in patients with type 2 diabetes. Endocrinol Metab (Seoul). (2019) 34:106–16. doi: 10.3803/EnM.2019.34.2.106
55. Boshchenko AA, Maslov LN, Mukhomedzyanov AV, Zhuravleva OA, Slidnevskaya AS, Naryzhnaya NV, et al. Peptides are cardioprotective drugs of the future: the receptor and signaling mechanisms of the cardioprotective effect of glucagon-like peptide-1 receptor agonists. Int J Mol Sci. (2024) 25:4900. doi: 10.3390/ijms25094900
56. Rodriguez H, El-Osta A. Epigenetic contribution to the development and progression of vascular diabetic complications. Antioxid Redox Signal. (2018) 29:1074–91. doi: 10.1089/ars.2017.7347
57. Song Z, Wang J, Zhang L. Ferroptosis: A new mechanism in diabetic cardiomyopathy. Int J Med Sci. (2024) 21:612–22. doi: 10.7150/ijms.88476
Keywords: dipeptidyl peptidase-4 inhibitors(DPP4i), type 2 diabetes mellitus(T2DM), cardiac magnetic resonance(CMR), cardiac structure and function, meta-analysis
Citation: Wang H, Guo S, Gu S, Li C, Wang F and Zhao J (2024) The effects of dipeptidyl peptidase-4 inhibitors on cardiac structure and function using cardiac magnetic resonance: a meta-analysis of clinical studies. Front. Endocrinol. 15:1428160. doi: 10.3389/fendo.2024.1428160
Received: 05 May 2024; Accepted: 26 August 2024;
Published: 11 September 2024.
Edited by:
Ramoji Kosuru, Versiti Blood Research Institute, United StatesReviewed by:
Alexander E. Berezin, Paracelsus Medical University, AustriaLilia M. M. Sierra-Galan, The American British Cowdray Medical Center, Mexico
Copyright © 2024 Wang, Guo, Gu, Li, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junyu Zhao, MTM1ODkwNjY0MzVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Haipeng Wang1†
Haipeng Wang1† Shuo Gu
Shuo Gu Chunyu Li
Chunyu Li Junyu Zhao
Junyu Zhao