
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 17 July 2024
Sec. Cancer Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1414968
This article is part of the Research Topic Endocrine Aspects of Gynaecological Cancers, volume II View all 5 articles
Background: With the increasing use of hormone replacement therapy (HRT), there is a need to understand its impact on the occurrence of female malignant tumors. This systematic review and meta-analysis aimed to assess the risk of ovarian cancer associated with HRT and its related risk factors.
Methods: PUBMED, OVID, Embase, Cochrane, and Web of Science were searched from 1980 to April 2022 to identify studies on the risk of ovarian cancer and hormone replacement therapy. The random-effects model was used to estimate the pooled risk of HRT in ovarian cancer, both in cohort studies and case-control studies. Additionally, the analysis examined the outcomes associated with different types of estrogen plus progesterone regimens. Meta-regression and sensitive analysis were performed to evaluate the heterogeneity.
Results: 21 cohort studies (involving 15,313 cases and 4,564,785 participants) and 30 case-control studies (including 18,738 cases and 57,747 controls) were analyzed. The pooled risks of ovarian cancer for HRT users were 1.20 (95% confidence interval [CI] 1.01–1.44) from cohort studies and 1.13 (95%CI 1.04–1.22) from case-control studies. However, after restricting the study period to recent decades, the significant results indicating a higher risk disappeared in cohort studies conducted after 2010 and in case-control studies conducted after 2006. Furthermore, the continuous use of estrogen-progesterone replacement therapy (EPRT) was associated with a risk comparable to that of sequential use. Subgroup analysis showed that both estrogen replacement treatment (ERT) and EPRT had minor risks; The risk further increased with prolonged exposure time, particularly for durations exceeding 10 years. Additionally, serous ovarian cancer appeared to be more susceptible than other pathological types.
Conclusion: The risk of ovarian cancer associated with HRT has been decreasing over time. However, ERT may increase this risk, particularly when used for an extended period. It is recommended that long-time users consider continuous EPRT as a safer alternative.
Systematic review registration: www.crd.york.ac.uk/prospero/, identifier CRD42022321279.
Ovarian cancer is known as the most lethal disease among malignant tumors affecting the female genital system. It is challenging to treat because most patients are diagnosed at a late stage. Epithelial ovarian cancer encompasses various histologic types, such as serous tumor, mucinous tumor, endometrioid tumor, clear cell tumor, and others. Among them, serous tumor is the most prevalent. Although the exact causes of ovarian cancer are not entirely clear, factors like persistent ovulation and gonadotropin stimulation are often reported as tumor pathogenic factors (1, 2). However, using oral contraceptives, pregnancy, and breastfeeding have been considered as protective factors. In addition, there are other risk factors to be aware of, such as smoking, obesity, and family history.
Hormone replacement therapy has been widely used to treat menopause syndrome in women. The main HRT regimens include estrogen alone or a combination of estrogen and progesterone. It is believed to have cardiovascular benefits and a therapeutic effect on osteoporosis. Women can experience the benefits of HRT long after the menstrual cycle has stopped. However, the optimal time to start therapy is within ten years of menopause or before the age of 60. According to the North American Menopause Society (NAMS), for women who begin hormone therapy more than 10 years after the onset of menopause or who are over 60 years old, the benefit-risk ratio is less favorable due to the higher absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia (3). Initiating HRT in mid-life may protect against cognitive impairment, whereas starting it in late-life could have deleterious effects (4).
However, the Women’s Health Initiative (WHI) study found a higher hazard ratio (HR) of 1.58 for invasive ovarian cancer in users of estrogen plus progestin compared to the control group (5). Some epidemiological studies have found a significant link between HRT and the risk of female cancers, especially breast cancer. However, when it comes to ovarian cancer, the outcomes of these studies have been conflicting. Some researchers have reported an increased risk of ovarian cancer associated with postmenopausal hormone use (6–12), while other studies have found contradictory results (13).
Beyond that, three meta-analyses have also reported conflicting findings regarding the risk of HRT and ovarian cancer (14–16). Therefore, we conducted this current meta-analysis to further investigate the potential association between hormone use and ovarian cancer, taking into consideration the period of research, specific type of hormone and duration of use. Moreover, we aim to examine whether certain histological subtypes of ovarian cancer are more susceptible to being influenced by hormone use.
This study adhered to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Our registration number in the CRD is 42022321279.
Literature from databases including PUBMED, OVID, Embase, Cochrane, and Web of Science published after 1980 was searched to identify relevant studies. We conducted a search using keywords such as “ hormone replacement therapy”, “estrogen replacement therapy” and “non-contracept hormones” in combination with “ovarian cancer” and “ovarian tumor”.
We excluded unrelated studies by checking their title and abstract. Then, we carefully reviewed the remaining articles to ensure they were relevant to our analysis. Finally, we manually scanned the references of previous review articles and meta-analyses to identify any additional published studies.
Each research has been individually checked and reviewed by three authors (HQ.X, LY.W, LP.S). In case of any conflicts, a group discussion would be conducted to resolve them. Our primary screening was based on the titles and abstracts of the research papers.
All the studies included in our analysis should meet the following criteria: (1) prospective or compared retrospective studies. (2) contain data on HRT use and ovarian cancer incidence confirmed by pathological examination. (3) the index of survival analysis, such as relative risk (RR) or odds ratio (OR), along with their corresponding 95% confidence intervals (CI), should be available.
