
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 19 July 2024
Sec. Cardiovascular Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1417228
This article is part of the Research Topic TRLs (Triglycerides-Rich Lipoproteins): A New Target for Atherosclerosis View all 8 articles
Aims: Cholesterol carried in triglyceride-rich lipoproteins, also called remnant cholesterol, is increasingly acknowledged as an important causal risk factor for atherosclerosis. Elevated remnant cholesterol, marked by elevated plasma triglycerides, is associated causally with an increased risk of atherosclerotic cardiovascular disease. However, the association with all-cause mortality and cause-specific mortality is inconclusive. This study aimed to test the hypothesis that remnant cholesterol levels and plasma triglycerides are associated with increased all-cause mortality and mortality from cardiovascular disease, cancer, and other causes.
Methods and results: Using a contemporary population-based cohort, 7,962 individuals from the National Health and Nutrition Examination Survey (NHANES) aged over 40 years at baseline in 2003–2015 were included. During up to 109.2 (± 1.44) months of follow-up, 1,323 individuals died: 385 individuals died from cardiovascular disease, 290 from cancer, 80 from cerebrovascular disease, and 568 from other causes. Compared with the middle tertile remnant cholesterol level, multivariable-adjusted mortality hazard ratios were 1.20 (95% confidence interval 1.02–1.40) for all-cause mortality. For the highest tertile remnant cholesterol level, multivariable-adjusted mortality hazard ratios were 1.21 (95% confidence interval 1.05,1.40). Our conclusions remained stable in subgroup analyses. Exploratory analysis of the cause of death subcategories showed corresponding hazard ratios of 1.25 (1.13–1.38) for Non-cardiovascular and Non-cerebrovascular Death for lower remnant cholesterol individuals, 1.47 (1.01–2.15) for cancer death for lower remnant cholesterol (RC) individuals, and 1.80 (1.36–2.38) for cancer death for higher RC individuals.
Conclusion: RC levels were associated with U-shaped all-cause mortality. RC was associated with mortality from non-cardiovascular, non-cerebrovascular, and cancer, but not from cardiovascular causes. This novel finding should be confirmed in other cohorts.
Remnant cholesterol (RC), a marker of cholesterol that remains undetected by standard lipid assays, has recently received increasing attention as a potential risk factor for CVDs (1). RC refers to the cholesterol contained in RLP particles, the cholesterol in the VLDL and IDL in the fasting state, and the cholesterol in the CM remnants in the postprandial state (2). The calculated RC (CRC) was defined as total cholesterol (TC) minus LDL-C minus HDL-C (3), which did not require special detection equipment and was more convenient and more accessible to implement in clinical diagnosis and therapy.
Studies have suggested a strong association between elevated RC levels and an increased risk of CVD events (4–6). Previous studies reveal that RC is associated with cardiovascular disease, inflammation, diabetes, frailty, and metabolism syndrome (7–10), but there are few studies on RC and all-cause mortality. In addition, RC is also associated with frailty, metabolic syndrome, and hepatic steatosis in middle-aged and elderly individuals (9–11). A cohort study involving 90,000 people showed that RC levels were associated stepwise with all-cause mortality. The FAVORIT study associated higher RC levels with higher all-cause mortality. A study has shown that the mortality rate of heart failure patients decreases as the level of RC increases. A study has shown that the mortality rate of heart failure patients decreases as the level of RC increases.
Our study aimed to determine whether RC is connected to mortality and causes of death in the middle-aged and elderly individuals by exploring the relationship between RC and all-cause mortality and specific causes of death in the middle-aged and elderly individuals.
The NHANES, applying a stratified, multistage probability sample of non-institutionalized civilians, is a cross-sectional investigation to obtain basic information and healthy conditions. The study design and data collection methods have been previously documented (12). The National Center for Health Statistics (NCHS) implements NHANES. NCHS Ethics Review Board had approved the protocols, and subjects had signed the informed consent (13).
Data were extracted from the 2003–2015 cycles. We included participants with available data on TC, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG). After excluding participants aged < 40 years (14), those with cancer at baseline, those with pregnant at baseline, those with incomplete covariate, and those with missing follow-up data, 7,962 individuals were enrolled for further analysis (Figure 1). All participants provided informed consent, and the program passed ethical review. Multiple imputation was used to replace missing values.
RC was determined by subtracting TC from LDL-C and HDL-C. LDL-C levels were calculated from directly measured TC, TG, and HDL-C via the Friedewald equation, widely used in clinical practice and research settings (1, 3, 15). Enzymatic assays were used to measure TC in the laboratory. A direct immunoassay or heparin-manganese (Mn) precipitation method was used to measure HDL-C levels. The lipoprotein lipase technique was employed to quantify TG. The NHANES Laboratory/Medical Technologies Procedures Manual provided detailed instructions for collecting and processing specimens (12). The quality assurance and quality control (QA/QC) processes in the NHANES comply with the 1988 Clinical Laboratory Improvement Act requirements.
The study’s primary endpoint was all-cause mortality, and the population was followed for a mean of 109.2 (± 1.44) months. The primary endpoint is death from any cause during the follow-up, referred to as all-cause mortality. Professionals performed follow-ups. Secondary endpoints were non-cardiovascular and non-cerebrovascular death and cancer death.
