- 1Department of Geriatric, Zhejiang Hospital of Integrated Traditional Chinese and Western Medicine, Hangzhou, Zhejiang, China
- 2Department of Hepatobiliary and Pancreatic Surgery, Affiliated Hangzhou First People’s Hospital, School of Medicine, Westlake University, Hangzhou, Zhejiang, China
Background: Limited data are available regarding the association between serum transferrin saturation (TSAT) levels and heart failure (HF).
Methods: We utilized data from National Health and Nutrition Examination Survey (NHANES) 2017- 2020.03 for analysis. Data on TAST, HF and covariates were extracted and analyzed. Weighted logistic regression and subgroup analysis were used to explore the independent association between TSAT and HF. Furthermore, interaction tests were also carried out to evaluate the strata differences. We subsequently assessed whether there was a non-linear relationship between the 2 using Restricted cubic spline (RCS) and threshold effect models.
Result: A total of 282 (3.87%) participants were identified to have HF. Among the total population, participants with HF had significantly lower TSAT levels compared to those without HF (24.63 vs. 27.95, P = 0.001). After fully adjusting for potential confounders, weighted multiple logistic regression models revealed a 2.6% reduced in the risk of HF when each unit of TSAT increased. There was also a negative association between elevated TSAT and developed risk of HF in the quartile groups (Q1 OR:1.00; Q2 OR: 0.924 [95%CI:0.593,1.440]; Q3 OR: 0.515 [95%CI:0.298,0.891]; Q4 OR:0.411 [95%CI:0.201,0.839]). The subgroup analysis results remained consistent across strata, with a strong negative correlation between TSAT and HF. Interaction tests showed no dependence on gender, age, Body Mass Index, race, diabetes, hypertension, hyperlipidemia, ratio of family income to poverty and education for this negative association between TSAT and HF (all p for interaction >0.05). The RCS and threshold effect models indicated a linear negative correlation between TSAT and HF, which was more pronounced when TSAT under 40%.
Conclusion: Overall, these findings suggest a consistent and negative association between TSAT levels and the presence of HF among middle-aged and older adults in the United States.
1 Introduction
Heart failure (HF) is the terminal stage of numerous cardiovascular diseases, leading to diminished functional capacity, poorer quality of life, higher mortality rates, thus posing a significant public health concern (1). Despite considerable advancements in HF treatment in recent years, patient mortality and re-hospitalization rates remain alarmingly high.
Iron deficiency (ID) is a common comorbidity in patients with HF and is independently linked to adverse outcomes (2–4). The European Society of Cardiology (ESC) guidelines for ID primarily focus on ferritin or transferrin saturation (TSAT) combined with ferritin (5). However, ferritin levels can be influenced by inflammatory pathways activation, especially in patients with chronic inflammatory diseases, and TSAT is less likely to fluctuate than ferritin, so TSAT is most associated with diagnostic ID (6, 7). Based on this, we speculate that TSAT has a greater impact on adverse outcomes in patients with HF. Current studies have mainly focused on adverse outcomes in patients with HF (8, 9), and the relationship between TSAT and the incidence of HF remains unknown.
Therefore, we performed a cross-sectional analysis to investigate the potential association between TSAT and HF in the United States adults.
2 Materials and methods
2.1 Data source
The National Health and Nutrition Examination Survey (NHANES) is a rigorous and scientific database, which provides a large amount of information on the nutrition and health of the non-institutionalized the United States population. Employing a stratified multistage probability sampling method, NHANES ensures the selection of a highly representative sample. Approved by the Ethic Review Board of the National Center for Health Statistics (NCHS), NHANES protocols involve both interviews and examinations, with all participants providing consent for their information to be used in research. The Institutional Review Board at the Zhejiang Hospital of Integrated Traditional Chinese and Western Medicine determined that this analysis used public datasets thus human subject approval was not needed.
2.2 Study population
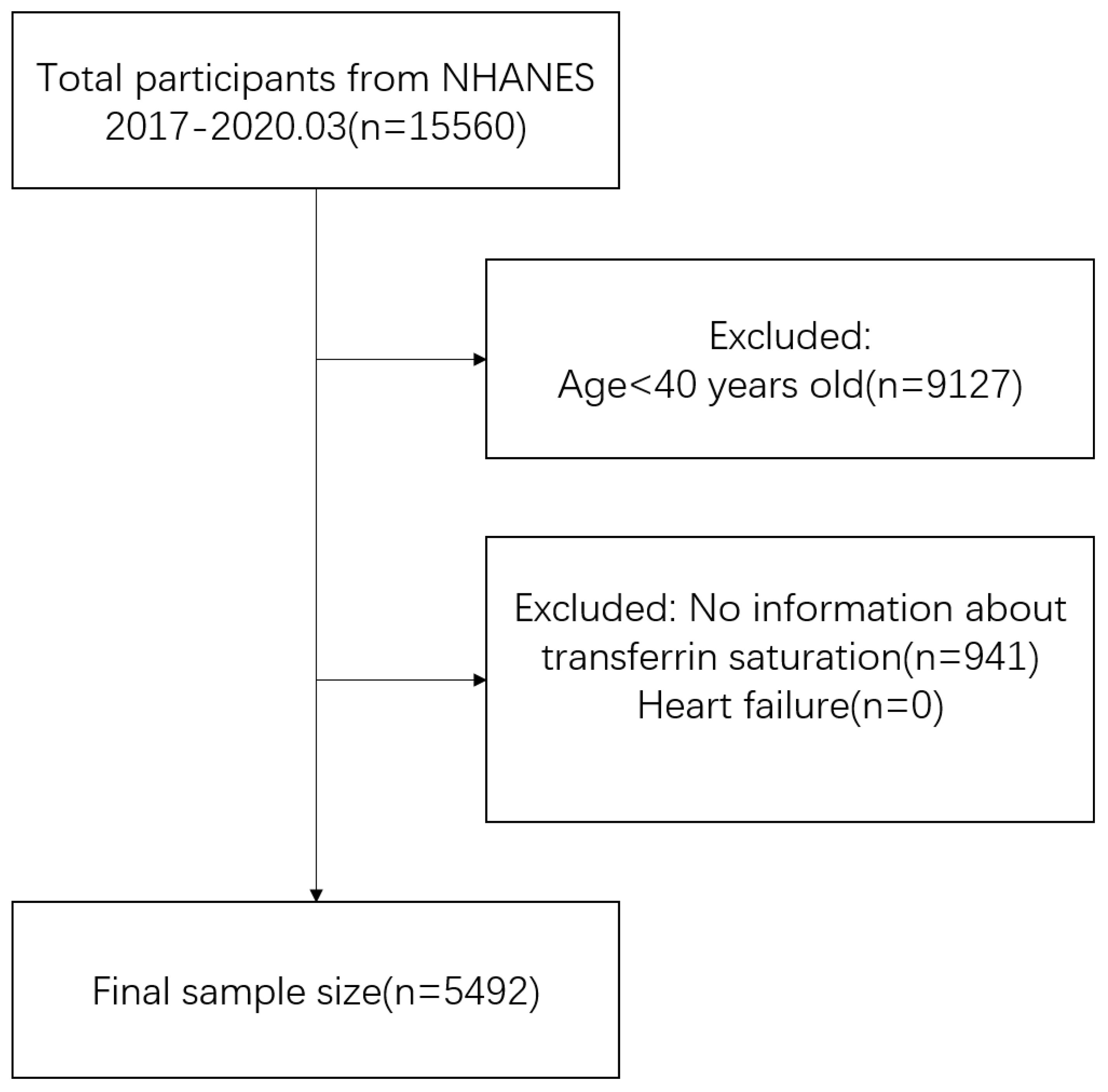
The study incorporated data from a single cross-sectional cycle of NHANES, which began in 2017 and ended in March 2020. Inclusion criteria comprised middle-aged and elderly (aged ≥40 years) individuals with complete TSAT and HF data (10, 11). Participant selection details are illustrated in Figure 1. Data on TSAT, HF and potential confounding factors were extracted and aggregated for each participant. Ultimately, the analysis included a total of 5,492 participants who met the specified inclusion and exclusion criteria.
2.3 Heart failure
