- 1Department of Epidemiology and Health Statistics, College of Public Health, Zhengzhou University, Zhengzhou, Henan, China
- 2Department of Orthopedics, Hongxing Hospital, 13th Division, Xinjiang Production and Construction Corps, Hami, Xinjiang, China
- 3Department of Epidemiology and Health Statistics, College of Public Health and Management, Wenzhou Medical University, Wenzhou, Zhejiang, China
Background: The impact of baseline triglyceride-glucose (TyG) index and abnormal low or high-density lipoprotein cholesterol (LDL-C or HDL-C) levels on all-cause and cardiovascular disease (CVD) mortality remains unclear. This study aimed to investigate the relationship between TyG index and LDL-C or HDL-C and all-cause and CVD mortality.
Methods: This retrospective cohort study analyzed data from health examinations of 69,068 older adults aged ≥60 in Xinzheng City, Henan Province, China, between January 2013 and January 2023. Cox proportional risk regression models were used to estimate the hazard ratio (HR) and 95% confidence interval (CI) of the TyG index and LDL-C or HDL-C about all-cause and CVD mortality. Restricted cubic spline was used to assess the dose-response relationship.
Results: During 400,094 person-years of follow-up (median follow-up 5.8 years [interquartile range 3.0-9.12]), 13,664 deaths were recorded, of which 7,045 were due to CVD. Compared with participants in the second quartile of the TyG index, participants in the fourth quartile had a 16% increased risk of all-cause mortality (HR: 1.16, 95% CI: 1.12,1.22), and an 8% increased risk of CVD mortality (HR: 1.08, 95% CI: 1.01,1.16). Similar results were observed in LDL-C and HDL-C, with all-cause and CVD mortality risks for participants in the fourth quartile compared with participants in the third quartile for LDL-C of (HR: 1.07, 95% CI: 1.02,1.12) and (HR: 1.09, 95% CI: 1.01,1.17), respectively. The risk of all-cause and CVD mortality in participants in the fourth quartile group compared with those in the second HDL-C quartile group was (HR: 1.10, 95% CI: 1.05,1.16) and (HR: 1.11, 95% CI: 1.04,1.18), respectively. We found that the TyG index was nonlinearly associated with all-cause and CVD mortality (P non-linear <0.05), and LDL-C was nonlinearly associated with all-cause mortality (P non-linear <0.05) but linearly associated with CVD mortality (P non-linear >0.05). HDL-C, on the other hand, was in contrast to LDL-C, which showed a non-linear association with CVD mortality. We did not observe a significant interaction between TyG index and LDL-C or HDL-C (P >0.05).
Conclusion: TyG index and LDL-C or HDL-C increased the risk of all-cause and CVD mortality, especially a high TyG index combined with abnormal LDL-C.
1 Background
Cardiovascular diseases (CVD) are the major cause of death and premature mortality in China (1, 2). The burden of CVD continues to increase annually, with approximately 330 million CVD patients in China. CVD is attributable to 2 out of every 5 deaths in China (3). The Global Burden of Disease (GBD) Study reports that the total prevalence of CVD worldwide has increased from 271 million in 1990 to 523 million in 2019. Additionally, the number of deaths has increased from 12.1 million to 18.6 million, and this trend is continuing (4).
Insulin resistance (IR), physiologically defined as a state of reduced responsiveness of insulin-targeted tissues to high physiologic insulin levels, is recognized as a causative driver of many modern diseases, including metabolic syndrome (Mts), type 2 diabetes mellitus (T2DM), and CVD complications (5). The main factors contributing to the development of IR are increased oxidative stress, hyperglycemia, and elevated lipid levels (6). Although advances have been made in therapies to help control blood glucose levels, CVD complications remain a major cause of morbidity and mortality in this population (7). The Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) is the most widely used surrogate indicator of IR but has limitations due to its complexity and cost, and it cannot be used in populations receiving insulin therapy (7). The triglyceride glucose (TyG) index is a low-cost and convenient tool for assessing IR in diabetic and non-diabetic patients (8, 9). Using HIEC and HOMA-IR as reference methods, the diagnostic accuracy of the TyG index in identifying IR has been tested in several studies. The highest sensitivity for HIEC was 96% and the highest specificity for HOMA-IR was 99% (8). The TyG index has also shown good performance in the estimation of IR in diabetic and non-diabetic patients compared to HOMA-IR (8). In addition, the TyG index does not require insulin quantification and can be used in all people, regardless of their insulin therapy status (9). However, fewer studies have been conducted on the association between the TyG index and CVD mortality.
IR not only makes individuals susceptible to CVD, it also enhances the effects of dyslipidemia (10). Dyslipidemia, mainly low or high-density lipoprotein cholesterol (LDL-C or HDL-C) in the abnormal range, is considered one of the major risk factors for CVD (11). Previous epidemiological studies have suggested that a higher TyG index is an important risk factor for all-cause and, in particular, CVD mortality (12, 13). However, these studies were all conducted in the general population. Furthermore, while a causal association between LDL-C and CVD mortality has been demonstrated (14), there are conflicting results regarding the relationship between HDL-C and CVD mortality. For example, some studies have suggested that higher HDL-C may be a better preventive factor for CVD (15), while others have found that high HDL-C levels are associated with an increased risk of CVD (16). Some studies have suggested a U-shaped pattern of all-cause and CVD mortality associated with LDL-C (17, 18), with some studies showing a linear relationship (19). It is also worth noting that there is limited research on the effect of the interaction between the TyG index and LDL-C or HDL-C on mortality risk.
To our knowledge, over the past 20-30 years, the health status of China’s total population has improved dramatically, with a significant increase in life expectancy, which has also meant a rapid and sustained increase in the aging population. Aging is considered to be an immutable factor that cannot be analyzed as a major influencing factor, so studies focusing on the elderly population are crucial. At the same time, the elderly are at high risk for CVD (20). Therefore, we wanted to explore the relationship between TyG index and LDL-C or HDL-C with all-cause and CVD mortality and to investigate the interaction between TyG index and LDL-C or HDL-C on risk in the elderly population.
