- 1Division of Pediatric Endocrinology, Cohen Children’s Medical Center, NorthwellHealth, New Hyde Park, NY, United States
- 2Biostatistics Unit, Office of Academic Affairs, Northwell Health, New Hyde Park, NY, United States
Introduction: Reports in adults indicate that Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2) infection and vaccination trigger the expression of autoimmune disease such as Graves’ disease, but the incidence of new onset Graves’ disease and its temporal relationship to the peaks of COVID-19 cases in children are unclear.
Methods: This is a retrospective study of children and adolescents with new-onset Graves’ disease diagnosed between September 2017 and August 2022, N=156, mean age of 12.5 ± 4 year (y), with a range of 2.9-17.9y. There were 119 female (76.3%) and 37 male (23.7%) subjects. Subjects were categorized into 2 groups: pre-COVID-19 era Graves’ disease (n=63, age 12.5 ± 3.3y), and COVID-19 era Graves’ disease (n=93, age 12.4 ± 4.4y). We calculated incidence rate based on new cases of Graves’ disease and total number of new patient referrals to our endocrine clinic. We first compared the demographic, clinical and biochemical data between the above 2 groups; and also, between subjects with either a history of COVID-19 infection (n=23) or vaccination (n=17) to a control group (n=63).
Results: The incidence of Graves’ disease was significantly higher during the pandemic: pre-COVID-19 versus the COVID-19 era, n=55, 0.56% vs n=93, 0.9%, p=0.005, after accounting for the total number of annual new patient referrals during the study period. The rise in the cases of Graves’ disease followed the spikes in the number of cases of COVID-19 in NY. There was also a statistically significant difference in the race distribution between the pre-COVID-19 and the COVID-19 era (p=0.026).
Discussion: The incidence of Graves’ disease increased significantly in children living in New York during the COVID-19 pandemic. The temporal relationship between the peaks of COVID-19 cases and the increased cases of new onset Graves’ disease suggest possible autoimmune triggering by SARS-CoV-2.
Introduction
Autoimmune disorders are characterized by the presence of autoantibodies and inflammatory reactions from the loss of immune tolerance and subsequent dysregulation of the immune system, leading to target organ damage and dysfunction (1). Graves’ disease results from a complex interaction between genetic predisposition, environmental factors, and the immune system. Thyroid stimulating hormone receptor antibody (TRAb) and/or thyroid stimulating immunoglobulin (TSI) stimulate the thyroid gland to produce excess thyroid hormone in Graves’ disease (2). Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2) infection and vaccination have been associated with the triggering of autoimmune diseases (1, 3, 4), particularly thyroid diseases (5, 6) such as Graves’ disease, following SARS-CoV-2 infection (7–9) or vaccination (10–14). These reports are mostly in adult patients, with very few publications in children (15, 16). Graves’ disease is rare in children and accounts for about 10% to 15% of cases of thyroid disorders in childhood. While there have been increasing reports of autoimmune thyroid disease following COVID-19 infection or vaccination, there is a dearth of knowledge on the incidence of new onset Graves’ disease and its temporal relationship to the peaks of COVID-19 cases in children during the pandemic.
The aim of this study was to determine possible changes in the incidence of Graves’ disease in children and adolescents living in New York before and during the COVID-19 pandemic era, and any association with the peaks of COVID-19 cases.
Materials and methods
Ethical approval
This study’s protocol was approved by the Feinstein Institute for Medical Research IRB (Docket #: 22-0728).
Study design and subjects
Subjects were included in the study if they were <18 years of age and had a new diagnosis of Graves’ disease at the Division of Pediatric Endocrinology of the Cohen Children’s Medical Center, Northwell Health, between September 2017 and August 2022. The diagnosis of Graves’ disease was made based on features of autoimmune hyperthyroidism as marked by a suppressed thyroid stimulating hormone (TSH), elevated total thyroxine (TT4) or free thyroxine (free T4) level, and/or total triiodothyronine (TT3) concentration with a positive TSI and/or TRAb. Data was collected on subjects’ sex, race, date of diagnosis, age at diagnosis, individual and family history of either autoimmune diseases and/or thyroid disease, presenting signs and symptoms and the duration of the symptoms. Further biochemical and laboratory data included TSH, T4, free T4, TT3, anti-thyroid peroxidase antibody (TPOAb), anti-Thyroglobulin antibody (TGAb), TSI and TSH receptor antibodies, liver enzymes. We collected data on treatment with atenolol and methimazole, along with the duration and doses of each therapy. For subjects diagnosed in the COVID-19 era (starting from March 2020) we also collected information on COVID-19 infection and vaccine status in the year prior to their clinical presentation.
Statistical analysis
Descriptive statistics were performed (e.g., frequency and proportion for categorical variables and means and standard deviations or median and interquartile range for continuous variables). We compared the demographic, clinical, and biochemical parameters of subjects between the groups, i.e., those diagnosed before COVID-19 pandemic (September 2017- February 2020) versus during the pandemic (March 2020-August 2022). We also compared subjects with a history of COVID-19 infection or COVID -19 vaccination in the year prior to their diagnosis with Graves’ disease to a control group, i.e., subjects diagnosed before the COVID-19 pandemic. A chi-square or Fisher’s exact test was used for categorical variables, and either a two-sample t-test or Wilcoxon rank sum test for continuous variables. The incidence rate was calculated as the number of new cases of Graves’ disease that presented to the Division of Pediatric Endocrinology divided by the total number of new-patient referrals made to the division. We computed the 95% exact binomial confidence interval and set the level of significance at p<0.05. All analyses were conducted using SAS software Version 9.4 (Cary, NC).
We reviewed the overall number of individuals that our division served for consultations, which includes Nassau and Queens Counties in New York, between 2018 and 2022. Population-level statistics for the 2 counties during the same period, specifically for the 5-19 year old sub-population, were equally reviewed and compared from census data (17, 18). The overall population decreased from 3.8 to 3.6 million individuals (17). Review of the percentile of pediatric population (5-19 years old) showed a similar proportion before and after the pandemic, with 15.8% in 2018 to 16% in 2022 in Queens County and 18.5% in 2018 to 18.4% in 2022 in Nassau County (18).We compared the number of new patients entering the clinic out of the total population of interest during that study period (by year) for Nassau and Queens Counties and found that in 2020 our Division of Endocrinology had a decline in the proportion of new patients seen as a percentage of the pediatric population in Nassau and Queens counties, when compared to each of the other years: 0.59% in 2020 vs 0.7% in 2018, 0.78% in 2019, 0.72% in 2021 and 0.76% in 2022. Therefore, we focused our calculation of incidence of Graves’ disease in the pediatric population on the subset of all new patients who presented to our division. Incidence for 2017 was not able to be computed as census data on population are reported by year, not month, and we only had 4 months of data for 2017.