Studies were excluded if the patients did not have a history of malignant ovarian tumor, as well as duplicate data or repeat analysis.
Two authors independently extracted risk data from these studies and conducted our meta-analysis. We also collected additional information such as author, year of publication, country, patients’ characteristics, type and duration of hormone use, cancer type, and adjusted variables in the analysis. The majority of patients had epithelial ovarian cancer, including subtypes such as serous, mucinous, endometrioid, and others.
The article would combine the result to calculate the use of ERT and EPRT based on the provided OR or RR value. If the hormone species were available, the risk estimates would be evaluated based on their grouping. The methods of HRT use were categorized as ERT and EPRT, further classified as continuous or sequential use. The adjusted estimate would be given preference over the unadjusted OR and RR. There were no restrictions on the length of follow-up. Any conflicts in this process would be resolved through group discussion.
We performed this meta-analysis to investigate the correlation between HRT use and the risk of ovarian cancer. We obtained a pooled risk estimate by statistically analyzing the data extracted from each individual study. The results were combined by the DerSimonian and Laird random-effects model.
We assessed the association between HRT use and ovarian cancer risk by comparing patients who had ever used it to those who had never used it. Consider the evolution of HRT regimens in recent years, we specifically analyzed the pooled RR of cohort studies conducted after 2010 and the pooled OR of case-control studies conducted after 2006. We used a random-effects model for analysis and combined the adjusted OR or RR with 95% CIs. To examine the homogeneity of the studies, we used the I2 statistics. The statistically significant heterogeneity was noted when the I2 value exceeded 50% or the P value of Q statistics was less than 0.10. We set the significance level at 0.10 to avoid type II errors.
Sensitivity analyses were conducted to evaluate publication bias using Egger’s regression asymmetry test, considering a P-value less than 0.10 as statistically significant. The results were presented with forest plots, showing each outcome as proportions with 95% confidence intervals (CI). Funnel plot asymmetry was used to assess publication bias, and all procedures were carried out using Stata 12.0 software (STATA Corporation, College Station, TX).
Our primary search policy yielded a total of 4996 citations. We carefully selected the studies that focused on HRT and ovarian cancer by reading their abstracts. After a thorough review, we identified 72 articles that met our criteria. Out of these, we excluded 21 articles for reasons such as: lack of statistical data necessary for calculations, non-compliance with our research design, and outdated versions of the studies.
Ultimately, we found 21 cohort studies (5–7, 9, 10, 17–32) and 30 case-control studies (8, 11, 12, 33–59). The selection and exclusion process of PRISMA flow diagram is shown in Figure 1. Based on the Newcastle-Ottawa Quality Assessment Form, each study got a moderate or high-quality score. The analysis of different designs as cohort studies and case-control studies is as follows.
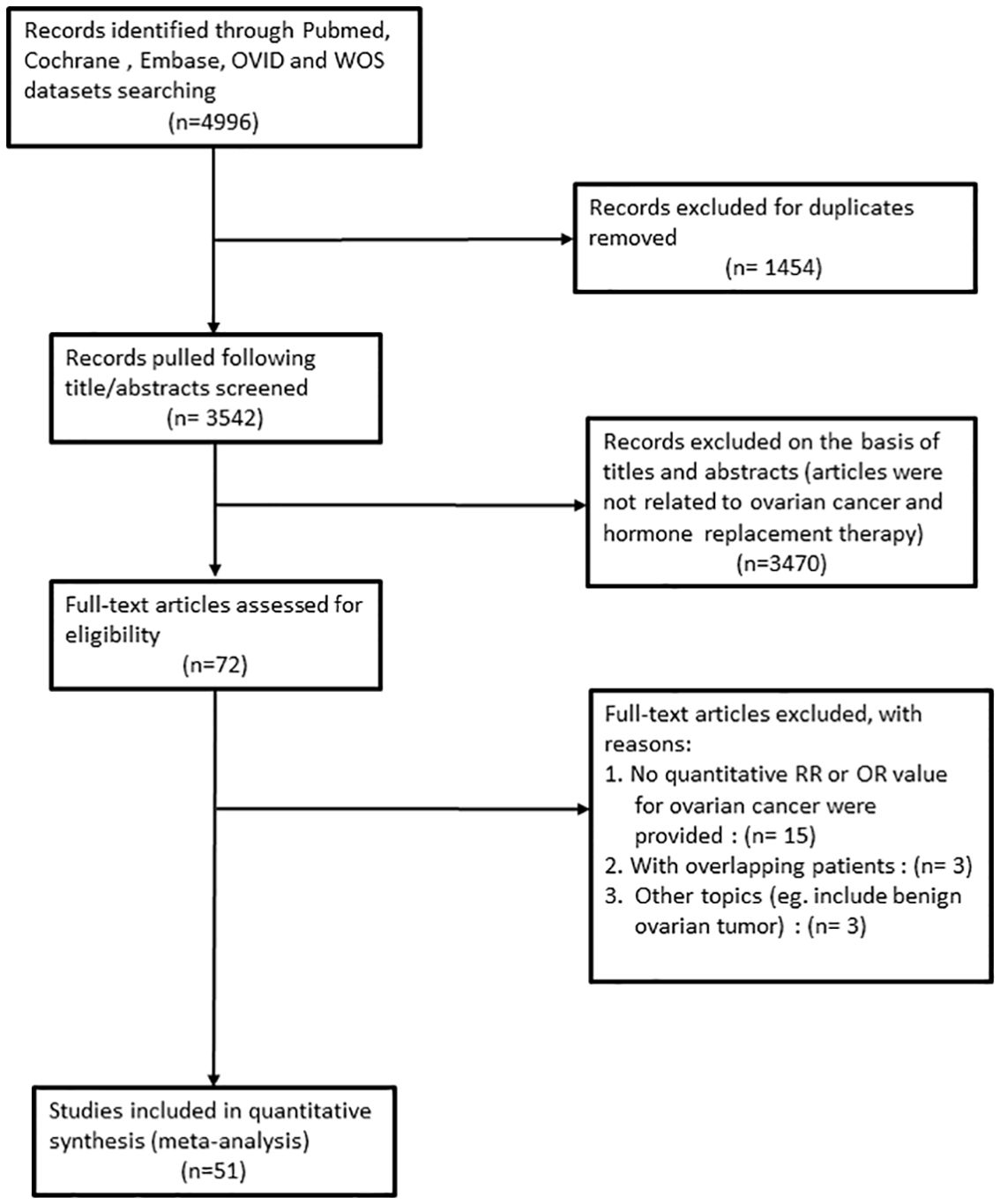
Figure 1 Flowchart of the study selection process according to PRISMA guidelines in this meta-analysis.
