- 1Department of Pediatric Diabetes and Endocrinology, Clinique Pédiatrique, Centre Hospitalier, Luxembourg, Luxembourg
- 2Deep Digital Phenotyping Research Unit, Department of Precision Health, Luxembourg Institute of Health, Strassen, Luxembourg
- 3Competence Center for Methodology and Statistics, Luxembourg Institute of Health, Strassen, Luxembourg
- 4Southern Health NHS Foundation Trust, Southampton, United Kingdom
- 5Institute of Endocrinology, Sheba Medical Center, Tel Hashomer, Israel
- 6Division of Pediatric Endocrinology, Department of Pediatrics, University Hospital Brussels, Brussels, Belgium
- 7Research Unit Research Group Growth and Development (GRON), Vrije Universiteit Brussel, Brussels, Belgium
Aims: To compare impact of pump treatment and continuous glucose monitoring (CGM) with predictive low glucose suspend (SmartGuard) or user initiated CGM (iscCGM) on sleep and hypoglycemia fear in children with type 1 Diabetes and parents.
Methods: Secondary analysis of data from 5 weeks pump treatment with iscCGM (A) or SmartGuard (B) open label, single center, randomized cross-over study was performed. At baseline and end of treatment arms, sleep and fear of hypoglycemia were evaluated using ActiGraph and questionnaires.
Results: 31 children (6-14 years, male: 50%) and 30 parents (28-55 years) participated. Total sleep minutes did not differ significantly for children (B vs. A: -9.27; 95% CI [-24.88; 6.34]; p 0.26) or parents (B vs. A: 5.49; 95% CI [-8.79; 19.77]; p 0.46). Neither daytime sleepiness nor hypoglycemia fear in children or parents differed significantly between the systems. Neither group met recommended sleep criteria.
Conclusion: Lack of sleep and fear of hypoglycemia remain a major burden for children with diabetes and their parents. Whilst no significant differences between the systems were found, future technology should consider psychosocial impacts of diabetes and related technologies on children and parents’ lived experience to ensure parity of esteem between physical and mental health outcomes.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier NCT03103867.
1 Introduction
The daily management of type 1 diabetes (T1D) is a 24/7 challenge for children and their caregivers and may have a major negative impact on their sleep and quality of life (1–3).
Fear of nocturnal hypoglycemia is common and a significant concern amongst parents of children with T1D (4–6), and is associated with enhanced attention to frequent checking of their children’s glycemia or sensor values or to get up during the night (7, 8). Data show that fear of hypoglycemia can lead to chronic sleep disturbance for the parents as for their children with diabetes (9–11). This highly prevalent chronic sleep interruption can affect caregivers of children with T1D with negative effects on their daily functioning and well-being (12, 13).
New technologies have been introduced to facilitate and improve care with automated sensor-augmented pump (SAP) and predictive low glucose suspend and alerts (SmartGuard) or with user-initiated intermittently scanned continuous glucose monitoring (iscCGM, Freestyle libre).
SAP treatment leads to improved metabolic outcome (14). Alerts about hypo-and hyperglycemia are programmed in SAP in order to enable patients and their caregivers to react quickly to such information. The Minimed 640G pump with SmartGuard feature combines alerts with an automated insulin suspension to prevent hypoglycemia. The pump suspends insulin infusion when the sensor glucose (SG) is within 3.9 mmol/l (70 mg/dl) above the low limit and predicted to be 1.1 mmol/l (20 mg/dl) or lower above the low limit in 30 min. Glucose values and glucose trends are available on the pump screen (15).
A multicenter evaluation shows that SmartGuard technology significantly reduces the risk for hypoglycemia in pediatric diabetes patients without increasing HbA1c (16).
However, alerts may be perceived as intrusive and anxiety-inducing which can lead to diabetes distress and alert fatigue as well as nocturnal awakenings (8, 17).
Freestyle Libre 1 is a device measuring the interstitial glucose levels continuously. The results can be obtained when the patient/caregiver actively scans the sensor (iscCGM): no alerts are given for hypo-or hyperglycemic events, nor is information available when the sensor is not scanned. Data is lost when more than 8 hours elapse between scans. No communication exists between this glucose measurement and the insulin pump (15).
The evaluation of iscCGM being as safe as self-monitoring of blood glucose (SMBG) and having a better metabolic outcome than SMBG is demonstrated in children (18, 19).
The impact of these technologies on metabolic control has been studied before (20).
To our knowledge, no study has yet addressed the focus on comparing the impact of these two technologies on fear of hypoglycemia, quality and quantity of sleep in children and their caregivers. In this report we analyze these questions using questionnaires, sleep diaries and ActiGraph data in the QUEST trial (15).
2 Materials and methods
2.1 Ethics committee statement
This study was approved by the National Luxembourgish Ethics committee (CNER). Only pseudonymized data was used for the analysis.
2.2 Study design and randomization
The study had an open-label, single-center, randomized, two-period crossover design.
Each patient was randomly allocated; the sequence codes (A-B or B-A) were determined in advance (15).