The outcome was HF. We defined HF by a synthesis of self-reported physician diagnoses and standardized medical condition questionnaires completed during personal interviews. HF was defined as individuals who responded affirmatively to the question MCQ160b ‘Has a doctor or other health professional ever told {you/SP} that {you/s/he}…had congestive heart failure?’
2.4 Transferrin saturation
Serum TSAT levels was used as the primary exposure variable of interest. TSAT(%sat) value was calculated using Iron (frozen), serum and calculated Total Iron Binding Capacity (TIBC): calculated %Sat= [Iron (frozen), serum/TIBC] x 100.
2.5 Other covariates
Theoretically, factors with potential effects on TSAT and HF should be adjusted for as covariates in a multiple logistic regression model, but such study conditions may not be met in practice. Therefore, based on previous studies, we have used well known variables as covariates in this study (12, 13). Demographic information included age (years), Body Mass Index (BMI, <25/25-30/>30), gender (Male/Female), race (Mexican American/Non-Hispanic White/Non-Hispanic Black/Other Race), education level (Less than high school/High school/More than high school), marital status (married or living with partner/living alone) and ratio of family income to poverty (PIR, ≤1.30/1.31-3.5/>3.5). Smoking and alcohol consumption were obtained from the questionnaire. Smoking status were categorized as never, former, current), and drink status were categorized as never, sometimes, often. Medical conditions were obtained from questionnaires, including hypertension (yes/no), diabetes (yes/no) and hyperlipoidemia (yes/no). All demographic information were used as covariates in the model and adjusted to exclude the effect of disease on the independent and dependent variables. Participants’ daily intake of total nutrients (energy, iron, sodium) was obtained through a questionnaire and calculated as the average of the sum of nutrient values answered on the first and second day. Sedentary minutes were obtained from the physical activity questionnaire, where we directly collected the amount of time (mins) participants were sedentary and inactive. HF can lead to liver and kidney dysfunction (14–16), so we obtained data on aspartate aminotransferase (AST, U/L), alanine aminotransferase (ALT, U/L), albumin (g/dL), blood urea nitrogen/creatinine (BUN/Cr) from the laboratory data. In addition, HF has been associated with chronic inflammation (17, 18), so we obtained high-sensitivity C-reactive protein data (HS-CRP, mg/L) from the laboratory data. Serum iron (ug/dL) and hemoglobin (g/dL) have been clearly associated with TSAT (19, 20), so we collected data on these two. All covariates are listed in Table 1.
2.6 Statistical analysis
Sample weights were incorporated into all estimates. Continuous variables were presented as mean (standard error), while categorical variables were represented as percentages.
The statistical analysis consisted of four main steps to investigate the association between TSAT and HF among the selected participants. Firstly, participants were divided into HF and non-HF groups. Weighted Student t test (for continuous variables) and weighted chi-square tests (for categorical variables) were conducted to compare the baseline characteristics. Secondly, weighted multivariable logistic regression analysis was performed to assess the relationship between TSAT and HF. Three models were utilized: Model 1 without covariate adjustments. Model 2 adjusted for gender, age and race. Model 3 adjusted all demographic information. Thirdly, a subgroup analysis was conducted to explore the impact of different subgroups on the results. Interaction tests were employed to explore potential heterogeneity between these subgroups. Additionally, Restricted cubic spline (RCS) was applied to examine linear or nonlinear relationship between them. Threshold effect analysis was conducted using two-piecewise linear regression model.
The presence of missing covariates in cross-sectional studies is inevitable, and in order to retain more information about the independent and respondent variables, missing covariates were processed. If the missing value of a continuous variable was within 10% of the total sample, we used the mean instead, otherwise we grouped the continuous variable according to certain rules, setting the missing value in a separate group. If the missing value of the categorical variable did not exceed a sample size of 20, we were directly regard as ‘no’ or ‘intermediate column’, otherwise we group them separately (21). If only the nutrient (energy, iron, sodium) of the second day is missing, the average nutrient (energy, iron, sodium) is replaced with the nutrient (energy, iron, sodium) of the first day. If the nutrients (energy, iron, sodium) are missing for both days, then proceed in the same way as the continuous variable.
Data processing and analysis were performed using R version 4.3.0, along with Storm Statistical Platform (http://www.medsta.cn/software), with P<0.05 considered to be statistically significant.