2 Methods
2.1 Study design and population
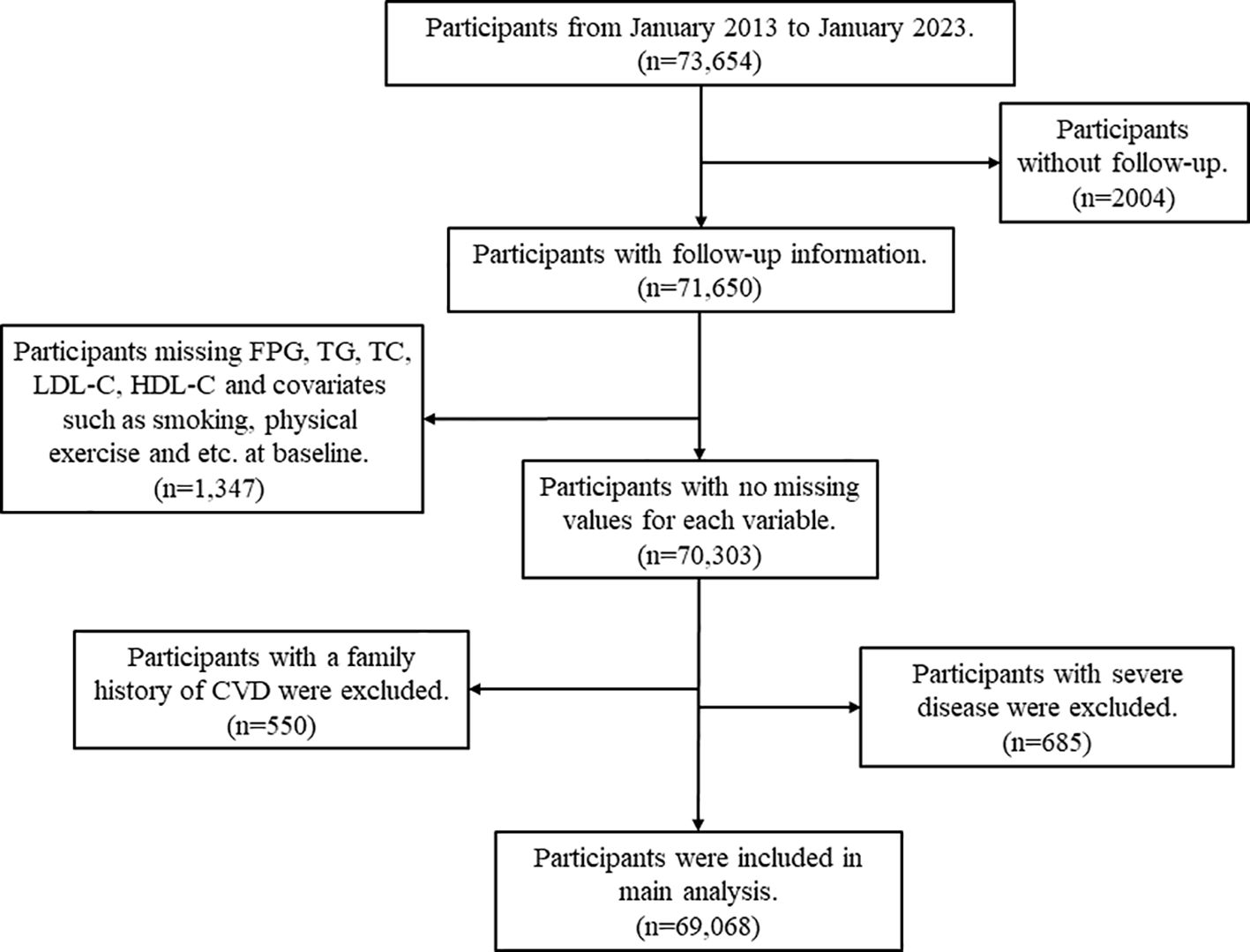
The data analyzed were obtained from the Resident Health Examinations Database of Xinzheng City, Henan Province, Central China, which is a large-scale cohort study of older adults aged 60 years and older conducted by the Centers for Disease Control (CDC) and Prevention of Xinzheng City and contains sociodemographic and mortality information on the population of Xinzheng City. Since January 1, 2011, Xinzheng City has been providing free annual health examinations to senior citizens aged 60 and older. At the initial examination, the physician creates a health profile for each resident, which includes basic demographic information (age, gender, marital status, etc.), blood indicators (fasting plasma glucose (FPG), triglycerides (TG), total cholesterol (TC), etc.), urinalysis, eye examination, chest X-ray, and other functions. For this study, we obtained follow-up information from 2013 to 2023 from a total of 56,069 eligible older adults. The Framingham study revealed that premature development of CVD in first-degree relatives, such as parents or siblings, is associated with an elevated risk of subsequent development in the offspring. The presence of genetic factors may lead to the occurrence of heart disease, stroke, heart failure, and other serious illnesses (serious mental illness and cancer) in several family members (21). Consequently, individuals with a family history of CVD such as coronary heart disease (CHD), stroke, and myocardial infarction (MI) at the start of the study were excluded. Participants with any of the following were excluded: (1) Exclude participants with a family history of CVD (n=550); (2) Exclude those with missing FPG, TG, TC, LDL-C, and HDL-C at baseline (n=1066); (3) Exclude those with missing one or more of the covariates of smoking, alcohol consumption, physical activity, waist circumference (WC), body mass index (BMI) at baseline (n=281); (4) Exclude participants with serious illnesses, including serious mental illness and cancer (n=685); and (5) Exclude participants with no follow-up records (n=2004). The process of screening the data is presented in Figure 1.
2.2 Statement
The study was approved by the Ethics Committee of Zhengzhou University (ID: ZZUIRB2019-019), and the research team obtained permission to use the data from the Zhengzhou Health Commission. All studies were conducted by the Declaration of Helsinki, and informed consent was obtained from all participants or their legal guardians.
2.3 Data collection
Standardized questionnaires were administered by trained researchers, and participants completed a questionnaire on sociodemographic characteristics, personal disease history, and lifestyle information at each health examination. Sociodemographic information included participants’ age, gender (male/female), and marital status (married/unmarried/widowed/divorced); disease history information included hypertension (yes/no), T2DM (yes/no), CHD (yes/no), stroke (yes/no) and cancer (yes/no). Lifestyle information included smoking status (never/ever/current), drinking status (never/occasional/more than once a week/daily), and physical activity status (never/occasional/more than once a week/daily). Current smoking was defined as having smoked more than 100 cigarettes in a lifetime and currently smoking (22). Alcohol consumption is defined as drinking more than 30 grams of alcohol in a single sitting, and more than 30 gram of alcohol per day is considered to be alcohol consumption for the day (23). Regular exercise is defined as 30 minutes of moderate-intensity exercise or 20 minutes of vigorous-intensity exercise three or more times per week (24). Participants’ height, weight, blood lipids, WC, FPG, systolic blood pressure (SBP), diastolic blood pressure (DBP), and resting heart rate (RHR) were measured by trained health professionals. Blood samples taken after participants fasted for 8 hours were used to measure FPG and blood lipids. Blood pressure (BP) was measured using an electronic sphygmomanometer (Omron HEM-7125, Kyoto, Japan). The subjects were instructed to rest quietly for five minutes in a standard supine position. Two measurements of SBP and DBP were then taken in the right brachial artery, with an interval of 30 minutes between each measurement. The average level was taken as the result of the BP measurements. Hypertension was defined as SBP ≥140 mmHg and DBP ≥90 mmHg or the use of antihypertensive medication (25). T2DM was defined as FPG ≥7.0 mmol/L or use of insulin or oral hypoglycemic agents, or a self-reported history of T2DM diagnosis (26). BMI was calculated as weight (kilograms) divided by the square of height (meters). A scoring scale consistent with Chinese body mass was used. The TyG index, calculated as TyG index = ln [Fasting TG (mg/dl) × FPG (mg/dl)]/2, is a composite indicator composed of TG and FPG levels (27). The diagnostic criteria for abnormal LDL-C is a level of greater than 130 mg/dl, and for HDL-C, a level of less than 40 mg/dl for men or less than 50 mg/dl for women is considered abnormal (28).
2.4 Outcomes
The outcome of interest was all-cause and CVD mortality. We defined CVD as a composite of CHD and stroke. Mortality causes were recorded using the international Classification of Diseases (ICD-10) codes. The study utilized ICD-10 codes I20-I25 for CHD and ICD-10 codes I60-I69 for stroke. All-cause mortality was defined as deaths resulting from any cause, while CVD mortality was defined as deaths resulting from either CHD or stroke.
2.5 Statistical analysis
Baseline characteristics of participants were presented based on their grouping by all-cause or CVD mortality, and the Kolmogorov-Smirnov test was used to verify the normal distribution of the data. Continuous variables that followed a normal distribution were described as mean (standard deviation), while non-normal variables were described as median (interquartile range). Descriptive variables were presented as frequencies and percentages. To compare baseline characteristics, categorical variables were analyzed using the Pearson chi-square test and continuous variables were analyzed using the Kruskal-Wallis H test.
Cox proportional risk regression models were used to estimate the hazard ratio (HR) and 95% confidence interval (CI) of the TyG index, LDL-C, and HDL-C for all-cause or CVD mortality. Model 1 was not adjusted, while model 2 was adjusted for age and gender at baseline. Finally, model 3 was adjusted for marital status, smoking, alcohol consumption, physical activity, SBP, DBP, BMI, WC, history of T2DM, and history of hypertension, based on model 2.
Restricted cubic spline plots were used to characterize the dose-response associations and to examine potential linear or non-linear associations between the TyG index, LDL-C, and HDL-C as continuous variables, and all-cause or CVD mortality. The three nodes of the cubic spline curve were set at the 10th, 50th, and 90th percentiles, respectively. The overall association was initially assessed for significance, and if significant, the results of the linear and non-linear tests were examined. A significance level of P <0.05 was reached for both the overall association test, which indicated that the overall association was significant, and a non-linear level of P <0.05, which indicated the presence of a non-linear association.