Results
One hundred and fifty-six (156) subjects were diagnosed with new-onset Graves’ disease between September 2017 and August 2022. The subjects’ demographic characteristics are described in Table 1, their clinical presentation profiles are summarized in Table 2, and their biochemical presentation summarized in Table 3. In order to analyze the thyroid function tests (TFT’s), we categorized each variable based on the free T4, Total T3 and Total T4 levels. If there was a discrepancy between the categorization, we prioritized the free T4 value, followed by the Total T3 and then Total T4 levels (Table 4). Of the 156 subjects diagnosed with Graves’ disease, 63 (40%) were diagnosed in the 30 months prior to the start of the pandemic (i.e., September 2017- February 2020), and 93 (60%) were diagnosed in the subsequent 30 months (March 2020-August 2022) during the pandemic era. The monthly rate of increase in Graves’ disease cases rose from 2 to 2.5 cases/month in the pre-COVID-19 era to 3.3 to 3.5 cases/month in the COVID-19 era. The incidence of Graves’ disease in this cohort in the COVID-19 era was significantly higher than the incidence in the pre-COVID-19 era, 0.90% vs 0.56%, p=0.005. (Table 5).
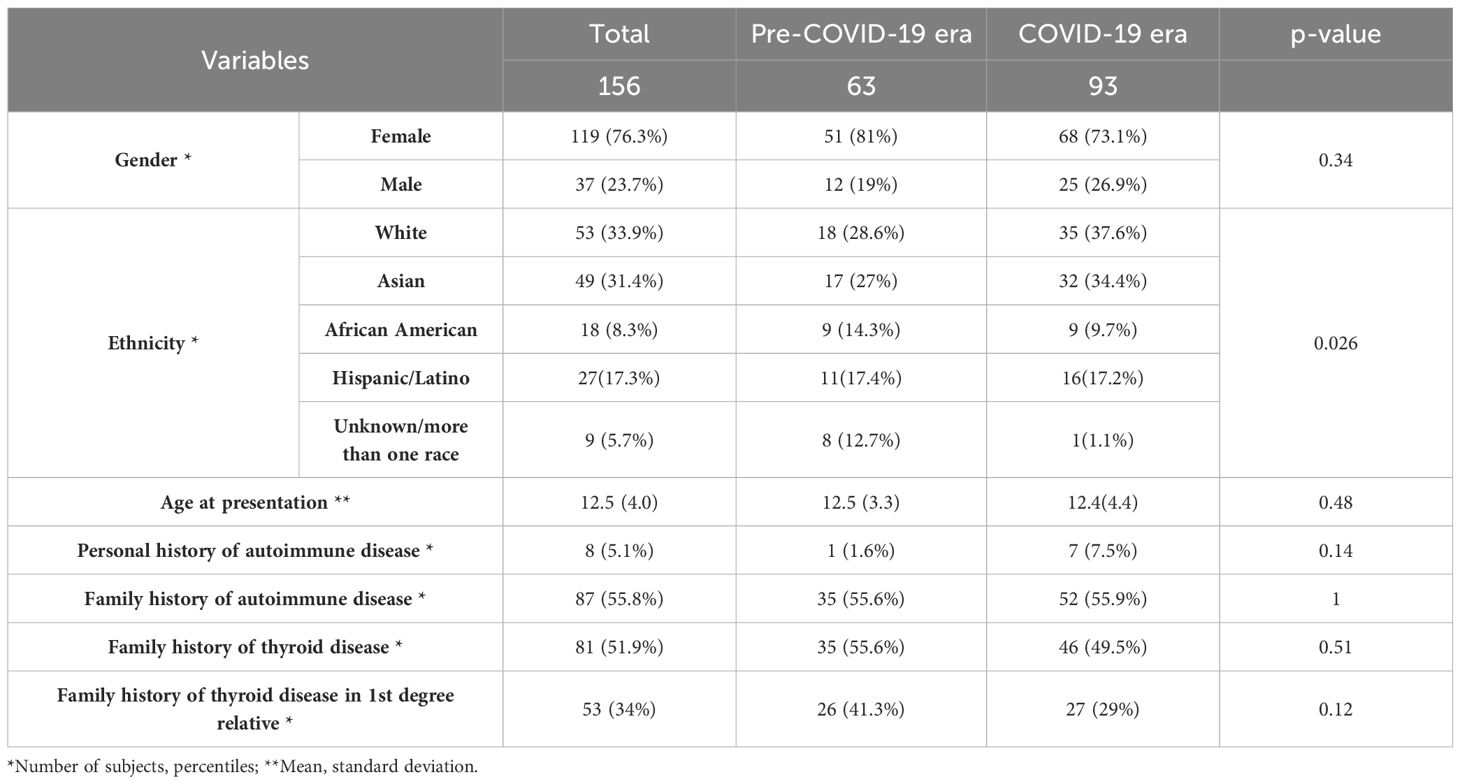
Table 1. Baseline demographic characteristics of the subjects stratified by pre-COVID-19 era and COVID-19 era.