3 Results
3.1 Characteristics of participants
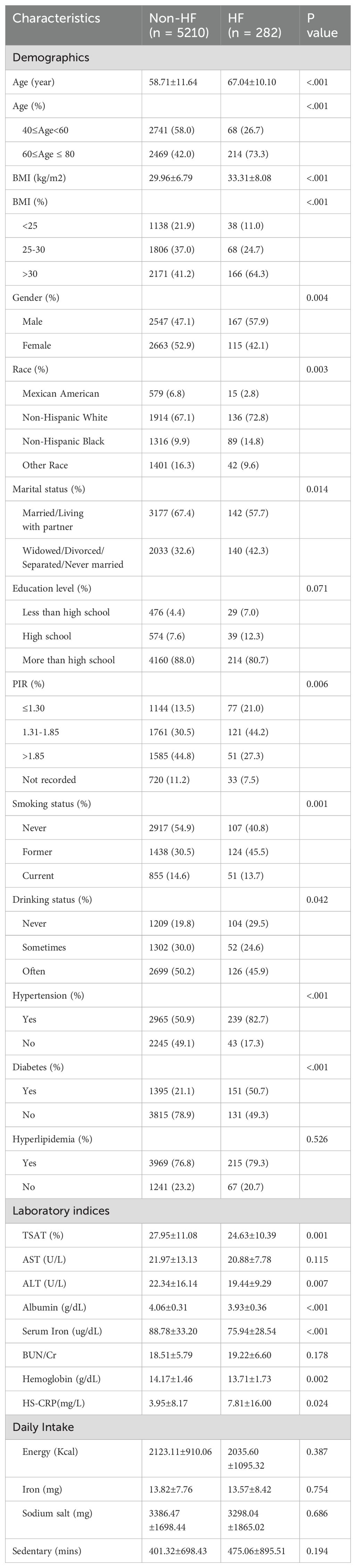
Table 1 presents the weighted baseline characteristics of participants selected from NHANES 2017 to 2020.03, categorized by the presence or absence of HF. The analysis encompassed 282 participants diagnosed with HF, constituting 3.87% of the total sample. The average age of the HF group was 67.04 years, with 57.9% being male and 42.1% female. In contrast, the non-HF group comprised 5,210 participants with an average of 58.71 years, of which 47.1% were male and 52.9% were female.
Significant differences between the two groups were noted in several including TSAT, ALT, albumin, serum iron, hemoglobin, HS-CRP, age, BMI, gender, race, PIR, marital status, smoking status, drinking status, hypertension and diabetes (all p < 0.05). There were no differences in education, hyperlipidemia, AST, BUN/Cr, daily intake and sedentary time (p > 0.05).
3.2 Association between TSAT and HF
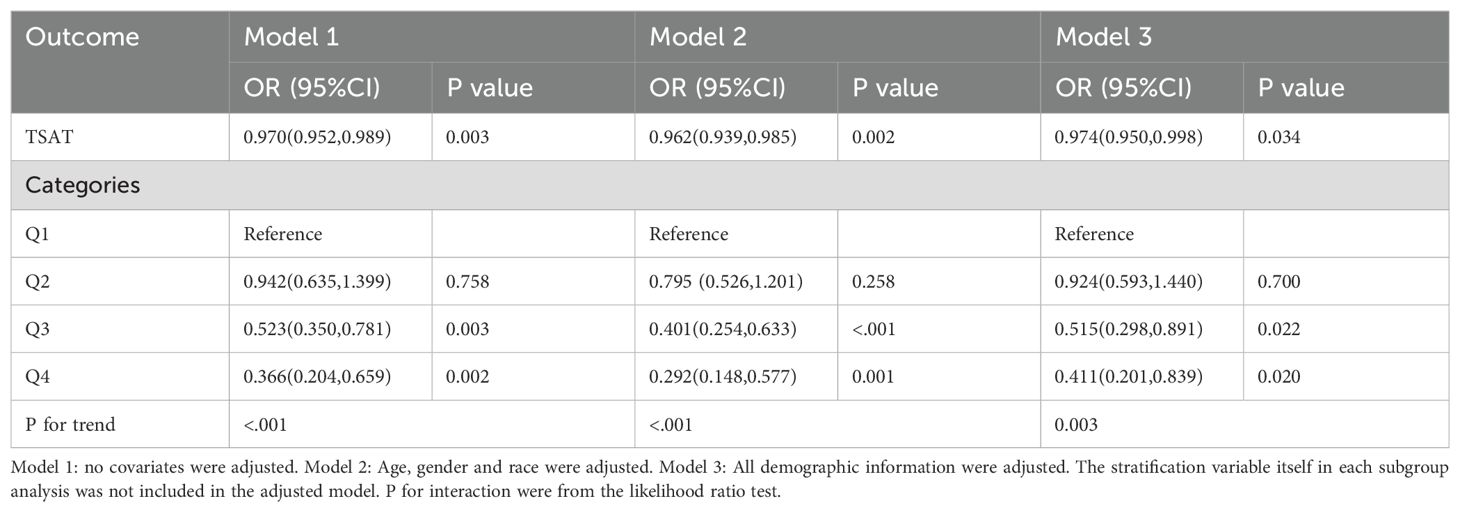
Table 2 presents the association between TSAT and the risk of HF. Three multivariate logistic regression models were employed to assess this association. In Model1, TSAT exhibited a negative correlation with the risk of HF (OR=0.970, 95%CI:0.952-0.989, P<0.05). This negative association remained significant after adjusting for confounding factors in both Model2 (OR=0.962, 95%CI:0.939-0.985, P<0.05) and Model3 (OR=0.974, 95%CI:0.950-0.998, P<0.05).
To delve deeper into the relationship between TSAT and HF, TSAT was categorized into quartiles. In Model3, the third quartiles (Q3: OR=0.515(0.298,0.891), 0.022) and highest (Q4: OR=0.411(0.201,0.839),0.020) showed a more pronounced negative correlation with the risk of HF compared to the lowest quartile (Q1). However, the second quartile (Q2: OR=0.924(0.593,1.440),0.700) did not exhibit statistical significance compared to the lowest quartile(Q1). These findings were consistent across all models. Additionally, when stratified by TSAT, the trend test between them remained significant in all models (P for trend<0.05).
