- 1Department of Rheumatology, Wangjing Hospital, China Academy of Chinese Medicine Science, Beijing, China
- 2Robotics Movement Department, Amazon, Boston, MA, United States
Background: The evidence supporting a connection between elevated serum uric acid (SUA) levels and diabetic peripheral neuropathy (DPN) is controversial. The present study performed a comprehensive evaluation of this correlation by conducting a systematic review and meta-analysis of relevant research.
Method: PubMed, Web of Science (WOS), Embase, and the Cochrane Library were searched for published literature from the establishment of each database to January 8, 2024. In total, 5 cohort studies and 15 cross-sectional studies were included, and 2 researchers independently screened and extracted relevant data. R 4.3.0 was used to evaluate the included literature. The present meta-analysis evaluated the relationship between SUA levels and the risk of DPN in type 2 diabetes (T2DM) by calculating the ratio of means (RoM) and 95% confidence intervals (CIs) using the method reported by JO Friedrich, and it also analyzed continuous outcome measures using standardized mean differences (SMDs) and 95% CIs to compare SUA levels between DPN and non-DPN groups. Funnel plot and Egger’s test were used to assess publication bias. Sensitivity analysis was conducted by sequentially removing each study one-by-one.
Results: The meta-analysis included 20 studies, with 12,952 T2DM patients with DPN and 16,246 T2DM patients without DPN. There was a significant correlation between SUA levels and the risk of developing DPN [odds ratio (OR) = 1.23; 95% CI: 1.07-1.41; p = 0.001]. Additionally, individuals with DPN had higher levels of SUA compared to those without DPN (SMD = 0.4; 95% CI: -0.11-0.91; p < 0.01).
Conclusion: T2DM patients with DPN have significantly elevated SUA levels, which correlate with a heightened risk of peripheral neuropathy. Hyperuricemia (HUA) may be a risk indicator for assessing the risk of developing DPN in T2DM patients.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42024500373.
1 Introduction
Serum uric acid (SUA) is the product of purine catalysis by xanthine oxidase 1 (1). Hyperuricemia (HUA), a metabolic disorder related to purine metabolism, is increasingly becoming a significant global health concern. HUA was initially primarily recognized for its association with gout and the resulting impact on quality of life (2). However, recent research has provided evidence linking HUA to various other conditions, such as cardiovascular diseases, renal dysfunction, and cancers (3–5). Previous studies have suggested a high prevalence of HUA among type 2 diabetes mellitus (T2DM) patients (6–10). HUA in diabetic patients can be attributed to a range of factors, such as elevated body mass, larger waist circumference, abnormal lipid levels, lack of physical activity, high blood pressure, and insulin resistance (11, 12), resulting in an unfavorable prognosis and an increase in complications associated with diabetes, such as neuropathy, retinopathy, and nephropathy (13).
The prevalence of diabetes-related complications is increasing worldwide. The influence of uric acid levels on the development of vascular complications has been assessed in individuals diagnosed with DM (14), and the correlation between uric acid levels and complications related to diabetes mellitus (DM) has garnered significant interest. Diabetic peripheral neuropathy (DPN) is a long-term complication linked to DM, with a prevalence ranging from 60% to 90%. Approximately half of patients do not exhibit any symptoms (15), and there is a significant incidence of disability and mortality associated with this condition (16). DPN is a condition of irreversible damage to the nerves, resulting in a gradual decline in sensory function starting from the lower limbs. DPN is further distinguished by significant morbidity and is accompanied by pain (17). There is an incomplete understanding of the factors contributing to the formation of DPN, but numerous hypotheses have proposed a multifactorial mechanism (18) involving various factors, such as elevated condensation levels, length of diabetes diagnosis, presence of high blood pressure, tobacco use, alcohol consumption, excessive weight gain, and HUA (19–22). Because the impact of uric acid on the progression of DPN is unknown, it is necessary to investigate the correlation between uric acid and DPN. There is currently a lack of effective treatments available for DPN that can reverse neuronal damage. Symptom management, such as pain relief, is primarily achieved through pharmacotherapy; however, this approach improves quality of life for some patients but is not effective for all (23–28). Early screening of risk factors will help explore new therapeutic approaches for DPN (27). Considering that SUA levels can be modified, interventions aimed at reducing uric acid may potentially serve as preventive or treatment strategies for DPN in individuals with DM.
The correlation between HUA and neurological disorders is two-sided, in which both low and high SUA may have adverse effects. Given the crucial role of SUA as an antioxidant, maintaining excessively low levels of SUA for an extended period may potentially expose individuals with DM to increased oxidative stress (OS) and disorders related to nerve damage (29). Conversely, HUA may facilitate the movement of smooth muscle cells into blood vessels and block the release of nitric oxide (NO) by endothelial cells, which may result in impaired blood vessel performance, inadequate blood supply to tissues, and impaired functionality of nerves in the peripheral region (30). Multiple studies have suggested a relationship between HUA concentrations and the heightened prevalence of DPN (31–33). A meta-analysis published in 2016 has indicated that patients diagnosed with DM accompanied by DPN exhibit noticeable increases in UA levels and that the presence of HUA is linked to an augmented risk of developing peripheral neuropathy (8). After 2016, additional cross-sectional studies, case-control studies, and other large-sample studies have been published, and some of the results are inconsistent with those of previous studies. For example, a population-based cross-sectional study in China has reported that patients with a lower level of uric acid have a higher risk of DPN compared to those with a normal level of uric acid, disagreeing with earlier findings (34). Because the evidence supporting the correlation between elevated SUA levels and DPN is controversial, the present study analyzed relevant studies to provide a comprehensive evaluation of this correlation to update and supplement existing research.
2 Materials and methods
2.1 Study registration
The present study was conducted following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The PRISMA checklist was presented as Supplementary Material 1. Additionally, the present protocol for the systematic review and meta-analysis was registered with the Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42024500373).
2.2 Search strategy
A comprehensive systematic search was conducted using the following four electronic databases: PubMed, Web of Science (WOS), Embase, and the Cochrane Library. This search spanned from the inception of each database to January 8, 2024. The following keywords were used to search the databases: uric acid or urate or hyperuricemia and diabetes and neuropathy or peripheral neuropathy. The complete search strategy is presented in Supplementary Material 2. The references of the included studies were further examined to identify additional relevant papers. Relevant systematic review or meta-analysis studies were identified and evaluated for inclusion into the present analysis.
2.3 Selection criteria
The inclusion criteria for the meta-analysis were as follows: (1) cohort, cross-sectional, or case-control studies; (2) examined the potential association between SUA levels and DPN among individuals with T2DM or compared SUA levels in patients with DPN versus a control group without DPN; (3) control groups consisted of T2DM patients without neuropathy; (4) utilized accurate and precise methodologies for uric acid level measurement; and (5) provided relevant data applicable for meta-analysis. The exclusion criteria were as follows: reviews, letters to the editor, conference papers, editorials, comments, case reports, and any articles not available in full text. Following the systematic search, two researchers (Xinwen Zhang and Xieyu Zhang) assessed the eligibility of the studies based on the titles and abstracts of all identified records. In case of any disagreement during the assessment process, a third researcher, Xiaoxu Li, was consulted to help reach a consensus.
2.4 Data extraction
For each included study, two investigators (Xinwen Zhang and Xieyu Zhang) independently extracted the following information using a predefined data extraction form: first author, publication year, study design, country of study, sample size, age (mean and standard deviation), gender, SUA levels (mean and standard deviation), odds ratio (OR) estimates, corresponding 95% confidence intervals (Cis), and matched or adjusted factors. OR estimates were extracted from the most comprehensively adjusted model in each study, aiming to minimize the impact of non-measured confounding factors.
To standardize the analysis, SUA levels reported in mg/dL were uniformly converted to μmol/L using the following conversion formula: 1 mg/dL = 59.48 μmol/L. If the levels of SUA were presented as medians and interquartile ranges, they were converted to means and standard deviations (35). All discrepancies were solved by a third investigator (Xiaoxu Li).
2.5 Risk of bias assessment
The risk of bias in the included articles was assessed by two reviewers (Xinwen Zhang and Xieyu Zhang) utilizing two distinct tools. The analytical cross-sectional studies meeting the inclusion criteria were assessed using the Joanna Briggs Institute (JBI) checklist (36). The JBI checklist was scored on a scale from 0 to 8, with each “yes” response to questions receiving 1 point. Conversely, responses marked as “no” or “unclear” were assigned 0 points. Cross-sectional studies achieving scores of 5 or higher on this scale were categorized as high quality. The included cohort and case-control studies were evaluated for the quality of selection, comparability, and outcome based on the Newcastle-Ottawa Quality Assessment Scale (NOQAS). The NOQAS scores ranged from 0 (poor) to 9 (excellent), with a score of 6 or higher indicating high quality studies. In instances of disagreement during the assessment process, a third researcher (Xiaoxu Li) was consulted to reach consensus.
