
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 13 February 2025
Sec. Bone Research
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1418932
 Xi Chen1
Xi Chen1 Ziyuan Wang1
Ziyuan Wang1 Wenzhen Fu1
Wenzhen Fu1 Zhe Wei1
Zhe Wei1 Jiemei Gu1
Jiemei Gu1 Chun Wang1
Chun Wang1 Zhenlin Zhang1,2*
Zhenlin Zhang1,2* Xiangtian Yu2*
Xiangtian Yu2* Weiwei Hu1*
Weiwei Hu1*Objective: CLCN7 mutation caused abnormal osteoclasts, resulting in osteopetrosis. Depending on the type of mutation, CLCN7 mutations can lead to severe or relatively benign forms of osteopetrosis. However, the serum metabolic alterations in osteopetrosis caused by CLCN7 mutation are still unknown. We aimed to investigate the differences in the metabolome of osteopetrosis patients caused by CLCN7 mutation versus healthy controls (HC), uncovering potential subtype diagnosis biomarkers.
Methods: 19 osteopetrosis patients caused by CLCN7 mutation and 19 HC were recruited for liquid chromatography–tandem mass spectrometry analysis. The screened pathway was validated in the myeloid cell specific Clcn7G763R mutant mouse model by quantitative real-time PCR analysis.
Results: Three metabolic pathways were significantly enriched, including glycerophospholipid metabolism (P=0.036948), arachidonic acid metabolism (P=0.0058585) and linoleic acid metabolism (P=0.032035). Ten differential expressed metabolites were located in these three pathways and classified ability with areas under the curve over 0.7 in receiver operating characteristic analysis, suggesting a certain accuracy for being the potential biological markers. Especially, we found that the proteins in glycerophospholipid metabolism were predicted to interact with ClC-7 and further verified that the expression of coding genes were significantly up-regulated in myeloid cell specific Clcn7G763R mutant mouse.
Conclusion: This study provides data on serum metabolomics in osteopetrosis caused by CLCN7 mutation and provides new potential metabolic markers and pathways for diagnosis and pathogenesis of osteopetrosis.
Osteopetrosis is a rare metabolic bone disease characterized by abnormally increased bone mineral density that leads to bone marrow failure, compressive neuropathy, and skeletal dysmorphism (1). According to genetic patterns, it can be divided into autosomal dominant osteopetrosis (ADO), autosomal recessive osteopetrosis (ARO), and X-linked osteopetrosis (XLO) (1–3). ADO is the most common form of osteopetrosis, which incidence is estimated at 1:20,000 (4). Early on, ADO was thought to include two phenotypes, ADO I (OMIM 607634) and ADO II (OMIM 166600) (2). ADO I is characterized by a mutation in the LDL receptor related protein 5 (LRP5) gene that leads to high bone mass but does not result in fractures (5). ADO II is caused by impaired osteoclastic bone resorption that usually results from heterozygous missense mutations in the chloride channel 7 (CLCN7) gene (6, 7).
CLCN7 is the gene that can not only cause severe recessive form of osteopetrosis, namely ARO, but also relatively benign form of osteopetrosis, namely ADO, depending on the type of CLCN7 mutation (8). Furthermore, ADO II caused by CLCN7 mutation accounts for 70% of ADO, which was the most common type of osteopetrosis (9). Therefore, this study focused on osteopetrosis caused by CLCN7 mutations. CLCN7 encodes the Cl-/H+ exchange transporter 7, also known as ClC-7, which generally localizes to the lysosomal compartment and the ruffled membrane of bone-resorbing osteoclasts (10). CLCN7 mutations cause the abnormal osteoclasts cannot secrete acid and thus cannot dissolve the bone, resulting in osteopetrosis. This disease displays heterogeneity, with phenotypes exhibiting various levels of severity, spanning from asymptomatic to life-threatening (11–13). In the absence of genetic testing or typical radiographic findings, elevated levels of lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and creatine kinase BB isozymes (CK-BB) are associated with osteopetrosis caused by CLCN7 mutations (14, 15). Nevertheless, levels of these biomarkers have not been shown to correlate with disease severity, and normal values do not exclude the presence of mutations in the CLCN7 gene (4). Therefore, there is an urgent need to find more specific and sensitive biomarkers.
There are many genetic studies on osteopetrosis, but currently, no serum metabolic research has been found on osteopetrosis caused by CLCN7 mutations. By illuminating specific characteristics that differentiate between health and disease phenotypes, metabolome has become the cornerstone of understanding the differences between physiological and pathological processes, potentially allowing us to search for biomarkers with better sensitivity and specificity and new insights for researching osteopetrosis. Herein, we utilized the liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis to investigate the metabolic differences between healthy controls (HC) and osteopetrosis patients caused by CLCN7 mutation, help decipher the primary metabolic pathway(s) that were altered in osteopetrosis and provide potential more specific and sensitive biomarkers.