Baseline demographic variables including age, gender, education levels (below high school, high school, above high school), race/ethnicity (Mexican, non-Hispanic White, non-Hispanic Black, other), poverty index ratio (< 1.3, 1.3 to 3.49, or ≥ 3.5) (16), marital status (married, alone), body weight, and height were collected from the household interview. Body mass index (BMI) was calculated as body weight divided by height squared (kg/m2) and classified participants into three weight-status groups: Normal (BMI < 25), overweight (BMI 25 to < 30), or obese (BMI ≥ 30) (17). The prevalence of the comorbidity (hypertension, diabetes) was recorded by a standardized medical condition questionnaire administered by trained interviewers. Information on smoking, alcohol use, medication (including lipid-lowering agents), and history of comorbidities had been obtained from the physical examination and associated questionnaire. Smoking habit was identified as someone who smoked 100 cigarettes in their lifetime. Alcohol user was defined as those who drank at least 12 alcoholic drinks in any one year. Blood pressure (BP) was calculated by averaging three consecutive BP readings after the participants rested calmly for five min. Hypertension was defined as systolic BP ≥140 mmHg and/or diastolic BP ≥ 90 mmHg, self-reported diabetes, or use of antihypertensive drugs (18). Diabetes was defined as fasting blood glucose ≥7.0 mmol/L, hemoglobin A1c (HbA1c) ≥ 6.5%, OGTT ≥ 11.0bmmol/L, or taking hypoglycemic drugs or self-reported diabetes (19). CKD-EPI Equation was used to calculate estimated glomerular filtration Rate (eGFR) (20), and the internationally recommended CKD staging based on eGFR level was used to assess the level of renal function of an individual (21). Individual liver function was assessed using The albumin-bilirubin (ALBI) grade (22). The albumin-bilirubin (ALBI) grade has emerged as an alternative, reproducible and objective measure of liver functional reserve in patients, defining worsening liver impairment across 3 grades (23).
The demographic data can be found in the demographics section of NHANES. Laboratory and questionnaire data can provide information on metabolic indicators and lifestyles. All the above variables are available for free at www.cdc.gov/nchs/nhanes/.
Weighted analysis was used as recommended by the NCHS, considering the oversampling of minorities to provide an accurate estimate of effects for the population because of NHANES’s complex multi-stage cluster survey design. Continuous variables were shown as population-weighted means with standard error (SE), while categorical variables were presented as frequency (percentage) (24).
The difference between the baseline characteristics was calculated using a population-weighted chi-square test (categorical variables) and a population-weighted linear regression model (continuous variables).
Cox regression hazard models were constructed to explore the association between the tertiary quantile RC levels (T1, T2, T3) and all-cause mortality, adjusted by several risk factors, including age, sex, race, smoking, education level, BMI, diabetes, hypertension, marital status, and alcohol consumption status, used to explore the potential non-linear relationship of RC with all-cause mortality among middle-aged and elderly and different subgroups.
Restricted cubic splines were used to explore the potential non-linear relationship of RC with all-cause mortality among US middle-aged and elderly and different subgroups. Multiple imputation was used to replace missing values.
Statistical analyses were performed via R version 4.3.2. P < 0.05 was regarded as statistically significant.
The weighted demographic features of the 7,962 subjects in the study grouped by RC are shown in Table 1, representing 104,991,870 U.S. people, for analysis and found associations between RC and mortality. The mean age was 56.30 ± 0.18 years. Of these subjects, 52.07% were men, and 72.43% were non-Hispanic white. The mean RC was 25.45 (± 0.24) mg/dl. During up to 109.2 (± 1.44) months of follow-up, 1,323 individuals died: 385 individuals died from cardiovascular disease, 290 from cancer, 80 from cerebrovascular disease, and 568 from other causes.
Individuals with a higher RC tended to be older, male, frequent smokers, obese, diabetic, non-Hispanic White, hypertensive, less educated, and with a lower income.
As we can see from Table 2, T2 has no significant association with T1 (0.95 [0.80, 1.13], P = 0.57) but has significantly lower all-cause mortality than T3 (1.27 [1.09, 1.49], P = 0.003) in the crude model. After adjusting for sex, age, and race (model 2), T2 has no significant association with T1 (1.10 [0.94, 1.28], P = 0.22) but has significantly lower all-cause mortality than T3 (1.35 [1.19, 1.54], P < 0.001). Model 3 adjusted for marital status, BMI, education level, smoking, alcohol consumption, diabetes, CKD, ALBI_Grade and hypertension based on model 2. T2 has significantly lower all-cause mortality than T1 (1.19 [1.02, 1.39], P = 0.02) and T3 (1.24 [1.07, 1.43], P = 0.004). Figure 2 shows the different levels of RC groups of KM survival curve. Figure 3 shows the relationship between RC and all-cause mortality.
