- Department of Cardiology, Minzu Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
Background: Prior research has indicated the importance of insulin resistance in the development of heart failure (HF). The metabolic score for insulin resistance (METS-IR), a novel measure for assessing insulin resistance, has been found to be associated with cardiovascular disease (CVD). Nevertheless, the relationship between METS-IR and heart failure remains uncertain.
Methods: This cross-sectional study collected data from the 2007–2018 National Health and Nutrition Examination Survey (NHANES). Multivariable logistic regression analysis and smoothing curve fitting were performed to explore the relationship between METS-IR and the risk of heart failure. Subgroup analysis and receiver operating characteristic (ROC) curve analysis were also conducted.
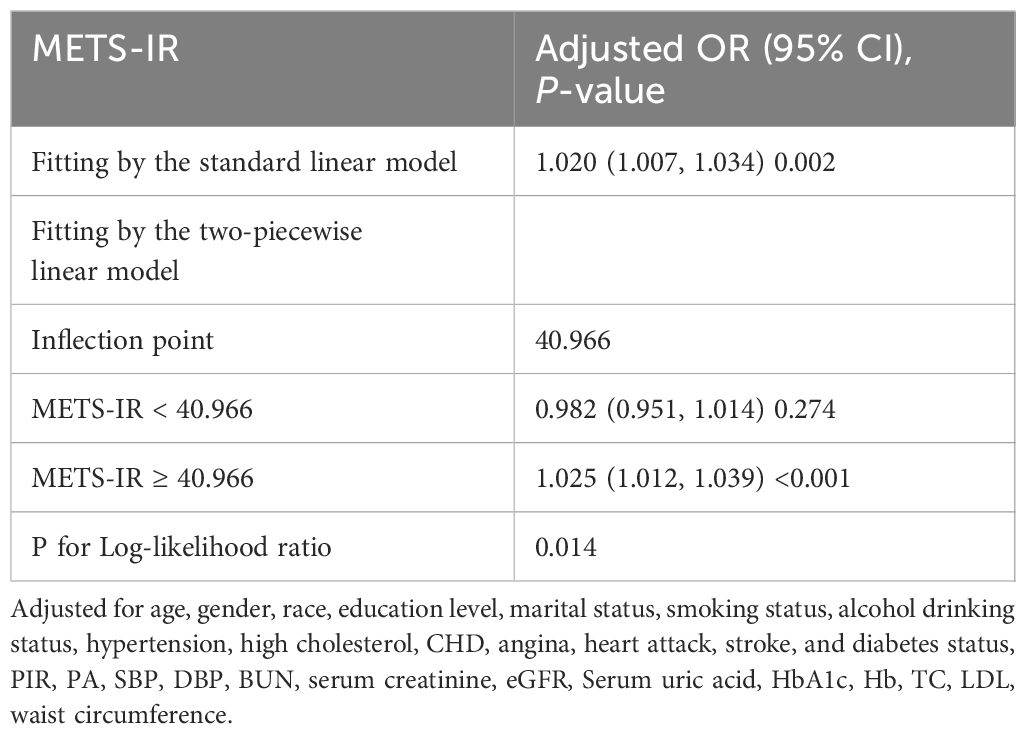
Results: A total of 14772 patients were included, of whom 485 (3.28%) had heart failure. We observed a significant positive association between METS-IR and the risk of heart failure in a fully adjusted model (per 1-unit increment in METS-IR: OR: 2.44; 95% CI: 1.38, 4.32). Subgroup analysis and interaction tests revealed no significant influence on this relationship. A saturation effect and nonlinear relationship between METS-IR and heart failure risk were found using a smoothing curve fitting analysis. The relationship was represented by a J-shaped curve with an inflection point at 40.966.
Conclusions: The results of our study indicated a J-shaped association between METS-IR and HF in adults in the United States. METS-IR may be a promising novel index for predicting the risk of heart failure. More longitudinal studies are needed to further verify causal relationships and validate the results in different classifications of heart failure populations.
1 Introduction
Heart failure affects approximately 65 million adults worldwide, and the incidence and prevalence are expected to continue increasing in the coming decades (1). Heart failure represents a significant global health problem, with high morbidity and mortality rates. It places a significant burden on healthcare systems and patients’ quality of life, leading to both health risks and economic pressure. Consequently, the timely detection and intervention of individuals at high risk of developing heart failure is of critical importance. The prognosis of heart failure is influenced by a number of factors, including age, gender, etiology, left ventricular ejection fraction, and comorbidities. Comorbidities have been demonstrated to have a significant impact on the development and progression of heart failure (2). Diabetes mellitus (DM) is a well-known risk factor that contributes to a poorer prognosis in heart failure and leads to increased hospitalizations and mortality rates (3). Type 2 diabetes mellitus (T2DM) and heart failure often co-occur, with approximately 15–25% of individuals with heart failure also having diabetes. In fact, the prevalence of heart failure in diabetics is four times higher than in the general population. According to reports, approximately 6% of individuals diagnosed with diabetes will develop heart failure at some point in their lives. The results of the Framingham Heart Study indicate that the occurrence of heart failure is two to five times more common in people with diabetes compared to healthy individuals, and that this is associated with poor outcomes (4).
Insulin resistance (IR) is a common feature observed in individuals with metabolic syndrome and T2DM. It is considered a key indicator of diabetes-related heart disease (DHD) (5), which encompasses coronary artery disease, autonomic heart disease, and diabetes cardiomyopathy (DCM) (6). DCM increases mortality in diabetes patients (7). Increasing evidence suggests that IR is the primary etiological factor in the development of nonischemic HF and post-ischemic HF (8–10). Several research studies have demonstrated that insulin resistance (IR) can affect blood circulation and the myocardium, myocardial fibrosis, cardiac hypertrophy, and ventricular remodeling. These effects contribute to the development of DHD and may ultimately lead to cardiac diastolic dysfunction and progression to HF (5).
Although the hyperinsulinemic-euglycemic clamp method (HEC) is considered to be the best for determining IR, it is not practical to use in clinical settings. Consequently, alternative non-insulin-based markers, including the homeostasis model assessment for insulin resistance (HOMA-IR), triglyceride-glucose (TyG) index, TyG-body mass index (TyG-BMI), and triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio, have been devised as replacements for evaluating IR (11, 12). Nevertheless, HOMA-IR also requires additional blood samples and analytical costs. Furthermore, there is conflicting evidence regarding the role of these indicators in the screening, diagnosis and prognosis of CHD, particularly in patients with coexisting various metabolic diseases.
The metabolic score for insulin resistance (METS-IR), which demonstrates a higher concordance with the HEC. A significant association between METS-IR and the development of T2DM (13), hypertension, and ischemic heart disease (14) in some studies. In comparison to other indicators, METS-IR is more effective for identifying individuals at high risk of CVD (15). However, previous studies only focused on the correlation between METS-IR and many CVDs, without examining its involvement in heart failure. In this study, we conducted a cross-sectional analysis using data from NHANES to examine the association between METS-IR and the risk of HF. In addition, we further explored the interactions and stratified confounders in the association between METS-IR and the risk of HF in different subgroups.
2 Materials and methods
2.1 Study population
The study utilized data from the NHANES, which is conducted by the National Center for Health Statistics (NCHS). NHANES is a comprehensive survey that aims to collect representative information on the health and nutrition of the non-institutionalized civilian population in the United States. To ensure a diverse sample, NHANES uses a stratified, multistage probability approach to select participants from across the country. The survey collects data through standardized in-home interviews, physical examinations, and laboratory tests carried out at mobile examination centers. All NHANES studies passed the National Center for Health Statistics (NCHS) Ethics Review Board, and written informed consent was obtained from all participants (https://www.cdc.gov/nchs/nhanes/irba98.htm).