In total, there were 4,564,785 participants and 15,313 patients included in 21 cohort studies. The characteristics of the studies are presented in Table 1. The research was conducted in Israel (n=1), several European countries (n=9), and the United States of America (n=11). The participants’ mean age ranged from 25 years to 79 years, and the average follow-up period spanned from 5 years to 26 years. We included thirty case-control studies, which involved 18,738 patients and 57,747 controls. Table 2 shows their characteristics. These studies were conducted in various countries: Australia (n=1), Canada (n=1), China (n=1), Europe (n=11), Mexico (n=1), and the United States of America (n=15). The mean age of the participants ranged from 20 to 79 years, with an average follow-up period of 1 to 13 years.
The individual and summary risk estimates for ovarian cancer with HRT use were presented in Figures 2, 3, classified by different study designs. Cohort studies found a 1.20 (95% CI 1.01–1.44) increased risk in patients with a history of HRT use, while case-control studies showed a 1.13 (95% CI 1.04–1.22) increased risk. The summary result indicated a higher risk in cohort studies. However, after restricting the studies to more recent years, the associated risks became negligible. As shown in Figures 4, 5, the pooled RR of cohort studies conducted after 2010 was 1.15 (95% CI 0.82–1.61), while the pooled OR of case-control studies conducted after 2006 was 1.09 (95% CI 0.93–1.27). Currently, the use of EPRT is more prevalent in HRT than single estrogen to mitigate endometrial stimulation. EPRT can be administered through either continuous or sequential use. As shown in Table 3, we examined nine cohort and case-control studies with data on different EPRT regimens and found that result in continuous hormone use (1.14, 95% CI 1.00–1.31) appeared to be similar with sequential use (1.33, 95% CI 1.13–1.57) (Figure 6).
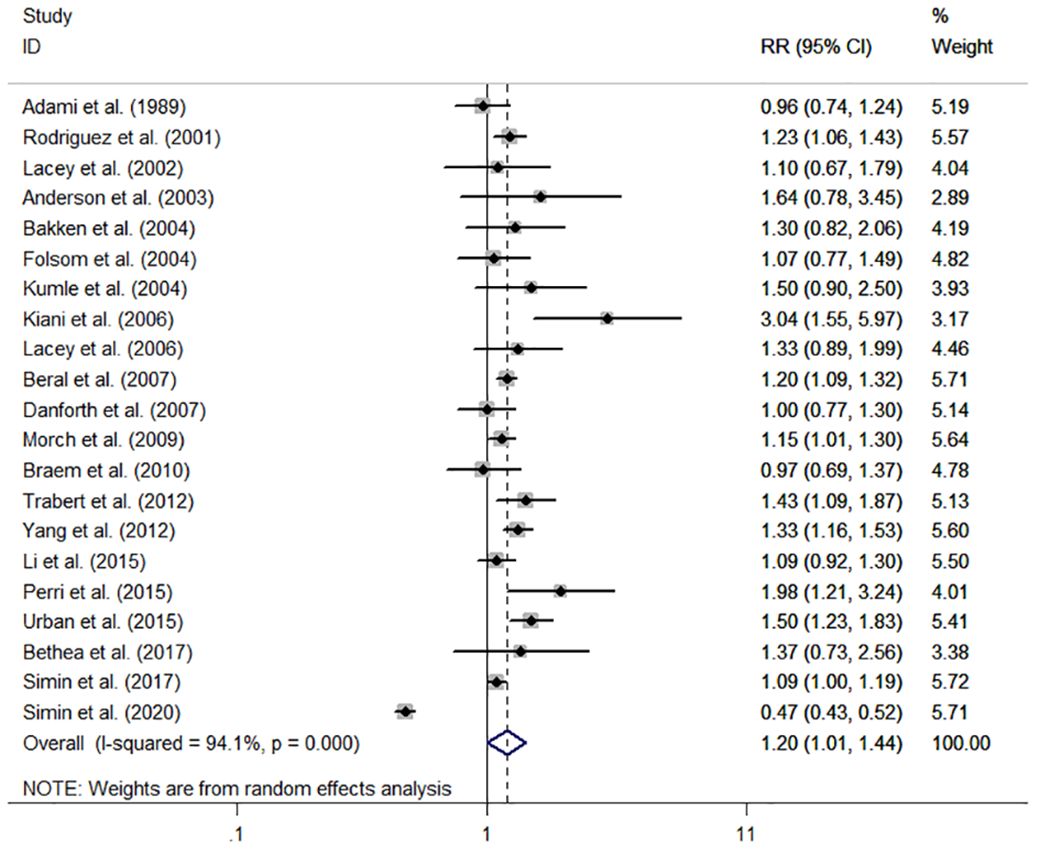
Figure 2 Forest plot of the association between HRT and the risk of ovarian cancer in cohort studies. The size of each gray box is proportional to the weight assigned to the respective study, with horizontal lines representing the 95% confidence intervals (CIs).