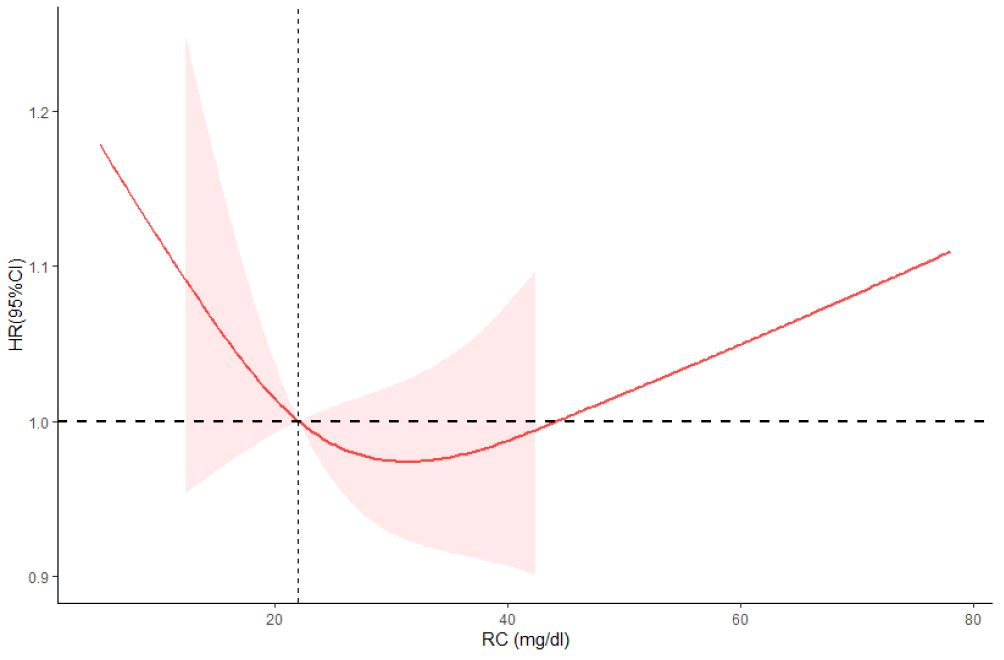
Figure 3 Restricted cubic spline plots between RC and all-cause mortality. Analysis was adjusted for Age + Sex + Race/Ethnic + Smoking + Hypertension + Diabetes + CKD + ALBI_Grade + Alcohol + Poverty + Marital Status + BMI. HR, hazard ratio; CI, confidence interval. Non-linear-P = 0.21.
In subgroup analysis, there was no significant difference in the association between RC levels and all-cause mortality among different subgroups (Figure 4). Based on the causes of death of different subgroups and populations, we plotted the proportion of causes of death for each subgroup (Figure 5).
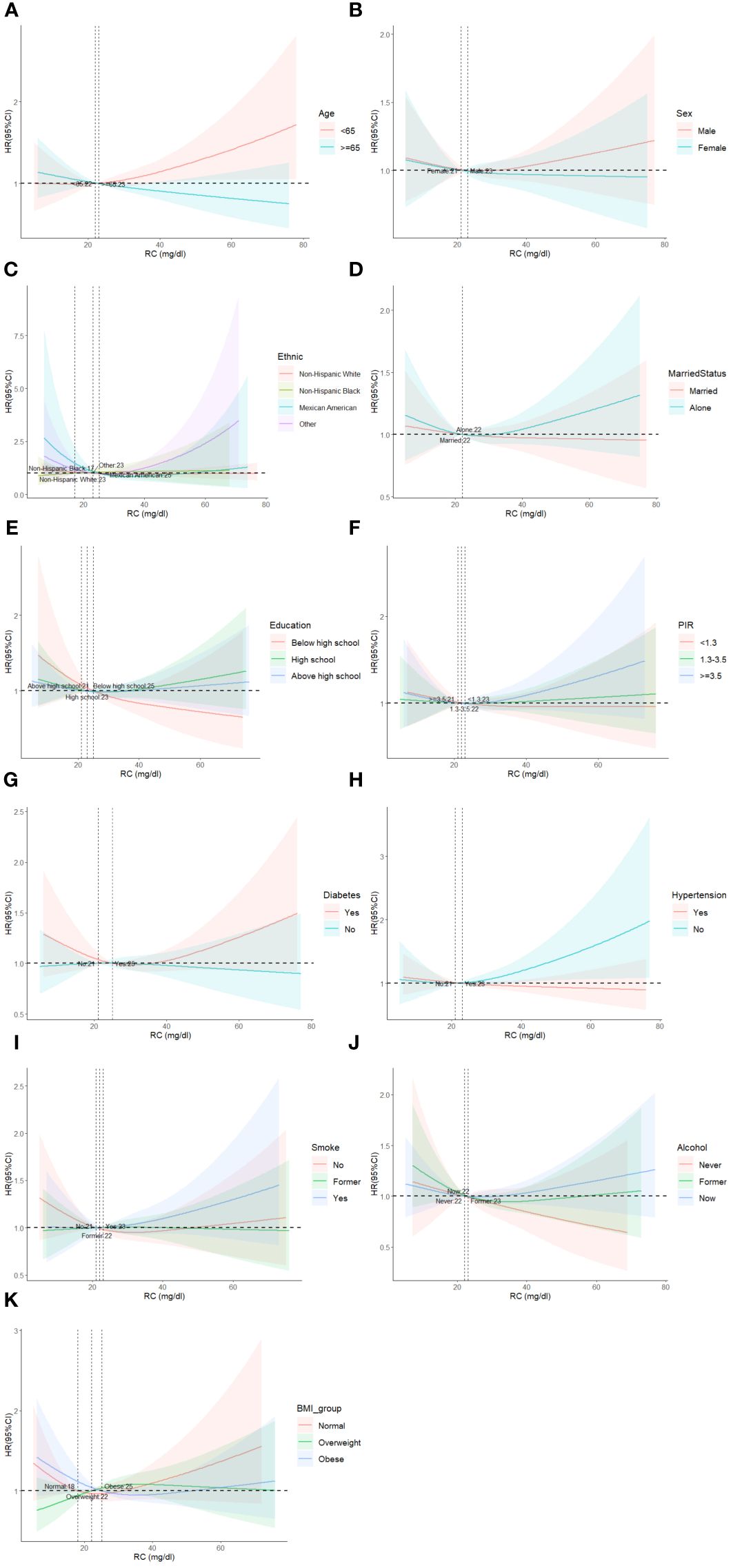
Figure 4 Restricted cubic spline plots of the association between RC and cardiovascular mortality in subgroups. Analyses by Age (A), Sex (B), Race/Ethnic (C), Marital Status (D), Education (E), Poverty ratio index (F), Diabetes (G), Hypertension (H), Smoke (I), Alcohol (J) and BMI (K). Analysis was adjusted for Age + Sex + Race + Smoking + Hypertension + Diabetes + CKD + ALBI_Grade + Alcohol + Poverty + Marital Status + BMI. HR, hazard ratio; CI, confidence interval.