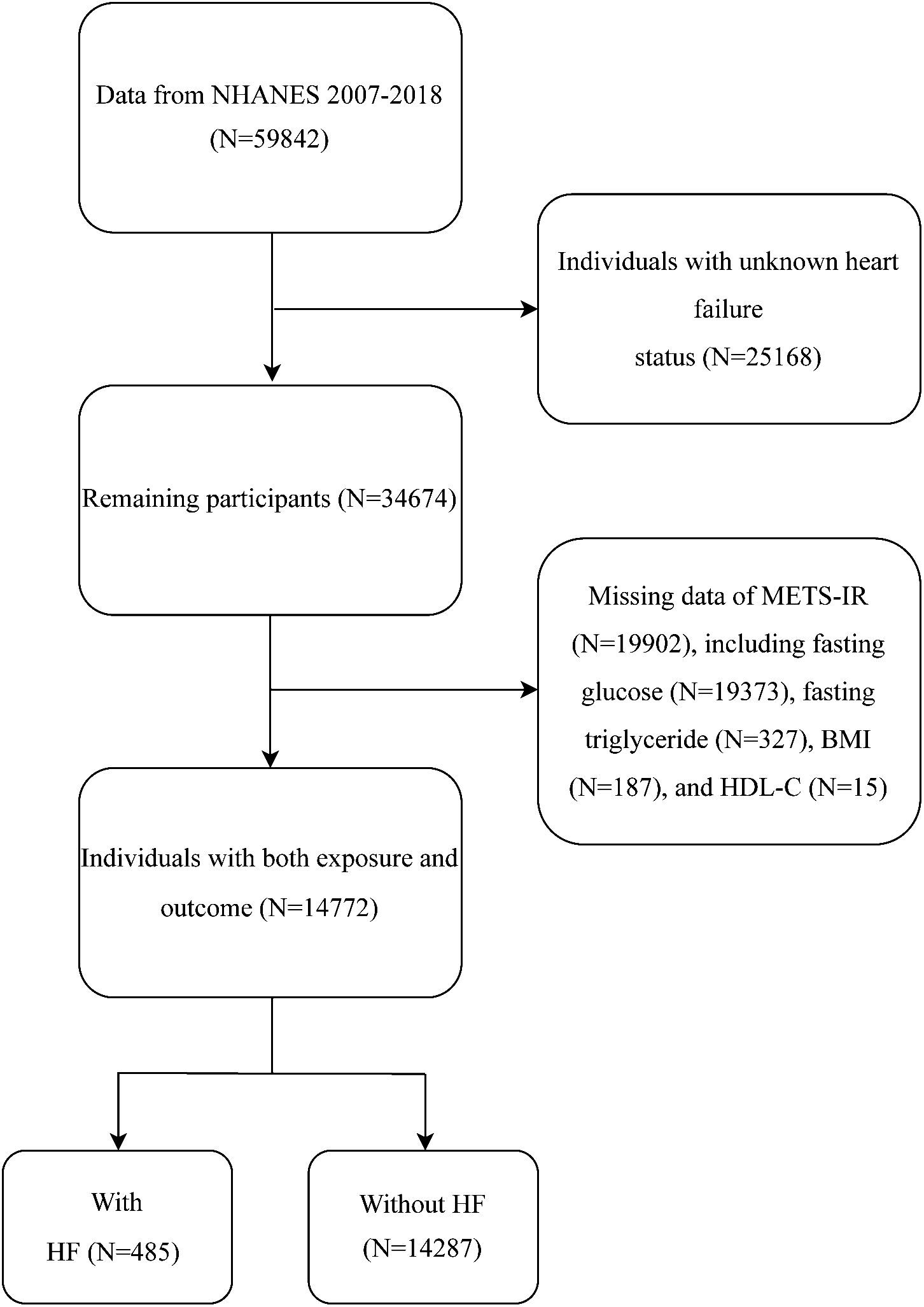
To explore the potential association between METS-IR and the risk of HF, our analysis was conducted using the 2007–2018 NHANES dataset. As shown in Figure 1, the initial sample consisted of 59842 participants. We then excluded participants who had no information on heart failure (n = 25168) and could not calculate METS-IR (n = 19902). A final total of 14772 participants were enrolled in this study.
2.2 Definitions of METS-IR and heart failure
METS-IR was calculated as ln [(2 × fasting plasma glucose (FPG) (mg/dL) + fasting triglycerides (TG) (mg/dL)] × body mass index (BMI) (kg/m2))/(ln[high-density lipoprotein cholesterol (HDL-c) (mg/dL]) (16). Subsequently, all participants were divided into four groups according to METS-IR quartiles: Group Q1 (<34.288), Group Q2 (34.288, 41.483), Group Q3 (41.483, 50.144), and Group Q4 (>50.144). Quality control of laboratory tests is available at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. Heart failure is defined in the study by asking participants a specific question regarding their medical history: “Have you ever been diagnosed with congestive heart failure by a doctor or other healthcare professional?” If participants respond affirmatively, their response is recorded as indicating the presence of congestive heart failure.
2.3 Covariates
Our study also selected possible factors that may affect the association between clinical relevance based METS-IR and heart failure, including age (year), gender (male/female), race (Mexican American/Other Hispanic/Non-Hispanic White/Non-Hispanic Black/Other Race), education level (below high school/high school or equivalent/college or above), marital status (married/unmarried), smoking status, drinking status, physical activity (PA), family poverty income ratio (PIR), waist circumference, body mass index (BMI, kg/m2), systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting glucose, LDL-c (low-density lipoprotein cholesterol), total cholesterol (TC), hemoglobin (Hb), hemoglobin A1c (HbA1c), blood urea nitrogen (BUN), serum creatinine, eGFR (estimated glomerular filtration rate), serum uric acid, hypertension, high cholesterol, diabetes, coronary heart disease (CHD), angina pectoris, heart attack, stroke. Smoking status was grouped into “never” (never smoked or less than a hundred cigarettes in life), “current” (more than a hundred cigarettes in life and is also ongoing currently), or “former” (more than a hundred cigarettes in life but currently not smoking). Drinking status was grouped into “never” (never consumed alcohol more than 12 times in a year), “current” (more than 12 times in a year and is also ongoing currently), or “former” (consumed alcohol more than 12 times in a year but currently not drinking). Physical activity (PA) was determined according to answers to a survey about whether a subject was vigorously or moderately active in recreational activities. Hypertension was defined as a systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg, or a self-reported history of hypertension or oral antihypertensive medications. High cholesterol was defined as a fasting TC ≥ 240 mg/dL or taking lipid-lowering agents. For diabetes, we adopted a comprehensive definition encompassing a fasting blood glucose level ≥126 mg/dL, a HbA1c ≥ 6.5%, use of oral hypoglycemic agents, insulin use, or self-reported history of diabetes. The formula used to eGFR was the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (17). All detailed measurement processes of the study variables are publicly available at www.cdc.gov/nchs/nhanes/.
2.4 Statistical analysis
Statistical analysis of the current study was rigorously conducted according to the design recommended by NHANES, taking into account sample weights, clustering, and stratified analysis. We used the weights of the fasting subsample and adjusted the weights to account for multiple cycles. Since we merged six cycles of NHANES datasets for data from 2007 to 2018, the SAF sample weights (WTSAF2YR/6) were applied for weighted analyses (https://wwwn.cdc.gov/nchs/nhanes/tutorials/default.aspx). Continuous variables were expressed as means ± standard deviations, and categorical variables were expressed as percentages. Based on their METS-IR levels, the study participants were divided into four groups or quartiles. The Chi-squared test or Kruskal-Wallis H test was employed to compare differences in population characteristics by METS-IR quartiles. In order to investigate the relationship between METS-IR and HF, a multivariate logistic regression analysis was used in the analysis to explore the relationship between METS-IR and HF. Depending on the adjustment for covariates, four models were generated. Model 1: no adjustment for covariates; Model 2: age, gender, and race were adjusted; Model 3: adjusted for covariates in Model 2 plus education level, marital status, smoking status, drinking status, hypertension, high cholesterol, CHD, angina, heart attack, stroke, and diabetes status; Model 4: adjusted for covariates in Model 3 plus PIR, PA, SBP, DBP, BUN, serum creatinine, eGFR, serum uric acid, HbA1c, Hb, TC, LDL-C, waist circumference. Subgroup analyses were carried out to examine the potential impacts of gender, age, BMI, eGFR, education level, smoking status, drinking status, CHD, hypertension, and diabetes status on the relationship between METS-IR and HF. In addition, smoothed curve fitting and threshold effect models in this study were used to verify the existence of non-linear relationships between the independent and response variables. A non-linear effects model was used when the likelihood ratio (LLR) was <0.05. The statistical analyzes of this study were performed via R, version 4.2.0 (R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). Statistical significance was determined by two-sided p values less than 0.05.
