- 1Department of Obstetrics and Gynecology, The First Affiliated Hospital of Ningbo University, Ningbo, China
- 2Department of Urology, The First Affiliated Hospital of Ningbo University, Ningbo, China
Background: Observational studies suggest the risk of primary ovarian insufficiency (POI) is increased in autoimmune disorders (AIDs), but it is unclear whether there is a causal relationship. Therefore, we aimed to investigate the bidirectional causality between 20 AIDs and POI using Mendelian randomization (MR) analysis.
Methods: A bidirectional two-sample MR investigation was designed by using publicly accessible summary-level data from genome-wide association studies (GWAS). The inverse variance weighted (IVW) method was performed as the main analysis, supplemented by several sensitivity analyses. Cochran Q test was used to evaluate SNP estimate heterogeneity. MR-Egger and MR-PRESSO methods were utilized to detect horizontal pleiotropy.
Results: The MR analyses revealed that genetically determined coeliac disease (CeD) (OR = 1.124, 95% CI 1.033-1.224, P = 0.007), vitiligo (OR = 1.092, 95% CI 1.003-1.188; P = 0.042), systemic lupus erythematosus (SLE) (OR = 1.122, 95% CI 1.030-1.223, P = 0.008), and selective immunoglobulin A deficiency (SIgAD) (OR = 0.866, 95% CI: 0.776-0.967, P = 0.011) exhibited significant causal relationships with POI. We also found suggestive evidence of positive effect of Addison’s disease (AD) towards POI (OR5e-6 = 1.076, 95% CI 1.002-1.154, P = 0.043).
Conclusion: This comprehensive MR analysis indicated that SLE, CeD, vitiligo, and AD caused an increased risk of POI, SIgAD was associated with a decreased risk of POI. These insights carry profound clinical implications, particularly emphasizing the early intervention for women with AIDs/POI who wish to preserve their reproductive potential or plan for future pregnancies.
Introduction
Primary ovarian insufficiency (POI) is a disorder characterized by the premature cessation of ovarian function before the age of 40, leading to amenorrhea, low estrogen levels, and elevated follicle-stimulating hormone (FSH) levels (1). The incidence of POI within the general female demographic is approximated to range from 1% to 3.7% (2–5). The state of early estrogen deficiency caused women facing an elevated risk for a spectrum of health complications, including cardiovascular disease, dementia, declined neurocognitive function, osteoporosis, and augmented overall mortality rate (6–11). POI arises from a complex interplay of genetic and immunological determinants, yet a substantial proportion of instances are idiopathic (12). The contribution of autoimmunity to POI is variably estimated between 5-30%, highlighting the cohort heterogeneity in the studied populations (12–15).
Autoimmune diseases (AIDs), such as thyroid disorders (Hashimoto thyroiditis and Grave’s disease) (16), Addison’s disease (17), and systemic lupus erythematosus (18), have been implicated in the pathogenesis of POI. However, the complex interplay between these conditions and POI remains incompletely understood, and the causal relationships are not fully elucidated. Traditional observational studies have been limited by potential confounders and reverse causation, which complicate the determination of causality in the relationship between autoimmune diseases and POI. Additionally, for patients presenting with POI in gynecological endocrinology clinics, the European Society of Human Reproduction and Embryology (ESHRE) has issued guidelines in screening for 21OH-Ab/ACA or TPO-Ab/TSH tests to identify certain immune disorders (1). However, in the case of women of reproductive age attending rheumatology departments due to autoimmune diseases, there currently exist no consensus or guidelines specifically dedicated to the screening of POI within this patient population. Early detection and diagnosis of POI is crucial for timely intervention, preservation of fertility, and management of long-term health risks. Indiscriminate surveillance and monitoring are anticipated to contribute to an augmented workload in the management of POI.
The current study employs Mendelian randomization (MR), a powerful epidemiological tool, to overcome the limitations of traditional observational research and provide novel insights into the causal interplay between a spectrum of autoimmune disorders and POI (19–21). By utilizing genetic variants as instrumental variables, MR allows for the circumvention of confounding factors and the establishment of causal inferences. This robust design ensures that the observed associations are less likely to be influenced by lifestyle factors, environmental exposures, or reverse causation, thereby offering a more accurate representation of the relationship between autoimmune disorders and the risk of POI. To our knowledge, comprehensive overview of the primary ovarian insufficiency consequence of autoimmune diseases is currently lacking. In this context, we have conducted a two-sample bidirectional MR study to systematically investigate the causal association between autoimmune diseases and POI. The significance of this research lies in its potential to inform clinical practice, particularly in the early identification and intervention for women with autoimmune diseases who wish to preserve their reproductive potential or are planning for future pregnancies.
Methods
Study design
The bidirectional two-sample MR investigation was designed to elucidate the causal association of 20 autoimmune diseases on the susceptibility to POI (Figure 1) following the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) guidelines (19). The investigation relied upon publicly accessible summary-level data derived from genome-wide association studies (GWAS), specifically incorporating datasets from the websites of FinnGen study (https://www.finngen.fi/en/access_results), GWAS Catalog (https://www.ebi.ac.uk/gwas/) (22), and other expansive consortia, ensuring the absence of overlap in study populations. Subsequently, we identified and utilized an additional contemporary dataset on autoimmune diseases from the IEU GWAS repository (https://gwas.mrcieu.ac.uk/datasets/) to corroborate our analytical findings. All incorporated studies underwent ethical scrutiny and received approval from pertinent review boards, with participants providing informed consent.