2.3 Participants
Patients fulfilling the following inclusion criteria got included: age between 6 and 14 years, type 1 diabetes and on insulin pump treatment for at least 6 months and HbA1c ≤ 11% (≤ 96.72 mmol/mol) (30).
Exclusion criteria were physical or psychological disease likely to interfere with an appropriate conduct of the study and chronic sleep medication used by the patient or by the participant primary caregiver. Prior to enrollment, written informed consent was obtained from the parents and all children gave their informed assent (30).
2.4 Procedures
The participants were randomized either to treatment A, insulin pump Minimed 640G and independent iscCGM (Freestyle libre 1) or to treatment B, SAP with SmartGuard feature (Minimed 640G), each for 5 weeks. Following a 3 weeks washout period the participants crossed over to the other study arm for another 5 weeks.
No specific dietary advice was given.
The week before randomization as well as during the last 7 days of each treatment the participants and one of their caregivers (same reference person throughout the course of the study) wore a sleep device on the wrist (ActiGraph) and completed a sleep diary. Before the start and at the end of each treatment arm the subject and his caregiver were asked to fill in the questionnaires.
To evaluate the hypoglycemia fear, the Children’s Hypoglycemia Survey (CHS) and Hypoglycemia Fear survey for parents were used. The Children’s Hypoglycemia Survey (24 items) measures 3 areas of hypoglycemia fear: their general fear of hypoglycemia and its consequences, the children’s fear of hypoglycemia in a specific situation, and the children’s behavior to avoid hypoglycemia. The survey for parents is divided into 2 subscales-scores, one asking about parental worry about their child’s hypoglycemia (15 items), and the other about behavior to prevent hypoglycemia for their child (11 items) (21–25).
Daytime sleepiness in the children and their caregiver were evaluated using the Epworth sleepiness scale, a self-administered questionnaire which provides a measurement of the subject’s general level of daytime sleepiness (26). The Epworth Sleepiness Scale is defined based on questions about the chances to fall asleep in different situations. The score ranges from 0-24 with the following interpretation: score 0-5: lower normal daytime sleepiness/6-10: higher normal daytime sleepiness/11-12: mild excessive daytime sleepiness/13-15: moderate excessive daytime sleepiness/16-24: severe excessive daytime sleepiness (26).
The detailed conduct of the study was previously published (15).
The use of the two glucose measurement tools and the features of the Minimed 640G pump were explained during the dedicated training visit. All participants had access to a 24/7 diabetes hotline in case of technical or any other issues. Settings of the SmartGuard were standardized based on the current experience (20). The low limit was set at 3.4 mmol/l, with an insulin suspension at ≤7.3 mmol/l if the predicted value within 30 minutes was 4.5 mmol/l (15).
2.5 Methods
Randomization (ratio 1:1) was performed by a statistician with 4 blocks of 8 participants and equal treatment allocation based on prepared envelopes with the sequence code (A-B or B-A). In this randomized block design the sequence codes were randomly allocated to each block. This kind of design is used to minimize the effects of systematic error.
After consenting, the envelope was opened by the medical team to provide the participant with the allocated treatment sequence (15, 30).
Blinding was not possible for the participant nor the medical team.
A sample size of 36 patients with a minimum of 31 patients was calculated for a power of 80% (15).
To ensure data quality, double data entry was performed within Ennov Clinical software, and online logical controls were performed with correction of erroneous data values.
Hypoglycemia Index in children (subscales and Hypoglycemia Fear Survey in parent/caregiver (subscales for hypoglycemia worry and behavior) at baseline and at the end of each treatment arm were also analyzed by using a linear mixed model with the same independent parameters as described previously.
Total sleep (minutes) and total wake time (minutes) and number of awakenings during the last 7 days of each treatment arm were measured by ActiGraph, in children and in one of their caregivers. Sleep analysis was performed using ActiLife data analysis software. The detailed assessment of sleep patterns was previously published (15). The average sleep time per night for each visit was used as the outcome to compare the two different treatments. Additionally, the average number of awakenings and the average length of total wake time per night and visit was compared between the two devices. Where Actigraph measurement of sleep was divided into more than one sleep period (due to being awake and getting out of bed for more than 10 minutes), total sleep time (defined by ActiLife), time and number of awakenings (defined by ActiLife), number of get-ups (number of sleep periods - 1), and time of being out of bed (time from out of bed till sleep onset) was added up to have one measurement per night. Sleep time during the day (nap; went to bed between 12pm and 7pm) was excluded from the analysis. For number of awakenings and total wake time, the estimations of the ActiLife algorithm (Sadeh for children (10-25 years) and Cole-Kripke for adults) were used as outcomes (27, 28).
Time to bed, time out of bed and number of awakenings were also compared with the sleep diary and some parameters were adjusted according to the sleep diary if they seemed too unrealistic when calculated by ActiLife. We used the ActiLife settings for bedtime (5 consecutive asleep minutes) and wake time (first 10 consecutive minutes of awake time following a sleep period). The definition of sleep is based on the accelerometer data. If there is no movement for at least 5 minutes, the period is defined as sleep. Vacation time was not taken into account.