3.3 Relationships of TSAT with HF in subgroups
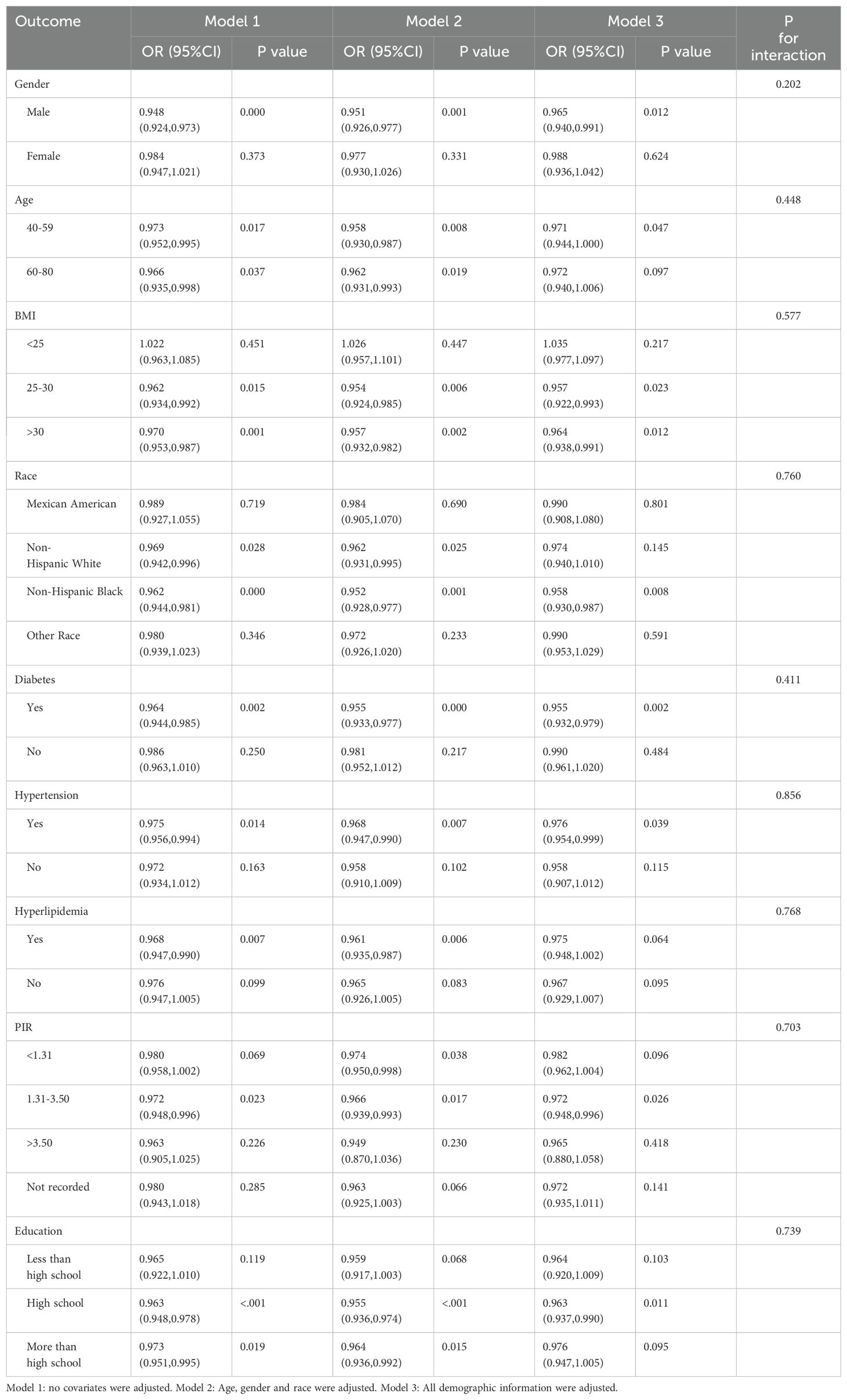
We investigated the potential relationships between TSAT with HF across various subgroups to further assess the robustness of the association (Table 3, Figure 2). Significant associations between TSAT and HF were observed in the following subgroups: male sex(OR = 0.965, 95%CI 0.940,0.991), 40-59 age (OR = 0.971, 95%CI 0.944,1.000),25≤BMI ≤ 30(OR = 0.957, 95%CI 0.922,0.993), BMI>30(OR = 0.964, 95%CI 0.938,0.991), non-Hispanic black(OR = 0.958, 95%CI 0.930,0.987), diabetes(OR = 0.955, 95%CI 0.932,0.979), hypertension (OR = 0.976, 95%CI 0.954,0.999), 1.3≤PIR ≤ 3.5(OR = 0.972, 95%CI 0.948,0.996), and high school education level (OR = 0.963, 95%CI 0.937,0.990). Notably, no significant interactions were observed for gender, age, BMI, race, diabetes, hypertension, hyperlipidemia, PIR and education suggesting that the association was not dependent on these variables (all p for interaction > 0.05).