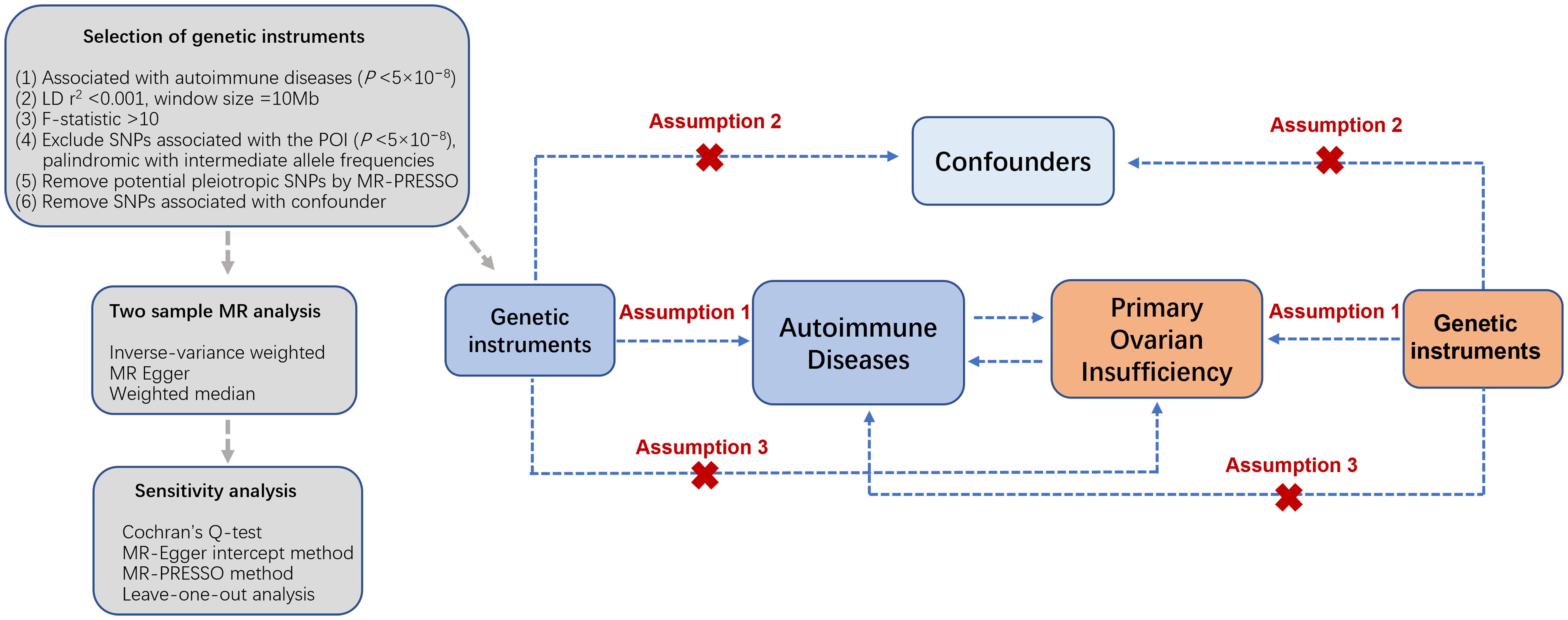
Figure 1. Overview of the bidirectional two-sample MR analysis. Assumption 1: genetic instrumental variables are strongly related to the exposure; Assumption 2: genetic instrumental variables are independent from confounding factors of the exposure-outcome; Assumption 3: genetic instrumental variables influence outcomes solely through the exposure. SNPs, single nucleotide polymorphisms; POI, primary ovarian insufficiency; LD, linkage disequilibrium.
Instrumental variables
Genetic variants associated with autoimmune diseases
Our investigation primarily aimed to explore the causal associations between 20 of the most common autoimmune disorders and POI. Autoimmune diseases included type 1 diabetes mellitus (T1DM) (23), coeliac disease (CeD) (24), Crohn’s disease (CD) (25), primary biliary cholangitis (PBC) (26), ulcerative colitis (UC) (27), Hashimoto’s thyroiditis (HT) (27), Grave’s disease (GD) (27), hypothyroidism (28), Addison’s disease (AD) (29), polymyalgia rheumatica (PMR) (30), myasthenia gravis (MG) (31), rheumatoid arthritis (RA) (27), multiple sclerosis (MS) (32), Sjogren’s syndrome (SS) (33, 34), psoriasis (35), vitiligo (36), systemic sclerosis (SSc) (37), idiopathic thrombocytopenic purpura (ITP) (27), systemic lupus erythematosus (SLE) (38), and selective immunoglobulin A deficiency (SIgAD) (39). To obtain the comprehensive information of the exposures among the European population, we identified and selected the most extensive and contemporaneously updated GWAS meta-analysis study for the initial MR analysis, as delineated in Table 1.
Genetic variants associated with POI
The GWAS data for primary ovarian insufficiency was obtained from the European FinnGen project, a study identifying genotype-phenotype correlations in 500,000 Finnish biobank participants (40). We used the recently updated R10 data release of FinnGen, encompassing 542 cases of POI and 218,970 controls. The inclusion criteria of POI came from the codes of the International classification of diseases 10th Revision (ICD-10), ICD-9, and ICD-8 with a specific phenotypic code being “E4_OVARFAIL”. The European Society of Human Reproduction and Embryology (ESHRE) criteria are employed for the diagnosis of POI. These criteria comprise the individual under 40 years, getting rare or absent menstruation for at least four months, and with at least two serum basic follicle-stimulating hormone (FSH) tests with levels exceeding 25 U/L, executed at least four weeks out (1).
Instrumental variables selection
Single-nucleotide polymorphisms (SNPs) associated with autoimmune disorders, reaching genome-wide significance (P < 5×10−8), were meticulously curated from the extensive GWAS studies. Linkage disequilibrium (LD) among SNPs associated with each autoimmune disease was computed utilizing the 1000 Genomes European panel and the clumping method. SNPs exhibiting linkage disequilibrium (defined as r2 > 0.001 and clump distance <10,000 kb) were excluded from the analysis (41). The strength of the instrumental variables was evaluated through the utilization of F-statistic. , wherein R2 denotes the proportion of variability that can be explained by IVs in the exposure, n signifies the sample size, and k represents the number of SNPs (42). IVs were designated as weak if the F-statistic was below 10 and were consequently omitted (43). We also excluded IVs that had a pronounced correlation with the POI (P < 5×10−8). Additionally, IVs that were palindromic with intermediate allele frequencies were disregarded. SNPs flagged as outliers by MR-PRESSO were similarly excluded to maintain the robustness of the MR analysis (44). Cigarette smoking, a common environmental determinant and established risk factor for POI development (45), had its associated SNPs removed (46). In the forward and reverse MR analyses for genetic variation selection, the standard practice is to incorporate all genome-wide significant variants (P < 5×10−8) to minimize false positives. In the absence of such variants or in the cases with limited number of SNPs, alternative thresholds (e.g., 5×10−6, 1×10−5) was employed, provided they adhere to the instrumental variable criteria.
Statistical analysis
Two-sample MR analysis was executed bidirectionally, initially considering the associations between genetic predisposition to 20 common autoimmune disorders with the risk of POI. The second set of MR analyses handled POI as the exposure and autoimmune disorders as the outcomes. A comparative assessment across three distinct methods was performed, encompassing inverse-variance weighted (IVW) (47), MR Egger (48), and weighted median (47). Each method incorporates slightly different assumptions regarding the nature of pleiotropy, thus a consistent point estimate across multiple methods enhances the robustness of causal inference, with the IVW method serving as primary analysis and other two methods contributing as sensitivity analyses. Cochran Q-test was utilized to evaluate heterogeneity across MR analysis (49). In instances where considerable heterogeneity was observed, we employed the IVW random-effects model to address this variability. We conducted a suite of sensitivity analyses encompassing the weighted median, MR-Egger, and MR-PRESSO methods to ascertain the stability of our findings and to detect potential horizontal pleiotropy. The weighted median estimation in MR analysis is reliable when a majority of the contributing weight is attributed to SNPs without pleiotropic influences. The MR-Egger regression, equipped with an intercept test, discerns and rectifies horizontal pleiotropy, yielding adjusted effect estimates. The MR-PRESSO approach identifies and rectifies pleiotropic outliers and employs a global test to assess SNP heterogeneity indicative of pleiotropy. A p-value threshold of > 0.05 was established to denote a lack of heterogeneity or pleiotropy among the SNPs. Additionally, we conducted a leave-one-out analysis to scrutinize the influence of outlier and pleiotropic SNPs on the causal estimates (50). Analyses of the POI against autoimmune diseases used a Bonferroni multiple testing corrected P-value of 0.0025 (0.05/20) because twenty autoimmune disorders were examined. Threshold values ranging from 0.0025 to 0.05 were interpreted as suggestive of potential causal relationships while adjusted P values < 0.0025 were deemed significant. All analyses were conducted using R packages TwoSampleMR and MRPRESSO in R version 4.1.3.