Totally, 19 osteopetrosis patients caused by CLCN7 mutation and 19 HC were enrolled in this study. Osteopetrosis patients were diagnosed by laboratory examination, imaging examination and gene testing. HC were recruited from the Health Examination Center. HC inclusion criteria were as follows (1) No metabolic or genetic bone diseases, such as hypoparathyroidism or hyperparathyroidism, hypothyroidism or hyperthyroidism, Paget’s disease, osteomalacia (2); No other medical history of liver, kidney, rheumatism, malignant tumor, etc. (3); No serious or active infection (4); Those who did not use any drugs for the treatment of metabolic bone disease or affecting bone metabolism. The descriptions of the participants are given in Table 1.
All subjects or their guardians (for participants who were under 18 years old) provided informed consents and this study was approved by the Ethics Committee of Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine.
38 fasting venous blood samples were collected and centrifuged at 3000 rpm for 15 min at 4°C. The supernatants were collected as serum samples. The quality control (QC) sample was prepared by mixing an equal aliquot of the supernatants from all of the serum samples.
LC-MS/MS analyses were performed using an UHPLC system (Vanquish, Thermo Fisher Scientific) with a UPLC BEH Amide column (2.1 mm × 100 mm, 1.7 μm) coupled to Orbitrap Exploris 120 mass spectrometer (Orbitrap MS, Thermo). The mobile phase consisted of 25mmol/L ammonium acetate and 25 ammonia hydroxide in water (pH = 9.75) (A) and acetonitrile (B). The Orbitrap Exploris 120 mass spectrometer was used for its ability to acquire MS/MS spectra on information-dependent acquisition mode in the control of the acquisition software (Xcalibur, Thermo).
For clinical parameters, statistical analyses were using IBM SPSS Statistics version 25.0 software (IBM, Armonk, NY, USA). Normally distributed variables, skewed variables, and categorical variables were described using mean ± standard deviation, median (upper quartile, lower quartile), and frequency (percentage), respectively. Between-group comparisons of age between the osteopetrosis group and the HC group were made using Mann-Whitney U test, and of sex were made using chi-square test.
The raw data were processed with relative standard deviation de-noising. Then, the missing values were filled up by half of the minimum value. Also, internal standard normalization method was employed in this data analysis. The final dataset was scaled and logarithmic transformed to minimize the impact of both noise and high variance of the variables using SIMCA (V16.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden). After these normalizations, an unsupervised principal component analysis (PCA) and a supervised model of orthogonal projections to latent structures discriminate analysis (OPLS-DA) were performed. Then, OPLS-DA permutation test was used to test the validity of the model, using R (ggplot2, V 3.3.5). Metabolites which meet the P<0.05 (Student’s t-test) and the OPLS-DA model’s variable importance in the projection (VIP)>1 are considered as differentially expressed metabolites (DEMs). We visualize the results of DEMs in the form of volcano plots, using R (ggplot2, V 3.3.5). For each set of comparisons, we calculated the Euclidean distance matrix for the quantitative values of DEMs and presented them in a heatmap, using R (pheatmap, V 1.0.12).
Then, we annotated all DEMs through the Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Database (http://www.kegg.jp/kegg/pathway.html), using R (base, V3.6.3), and calculated enrichment factor (the ratio of the number of DEMs annotated in a pathway to the number of all metabolites in that pathway, higher values indicate greater enrichment) by enrichment analysis with P-value (Fisher’s test), using R (ggplot2, V3.3.5). We analyzed the interactions between proteins in the key pathways enriched by DEMs and ClC-7 with the STRING database (http://string-db.org).
We also calculated the corresponding ratio for the quantitative values of DEMs, and took the logarithmic conversion at base 2. The receiver operating characteristic (ROC) curves were plotted for DEMs located in the differential key pathways, using R (plotROC V2.2.1, pROC V1.16.2), and the areas under the curve (AUC) were calculated to evaluate the accuracy of diagnosis model based on these DEMs.
Total RNA was isolated from the bone tissues of both upper limbs from the myeloid cell specific Clcn7G763R mutant mouse model (16) according to the protocol supplied with TRIzol Reagent (Sigma, St. Louis, MO, USA). 1 ug total RNA was used for cDNA synthesis using the cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). Then quantitative real-time PCR analysis was performed (Roche LightCycler480II). All the primers were purchased from Beijing Tsingke Biotech Co., Ltd. The sequences of the primers were listed in Supplementary Table S1. mRNA expression levels were normalized to the respective β-actin mRNA and the 2-ΔΔCt method was used to determine the relative amounts of mRNA transcribed. Independent samples t-test for normally distributed variables, Mann-Whitney U test for non-normally distributed variables.
The clinical features of 19 osteopetrosis patients with CLCN7 mutation and 19 HC matched by age and sex are shown in Table 1. The results of Sanger sequencing in osteopetrosis patients with CLCN7 heterozygous mutation are shown in Table 2.
The score scatter plot obtained by PCA of QC samples and experimental samples was shown in Supplementary Figure S1. As shown in the PCA score plot, all QC samples exhibited good overlap, indicating satisfactory stability in the analytical system, and the samples were essentially within the 95% confidence interval.
OPLS-DA analysis and OPLS-DA permutation test were used to characterize the differences between the two groups and evaluated the statistical significance of the model, as shown in Figures 1A, B. From the score scatter plot of OPLS-DA model, it can be seen that the osteopetrosis group and the HC group are very different on serum metabolites, and the within-group reproducibility of each group is good. The Q2 = 0.969, R2Y=0.994 and P-values of Q2 and R2Y are less than 0.05, indicating that the OPLS-DA model was not overfitting and had high separating capacity.