Thresholds were estimated by testing all possible values and selecting the threshold point with the highest likelihood. Additionally, a two-segment Cox proportional risk model was used to examine the relationship between TyG index, LDL-C or HDL-C, and the risk of all-cause or CVD mortality on both sides of the inflection point.
In subgroup analyses, participants were stratified based on gender (male/female), and age (<65/≥65) at baseline to test for differences in outcomes across subgroups. To test the robustness of the current study, we performed a sensitivity analysis. Participants with less than two years of follow-up were excluded from the principal component analysis.
Statistical analyses were conducted using R software, version 4.1.3. All P-values were 2-sided and a P < 0.05 was considered statistically significant unless otherwise stated.
3 Result
3.1 Baseline characteristics of study participants
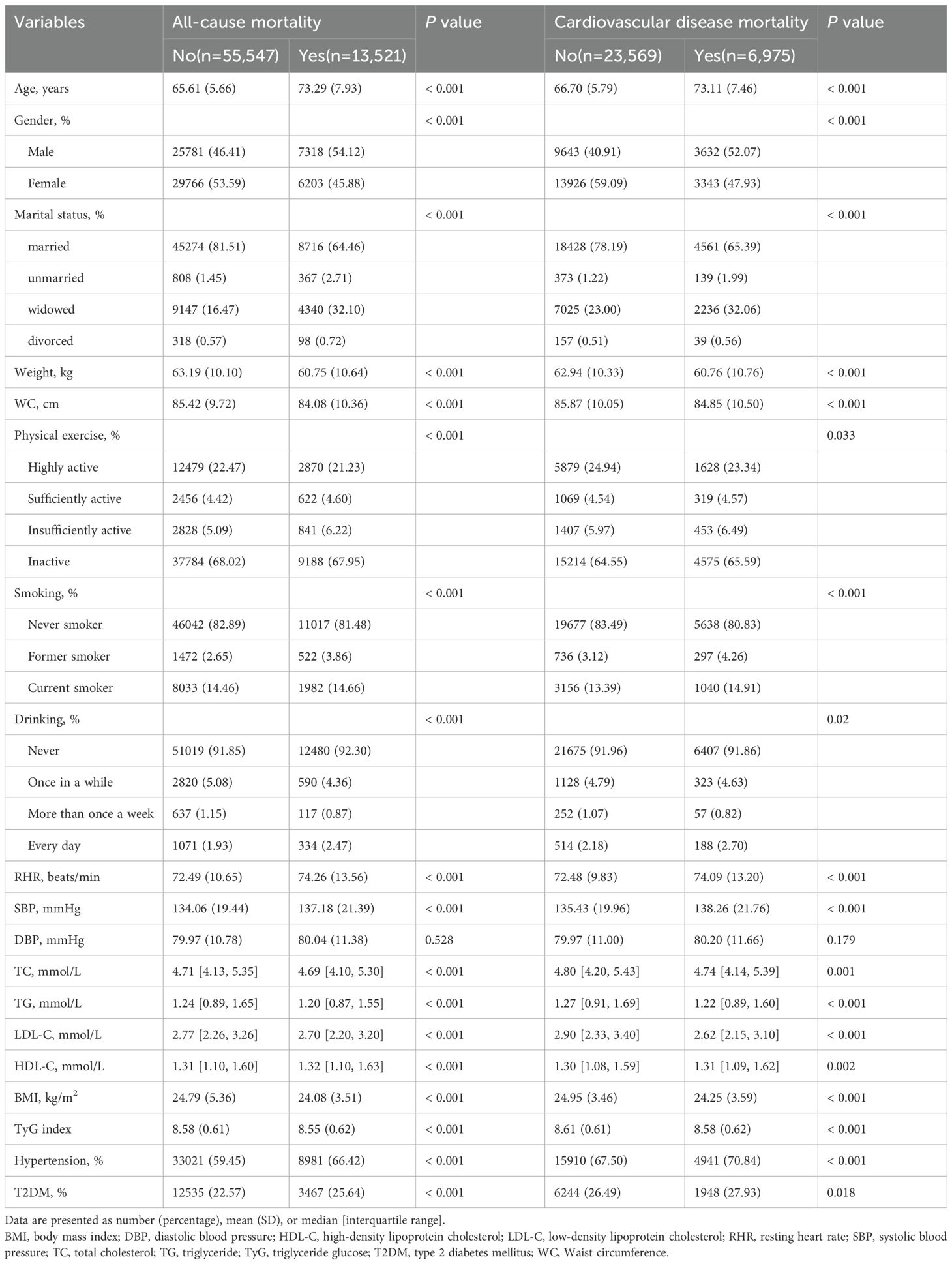
Table 1 shows baseline characteristics for all participants. The baseline data were analyzed for 69,068 older participants (median age 65 years [interquartile range 61-71]). During the 400,094 person-year follow-up period (median follow-up time 5.8 years [interquartile range 3.0-9.12]), 13664 deaths were recorded, of which 7045 were due to CVD. During the follow-up period, individuals who died were more likely to be male, had no spouse, were less physically active, were former or current smokers, drank alcohol daily, had lower weight, WC, BMI, TyG index, TC, TG, LDL-C, and higher RHR, SBP, DBP, HDL-C, and were more likely to have hypertension and T2DM. Similarly, individuals who died from CVD had similar baseline characteristics. In addition, according to the TyG index, LDL-C, and HDL-C, there were significant differences between the four groups in terms of age, gender, marital status, WC, BMI, smoking, alcohol consumption, physical activity, TC, TG, hypertension, and T2DM (Supplementary Tables S1-S3).
3.2 Association of TyG index and LDL-C or HDL-C with all-cause and CVD mortality
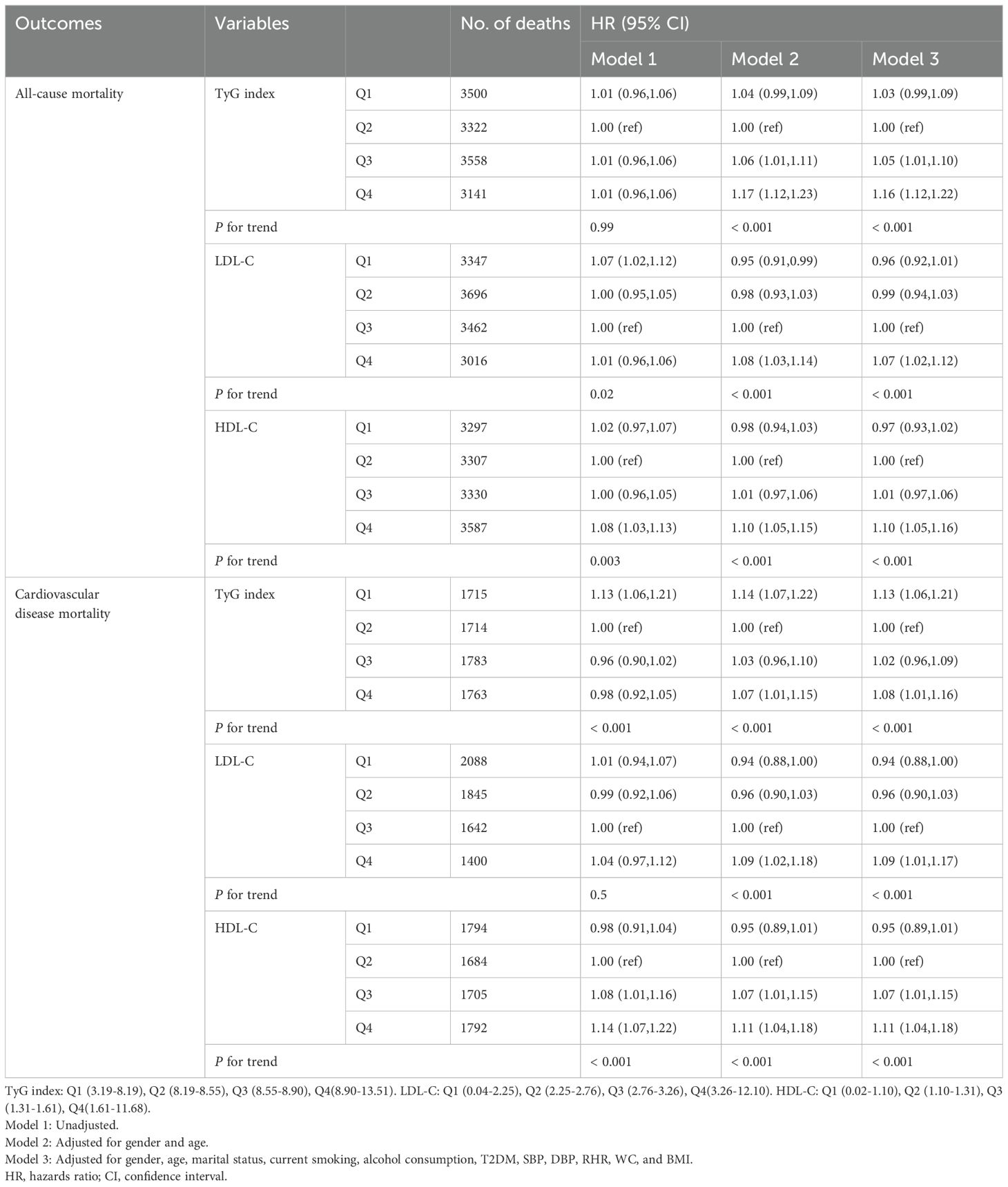
After adjusting for covariates such as age, gender, marital status, physical activity, smoking, alcohol consumption, BMI, WC, hypertension, and T2DM, Cox proportional risk analyses showed that the multivariate-adjusted HR (95% CI) for all-cause mortality in the first, third, and fourth quartiles of the TyG index compared with the second quartile were 1.03 (0.99,1.09), 1.05 (1.01,1.10), and 1.16 (1.12,1.22), and the multivariable-adjusted HR (95% CI) for CVD mortality was 1.13 (1.06,1.21), 1.02 (0.96,1.09), and 1.08 (1.01,1.16), respectively.