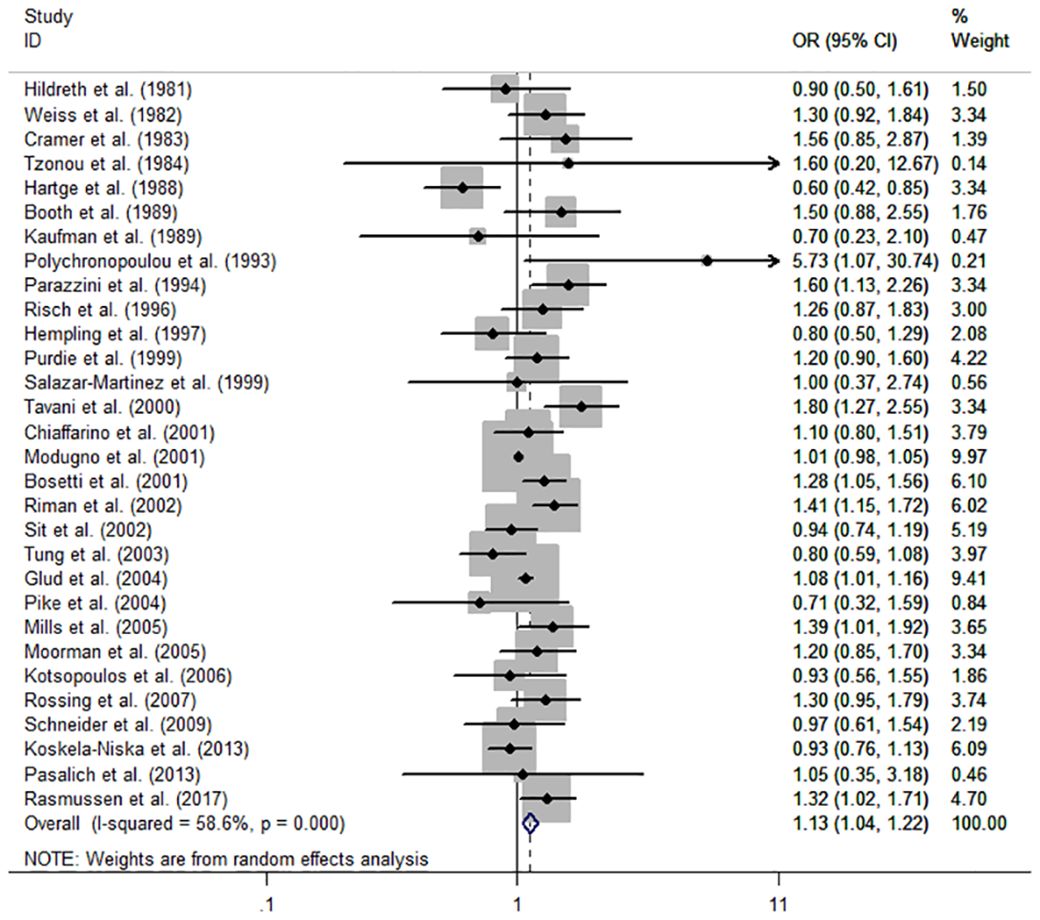
Figure 3 Forest plot of the association between HRT and the risk of ovarian cancer in case-control studies. The size of each gray box is proportional to the weight assigned to the respective study, with horizontal lines representing the 95% confidence intervals (CIs).