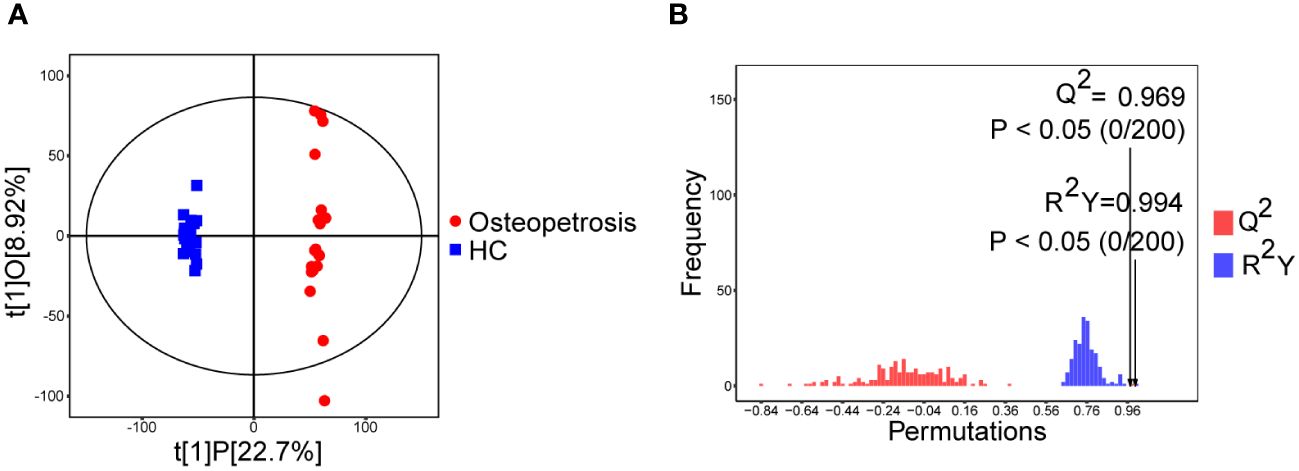
Figure 1 Multivariate analyses of serum metabolites. (A) Score scatter plot of OPLS-DA model; (B) Permutation plot test of OPLS-DA model. HC, healthy controls.
After the above analysis, combined with the results of univariate analysis and multivariate analysis, the metabolites between osteopetrosis patients and HC were screened. A total of 139 DEMs were screened based on the criteria of Student’s t-test P<0.05 and OPLS-DA model VIP>1, as shown in Figure 2. For each set of comparisons, we calculated the Euclidean distance matrix for the quantitative values of the DEMs, clustered them in a complete linkage method, and presented them in a heat map, showed in Supplementary Figure S2 in Supplementary materials. As shown, there was a significant global difference in metabolic profiles between osteopetrosis patients and HC.
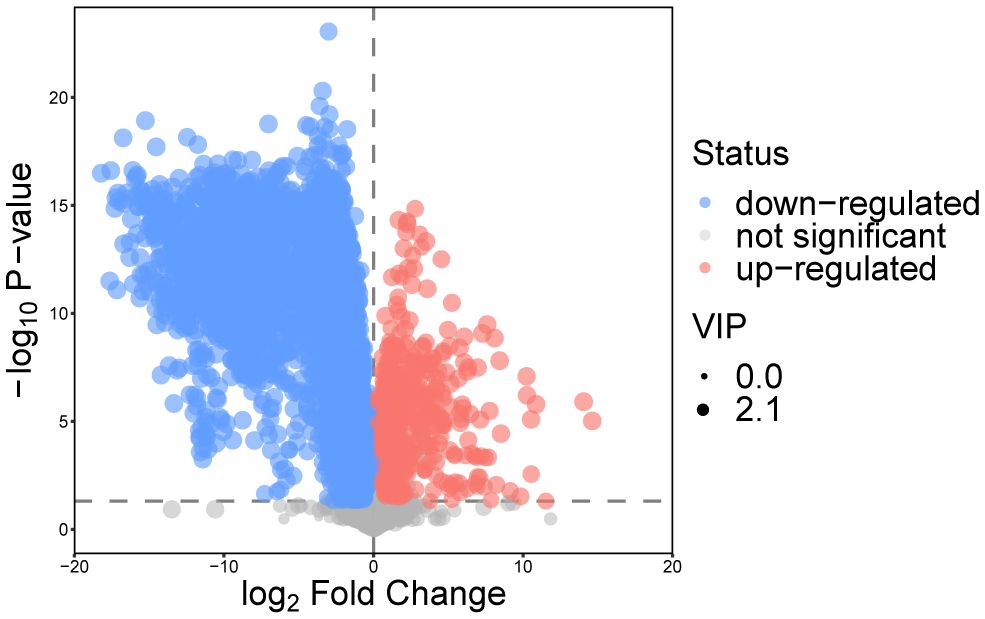
Figure 2 Volcano plot for differentially expressed metabolites for the osteopetrosis group and the healthy control group. VIP, variable importance in projection.