Results
Genetic instruments and strength
Each single SNP exhibited an F-statistic value that surpassed the critical threshold of 10, thereby mitigating the potential confounding effects of weak instrumental variables on the analytical outcomes. Detailed information of the individual SNP characteristics is provided in Supplementary Tables 1–20. The synthesized MR analyses, employing the IVW method, are graphically depicted in Figure 2, with the comprehensive results articulated in Supplementary Table 21.
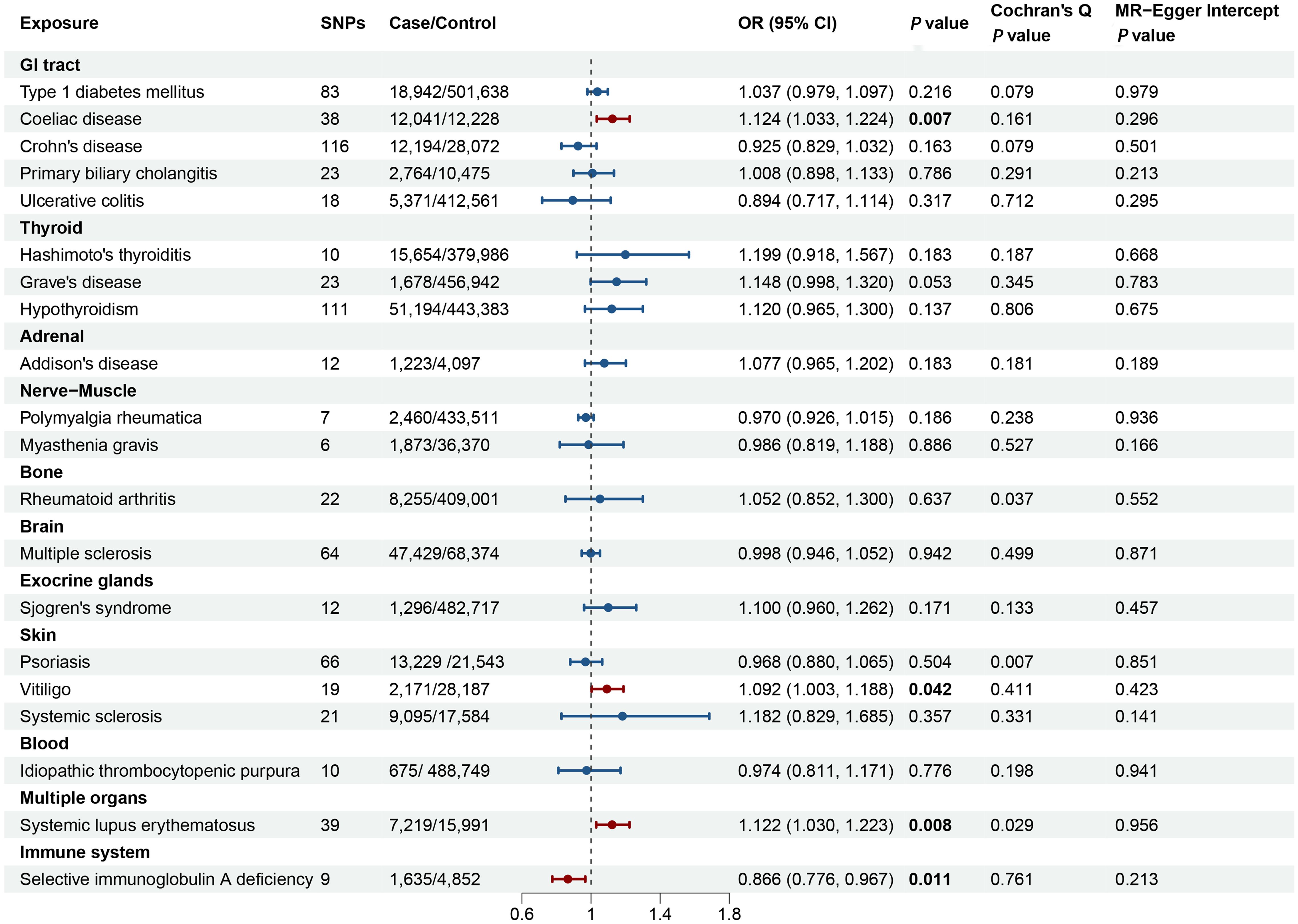
Figure 2. Forest plot illustrating the causal effect of autoimmune diseases on primary ovarian insufficiency. The causal effect of different autoimmune diseases on primary ovarian insufficiency was expressed as OR per unit. Error bars represent the 95% CIs of the estimates. GI, gastrointestinal; SNPs, single nucleotide polymorphisms; OR, odds ratio; CI, confidence interval.
Two-sample MR analysis
Figure 2 and Supplementary Table 21 delineated the genetic correlations between AIDs and POI. The MR analyses revealed that genetically determined CeD, vitiligo, SLE, and SIgAD exhibited significant causal relationships with POI. Conversely, no discernible causal links were identified between genetically determined T1DM, CD, PBC, UC, HT, GD, hypothyroidism, AD, PMR, MG, RA, MS, SS, SSc, psoriasis, and ITP with POI.
Specifically, the causal relationship between CeD and POI was explored further. The IVW method revealed a significant positive association, where genetically-inferred predisposition to CeD was linked to an increased likelihood of POI (odds ratio [OR] = 1.124, 95% confidence interval [CI] 1.033-1.224, P = 0.007). This causal effect was corroborated by the weighted median and MR-Egger approaches, which yielded consistent results (Supplementary Table 21). Cochran’s Q-test, MR-Egger intercept, and MR-PRESSO analyses, did not indicate the presence of substantial heterogeneity or horizontal pleiotropy (Supplementary Figure 1; Supplementary Table 21). The leave-one-out sensitivity analysis further confirmed the robustness of the observed causal relationship between genetically-predicted CeD and POI (Supplementary Figure 2).
An increased genetic predisposition to vitiligo was associated with a heightened risk of POI, with each 1-unit increase in the log-transformed odds of vitiligo corresponding to an OR of 1.092 (95% CI 1.003-1.188; P = 0.042) for POI. Assessments of horizontal pleiotropy, including MR-Egger intercept and MR-PRESSO, did not reveal any significant biases (Figure 2; Supplementary Table 21).