3 Results
3.1 Baseline characteristics
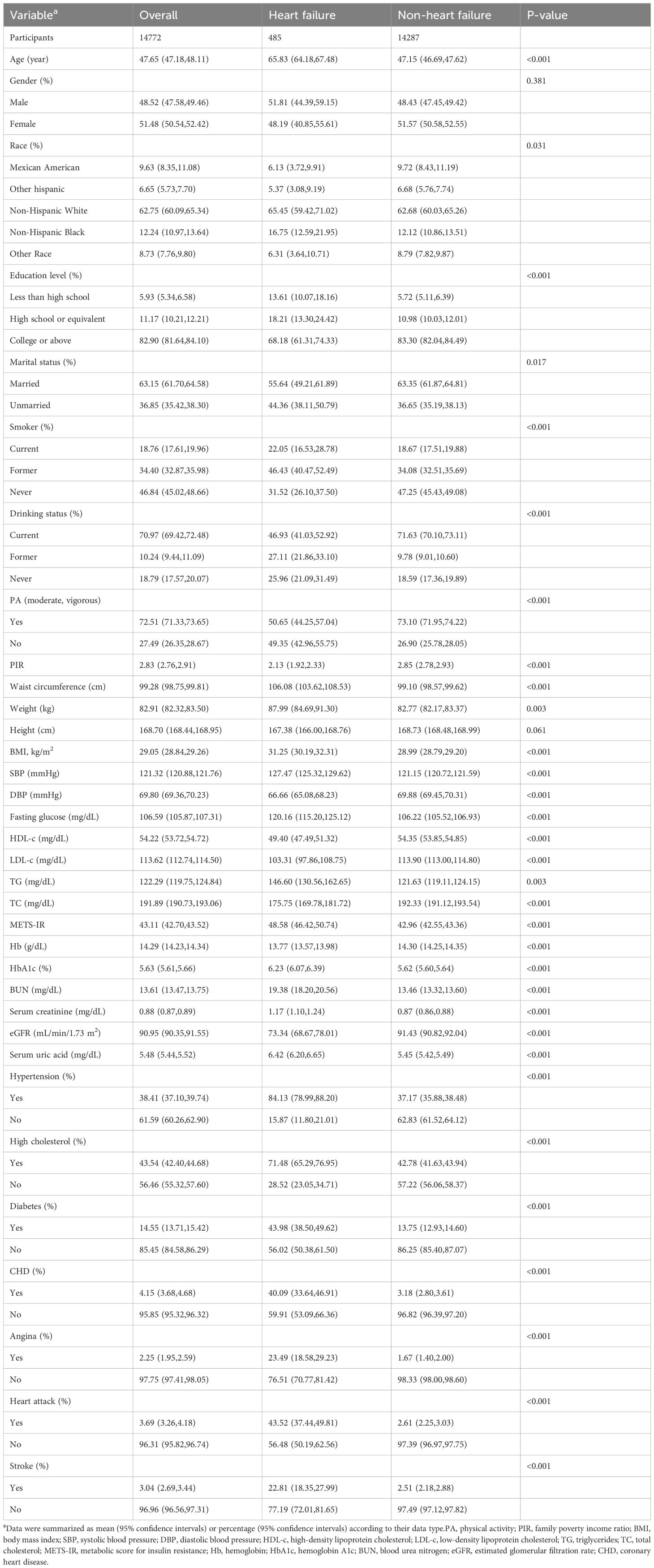
A total of 14772 participants were included in this study (Figure 1). Among them, 485 (3.28%) had heart failure. The mean age (95% CI) was 47.65 (47.18, 48.11) years, and the mean METS-IR was 43.11 (42.70, 43.52). As shown in Table 1, compared to non-HF individuals, the included HF population was more likely to be older, smokers, have hypertension, high cholesterol, diabetes, CHD, angina, heart attack, and stroke, individuals with lower education levels, income levels, and eGFR, and higher waist circumference, body mass index, SBP, fasting glucose, and HbA1c (p < 0.001). Notably, the HF group exhibited a significantly higher METS-IR than the non-HF group (48.58 vs. 42.92, p < 0.001).
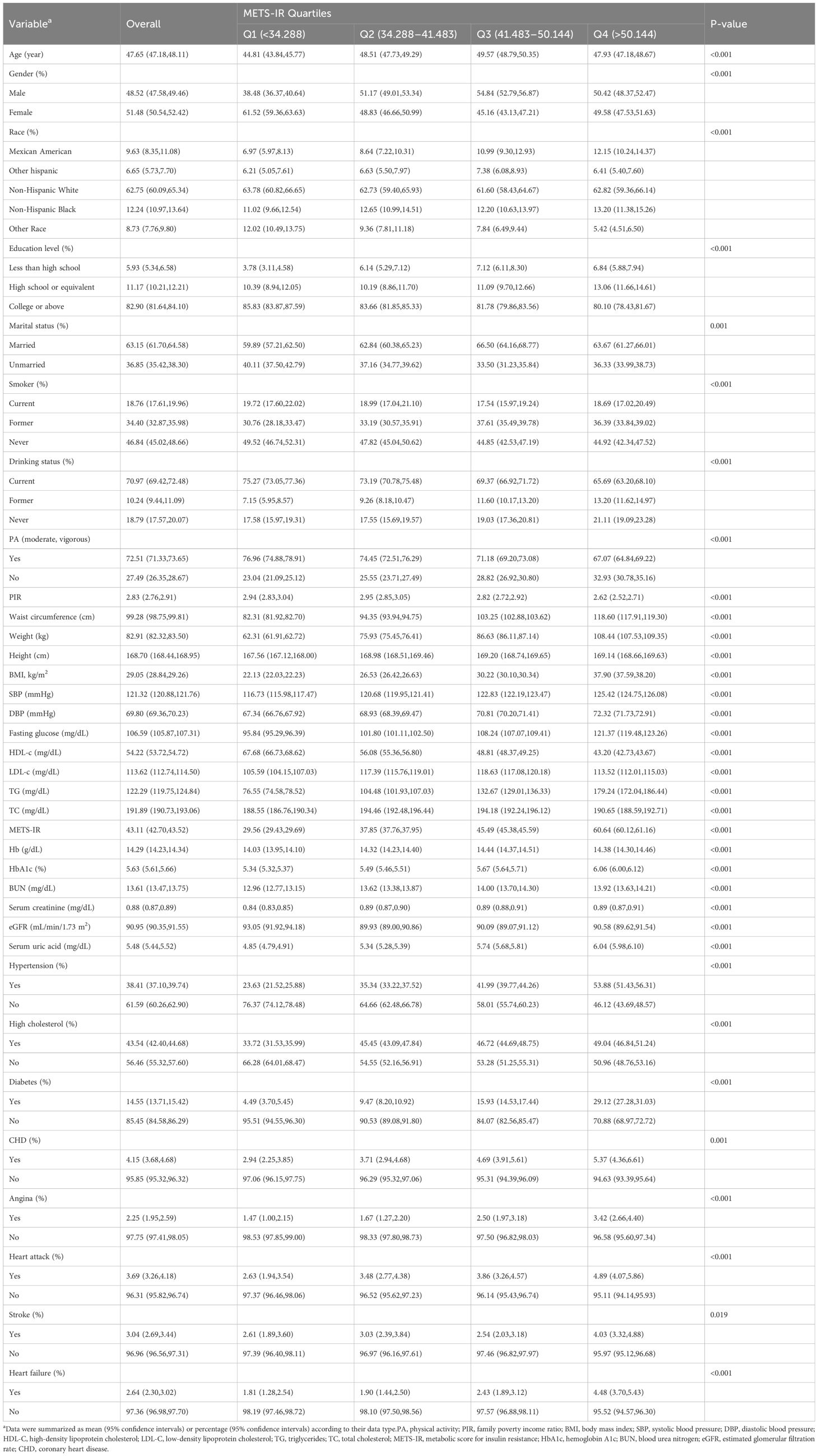
Table 2 displays the participants’ clinical characteristics according to METS-IR quartiles. The values for the different quartiles were as follows: quartile 1 (<34.288), quartile 2 (34.288, 41.483), quartile 3 (41.483, 50.144), and quartile 4 (>50.144). Compared to participants in the lower METS-IR group, participants in the METS-IR Q4 group were more likely to be Mexican Americans, smokers, hypertension, high cholesterol, diabetes, CHD, angina, heart attack, individuals with lower education levels, less physical activity, higher levels of waist circumference, weight, BMI, blood pressure, fasting glucose, TG, HbA1c, serum uric acid, and lower HDL-c (all P < 0.01). Importantly, participants with high levels of METS-IR had a higher prevalence of HF (P < 0.01).