Characteristics of children and parents were presented using mean and standard deviations (SD) for continuous variables, median, 25% and 75% quartiles (Q1, Q3) for count variables and frequency and percentage for categorical variables. Characteristics for children are shown for the total study population and separated by treatment sequences. Z score BMI are calculated with the formula Z score = (X-m)/SD; X = BMI, m = mean, SD = standard deviation of BMI of the reference population (WHO growth reference (2006) data) with same sex and age (29).
Total sleep, quality of sleep (Epworth sleepiness scale and sleep diary) and number of awakenings were analyzed by using a linear mixed model and a naïve Fisher’s Exact test with treatment given (A vs. B), treatment sequence (A-B vs. B-A) and period of treatment (week 5 vs. week 13) as fixed effects factors and patient as a random effect.
Least square means and their 95% confidence intervals (CI) from the linear mixed models were reported as adjusted mean average sleep time and adjusted average number of awakenings in children and parents.
All test were two-tailed and a p-value<0.05 was claimed statistically significant. RStudio 2021.09.2 was used for statistical analysis.
3 Results
32 children, 16 male (50%), between 6 -14 years with a mean HbA1c of 7.47%, (58.14 mmol/mol), SD 0.59, a mean diabetes duration of 5.91 years (SD 3.29), being on insulin pumps for 5.07 (SD 3.87) years, were included in this study. Metabolic outcome as primary endpoint was reported previously (30). One child dropped out of the study after the first visit at baseline, without wearing neither the ActiGraph nor filling out any of the questionnaires and sleep diaries. 31 children (16 males) completed the study.
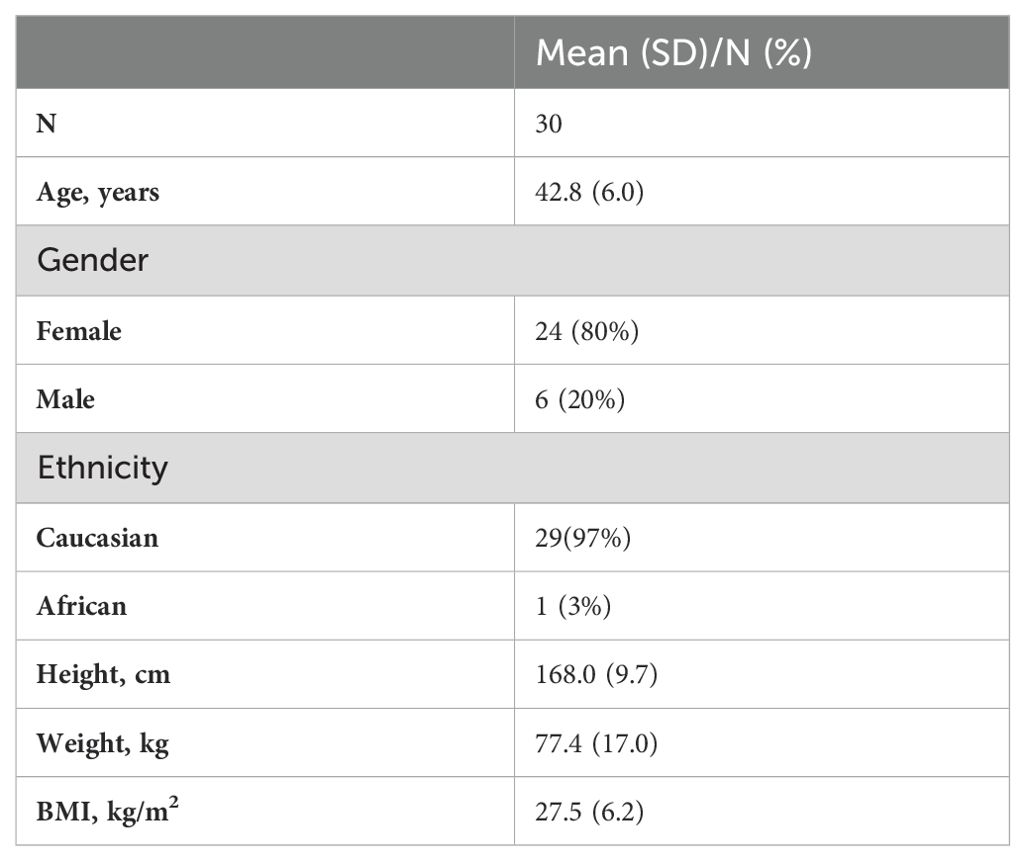
30 caregivers (24 females, 28 – 55 years (mean 42.77 years, SD 5.96)) participated in the study.
One parent had two children included in the study, therefore only one questionnaire and sleep diary was filled out and one Actigraph was worn by the parent. 28 parents and children answered all the questionnaires.
Table 1 (30) shows the demographic baseline values for study participants (31 children), Table 2 the data for the participating parents (30 caregivers).