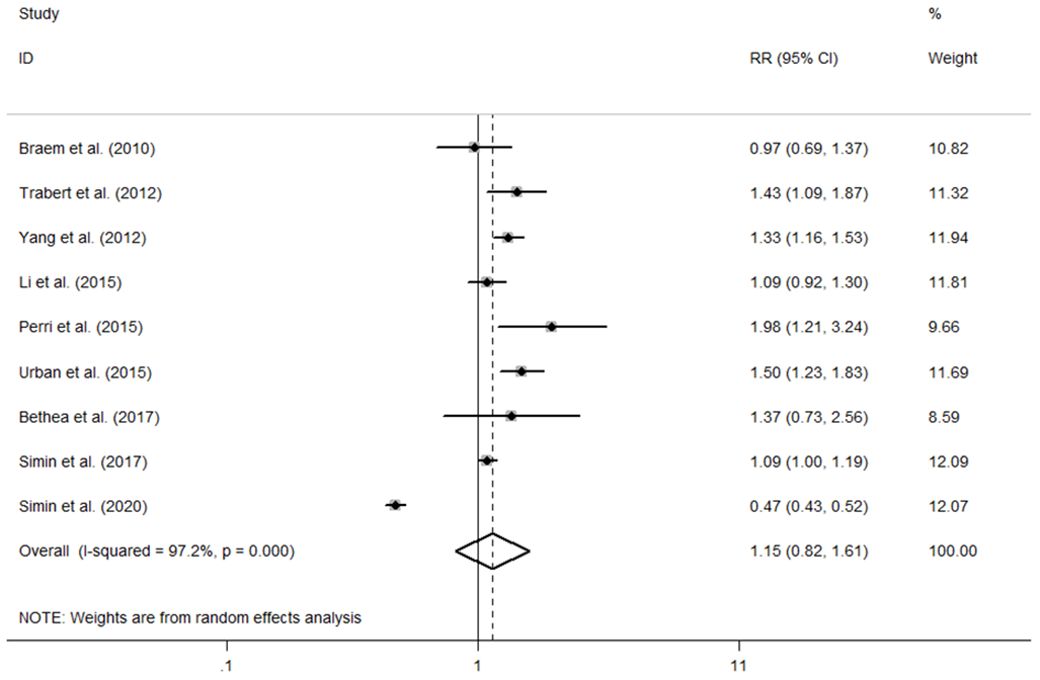
Figure 4 Forest plot of the association between HRT and the risk of ovarian cancer in cohort studies after 2010. The size of each gray box is proportional to the weight assigned to the respective study, with horizontal lines representing the 95% confidence intervals (CIs).
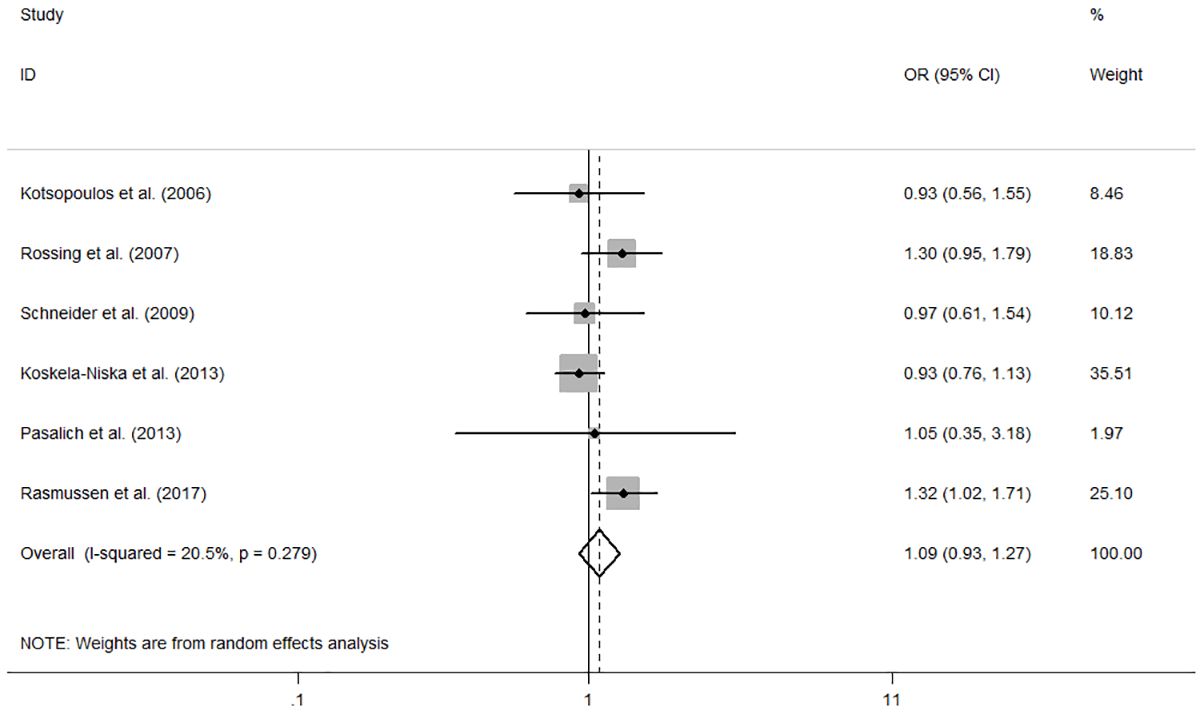
Figure 5 Forest plot of the association between HRT and the risk of ovarian cancer in case-control studies after 2006. The size of each gray box is proportional to the weight assigned to the respective study, with horizontal lines representing the 95% confidence intervals (CIs).