Due to the specificity of CLCN7 induced osteopetrosis, which means that biallelic CLCN7 pathogenic mutations lead to ARO and heterozygous pathogenic mutations lead to ADO, we analyzed the concordance between biallelic pathogenic mutations and heterozygous mutation samples, as shown in Figure 3 and Table 2. We actually found ARO patient (patient 7) was biallelic CLCN7 mutations and had significant different metabolic levels from ADO patients caused by CLCN7 heterozygous mutations. This suggests that the differential metabolism characteristics of individuals can not only distinguish osteopetrosis patients and HC, but also have potential ability to further discriminate different osteopetrosis subtypes induced by biallelic or heterozygous mutations of CLCN7.
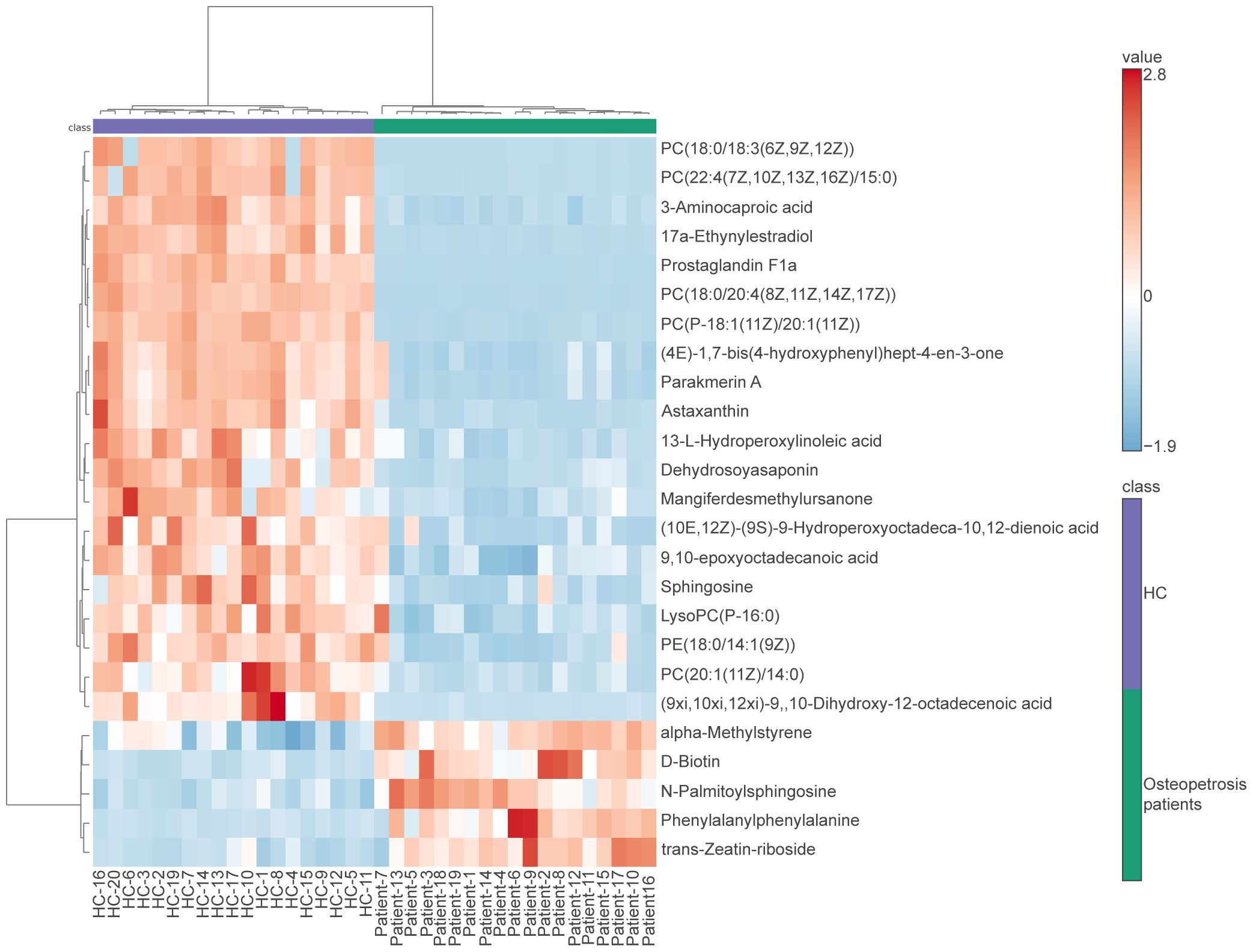
Figure 3 Heatmap of differentially expressed metabolites for subjects enrolled in this study. In the osteopetrosis group, patient 7 carried CLCN7 biallelic pathogenic mutations and the rest of the patients carried CLCN7 heterozygous mutation. HC, healthy controls.
According to the KEGG pathway database, all pathways mapped by DEMs were sorted out, and comprehensive analyses (including enrichment analysis and topology analysis) were used to find the key pathways with the highest correlation with metabolism differences between two groups. A total of 26 pathways were enriched, three of which were statistically significant, namely arachidonic acid (AA) metabolism (P=0.0058585), linoleic acid (LA) metabolism (P=0.032035), glycerophospholipid (GP) metabolism (P=0.036948), as shown in Table 3.