3.2 Association between METS-IR and HF
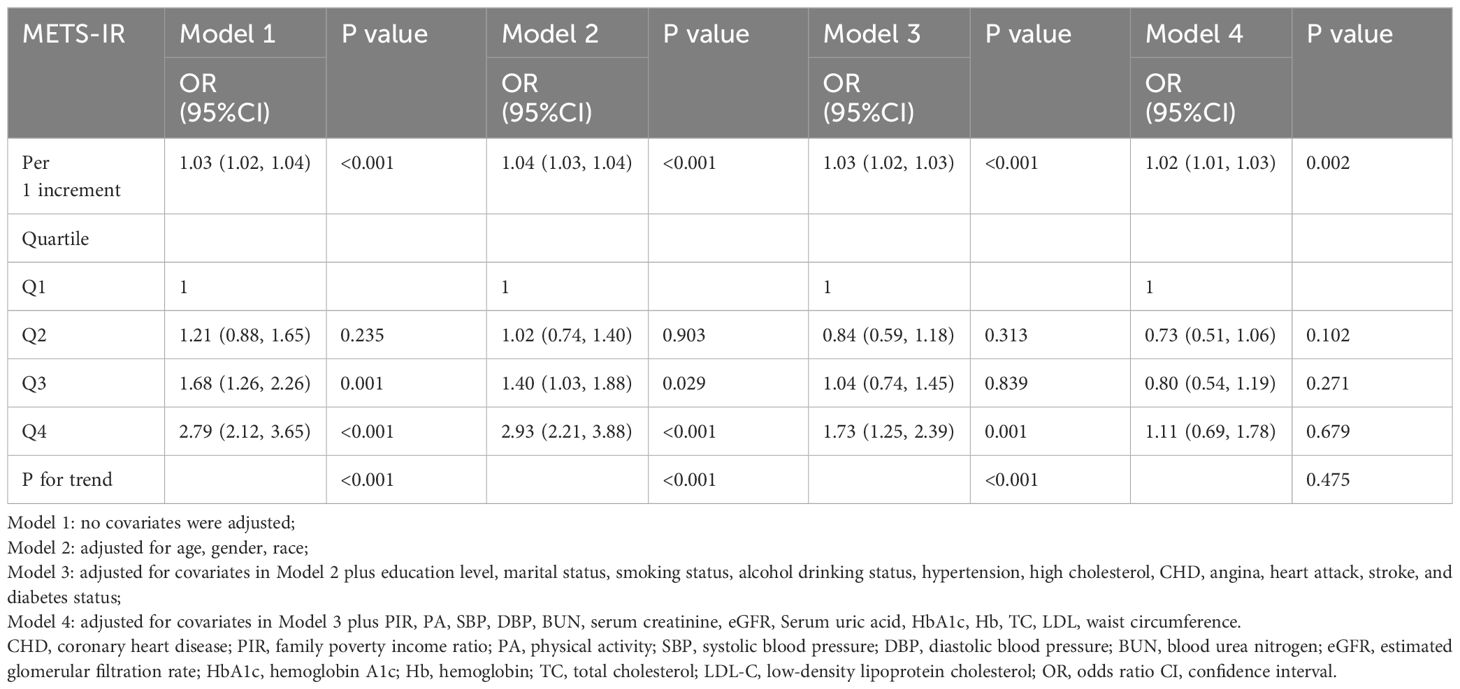
The continuous variables analysis showed a positive association. For each unit increase (1%) in METS-IR, the risk of heart failure increased by 3% (OR = 1.03, 95% CI: 1.02–1.04) in the unadjusted model and in the fully adjusted model by 2% (OR = 1.02, 95% CI: 1.01–1.63). Furthermore, we transformed the METS-IR from a continuous variable into a categorical variable (quartiles) for sensitivity analysis (Table 3). In Model 1 (not adjusted), the risk of HF in the highest quartile increased by 1.79 compared with the lowest quartile (OR: 2.79, 95% CI 2.12, 3.65, p < 0.001). The OR (95% CI) in Model 2 was 2.93 (2.21, 3.88) after adjusting for age, gender, and race. Model 3 was adjusted for covariates in Model 2 plus education level, marital status, smoking status, alcohol drinking status, hypertension, high cholesterol, CHD, angina, heart attack, stroke, and diabetes status, and the OR for HF increased with a higher METS-IR, showing a significant association (OR: 1.73, 95% CI 1.25, 2.39, p < 0.001). There was statistical significance in the test for trend in Model 1, 2, and 3. After adjusting for all covariates in Model 4, the fully adjusted ORs and 95% CIs of Q2, Q3 and Q4 compared with Q1 were 0.73 (0.51, 1.06), 0.80 (0.54, 1.19) and 1.11 (0.69, 1.78) (p for trend = 0.475), respectively.
3.3 Non-linearity and threshold effect analysis between METS-IR and HF
To illustrate the nonlinear relationship and saturation effect between METS-IR and HF, a smooth curve fitting technique was employed, as shown in Figures 2 and 3. Among all participants, the correlation between METS-IR and HF exhibited a J-shaped curve after adjustments, with inflection points observed at 40.966 (Table 4). When the METS-IR measurements were below 40.966, the effect values were not statistically significant. However, when the METS-IR exceeded 40.966, there was a significant effect value of 1.025.

Figure 2 The smooth curve fit for the association between METS-IR and the prevalence of heart failure.