2.6 Data synthesis and analysis
Meta-analyses were conducted using R 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/). To facilitate a more effective analysis and comparison of studies reporting different types of outcomes (continuous and binary), the present meta-analysis was divided into two main parts. First, the relationship between SUA levels and the risk of DPN in T2DM patients was assessed using ORs and 95% CIs. Among the included studies, some studies reported continuous outcomes. For these studies, the method proposed by JO Friedrich was used to calculate the ratio of means (RoM) and 95% CIs (37), which were then integrated into the broader analysis. Second, to compare SUA levels between T2DM patients with DPN and those without DPN, studies reporting continuous outcome measures were analyzed using standardized mean differences (SMDs) and 95% CIs in a meta-analytic approach.
Random effects models were used based on the assumption that there was true heterogeneity among studies due to variations in populations and settings where the studies were conducted. Heterogeneity in outcomes was assessed through multiple methods. Although it may be overly sensitive in meta-analyses encompassing a large number of studies, Cochran’s Q test and its associated p-value were reported (38). Furthermore, the I2 statistic, which quantifies the percentage of variance attributable to true effect differences rather than sampling error (39), was also reported. Given that meta-analyses of prevalence often result in elevated I2 values, which may not accurately reflect true heterogeneity, prediction intervals were additionally presented (40). These intervals forecast the expected range of outcomes in 95% of comparable studies, thereby elucidating the extent of uncertainty in the estimated outcomes (41). Additionally, to account for the impact of variables, such as study design, study region, and sample size, on heterogeneity within the study literature, subgroup analyses were conducted. The potential publication bias was assessed by a funnel plot and Egger’s test. Sensitivity analysis was performed by removing each study one-by-one to verify the robustness of the pooled value.
3 Results
3.1 Selection of studies
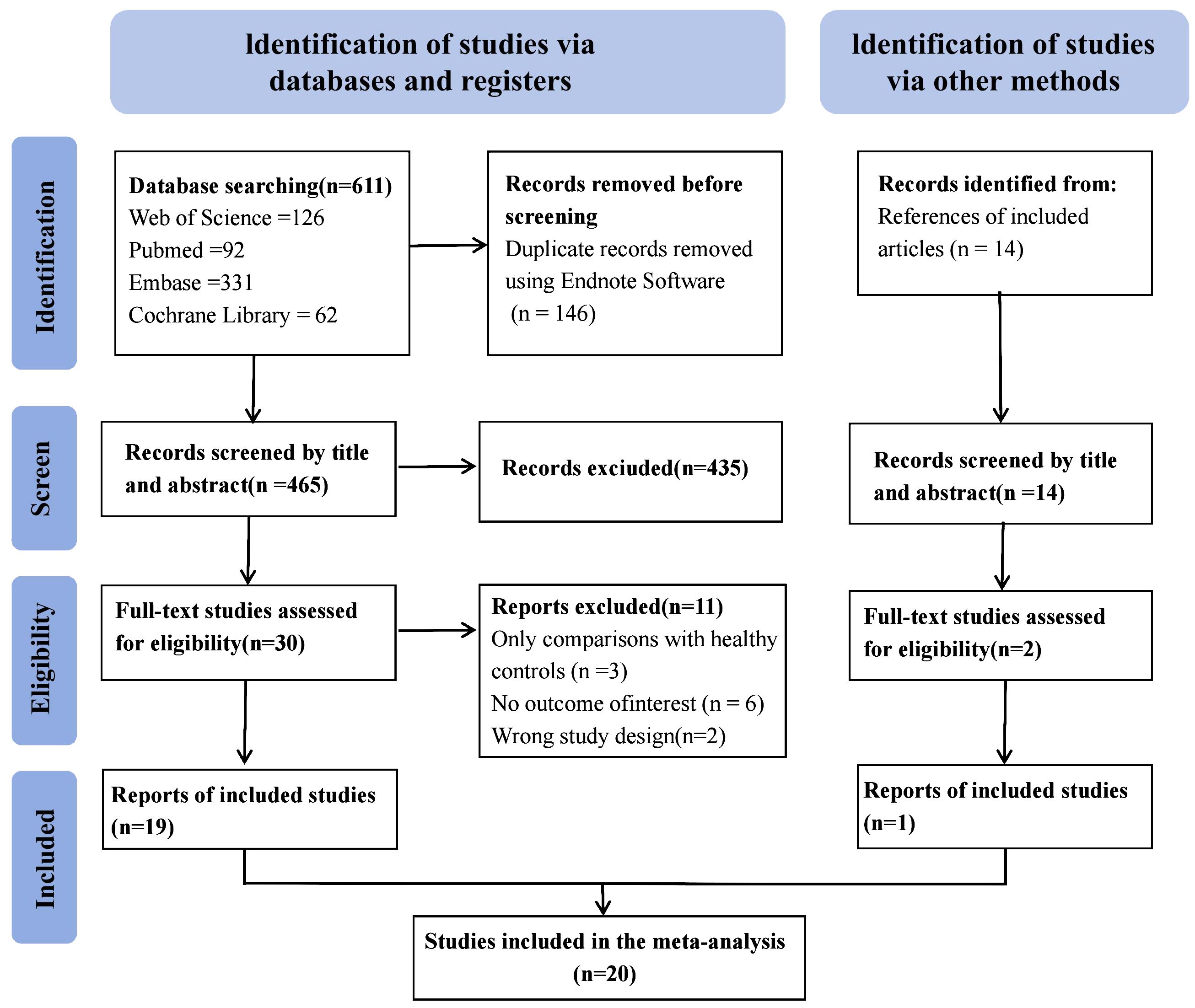
The electronic search of 4 databases yielded 549 studies. Additionally, a manual review of the references from these studies identified one additional eligible study, resulting in 550 studies. Ultimately, 20 articles met the inclusion criteria for the present meta-analysis. The search process is summarized in Figure 1.
3.2 Basic characteristics of the included studies
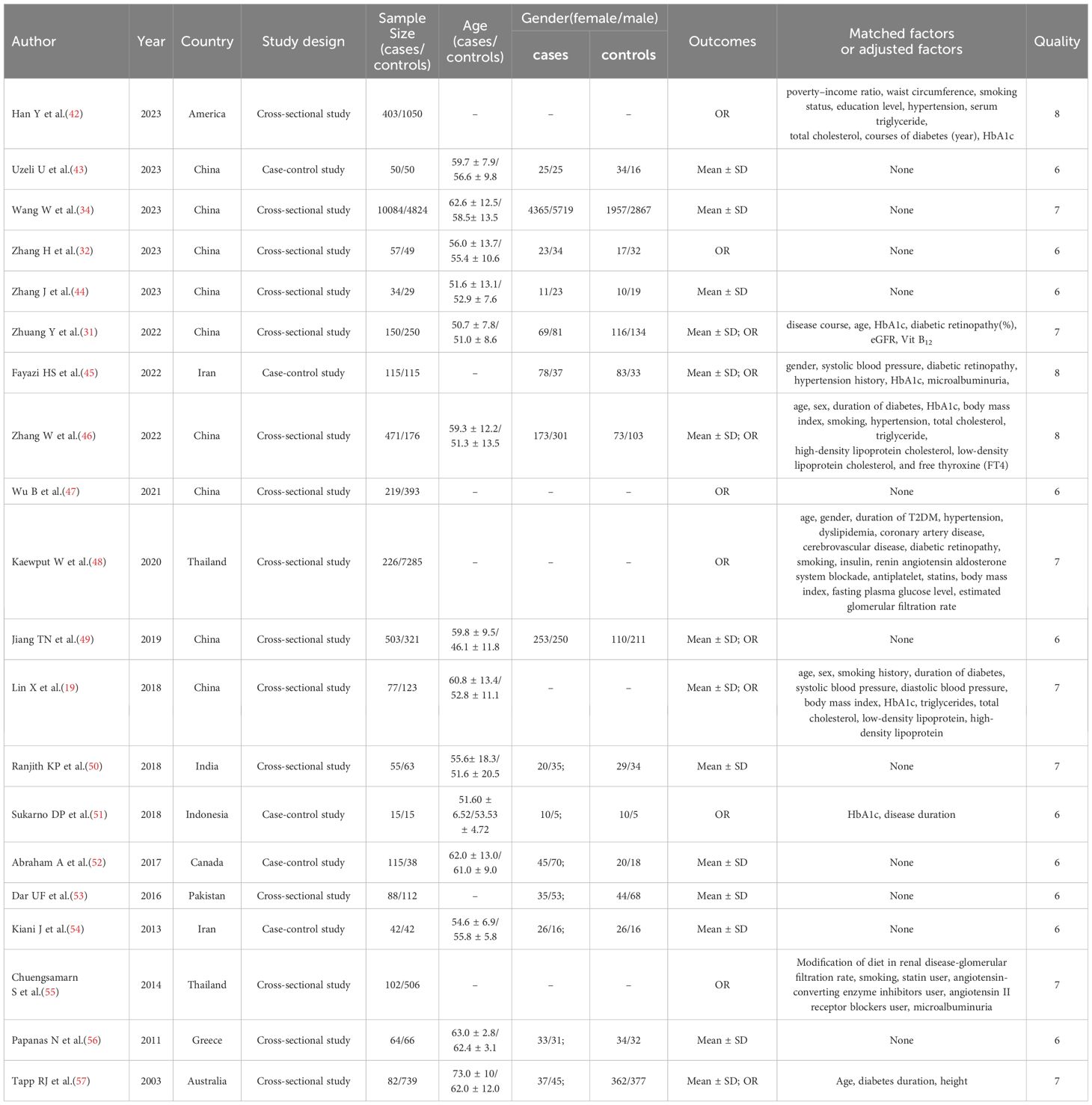
Table 1 shows the essential characteristics of the 20 articles incorporated into the present meta-analysis. The present analysis comprised 29,198 patients, including 12,952 patients diagnosed with DPN and 16,246 patients without DPN. Moreover, there were 15 cross-sectional studies and 5 cohort studies. The included literature originated from the following locations: nine articles from China; two articles from Iran and Thailand; and one article from the United States, Canada, India, Indonesia, Pakistan, Greece, and Australia.