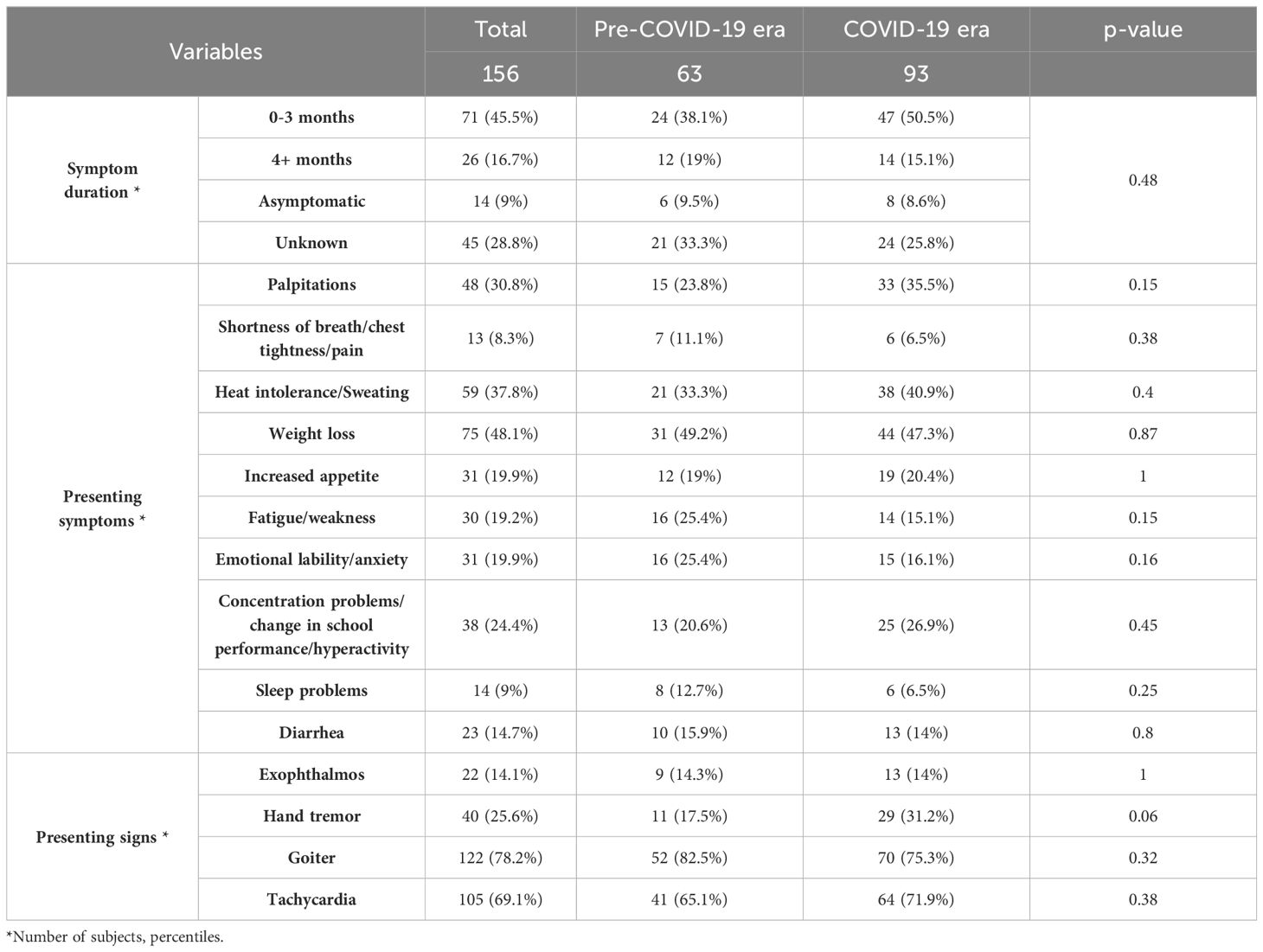
Table 2. Comparative analysis of the subjects’ presenting symptoms and signs stratified by pre-COVID-19 era and COVID-19 era.
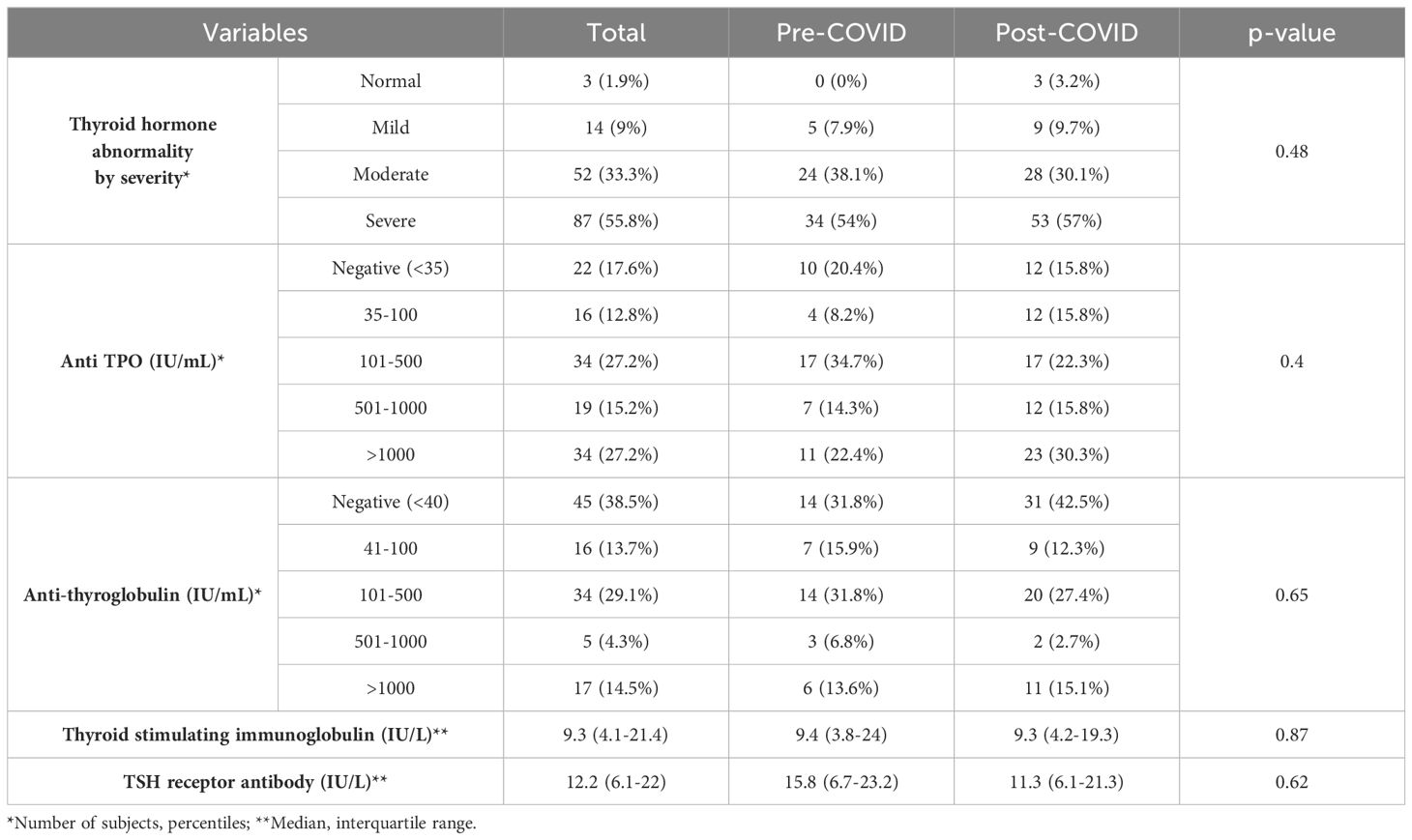
Table 3. Comparative analysis of the subjects’ biochemical presentation stratified by pre-COVID-19 era and COVID-19 era.