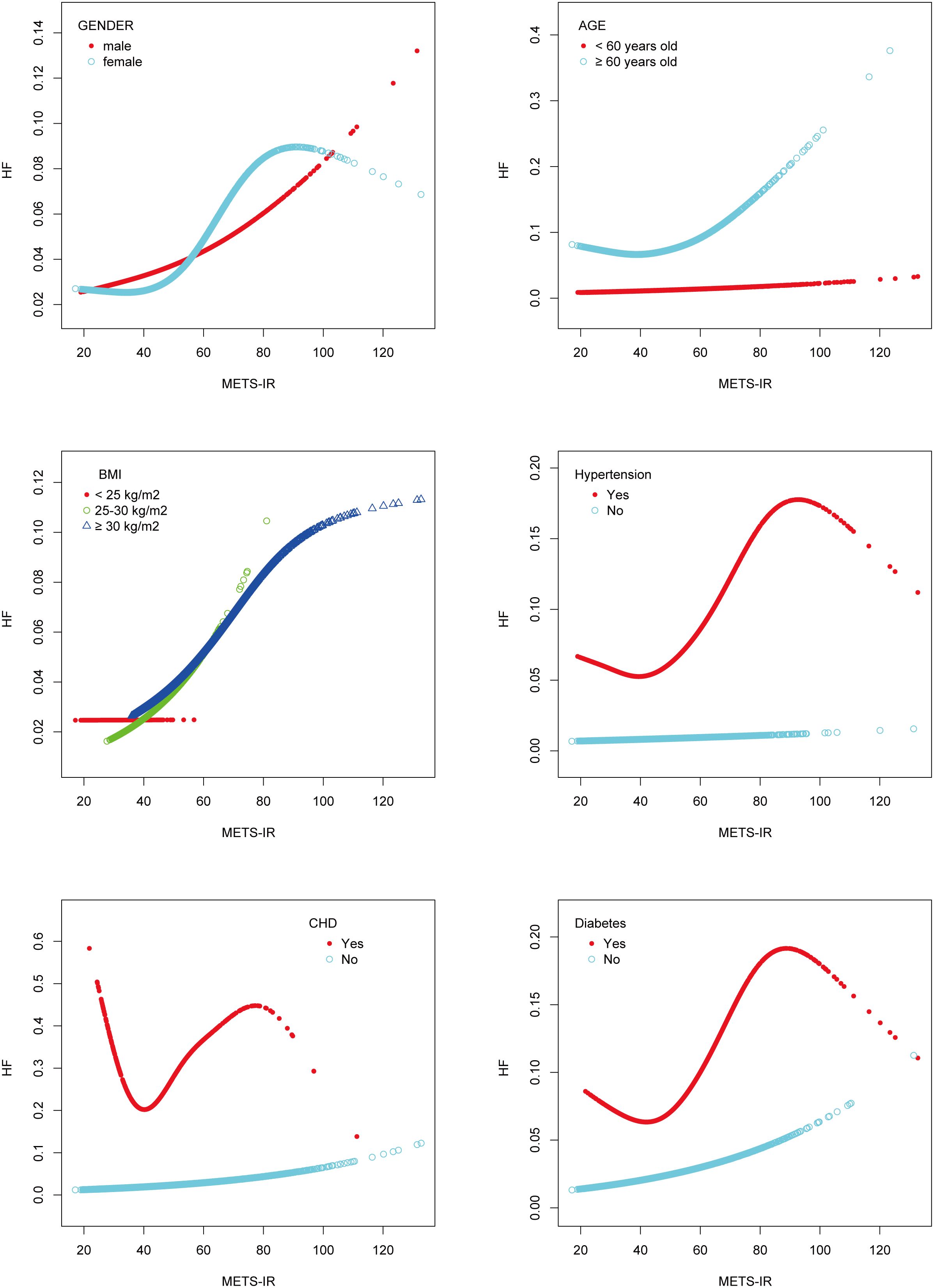
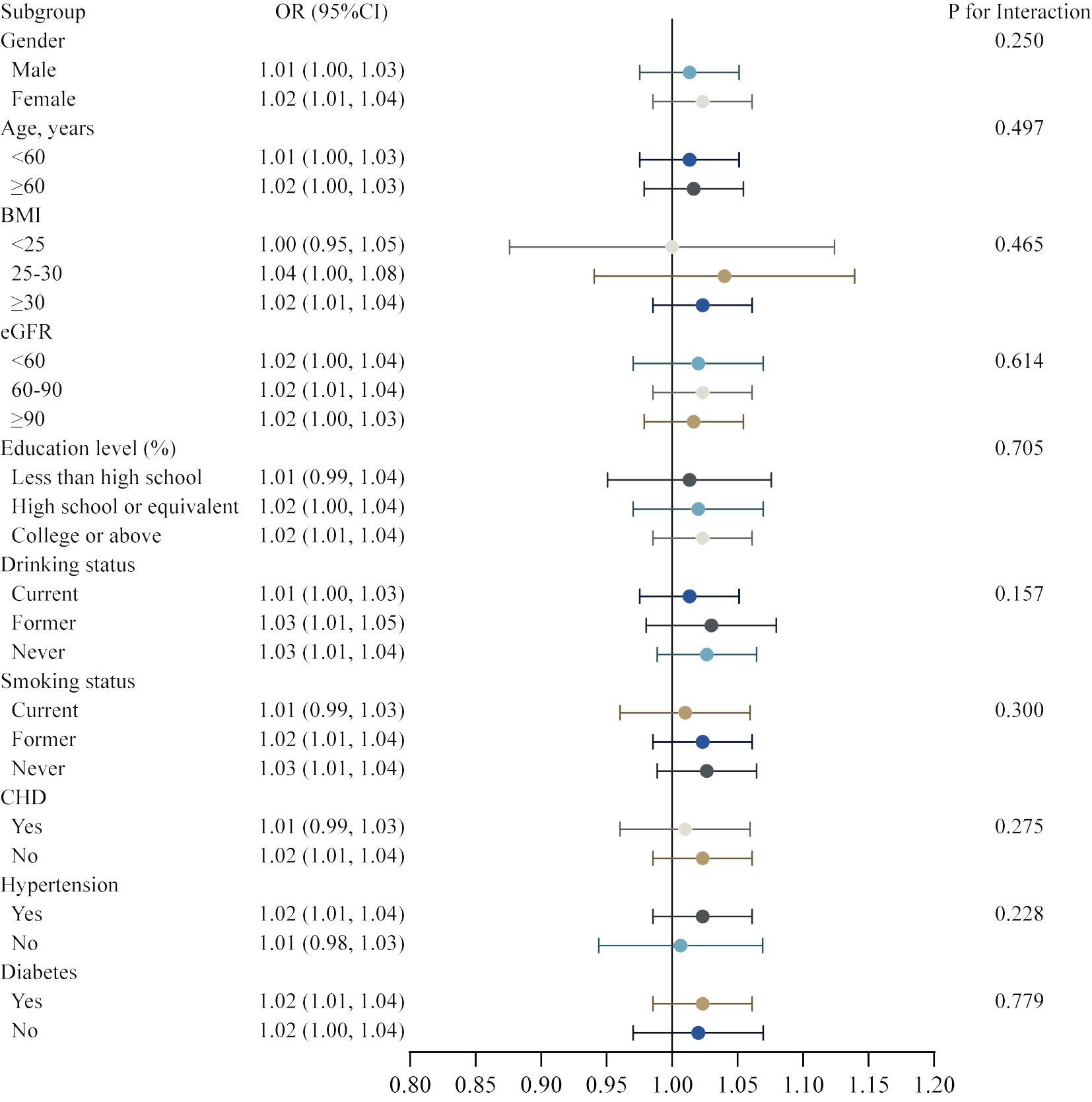
Figure 3 Subgroups analysis for the association between METS-IR and the prevalence of heart failure by gender, age, BMI, hypertension, CHD, and diabetes.
Additionally, the study was carried out using smoothing curve fitting stratified by gender, age, body mass index, hypertension, CHD, and diabetes in order to confirm whether the positive correlation between METS-IR and HF was non-linear across various cohort characteristics (Figure 3). The final results indicated that the METS-IR association with HF was curvilinear in female, aged ≥ 60 years, BMI <25 kg/m2, hypertension, CHD, and diabetes.
3.4 Subgroup analysis
Subsequently, we conducted a subgroup analysis with interaction test to investigate whether the association between METS-IR and the risk of HF was robust in various demographic settings. In Model 4, all covariates were taken into account, with the exception of the covariates that were utilized to establish subgroups. The robustness of our main finding between the METS-IR and gender, age, BMI, eGFR, education level, drinking status, smoking status, CHD, hypertension, and diabetes was illustrated in the figure, with no significant interactions and all P for interaction were > 0.05 (Figure 4).