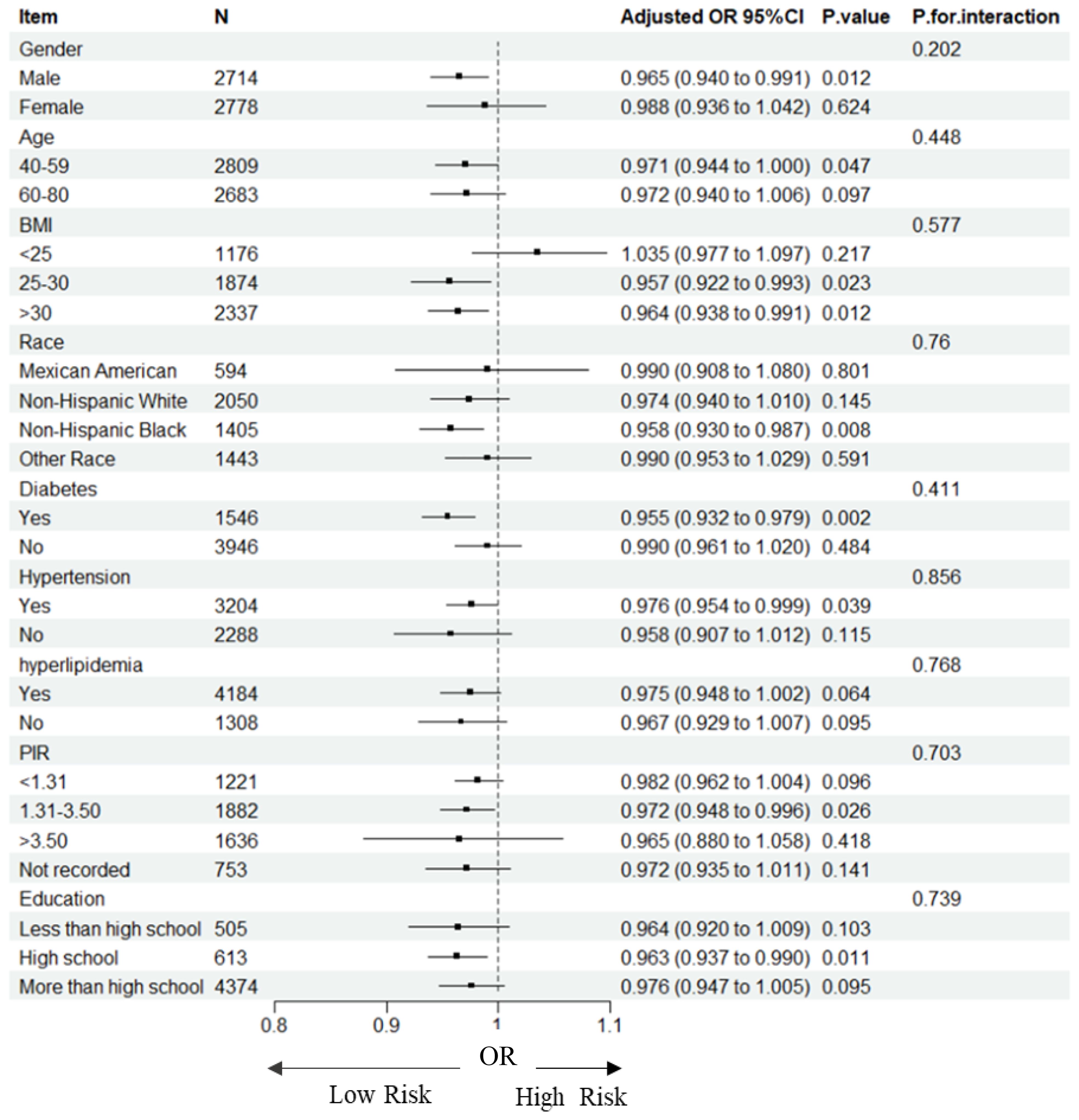
Figure 2. Forest plot for subgroup analysis of association between TSAT and HF. Odds ratios (ORs) were calculated using multivariate Logistic regression models adjusted in Model3. In each case, the stratification variable itself in each subgroup analysis was not included in the adjusted model. The square represents the OR value; the solid line represents 95% confidence interval (CI); the dotted line represents invalid lines.
3.4 Identification of non-linear relationship
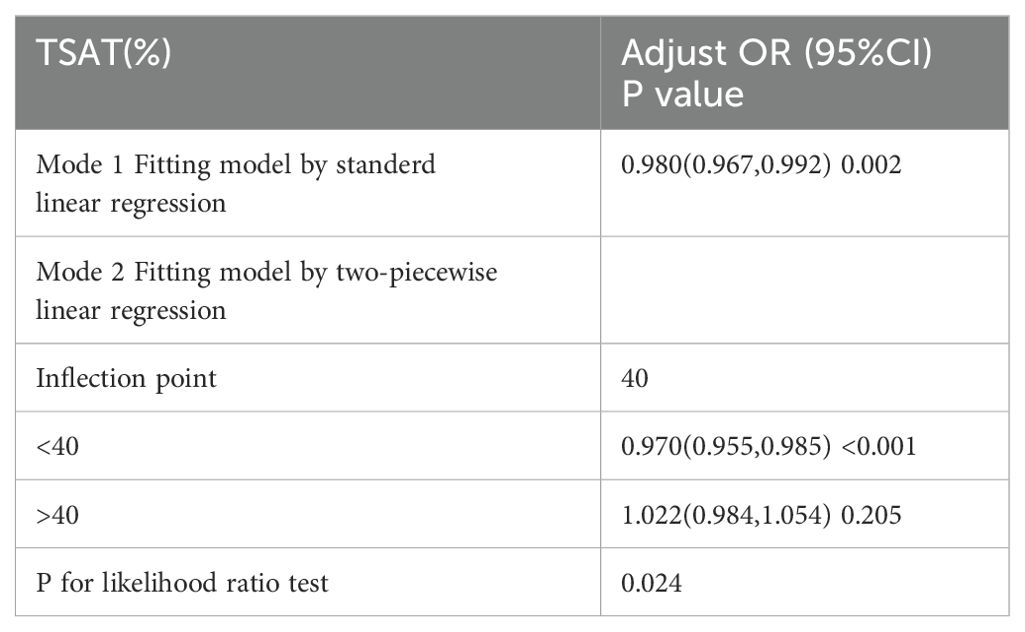
We further explored the dose–response relationship between TSAT and HF using RCS plots, and the results are shown in Figure 3. We built three RCS plots according to Model 1, Model 2 and Model 3. Figures 3A, B showed a nonlinear dose–response relationship between TSAT and HF (P for nonlinearity = 0.033, P for nonlinearity = 0.013), while Figure 3C showed a linear dose–response relationship between TSAT and HF (P for nonlinearity = 0.065). A threshold effect analysis of TSAT on HF was further performed by the two-piecewise linear regression. As shown in Table 4, the inflection point was 40. Each unit increase of TSAT was associated with a 3.0% decrease of the risk of HF below 40, and the relationship was not statistically significant above 40.