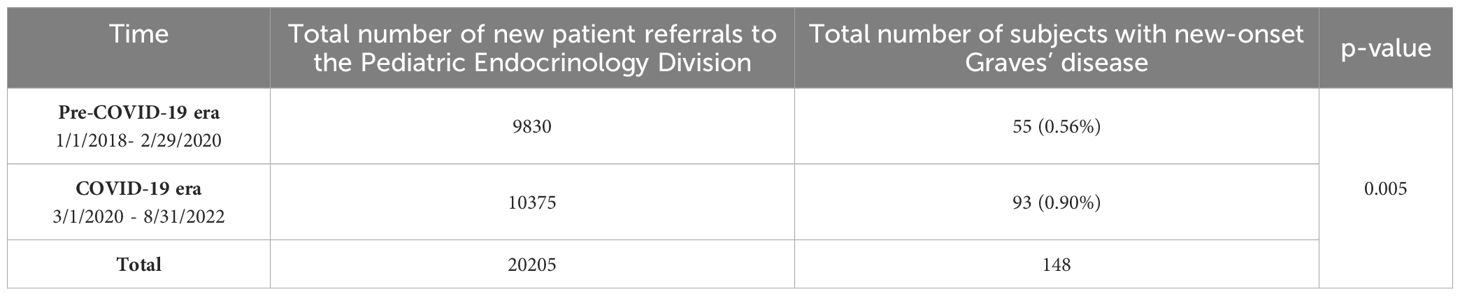
Table 5. Incidence of new onset Graves’ disease cases in the pre-COVID-19 era versus the COVID-19 era.
More White and Asian subjects were diagnosed with new-onset Graves’ disease than African American subjects during the pandemic, (p=0.026), (Table 1). Additionally, more subjects presented with fatigue/weakness (p=0.15) and emotional lability/anxiety (p=0.16) in the pre-COVID-19 era, while more individuals presented with palpitations (p=0.15) and tremor (p=0.06) in the COVID-19 era (Table 2).
Positive Versus Negative History of COVID-19 Infection/vaccination
When evaluating for a prior COVID-19 infection/vaccination, 23/93 (24.7%) subjects reported a previous COVID-19 infection, and 17/93 (18.2%) subjects reported COVID-19 vaccination, in the year prior to diagnosis. Out of these subjects, 5 reported both. 63 subjects were diagnosed with Graves’ disease before the pandemic and served as the control group.
Some differences in the clinical presentation were noted between the groups as shown in Table 6. In the group with a reported prior COVID-19 infection, a higher percentage of subjects reported concentration problems, irritability or changes in school performance (p=0.03). Similar increases were seen in the vaccinated group. Those vaccinated had a higher percentage of exophthalmos at presentation, and a lower percentage of goiter.
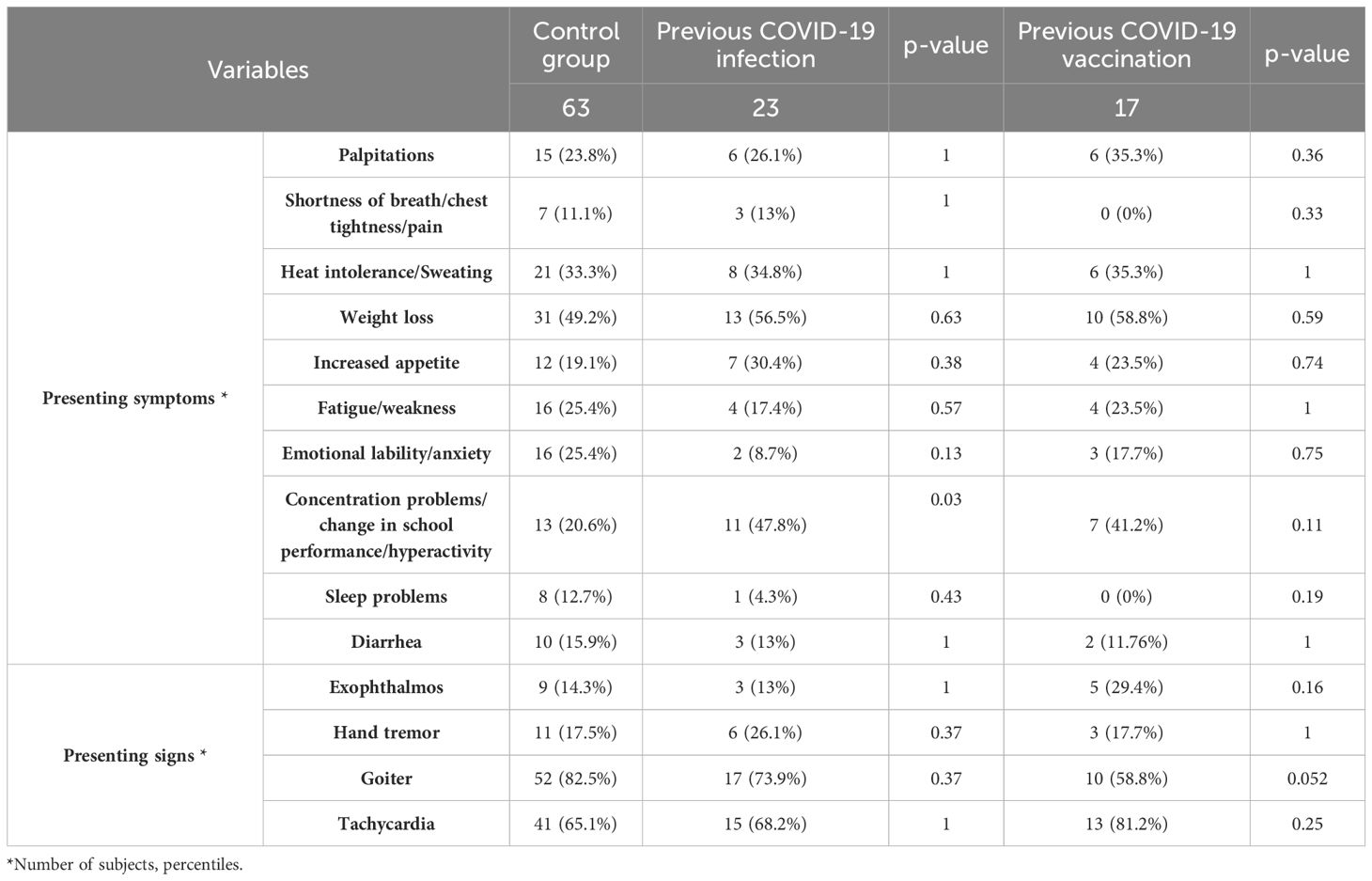
Table 6. Comparative analysis of the subjects’ presenting symptoms and signs between subjects with prior infection/vaccination status and the controls.