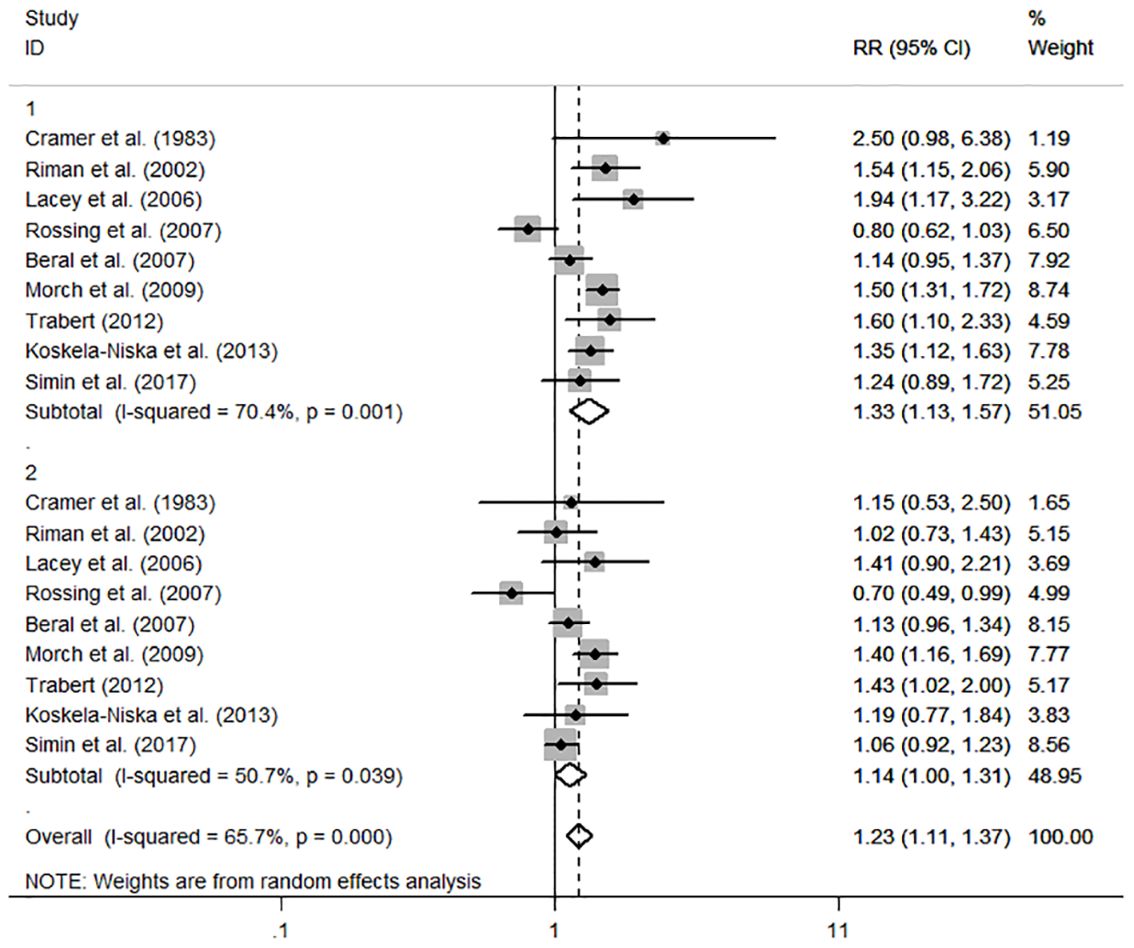
Figure 6 Forest plot of the association between HRT and the risk of ovarian cancer in subgroup analysis stratified by different EPRT regimens. The size of each gray box is proportional to the weight assigned to the respective study, with horizontal lines representing the 95% confidence intervals (CIs).
In subgroup analysis based on hormone types, we examined the effects of estrogen or estrogen plus progesterone respectively. Among eight cohort studies, we conducted a synthetic calculation and found that both users of ERT (RR=1.29, 95%CI 1.15–1.45) and EPRT (RR=1.25, 95%CI 1.11–1.41) had minor risk of ovarian cancer (Table 4). However, in the six case-control studies, the differences were not significant for the ERT (RR=1.34, 95% CI 0.95–1.88) and EPRT (RR=0.95, 95% CI 0.80–1.13) groups (Table 4).
Additionally, the risk was higher for long-term HRT users, especially those using it for more than five years, and even more so for over ten years. In six cohort studies, the summary risk estimates for different durations were 1.07 (95% CI 0.96–1.19) for less than 5 years, 1.39 (95% CI 1.20–1.62) for 5–9 years, and 1.52 (95% CI 1.31–1.77) for more than 10 years. In eleven case-control studies assessing the risk of ovarian cancer for varying hormone durations, the summary risk estimates were 1.04 (95% CI 0.82–1.33) for less than 5 years, 1.13 (95% CI 0.99–1.29) for 5–9 years, and 1.37 (95% CI 1.02–1.85) for more than 10 years. Only the CI for the longest duration crossed 1.0 (Table 4).
The influence of HRT varies among different histological subtypes. In cohort studies, there is a significant association between HRT and serous cancer (RR=1.57, 95% CI 1.43–1.72), as well as in case-control studies (OR=1.17, 95% CI 1.00–1.35). Additionally, the use of HRT is linked to an increased incidence of endometrioid cancer in cohort studies (RR=1.44, 95% CI 1.00–2.06) (Table 4). This may be due to the fact that endometrial cells are more sensitive to estrogen stimulation. Subgroup analysis results are provided in Table 4.
We conducted a multivariate meta-regression to assess the heterogeneity between the studies included. The heterogeneity was found to be moderate in case-control studies and high in cohort studies, with minimal change even after excluding studies with high RR or OR. The covariates examined were study design, publication year, and study region. However, none of them seemed to have an impact on the between-study heterogeneity.