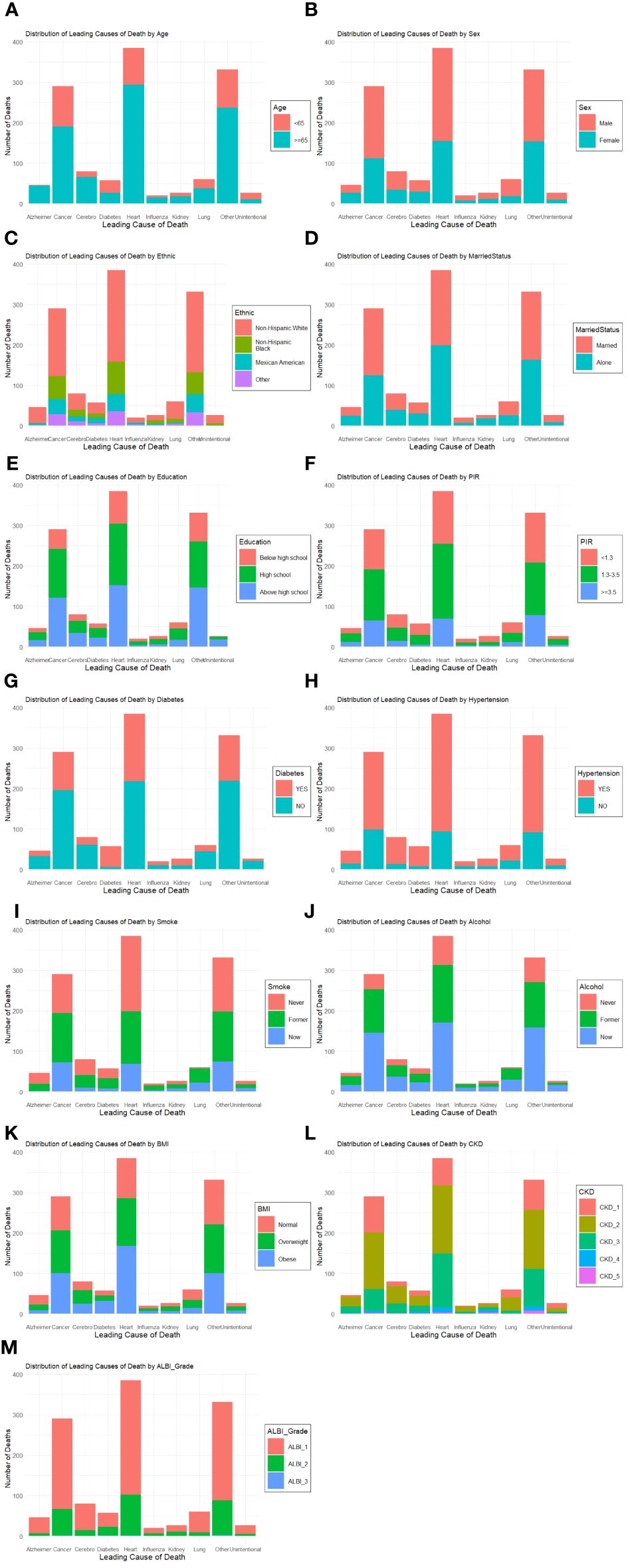
Figure 5 Plots of the proportion of deaths in each subgroup. Analyses by Age (A), Sex (B), Race/Ethnic (C), Marital Status (D), Education (E), Poverty ratio index (F), Diabetes (G), Hypertension (H), Smoke (I), Alcohol (J), BMI (K), CKD (L) and ALBI_Group (M).
RC in all models was not significantly associated with cardiovascular death. In model 1, T2 exhibits no significant association with T1 (0.82 [0.28, 1.18], P = 0.28), while its association with T3 (1.09 [0.82, 1.45], P = 0.55) is not statistically significant. In model 2, T2’s association with T1 (0.95 [0.65, 1.37], P = 0.77) and T3 (1.19 [0.90, 1.57], P = 0.22) indicates no significant association. Finally, in model 3, T2 correlates with T1 (1.10 [0.76, 1.60], P = 0.62), while its association with T3 (1.06 [0.79, 1.42], P = 0.69) remains no statistically insignificant.
Table 3 demonstrates the association between RC levels and non-cardiovascular and non-cerebrovascular death. Models are adjusted in the same as in Table 2. In model 1, T2 exhibits a significant association with T1 (1.06 [0.86, 1.30], P = 0.58), while its association with T3 (1.40 [1.16, 1.68], P < 0.001) is statistically significant. In model 2, T2’s association with T1 (1.22 [1.01, 1.48], P = 0.04) and T3 (1.46 [1.25, 1.70], P < 0.001) indicates a significant association. Finally, in model 3, T2 significantly correlates with T1 (1.29 [1.06, 1.55], P = 0.01), while its association with T3 (1.34 [1.13, 1.58], P < 0.001) remains statistically insignificant.
Table 3 shows a significant link between RC levels and cancer death. Models are adjusted in the same as in Table 2. In model 1, T2 has no significant association with T1 (1.27[0.87, 1.84], P = 0.21) and T3 (1.81 [1.33, 2.47], P < 0.001), indicating a significant association. In model 2, T2 has no significant association with T1 (1.42 [0.98, 2.06], P = 0.06) but has significantly higher cancer mortality than T3 (1.89 [1.43, 2.51], P < 0.001). In model 3, T2 has significantly lower cancer mortality than T1 (1.43 [0.99, 2.08], P = 0.06 and T3 (1.83 [1.38, 2.43], P < 0.001). Figure 5 shows the KM survival curve with significant differences in cancer survival rates and non-cardiovascular and non-cerebrovascular survival between RC levels (P = 0.004). The T2 group had a higher cancer survival probability than T1 and T3 groups (P = 0.001).