Discussion
This study found a statistically significant increase in the incidence of new-onset Graves’ disease between the pre-COVID-19 and COVID-19 era (p=0.005), when compared with the total number of new patient referrals to the pediatric endocrine clinic. This is the largest study investigating the incidence of new-onset Graves’ disease during the COVID-19 pandemic in the pediatric population, as well as the longest in duration in both the pediatric and adult population.
The mechanism of this increased incidence in Graves’ disease during the COVID-19 era is unclear. However, several mechanisms have been proposed to lead to autoimmunity following a pathogenic virus infection or vaccination in different mechanisms, mainly molecular mimicry (1, 3, 19–21). A systematic review also suggests that cytokine storm, and the expression of angiotensin-converting enzyme 2 (ACE2) may play a significant role in the pathogenesis of Graves’ disease (22).
SARS-CoV-2 is an enveloped virus that can invade the cell by attaching to the host cell receptor, ACE2 (1). A recent study investigating expression analysis of ACE2 in 31 tissues derived from healthy individuals found that ACE2 protein expression is widely detected in various types of tissues and upregulated in thyrocytes (5). In a different study, ACE-2 messenger ribonucleic acid (mRNA) was detected in all surgical samples of 15 thyroid glands of patients with nodular goiter (23). It is plausible that the thyroid gland is affected by SARS-CoV-2 and can lead to thyroid dysfunction. Therefore, it is possible that the increased incidence found in our study during COVID-19 era was due to the triggering of the expression of autoimmune thyroid disease by exposure to SARS-CoV-2.
Additionally, vaccines work by triggering the immune response, which may stimulate a hyperinflammatory condition (4). The COVID-19 vaccines approved for children and adolescents >6 months of age in the United States are mRNA vaccines consisting of the genetic code for the spike protein antigen. Their approvals were gradual, as shown in Figure 1 (24). There had been reports of cases of new-onset Graves’ disease following COVID-19 infection and or vaccination (7–14, 25).
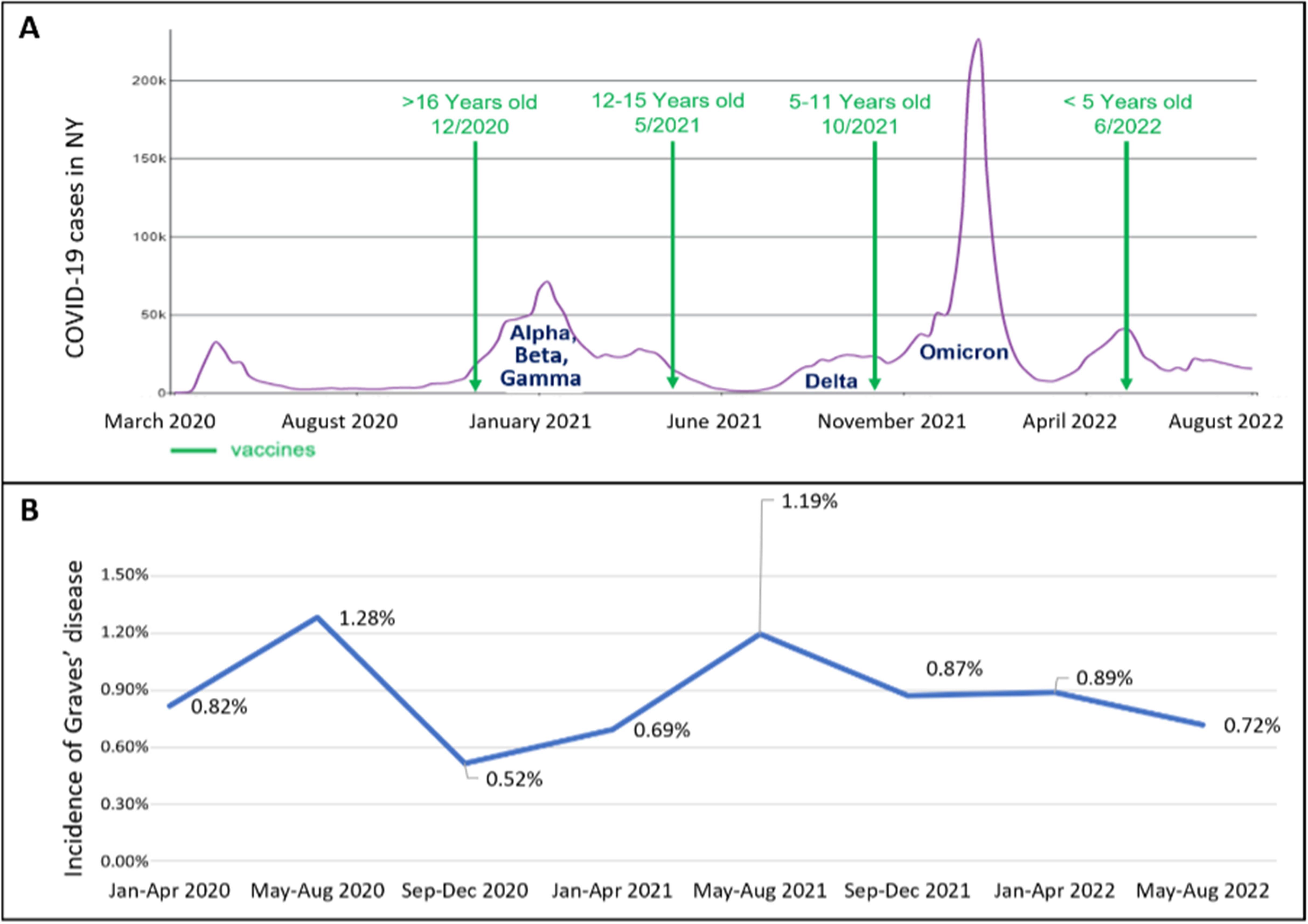
Figure 1. (A, B) The timeline of the COVID-19 era. (A) shows the number of COVID-19 cases diagnosed overtime and the SARS-COV-2 variants in NY as extracted from the CDC database (24), and the dates vaccinations were approved for use in children and adolescents. (B) shows the incidence of new onset Graves’ disease cases in the pediatric endocrinology division. There is a temporal relationship between the spikes in COVID-19 cases and subsequent increase in incidence of cases of new-onset Graves’ disease.