Sensitivity analysis was conducted to determine if any particular study had significant implications for the results (Figures 7A, B). In cohort studies, the pooled RRs ranged from 1.16 (95%CI 0.97–1.39) to 1.22 (95%CI 1.01–1.47); For case-control studies, the pooled ORs varied from 1.11 (95%CI 1.03–1.19) to 1.15 (95%CI 1.70–1.24). No individual study exhibited this effect.
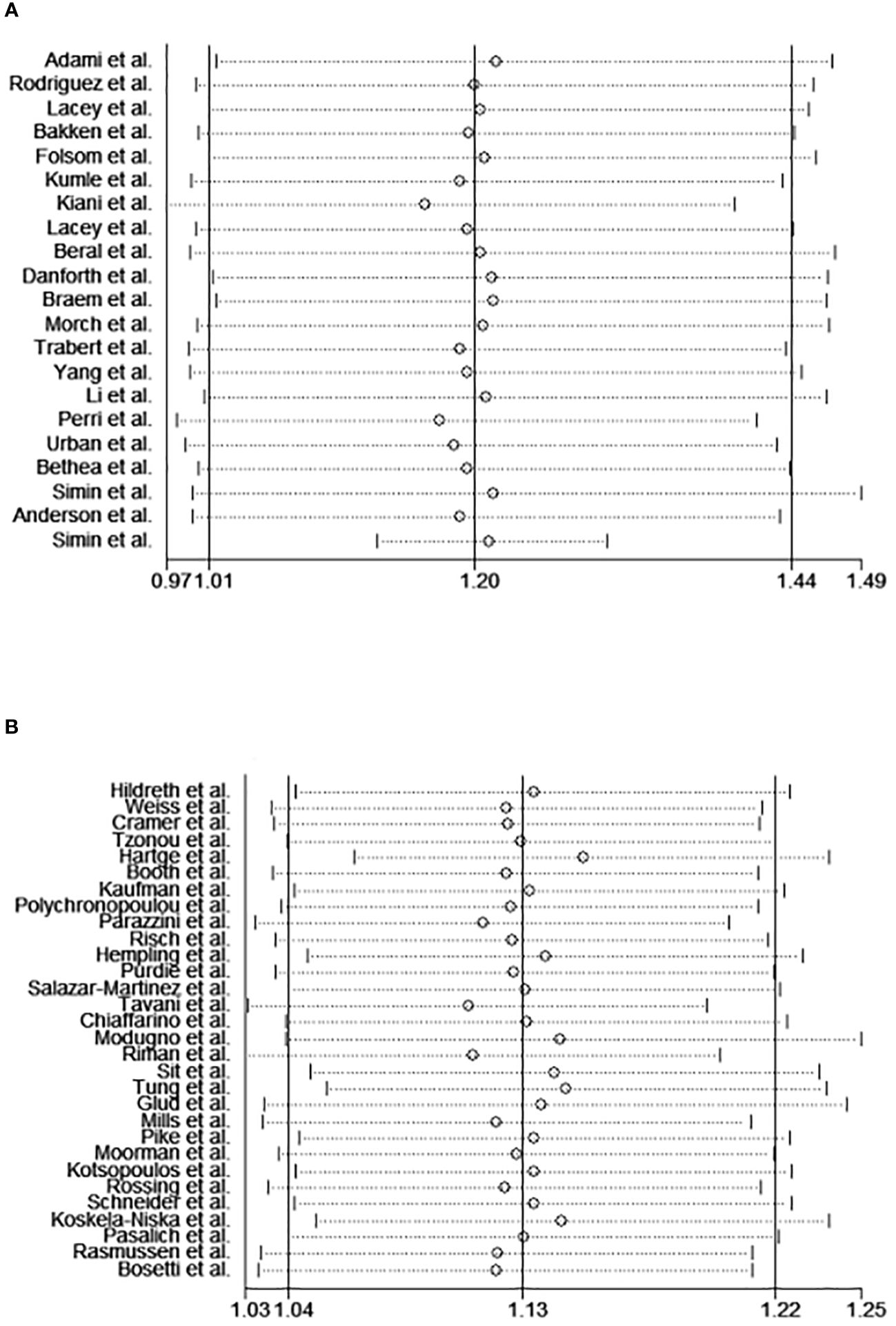
Figure 7 The sensitivity analysis of the meta-analysis of cohort studies (A) and case-control studies (B).
The funnel plot, Begg’s test, and Egger’s test results are displayed in Figures 8A–C. These tests indicated no significant publication bias (Begg’s test P=0.407, Egger’s test P=0.070) in our included articles on the association between HRT and ovarian cancer risk.