For DEMs located in the screened three metabolic pathways, we evaluated their accuracy for the diagnosis of osteopetrosis using ROC analysis and calculating the AUC values, as shown in Table 4. All of them, a total of 10 DEMs meet AUC>0.7, which means they have a certain accuracy for being the potential biological markers for osteopetrosis with CLCN7 mutation. In addition, there are 7 DEMs, Prostaglandin G2 (PGG2), Prostaglandin B2 (PGB2), 6-Ketoprostaglandin E1, 13-oxo-octadecadienoic acid (13-oxo-ODE), 13-L-Hydroperoxylinoleic acid (LOOH), acetylcholine and glycerophosphocholine, which satisfy AUC>0.9 and are of great value for diagnosis.
To better understand the physiological processes in which these ten DEMs are involved, we analyzed the top 25 metabolites with the strongest correlation to each of the ten DEMs, as shown in Supplementary Figure S3. Subsequently, for the 3 DEMs in the GP metabolism, the most relevant metabolites for each DEM, a total of 75, were included in the enrichment analysis, as shown in Supplementary Table S2. As can be seen in Supplementary Table S2, the raw P values of both the LA metabolism and the AA metabolism were less than 0.05, suggesting that the three pathways are closely linked.
To further understand the role of the screened three pathways in osteopetrosis caused by CLCN7 mutation, we analyzed the interactions of proteins in the three pathways with ClC-7 through the STRING database. Only proteins in GP metabolism are predicted to interact with ClC-7, which increases our interest in the role of this pathway in the pathogenesis of osteopetrosis caused by CLCN7 mutation. As shown in Figure 4A, proteins predicted to interact with ClC-7 include diacylglycerol kinase theta (DGKQ), diacylglycerol kinase zeta (DGKZ), phosphatidylserine synthase 1 (PTDSS1), phosphoethanolamine/phosphocholine phosphatase 1 (PHOSPHO1), glycerol-3-phosphate acyltransferase 4 (GPAT4), lysophospholipase 1 (LYPLA1), lysophospholipase 2 (LYPLA2), and patatin like phospholipase domain containing 6 (PNPLA6). In the above proteins, PHOSPHO1 is the enzyme for the degradation of DEM phosphorylcholine, and LYPLA1 and LYPLA2 are the enzymes for the production of DEM glycerophosphocholine. In addition, we found that the production and degradation of DEMs located in GP metabolism were equally dependent on enzymes through the KEGG pathway database, including choline-phosphate cytidylyltransferase 1A and 1B (PCYT1A and PCYT1B; enzymes for the degradation of phosphorylcholine), choline O-acetyltransferase (CHAT; enzyme for the production of acetylcholine), acetylcholinesterase (ACHE; enzyme for the degradation of acetylcholine) and glycerophosphocholine phosphodiesterase 1 (GPCPD1; enzyme for the degradation of glycerophosphocholine).
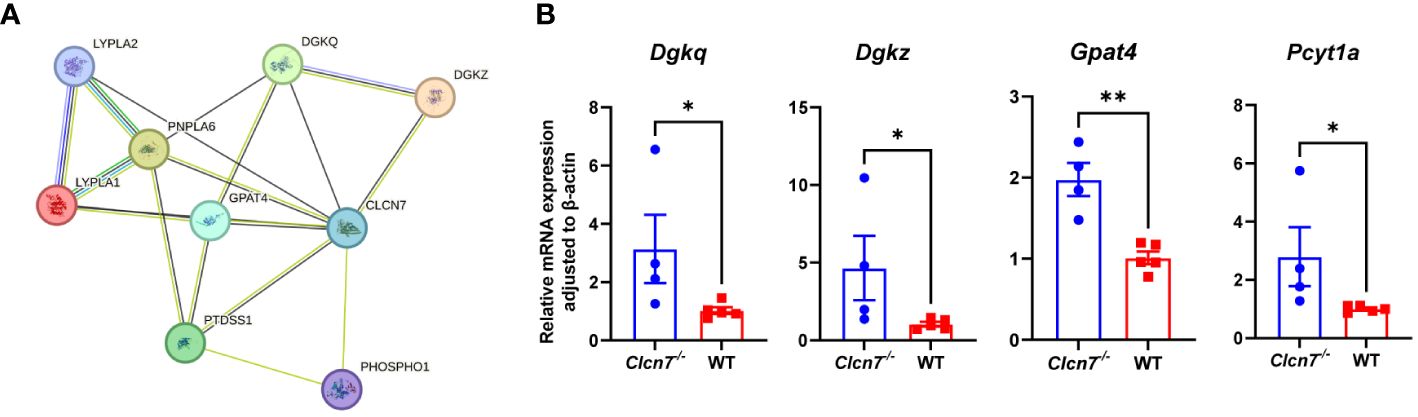
Figure 4 Validation of differential metabolic pathways. (A) proteins predicted to interact with ClC-7 in glycerophospholipid metabolism through the STRING database (version 12.0) with minimum required interaction score (0.15); (B) quantitative real-time PCR analysis results in the myeloid cell specific Clcn7G763R mutant mouse model. *P<0.05; **P<0.01; Clcn7-/-, Clcn7fl/fl; LysM-Crecre/-; WT, Clcn7fl/fl; LysM-Cre-/-.