A matched case control study done in adult population did not find an association between COVID-19 vaccination and the incidence of Graves’ disease (26). One publication reported an increased incidence of Graves’ disease in 2021 vs 2017-2019 in adults (27), and another smaller pediatric study reported an increased incidence as well as more patients with palpitations and tremor (28). Our data is similar to the above clinical finding of increased palpitations (p=0.15) and tremor (p=0.06) during the pandemic.
We found that an increase in the number and incidence of new-onset Graves’ disease cases occurred a few months after the peak of COVID-19 cases, specifically after the first peak in March-April 2020 and after the peak in January 2021 that included the Alpha, Beta and Gamma variants as detailed in Figure 1. This again suggests the possibility that SARS-CoV-2 could trigger autoimmune thyroid disease, especially Graves’ disease. We further examined the incidence rates in the years preceding the COVID-19 era to provide robust comparative data for the COVID-19 era incidence. There was no additional peak in new Graves’ disease cases after the peak in COVID-19 cases in January 2022 due to the Omicron variant which may imply an altered immune response to new variants or a protective effect of vaccination. One study demonstrates that individuals who contracted SARS-CoV-2 in the early stages of the pandemic exhibited a higher prevalence of immunological abnormalities compared to those infected during later phases of the pandemic (29).
There was a statistically significant difference in the ethnicity/race distribution between the pre-COVID-19 era and the COVID-19 era (Table 1). The percentage of African American subjects with new onset Graves’ disease decreased during the pandemic, and the percentage of White and Asian subjects increased, despite the race distribution remaining stable in our catchment area (18). A possible explanation can be genetic polymorphisms in the ACE2 gene which is suggested as one of the factors that changes the receptor stability and vulnerability to the virus and the clinical presentation after COVID-19 infection (30–33).
Specifically in the African American population, reduced molecular expression of ACE2 has been reported (30), which might affect the virus’ invasion of the thyrocytes. This may suggest important clinical implications as to whether certain polymorphisms may have a protective effect against viral invasion as opposed to others. Further research is needed to evaluate for these racial differences due to small number of African American patients in the current study.
A statistically significant increase in concentration problems, irritability or change in school performance was seen in those with a prior COVID-19 infection (Table 6). A confounder might be the effect of the pandemic on school performance in general as described in a meta-analysis (34), but when comparing the pre-pandemic to the post-pandemic group, there was no such difference (Table 2). A possible explanation can be long-COVID, as one of the symptoms described is concentration problems (35).
Exophthalmos is the most common extrathyroidal manifestation of Graves’ disease (25%). Other extrathyroidal manifestations include thyroid dermopathy (1.5%) and acropachy (0.3%) (36). We found a higher percentage of subjects presenting with exophthalmos in those that were vaccinated (p=0.16), (Table 6). Review of the literature shows that multiple case reports were published reporting thyroid eye disease following COVID-19 vaccination (37–40). No patients in the current study reported other extrathyroidal manifestation.
One study (41) showed an increase of severity during the COVID-19 era in patients with new onset autoimmune hypothyroidism, mostly related to difficulties in access to healthcare services. We did not find differences in the severity of clinical or biochemical presentation between the pre COVID-19 and the COVID-19 era.
Our study has several limitations which include its retrospective design, which precludes causation. Since the population of patients who attend our endocrine clinic is not generalizable, the incidence estimates used in our study may not reflect true incidence in the population. However, the fact that the proportion of the general population who were seen as new patients in our endocrine clinic remained relatively stable from 2018 to 2022 (≈0.7 to 0.78%) supports the premise for an increased incidence of new cases of Graves’ disease in Queens and Nassau County in the COVID-19 era. Some confounders need to be taken into consideration. As discussed in the methods section, there was no significant population change (17, 18) or change in the healthcare seeking behavior to our practice (Table 5) that can explain the change in incidence. Another cofounder is physical and psychological stress, which is a risk factor in the pathogenesis of different autoimmune disorders and can trigger new onset or exacerbation of those diseases (42, 43). It is possible that the sedentary lifestyle and dietary change during the pandemic had an effect as well. Studies showed an increased incidence of different autoimmune diseases in patients less engaged in physical activity (44). The relationship between diet and autoimmunity was also investigated. The temporal relationship we found between the number of COVID-19 cases and the number of new onset Graves’ disease cases is less likely to be explained by those confounders (45, 46).
This study was performed on data from a specific geographic area. Even though New York’s population is relatively diverse as shown in the race/ethnicity distribution, this may limit the generalizability of the findings to other regions. Multicenter study across different populations can help validate the findings.
Further limitation includes the relatively small sample of patients with previous COVID-19 infection/vaccine. A prospective study comparing patients with new onset Graves’ disease after a SARS-CoV-2 infection or vaccination versus unexposed patients is now in progress by our group and could provide a better understanding of risk factors and presenting clinical characteristics.