At the same time, the risk of all-cause and CVD mortality increased significantly with increasing quartiles of LDL-C and HDL-C. Compared with the third quartile of the LDL-C, the multivariable-adjusted HR (95% CI) for all-cause mortality in the first, second, and fourth quartiles of LDL-C was 0.96 (0.92,1.01), 0.99 (0.94,1.03), and 1.07 (1.02,1.12), and that for CVD mortality was were 0.94 (0.88,1.00), 0.96 (0.90,1.03), and 1.09 (1.01,1.17), respectively. Compared with the second quartile of the HDL-C, the multivariable-adjusted HR (95% CI) for all-cause mortality in the first, third, and fourth quartiles of HDL-C was 0.97 (0.93,1.02), 1.01 (0.97,1.06), and 1.10 (1.05,1.16), and that for CVD mortality was were 0.95 (0.89,1.01), 1.07 (1.01,1.15), and 1.11 (1.04,1.18), respectively. In models 2 and 3, LDL-C and HDL-C were associated with an increasing trend in all-cause and CVD mortality (Table 2, P for trend all <0.001).
Cox proportional risk regression models with restricted cubic spline were used to estimate the dose-response relationships of TyG index, LDL-C, and HDL-C with all-cause and CVD mortality. The three nodes of the cubic spline curve were set at the 10th, 50th, and 90th percentiles, respectively. The results showed non-linear associations between TyG index and all-cause (Figure 2A, P non-linear <0.001) and CVD mortality (Figure 2D, P non-linear <0.001) after adjusting for covariates in model 3. The study found J-shaped associations between both LDL-C and all-cause mortality (Figure 2B, P non-linear <0.05) and HDL-C and CVD mortality (Figure 2F, P non-linear <0.05). However, no non-linear associations were found between LDL-C and all-cause mortality, and HDL-C and CVD mortality (Figures 2C, E, all P non-linear >0.05). It is important to note that the TyG index and CVD mortality had a U-shaped association.
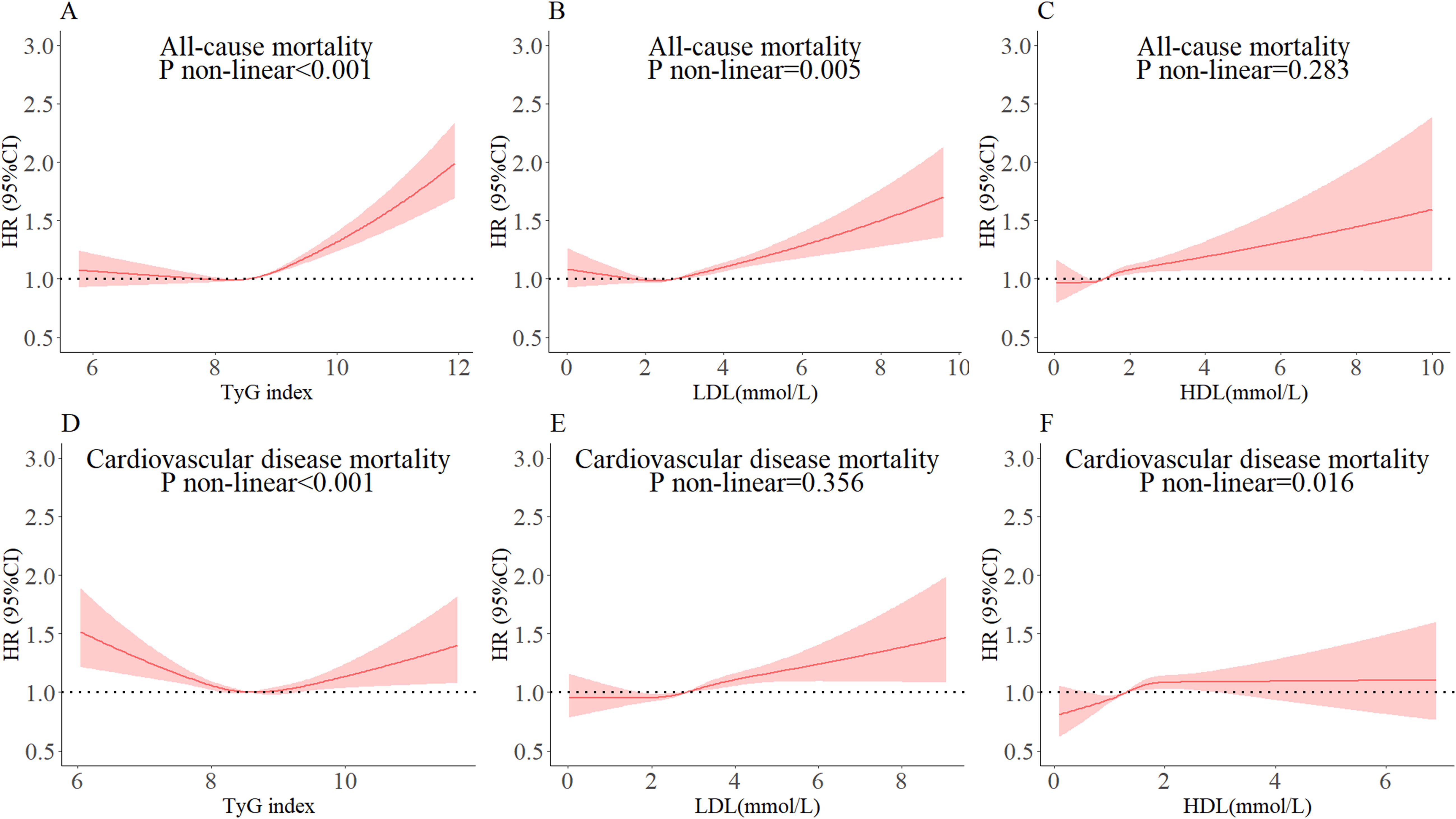
Figure 2. Dose-response relationships of TyG index, LDL-C, and HDL-C with all-cause and cardiovascular mortality. The cut-off levels for TyG index in all-cause and cardiovascular mortality were 8.56 (A) and 8.74 (D), for LDL-C 1.66 (B) and 2.84 (E), and for HDL-C 1.36 (C) and 1.30 (F), respectively. The circles represent the points (5, 25, 50, 75, and 95 percentiles) where the nodes were placed. The region between the two dotted lines represents the 95% confidence interval (95% CI). The model was adjusted for gender, age, marital status, current smoking, alcohol consumption, T2DM, SBP, DBP, RHR, WC, and BMI.