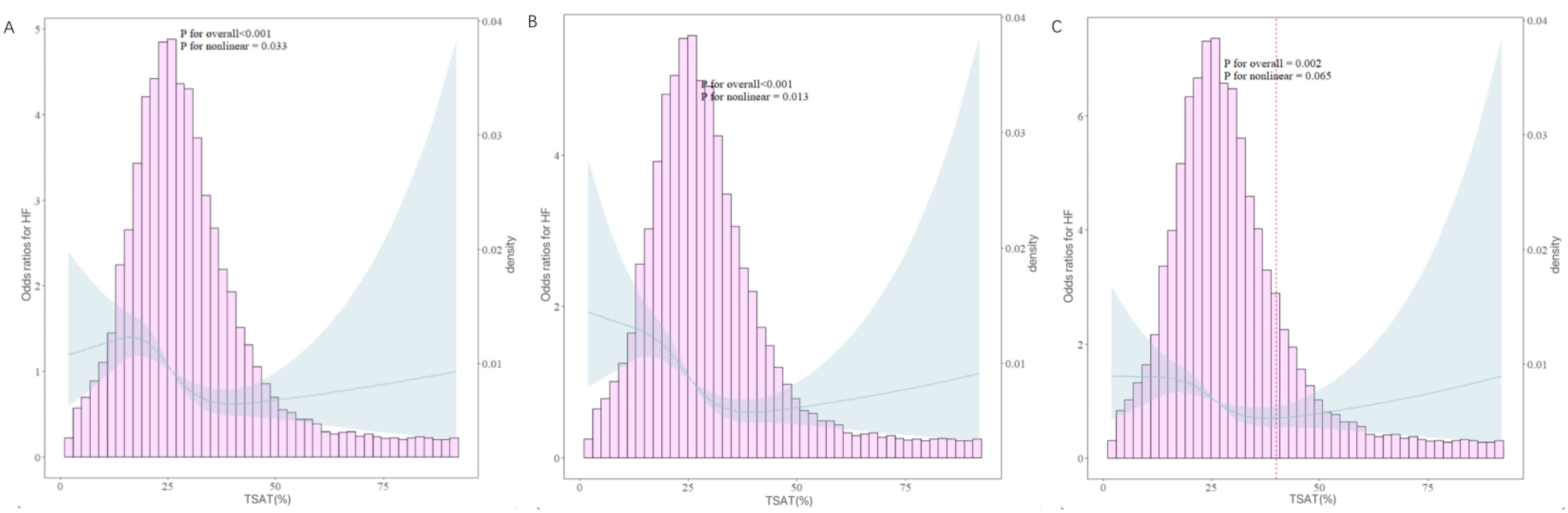
Figure 3. RCS curves between the TSAT and HF. (A: adjusted in Model 1; B: adjusted in Model 2; C: adjusted in Model 3). The solid blue lines represented the ORs of HF, the blue region indicated corresponding 95% Cis, the pink histogram showed the distribution of TSAT. The left Y-axis represents the OR of HF, the right Y-axis represents the frequency distribution of TSAT, the X-axis is the TSAT value. The picture show both the frequency distribution of data and the fitting curve of RCS model. The P-value for overall association <0.05 manifested a significant association, whatever the shape of the dose-response curve was. The P-value for non-linear association <0.05 indicated a nonmonotonic dose-response curve.
4 Discussion
To illuminate the correlation between TSAT and HF incidence, we conducted a cross-sectional analysis involving 5,492 individuals from NHANES 2017-2020.03. Our study revealed an inverse association between TSAT and HF. Specifically, for each 1% increase in TSAT, the risk of HF decreased by 2.6% after adjusting for covariates. This negative and significant association persisted in approximately half of the subgroups examined. To assess the linearity of this relationship, we employed restricted cubic spline regression and identified a negative linear relationship between TSAT and HF. Threshold effect analysis suggested that the inflection point was 40.
In addition, our study found that compared with non-HF patients, patients with HF had lower ALT, albumin, serum iron, hemoglobin and higher CRP. This is consistent with previous studies that low ALT, albumin, hemoglobin and serum iron are associated with adverse outcomes in patients with HF (22–25). However, no significant differences were found between the two groups in terms of dietary intake and sedentary time, which we think may be due to the fact that these data were obtained through recall. More RCT studies or cohort studies are needed to verify the effects of dietary intake and sedentary time on patients with HF.
TSAT serves as a measure of the percentage of transferrin saturated with iron, reflecting the availability of iron for erythropoiesis and myocardial tissue (26). Although existing research on the relationship between TSAT and HF incidence is still relatively rare, the use of intravenous iron supplementation to treat HF has become a hot topic of research today (27–29). While ID is known for its associations with clinical consequences related to erythropoiesis, chronic ID by itself, independent of anemia, poses detrimental effects on oxidative metabolism, cellular energetics, and immune mechanisms, consequently leading to structural and functional changes in the myocardium. This includes reductions in oxygen storage within myoglobin and diminished tissue oxidative capacity, ultimately resulting in mitochondrial and left ventricular (LV) dysfunction (30). Consequently, it’s not surprising that ID correlates with LV hypertrophy, dilatation, compromised hemodynamics and symptomatic HF (31).
The underlying mechanism linking TSAT and HF remains unclear. Some animal studies have demonstrated that ID induces diastolic dysfunction and HF, characterized by pulmonary congestion, cardiac fibrosis, and increased cardiac inflammation (32). For instance, Toblli et al. established an ID model in rats by feeding them a low-iron diet, leading to significantly reduced serum iron and TSAT levels compared to rats fed normal diets. Echocardiography and tissue Doppler evaluations revealed significantly increased left ventricular end-diastolic diameter, left ventricular end-systolic diameter, left ventricular posterior wall thickness, and decreased S and E waves velocities (33). Similarly, Chung et al. found that iron-deficient animals exhibited reduced ventricular ejection fraction measured by magnetic resonance imaging, and disturbances in Ca 2+ signaling, a pathway linked to the contractile deficit in failing hearts. This was attributed to downregulated RyR2 channels, decreased sarcoplasmic reticulum release, dephosphorylation of phosphoprotein, and suppressed SERCA pump activity (34). ID has also been implicated in increasing oxidative/nitrosative stress in the heart, leading to aberrant mitochondrial and irregular sarcomere organization, and cytochrome c release. This oxidative/nitrosative stress arises from increased mitochondrial and enzymatic superoxide (O2• −) production, enhanced inducible nitric oxide synthase expression, and subsequent peroxynitrite (ONOO−) formation (29). Clinical studies have further supported these findings. For example, Zhang et al. reported lower concentrations of cardiac iron in heart tissue of patients with HF compared to those without HF. This depletion of cardiac iron is believed to be associated with inhibited respiratory chain and Krebs cycle enzyme activity, depletion of iron storage, and an increase in malondialdehyde concentration (35).