Figure 6 shows the Restricted cubic spline plots between non-cardiovascular and non-cerebrovascular survival (A), cancer survival (B), and RC. In Figure 6, the Non-linear-P for non-cardiovascular and non-cerebrovascular death (A) was 0.09, the Non-linear-P for cancer death (B) was 0.67. Figure 7 shows the restricted cubic spline plots between Non-cardiovascular and Non-cerebrovascular Death (A) and Cancer Death (B) and RC.
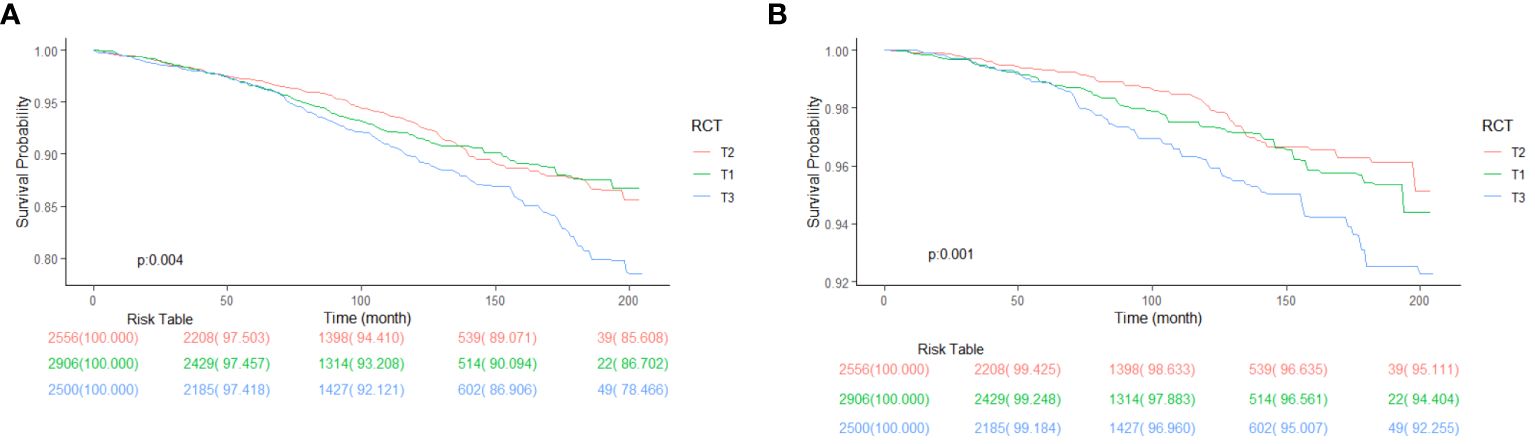
Figure 6 Kaplan-Meier survival curve between Non-cardiovascular and Non-cerebrovascular Survival (A), Cancer Survival (B) and RC, classified by RC levels.
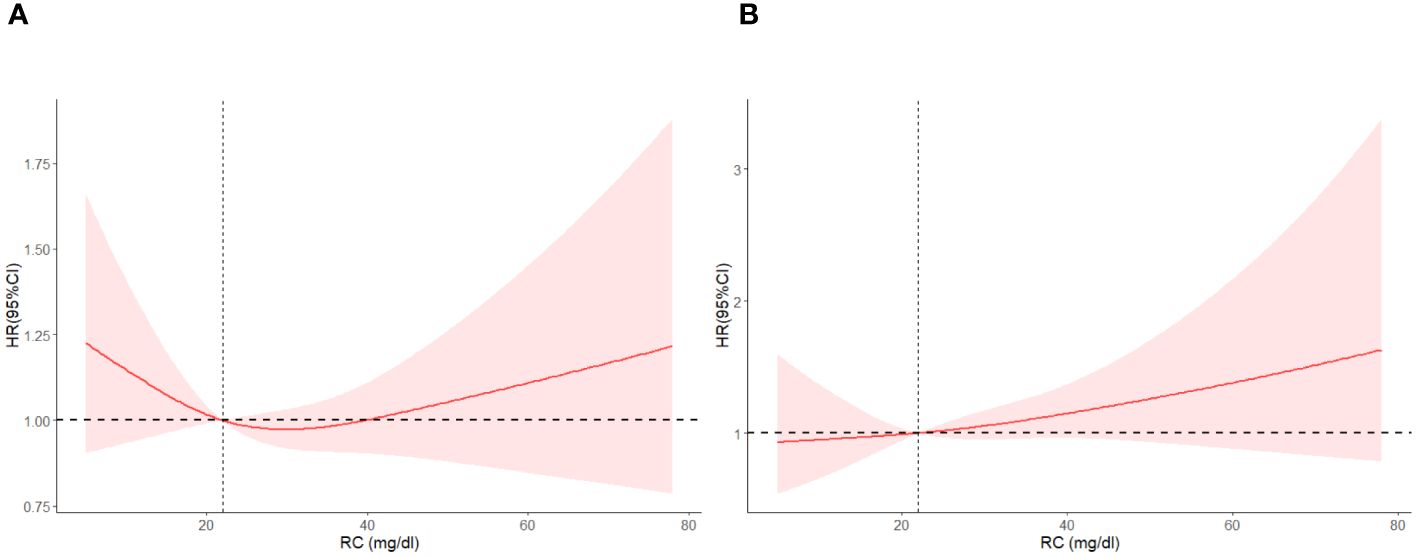
Figure 7 Restricted cubic spline plots between Non-cardiovascular and Non-cerebrovascular Death (A) and Cancer Death (B) and RC.