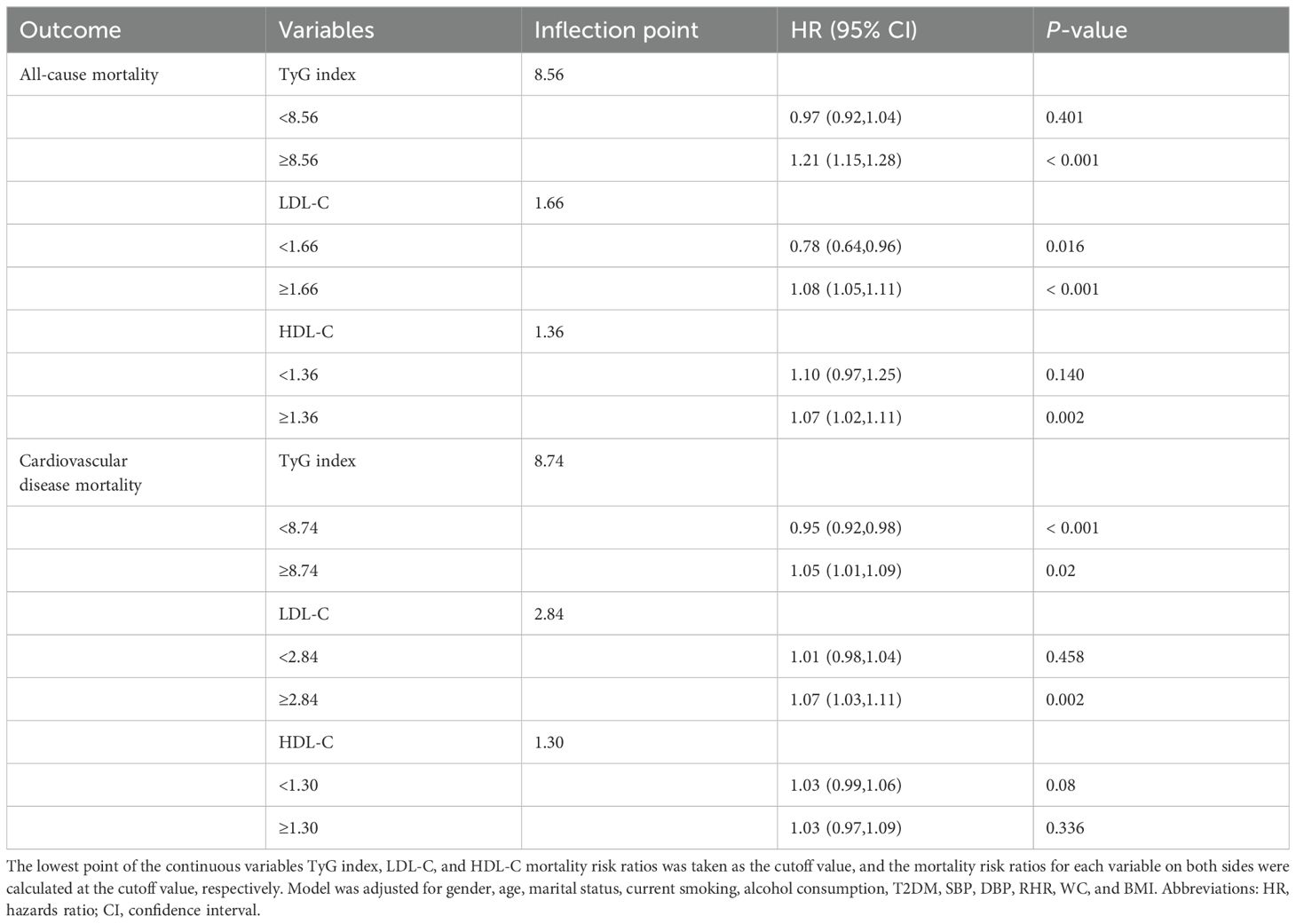
The inflection points of TyG index, LDL-C, or HDL-C for all-cause and CVD mortality were determined using a two-segment Cox proportional risk-based regression model (Table 3). After adjusting for covariates in model 3, both TyG index, LDL-C, and HDL-C were found to be significantly and positively associated with all-cause and CVD mortality when above the inflection point. It should be noted that CVD mortality decreased by 5% when the TyG index was below the inflection point (HR: 0.95, 95% CI: 0.92, 0.98). Additionally, when LDL-C was below the inflection point, all-cause mortality decreased by 22% (HR: 0.78, 95% CI: 0.64, 0.96).
To examine the relationship between the TyG index and LDL-C or HDL-C and the risk of all-cause and CVD mortality, we divided the participants into four subgroups based on their baseline TyG index levels and whether their LDL-C or HDL-C levels were within the normal range (Table 4). Compared to the low TyG index combined with LDL-C normal group, participants in the high TyG index combined with LDL-C abnormal group had a 14% (adjusted HR: 1.14, 95% CI: 1.08,1.20), and 13% (adjusted HR: 1.13, 95% CI: 1.04,1.22) increase in all-cause and CVD mortality, respectively. No significant interactive effects of TyG index and LDL-C on the risk of all-cause and CVD mortality. Participants in the high TyG index combined with the abnormal HDL-C group had an 11% increase in all-cause mortality compared to the low TyG index combined with the normal HDL-C group (adjusted HR: 1.11, 95% CI: 1.04,1.17). However, a high TyG index combined with HDL-C abnormality did not significantly increase CVD mortality. Similarly, there were no significant interactive effects of TyG index and HDL-C on the risk of all-cause and CVD mortality.
3.3 Subgroup analyses and sensitivity analyses
Subgroup analyses showed that TyG index and LDL-C or HDL-C were more consistently positively associated with all-cause mortality by gender, and age (Figure 3). Sensitivity analyses produced results consistent with the main analysis (Supplementary Table S4, Supplementary Figure S1).