Genetically-predicted SLE demonstrated a positive correlation with POI risk using the IVW method (OR = 1.122, 95% CI 1.030-1.223, P = 0.008). Consistent effect directions were observed with the MR-Egger and weighted median approaches, despite some evidence of potential horizontal pleiotropy (MR-PRESSO Global test = 0.044) and heterogeneity (Cochran’s Q test = 0.029). Further investigation identified two outliers (rs150180633 and rs35000415), and after excluding these, the association remained significant (OR = 1.156, 95% CI 1.066-1.254, P < 0.001). Leave-one-out analyses did not reveal any other outliers among the instrumental variables (Supplementary Figure 3).
For SIgAD, the MR analysis that was associated with a decreased risk of POI (OR = 0.866, 95% CI: 0.776-0.967, P = 0.011) (Figure 2). The result was consistently replicated using the weighted median and MR-Egger approaches, as shown in Supplementary Table 21. The causal association between SIgAD and POI demonstrated resilience to biases from horizontal pleiotropic effects (MR-PRESSO Global test = 0.677), and the leave-one-out analysis further validated the reliability of these results (Supplementary Figure 4).
Additional methodological replications using expanded GWAS data corroborated the significant causal relationships between genetically-predicted CeD (IVW method: OR = 1.096, 95%CI 1.017-1.182; P = 0.016) (51), SLE (IVW method: OR = 1.220, 95% CI 1.025-1.452, P = 0.025) (27), and vitiligo (IVW method: OR=1.560, 95% CI 1.155-2.107, P = 0.004) (52) with POI (Supplementary Table 22). The lack of significant heterogeneity observed in the Cochran’s Q-test and the absence of evidence for horizontal pleiotropy based on the MR-Egger intercept test suggested consistency across the instrumental variables. Furthermore, the leave-one-out sensitivity analysis validated the stability of the results (Supplementary Figures 5-7).
The MR analyses in the reverse direction did not reveal any notable causal effect of genetic predisposition to POI on the susceptibility to AIDs (Figure 3). This was observed across various autoimmune diseases, additional details for MR Egger and weighted median methods are provided in the Supplementary Table 23.
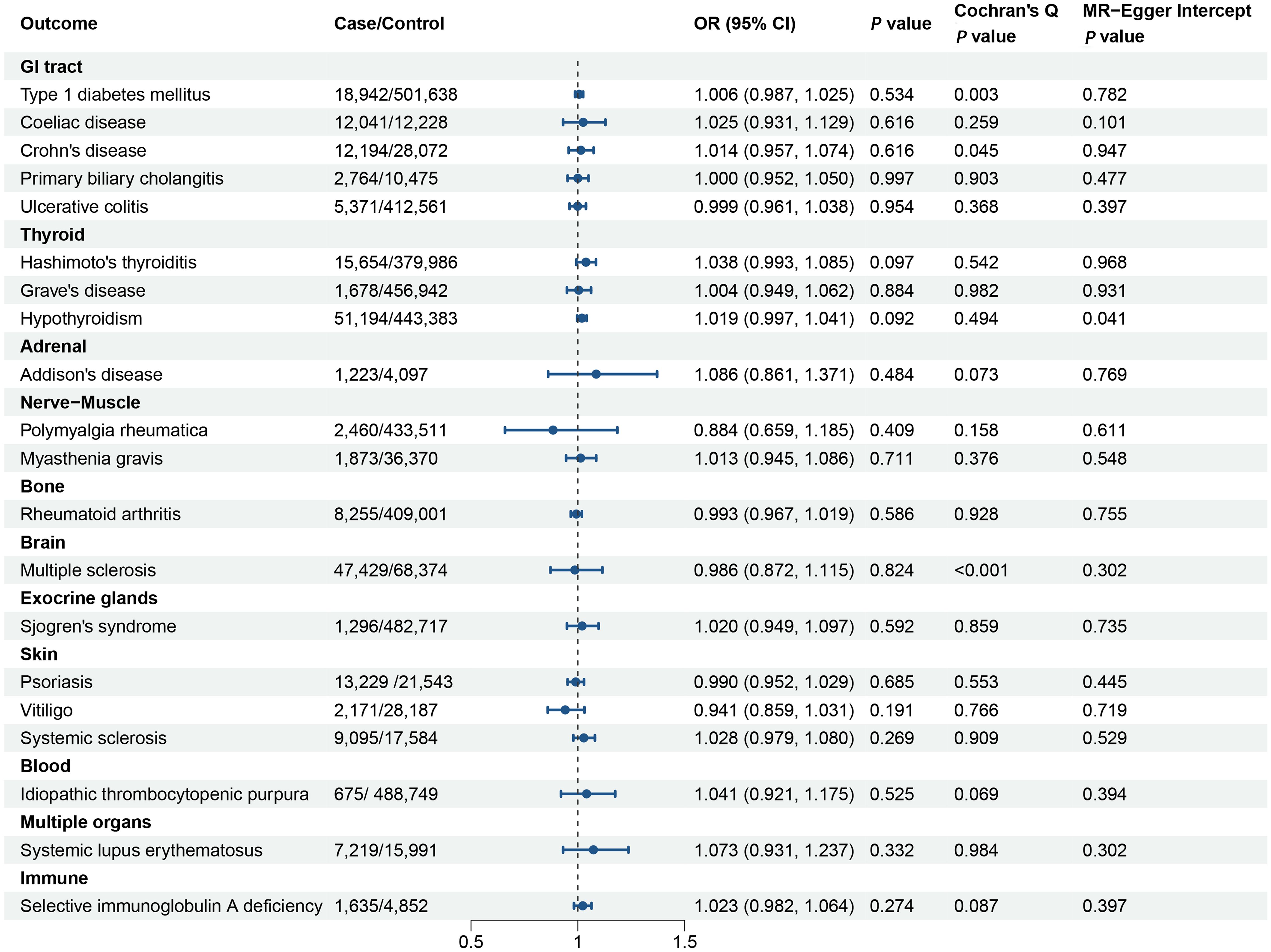
Figure 3. Forest plot illustrating the associations of the genetically predicted primary ovarian insufficiency with the risk of autoimmune diseases. The causal effect of primary ovarian insufficiency on different autoimmune diseases was expressed as OR per unit. Error bars represent the 95% CIs of the estimates. GI, gastrointestinal; OR, odds ratio; CI, confidence interval.
Discussion
The present study’s findings from the bidirectional two-sample MR investigation provide novel insights into the causal interplay between a spectrum of autoimmune disorders and primary ovarian insufficiency. By leveraging the robust design of MR, which circumvents the limitations of traditional observational studies, we have delineated a genetic association between certain autoimmune diseases and the susceptibility to POI. Our results revealed a significant genetic predisposition in individuals with coeliac disease, vitiligo, systemic lupus erythematosus, and suggestive evidence of positive effect of Addison’s disease towards POI, indicating that these conditions may predispose to POI at a genetic level. Conversely, selective IgA deficiency was correlated with a decreased incidence of POI, suggesting the presence of a potentially protective genetic factor.