3.5 ROC analysis
The receiver operating characteristic curves (ROC) for METS-IR to detect heart failure in four models are presented in Figure 5. METS-IR (continuous) predicted the area under the ROC curve of 0.616 (95% CI: 0.589–0.642, p < 0.001) in the unadjusted model. The optimal cutoff value was 48.11, with a sensitivity of 47.0% and a specificity of 70.8%. Statistical significances were found in predicting the area of the ROC curves in Model 2 (AUC: 0.807, 95% CI: 0.789–0.825, p < 0.001), Model 3 (AUC: 0.903, 95% CI: 0.889–0.917, p < 0.001), and Model 4 (AUC: 0.916, 95% CI: 0.904–0.928, p < 0.001).
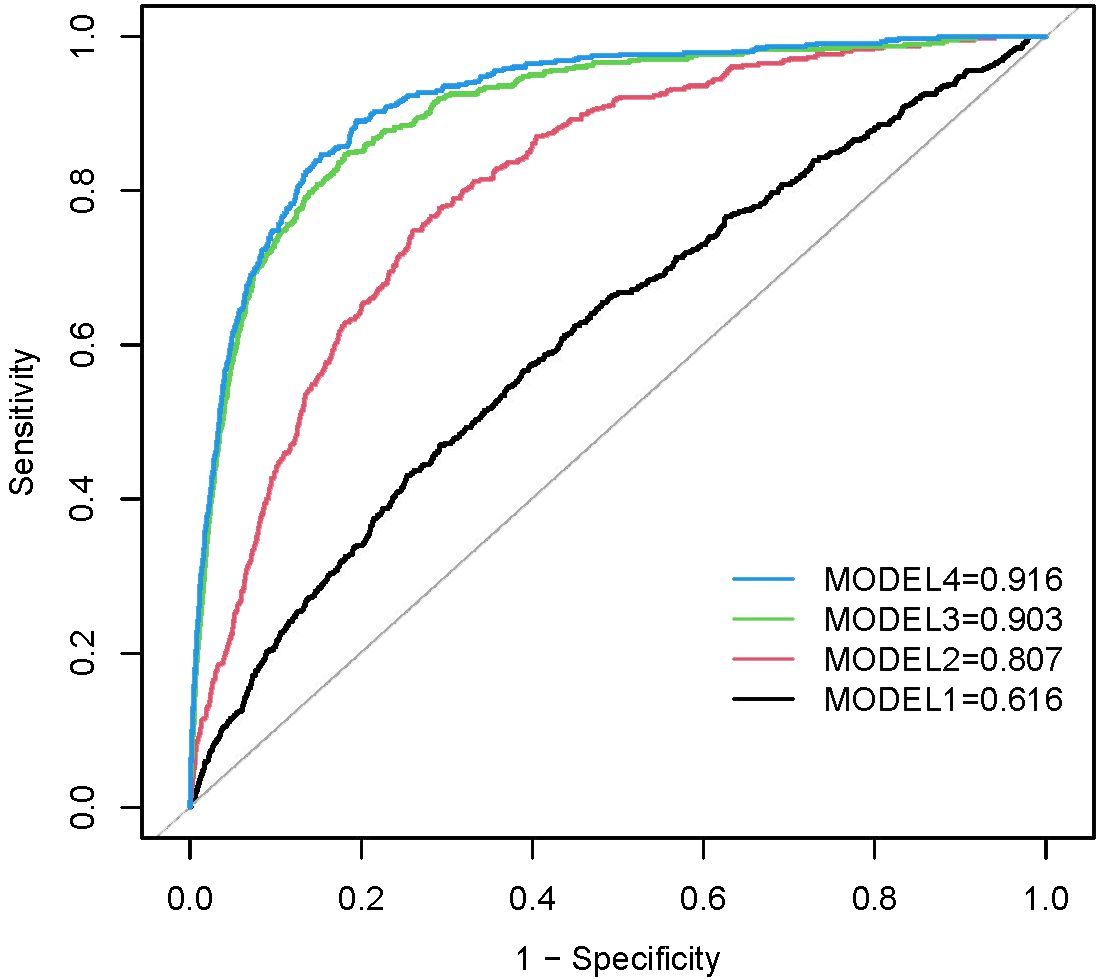
Figure 5 ROC curves between METS-IR and HF in Model 1, Model 2, Model 3, and Model 4. Model 1 was unadjusted. Model 2 was adjusted for age, gender, and race. Model 3 was adjusted for covariates in Model 2 plus education level, marital status, smoking status, alcohol drinking status, hypertension, high cholesterol, CHD, angina, heart attack, stroke, and diabetes status. Model 4 was adjusted for covariates in Model 3 plus PIR, PA, SBP, DBP, BUN, serum creatinine, eGFR, Serum uric acid, HbA1c, Hb, TC, LDL, waist circumference.
4 Discussion
The results of this large cross-sectional study indicate that METS-IR and the risk of heart failure were positively correlated after adjusting for potential confounders. These connections were similar across populations, according to the findings of subgroup analyses and interaction testing. Notably, a non-linear relationship was observed between METS-IR and HF risk, with a distinct inflection point occurring at a METS-IR measurement of 40.966, characterized by a J-shaped curve. METS-IR may be a significant predictor of heart failure and a potential therapeutic target.
In comparison to other non-insulin-based indices, recent research has demonstrated that METS-IR is a viable and trustworthy alternative biomarker for IR. As a valid surrogate for insulin sensitivity that includes both laboratory and anthropometric measurements, METS-IR has been proposed as a tool to identify individuals at an elevated risk of developing T2DM, metabolic syndrome, and other cardiovascular diseases (CVDs) at an early age (16, 18). To the best of our knowledge, our study is the first to examine the link between METS-IR and HF. Previous studies have investigated the correlation between METS-IR and cardiovascular risk factors and CVD in participants with or without diabetes. Han et al. conducted an analysis on a cohort of 15,453 normoglycemic participants and found that METS-IR was a significant risk indicator for pre-hypertension (pre-HTN) and hypertension (HTN). Their findings indicated that for each unit increase in METS-IR, there was a 7% rise in the prevalence of pre-HTN (adjusted odds ratio [OR] = 1.07, 95% confidence interval [CI]: 1.06–1.08) and a 13% increase in the prevalence of HTN (adjusted OR = 1.13, 95% CI: 1.10–1.16) (19). A stronger positive correlation between METS-IR and pulse wave velocity (PWV) was observed in individuals with high cardiovascular risk conditions after adjusting for sex, age, treatment for hypertension, and smoking status (β=0.350, 95% CI: 0.204–0.418), compared to other non-insulin-based IR indices such as the TG/HDL-C index and the TyG index (20). Another long-term prospective cohort involving 14220 individuals diagnosed with H-Type Hypertension in China, with a median follow-up of 3.94 years, found that METS-IR was not only significantly positively associated with the development of CVD (HR=1.66, 95% CI: 1.30–2.12), but also with an increased risk of cardiovascular mortality (HR=1.57; 95% CI, 1.22–2.02) and all-cause mortality (HR=1.33; 95% CI, 1.11–1.60) (21). Furthermore, another longitudinal cohort study involving 17,943 Korean participants without diabetes found that elevated METS-IR was positively and independently associated with the incidence of ischemic heart disease (IHD). During a 50-month follow-up period, HRs of IHD for METS-IR quartiles 1–4 were 1.00, 1.62 (95% CI 1.04–2.53), 1.87 (95% CI 1.20–2.91), and 2.11 (95% CI 1.35–3.30) after adjusting for potential confounding variables (15). However, the evidence for the association between METS-IR and heart failure among the general adult population in the United States is limited. Based on a well-designed national survey, our study first demonstrated that METS-IR was significantly higher in the heart failure group than in the non-heart failure group. Furthermore, higher METS-IR predicted a higher risk of heart failure, which was consistent with the adverse cardiovascular health effects of METS-IR described in previous research (15, 31). Analysis of the smoothed curve fit revealed a J-shaped relationship between METS-IR and heart failure, with an inflection point at 40.966. By being able to identify the threshold value at which the risk of heart failure begins to increase, physicians are able to target specific patient subpopulations for closer monitoring and intervention. This personalized approach to heart failure management can provide a new foundation for the prevention and control of heart failure, leading to improved patient outcomes and reduced healthcare costs. Furthermore, subgroup analysis indicates that the relationship between METS-IR and HF is not potentially modified by variables such as gender, age, BMI, hypertension, coronary heart disease, or diabetes.