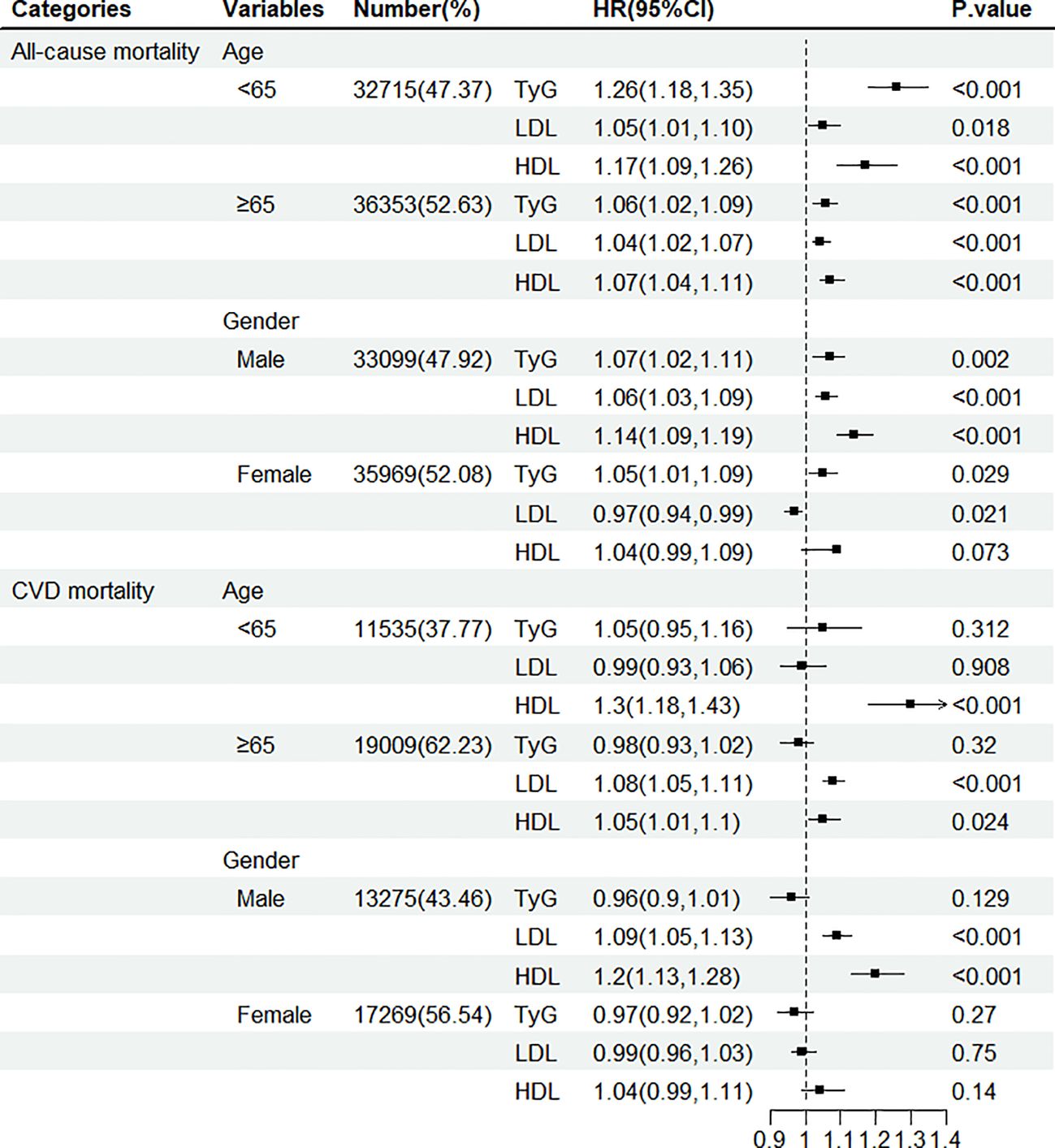
Figure 3. Hazard ratio (95% confidence interval) of all-cause and CVD mortality for per 1-SD increase in TyG index, LDL-C, and HDL-C according to gender and age.
4 Discussion
In this study, we found that after adjusting for various covariates, the TyG index had a non-linear association with the risk of all-cause and CVD mortality. LDL-C had a non-linear association with the risk of all-cause mortality and a linear with CVD mortality. HDL-C had a linear association with the risk of all-cause mortality and a non-linear with CVD mortality. Additionally, through threshold effect analysis, we have identified a turning point for the TyG index (8.56 for all-cause mortality and 8.74 for CVD mortality). At this point, a high TyG index combined with abnormal LDL-C significantly increases the risk of all-cause and CVD mortality. Furthermore, the combination of abnormal HDL-C significantly increases the risk of all-cause mortality only. It is noteworthy that the fourth quartile of the TyG index is associated with lower mortality than the third quartile. This finding is inconsistent with the j-shaped curve observed in the restricted cubic spline plot. This apparent incongruity may have arisen because the value of the vertical coordinate of the restricted cubic spline plot plotted under the survival analysis condition is the risk ratio, whereas the number of outcome events presented in the table represents only one of the two variables of the survival analysis, i.e., it is the survival outcome. However, in the context of survival analysis, it is essential to consider the survival time of the subjects. The data demonstrated that individuals who died in the fourth quartile of the TyG index survived for a significantly longer period than those who died in the other three quartiles. Although the risk ratio increased in the fourth quartile group, the magnitude was less pronounced, suggesting that fewer individuals died in the fourth quartile group than in the third quartile group.
Some aspects of our findings are consistent with previous studies that have shown a positive correlation between higher TyG index and higher all-cause and CVD mortality. For instance, a cohort study conducted by the National Health and Nutrition Examination Survey, which included 20,194 participants and had a follow-up period of 9.82 years, demonstrated a non-linear relationship between the TyG index and all-cause and CVD mortality in the general population. The study found that the lowest risk of all-cause or CVD mortality occurred when the TyG index was 9.36 or 9.52 (29). Another recent study, which only included adults 18 years of age and older, similarly demonstrated a non-linear association between the TyG index and all-cause and CVD mortality. However, this study found a shift from a non-linear association to a linear positive association between the TyG index and CVD mortality when focusing on study participants aged 45-64 years (30). One possible explanation for the association between age and the TyG index is that younger people are believed to be more susceptible to IR (31), which is closely linked to the TyG index (32). As a result, as the TyG index increases, this group is more likely to develop concomitant metabolic diseases that contribute to CVD mortality. Additionally, studies investigating the relationship between the TyG index and cardiovascular metabolic multimorbidity (CMM) have discovered U-shaped associations between the TyG index and all-cause and CVD mortality (33). However, conflicting results exist regarding the effect of the TyG index on all-cause and CVD mortality in older adults. A meta-analysis that included 12 cohort studies found no statistical correlation between the TyG index and all-cause and CVD mortality (34). This lack of correlation may be due to the small number of studies included in the analysis.
Previous studies have found a strong association between lower or higher elevations of FPG and CVD morbidity and mortality even in nondiabetic patients, with a J-shaped association between blood glucose levels and CVD mortality (35). A study of TG reported that elevated TG was associated with a reduced risk of CVD mortality (36). Low TG and blood glucose may represent individuals in a poorer nutritional state, and in addition, hypoglycemia-induced thrombosis contributes to increased CVD mortality (37). Therefore, we need to maintain normal TyG index levels.
A cohort study in Denmark found that higher LDL-C was associated with an increased risk of all-cause and CVD mortality (18). Similarly, a meta-analysis of 14 studies found that LDL-C is associated with a higher risk of CVD mortality (38). These findings support the view that LDL-C is the ‘bad cholesterol’ and that higher levels of LDL-C are associated with an increased risk of death (14). Our study is similar to these studies and similarly demonstrates the association of higher LDL-C with death from CVD. Also, the fact that LDL-C collection preceded and the outcome of death appeared later in the study population confirms the causal relationship between LDL-C and CVD mortality. Regarding the evidence for HDL-C, it was found that higher levels of HDL-C were associated with an increased risk of all-cause mortality (39). This is consistent with the findings of a cohort study conducted in Copenhagen, where HDL-C is known as an ‘anti-atherogenic lipoprotein’ that prevents atherosclerosis and slows the onset of CVD (40). However, our study found the opposite. Individuals who died of all-cause and cardiovascular disease during the follow-up period were, on average, approximately seven to eight years older than those who survived. However, individuals with lower high-density lipoprotein cholesterol (HDL-C) may have died during the 5.8-year follow-up period and therefore could not be included in the study. Due to the survival effect, high-density lipoprotein cholesterol (HDL-C) was observed to be higher in the deceased cohort than in the surviving cohort. Consequently, it is plausible that elevated HDL-C levels may be associated with an increased risk of all-cause mortality.