SLE is an autoimmune disease that affects the majority of women in their reproductive years, characterized by an immune system that mistakenly attacks the body’s own tissues, including the ovaries. The relationship between SLE and POI is multifaceted and can involve several mechanisms. The chronic inflammation associated with SLE can lead to tissue damage in the ovaries, potentially resulting in POI. Inflammatory cytokines and immune cells can infiltrate the ovarian tissue and disrupt normal follicular development and function. Certain medications used to treat SLE, particularly alkylating agents like cyclophosphamide (CYC), are known to be gonadotoxic. These drugs can cause a reduction in ovarian reserve and lead to POI. Furthermore, women with SLE frequently possess a variety of autoantibodies that can, in some cases, cross-react with ovarian antigens, potentially triggering an autoimmune assault on the ovaries and resulting in POI. Moreover, there may be genetic and hormonal factors that predispose individuals with SLE to develop POI, certain gene polymorphisms associated with both SLE and POI may contribute to the co-occurrence of these conditions. Additionally, the systemic nature of SLE can lead to a variety of complications that may indirectly affect ovarian function, such as cardiovascular disease, which can influence blood flow to the ovaries. A study by Akawatcharangura et al. reported a heightened incidence of POI within the SLE patient cohort in comparison to that observed in the general population (18). The research reported that 12% of SLE patients treated with immunosuppressive agents developed POI, and a cumulative dose of CYC exceeding 10 grams was the only independent risk factor for POI. The study suggests that systematic evaluation and early recognition of POI should be promoted in the care of SLE patients to prevent these events. Another study also pointed out that in the absence of CYC treatment, the prevalence of POF in SLE patients is consistent with that of the general population (53). This indicates that most SLE patients are unlikely to have autoimmune processes in the ovaries. Consistent with these researches, our study revealed that SLE as a risk factor for POI among women of reproductive age. These findings underscore the importance of monitoring ovarian function in SLE patients, especially those who are undergoing treatments with potential gonadotoxic medications that may affect ovarian reserve (54). For women with SLE, particularly those planning for future fertility, understanding and assessing the risk of POI is crucial.
Vitiligo is a chronic skin condition characterized by the appearance of white patches due to the loss of melanocytes, the pigment-producing cells in the skin. It affects both genders and involves complex etiological factors, including genetics, autoimmune responses, oxidative stress, and melanocyte destruction. Both innate and adaptive immune systems play a significant role in the pathogenesis of vitiligo. Melanocytes, the pigment-producing cells in the skin, are destroyed through mechanisms involving molecules released from these cells and possibly keratinocytes, which trigger an immune response and target various organs, including the ovaries, leading to a decrease in ovarian reserves. Ünal et al. investigated the relationship between vitiligo and its potential effects on ovarian reserves in women (55). The study found a negative correlation between the duration of vitiligo and AMH, prolactin, and ovarian volume, indicating that longer exposure to the disease may be associated with reduced ovarian reserve. A positive correlation was also observed between disease duration and LH and POI rates. We found a positive causal association between vitiligo and POI in our MR study (OR=1.092, 95% CI 1.003-1.188, P=0.042). The result is consistent with forementioned case-control study (55). Given the potential autoimmune link between vitiligo and POI, it is recommended that once vitiligo is diagnosed, the ovarian reserve should be evaluated and monitored routinely in all reproductive-aged women. Early detection and appropriate management of these conditions can help improve the quality of life and reproductive health for affected individuals.
Celiac disease, characterized by an immune-mediated intolerance to gluten, can lead to systemic effects that extend beyond the gastrointestinal tract, including potential endocrinopathies that may affect ovarian function (56). The potential impact of celiac disease on ovarian reserve may be mediated through several mechanisms. Malnutrition, particularly deficiencies in vitamins and minerals such as iron, zinc, and vitamin D, which are common in celiac disease, can disrupt the hypothalamic-pituitary-ovarian axis, leading to hormonal imbalances and potentially affecting follicular development and ovulation. Additionally, chronic inflammation and the immune response associated with celiac disease may have direct effects on ovarian tissue. However, research on this topic has yielded mixed results. Cakmak et al. found that AMH level and ovarian reserve was decreased and their decline is correlated with the duration of the celiac disease in patients of reproductive age (57), while Comba et al. reported no significant impact in adolescent girls (58). It is possible that the influence of celiac disease on ovarian reserve may vary depending on factors including the duration and severity of the disease, the adequacy of dietary management, and individual genetic predispositions. In our study, we observed that celiac disease increased the risk of POI (OR=1.124, 95% CI: 1.033 to 1.224, P=0.007), and this result was similar upon validation in a larger dataset (OR=1.096, 95% CI: 1.017 to 1.182, P=0.015). In light of these considerations, it is prudent for clinicians to be vigilant in assessing risk of POI in women with celiac disease of reproductive age, particularly those with a history of poor disease control or nutritional deficiencies. Early detection and appropriate management of celiac disease, including the implementation of a gluten-free diet and supplementation to correct nutritional deficiencies, may be crucial in preserving ovarian reserve and optimizing fertility outcomes.
Selective IgA Deficiency (SIgAD) is a primary immunodeficiency disorder characterized by the near absence or significantly reduced levels of immunoglobulin A in the blood. It is the most common form of primary immunodeficiency. While the majority of individuals with SIgAD exhibit an asymptomatic course, a subset of patients may experience a spectrum of clinical sequelae, including respiratory infections, allergic reactions, gastrointestinal tract pathologies, neoplastic conditions, and autoimmune disorders. Magen et al. reported that three patients were diagnosed with SIgAD and presented with amenorrhea and desired fertility and suggested that POI may be one of the concomitant autoimmune diseases in SIgAD patients (59). Our MR study indicated a negative association between SIgAD and POI. These studies highlighted the potential link between SIgAD and POI, emphasizing the importance of considering autoimmune etiologies in the differential diagnosis of POI, especially in patients with a history of primary immunodeficiencies.
Addison’s disease is characterized by the insufficient production of glucocorticoids by the adrenal cortex, leading to a range of symptoms including fatigue, hypotension, and hyperpigmentation. The coexistence of AD and POI is more frequent than would be expected by chance, suggesting a shared pathophysiological mechanism. Both conditions are part of the spectrum of autoimmune endocrinopathies and are associated with the presence of specific autoantibodies. For instance, antibodies against 21-hydroxylase, an enzyme crucial for steroid synthesis in the adrenal and ovarian tissues, are commonly found in patients with AD and can also be present in those with POI (60). Recent research has further highlighted the association between AD and POI. Vogt et al. found that women with AD have a higher prevalence of POI compared to the general population (61). This study underscores the importance of screening women with AD for POI and vice versa, given the potential impact on fertility and long-term health. Another study offered insights into the disease progression of autoimmune POI in individuals with AD, delineating the transition from normal ovarian function to manifest ovarian dysfunction (62). This research emphasizes the dynamic nature of autoimmune endocrinopathies and the need for longitudinal follow-up to monitor changes in ovarian function. In our investigation, we observed that the suggestive evidence of adverse effect of AD on the susceptibility to POI were generally in line with existing observational research; however, this association did not remain significant following the implementation of more rigorous selection criteria for instrumental variables. In conclusion, the relationship between AD and POI is complex and multifaceted, with autoimmune mechanisms playing a pivotal role in the pathogenesis of both conditions. The increased prevalence of POI in women with AD highlights the importance of a comprehensive endocrine evaluation and appropriate management strategies to preserve reproductive health and overall well-being.