Our study included 7,962 middle-aged and older adults, representing 104,991,870 U.S. people, and found associations between RC and mortality. Firstly, we discovered that the lower RC group T1 (1.20 [1.02, 1.40], P = 0.03) and the higher RC group T3 (1.21[1.05,1.40], P = 0.01) are both associated with a higher risk of all-cause mortality. Secondly, RC levels are not associated with cardiovascular death. Moreover, the lower RC level T1 is associated with higher non-cardiovascular and non-cerebrovascular death (1.25[1.13,1.38], P<0.001). Furthermore, lower and higher RC levels are associated with higher cancer deaths. The lower group T1 (1.47 [1.01, 2.15], P = 0.04), the higher group T3 (1.80 [1.36, 2.38], P < 0.001).
The study shows that older, male, non-Hispanic white, obese, less educated, frequent smokers, diabetic, hypertensive, low income, and never-drink-alcohol people tend to have higher RC. Similar to our findings, the Castaner study showed that people with obesity and hypertension are more likely to have higher levels of RC (4); Hu et al. found that people with low education and low income had higher RC levels (9). Tian et al. found that people with hypertension and diabetes had a higher level of RC (25). Interestingly, the study showed that people who never drank alcohol people are more likely to have higher RC. That might be because of moderate alcohol consumption. Although, there was no confirmed evidence of protection from moderate drinking for all-cause and cause-specific mortality (26, 27).
We found a U-shaped curve between RC and mortality; all-cause mortality was lowest in the middle tertile (T2) of RC and increased in the lowest (T1) or highest tertile (T3). A cohort study, which included approximately 90,000 Danish general population, showed lower all-cause mortality in people with intermediate RC levels, similar to our results (15). Tian et al. included 3,403,414 community-based participants from ChinaHEART, an ongoing government-funded public health program throughout China. They found that the second percentile levels of RC concentrations were associated with the lowest all-cause mortality (28).
A study included 2823 patients with heart failure shows that low remnant cholesterol levels are associated with increased all-cause mortality in HF patients. And speculated that high cholesterol levels and higher CRP level can improve myocardial energy level and ability to resist infection in patients with heart failure, thus improved the prognosis of patients (15). But there is no clear research and clinical evidence of this association, their relationship still needs further research. A cohort study including 5,414 Danish patients diagnosed with ischemic heart disease found that the risk of all-cause mortality increased with higher RC concentrations (29). Horace et al. also found that increasing RC levels increased the risk of all-cause mortality (30). A Mendelian randomization design genetically determined that increased remnant cholesterol concentrations were associated with increased IHD risk (4, 31). Furthermore, genetically increased RC was also associated with low-grade inflammation (3). This mechanism might explain how RC contributes to increased all-cause mortality. A systematic review including 30,605 patients suggests that elevated concentration RC may independently predict MACEs in patients with CHD (32). Our study showed that the middle but not the lowest RC levels were associated with the lowest all-cause mortality in middle-aged and older adults. There might be extreme concentrations of HDL-C or LDL-C, resulting in higher all-cause mortality (14, 33, 34). RC levels are inversely associated with HDL-C and LDL-C. The findings suggested that people may benefit from the middle RC levels ranging from 20–30 mg/dl.
In our study, cardiovascular mortality was not statistically associated with RC level. On the contrary, a contemporary population-based cohort including 87,192 individuals from the Copenhagen General Population Study aged 20–69 years found that elevated RC is associated with increased risk of mortality from cardiovascular and other causes but not from cancer (35). Differences in results may be due to differences in the included populations and the covariates, which we additionally adjusted for hypertension. Additionally, LDL-C, which is inversely associated with RC, is more strongly associated with cardiovascular outcomes (34). LDL-C levels are the target of lipid-lowering for ASCVD prevention and treatment (36).
These findings suggest that extreme RC levels were associated with higher cancer mortality. Similarly, an analysis of the US population showed higher cancer mortality all-cause mortality at higher RC (37). Observational studies have shown that lower HDL-C levels are associated with higher cancer mortality (34), and lower HDL-C may cause higher cancer mortality at higher RC levels. On the contrary, a Chinese cohort suggested that higher levels of RC were associated with lower cancer mortality (25). Because cholesterol is essential in regulating immune cell function, high serum cholesterol levels enhance the antitumor function of natural killer cells (38). There may be a weaker inhibitory effect on the tumor at low RC levels. A recent analysis of residual cholesterol and cancer death in a limited Nordic population was negative (35). The association and mechanism between RC level and cancer mortality have not been concluded, and further study is needed.
The study indicated that lower RC levels were significantly associated with higher non-cardiovascular and non-cerebrovascular mortality. However, the association with increased mortality from other causes is complex to explain since the relationship between elevated RC, plasma triglycerides, and most non-cardiovascular diseases is unclear (35).
We demonstrated stability between RC and mortality across population subgroups. The results were similar across multiple subgroup analyses, indicating that the association between RC and all-cause mortality was consistent across gender, age, race, health status, and social relationships. Tian et al. (25) and Wadstrom et al. (35). obtained similar results in the subgroup analysis, with no significant differences in age, gender, smoking, diabetes, and BMI levels between the different groups.