In this study, we analyzed the interaction between the TyG index and LDL-C or HDL-C about the risk of all-cause and CVD mortality. Our findings suggest that an elevated TyG index is more strongly associated with all-cause and CVD mortality in subjects with abnormal LDL-C, and with all-cause mortality in those with abnormal HDL-C. It is important to note that the TyG index and serum cholesterol level are two independent measurements. However, the TyG index is obtained from the combined measurement of TG and FPG. TG and LDL-C or HDL-C levels are often considered interrelated biomarkers of the underlying state of the circulatory and cardiovascular systems. Hypertension, hyperlipidemia, and hyperglycemia have been referred to as the ‘three highs.’ Studies have demonstrated that they interact with each other (41). It is worth mentioning that previous researchers have used the ratio of TG to HDL-C in combination with the TyG index to explore associations with the development of CVD (42). The potential biological mechanisms through which the TyG index and LDL-C or HDL-C are linked to all-cause mortality and CVD mortality, respectively, are outlined below. A reduction in NO results in impaired endothelial-dependent vasodilation. Moreover, impaired vasodilation is not a cause or risk of diabetes; on the contrary, endothelial dysfunction is a frequent consequence of diabetes (43, 44). Furthermore, evidence indicates that IR serves as a marker for cardiovascular metabolic diseases (CMD) and dyslipidemia (45). Elevated blood lipids have been demonstrated to promote the formation of atherosclerotic plaques (46). Furthermore, dyslipidemia is associated with an increased risk of thrombotic events and, consequently, mortality from cardiovascular causes (47). The aforementioned mechanisms may partially elucidate the potential correlation between the TyG index and lipids and the risk of mortality in patients with cardiovascular disease (48). There was no consistency found between the TyG index and LDL-C or HDL-C level to all-cause mortality across different age and sex groups. The effect of the TyG index and LDL-C or HDL-C level on all-cause mortality decreased with age among females. However, a positive correlation still reached a significant level even among those over 65 years of age, which is consistent with previous findings (49–51). It is worth noting that the risk of all-cause mortality was greater for each 1 SD increase in HDL-C in participants younger than 65 years of age compared with participants older than 65 years of age. This suggests that older adults, especially those in the early stages of aging, should pay particular attention to HDL-C metrics. The reason for this phenomenon may be related to the fact that the participants were in a degenerative stage of body functions when they first entered old age.
The study has several strengths. Firstly, the data were obtained from the records of annual health checkups in Xinzheng City, Henan Province, China, focusing on adults aged 60 years and older, which represents a large sample size. Secondly, instruments were used to measure participants’ serum cholesterol levels rather than relying on self-reporting, reducing information bias. Additionally, our mortality data were obtained from the Centers for Disease Control and Prevention, and the causes of death were reviewed by at least three clinical experts. Finally, no previous study has explored the interaction between the TyG index and serum cholesterol levels, and we explored whether there was an interaction between the two. However, this study has some limitations. Firstly, individuals who died of all-cause and CVD during the follow-up period were approximately 7 to 8 years older than those who did not die. However, during the follow-up time of 5.8 years, some of their peers with higher TyG index, LDL-C, and lower HDL-C may have died, and thus they could not be recruited into the study. Due to survival effects, the TyG index and LDL-C were lower in those who died than in those who did not die, and HDL-C was higher than in those who died. It is therefore possible that the data presented in the study may be biased. Secondly, the latent category trajectory model (LCGM) analysis highlights the importance of considering dynamic changes in participant measures during follow-up, regardless of baseline. Furthermore, the questionnaire did not include information on diet and cardiorespiratory fitness, which would have helped to eliminate the possibility of reverse causation and residual confounding. Additionally, the study focused on individuals aged 60 years and older, which may limit generalizability to the entire population.
5 Conclusion
In this study, we found that TyG index and LDL-C or HDL-C were significantly associated with an increased risk of all-cause and CVD mortality in a Chinese population of older adults. Furthermore, a high TyG index combined with abnormal LDL-C levels was also associated with an elevated risk. These findings suggest that routine monitoring and control of TyG index and lipids should be strengthened, and these indices should be included in risk assessment as risk factors for all-cause and CVD mortality.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Zhengzhou University (ID: ZZUIRB2019-019). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DS: Writing – review & editing, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization, Writing – original draft. ZA: Writing – review & editing, Conceptualization. LC: Writing – review & editing, Funding acquisition. XC: Writing – review & editing, Investigation. WW: Writing – review & editing, Data curation, Conceptualization. YFC: Writing – review & editing, Validation. YLC: Writing – review & editing, Resources. SS: Writing – review & editing, Resources.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Key Research and Development Program “Research on prevention and control of major chronic non-communicable diseases” of China (Grant No:2017YFC1307705).
Acknowledgments
The investigators are grateful to the dedicated participants and all research staff of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1422086/full#supplementary-material
Abbreviations
ASCVD, Atherosclerotic Cardiovascular Disease; BMI, Body mass index; CDC, Center for Disease Control and Prevention; CHD, Coronary heart disease; CMD, Cardiovascular multimorbidity disease; CMM, Cardiovascular metabolic multimorbidity; CVD, Cardiovascular disease; DBP, Diastolic blood pressure; FPG, Fasting plasma glucose; GBD, Global Burden of Disease; HDL-C, High-density lipoprotein cholesterol; HOMA-IR, Homeostasis model assessment of insulin resistance; IQR, Interquartile range; IR, Insulin resistance; LDL-C, Low-density lipoprotein cholesterol; MI, Myocardial infarction; Mts, Metabolic syndrome; NO, Nitric oxide; RHR, Resting heart rate; SBP, Systolic blood pressure; SD, Standard deviation; TC, Total cholesterol; TG, Triglyceride; TyG, Triglyceride-glucose; T2DM, Type 2 diabetes mellitus; WC, Waist circumference.
References
1. Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet (London England). (2016) 387:251–72. doi: 10.1016/s0140-6736(15)00551-6
2. Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, et al. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet (London England). (2013) 381:1987–2015. doi: 10.1016/s0140-6736(13)61097-1
3. National Centre For Cardiovascular Diseases The Writing Committee of The Report on Cardiovascular Health And Diseases In China. The W: report on cardiovascular health and diseases in China 2022: an updated summary. Biomed Environ sciences: BES. (2023) 36:669–701. doi: 10.3967/bes2024.162
4. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
5. Lee SH, Park SY, Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. (2022) 46:15–37. doi: 10.4093/dmj.2021.0280
6. Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. (2015) 6:456–80. doi: 10.4239/wjd.v6.i3.456
7. Htay T, Soe K, Lopez-Perez A, Doan AH, Romagosa MA, Aung K. Mortality and cardiovascular disease in type 1 and type 2 diabetes. Curr Cardiol Rep. (2019) 21:45. doi: 10.1007/s11886-019-1133-9
8. Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC, et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: A systematic review. Int J Endocrinol. (2020) 2020:4678526. doi: 10.1155/2020/4678526
9. Minh HV, Tien HA, Sinh CT, Thang DC, Chen CH, Tay JC, et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J Clin hypertension (Greenwich Conn). (2021) 23:529–37. doi: 10.1111/jch.14155
10. Wang T, Li M, Zeng T, Hu R, Xu Y, Xu M, et al. Association between insulin resistance and cardiovascular disease risk varies according to glucose tolerance status: A nationwide prospective cohort study. Diabetes Care. (2022) 45:1863–72. doi: 10.2337/dc22-0202
11. Junren Z, Runlin G. Chinese guidelines for prevention and control of dyslipidemia in adults (2016 revision). China Circ Mag. (2016) 31.
12. Zhao M, Xiao M, Tan Q, Lu F. Triglyceride glucose index as a predictor of mortality in middle-aged and elderly patients with type 2 diabetes in the US. Sci Rep. (2023) 13:16478. doi: 10.1038/s41598-023-43512-0
13. Yu Y, Wang J, Ding L, Huang H, Cheng S, Deng Y, et al. Sex differences in the nonlinear association of triglyceride glucose index with all-cause and cardiovascular mortality in the general population. Diabetol Metab Syndrome. (2023) 15:136. doi: 10.1186/s13098-023-01117-7
14. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. (2017) 38:2459–72. doi: 10.1093/eurheartj/ehx144
15. Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arterioscler (Dallas Tex). (1988) 8:737–41. doi: 10.1161/01.atv.8.6.737
16. Liu C, Dhindsa D, Almuwaqqat Z, Ko YA, Mehta A, Alkhoder AA, et al. Association between high-density lipoprotein cholesterol levels and adverse cardiovascular outcomes in high-risk populations. JAMA Cardiol. (2022) 7:672–80. doi: 10.1001/jamacardio.2022.0912
17. Sung KC, Huh JH, Ryu S, Lee JY, Scorletti E, Byrne CD, et al. Low levels of low-density lipoprotein cholesterol and mortality outcomes in non-statin users. J Clin Med. (2019) 8. doi: 10.3390/jcm8101571
18. Johannesen CDL, Langsted A, Mortensen MB, Nordestgaard BG. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ (Clinical Res ed). (2020) 371:m4266. doi: 10.1136/bmj.m4266
19. Ke C, Shen Y. Letter by ke and shen regarding article, “Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease: results from the cooper center longitudinal study. Circulation. (2019) 139:2190–1. doi: 10.1161/circulationaha.118.038328
20. Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. (2019) 16:203–12. doi: 10.1038/s41569-018-0119-4
21. Evers IM, de Valk HW, Visser GHA. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. Obstetrics Gynecol. (2004) 104. doi: 10.1136/bmj.38043.583160.EE
22. Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J periodontol. (2000) 71:743–51. doi: 10.1902/jop.2000.71.5.743
23. Choi YJ, Lee DH, Han KD, Kim HS, Yoon H, Shin CM, et al. The relationship between drinking alcohol and esophageal, gastric or colorectal cancer: A nationwide population-based cohort study of South Korea. PloS One. (2017) 12:e0185778. doi: 10.1371/journal.pone.0185778
24. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci sports Exercise. (2000) 32:S498–504. doi: 10.1097/00005768-200009001-00009