3.3 Evaluation of the methodological quality of the studies
The JBI checklist scores for the included cross-sectional studies varied from 6 to 8, while the NOQAS scores for the included case-control studies ranged from 6 to 8. These scores indicated that the quality of the studies was medium to high.
3.4 Meta-analysis of SUA levels and DPN risk in T2DM patients
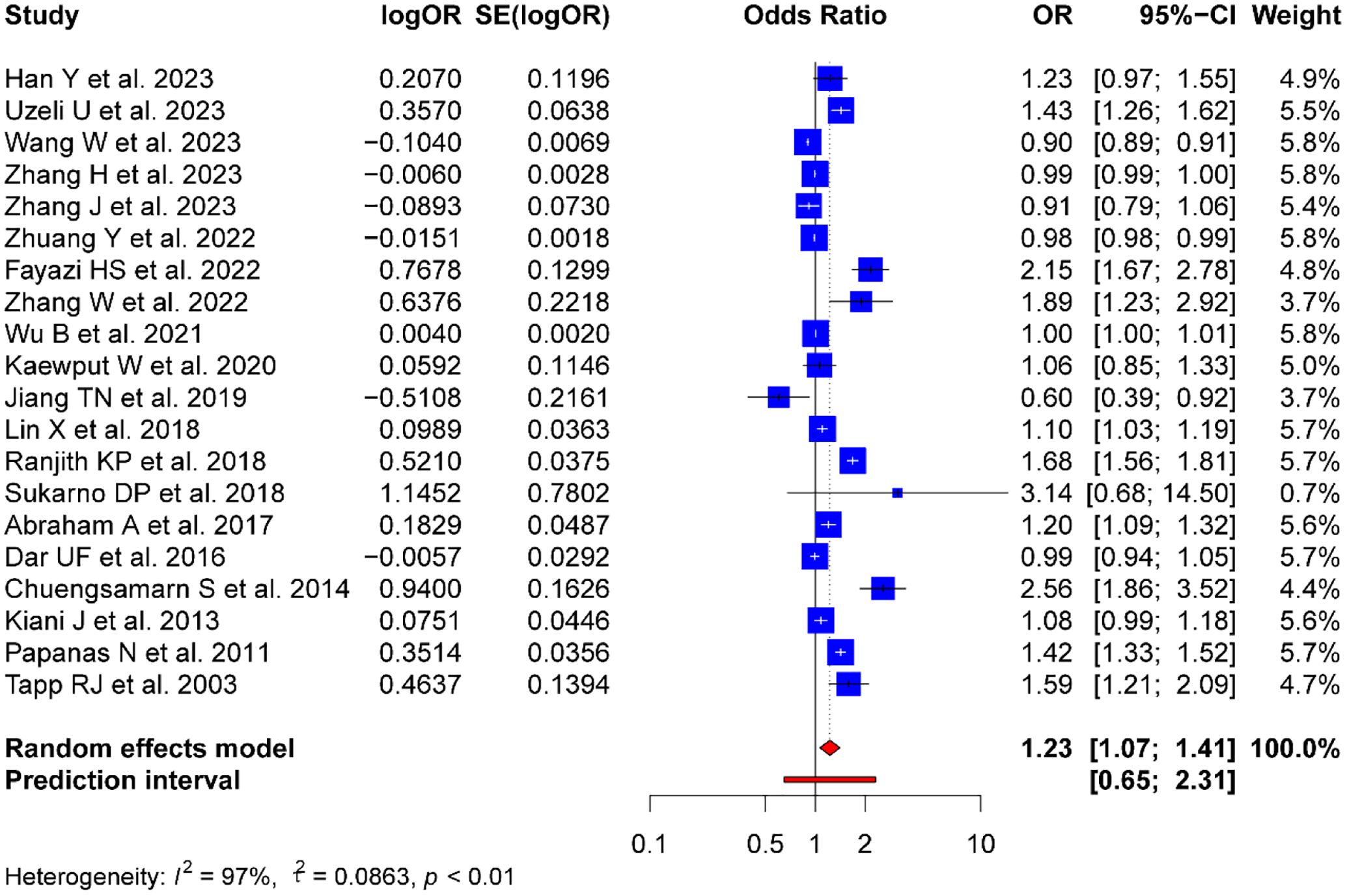
The meta-analysis of SUA levels and the risk of DPN yielded a combined effect size OR of 1.23 (95% CI: 1.07-1.41; Prl: 0.65-2.31). Moreover, the heterogeneity of the included studies was high (p = 0.001, I2 = 97%, τ2 = 0.0863) (Figure 2).
3.4.1 Subgroup analysis
To further explore the sources of heterogeneity of the relationship between SUA levels and the risk of DPN in T2DM patients, subgroup analyses based on study type, country, and sample size were conducted.
Among the 15 cross-sectional studies, the combined OR was 1.17 (95% CI: 1.00-1.38), with significant heterogeneity (p < 0.01, I2 = 98%, τ2 = 0.0890). In the five case-control studies, the combined OR was 1.23 (95% CI: 1.07-1.41), also showing substantial heterogeneity (p < 0.01, I2 = 88%, τ2 = 0.076).
Subgroup analysis by country revealed the following outcomes: the combined OR for the nine Chinese studies was 1.04 (95% CI: 0.9-1.19, p < 0.01, I2 = 96%, τ2 = 0.0374); the combined OR for the Iranian studies (n=2) was 1.51 (95% CI: 0.77-2.97, p < 0.01, I2 = 95%, τ2 = 0.2305); and the combined OR for the two Thai studies was 1.64 (95% CI: 0.69-3.88, p < 0.01, I2 = 95%, τ2 = 0.3681). The studies from the other regions were insufficient for subgroup analysis.
A subgroup analysis was conducted based on sample size. The quartile method was used to divide the studies into four groups according to their sample sizes, ensuring that each group contained a relatively uniform amount of data to reduce the impact of subjective division. The following four groups based on sample size were analyzed: Group 1 (Q1), which included sample sizes less than 115; Group 2 (Q2), which included sample sizes between 115 and 215; Group 3 (Q3), which included sample sizes between 215 and 718; and Group 4 (Q4), which included sample sizes greater than 718. Meta-analyses were conducted for each sample size group, yielding the following results: Q1: OR = 1.40 (95% CI: 0.91-2.14, p < 0.01, I2 = 94%, τ2 = 0.1926); Q2: OR = 1.26 (95% CI: 1.05-1.51, p < 0.01, I2 = 97%, τ2 = 0.0426); Q3: OR = 1.30 (95% CI: 0.94-1.79, p < 0.01, I2 = 96%, τ2 = 0.1223); and Q4: OR = 1.04 (95% CI: 0.79-1.38, p < 0.01, I2 = 86%, τ2 = 0.0866). Based on the analysis results of these four groups, the sample size impacted the effect size and heterogeneity. The groups with smaller sample sizes (Q1 and Q2) showed larger effect sizes and heterogeneity, which may be related to the instability and more significant variability of results in studies with small sample sizes. The groups with larger sample sizes (Q3 and Q4) exhibited smaller effect sizes that were not statistically significant and slightly lower heterogeneity. The forest plots for these subgroup analyses are shown in Supplementary Material 3.
Despite conducting subgroup analyses, there was still persistent high heterogeneity across the groups, which suggested that the included factors, such as study type, country, and sample size, may not fully explain the variability observed in the relationship between uric acid levels and the risk of DPN in T2DM patients. Thus, other factors that affect heterogeneity may be involved, including differences in experimental conditions (such as the stage of DPN among participants), demographic variations (such as age and gender), varying diagnostic criteria, and different measurement methods for determining uric acid levels and DPN status.