In our subgroup analysis, we found that the association between TSAT and HF was more pronounced in participants with high school education compared to other education. The possible reason is that people with high school education have stronger self-management skills than people with less than high school education, allowing them to more effectively adjust their lifestyle, follow medical advice or take other preventive measures to reduce the risk of HF (36); while people with high school education have lower psychological stress than those with more than high school education, which reduce the occurrence of HF (37). This result is also consistent with the PIR in this study. However, the underlying mechanisms for this education-related effect of TSAT on HF are not yet well understood and more research is needed.
However, our study had several limitations: (1) the specific types and NYHA classification of HF were unknown, and the data of NT-pro brain natriuretic peptide (BNP) or BNP was not found. (2) The participants included in our study were middle-aged and older individuals from the United States, and therefore, the generalizability of our findings to other age groups and national populations requires further investigation. (3) the factors influencing both TSAT and HF are multifaceted, and despite adjusting for relevant covariates in our models based on existing literature, the possibility of bias from unaccounted potential covariates cannot be ruled out. (4) The cross-sectional nature of our study limits our ability to establish causality. Further prospective studies and randomized controlled trials are needed to confirm the link between TSAT and HF.
5 Conclusions
Our study found a linear negative association between TSAT and the presence of HF among middle-aged and older adults, after being adjusted for potential confounders. This finding highlights the importance of improve TSAT in HF patients to prevent disease progression. In the future, additional randomized controlled trials or cohort studies are urgently needed to validate our conclusions and further explore the underlying mechanisms of this association.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by National Center for Health Statistics (NCHS). The patients/participants provided their written informed consent to participate in this study.
Author contributions
MW: Writing – original draft. DZ: Software, Writing – review & editing. LJ: Data curation, Writing – review & editing. MY: Validation, Writing – review & editing. JN: Supervision, Writing – review & editing. JY: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. (2020) 141:e139–596. doi: 10.1161/CIR.0000000000000757
2. Amaechi UM, Aiwuyo HO, Ewelukwa C, Ilerhunmwuwa N, Osarenkhoe JO, Kweki AG, et al. Assessing Iron Status in Chronic Heart Failure Patients by Using Serum Ferritin and Transferrin Saturation Levels: A Cross-Sectional Descriptive Study. Cureus. (2023) 15:e39425. doi: 10.7759/cureus.39425
3. Loncar G, Obradovic D, Thiele H, von Haehling S, Lainscak M. Iron deficiency in heart failure. ESC Heart Fail. (2021) 8:2368–79. doi: 10.1002/ehf2.13265
4. Beavers CJ, Ambrosy AP, Butler J, Davidson BT, Gale SE, Piña IL, et al. Iron Deficiency in Heart Failure: A Scientific Statement from the Heart Failure Society of America. J Card Fail. (2023) 29:1059–77. doi: 10.1016/j.cardfail.2023.03.025
5. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2022) 24:4–131. doi: 10.1002/ejhf.2333
6. Cappellini MD, Comin-Colet J, de Francisco A, Dignass A, Doehner W, Lam CS, et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am J Hematol. (2017) 92:1068–78. doi: 10.1002/ajh.24820
7. Cacoub P, Vandewalle C, Peoc’h K. Using transferrin saturation as a diagnostic criterion for iron deficiency: A systematic review. Crit Rev Clin Lab Sci. (2019) 56:526–32. doi: 10.1080/10408363.2019.1653820
8. Fitzsimons S, Yeo TJ, Ling LH, Sim D, Leong KTG, Yeo PSD, et al. Impact of change in iron status over time on clinical outcomes in heart failure according to ejection fraction phenotype. ESC Heart Fail. (2021) 8:4572–83. doi: 10.1002/ehf2.13617
9. Ueda T, Kawakami R, Nogi K, Nogi M, Ishihara S, Nakada Y, et al. Serum iron: a new predictor of adverse outcomes independently from serum hemoglobin levels in patients with acute decompensated heart failure. Sci Rep. (2021) 11:2395. doi: 10.1038/s41598-021-82063-0
10. Wang Y, Xiao Y, Zhang Y. A systematic comparison of machine learning algorithms to develop and validate prediction model to predict heart failure risk in middle-aged and elderly patients with periodontitis (NHANES 2009 to 2014). Med (Baltimore). (2023) 102:e34878. doi: 10.1097/MD.0000000000034878
11. Kałużna-Oleksy M, Wawrzyniak M, Klimkowska M, Dudek M, Migaj J, Straburzyńska-Migaj E. Awareness of heart failure and perception of the problem in the general population. Cardiovasc J Afr. (2022) 33:180–5. doi: 10.5830/CVJA-2021-059
12. Cohen-Solal A, Philip JL, Picard F, Delarche N, Taldir G, Gzara H, et al. Iron deficiency in heart failure patients: the French 207 CARENFER prospective study. ESC Heart Fail. (2022) 9:874–84. doi: 10.1002/ehf2.13850
13. Parikh A, Natarajan S, Lipsitz SR, Katz SD. Iron deficiency in community-dwelling US adults with self-reported heart failure in the National Health and Nutrition Examination Survey III: prevalence and associations with anemia and inflammation. Circ Heart Fail. (2011) 4:599–606. doi: 10.1161/CIRCHEARTFAILURE.111.960906
14. Verbrugge FH, Guazzi M, Testani JM, Borlaug BA. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation. (2020) 142:998–1012. doi: 10.1161/CIRCULATIONAHA.119.045409