The strength of this study is the use of a large sample size, long-term follow-up cohort data, and comprehensive consideration and adjustment of the risk factors involved. Also, sampling weights developed by the NCHS to minimize nonresponse bias were used in our analyses; we used weights, and the data better represent the actual population. Lipid profile levels were from standard laboratory measurements, which makes the results easily translatable to real-world clinical settings. Furthermore, the subgroup analyses were consistent, indicating stable results. Besides, the comprehensive collection of baseline information made extensive adjustment and stratification possible, which clarifies and strengthens the results. Most importantly, this study showed a U-shaped association between RC and all-cause mortality, suggesting we should focus on lowering LDL cholesterol concentrations and keeping RC at moderate levels.
Our study has several limitations. Firstly, as an observational study, it is prone to confounding and cannot conclude causality. Given the variability of RC concentrations, the use of a single measurement of a patient’s lipid profile at study enrollment without repeated sampling will lead to regression dilution bias; thus, the present results should be viewed as minimal estimates, whereas the actual risk increase in all-cause mortality for increased remnant cholesterol likely is higher than that observed in the present study. Thus, future studies are needed to focus on the incidence of all-cause mortality and causes of death based on Mendelian randomization or other methods to address causal questions better.
In conclusion, both lower or higher RC levels were associated with higher mortality. These results suggest that individuals with RC at the extremes may have a higher mortality risk and may require more attention. Maintaining RC may be a target for regulating cholesterol to reduce mortality risk. Our study highlights the predictive value and uniqueness of RC in estimating all-cause mortality. By recognizing the importance of RC, clinicians can improve risk stratification, enhance patient management, and ultimately improve patient outcomes.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by National Center for Health Statistics Institutional Review Board of the United States. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MB: Writing – original draft, Data curation, Formal analysis, Software. JL: Data curation, Formal analysis, Writing – original draft. YW: Writing – original draft, Visualization. ML: Visualization, Writing – original draft. CW: Supervision, Writing – review & editing. JZ: Writing – review & editing, Supervision. MS: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National High Level Hospital Clinical Research Funding (2023-HLHCRF-BQ-45), the Elite Medical Professionals Project of China-Japan Friendship Hospital (ZRJY2024-QMPY18, ZRJY2023-QM24), and the Undergraduate Scientific Research Training Program of Capital Medical University (XSKY2023319).
Thanks to Zhang Jing (Shanghai Tongren Hospital) for his work on the NHANES database. His outstanding work, nhanesR package and webpage, makes it easier for us to explore NHANES database.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hoogeveen RC, Ballantyne CM. Residual cardiovascular risk at low LDL: remnants, lipoprotein(a), and inflammation. Clin Chem. (2021) 67:143–53. doi: 10.1093/clinchem/hvaa252
2. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. (2007) 298:299–308. doi: 10.1001/jama.298.3.299
3. Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. (2013) 128:1298–309. doi: 10.1161/CIRCULATIONAHA.113.003008
4. Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. (2013) 61:427–36. doi: 10.1016/j.jacc.2012.08.1026
5. Joshi PH, Khokhar AA, Massaro JM, Lirette ST, Griswold ME, Martin SS, et al. Lipoprotein investigators collaborative (LIC) study group. Remnant lipoprotein cholesterol and incident coronary heart disease: the jackson heart and framingham offspring cohort studies. J Am Heart Assoc. (2016) 5:e002765. doi: 10.1161/JAHA.115.002765
6. Varbo A, Nordestgaard BG. Remnant cholesterol and risk of ischemic stroke in 112,512 individuals from the general population. Ann Neurol. (2019) 85:550–9. doi: 10.1002/ana.25432
7. Hu X, Liu Q, Guo X, Wang W, Yu B, Liang B, et al. The role of remnant cholesterol beyond low-density lipoprotein cholesterol in diabetes mellitus. Cardiovasc Diabetol. (2022) 21:117. doi: 10.1186/s12933-022-01554-0
8. Henein MY, Vancheri S, Longo G, Vancheri F. The role of inflammation in cardiovascular disease. Int J Mol Sci. (2022) 23:12906. doi: 10.3390/ijms232112906
9. Hu Y, Wang X, Lin L, Huan J, Li Y, Zhang L, et al. Association of remnant cholesterol with frailty: findings from observational and Mendelian randomization analyses. Lipids Health Dis. (2023) 22:115. doi: 10.1186/s12944-023-01882-4
10. Zou Y, Kuang M, Zhong Y, Jiang C. Remnant cholesterol can identify individuals at higher risk of metabolic syndrome in the general population. Sci Rep. (2023) 13:5957. doi: 10.1038/s41598-023-33276-y
11. Huang H, Wang J, Wu L, Ruan J, Hou L, Shen C, et al. Remnant cholesterol and severity of nonalcoholic fatty liver disease. Diabetol Metab Syndr. (2023) 15:238. doi: 10.1186/s13098-023-01220-9
12. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat 1. (2013) (56):1–37.
13. National Center for Health Statistics. 2015 Public-Use Linked Mortality Files (2019). Available online at: https://www.cdc.gov/nchs/data-linkage/mortality-public.htm#.