To further investigate the role of the GP pathway in the pathogenesis of CLCN7 mutations, we validated the expression of the genes encoding the above proteins in the myeloid cell specific Clcn7G763R mutant mouse model by quantitative real-time PCR analysis (Figure 4B). As shown in Figure 4B, the expression of Dgkq (P=0.027), Dgkz (P=0.027), Gpat4 (P=0.002) and Pcyt1a (P=0.014) were significantly higher in Clcn7-/- (Clcn7fl/fl; LysM-Crecre/-) mice than in the litter mate control (WT; Clcn7fl/fl; LysM-Cre-/-).
This study presented a comprehensive metabolomics evaluation for osteopetrosis caused by CLCN7 mutation in Chinese populations. We investigated the associations of metabolites with osteopetrosis to find specific and sensitive biomarkers and provide insights of osteopetrosis pathogenesis. To the best of our knowledge, this is the first study to analyze serum metabolic characteristics of osteopetrosis patients with CLCN7 mutation by LC-MS/MS. In this study, we found that 10 metabolites including PGG2, Delta-12-prostaglandin J2 (D12-PGJ2), 15-KETE, PGB2, 6-Ketoprostaglandin E1, 13-oxo-ODE, LOOH, phosphorylcholine, acetylcholine and glycerophosphocholine, were significantly down-regulated in osteopetrosis patients caused by CLCN7 mutation. Besides, we found GP metabolism was significantly enriched and probably involved in the pathogenesis of osteopetrosis.
GP metabolism, especially phosphorylcholine, acetylcholine, and glycerophosphocholine, were remarkably different between the two groups. GP is one of the important components of the bilayer membrane structure of the cell membrane, and play a vital role in cellular functions, including the regulation of transport processes, protein function and signal transduction (17–19). In previous studies, acetylcholine and glycerophosphocholine were shown to be associated with bone metabolism (20–22). Recent study has shown that ACHE as a hydrolytic enzyme will decrease acetylcholine concentrations, suppressing bone formation and increasing bone resorption (20). And Lips et al. confirmed that mice with gene deficiency of muscarinic acetylcholine receptor M3 had a declined trabecular bone volume, bone surface, and a higher trabecular pattern factor compared to wild type (21). This also means that acetylcholine may play an important role in bone metabolism. Øyen et al. have reported that subjects with low dietary total glycerophosphocholine are at higher risk of lower femoral neck bone mineral density compared to subjects with high levels, especially middle-aged men and elderly women (22).
Our group has previously reported 6 novel mutations of CLCN7, including G741R, a more severe mutation, with multiple fractures occurring in the patient carrying this mutation (23). It is now widely accepted that the pathogenesis of CLCN7 mutations is due to the disruption of the acid environment necessary to dissolve the mineral component of bone, which prevents osteoclasts from functioning and leads to osteopetrosis (24). We therefore constructed the myeloid cell specific Clcn7G763R mutant mouse model to better explore the pathogenesis of CLCN7. And Clcn7-/- mice (Clcn7fl/fl; LysM-Crecre/-) do have significantly reduced body weight, shorter femur length, and increased bone mineral density than the litter mate control (WT; Clcn7fl/fl; LysM-Cre-/-) (16). In this study, we found that the expression of Pcyt1a encoding the degradation enzyme of phosphorylcholine is statistically increased in Clcn7 -/- mice compared to WT. We therefore hypothesize that there was the same increased expression of the PCYT1A gene in osteopetrosis patients caused by CLCN7 mutation, which might account for the decrease level of phosphorylcholine. In addition, we identified some of the proteins in GP metabolism that may interact with ClC-7 through the STRING database, and subsequently verified the expression of genes encoding these proteins in Clcn7-/- mice. We found that the expressions of Dgkq, Dgkz and Gpat4 were upregulated in Clcn7-/- mice. Dgkq and Dgkz encode DGKQ and DGKZ proteins, respectively, which are isozymes of diacylglycerol kinases (DGK) that convert diacylglycerol (DAG) to phosphatidic acid (25). Both DGKQ and DGKZ are expressed in bone residing cells, such as osteoclasts (26). Recent studies have shown that Dgkz-/- mice have increased osteoclast number in the trabecular and cortical bone and an osteoporotic bone phenotype due to the accumulation of DAG contributing to upregulation of c-Fos (26, 27). Therefore, we speculate that DGKQ and DGKZ may be involved in the pathogenesis of osteopetrosis by regulating the level of DAG. Taken together, GP metabolism probably plays an important role in the pathogenesis of osteopetrosis caused by CLCN7 mutation.
Some limitations should be considered when interpreting our study. First, as osteopetrosis is a rare disease, the sample size of this study is inevitably small. Further studies with larger sample sizes are needed, especially to increase the number of biallelic pathogenic mutations to better analyze metabolic differences between biallelic pathogenic mutations, heterozygous mutation, and normal individuals. Second, the present statistical method of metabolomics cannot exclude other confounding factors which could influence osteopetrosis metabolism, such as age and gender. However, we balanced the age and gender between the two groups, which may reduce this impact to some extent. Third, due to the long-term sample collection in this study and the fact that the HC samples were collected from Health Examination Center, some biochemical test results were missing or incomplete.