Several potential mechanisms may explain this positive correlation between METS-IR and HF. Accumulating evidence confirms that IR plays a crucial role as a risk factor for HF. First, IR can cause energy metabolism in the myocardium, resulting in a shift towards using fatty acid metabolism rather than glucose for myocardial energy acquisition. One way that the degree of IR affected the heart was by reducing its capacity to utilise fatty acids, which can result in the accumulation of lipids and an elevation in their concentration. Ultimately, this will result in the occurrence of HF due to mitochondrial dysfunction and apoptosis (22). Conversely, in diabetes, elevated reactive oxygen species (ROS) are produced as a result of progressive mitochondrial impairment. This exacerbates diabetic cardiomyopathy (DCM) and worsens oxidative stress, further impairing cardiac function (23). Furthermore, the presence of excess circulating free fatty acids can lead to the development of lipotoxicity, which in turn can result in the impairment of pancreatic and myocardial β-cell function, as well as glucose intolerance and heart failure (24). Second, the nucleotide-binding oligomerization domain-like receptor family, pyrin domain-containing 3 (NLRP3), can be activated by IR, which results in the production of interleukin-18 (IL-18) and interleukin-1 beta (IL-1β), as well as local tissue inflammation. Studies have shown that therapeutic interventions involving silencing of the NLRP3 gene have been effective in alleviating cardiac inflammation, pyroptosis, and fibrosis, as well as improving cardiac function (25, 26). Third, calcium plays a crucial role in regulation of mitochondrial function. However, cardiac insulin resistance impairs calcium homeostasis via the PKB–SPEG–SERCA2a pathway, which can diminish the cardiac capacity to process calcium (27) and impair cardiac diastolic function (28), and contributes to the development of DCM (29). Fourth, IR and the resulting hyperinsulinemia could lead to an increase in blood pressure by activating the sympathetic nervous system and the renin-angiotensin-aldosterone system, ultimately resulting in myocardial fibrosis and cardiac dysfunction (30).
Conversely, there is evidence that extremely low levels of TG, FPG, or BMI have a negative impact on health and may even accelerate the onset of certain diseases. Notably, lower IR levels were associated with lower fasting glucose levels. It has been demonstrated that hypoglycemia and rapid fluctuations in blood glucose levels increase levels of counter-regulatory hormones such as epinephrine and norepinephrine. These hormones have the potential to cause platelet aggregation and vasoconstriction, which would accelerate the development of ischaemia in the cardiovascular system (31). A J-shaped relationship between blood glucose levels and cardiovascular events or all-cause mortality was confirmed by a study, with lower fasting blood glucose levels being associated with an increase in adverse events (32). Similarly, low TG levels were identified as an elevated risk of hemorrhagic stroke (33), cardiovascular outcomes and all-cause mortality in patients with type 2 diabetes (34). Previous studies have shown that low BMI is inversely related to several common risk factors for atherosclerosis. However, some large Western population studies have shown a U-shaped association between BMI and CVD mortality (35). According to studies, BNP and NT-proBNP concentrations are inversely correlated with BMI, and particularly high levels have been reported in patients with cardiac cachexia. Low BMI has been linked to a worse prognosis in patients with chronic heart failure, and survival is further impaired if CHF progresses to cardiac cachexia (36). The causality of low body weight and poor prognosis is debated, with some arguing that it is a consequence and others suggesting that it is a cause, but there’s no denying that the obesity paradox exists.
The main advantage of this study is that it was conducted using data from the NHANES, which were collected using a stratified multistage probability sampling strategy. Our study has a large sample size with 14,772 enrolled participants, which increases the representativeness and reliability of the study. Furthermore, we adjusted for potential confounding variables, including age, gender, race, education level, marital status, smoking status, drinking status, hypertension, high cholesterol, CHD, angina, heart attack, stroke, and diabetes status, PIR, PA, SBP, DBP, BUN, serum creatinine, eGFR, serum uric acid, HbA1c, Hb, TC, LDL-C, and waist circumference to reduce the impact of confounding factors and obtain more reliable results. The relationship between METS-IR and heart failure among US adults was initially investigated, revealing a J-shaped association with an inflection point at 40.966. The inflection point identified in our study serves to further enhance the clinical utility of METS-IR measurements.
It should be noted that there are some limitations to our study. First, due to the cross-sectional design of NHANES, we were unable to determine a causal relationship between METS-IR and heart failure. Second, despite our efforts to account for potential confounding variables, the influence of unidentified or unmeasured confounders could not be completely ruled out. However, the existing association was robust enough that it was unlikely to be significantly altered by unaccounted for confounding variables. Third, the NHANES database does not provide relevant questionnaire in this database that mentions the classification of heart failure, as well as brain natriuretic peptide, ultrasound and imaging studies such as cardiac ultrasound and cardiac magnetic resonance that reflect the details information of heart failure. Therefore, this study cannot further evaluate the relationship between METS-IR and the severity and classification of heart failure. Fourth, it is important to note that the study population is limited to the United States. Therefore, further prospective cohort studies are necessary to confirm and promote the current findings in a larger population.
5 Conclusions
In summary, METS-IR was significantly associated with the risk of HF positively. This cross-sectional study revealed that METS-IR had a J-shaped relationship with the risk of HF among a nationally representative sample of adults in the United States. The METS-IR cut-off (40.966) has a certain clinical application value. However, more longitudinal studies are still needed to further verify causal relationships and validate the results in different classifications of heart failure populations.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cdc.gov/nches/nhanes.