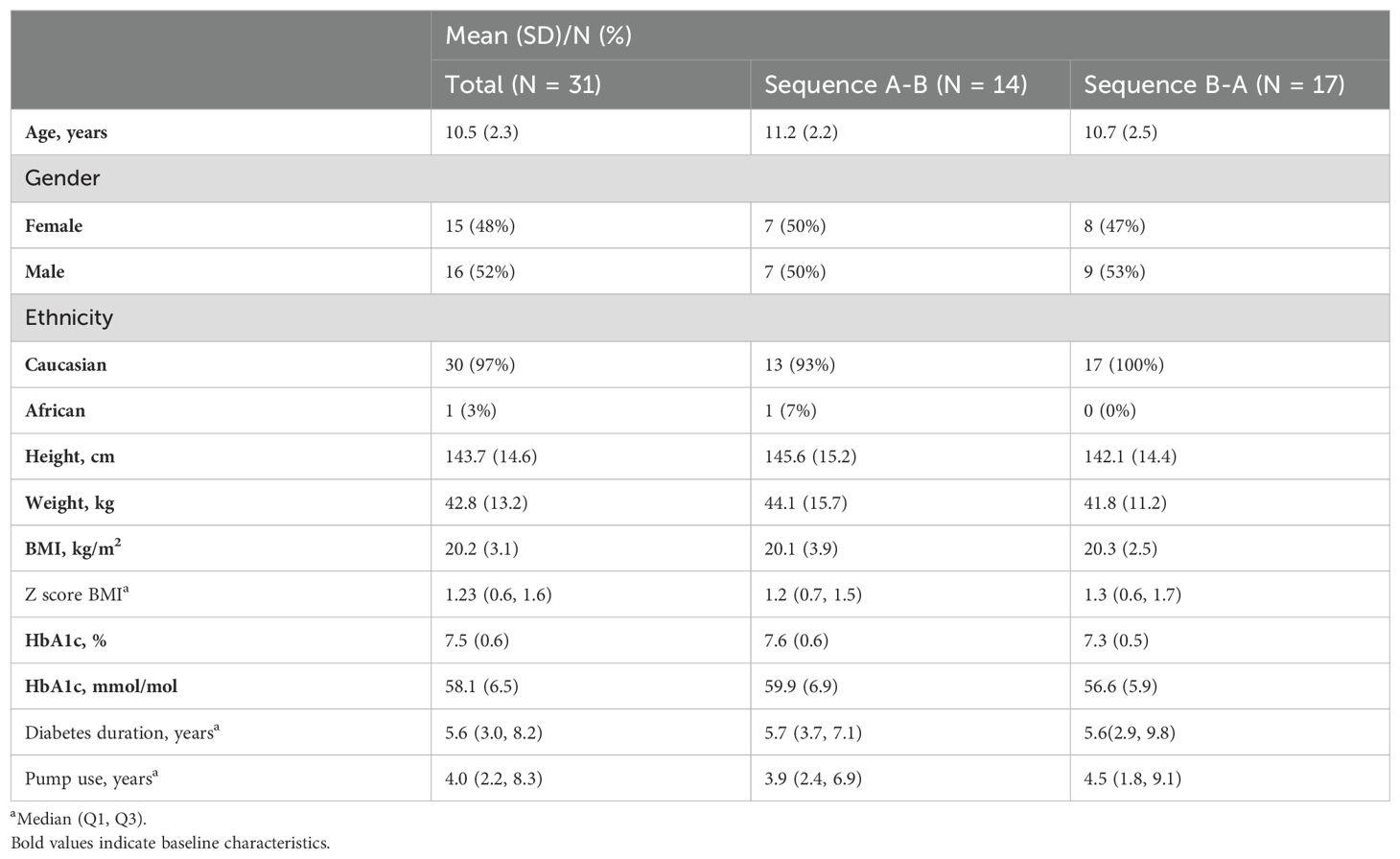
Table 1. Descriptive baseline characteristics of the participating children (30).
3.1 Description of missing data
3.1.1 Children
For one child the glucose sensor values of the last visit are missing. For another child the questionnaire, sleep diary and ActiGraph data of the wash-out period are missing for visit 3. Two children did not return/fill out the questionnaire of visit 2. Two children did not completely fill out all questions at visit 3. The maximum percentage of missing data in the models was 6.5% for children (model including Epworth Sleepiness Scale).
3.1.2 Parents
The parent of the child whose data were missing for visit 3, had also no data for visit 3. One parent had missing data for the ActiGraph and the sleep diary for visit 2, 3 and 4. Two parents did not fill out/return the questionnaire at visit 2. One parent did not answer one question at visit 3. The maximum percentage of missing data in the models was 10% for parents (model including Epworth Sleepiness Scale).
3.2 Sleep data results
3.2.1 Total sleep time
Adjusted average sleep time for children in treatment A (pump and iscCGM) was 449.3 (95% CI [432.8; 465.7]) minutes per night (7.5 hours per night) and 440.0 (95% CI [423.6; 456.5]) minutes per night (7.3 hours per night) for treatment B (pump plus SmartGuard). No significant difference of total sleep time between devices was found (p-value 0.255).