14. Yang Z, Yang K, Shi J, Yang Q, Zhang Y, Gao J, et al. The association between remnant cholesterol and the estimated 10-year risk of a first hard cardiovascular event. Front Cardiovasc Med. (2022) 9:913977. doi: 10.3389/fcvm.2022.913977
15. Varbo A, Freiberg JJ, Nordestgaard BG. Extreme nonfasting remnant cholesterol vs extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90000 individuals from the general population. Clin Chem. (2015) 61:533–43. doi: 10.1373/clinchem.2014.234146
16. Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. Adults, 1999-2018. N Engl J Med. (2021) 384:2219–28. doi: 10.1056/NEJMsa2032271
17. Obesity and overweight . Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
18. Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. (2023) 41:1874–2071. doi: 10.1097/HJH.0000000000003480
19. American Diabetes Association. 2. Classification and diagnosis of diabetes; Standards of Medical Care in Diabetes—2020. Diabetes Care. (2020) 43(Suppl 1):S14–31. doi: 10.2337/dc20-S002
20. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
21. Andrassy KM. Comments on 'KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease'. Kidney Int. (2013) 84:622–3. doi: 10.1038/ki.2013.243
22. Toyoda H, Johnson PJ. The ALBI score: From liver function in patients with HCC to a general measure of liver function. JHEP Rep. (2022) 4:100557. doi: 10.1016/j.jhepr.2022.100557
23. Demirtas CO, D'Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: Evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. (2021) 3:100347. doi: 10.1016/j.jhepr.2021.100347
24. Feng G, Qin G, Zhang T, Chen Z, Zhao Y. Common statistical methods and reporting of results in medical research. Cardiovasc Innov Appl. (2022) 6(3):117–25. doi: 10.15212/CVIA.2022.0001
25. Tian Y, Wu Y, Qi M, Song L, Chen B, Wang C, et al. Associations of remnant cholesterol with cardiovascular and cancer mortality in a nationwide cohort. Sci Bull. (2024) 69:526–34. doi: 10.1016/j.scib.2023.12.035
26. Millwood IY, Im PK, Bennett D, Hariri P, Yang L, Du H, et al. Alcohol intake and cause-specific mortality: conventional and genetic evidence in a prospective cohort study of 512 000 adults in China. Lancet Public Health. (2023) 8:e956–e67. doi: 10.1016/S2468-2667(23)00217-7
27. Im PK, Wright N, Yang L, Chan KH, Chen Y, Guo Y, et al. Alcohol consumption and risks of more than 200 diseases in Chinese men. Nat Med. (2023) 29:1476–86. doi: 10.1038/s41591-023-02383-8
28. Zhao L, Zhao X, Tian P, Liang L, Huang B, Huang L, et al. Predictive value of remnant cholesterol level for all-cause mortality in heart failure patients. Front Cardiovasc Med. (2023) 10:1063562. doi: 10.3389/fcvm.2023.1063562
29. Jepsen AM, Langsted A, Varbo A, Bang LE, Kamstrup PR, Nordestgaard BG. Increased remnant cholesterol explains part of residual risk of all-cause mortality in 5414 patients with ischemic heart disease. Clin Chem. (2016) 62:593–604. doi: 10.1373/clinchem.2015.253757
30. Horace RW, Roberts M, Shireman TI, Merhi B, Jacques P, Bostom AG, et al. Remnant cholesterol is prospectively associated with cardiovascular disease events and all-cause mortality in kidney transplant recipients: the FAVORIT study. Nephrol Dial Transplant. (2022) 37:382–9. doi: 10.1093/ndt/gfab068
31. Jørgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjærg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. (2013) 34:1826–33. doi: 10.1093/eurheartj/ehs431
32. Tian Y, Wu W, Qin L, Yu X, Cai L, Wang H, et al. Prognostic value of remnant cholesterol in patients with coronary heart disease: A systematic review and meta-analysis of cohort studies. Front Cardiovasc Med. (2022) 9:951523. doi: 10.3389/fcvm.2022.951523
33. Johannesen CDL, Langsted A, Mortensen MB, Nordestgaard BG. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. Bmj. (2020) 371:m4266. doi: 10.1136/bmj.m4266
34. von Eckardstein A, Nordestgaard BG, Remaley AT, Catapano AL. High-density lipoprotein revisited: biological functions and clinical relevance. Eur Heart J. (2023) 44:1394–407. doi: 10.1093/eurheartj/ehac605
35. Wadström BN, Pedersen KM, Wulff AB, Nordestgaard BG. Elevated remnant cholesterol, plasma triglycerides, and cardiovascular and non-cardiovascular mortality. Eur Heart J. (2023) 44:1432–45. doi: 10.1093/eurheartj/ehac822
36. Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, et al. AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: A report of the american heart association/american college of cardiology joint committee on clinical practice guidelines. Circulation. (2023) 148:e9–e119. doi: 10.1161/CIR.0000000000001168
37. Huang H, Guo Y, Liu Z, Zeng Y, Chen Y, Xu C. Remnant cholesterol predicts long-term mortality of patients with metabolic dysfunction-associated fatty liver disease. J Clin Endocrinol Metab. (2022) 107:e3295–e303. doi: 10.1210/clinem/dgac283
Keywords: remnant cholesterol, all-cause mortality, cardiovascular disease, cause-specific mortality, NHANES
Citation: Bai M, Liao J, Wang Y, Liang M, Wang C, Zhang J and Shao M (2024) Remnant cholesterol and all-cause mortality risk: findings from the National Health and Nutrition Examination Survey, 2003-2015. Front. Endocrinol. 15:1417228. doi: 10.3389/fendo.2024.1417228
Received: 14 April 2024; Accepted: 08 July 2024;
Published: 19 July 2024.
Edited by:
Kayoko Hosaka, Karolinska Institutet (KI), SwedenReviewed by:
Erik Josef Behringer, Loma Linda University, United StatesCopyright © 2024 Bai, Liao, Wang, Liang, Wang, Zhang and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingjing Shao, c2hhb21pbmdqaW5nQHpyeWh5eS5jb20uY24=; Jie Zhang, emhhbmdqaWU5NDk2QGJ1Y20uZWR1LmNu; Chuan Wang, dHNfa2V2aW5AaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.