Author contributions
XS: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Formal analysis, Data curation, Conceptualization. CZ: Writing – review & editing, Supervision. XZ: Writing – review & editing, Supervision, Methodology.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to extend our appreciation to all of the NHANES participants and staff.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: A report from the american heart association. Circulation. (2023) 147:e93–e621. doi: 10.1161/CIR.0000000000001123
2. Masarone D, Pacileo R, Pacileo G. Use of disease-modifying drugs in diabetic patients with heart failure with reduced ejection fraction. Heart failure Rev. (2023) 28:657–65. doi: 10.1007/s10741-021-10189-4
3. Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: A scientific statement from the american heart association. Circulation. (2016) 134:e535–78. doi: 10.1161/CIR.0000000000000450
4. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the framingham study. JAMA. (1979) 241:2035–8. doi: 10.1001/jama.1979.03290450033020
5. Fan Y, Yan Z, Li T, Li A, Fan X, Qi Z, et al. Primordial drivers of diabetes heart disease: comprehensive insights into insulin resistance. Diabetes Metab J. (2024) 48:19–36. doi: 10.4093/dmj.2023.0110
6. Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res. (2020) 126:1501–25. doi: 10.1161/CIRCRESAHA.120.315913
7. Tian J, Liu Y, Liu Y, Chen K, Lyu S. Ginkgo biloba Leaf Extract Protects against Myocardial Injury via Attenuation of Endoplasmic Reticulum Stress in Streptozotocin-Induced Diabetic ApoE(-/-) Mice. Oxid Med Cell Longevity. (2018) 2018:2370617. doi: 10.1155/2018/2370617
8. Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. (2008) 51:93–102. doi: 10.1016/j.jacc.2007.10.021
9. Qi Y, Xu Z, Zhu Q, Thomas C, Kumar R, Feng H, et al. Myocardial loss of IRS1 and IRS2 causes heart failure and is controlled by p38α MAPK during insulin resistance. Diabetes. (2013) 62:3887–900. doi: 10.2337/db13-0095
10. Fu F, Zhao K, Li J, Xu J, Zhang Y, Liu C, et al. Zhang H et al: Direct Evidence that Myocardial Insulin Resistance following Myocardial Ischemia Contributes to Post-Ischemic Heart Failure. Sci Rep. (2015) 5:17927. doi: 10.1038/srep17927
11. Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med Res Method. (2011) 11:158. doi: 10.1186/1471-2288-11-158
12. Zhang Z, Zhao L, Lu Y, Meng X, Zhou X. Association between non-insulin-based insulin resistance indices and cardiovascular events in patients undergoing percutaneous coronary intervention: a retrospective study. Cardiovasc Diabetol. (2023) 22:161. doi: 10.1186/s12933-023-01898-1
13. Cheng H, Yu X, Li YT, Jia Z, Wang JJ, Xie YJ, et al. Association between METS-IR and prediabetes or type 2 diabetes mellitus among elderly subjects in China: A large-scale population-based study. Int J Environ Res Public Health. (2023) 20(2):1053. doi: 10.3390/ijerph20021053
14. Kim SS, Cheong JY. Screening and prediction of nonalcoholic fatty liver disease using a peripheral insulin resistance index: Potential benefits and limitations. Clin Mol Hepatol. (2022) 28:802–5. doi: 10.3350/cmh.2022.0249
15. Yoon J, Jung D, Lee Y, Park B. The Metabolic Score for Insulin Resistance (METS-IR) as a Predictor of Incident Ischemic Heart Disease: A Longitudinal Study among Korean without Diabetes. J personalized Med. (2021) 11(8):742. doi: 10.3390/jpm11080742
16. Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. (2018) 178:533–44. doi: 10.1530/EJE-17-0883
17. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Internal Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
18. Tucker LA. Macronutrient intake and insulin resistance in 5665 randomly selected, non-diabetic U.S. Adults. Nutrients. (2022) 14(5):918. doi: 10.3390/nu14050918
19. Han KY, Gu J, Wang Z, Liu J, Zou S, Yang CX, et al. Association between METS-IR and prehypertension or hypertension among normoglycemia subjects in Japan: A retrospective study. Front Endocrinol. (2022) 13:851338. doi: 10.3389/fendo.2022.851338
20. Bello-Chavolla OY, Antonio-Villa NE, Vargas-Vázquez A, Martagón AJ, Mehta R, Arellano-Campos O, et al. Prediction of incident hypertension and arterial stiffness using the non-insulin-based metabolic score for insulin resistance (METS-IR) index. J Clin hypertension (Greenwich Conn). (2019) 21:1063–70. doi: 10.1111/jch.13614
21. Zhang L, Yu C, Wang T, Zhou W, Bao H, Cheng X. Association of the metabolic score for insulin resistance with cardiovascular diseases, cardiovascular and all-cause mortality in Chinese hypertensive population. Front Endocrinol. (2023) 14:1326436. doi: 10.3389/fendo.2023.1326436
22. Zheng H, Zhu H, Liu X, Huang X, Huang A, Huang Y. Mitophagy in diabetic cardiomyopathy: roles and mechanisms. Front Cell Dev Biol. (2021) 9:750382. doi: 10.3389/fcell.2021.750382
23. Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-MaChado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. (2018) 9:119. doi: 10.1038/s41419-017-0135-z
24. Nakamura K, Miyoshi T, Yoshida M, Akagi S, Saito Y, Ejiri K, et al. Pathophysiology and treatment of diabetic cardiomyopathy and heart failure in patients with diabetes mellitus. Int J Mol Sci. (2022) 23(7):3587. doi: 10.3390/ijms23073587
25. Luo B, Li B, Wang W, Liu X, Xia Y, Zhang C, et al. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PloS One. (2014) 9:e104771. doi: 10.1371/journal.pone.0104771
26. Pal PB, Sonowal H, Shukla K, Srivastava SK, Ramana KV. Aldose reductase mediates NLRP3 inflammasome-initiated innate immune response in hyperglycemia-induced thp1 monocytes and male mice. Endocrinology. (2017) 158:3661–75. doi: 10.1210/en.2017-00294
27. Boyman L, Karbowski M, Lederer WJ. Regulation of mitochondrial ATP production: ca(2+) signaling and quality control. Trends Mol Med. (2020) 26:21–39. doi: 10.1016/j.molmed.2019.10.007
28. Eisner DA, Caldwell JL, Trafford AW, Hutchings DC. The control of diastolic calcium in the heart: basic mechanisms and functional implications. Circ Res. (2020) 126:395–412. doi: 10.1161/CIRCRESAHA.119.315891
29. Quan C, Du Q, Li M, Wang R, Ouyang Q, Su S, et al. A PKB-SPEG signaling nexus links insulin resistance with diabetic cardiomyopathy by regulating calcium homeostasis. Nat Commun. (2020) 11:2186. doi: 10.1038/s41467-020-16116-9
30. Bradberry S, Sheehan T, Vale A. Use of oral dimercaptosuccinic acid (succimer) in adult patients with inorganic lead poisoning. QJM: monthly J Assoc Physicians. (2009) 102:721–32. doi: 10.1093/qjmed/hcp114
31. Galassetti P, Davis SN. Effects of insulin per se on neuroendocrine and metabolic counter-regulatory responses to hypoglycaemia. Clin Sci (Lond). (2000) 99:351–62. doi: 10.1042/cs0990351
32. Lee JH, Han K, Huh JH. The sweet spot: fasting glucose, cardiovascular disease, and mortality in older adults with diabetes: a nationwide population-based study. Cardiovasc Diabetol. (2020) 19:44. doi: 10.1186/s12933-020-01021-8
33. ZKozdag G, Ertas G, Emre E, Akay Y, Celikyurt U, Sahin T, et al. Low serum triglyceride levels as predictors of cardiac death in heart failure patients. Tex Heart Inst J. (2013) 40:521–8. doi: 10.1097/FJC.0b013e3182a50c45
34. Yun JS, Park YM, Han K, Cha SA, Ahn YB, Ko SH. Severe hypoglycemia and the risk of cardiovascular disease and mortality in type 2 diabetes: a nationwide population-based cohort study. Cardiovasc Diabetol. (2019) 18:103. doi: 10.1186/s12933-019-0909-y
35. Zaccardi F, Dhalwani NN, Papamargaritis D, Webb DR, Murphy GJ, Davies MJ, et al. Nonlinear association of BMI with all-cause and cardiovascular mortality in type 2 diabetes mellitus: a systematic review and meta-analysis of 414,587 participants in prospective studies. Diabetologia. (2017) 60:240–8. doi: 10.1007/s00125-016-4162-6
Keywords: metabolic score for insulin resistance, heart failure, NHANES, nonlinear associations, cross-sectional study
Citation: Su X, Zhao C and Zhang X (2024) Association between METS-IR and heart failure: a cross-sectional study. Front. Endocrinol. 15:1416462. doi: 10.3389/fendo.2024.1416462
Received: 12 April 2024; Accepted: 17 June 2024;
Published: 01 July 2024.
Edited by:
Zhengyang Bao, Wuxi Maternity and Child Health Care Hospital, ChinaCopyright © 2024 Su, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianwei Zhang, enh3MDYwOUAxNjMuY29t
 Xiaozhou Su
Xiaozhou Su Chunli Zhao
Chunli Zhao Xianwei Zhang
Xianwei Zhang