For parents the adjusted average sleep time in treatment A was 413.8 (95% CI [395.4; 432.2]) minutes per night (6.9 hours) with a non-significant increase in sleep time of 5.5 minutes (95%CI [-8.8; 19.8]); p-value 0.46) for treatment B (419.3 (95% CI [400.9; 437.7])).
3.2.2 Number of awakenings
The adjusted average number of nocturnal awakenings in children in treatment A (pump and iscCGM) was 24.7 (95% CI [22.5; 26.9]) and 25.2 (95% CI [23.0; 27.3]) in treatment B (pump plus SmartGuard); no significant difference between the devices was found (p-value 0.64).
For parents the adjusted average number of nocturnal awakenings in treatment A was 16.3 (95% CI [14.5; 18.1]) compared to 16.1 (95% CI [14.3; 17.9]) in treatment B; no significant difference between the devices and number of awakenings was found (p-value 0.76).
3.2.3 Number of nocturnal get-ups
The adjusted average number of nocturnal get-ups in children did not show a significant difference between the two devices: 0.35 (95% CI [0.23; 0.48]) in treatment A compared to 0.41 (95% CI [0.28; 0.54]) in treatment B; p-value: 0.25. The number of nocturnal get-ups in parents was 0.58 (95% CI [0.36; 0.80]) in device A compared to 0.64 (95% CI [0.43; 0.86]) in device B; no significant difference was found (p- value 0.35).
3.3 Questionnaire results
3.3.1 Hypoglycemia fear questionnaire
The score (Hypoglycemia Survey for children and for parent/caregiver with subscales for hypoglycemia worry and behavior) ranges from 0 = no fear to 104 high fear.
In the participating children, the adjusted mean for the hypoglycemia score was 55.1 (95% CI [51.7; 58.8]) for children in arm A. In treatment B, the score of hypoglycemia fear decreased by -0.8 (95% CI [-4.5; 2.9]), but no significant difference was observed between both devices (p-value = 0.67). In parents, the adjusted mean score was 40.67 (95% CI [33.1; 48.3]) in treatment A and decreased by -2.9 (95% CI [-7.0; 1.3]) points for device B. No significant difference for hypoglycemia fear was found between the devices (p= 0.18).
3.3.2 Epworth sleepiness scale
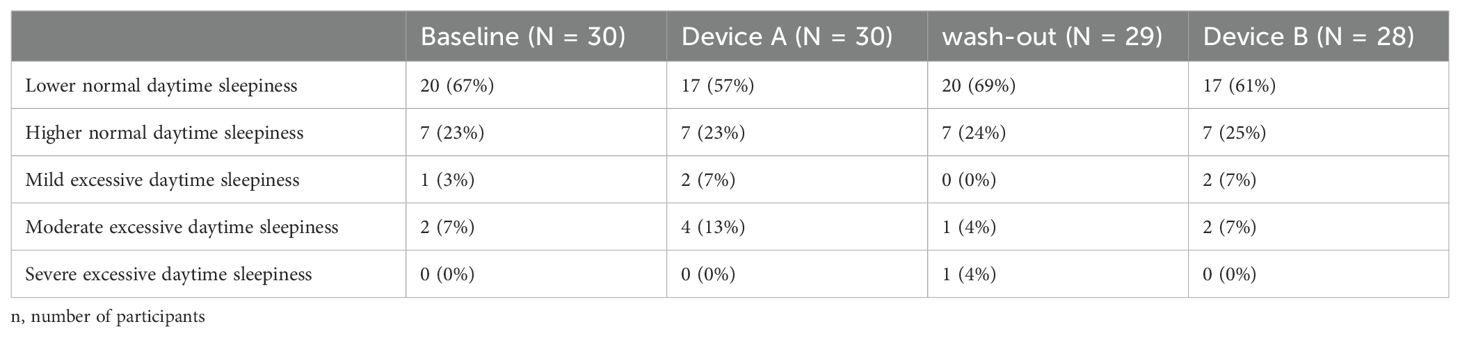
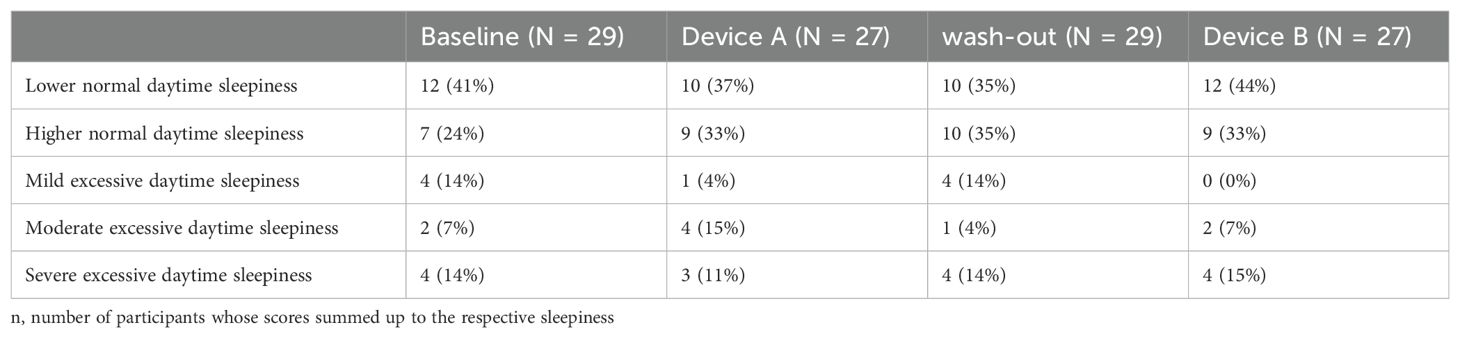
The participating children showed on average a less normal daytime sleepiness during baseline, device A, B and washout period (summary in Table 3) than their parents (Table 4). No significant difference of the Epworth’s Sleepiness Scale and between device groups was found (p-value = 0.54). Also when only considering the 5-level interpretation scale of Epworth’s Sleepiness Scale with a naive Fisher’s Exact test, no significant differences was found between the devices (p = 0.90).