3.4.2 Sensitivity analysis
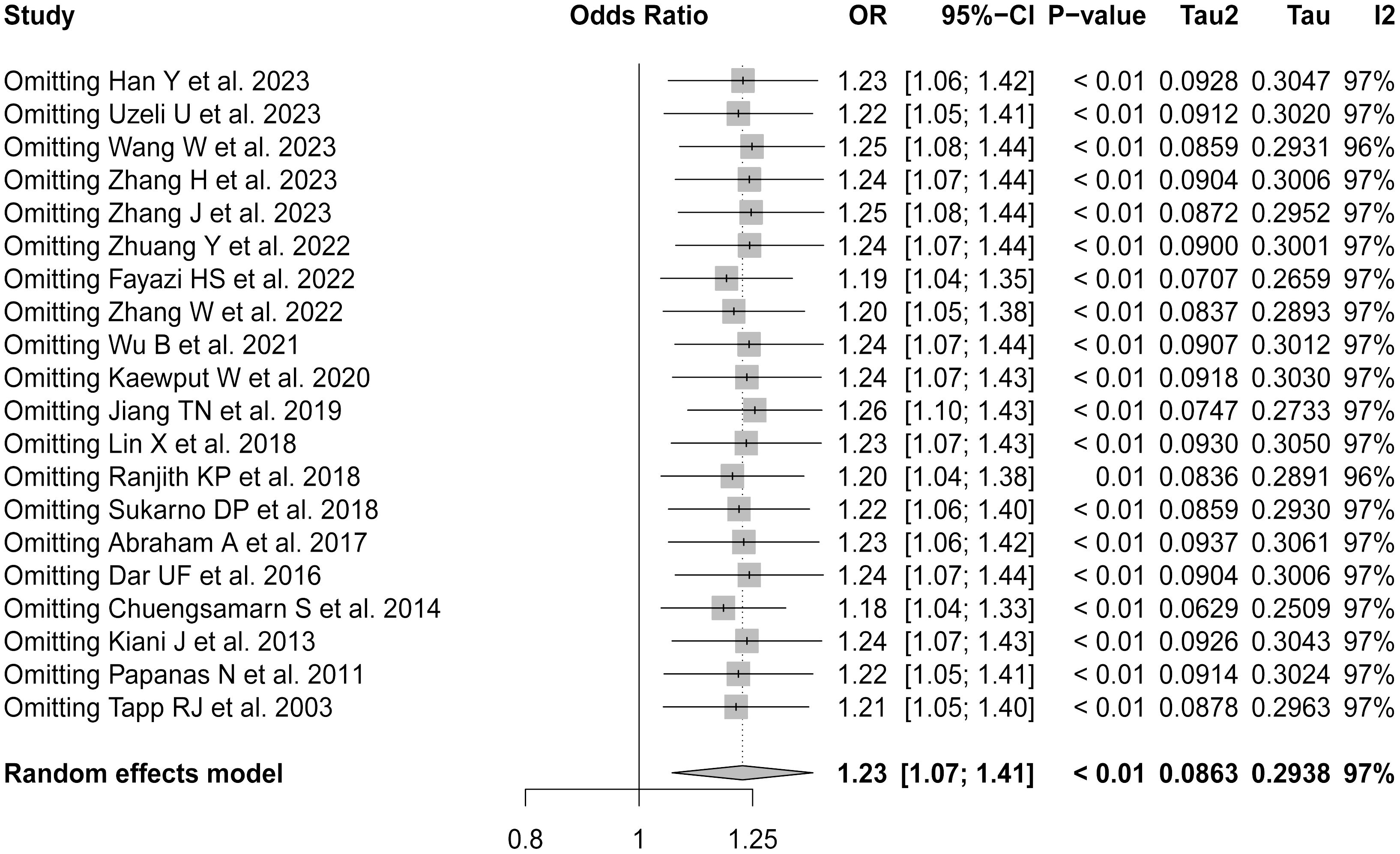
Figure 3 illustrates the robustness of the present analyses. For the sensitivity analysis, each study was sequentially excluded from the meta-analysis to assess the impact on the overall results. Excluding individual studies did not significantly alter the combined ORs. This lack of substantial variation in the outcomes with the exclusion of each study validated the stability and reliability of the present findings. Despite the high heterogeneity observed in the primary analysis, these results confirmed the reported associations between uric acid levels and the risk of DPN in T2DM patients, supporting their relevance in the broader context of diabetes research.
3.4.3 Publication bias
The contour-enhanced funnel plot for the meta-analysis suggested no evidence of publication bias. In addition, the Begg’s test (p = 0.7952) and Egger’ test (p=0.0552) results were not statistically significant, indicating no publication bias (Supplementary Material 4).
3.5 Comparison of SUA levels between DPN and non-DPN patients
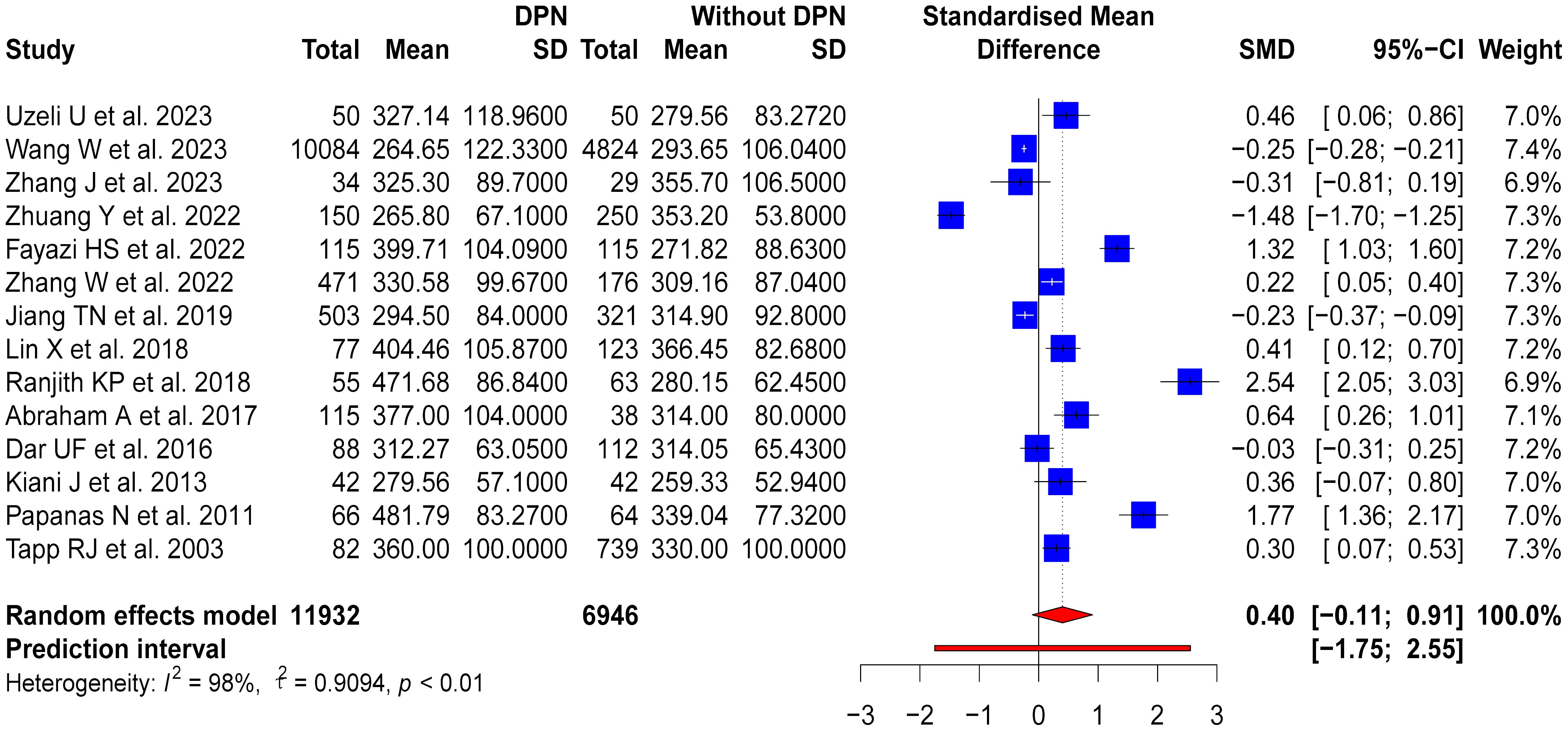
Fourteen studies compared SUA levels between DPN patients and non-DPN patients, which included 11,932 DPN patients and 6,946 non-DPN patients. DPN patients had higher SUA levels than non-DPN patients, with an SMD of 0.4 (95% CI: -0.11-0.91; Prl: -1.75-2.55). However, high heterogeneity was observed in these studies (p < 0.01, I2 = 98%, τ2 = 0.9094) (Figure 4).