Among autoimmune-related etiologies, thyroid dysfunctions, specifically Hashimoto’s thyroiditis, hypothyroidism, and Graves’ disease, exhibit the correlation with POI (16, 63), but the findings have differed considerably across studies, and conclusive evidence has remained elusive (64, 65). Recently, Hsieh et al. analyzed 21,325 subjects with autoimmune thyroid diseases (Hashimoto’s and Grave’s disease) and found that they are associated with higher risk of POI (66). However, Polyzos et al. conducted a study recruiting 5000 women and reported that neither thyroid autoimmunity (TAI) nor hypothyroidism was associated with poor ovarian reserve (67). In our MR study, we observed that hypothyroidism, Hashimoto’s thyroiditis, and Graves’ disease increased the risk of POI but the results were not statistically significant.
To the best of our knowledge, this investigation marks the first application of MR to elucidate the causal impact of autoimmune diseases on the POI, utilizing the most contemporary and extensive GWAS data available, ensuring a broad representation of the European population. The MR approach was employed to circumvent potential confounding biases, thereby facilitating the derivation of robust causal inferences. The credibility of our findings is further bolstered by a series of sensitivity analyses. These analyses meticulously excluded SNPs that are correlated with cigarette smoking from the set of instrumental variables (68). This exclusionary approach ensures a more stringent assessment of the MR results, mitigating the potential influence of these SNPs on the observed associations. The MR analyses yielded consistent findings across various methodological approaches, including the application of simple median, weighted median, and MR-Egger regression models. The robustness of the associations was further substantiated by leave-one-out sensitivity analyses, which demonstrated stable relationships upon sequential exclusion of individual SNPs, although the specific data for these analyses are not presented. Despite the MR-Egger regression not revealing evidence of either beneficial or detrimental pleiotropy, the possibility of balanced pleiotropy remains an unresolved concern.
Nevertheless, the study acknowledges several limitations that warrant emphasis. Initially, the reliance on genetic data predominantly from individuals of European descent in GWAS may restrict the applicability of the research outcomes across heterogeneous populations. Furthermore, despite rigorous attempts to address pleiotropy, complete eradication of all forms of pleiotropic effects in MR studies remains unattainable. Consequently, unexplored pathways and confounding variables may persist, potentially introducing bias into the outcomes. Lastly, a notable constraint of this study is the inability to stratify the analysis according to the severity of autoimmune disorders and other critical factors, such as age, which could offer a more nuanced comprehension of the examined associations. Fourthly, sex-specific aggregated data from GWAS pertaining to autoimmune diseases were inaccessible. However, a population-based cohort study of 22 million individuals in the UK reported that most autoimmune disorders were more common in women than in men (69). Thyroid disorders, Sjögren’s syndrome, systemic lupus erythematosus, and systemic sclerosis had the highest incidence rate ratios in women compared with men (69). To rule out reversed causation, these analyses should be rerun in women and men separately.
In conclusion, the comprehensive MR analysis conducted in this study indicated that SLE, CeD, vitiligo, and AD caused an increased risk of POI, SIgAD was associated with a decreased risk of POI. These insights carry profound clinical implications, particularly emphasizing the early intervention for women with AIDs/POI who wish to preserve their reproductive potential or plan for future pregnancies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Software, Writing – original draft, Writing – review & editing. YH: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Methodology. YS: Data curation, Software, Writing – original draft, Writing – review & editing, Methodology. TZ: Data curation, Methodology, Writing – original draft, Writing – review & editing, Formal analysis. SL: Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Software. HD: Writing – original draft, Writing – review & editing, Funding acquisition, Resources, Supervision, Validation. YG: Writing – original draft, Writing – review & editing, Supervision, Validation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by Medical and Health Science and Technology Program of Zhejiang Province (2022KY303, 2023KY1066).
Acknowledgments
All datasets utilized in the present investigation were sourced from publicly accessible databases and collaborative consortia. The authors extend their gratitude to these entities for facilitating access to the data, which was instrumental in the execution of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1417896/full#supplementary-material
References
1. Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, et al. Eshre Guideline: management of women with premature ovarian insufficiency. Hum Reprod. (2016) 31:926–37. doi: 10.1093/humrep/dew027
2. Golezar S, Ramezani Tehrani F, Khazaei S, Ebadi A, Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric. (2019) 22:403–11. doi: 10.1080/13697137.2019.1574738
3. Lagergren K, Hammar M, Nedstrand E, Bladh M, Sydsjö G. The prevalence of primary ovarian insufficiency in Sweden; a national register study. BMC Womens Health. (2018) 18:175. doi: 10.1186/s12905-018-0665-2
4. Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod. (2003) 18:199–206. doi: 10.1093/humrep/deg005
5. Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. (1986) 67:604–6.