The study’s strengths include it being the largest and longest retrospective pediatric study on the incidence of Graves’ disease in the pediatric population associated with the COVID-19 pandemic. Additionally, there is a comparison of the timing of the peaks in the number of subjects presenting with new onset Graves’ disease with the peaks of new cases of COVID-19 in New York state as reported by the CDC (24).
In conclusion, our study provides evidence that the incidence of Graves’ disease increased significantly in our institution during the COVID-19 era in children and adolescents in the US. The temporal relationship between increases in the number of COVID-19 cases and the elevated incidence of new onset Graves’ disease could suggest the possibility of triggering of autoimmune thyroid disease by SARS-CoV-2 virus. However, mechanistic studies are needed to provide conclusive evidence.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Feinstein Institute for Medical Research IRB (Docket #: 22-0728). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
NP-S: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. JF: Formal analysis, Writing – original draft, Writing – review & editing, Methodology, Software, Validation. BN: Writing – review & editing. PS: Conceptualization, Supervision, Writing – original draft, Writing – review & editing, Investigation, Methodology.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu Y, Sawalha AH, Lu Q. Covid-19 and autoimmune diseases. Curr Opin Rheumatol. (2021) 33:155–62. doi: 10.1097/bor.0000000000000776
2. Léger J, Carel JC. Hyperthyroidism in childhood: causes, when and how to treat. J Clin Res Pediatr Endocrinol. (2013) 5 Suppl 1:50–6. doi: 10.4274/jcrpe.854
3. Yazdanpanah N, Rezaei N. Autoimmune complications of covid-19. J Med Virol. (2022) 94:54–62. doi: 10.1002/jmv.27292
4. Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, et al. New-onset autoimmune phenomena post-covid-19 vaccination. Immunology. (2022) 165:386–401. doi: 10.1111/imm.13443
5. Murugan AK, Alzahrani AS. Sars-cov-2: emerging role in the pathogenesis of various thyroid diseases. J Inflammation Res. (2021) 14:6191–221. doi: 10.2147/jir.S332705
6. Tutal E, Ozaras R, Leblebicioglu H. Systematic review of covid-19 and autoimmune thyroiditis. Travel Med Infect Dis. (2022) 47:102314. doi: 10.1016/j.tmaid.2022.102314
7. Ghareebian H, Mariash C. Covid-19-induced graves’ Disease. Cureus. (2022) 14:e22260. doi: 10.7759/cureus.22260
8. Murugan AK, Alzahrani AS. Sars-cov-2 plays a pivotal role in inducing hyperthyroidism of graves’ Disease. Endocrine. (2021) 73:243–54. doi: 10.1007/s12020-021-02770-6
9. Mateu-Salat M, Urgell E, Chico A. Sars-cov-2 as a trigger for autoimmune disease: report of two cases of graves’ Disease after covid-19. J Endocrinol Invest. (2020) 43:1527–8. doi: 10.1007/s40618-020-01366-7
10. Bostan H, Ucan B, Kizilgul M, Calapkulu M, Hepsen S, Gul U, et al. Relapsed and newly diagnosed graves’ Disease due to immunization against covid-19: A case series and review of the literature. J Autoimmun. (2022) 128:102809. doi: 10.1016/j.jaut.2022.102809
11. Lui DTW, Lee KK, Lee CH, Lee ACH, Hung IFN, Tan KCB. Development of graves’ Disease after sars-cov-2 mrna vaccination: A case report and literature review. Front Public Health. (2021) 9:778964. doi: 10.3389/fpubh.2021.778964
12. Chee YJ, Liew H, Hoi WH, Lee Y, Lim B, Chin HX, et al. Sars-cov-2 mrna vaccination and graves’ Disease: A report of 12 cases and review of the literature. J Clin Endocrinol Metab. (2022) 107:e2324–e30. doi: 10.1210/clinem/dgac119
13. Triantafyllidis KK, Giannos P, Stathi D, Kechagias KS. Graves’ Disease following vaccination against sars-cov-2: A systematic review of the reported cases. Front Endocrinol (Lausanne). (2022) 13:938001. doi: 10.3389/fendo.2022.938001
14. Vera-Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sánchez Valadez TI, Jara LJ. Two cases of graves’ Disease following sars-cov-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. (2021) 31:1436–9. doi: 10.1089/thy.2021.0142
15. Rockett J. A case report of graves’ Disease following sars-cov-2 infection. Int J Contemp Pediatr. (2021) 8:1260. doi: 10.18203/2349-3291.ijcp20212481
16. Qureshi NK, Bansal SK. Autoimmune thyroid disease and psoriasis vulgaris after covid-19 in a male teenager. Case Rep Pediatr. (2021) 2021:7584729. doi: 10.1155/2021/7584729
17. United States Census Bureau. Available online at: https://www.census.gov (accessed January 3, 2024).
18. USA Facts. Available online at: https://usafacts.org (accessed January 3, 2024).
19. Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. (2006) 19:80–94. doi: 10.1128/cmr.19.1.80-94.2006
20. Ercolini AM, Miller SD. The role of infections in autoimmune disease. Clin Exp Immunol. (2009) 155:1–15. doi: 10.1111/j.1365-2249.2008.03834.x
21. Ehrenfeld M, Tincani A, Andreoli L, Cattalini M, Greenbaum A, Kanduc D, et al. Covid-19 and autoimmunity. Autoimmun Rev. (2020) 19:102597. doi: 10.1016/j.autrev.2020.102597
22. Vamshidhar IS, Rani SSS, Kalpana M, Gaur A, Umesh M, Ganji V, et al. Impact of covid-19 on thyroid gland functions with reference to graves’ Disease: A systematic review. J Family Med Prim Care. (2023) 12:1784–9. doi: 10.4103/jfmpc.jfmpc_2246_22
23. Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, et al. Detection of sars-cov-2 receptor ace-2 mrna in thyroid cells: A clue for covid-19-related subacute thyroiditis. J Endocrinol Invest. (2021) 44:1085–90. doi: 10.1007/s40618-020-01436-w
24. Centers for Disease Control and Prevention (Cdc). Available online at: https://covid.cdc.gov/covid-data-tracker/datatracker-home (accessed March 2, 2023).