3.5.1 Subgroup analysis
To investigate factors contributing to variability, subgroup analyses based on study design, country of study, and sample size were conducted. For the ten cross-sectional studies, a moderate variation in SUA levels was observed between DPN and non-DPN patients (SMD = 0.28, 95% CI: -0.4-0.97), but there was high heterogeneity among these studies (p < 0.01, I2 = 98%, τ2 = 1.2096). The four case-control studies showed a more noticeable difference in SUA levels (SMD = 0.71, 95% CI: 0.27-1.15), but there was also significant heterogeneity (p < 0.01, I2 = 85%, τ2 = 0.1651). Moreover, notable geographical variations were observed in the subgroup analyses based on the country of study. The seven studies from China revealed a slight reduction in SUA levels in patients with DPN (SMD = -0.17, 95% CI: -0.66-0.32), but there was considerable heterogeneity (p < 0.01, I2 = 97%, τ2 = 0.4212). Conversely, the two Iranian studies indicated an increase in SUA levels in patients with DPN (SMD = 0.86, 95% CI: -0.08-1.79), but there was high heterogeneity (p < 0.01, I2 = 92%, τ2 = 0.4207). Further subgroup analysis was conducted based on the sample size, dividing the studies into four groups using quartiles. The first group (Q1) had a sample size of less than 165, the second group (Q2) had a sample size between 165 and 315, the third group (Q3) had a sample size between 315 and 778, and the fourth group (Q4) had a sample size greater than 778. The results of the subgroup analysis showed that the group with the smallest sample size, Q1, had an OR of 1.16 (95% CI: -0.06-2.38, p < 0.01, I2 = 96%, τ2 = 1.5005), Q2 had an OR of 0.57 (95% CI: -0.21-1.34, p < 0.01, I2 = 96%, τ2 = 0.4507), Q3 had an OR of -0.30 (95% CI: -1.46-0.86, p < 0.01, I2 = 99%, τ2 = 1.0308), and Q4 had an OR of 0.04 (95% CI: -0.31-0.38, p < 0.01, I2 = 91%, τ2 = 0.1113). These results suggest that the groups with smaller sample sizes (Q1 and Q2) exhibited higher heterogeneity and more significant effect sizes, but the results were unstable and not statistically significant. In contrast, the groups with larger sample sizes (Q3 and Q4) showed smaller effect sizes but still had some degree of heterogeneity. These results highlighted the diverse impacts of HUA on patients with DPN, which were influenced by study design, geographical location, and sample size, thereby warranting further investigation. The forest plots for these subgroup analyses are shown in Supplementary Material 5.
3.5.2 Sensitivity analysis
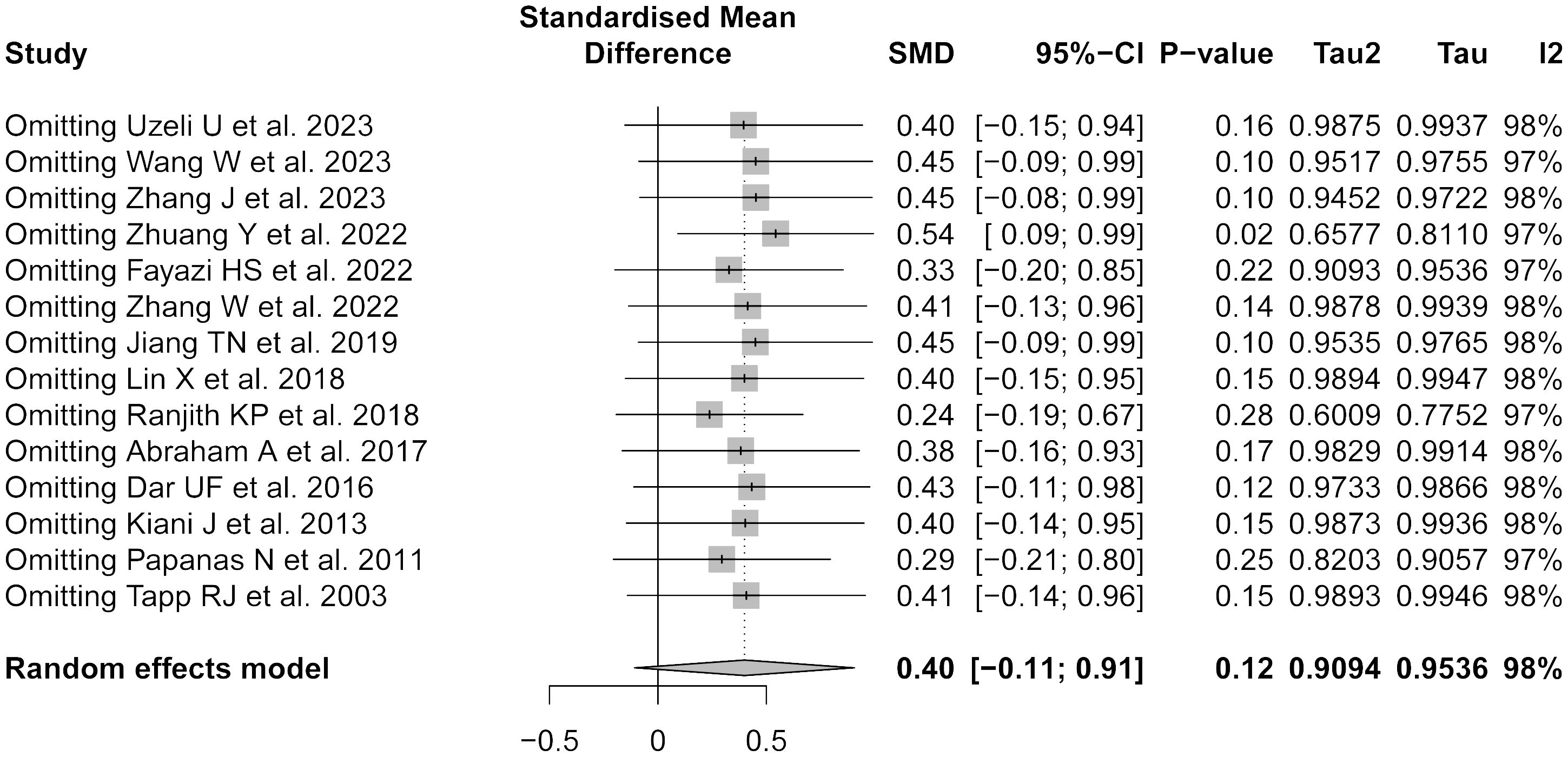
The sensitivity analysis confirmed the robustness of the meta-analysis (Figure 5). Exclusion of any single study did not significantly alter the overall effect size, underscoring the stability of the present findings.
3.5.3 Publication bias
A contour-enhanced funnel plot was utilized alongside Egger’s and Begg’s tests to assess publication bias in the present meta-analysis. The funnel plot was asymmetrical as indicated by the dispersion of studies outside the central area of the funnel (Supplementary Material 6). In addition, Egger’s test yielded a p-value of 0.0388, suggesting potential publication bias, but Begg’s test showed a p-value of 0.208, indicating no significant evidence of bias. The discrepancy between these tests may be attributed to the greater sensitivity of Egger’s tests to detect bias, especially in studies with smaller sample sizes. Overall, these findings suggested that the present results should be interpreted with caution, taking into account the possibility of publication bias, as suggested by Egger’s test.
4 Discussion
Until now, a comprehensive analysis of the association between HUA and DPN in individuals diagnosed with DM has been lacking. The present study performed a systematic review and meta-analysis, which was comprised of 20 studies, including 12,952 T2DM patients with DPN and 16,246 T2DM patients without DPN, to thoroughly examine the relationship between HUA and DPN development.
DPN is a severe and long-term complication, resulting from prolonged high blood sugar, which damages peripheral nerves. Approximately 50% of DM patients may experience neuropathy in their lifetime (58). Cross-sectional and cohort studies conducted since 2016 have reported a DPN incidence of approximately 8.8/1,000 person-years among individuals with type 1 diabetes mellitus (T1DM) (59) and 24–26.9/1,000 person-years among individuals with T2DM (59, 60). DPN prevalence is generally 30% (61, 62). A recent worldwide meta-analysis (29 studies with 50,112 participants) has reported that individuals with T2DM have higher DPN prevalence (31.5%, 95% CI 24.4–38.6%) compared to those with T1DM (17.5%, 95% CI 13.1–36.5%) (63). DPN is characterized by neuropathic pain, numbness, and sensory abnormality on symmetrical, bilateral distal limbs, which not only increases the risks of foot ulceration and even lower limb amputation but also affects the patient’s health, thereby causing a heavy financial burden (17). Moreover, DPN may heighten cardiovascular disease risks (17). Diabetes duration is a strong DPN determinant, and DPN prevalence varies by country and ranges from 1% to 80% (64). This large variation likely arises from multiple factors, including disease severity, diabetes duration, DPN definition, and comorbid conditions predisposing to neuropathy development, especially metabolic syndrome. Nerve conduction is often used as a standard diagnostic tool for DPN, but it is time-consuming, costly, and somewhat difficult to clinically diagnose (65, 66). As a metabolic disease, the occurrence of DPN has been suggested to be related to the imbalance of metabolic pathways caused by hyperglycemia, lipid metabolism disorders, and insulin abnormalities, which can lead to OS, inflammatory reaction, mitochondrial dysfunction, and nerve cell damage (67). Because there is no effective treatment for DPN, early glycemic control combined with exercise and a healthy diet is suggested to prevent and delay disease progression (26). The potential pathogenic mechanisms of DPN remain unclear, limiting the exploration of useful prevention and treatment strategies for DPN (65, 66).