6. Anagnostis P, Siolos P, Gkekas NK, Kosmidou N, Artzouchaltzi AM, Christou K, et al. Association between age at menopause and fracture risk: a systematic review and meta-analysis. Endocrine. (2019) 63:213–24. doi: 10.1007/s12020-018-1746-6
7. Li XT, Li PY, Liu Y, Yang HS, He LY, Fang YG, et al. Health-related quality-of-life among patients with premature ovarian insufficiency: a systematic review and meta-analysis. Qual Life Res. (2020) 29:19–36. doi: 10.1007/s11136-019-02326-2
8. Mishra SR, Chung HF, Waller M, Dobson AJ, Greenwood DC, Cade JE, et al. Association between reproductive life span and incident nonfatal cardiovascular disease: a pooled analysis of individual patient data from 12 studies. JAMA Cardiol. (2020) 5:1410–8. doi: 10.1001/jamacardio.2020.4105
9. Zhu D, Chung HF, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. (2019) 4:e553–e64. doi: 10.1016/S2468-2667(19)30155-0
10. Jacobsen BK, Heuch I, Kvåle G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 norwegian women. Am J Epidemiol. (2003) 157:923–9. doi: 10.1093/aje/kwg066
11. Tao XY, Zuo AZ, Wang JQ, Tao FB. Effect of primary ovarian insufficiency and early natural menopause on mortality: a meta-analysis. Climacteric. (2016) 19:27–36. doi: 10.3109/13697137.2015.1094784
12. Jiao X, Zhang H, Ke H, Zhang J, Cheng L, Liu Y, et al. Premature ovarian insufficiency: phenotypic characterization within different etiologies. J Clin Endocrinol Metab. (2017) 102:2281–90. doi: 10.1210/jc.2016-3960
13. Silva CA, Yamakami LY, Aikawa NE, Araujo DB, Carvalho JF, Bonfá E. Autoimmune primary ovarian insufficiency. Autoimmun Rev. (2014) 13:427–30. doi: 10.1016/j.autrev.2014.01.003
14. Bakalov VK, Anasti JN, Calis KA, Vanderhoof VH, Premkumar A, Chen S, et al. Autoimmune oophoritis as a mechanism of follicular dysfunction in women with 46,xx spontaneous premature ovarian failure. Fertil Steril. (2005) 84:958–65. doi: 10.1016/j.fertnstert.2005.04.060
15. Kirshenbaum M, Orvieto R. Premature ovarian insufficiency (POI) and autoimmunity-an update appraisal. J Assist Reprod Genet. (2019) 36:2207–15. doi: 10.1007/s10815-019-01572-0
16. Sharif K, Watad A, Bridgewood C, Kanduc D, Amital H, Shoenfeld Y. Insights into the autoimmune aspect of premature ovarian insufficiency. Best Pract Res Clin Endocrinol Metab. (2019) 33:101323. doi: 10.1016/j.beem.2019.101323
17. Reato G, Morlin L, Chen S, Furmaniak J, Smith BR, Masiero S, et al. Premature ovarian failure in patients with autoimmune addison’s disease: clinical, genetic, and immunological evaluation. J Clin Endocrinol Metab. (2011) 96:E1255–61. doi: 10.1210/jc.2011-0414
18. Akawatcharangura P, Taechakraichana N, Osiri M. Prevalence of Premature ovarian failure in systemic lupus erythematosus patients treated with immunosuppressive agents in Thailand. Lupus. (2016) 25:436–44. doi: 10.1177/0961203315617539
19. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: The STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
20. Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, et al. Recent developments in mendelian randomization studies. Curr Epidemiol Rep. (2017) 4:330–45. doi: 10.1007/s40471-017-0128-6
21. Davey Smith G, Hemani G. Mendelian Randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
22. Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The nhgri-ebi gwas catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. (2019) 47:D1005–D12. doi: 10.1093/nar/gky1120
23. Chiou J, Geusz RJ, Okino ML, Han JY, Miller M, Melton R, et al. Interpreting type 1 diabetes risk with genetics and single-cell epigenomics. Nature. (2021) 594:398–402. doi: 10.1038/s41586-021-03552-w
24. Trynka G, Hunt KA, Bockett NA, Romanos J, Mistry V, Szperl A, et al. Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nat Genet. (2011) 43:1193–201. doi: 10.1038/ng.998
25. de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. (2017) 49:256–61. doi: 10.1038/ng.3760
26. Cordell HJ, Fryett JJ, Ueno K, Darlay R, Aiba Y, Hitomi Y, et al. An international genome-wide meta-analysis of primary biliary cholangitis: novel risk loci and candidate drugs. J Hepatol. (2021) 75:572–81. doi: 10.1016/j.jhep.2021.04.055
27. Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. (2021) 53:1415–24. doi: 10.1038/s41588-021-00931-x
28. Mathieu S, Briend M, Abner E, Couture C, Li Z, Bossé Y, et al. Genetic association and mendelian randomization for hypothyroidism highlight immune molecular mechanisms. iScience. (2022) 25:104992. doi: 10.1016/j.isci.2022.104992
29. Eriksson D, Røyrvik EC, Aranda-Guillén M, Berger AH, Landegren N, Artaza H, et al. GWAS for autoimmune addison’s disease identifies multiple risk loci and highlights aire in disease susceptibility. Nat Commun. (2021) 12:959. doi: 10.1038/s41467-021-21015-8
30. Zorina-Lichtenwalter K, Bango CI, Van Oudenhove L, Čeko M, Lindquist MA, Grotzinger AD, et al. Genetic risk shared across 24 chronic pain conditions: identification and characterization with genomic structural equation modeling. Pain. (2023) 164:2239–52. doi: 10.1097/j.pain.0000000000002922
31. Chia R, Saez-Atienzar S, Murphy N, Chiò A, Blauwendraat C, Roda RH, et al. Identification of genetic risk loci and prioritization of genes and pathways for myasthenia gravis: a genome-wide association study. Proc Natl Acad Sci U.S.A. (2022) 119:e2108672119. doi: 10.1073/pnas.2108672119
32. International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. (2019) 365. doi: 10.1126/science.aav7188
33. Khatri B, Tessneer KL, Rasmussen A, Aghakhanian F, Reksten TR, Adler A, et al. Genome-Wide Association study identifies sjögren’s risk loci with functional implications in immune and glandular cells. Nat Commun. (2022) 13:4287. doi: 10.1038/s41467-022-30773-y
34. Taylor KE, Wong Q, Levine DM, McHugh C, Laurie C, Doheny K, et al. Genome-wide association analysis reveals genetic heterogeneity of sjögren’s syndrome according to ancestry. Arthritis Rheumatol. (2017) 69:1294–305. doi: 10.1002/art.40040
35. Chalitsios CV, Tsilidis KK, Tzoulaki I. Psoriasis and Covid-19: A bidirectional mendelian randomization study. J Am Acad Dermatol. (2023) 88:893–5. doi: 10.1016/j.jaad.2022.10.019
36. Jin Y, Roberts GHL, Ferrara TM, Ben S, van Geel N, Wolkerstorfer A, et al. Early-onset autoimmune vitiligo associated with an enhancer variant haplotype that upregulates class ii hla expression. Nat Commun. (2019) 10:391. doi: 10.1038/s41467-019-08337-4
37. López-Isac E, Acosta-Herrera M, Kerick M, Assassi S, Satpathy AT, Granja J, et al. GWAS for systemic sclerosis identifies multiple risk loci and highlights fibrotic and vasculopathy pathways. Nat Commun. (2019) 10:4955. doi: 10.1038/s41467-019-12760-y
38. Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. (2015) 47:1457–64. doi: 10.1038/ng.3434
39. Bronson PG, Chang D, Bhangale T, Seldin MF, Ortmann W, Ferreira RC, et al. Common variants at pvt1, atg13-ambra1, ahi1 and clec16a are associated with selective IgA deficiency. Nat Genet. (2016) 48:1425–9. doi: 10.1038/ng.3675
40. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. Finngen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
41. Birney E. Mendelian randomization. Cold Spring Harb Perspect Med. (2022) 12. doi: 10.1101/cshperspect.a041302
42. Burgess S, Thompson SG. Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036
43. Sanderson E. Multivariable Mendelian randomization and mediation. Cold Spring Harb Perspect Med. (2021) 11. doi: 10.1101/cshperspect.a038984
44. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
45. Vabre P, Gatimel N, Moreau J, Gayrard V, Picard-Hagen N, Parinaud J, et al. Environmental pollutants, a possible etiology for premature ovarian insufficiency: a narrative review of animal and human data. Environ Health. (2017) 16:37. doi: 10.1186/s12940-017-0242-4
46. Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. (2019) 51:237–44. doi: 10.1038/s41588-018-0307-5
47. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
48. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
49. Bowden J, Spiller W, Del Greco MF, Sheehan N, Thompson J, Minelli C, et al. Improving the visualization, interpretation and analysis of two-sample summary data mendelian randomization via the radial plot and radial regression. Int J Epidemiol. (2018) 47:2100. doi: 10.1093/ije/dyy265
50. Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing mendelian randomization investigations: update for summer 2023. Wellcome Open Res. (2019) 4:186. doi: 10.12688/wellcomeopenres
51. Jiang L, Zheng Z, Fang H, Yang J. A generalized linear mixed model association tool for biobank-scale data. Nat Genet. (2021) 53:1616–21. doi: 10.1038/s41588-021-00954-4
52. Jin Y, Andersen G, Yorgov D, Ferrara TM, Ben S, Brownson KM, et al. Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nat Genet. (2016) 48:1418–24. doi: 10.1038/ng.3680
53. Mayorga J, Alpízar-Rodríguez D, Prieto-Padilla J, Romero-Díaz J, Cravioto MC. Prevalence of premature ovarian failure in patients with systemic lupus erythematosus. Lupus. (2016) 25:675–83. doi: 10.1177/0961203315622824
54. Ceccarelli F, Orefice V, Perrone G, Pirone C, Perricone C, Truglia S, et al. Premature ovarian failure in patients affected by systemic lupus erythematosus: a cross-sectional study. Clin Exp Rheumatol. (2020) 38:450–4.