To our knowledge, we demonstrated the serum metabolic profile of osteopetrosis caused by CLCN7 mutation for the first time. In this study, a total of ten DEMs were found that can be used as potential diagnostic biological markers. Our study also highlighted that metabolic disorder in GP metabolism probably involved in the pathogenesis of osteopetrosis caused by CLCN7 mutation. Overall, our study underscores the value of metabolomics in exploring biomarkers and biological mechanisms of osteopetrosis.
The original contributions presented in the study are publicly available. This data can be found here: MetaboLights database (www.ebi.ac.uk/metabolights/MTBLS11383).
The studies involving humans were approved by the Ethics Committee of Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. The animal study was approved by Shanghai Sixth People’s Hospital Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
XC: Writing – review & editing, Writing – original draft, Project administration, Methodology, Formal analysis, Conceptualization. ZiW: Writing – review & editing, Data curation. WF: Writing – review & editing, Data curation. ZhW: Writing – review & editing, Data curation. JG: Writing – review & editing, Methodology. CW: Writing – review & editing, Methodology. ZZ: Writing – review & editing, Supervision. XY: Writing – review & editing, Supervision. WH: Writing – review & editing, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by the National Natural Science Foundation of China (NSFC) (82170895, 82100941, 82070903, 81770872 and 82270933), the National Key R&D Program of China (2022YFA1004800) and the Clinical Research Plan of SHDC (SHDC2022CRS044).
We are grateful to all participants in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1418932/full#supplementary-material
1. Tolar J, Teitelbaum SL, Orchard PJ. Osteopetrosis. N Engl J Med. (2004) 351:2839–49. doi: 10.1056/NEJMra040952
2. Whyte MP. Osteopetrosis: discovery and early history of "Marble bone disease". Bone. (2023) 171:116737. doi: 10.1016/j.bone.2023.116737
3. Villa A, Guerrini MM, Cassani B, Pangrazio A, Sobacchi C. Infantile Malignant, autosomal recessive osteopetrosis: the rich and the poor. Calcif Tissue Int. (2009) 84:1–12. doi: 10.1007/s00223-008-9196-4
4. Wu CC, Econs MJ, DiMeglio LA, Insogna KL, Levine MA, Orchard PJ, et al. Diagnosis and management of osteopetrosis: consensus guidelines from the osteopetrosis working group. J Clin Endocrinol Metab. (2017) 102:3111–23. doi: 10.1210/jc.2017-01127
5. Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Bénichou O, Scopelliti D, et al. Six novel missense mutations in the ldl receptor-related protein 5 (Lrp5) gene in different conditions with an increased bone density. Am J Hum Genet. (2003) 72:763–71. doi: 10.1086/368277
6. Stauber T, Wartosch L, Vishnolia S, Schulz A, Kornak U. Clcn7, a gene shared by autosomal recessive and autosomal dominant osteopetrosis. Bone. (2023) 168:116639. doi: 10.1016/j.bone.2022.116639
7. Zifarelli G. The role of the lysosomal cl(-)/H(+) antiporter clc-7 in osteopetrosis and neurodegeneration. Cells. (2022) 11(3):366. doi: 10.3390/cells11030366
8. Boudin E, Van Hul W. Sclerosing bone dysplasias. Best Pract Res Clin Endocrinol Metab. (2018) 32:707–23. doi: 10.1016/j.beem.2018.06.003
9. Del Fattore A, Fornari R, Van Wesenbeeck L, de Freitas F, Timmermans JP, Peruzzi B, et al. A new heterozygous mutation (R714c) of the osteopetrosis gene, pleckstrin homolog domain containing family M (with run domain) member 1 (Plekhm1), impairs vesicular acidification and increases tracp secretion in osteoclasts. J Bone Miner Res. (2008) 23:380–91. doi: 10.1359/jbmr.071107
10. Barvencik F, Kurth I, Koehne T, Stauber T, Zustin J, Tsiakas K, et al. Clcn7 and tcirg1 mutations differentially affect bone matrix mineralization in osteopetrotic individuals. J Bone Miner Res. (2014) 29:982–91. doi: 10.1002/jbmr.2100
11. Waguespack SG, Koller DL, White KE, Fishburn T, Carn G, Buckwalter KA, et al. Chloride channel 7 (Clcn7) gene mutations and autosomal dominant osteopetrosis, type ii. J Bone Miner Res. (2003) 18:1513–8. doi: 10.1359/jbmr.2003.18.8.1513
12. Waguespack SG, Hui SL, Dimeglio LA, Econs MJ. Autosomal dominant osteopetrosis: clinical severity and natural history of 94 subjects with a chloride channel 7 gene mutation. J Clin Endocrinol Metab. (2007) 92:771–8. doi: 10.1210/jc.2006-1986