25. UN Centre for Human Settlements (Habitat) WHO. Global status report on non-communicable diseases 2010. (2011).
26. Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes/metabol Res Rev. (2019) 35:e3158. doi: 10.1002/dmrr.3158
27. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1210/jc.2010-0288
28. Alavi Tabatabaei G, Mohammadifard N, Rafiee H, Nouri F, Maghami Mehr A, Najafian J, et al. Association of the triglyceride glucose index with all-cause and cardiovascular mortality in a general population of Iranian adults. Cardiovasc Diabetol. (2024) 23:66. doi: 10.1186/s12933-024-02148-8
29. Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The triglyceride-glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front Cardiovasc Med. (2020) 7:628109. doi: 10.3389/fcvm.2020.628109
30. Chen J, Wu K, Lin Y, Huang M, Xie S. Association of triglyceride glucose index with all-cause and cardiovascular mortality in the general population. Cardiovasc Diabetol. (2023) 22:320. doi: 10.1186/s12933-023-02054-5
31. Sun M, Guo H, Wang Y, Ma D. Association of triglyceride glucose index with all-cause and cause-specific mortality among middle age and elderly US population. BMC geriatrics. (2022) 22:461. doi: 10.1186/s12877-022-03155-8
32. Liu R, Li L, Wang L, Zhang S. Triglyceride-glucose index predicts death in patients with stroke younger than 65. Front Neurol. (2023) 14:1198487. doi: 10.3389/fneur.2023.1198487
33. Liu Q, Zhang Y, Chen S, Xiang H, Ouyang J, Liu H, et al. Association of the triglyceride-glucose index with all-cause and cardiovascular mortality in patients with cardiometabolic syndrome: a national cohort study. Cardiovasc Diabetol. (2024) 23:80. doi: 10.1186/s12933-024-02152-y
34. Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. (2022) 21:124. doi: 10.1186/s12933-022-01546-0
35. Lee JH, Han K, Huh JH. The sweet spot: fasting glucose, cardiovascular disease, and mortality in older adults with diabetes: a nationwide population-based study. Cardiovasc Diabetol. (2020) 19:44. doi: 10.1186/s12933-020-01021-8
36. Ambrosy AP, Yang J, Sung SH, Allen AR, Fitzpatrick JK, Rana JS, et al. Triglyceride levels and residual risk of atherosclerotic cardiovascular disease events and death in adults receiving statin therapy for primary or secondary prevention: insights from the KP REACH study. J Am Heart Assoc. (2021) 10:e020377. doi: 10.1161/jaha.120.020377
37. Li G, Zhong S, Wang X, Zhuge F. Association of hypoglycaemia with the risks of arrhythmia and mortality in individuals with diabetes - a systematic review and meta-analysis. Front Endocrinol. (2023) 14:1222409. doi: 10.3389/fendo.2023.1222409
38. Jung E, Kong SY, Ro YS, Ryu HH, Shin SD. Serum cholesterol levels and risk of cardiovascular death: A systematic review and a dose-response meta-analysis of prospective cohort studies. Int J Environ Res Public Health. (2022) 19. doi: 10.3390/ijerph19148272
39. Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. (2017) 38:2478–86. doi: 10.1093/eurheartj/ehx163
40. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. Framingham Study Am J Med. (1977) 62:707–14. doi: 10.1016/0002-9343(77)90874-9
41. Zanchetti A. Hyperlipidemia in the hypertensive patient. Am J Med. (1994) 96:3s–8s. doi: 10.1016/0002-9343(94)90225-9
42. Mirshafiei H, Darroudi S, Ghayour-Mobarhan M, Esmaeili H, AkbariRad M, Mouhebati M, et al. Altered triglyceride glucose index and fasted serum triglyceride high-density lipoprotein cholesterol ratio predict incidence of cardiovascular disease in the Mashhad cohort study. BioFactors (Oxford England). (2022) 48:643–50. doi: 10.1002/biof.1816
43. Molina MN, Ferder L, Manucha W. Emerging role of nitric oxide and heat shock proteins in insulin resistance. Curr hypertension Rep. (2016) 18:1. doi: 10.1007/s11906-015-0615-4
44. Trifunovic D, Stankovic S, Sobic-Saranovic D, Marinkovic J, Petrovic M, Orlic D, et al. Acute insulin resistance in ST-segment elevation myocardial infarction in non-diabetic patients is associated with incomplete myocardial reperfusion and impaired coronary microcirculatory function. Cardiovasc Diabetol. (2014) 13:73. doi: 10.1186/1475-2840-13-73
45. Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabol: Clin Exp. (2021) 119:154766. doi: 10.1016/j.metabol.2021.154766
46. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. (2018) 17:122. doi: 10.1186/s12933-018-0762-4
47. Zhao X, Wang Y, Chen R, Li J, Zhou J, Liu C, et al. Triglyceride glucose index combined with plaque characteristics as a novel biomarker for cardiovascular outcomes after percutaneous coronary intervention in ST-elevated myocardial infarction patients: an intravascular optical coherence tomography study. Cardiovasc Diabetol. (2021) 20:131. doi: 10.1186/s12933-021-01321-7
48. Adeva-Andany MM, Ameneiros-Rodríguez E, Fernández-Fernández C, Domínguez-Montero A, Funcasta-Calderón R. Insulin resistance is associated with subclinical vascular disease in humans. World J Diabetes. (2019) 10:63–77. doi: 10.4239/wjd.v10.i2.63
49. Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL. Assessment of pancreatic β-cell function: review of methods and clinical applications. Curr Diabetes Rev. (2014) 10:2–42. doi: 10.2174/1573399810666140214093600
50. Wu M, Liao S, Si J, Guo X, Kang L, Xu B, et al. Association of low-density lipoprotein-cholesterol with all-cause and cause-specific mortality. Diabetes Metab syndrome. (2023) 17:102784. doi: 10.1016/j.dsx.2023.102784
Keywords: triglyceride-glucose index, low density lipoprotein cholesterol, high density lipoprotein cholesterol, all-cause mortality, cardiovascular disease mortality
Citation: Su D, An Z, Chen L, Chen X, Wu W, Cui Y, Cheng Y and Shi S (2024) Association of triglyceride-glucose index, low and high-density lipoprotein cholesterol with all-cause and cardiovascular disease mortality in generally Chinese elderly: a retrospective cohort study. Front. Endocrinol. 15:1422086. doi: 10.3389/fendo.2024.1422086
Received: 23 April 2024; Accepted: 07 October 2024;
Published: 29 October 2024.
Edited by:
Matthias Blüher, Leipzig University, GermanyReviewed by:
Oscar Perez-Mendez, Monterrey Institute of Technology and Higher Education (ITESM), MexicoArianna Toscano, University Hospital of Policlinico G. Martino, Italy
Copyright © 2024 Su, An, Chen, Chen, Wu, Cui, Cheng and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songhe Shi, c3NoQHp6dS5lZHUuY24=
 Donghai Su
Donghai Su Zhantian An2
Zhantian An2 Yulin Cheng
Yulin Cheng Songhe Shi
Songhe Shi