55. Ünal Ö, Tüten N. Evaluation of ovarian reserves in women suffering from an autoimmune disease vitiligo. Eur Rev Med Pharmacol Sci. (2023) 27:4608–13. doi: 10.26355/eurrev_202305_32468
56. Iversen R, Sollid LM. The immunobiology and pathogenesis of celiac disease. Annu Rev Pathol. (2023) 18:47–70. doi: 10.1146/annurev-pathmechdis-031521-032634
57. Cakmak E, Karakus S, Demirpence O, Demet Coskun B. Ovarian reserve assessment in celiac patients of reproductive age. Med Sci Monit. (2018) 24:1152–7. doi: 10.12659/MSM.909033
58. Comba C, Comba A, Yılmaz H, Erdogan SV, Demir O. Celiac disease does not influence markers of ovarian reserve in adolescent girls. Arch Gynecol Obstet. (2020) 302:1263–9. doi: 10.1007/s00404-020-05666-4
59. Magen E, Masalha A, Zueva E, Vardy DA. Possible autoimmune primary ovarian insufficiency in patients with selective IgA deficiency. Isr Med Assoc J. (2018) 20:192–3.
60. Bensing S, Giordano R, Falorni A. Fertility and pregnancy in women with primary adrenal insufficiency. Endocrine. (2020) 70:211–7. doi: 10.1007/s12020-020-02343-z
61. Vogt EC, Breivik L, Røyrvik EC, Grytaas M, Husebye ES, Øksnes M. Primary ovarian insufficiency in women with addison’s disease. J Clin Endocrinol Metab. (2021) 106:e2656–e63. doi: 10.1210/clinem/dgab140
62. De Bellis A, Bellastella G, Falorni A, Aitella E, Barrasso M, Maiorino MI, et al. Natural history of autoimmune primary ovarian insufficiency in patients with addison’s disease: from normal ovarian function to overt ovarian dysfunction. Eur J Endocrinol. (2017) 177:329–37. doi: 10.1530/eje-17-0152
63. Hasegawa Y, Kitahara Y, Osuka S, Tsukui Y, Kobayashi M, Iwase A. Effect of hypothyroidism and thyroid autoimmunity on the ovarian reserve: A systematic review and meta-analysis. Reprod Med Biol. (2022) 21:e12427. doi: 10.1002/rmb2.12427
64. Ke H, Hu J, Zhao L, Ding L, Jiao X, Qin Y. Impact of thyroid autoimmunity on ovarian reserve, pregnancy outcomes, and offspring health in euthyroid women following in vitro fertilization/intracytoplasmic sperm injection. Thyroid. (2020) 30:588–97. doi: 10.1089/thy.2018.0657
65. Osuka S, Iwase A, Goto M, Takikawa S, Nakamura T, Murase T, et al. Thyroid autoantibodies do not impair the ovarian reserve in euthyroid infertile women: a cross-sectional study. Horm Metab Res. (2018) 50:537–42. doi: 10.1055/a-0637-9430
66. Hsieh YT, Ho JYP. Thyroid autoimmunity is associated with higher risk of premature ovarian insufficiency-a nationwide health insurance research database study. Hum Reprod. (2021) 36:1621–9. doi: 10.1093/humrep/deab025
67. Polyzos NP, Sakkas E, Vaiarelli A, Poppe K, Camus M, Tournaye H. Thyroid autoimmunity, hypothyroidism and ovarian reserve: a cross-sectional study of 5000 women based on age-specific amh values. Hum Reprod. (2015) 30:1690–6. doi: 10.1093/humrep/dev089
68. Penzias A, Azziz R, Bendikson K, Falcone T, Hansen K, Hill M, et al. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. (2020) 114:1151–7. doi: 10.1016/j.fertnstert.2020.09.134
69. Conrad N, Misra S, Verbakel JY, Verbeke G, Molenberghs G, Taylor PN, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the Uk. Lancet. (2023) 401:1878–90. doi: 10.1016/s0140-6736(23)00457-9
Keywords: autoimmune disease, primary ovarian insufficiency, Mendelian randomization, single-nucleotide polymorphisms, causal association
Citation: Du Y, Hu Y, Sheng Y, Zhu T, Liu S, Ding H and Guan Y (2024) Primary ovarian insufficiency consequence of autoimmune diseases: a bidirectional two-sample Mendelian randomization study. Front. Endocrinol. 15:1417896. doi: 10.3389/fendo.2024.1417896
Received: 15 April 2024; Accepted: 20 November 2024;
Published: 09 December 2024.
Edited by:
Gedis Grudzinskas, Consultant, London, United KingdomReviewed by:
Bunpei Ishizuka, Rose Ladies Clinic, JapanNuman Cim, Haseki Training and Research Hospital, Türkiye
Copyright © 2024 Du, Hu, Sheng, Zhu, Liu, Ding and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yutao Guan, Z3Vhbnl1dGFvQG5iZHl5eS5jb20=; Huiqing Ding, ZGluZ2h1aXFpbmdAbmJkeXl5LmNvbQ==
 Yongming Du1
Yongming Du1 Yuehua Sheng
Yuehua Sheng Tianhong Zhu
Tianhong Zhu Yutao Guan
Yutao Guan