25. França ÂS, Pinto SM, Vieira DC, Silva MT, Pinho PL. Graves disease: A new association with covid-19? Semergen. (2023) 49:101834. doi: 10.1016/j.semerg.2022.101834
26. Gorshtein A, Turjeman A, Duskin-Bitan H, Leibovici L, Robenshtok E. Graves’ Disease following covid-19 vaccination: A population-based, matched case-control study. J Clin Endocrinol Metab. (2024) 109:e508–e12. doi: 10.1210/clinem/dgad582
27. Barajas Galindo DE, Ramos Bachiller B, González Roza L, García Ruiz de Morales JM, Sánchez Lasheras F, González Arnáiz E, et al. Increased incidence of graves’ Disease during the sars-cov2 pandemic. Clin Endocrinol (Oxf). (2023) 98:730–7. doi: 10.1111/cen.14860
28. Donner JR, Has P, Topor LS. Increased incidence and severity of new graves disease diagnoses in youth during the covid-19 pandemic. Endocr Pract. (2023) 29(5):349–52. doi: 10.1016/j.eprac.2023.01.011
29. Oakes EG, Dillon E, Buhler KA, Guan H, Paudel M, Marks K, et al. Earlier vs. Later time period of covid-19 infection and emergent autoimmune signs, symptoms, and serologies. J Autoimmun. (2024) 148:103299. doi: 10.1016/j.jaut.2024.103299
30. Vinciguerra M, Greco E. Sars-cov-2 and black population: ace2 as shield or blade? Infect Genet Evol. (2020) 84:104361. doi: 10.1016/j.meegid.2020.104361
31. Hou Y, Zhao J, Martin W, Kallianpur A, Chung MK, Jehi L, et al. New insights into genetic susceptibility of covid-19: an ace2 and tmprss2 polymorphism analysis. BMC Med. (2020) 18:216. doi: 10.1186/s12916-020-01673-z
32. Vadgama N, Kreymerman A, Campbell J, Shamardina O, Brugger C, Research Consortium GE, et al. Sars-cov-2 susceptibility and ace2 gene variations within diverse ethnic backgrounds. Front Genet. (2022) 13:888025. doi: 10.3389/fgene.2022.888025
33. Mahase V, Sobitan A, Rhoades R, Zhang F, Baranova A, Johnson M, et al. Genetic variations affecting ace2 protein stability in minority populations. Front Med (Lausanne). (2022) 9:1002187. doi: 10.3389/fmed.2022.1002187
34. Di Pietro G. The impact of covid-19 on student achievement: evidence from a recent meta-analysis. Educ Res Rev. (2023) 39:100530. doi: 10.1016/j.edurev.2023.100530
35. Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. Bmj. (2021) 374:n1648. doi: 10.1136/bmj.n1648
36. Bartalena L, Fatourechi V. Extrathyroidal manifestations of graves’ Disease: A 2014 update. J Endocrinol Invest. (2014) 37:691–700. doi: 10.1007/s40618-014-0097-2
37. Rubinstein TJ. Thyroid eye disease following covid-19 vaccine in a patient with a history graves’ Disease: A case report. Ophthal Plast Reconstr Surg. (2021) 37:e221–e3. doi: 10.1097/iop.0000000000002059
38. Park KS, Fung SE, Ting M, Ozzello DJ, Yoon JS, Liu CY, et al. Thyroid eye disease reactivation associated with covid-19 vaccination. Taiwan J Ophthalmol. (2022) 12:93–6. doi: 10.4103/tjo.tjo_61_21
39. Im Teoh JH, Mustafa N, Wahab N. New-onset thyroid eye disease after covid-19 vaccination in a radioactive iodine-treated graves’ Disease patient: A case report and literature review. J ASEAN Fed Endocr Soc. (2023) 38:125–30. doi: 10.15605/jafes.038.01.19
40. Hung C-W, Hung C-H. Reactivation of graves’ Disease and thyroid eye disease following covid-19 vaccination – a case report. Ocular Immunol Inflammation. (2023) 31:1286–90. doi: 10.1080/09273948.2023.2176889
41. Munarin J, Tuli G. Differences in the clinical picture at the onset of diagnosis of severe autoimmune hypothyroidism in children depending on the time of diagnosis: before or during the sars-cov-2 pandemic. Pediatr Endocrinol Diabetes Metab. (2023) 29:253–8. doi: 10.5114/pedm.2023.133123
42. Stojanovich L, Marisavljevich D. Stress as a trigger of autoimmune disease. Autoimmun Rev. (2008) 7:209–13. doi: 10.1016/j.autrev.2007.11.007
43. Theoharides TC. Stress, inflammation, and autoimmunity: the 3 modern erinyes. Clin Ther. (2020) 42:742–4. doi: 10.1016/j.clinthera.2020.04.002
44. Sharif K, Watad A, Bragazzi NL, Lichtbroun M, Amital H, Shoenfeld Y. Physical activity and autoimmune diseases: get moving and manage the disease. Autoimmun Rev. (2018) 17:53–72. doi: 10.1016/j.autrev.2017.11.010
45. Gershteyn IM, Burov AA, Miao BY, Morais VH, Ferreira LMR. Immunodietica: interrogating the role of diet in autoimmune disease. Int Immunol. (2020) 32:771–83. doi: 10.1093/intimm/dxaa054
Keywords: Graves’ disease, hyperthyroidism, auto-immune thyroid disease, COVID-19, SARS-CoV-2
Citation: Pollack-Schreiber N, Fishbein JS, Nwosu BU and Salemi P (2024) Increased incidence of Graves’ disease during the COVID-19 pandemic in children and adolescents in the United States. Front. Endocrinol. 15:1426672. doi: 10.3389/fendo.2024.1426672
Received: 01 May 2024; Accepted: 14 November 2024;
Published: 05 December 2024.
Edited by:
Sally Radovick, The State University of New Jersey, United StatesReviewed by:
Gerdi Tuli, Regina Margherita Hospital, ItalyGiulia Lanzolla, University of Pennsylvania, United States
Copyright © 2024 Pollack-Schreiber, Fishbein, Nwosu and Salemi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naama Pollack-Schreiber, TlBvbGxhY2tTY2hyQG5vcnRod2VsbC5lZHU=
 Naama Pollack-Schreiber
Naama Pollack-Schreiber Joanna S. Fishbein
Joanna S. Fishbein Benjamin Udoka Nwosu
Benjamin Udoka Nwosu Parissa Salemi
Parissa Salemi