Elevated SUA levels are typically characterized by SUA levels greater than 6.8 mg/dL, indicating a higher likelihood of developing gout. Earlier research has indicated a potential association between elevated levels of uric acid in the blood and the development of cardiovascular conditions (68, 69). Studies have suggested that there is a potential association between HUA and several conditions, such as elevated glucose levels, reduced insulin sensitivity, irregular lipid profiles, and metabolic syndrome (70–75), which may contribute to the onset of diabetic neuropathy (76). Previous studies have suggested a high prevalence of HUA among T2DM patients (6–10), and there is an association between levels of uric acid and DPN, suggesting that uric acid may serve as an indicator for HUA-induced OS in the progression of diabetic neuropathy (77–80). Once inside cells, uric acid activates specific mitogen-activated protein kinases (MAPKs), leading to cyclooxygenase-2 (COX-2) induction and increased production of local thromboxane, and uric acid also upregulates the mRNA expression of platelet-derived growth factor A, C, and alpha receptors (81). Research has demonstrated that uric acid leads to impaired functioning of endothelial cells, potentially exacerbating the progression of diabetic neuropathy (73, 82). Thus, these potential pathological mechanisms suggest that elevated SUA levels are associated with an elevated likelihood of developing DPN. Two recent studies have reported that SUA levels vary among diabetes patients with and without neuropathy in the extremities, as well as those with and without sudomotor dysfunction (74).
The present meta-analysis indicated that there was a positive correlation between elevated HUA levels and an increased risk of DPN in individuals with T2DM, suggesting that uric acid may contribute to the development of DPN. The present meta-analysis also showed that the levels of SUA in patients with DPN were significantly higher compared to those without DPN, suggesting an association between elevated SUA levels and DPN in diabetes patients. Therefore, these findings suggested that an elevated concentration of uric acid in the bloodstream is associated with a higher likelihood of developing DPN.
Considering that SUA levels can be modified, interventions aimed at reducing uric acid may have potential preventive or therapeutic benefits for diabetic patients with DPN. At present, the clinical potential of uric acid-lowering interventions for DPN has been suggested by cross-section studies and case-control studies, highlighting the necessity for randomized controlled trials (RCTs) to test the efficacy of these interventions. Multi-center, blinded, randomized controlled trials, with strict inclusion and exclusion criteria, are needed. Unified uric acid determination and DPN detection, as well as DPN stage details, are required to eliminate multi-factor interference to objectively reflect the influence of uric acid-lowering intervention measures on DPN and provide new ideas for the prevention, diagnosis, and treatment of DPN.
4.1 Limitations and prospects
The present meta-analysis had several limitations. There is a crucial need for additional prospective cohort investigations to explore the impact of HUA on the susceptibility to DPN. While the present analysis included cross-sectional and case-control studies, conducting well-designed prospective cohort studies is essential for a more accurate assessment of the potential association between HUA and DPN risk. The present analysis focused solely on the correlation between HUA and DPN risk in patients with T2DM, as there was a lack of relevant research available for individuals diagnosed with T1DM. Therefore, further studies are necessary to evaluate this correlation, specifically in patients with T1DM.
Only one article in the literature analyzed the relationship between DPN severity and SUA levels (34). The results suggested that the prevalence of DPN decreases with increasing SUA levels, indicating that individuals with normal SUA levels may be at higher risk of developing DPN compared to those with lower SUA levels. However, the present analysis indicated that there were higher SUA levels in patients with DPN than those with mild DPN, suggesting the potential role of SUA in the progression or severity of DPN. Nonetheless, the exact mechanism underlying this relationship remains unclear and requires further investigation.
The present findings demonstrated that the effect of SUA levels on DPN patients varied depending on several factors, such as study location and sample size. For instance, a cross-sectional survey in China with a large sample size has suggested that high uric acid levels may reduce the risk of DPN (34). In contrast, a cross-sectional survey in Thailand has indicated that high uric acid levels are a risk factor for DPN (83). These discrepancies may reflect differences in lifestyle, genetic background, and healthcare systems among different regions. Moreover, studies with smaller samples showed larger effect sizes and higher heterogeneity, which may be due to greater instability and variability in the results of studies with small sample sizes. Conversely, studies with greater sample sizes showed smaller and non-significant effect sizes with reduced heterogeneity. These limitations emphasize the necessity for further prospective cohort studies and more rigorous study designs to mitigate heterogeneity and enhance the reliability of the findings.
4.2 Conclusion
In summary, the present study identified a correlation between increased SUA levels and an enhanced susceptibility to the development of DPN. Thus, high SUA may be a risk factor and potential predictor of DPN.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
XL: Writing – original draft. XYZ: Writing – review & editing, Supervision, Conceptualization. XWZ: Writing – review & editing, Writing – original draft, Software, Methodology. XZ: Writing – review & editing, Visualization. GW: Writing – review & editing, Visualization. JS: Writing – review & editing, Validation. YY: Writing – review & editing, Investigation, Conceptualization. SF: Writing – review & editing, Conceptualization. JZ: Writing – review & editing, Validation. KZ: Writing – review & editing. JD: Writing – review & editing, Validation. JG: Writing – review & editing, Formal Analysis. WC: Writing – review & editing, Project administration.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Key Research and Development Plan Project: Gout staging integrated Chinese and Western medicine treatment plan optimization and clinical assistant decision platform construction (project No. 2022YFC3501201).
Acknowledgments
We extend our appreciation to everyone who participated in this research.
Conflict of interest
Author JG was employed by the company Amazon.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1416311/full#supplementary-material
Abbreviations
CI, Confidence intervals; DM, Diabetes mellitus; DPN, Diabetic peripheral neuropathy; JBI, Joanna Briggs Institute; MAPK, Mitogen-activated protein kinases; NOQAS, Newcastle-Ottawa Quality Assessment Scale; OR, Odds ratio; OS, Oxidative stress; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, Randomized controlled trials; RoM, Ratio of means; SMD, Standardized mean differences; SUA, Serum uric acid WOS, Web of Science.
References
1. Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. (1986) 235:747–54. doi: 10.1042/bj2350747
2. Xia Y, Wu Q, Wang H, Zhang S, Jiang Y, Gong T, et al. Global, regional and national burden of gout, 1990–2017: a systematic analysis of the global burden of disease study. Rheumatology. (2020) 59:1529–10. doi: 10.1093/rheumatology/kez476
3. Mallat SG, Kattar S, Tanios BY, Jurjus A. Hyperuricemia, hypertension, and chronic kidney disease: an emerging association. Curr Hypertens Rep. (2016) 18:74. doi: 10.1007/s11906-016-0684-z
4. Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. (2017) 15:123. doi: 10.1186/s12916-017-0890-9
5. Xie Y, Xu P, Liu K, Lin S, Wang M, Tian T, et al. Hyperuricemia and gout are associated with cancer incidence and mortality: a meta-analysis based on cohort studies. J Cell Physiol. (2019) 234:14364–76. doi: 10.1002/jcp.28138
6. Verma S, Ji Q, Bhatt DL, Mazer CD, Al-Omran M, Inzucchi SE, et al. Association between uric acid levels and cardio-renal outcomes and death in patients with type 2 diabetes: A subanalysis of EMPA-REG OUTCOME. Diabetes Obes Metab. (2020) 22:1207–14. doi: 10.1111/dom.13991
7. Wan H, Wang Y, Chen Y, Fang S, Zhang W, Xia F, et al. Different associations between serum urate and diabetic complications in men and postmenopausal women. Diabetes Res Clin Pract. (2020) 160:108005. doi: 10.1016/j.diabres.2020.108005
8. Yu S, Chen Y, Hou X, Xu D, Che K, Li C, et al. Serum uric acid levels and diabetic peripheral neuropathy in type 2 diabetes: a systematic review and meta-analysis. Mol Neurobiol. (2016) 53:1045–51. doi: 10.1007/s12035-014-9075-0
9. Wang J, Yu Y, Li X, Li D, Xu C, Yuan J, et al. Serum uric acid levels and decreased estimated glomerular filtration rate in patients with type 2 diabetes: A cohort study and meta-analysis. Diabetes Metab Res Rev. (2018) 34:e3046. doi: 10.1002/dmrr.3046
10. Kodama S, Saito K, Yachi Y, Asumi M, Sugawara A, Totsuka K, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. (2009) 32:1737–42. doi: 10.2337/dc09-0288
11. Shi R, Niu Z, Wu B, Hu F. Study on the risk factors for hyperuricaemia and related vascular complications in patients with type 2 diabetes mellitus. Risk Manag Healthc Policy. (2020) 13:1661–75. doi: 10.2147/RMHP.S255042
12. Fennoun H, Haraj N, El Aziz S, Bensbaa S, Chadli A. Risk factors associated with hyperuricemia in patients with diabetes type 2: about 190 cases. Diabetes Research: Open Access. (2020) 2:12–6. doi: 10.36502/droa
13. Rao TMV, Vanukuri NK. A study on serum uric acid levels in type 2 diabetes mellitus and its association with cardiovascular risk factors. IAIM. (2016) 3:148–55.
14. Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat Rev Dis Primers. (2019) 5:42. doi: 10.1038/s41572-019-0092-1
15. Boulton AJM, Armstrong DG, Kirsner RS, Attinger CE, Lavery LA, Lipsky BA, et al. Diagnosis and management of diabetic foot complications. Arlington (VA: American Diabetes Association (2018). doi: 10.2337/db20182-1
16. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. (2017) 40:136–54. doi: 10.2337/dc16-2042
17. Sloan G, Selvarajah D, Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol. (2021) 17:400–20. doi: 10.1038/s41574-021-00496-z
18. Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. (2017) 93:1296–313. doi: 10.1016/j.neuron.2017.02.005
19. Lin X, Xu L, Zhao D, Luo Z, Pan S. Correlation between serum uric acid and diabetic peripheral neuropathy in T2DM patients. J Neurol Sci. (2018) 385:78–82. doi: 10.1016/j.jns.2017.11.034
20. Kazamel M, Stino AM, Smith AG. Metabolic syndrome and peripheral neuropathy. Muscle Nerve. (2021) 63:285–93. doi: 10.1002/mus.27086
21. Braffett BH, Gubitosi-Klug RA, Albers JW, Feldman EL, Martin CL, White NH, et al. Risk factors for diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) Study. Diabetes. (2020) 69:1000–10. doi: 10.2337/db19-1046
22. Liu X, Xu Y, An M, Zeng Q. The risk factors for diabetic peripheral neuropathy: a meta-analysis. PLoS One. (2019) 14:e0212574. doi: 10.1371/journal.pone.0212574
23. Callaghan BC, Gallagher G, Fridman V, Feldman EL. Diabetic neuropathy: what does the future hold? Diabetologia. (2020) 63:891–7. doi: 10.1007/s00125-020-05085-9
24. Cernea S, Raz I. Management of diabetic neuropathy. Metabolism. (2021) 123:154867. doi: 10.1016/j.metabol.2021.154867
25. Callaghan BC, Xia R, Banerjee M, de Rekeneire N, Harris TB. Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care. (2016) 39:801–7. doi: 10.2337/dc16-0081
26. Elafros MA, Andersen H, Bennett DL, Savelieff MG, Viswanathan V, Callaghan BC, et al. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol. (2022) 21:922–36. doi: 10.1016/S1474-4422(22)00188-0
27. Yang K, Wang Y, Li YW, Chen YG, Xing N, Lin HB, et al. Progress in the treatment of diabetic peripheral neuropathy. BioMed Pharmacother. (2022) 148:112717. doi: 10.1016/j.biopha.2022.112717
28. Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. (2019) 7:938–48. doi: 10.1016/S2213-8587(19)30081-6
29. Richette P, Doherty M, Pascual E, Bardin T. SUA levels should not be maintained <3mg/dL for several years. Response to 'EULAR gout treatment guidelines by Richette et al: uric acid and neurocognition by Singh et al'. Ann Rheum Dis. (2018) 77:e21. doi: 10.1136/annrheumdis-2017-211423
30. Kirca M, Oguz N, Cetin A, Uzuner F, Yeşilkaya A. Uric acid stimulates proliferative pathways in vascular smooth muscle cells through the activation of p38 MAPK, p44/42 MAPK and PDGFRβ. J Recept Signal Transduct Res. (2017) 37:167–73. doi: 10.1080/10799893.2016.1203941
31. Zhuang Y, Huang H, Hu X, Zhang J, Cai Q. Serum uric acid and diabetic peripheral neuropathy: a double-edged sword. Acta Neurol Belg. (2023) 123:857–63. doi: 10.1007/s13760-022-01978-1
32. Zhang H, Vladmir C, Zhang Z, Zhou W, Xu J, Zhao W, et al. Serum uric acid levels are related to diabetic peripheral neuropathy, especially for motor conduction velocity of tibial nerve in type 2 diabetes mellitus patients. J Diabetes Res. (2023) 2023:3060013. doi: 10.1155/2023/3060013
33. Zhang Y, Tang Z, Tong L, Wang Y, Li L. Serum uric acid and risk of diabetic neuropathy: a genetic correlation and mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1277984. doi: 10.3389/fendo.2023.1277984
34. Wang W, Ji Q, Ran X, Li C, Kuang H, Yu X, et al. Prevalence and risk factors of diabetic peripheral neuropathy: A population-based cross-sectional study in China. Diabetes Metab Res Rev. (2023) 39:e3702. doi: 10.1002/dmrr.3702
35. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
36. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetc R, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs Institute (2017) 5:217–69.
37. Friedrich JO, Adhikari NK, Beyene J. Ratio of means for analyzing continuous outcomes in meta-analysis performed as well as mean difference methods. J Clin Epidemiol. (2011) 64:556–64. doi: 10.1016/j.jclinepi.2010.09.016
38. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
39. Ioannidis JPA, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. (2007) 335:914–6. doi: 10.1136/bmj.39343.408449.80
40. Migliavaca CB, Stein C, Colpani V, Barker TH, Ziegelmann PK, Munn Z, et al. Meta-analysis of prevalence: I2 statistic and how to deal with heterogeneity. Res Synth Methods. (2022) 13:363–7. doi: 10.1002/jrsm.1547
41. IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. (2016) 6:e010247. doi: 10.1136/bmjopen-2015-010247
42. Han Y, Wang S, Zhao H, Cao Y, Han X, Di H, et al. Lower serum uric acid levels may lower the incidence of diabetic chronic complications in U.S. Adults aged 40 and over. J Clin Med. (2023) 12:725. doi: 10.3390/jcm12020725
43. Uzeli U, Doğan AG. The relationship between diabetic neuropathy and uric acid/high-density lipoprotein ratio in patients with type-2 diabetes mellitus. Cureus. (2023) 15:e45151. doi: 10.7759/cureus.45151
44. Zhang J, Ye L, Bai X, Huang Y, Lin J, Huang H. Cervical and ocular vestibular evoked myogenic potentials in patients with Diabetic Peripheral Neuropathy. Diabetol Metab Syndr. (2023) 15:100. doi: 10.1186/s13098-023-01068-z
45. Fayazi HS, Yaseri M, Mortazavi SS, Sharifhassan Z, Assadinia AS. The relation between serum uric acid levels and diabetic peripheral neuropathy in type 2 diabetes in Guilan, north of Iran. BMC Endocr Disord. (2022) 22:39. doi: 10.1186/s12902-022-00952-5
46. Zhang W, Chen L, Lou M. Association of elevated serum uric acid with nerve conduction function and peripheral neuropathy stratified by gender and age in type 2 diabetes patients. Brain Sci. (2022) 12:1704. doi: 10.3390/brainsci12121704
47. Wu B, Niu Z, Hu F. Study on risk factors of peripheral neuropathy in type 2 diabetes mellitus and establishment of prediction model. Diabetes Metab J. (2021) 45:526–38. doi: 10.4093/dmj.2020.0100
48. Kaewput W, Thongprayoon C, Rangsin R, Jindarat S, Narindrarangkura P, Bathini T, et al. The association between serum uric acid and peripheral neuropathy in patients with type 2 diabetes mellitus: A multicenter nationwide crossSectional study. Korean J Fam Med. (2020) 41:189–94. doi: 10.4082/kjfm.18.0205
49. Jiang TN, Li YF, Huo LL, Zhang Q, Wang LY, Zhao CL, et al. Association between serum uric acid and large-nerve fiber dysfunction in type 2 diabetes: a cross-sectional study. Chin Med J (Engl). (2019) 132:1015–22. doi: 10.1097/CM9.0000000000000223
50. Ranjith KP, Potu B, Anju M, Velladath SU, Hande M. Evaluation and comparison of blood parameters in diabetic patients with and without peripheral neuropathy. J Clin Diagn Res. (2018) 12. doi: 10.7860/JCDR/2018/37075.11842
51. Putri SD, Muhammad H, Fidiana F, Basuki M. Correlation between high serum uric acid levels with occurrence of diabetic peripheral neuropathy in patients with type 2 diabetes mellitus in Soetomo General Hospital. Сахарный диабет. (2018) 21:277–82. doi: 10.14341/DM9590
52. Abraham A, Breiner A, Barnett C, Katzberg HD, Lovblom LE, Perkins BA, et al. Uric acid levels correlate with the severity of diabetic sensorimotor polyneuropathy. J Neurol Sci. (2017) 379:94–8. doi: 10.1016/j.jns.2017.05.053
53. Dar UF, Hasan M, Umar N. Mean serum uric acid levels in type 2 diabetics with and without diabetic peripheral neuropathy. Age (years). (2016) 55:60–5.