13. Wang Z, Li X, Wang Y, Fu W, Liu Y, Zhang Z, et al. Natural history of type ii autosomal dominant osteopetrosis: A single center retrospective study. Front Endocrinol (Lausanne). (2022) 13:819641. doi: 10.3389/fendo.2022.819641
14. Whyte MP, Kempa LG, McAlister WH, Zhang F, Mumm S, Wenkert D. Elevated serum lactate dehydrogenase isoenzymes and aspartate transaminase distinguish albers-schönberg disease (Chloride channel 7 deficiency osteopetrosis) among the sclerosing bone disorders. J Bone Miner Res. (2010) 25:2515–26. doi: 10.1002/jbmr.130
15. Research CSoOaBM. Expert consensus on clinical diagnosis and treatment of osteopetrosis. Chin J Osteoporosis Bone Mineral Res. (2023) 16:413–28. doi: 10.3969/j.issn.1674-2591.2023.05.001
16. Ziyuan W, Shanshan L, Xiang L, Zhenlin Z, Chun W. Generation and phenotype of myeloid cell specific clcn7-mutation osteopetrosis mouse model. Chin J Endocrinol Metab. (2022) 38:313–21. doi: 10.3760/cma.j.cn311282-20211224-00813
17. Ecker J, Liebisch G. Application of stable isotopes to investigate the metabolism of fatty acids, glycerophospholipid and sphingolipid species. Prog Lipid Res. (2014) 54:14–31. doi: 10.1016/j.plipres.2014.01.002
18. Guido ME, Monjes NM, Wagner PM, Salvador GA. Circadian regulation and clock-controlled mechanisms of glycerophospholipid metabolism from neuronal cells and tissues to fibroblasts. Mol Neurobiol. (2022) 59:326–53. doi: 10.1007/s12035-021-02595-4
19. Zhu Q, Wu Y, Mai J, Guo G, Meng J, Fang X, et al. Comprehensive metabolic profiling of inflammation indicated key roles of glycerophospholipid and arginine metabolism in coronary artery disease. Front Immunol. (2022) 13:829425. doi: 10.3389/fimmu.2022.829425
20. Luo X, Lauwers M, Layer PG, Wen C. Non-neuronal role of acetylcholinesterase in bone development and degeneration. Front Cell Dev Biol. (2020) 8:620543. doi: 10.3389/fcell.2020.620543
21. Lips KS, Kneffel M, Willscheid F, Mathies FM, Kampschulte M, Hartmann S, et al. Altered ultrastructure, density and cathepsin K expression in bone of female muscarinic acetylcholine receptor M3 knockout mice. Int Immunopharmacol. (2015) 29:201–7. doi: 10.1016/j.intimp.2015.05.012
22. Øyen J, Gjesdal CG, Karlsson T, Svingen GF, Tell GS, Strand E, et al. Dietary choline intake is directly associated with bone mineral density in the hordaland health study. J Nutr. (2017) 147:572–8. doi: 10.3945/jn.116.243006
23. Wang C, Zhang H, He JW, Gu JM, Hu WW, Hu YQ, et al. The virulence gene and clinical phenotypes of osteopetrosis in the chinese population: six novel mutations of the clcn7 gene in twelve osteopetrosis families. J Bone Miner Metab. (2012) 30:338–48. doi: 10.1007/s00774-011-0319-z
24. Frattini A, Pangrazio A, Susani L, Sobacchi C, Mirolo M, Abinun M, et al. Chloride channel clcn7 mutations are responsible for severe recessive, dominant, and intermediate osteopetrosis. J Bone Miner Res. (2003) 18:1740–7. doi: 10.1359/jbmr.2003.18.10.1740
25. Shulga YV, Topham MK, Epand RM. Regulation and functions of diacylglycerol kinases. Chem Rev. (2011) 111:6186–208. doi: 10.1021/cr1004106
26. Zamani A, Decker C, Cremasco V, Hughes L, Novack DV, Faccio R. Diacylglycerol kinase Z (Dgkζ) is a critical regulator of bone homeostasis via modulation of C-fos levels in osteoclasts. J Bone Miner Res. (2015) 30:1852–63. doi: 10.1002/jbmr.2533
Keywords: osteopetrosis, CLCN7 mutation, metabolomics, liquid chromatography-tandem mass spectrometry, glycerophospholipid metabolism
Citation: Chen X, Wang Z, Fu W, Wei Z, Gu J, Wang C, Zhang Z, Yu X and Hu W (2025) Metabolomics study of osteopetrosis caused by CLCN7 mutation reveals novel pathway and potential biomarkers. Front. Endocrinol. 15:1418932. doi: 10.3389/fendo.2024.1418932
Received: 17 April 2024; Accepted: 13 May 2024;
Published: 13 February 2025.
Edited by:
Xiaofang Wang, Texas A&M University Baylor College of Dentistry, United StatesReviewed by:
Harry Blair, University of Pittsburgh, United StatesCopyright © 2025 Chen, Wang, Fu, Wei, Gu, Wang, Zhang, Yu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenlin Zhang, emhhbmd6bEBzanR1LmVkdS5jbg==; Xiangtian Yu, Z3JhY2V5dTE5ODVAMTYzLmNvbQ==; Weiwei Hu, aHV3ZWl3ZWkxOTgwQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.