4 Discussion
In our real-life study neither children with type 1 diabetes nor their parents show a significant difference in either hypoglycemia fear, quality or quantity of sleep during the use of two different glucose monitoring systems with or without the alarm function and predictive low glucose suspend. The lack of change in hypoglycemia fear may explain why we do not observe a change in sleep quality and quantity. Whether this depends on the short duration of our intervention or on other factors that were no taken into account is uncertain. The time to get used to a new system and develop a confidence in its function may vary between individuals and for some the 5 weeks may have been insufficient (31).
In our study, the mean sleep data outcome of our participants (children or caregivers) was below the recommended sleep duration as published by the American Academy of Sleep medicine (AASM). According to the Consensus Statement of the AASM children (6 to 12 years of age) should sleep 9 to 12 hours per 24 hours and teenagers (13 to 18 years) 8 to 10 hours per 24 hours to promote optimal health (32). Sleep deprivation occurs. when an individual fails to get enough sleep. In healthy children, sleep deprivation is associated with worse cognitive functioning, school performance and more behavioral problems (33).
In our study, the children, slept on average between 1.2 and 1.5 hours less than the minimum recommended sleep, in both treatment arms.
Per night, they slept an average of 9 minutes longer in treatment A (pump and iscCGM) compared to treatment B, which was not significant.
For adults, the AASM and the Sleep Research Society recommend in their Consensus Statement at least 7 or more hours per night on a regular basis to promote optimal health (34). The parents in our study missed on average the minimum of recommended sleep slightly (6.89 hours in treatment A and 6.98 in treatment B). Unlike their children, the parents in our study slept an average of 5.5 minutes longer per night in treatment B (pump and SmartGuard).
According to the consensus statements, all participants in our study are considered to be sleep deprived (children more than their parents).
Caregivers of children with T1D are known to be frequently sleep deprived and to worry about their child’s nighttime glucose (35). Sleep deprivation plays a role in different physiological processes influencing disease development (36). Treatment modalities, which can improve sleep quality and quantity, may have more impact on the general health and not only on diabetes outcome.
Sleep analysis and psycho-behavioral outcomes will have an added value in the evaluation of new technologies or new treatments and should be included as outcome parameter (37).
Future studies are needed to further explore the best use of new technologies and to offer a personalized medical approach.
5 Strength and limitations
The study is limited due to the constrained study duration and the number of participants. The study was powered for the primary outcome (percent of time spent in glucose target, TIT, (3.9 - 8 mmol/l) of treatment A and B during the final 7 days of the five-week device arm) (28).
The strength of the study derives from the fact that all data reflect the real world situation, as they were collected in free living at home. Another strength is the evaluation of sleep information with an objective method (Actigraphy) complemented with a sleep diary and not only based on self-reported data.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comité National d’Ethique de Recherche Luxembourg. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
US: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing – original draft. GA: Conceptualization, Data curation, Methodology, Project administration, Software, Validation, Writing – review & editing. MF: Investigation, Resources, Writing – review & editing. CM: Investigation, Resources, Writing – review & editing. AS: Formal analysis, Software, Validation, Writing – review & editing. MV: Formal analysis, Methodology, Software, Validation, Writing – review & editing. KB-K: Methodology, Validation, Writing – review & editing. OC: Methodology, Validation, Writing – review & editing. IG: Supervision, Validation, Writing – review & editing. CB: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This is an investigator-initiated study. Medtronic contributed in kind to the devices (insulin pumps, transmitter, I-Pro 2) and to the accessories (glucose sensors).
Acknowledgments
Medtronic contributed in kind to the devices (insulin pumps, transmitter, I-Pro 2) and to the accessories (glucose sensors).