54. Kiani J, Habibi Z, Tajziehchi A, Moghimbeigi A, Dehghan A, Azizkhani H. Association between serum uric acid level and diabetic peripheral neuropathy (A case control study). Caspian J Intern Med. (2014) 5:17–21.
55. Chuengsamarn S, Rattanamongkolgul S, Jirawatnotai S. Association between serum uric acid level and microalbuminuria to chronic vascular complications in Thai patients with type 2 diabetes. J Diabetes Complications. (2014) 28:124–9. doi: 10.1016/j.jdiacomp.2013.12.002
56. Papanas N, Katsiki N, Papatheodorou K, Demetriou M, Papazoglou D, Gioka T, et al. Peripheral neuropathy is associated with increased serum levels of uric acid in type 2 diabetes mellitus. Angiology. (2011) 62:291–5. doi: 10.1177/0003319710394164
57. Tapp RJ, Shaw JE, de Courten MP, Dunstan DW, Welborn TA, Zimmet PZ, et al. Foot complications in Type 2 diabetes: an Australian population-based study. Diabetes Med. (2003) 20:105–13. doi: 10.1046/j.1464-5491.2003.00881.x
58. Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diabetes Rep. (2019) 19:86. doi: 10.1007/s11892-019-1212-8
59. Amutha A, Ranjit U, Anjana RM, Shanthi RCS, Rajalakshmi R, Venkatesan U, et al. Clinical profile and incidence of microvascular complications of childhood and adolescent onset type 1 and type 2 diabetes seen at a tertiary diabetes center in India. Pediatr Diabetes. (2021) 22:67–74. doi: 10.1111/pedi.13033
60. An J, Nichols GA, Qian L, Munis MA, Harrison TN, Li Z, et al. Prevalence and incidence of microvascular and macrovascular complications over 15 years among patients with incident type 2 diabetes. BMJ Open Diabetes Res Care. (2021) 9. doi: 10.1136/bmjdrc-2020-001847
61. Aronson R, Chu L, Joseph N, Brown R. Prevalence and risk evaluation of diabetic complications of the foot among adults with type 1 and type 2 diabetes in a large Canadian population (PEDAL study). Can J Diabetes. (2021) 45:588–93. doi: 10.1016/j.jcjd.2020.11.011
62. Dabelea D, Stafford JM, Mayer-Davis EJ, D'Agostino R Jr, Dolan L, Imperatore G, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. (2017) 317:825–35. doi: 10.1001/jama.2017.0686
63. Sun J, Wang Y, Zhang X, Zhu S, He H. Prevalence of peripheral neuropathy in patients with diabetes: A systematic review and meta-analysis. Prim Care Diabetes. (2020). doi: 10.1016/j.pcd.2019.12.005
64. Lu Y, Xing P, Cai X, Luo D, Li R, Lloyd C, et al. Prevalence and risk factors for diabetic peripheral neuropathy in type 2 diabetic patients from 14 countries: estimates of the INTERPRET-DD study. Front Public Health. (2020) 8:534372. doi: 10.3389/fpubh.2020.534372
65. Javed S, Hayat T, Menon L, Alam U, Malik RA. Diabetic peripheral neuropathy in people with type 2 diabetes: too little too late. Diabetes Med. (2020) 37:573–9. doi: 10.1111/dme.14194
66. Yan P, Wan Q, Zhang Z, Tang Q, Wu Y, Xu Y, et al. Decreased physiological serum total bile acid concentrations in patients with type 2 diabetic peripheral neuropathy. Diabetes Metab Syndr Obes. (2021) 14:2883–92. doi: 10.2147/DMSO.S313488
67. Rosenberger DC, Blechschmidt V, Timmerman H, Wolff A, Treede RD. Challenges of neuropathic pain: focus on diabetic neuropathy. J Neural Transm (Vienna). (2020) 127:589–624. doi: 10.1007/s00702-020-02145-7
68. Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). (2010) 62:170–80. doi: 10.1002/acr.20065
69. Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheumatol. (2009) 61:885–92. doi: 10.1002/art.24612
70. Petta S, Cammà C, Cabibi D, Di Marco V, Craxì A. Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. (2011) 34:757–66. doi: 10.1111/apt.2011.34.issue-7
71. Yang T, Chu CH, Bai CH, You SL, Chou YC, Chou WY, et al. Uric acid level as a risk marker for metabolic syndrome: a Chinese cohort study. Atherosclerosis. (2012) 220:525–31. doi: 10.1016/j.atherosclerosis.2011.11.014
72. Gonçalves JP, Oliveira A, Severo M, Santos AC, Lopes C. Cross-sectional and longitudinal associations between serum uric acid and metabolic syndrome. Endocrine. (2012) 41:450–7. doi: 10.1007/s12020-012-9629-8
73. Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW, Kim KC, et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. (2014) 28:3197–204. doi: 10.1096/fj.13-247148
74. Nagahama K, Inoue T, Iseki K, Touma T, Kinjo K, Ohya Y, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res. (2004) 27:835–41. doi: 10.1291/hypres.27.835
75. Krishnan E, Akhras KS, Sharma H, Marynchenko M, Wu EQ, Tawk R, et al. Relative and attributable diabetes risk associated with hyperuricemia in US veterans with gout. QJM. (2013) 106:721–9. doi: 10.1093/qjmed/hct093
76. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. (2012) 11:521–34. doi: 10.1016/S1474-4422(12)70065-0
77. Martinon F. Mechanisms of uric acid crystal-mediated autoinflammation. Immunol Rev. (2010) 233:218–32. doi: 10.1111/j.0105-2896.2009.00860.x
78. Premkumar LS, Pabbidi RM. Diabetic peripheral neuropathy: role of reactive oxygen and nitrogen species. Cell Biochem Biophys. (2013) 67:373–83. doi: 10.1007/s12013-013-9609-5
79. Fabbrini E, Serafini M, Colic Baric I, Hazen SL, Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes. (2014) 63:976–81. doi: 10.2337/db13-1396
80. Stamp LK, Turner R, Khalilova IS, Zhang M, Drake J, Forbes LV, et al. Myeloperoxidase and oxidation of uric acid in gout: implications for the clinical consequences of hyperuricaemia. Rheumatol (Oxford). (2014) 53:1958–65. doi: 10.1093/rheumatology/keu218
81. Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. (2003) 41:1183–90. doi: 10.1161/01.HYP.0000069700.62727.C5
82. Papežíková I, Pekarová M, Kolářová H, Klinke A, Lau D, Baldus S, et al. Uric acid modulates vascular endothelial function through the down regulation of nitric oxide production. Free Radic Res. (2013) 47:82–8.
Keywords: meta-analysis, systematic review, diabetic peripheral neuropathy, hyperuricemia, uric acid
Citation: Zhang X, Zhang X, Li X, Zhao X, Wei G, Shi J, Yang Y, Fan S, Zhao J, Zhu K, Du J, Guo J and Cao W (2024) Association between serum uric acid levels and diabetic peripheral neuropathy in type 2 diabetes: a systematic review and meta-analysis. Front. Endocrinol. 15:1416311. doi: 10.3389/fendo.2024.1416311
Received: 12 April 2024; Accepted: 25 June 2024;
Published: 12 July 2024.
Edited by:
Dhiraj Kumar, National Eye Institute (NIH), United StatesReviewed by:
Dia Advani, Khalifa University, United Arab EmiratesSmita Kumari, The Ohio State University, United States
Copyright © 2024 Zhang, Zhang, Li, Zhao, Wei, Shi, Yang, Fan, Zhao, Zhu, Du, Guo and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Cao, YWNhZGVtaWNfY2Fvd2VpNjZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xieyu Zhang1†
Xieyu Zhang1† Xinwen Zhang
Xinwen Zhang Xiaoxu Li
Xiaoxu Li Guangcheng Wei
Guangcheng Wei Jiahe Zhao
Jiahe Zhao Wei Cao
Wei Cao