15. Grande D, Gioia MI, Terlizzese P, Iacoviello M. Heart Failure and Kidney Disease. Adv Exp Med Biol. (2018) 1067:219–38. doi: 10.1007/5584_2017_126
16. Xanthopoulos A, Starling RC, Kitai T, Triposkiadis F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. (2019) 7:87–97. doi: 10.1016/j.jchf.2018.10.007
17. Halade GV, Lee DH. Inflammation and resolution signaling in cardiac repair and heart failure. EBioMedicine. (2022) 79:103992. doi: 10.1016/j.ebiom.2022.103992
18. Schiattarella GG, Rodolico D, Hill JA. Metabolic inflammation in heart failure with preserved ejection fraction. Cardiovasc Res. (2021) 117:423–34. doi: 10.1093/cvr/cvaa217
19. Ribot-Hernández I, Martín-González C, Vera-Delgado V, González-Navarrete L, de Armas-González JF, Viña-Rodríguez J, et al. Prognostic Value of Serum Iron, Ferritin, and Transferrin in Chronic Alcoholic Liver Disease. Biol Trace Elem Res. (2020) 195:427–35. doi: 10.1007/s12011-019-01887-0
20. Ambrosy AP, Fitzpatrick JK, Tabada GH, Gurwitz JH, Artz A, Schrier SL, et al. A reduced transferrin saturation is independently associated with excess morbidity and mortality in older adults with heart failure and incident anemia. Int J Cardiol. (2020) 309:95–9. doi: 10.1016/j.ijcard.2020.03.020
21. Han H, Chen Y, Zhang S, Ji X, Zhu M, Ma W, et al. Association between serum ferritin and liver stiffness in adults aged ≥20 years: A cross-sectional study based on NHANES. Med (Baltimore). (2023) 102:e34838. doi: 10.1097/MD.0000000000034838
22. Segev A, Itelman E, Avaky C, Negru L, Shenhav-Saltzman G, Grupper A, et al. Low ALT Levels Associated with Poor Outcomes in 8700 Hospitalized Heart Failure Patients. J Clin Med. (2020) 9:3185. doi: 10.3390/jcm9103185
23. Liu L, Gong B, Wang W, Xu K, Wang K, Song G. Association between haemoglobin, albumin, lymphocytes, and platelets and mortality in patients with heart failure. ESC Heart Fail. (2024) 11:1051–60. doi: 10.1002/ehf2.14662
24. Palau P, Llàcer P, Domínguez E, Tormo JP, Zakarne R, Mollar A, et al. Iron deficiency and short-term adverse events in patients with decompensated heart failure. Clin Res Cardiol. (2021) 110:1292–8. doi: 10.1007/s00392-021-01832-z
25. Burger PM, Koudstaal S, Mosterd A, Fiolet ATL, Teraa M, van der Meer MG, et al. C-Reactive Protein and Risk of Incident Heart Failure in Patients With Cardiovascular Disease. J Am Coll Cardiol. (2023) 82:414–26. doi: 10.1016/j.jacc.2023.05.035
26. Augusto SN, Martens P. Heart Failure-Related Iron Deficiency Anemia Pathophysiology and Laboratory Diagnosis. Curr Heart Fail Rep. (2023) 20:374–81. doi: 10.1007/s11897-023-00623-z
27. Kalogeropoulos AP, Lewis GD. Benefits of intravenous iron supplementation in patients with heart failure: mounting evidence and open questions. Eur J Heart Fail. (2023) 25:538–40. doi: 10.1002/ejhf.2787
28. Mentz RJ, Ambrosy AP, Ezekowitz JA, Lewis GD, Butler J, Wong YW, et al. Randomized Placebo-Controlled Trial of Ferric Carboxymaltose in Heart Failure With Iron Deficiency: Rationale and Design. Circ Heart Fail. (2021) 14:e008100. doi: 10.1161/CIRCHEARTFAILURE.120.008100
29. Graham FJ, Pellicori P, Kalra PR, Ford I, Bruzzese D, Cleland JGF. Intravenous iron in patients with heart failure and iron deficiency: an updated meta-analysis. Eur J Heart Fail. (2023) 25:528–37. doi: 10.1002/ejhf.2810
30. Dong F, Zhang X, Culver B, Chew HG Jr, Kelley RO, Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci (Lond). (2005) 109:277–86. doi: 10.1042/CS20040278
31. Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. (2013) 34:816–29. doi: 10.1093/eurheartj/ehs224
32. Naito Y, Tsujino T, Matsumoto M, Sakoda T, Ohyanagi M, Masuyama T. Adaptive response of the heart to long-term anemia induced by iron deficiency. Am J Physiol Heart Circ Physiol. (2009) 296:H585–93. doi: 10.1152/ajpheart.00463.2008
33. Toblli JE, Cao G, Rivas C, Giani JF, Dominici FP. Intravenous iron sucrose reverses anemia-induced cardiac remodeling, prevents myocardial fibrosis, and improves cardiac function by attenuating oxidative/nitrosative stress and inflammation. Int J Cardiol. (2016) 212:84–91. doi: 10.1016/j.ijcard.2016.03.039
34. Chung YJ, Luo A, Park KC, Loonat AA, Lakhal-Littleton S, Robbins PA, et al. Iron-deficiency anemia reduces cardiac contraction by downregulating RyR2 channels and suppressing SERCA pump activity. JCI Insight. (2019) 4:e125618. doi: 10.1172/jci.insight.125618
35. Zhang H, Zhabyeyev P, Wang S, Oudit GY. Role of iron metabolism in heart failure: From iron deficiency to iron overload. Biochim Biophys Acta Mol Basis Dis. (2019) 1865:1925–37. doi: 10.1016/j.bbadis.2018.08.030
36. Toback M, Clark N. Strategies to improve self-management in heart failure patients. Contemp Nurse. (2017) 53:105–20. doi: 10.1080/10376178.2017.1290537
Keywords: transferrin saturation, heart failure, NHANES, weighted logistic regression analysis, subgroup analysis
Citation: Wang M, Zhang D, Jiang L, Ye M, Nie J and Yin J (2024) Association between serum transferrin saturation levels and heart failure in adults aged ≥40 years: a cross-sectional study based on NHANES (2017-2020.03). Front. Endocrinol. 15:1419064. doi: 10.3389/fendo.2024.1419064
Received: 03 May 2024; Accepted: 14 August 2024;
Published: 30 August 2024.
Edited by:
Lei Ye, University of Alabama at Birmingham, United StatesReviewed by:
Yu Jiang, University of Alabama at Birmingham, United StatesWei-Wen Lim, National Heart Centre Singapore, Singapore
Copyright © 2024 Wang, Zhang, Jiang, Ye, Nie and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Yin, WWluampALm5qbXUuZWR1LmNu
 Mian Wang
Mian Wang Dongyang Zhang1
Dongyang Zhang1 Junjie Yin
Junjie Yin