Conflict of interest
Author OC was employed by the company Medtronic. Author CB got speakers honorarium and was part of the Medtronic Psychology e-learning Board.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wood JR, Miller KM, Maahs DM, Beck RW, DiMeglio LA, Libman IM, et al. Most youth with type 1 diabetes in the T1 D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. (2013) 36:2035–7. doi: 10.2337/dc12-1959
2. Sinisterra M, Hamburger S, Tully C, Hamburger E, Jaser S, Streisand R. Young children with type 1 diabetes: sleep, health-related quality of life, and continuous glucose monitor use. Diabetes Technol Ther. (2020) 22:639–42. doi: 10.1089/dia.2019.0437
3. Whittemore R, Jaser S, Chao A, Jang M, Grey M. Psychological experience of parents of children with type 1 diabetes: a systematic mixed-studies review. Diabetes Educ. (2012) 38:562–79. doi: 10.1177/0145721712445216
4. Driscoll KA, Raymond J, Naranjo D, Patton SR. Fear of hypoglycemia in children and adolescents and their parents with type 1 diabetes. Curr Diabetes Rep. (2016) 16:77. doi: 10.1007/s11892-016-0762-2
5. Fernando A, Patel V. Fear of hypoglycaemia in pediatric diabetes: a literature review. Br J Diabetes. (2021) 21:36–42. doi: 10.15277/bjd.2021.286
6. Zhang L, Xu H, Liu L, Bi Y, Li X, Kan Y, et al. Related factors associated with fear of hypoglycemia in parents of children and adolescents with type 1 diabetes - A systematic review. J Pediatr Nurs. (2022) 66:125–35. doi: 10.1016/j.pedn.2022.05.022
7. Barnard K, Thomas S, Royle P, Noyes K, Waugh N. Fear of hypoglycaemia in parents of young children with type 1 diabetes: a systematic review. BMC Pediatr. (2010) 10:50. doi: 10.1186/1471-2431-10-50
8. Barnard KD, Oliver N, Adolfsson P, Aldred C, Kerr D. Is iatrogenic sleep disturbance worth the effort in Type 1 diabetes? Diabetes Med. (2015) 32:984–6. doi: 10.1111/dme.12699
9. Morrow T, Bhatia SH, Parmar AM, Baker L, Abadula F, Williamson D, et al. Sleep habits of early school-aged children with type 1 diabetes and their parents: family characteristics and diabetes management. Behav Sleep Med. (2022) 20(5):649–58. doi: 10.1080/15402002.2021.1977305
10. Barnard K, James J, Kerr D, Adolfsson P, Runion A, Sebedzija G. Impact of chronic sleep disturbance for people living with T1 diabetes. J Diabetes Sci Technol. (2016) 10:762–7. doi: 10.1177/1932296815619181
11. Jaser SS, Foster NC, Nelson BA, Kittelrud JM, DiMeglio LA, Quinn M, et al. T1D Exchange Clinic Network: Sleep in children with type 1 diabetes and their parents in the T1D Exchange. Sleep Med. (2017) 39:108–15. doi: 10.1016/j.sleep.2017.07.005
12. Lindström C, Aman J, Norberg AL. Parental burnout in relation to sociodemographic, psychosocial and personality factors as well as disease duration and glycaemic control in children with Type 1 diabetes mellitus. Acta Paediatr. (2011) 100:1011–7. doi: 10.1111/j.1651-2227.2011.02198.x
13. Carreon SA, Cao VT, Anderson BJ, Thompson DI, Marrero DG, Hilliard ME. [amp]]Igrave; don’t sleep through the night`: Qualitative study of sleep in type 1 diabetes. Diabetes Med. (2022) 39:e14763. doi: 10.1111/dme.14763
14. Battelino T, Conget I, Olsen B, Schütz-Fuhrmann I, Hommel E, Hoogma R, et al. SWITCH Study Group: The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. (2012) 55:3155–62. doi: 10.1007/s00125-012-2708-9
15. Schierloh U, Aguayo GA, Fichelle M, De Melo Dias C, Celebic A, Vaillant M, et al. Effect of predicted low suspend pump treatment on improving glycaemic control and quality of sleep in children with type 1 diabetes and their caregivers: the QUEST randomized cross-over study. Trials. (2018) 19:665. doi: 10.1186/s13063-018-3034-4
16. Biester T, Kordonouri O, Holder M, Remus K, Kieninger-Baum D, Wadien T, et al. Let the algorithm do the work”: reduction of hypoglycemia using sensor-augmented pump therapy with predictive insulin suspension (Smart Guard) in pediatric type 1 diabetes patients. Diabetes Technol Ther. (2017) 19:173–82. doi: 10.1089/dia.2016.0349
17. Shivers JP, Mackowiak L, Anhalt H, Zisser H. Turn it off!”: diabetes device alarm fatigue considerations for the present and the future. J Diabetes Sci Tecnol. (2013) 7:789–94. doi: 10.1177/193229681300700324
18. Edge J, Acerini C, Campbell F, Hamilton-Shield J, Moudiotis C, Rahman S, et al. An alternative sensor-based method for glucose monitoring in children and young people with diabetes. Arch Dis Child. (2017) 102:543–9. doi: 10.1136/archdischild-2016-311530
19. Al Hayek AA, Robert AA, Al Dawish MA. Effectiveness of freestyle libre flash glucose monitoring system on diabetes distress among individuals with type 1 diabetes: A prospective study. Diabetes Ther. (2020) 11:927–37. doi: 10.1007/s13300-020-00793-2
20. Abraham MB, de Bock M, Paramalingam N, O’Grady MJ, Ly TT, George C, et al. Prevention of insulin-induced hypoglycemia in type 1 diabetes with predictive low glucose management system. Diabetes Technol Ther. (2016) 18:436–43. doi: 10.1089/dia.2015.0364
21. Clarke WL, Gonder-Frederick A, Snyder AL, Cox DJ. Maternal fear of hypoglycemia in their children with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab. (1998) suppl 1:189–94. doi: 10.1515/jpem.1998.11.s1.189
22. Kamps JL, MC R, Varela RE. Development of a new fear of hypoglycemia scale: preliminary results. J Pediatr Psychol. (2005) 30:287–91. doi: 10.1093/jpepsy/jsi038
23. Gonder-Frederick L, Nyer M, Shepard JA, Vajda K, Clarke W. Assessing fear of hypoglycemia in children with Type 1 Diabetes and their parents. Diabetes Manag (Lond). (2011) 1:627–39. doi: 10.2217/dmt.11.60
24. Haugstvedt A, Wentzel-Larsen T, Aarflot M, Rokne B, Graue M. Assessing fear of hypoglycemia in a population-based study among parents of children with type 1 diabetes - psychometric properties of the hypoglycemia fear survey - parent version. BMC Endocr Disord. (2015) 15:2. doi: 10.1186/1472-6823-15-2
25. Patton SR, Noser AE, Clements MA, Dolan LM, Powers SW. Reexamining the hypoglycemia fear survey for parents of young children in a sample of children using insulin pumps. Diabetes Technol Ther. (2017) 19:103–8. doi: 10.1089/dia.2016.0389
26. Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540
27. Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. (1994) 17:201–7. doi: 10.1093/sleep/17.3.201
28. Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. (1992) 15:461–9. doi: 10.1093/sleep/15.5.461
29. World Health Organization. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. 1st ed. Acta Paediatr Suppl. (2006) 450:76–85.
30. Schierloh U, Aguayo GA, Schritz A, Fichelle M, De Melo Dias C, Vaillant MT, et al. Intermittent scanning glucose monitoring or predicted low suspend pump treatment: does it impact time in glucose target and treatment preference? The QUEST randomized crossover study. Front Endocrinol (Lausanne). (2022) 13:870916. doi: 10.3389/fendo.2022.870916
31. Lally P, Van Jaarsveld CHM, Potts HWW, Wardle J. How are habits formed: Modelling habit formation in the real world. Eur J Soc Psychol. (2010) 40:998–1009. doi: 10.1002/ejsp.v40:6
32. Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, et al. Recommended amount of sleep for pediatric populations: A consensus statement of the American academy of sleep medicine. J Clin Sleep Med. (2016) 12:785–6. doi: 10.5664/jcsm.5866
33. Astill RG, van der Heijden KB, Van Ijzendoorn MH, Van Someren EJ. Sleep, cognition, and behavioral problems in school-age children: a century of research meta-analyzed. Psychol Bull. (2012) 138:1109–38. doi: 10.1037/a0028204
34. Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Recommended amount of sleep for a healthy adult: A joint consensus statement of the American academy of sleep medicine and sleep research society. Sleep. (2015) 38:843. doi: 10.5665/sleep.4716
35. Feeley CA, Clougherty M, Siminerio L, Charron-Prochownik D, Allende AL, Chasens ER. Sleep in caregivers of children with type 1 diabetes. Diabetes Educ. (2019) 45:80–6. doi: 10.1177/0145721718812484
36. Luyster FS, Strollo PJ, Zee PC, Walsh JK. Sleep: A health imperative. Sleep. (2012) 35:727–34. doi: 10.5665/sleep.1846
Keywords: type 1 diabetes (T1D), children, parents, fear of hypoglycemia, sleep, sensor augmented pump, iscCGM
Citation: Schierloh U, Aguayo GA, Fichelle M, De Melo Dias C, Schritz A, Vaillant M, Barnard-Kelly K, Cohen O, Gies I and de Beaufort C (2024) Fear of hypoglycemia and sleep in children with type 1 diabetes and their parents. Front. Endocrinol. 15:1419502. doi: 10.3389/fendo.2024.1419502
Received: 18 April 2024; Accepted: 18 November 2024;
Published: 16 December 2024.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Hans-Michael Kaltenbach, ETH Zürich, SwitzerlandDaniela Lopes Gomes, Federal University of Pará, Brazil
Copyright © 2024 Schierloh, Aguayo, Fichelle, De Melo Dias, Schritz, Vaillant, Barnard-Kelly, Cohen, Gies and de Beaufort. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ulrike Schierloh, c2NoaWVybG9oLnVscmlrZUBtZS5jb20=
 Ulrike Schierloh
Ulrike Schierloh Gloria A. Aguayo
Gloria A. Aguayo Muriel Fichelle1
Muriel Fichelle1 Anna Schritz
Anna Schritz Michel Vaillant
Michel Vaillant Ohad Cohen
Ohad Cohen Inge Gies
Inge Gies Carine de Beaufort
Carine de Beaufort