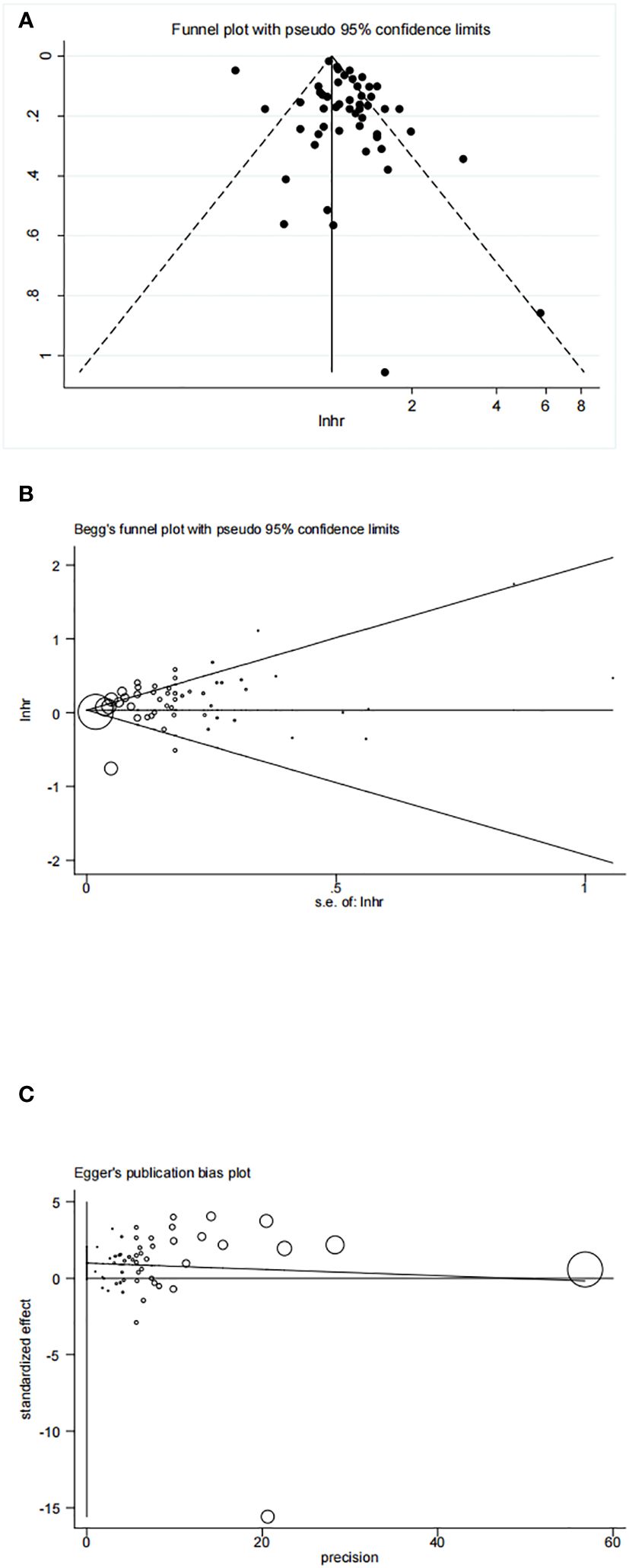
Figure 8 (A) Funnel plot of included studies to assess publications bias; (B) Begg’s test of included studies to assess publications bias; (C) Egger’s test of included studies to assess publications bias.
Previous Meta-analysis have yielded conflicting findings regarding the association between hormone use and ovarian cancer risk. Two studies found no association during the early period (15, 16), while another article published in 1998 reported positive findings (15), but both used the fixed effects model and encountered heterogeneity. In a recent article, the authors suggested a summary risk estimate of 1.29 (95%CI 1.11–1.38) for menopausal HRT. Its results were positive, as the authors selectively highlighted the most significant findings from each included study (60). A meta-analysis of 52 epidemiological studies since 1970 revealed that women initiating hormone therapy for 5 years around age 50 experience approximately one additional case of ovarian cancer per 1000 users (64). In 2016, The International Menopause Society (IMS) noted that the relationship between HRT and ovarian cancer remains unclear. The risks and benefits of hormone therapy varied between women undergoing menopausal transition and older women (65). In 2023, IMS published a practitioner’s toolkit for managing menopause. They suggested thorough evaluation and individually tailored drug regimens to ensure appropriate care for patients (66). The European Menopause and Andropause Society (EMAS) and North American Menopause Society (NAMS) have insufficient evidence linking HRT to ovarian cancer. However, EMAS advises caution regarding hormone therapy in women with serous epithelial ovarian cancer. NAMS emphasizes a level II finding indicating a slight but statistically significant ovarian cancer risk associated with hormone use in observational studies (3, 67).
In this meta-analysis, we aimed to assess the relationship between hormone use and ovarian cancer risk. Our key findings are as follows:
First, previously hormone use increased the risk of ovarian cancer in both cohort studies and case-control studies. However, these effects became trivial when we limited the study period to recent years. These findings imply that advancements in medication and adjustments in administration methods have potentially reduced the risk of HRT on ovarian cancer. Due to the limited number of studies from the past decade included in this research, the results are subject to certain limitations. It is conceivable that the influence of HRT on the incidence of ovarian cancer is declining.
Second, in subgroup analysis of hormone type, only cohort studies manifested that the use of either single estrogen or estrogen plus progesterone use could increase the risk of ovarian cancer, but this finding was not significant in case-control studies. In the WHI trial, Anderson et al. reported a non-significant hazard ratio of 1.64 in EPRT users (5). However, they did not compare the risk between ERT and EPRT, and there was no data available on estrogen alone (68). Some other studies indicated that EPRT had a lower ovarian cancer risk compared to ERT in HRT users (69, 70). This aligns with the findings of our research. The hormone types were classified in 8 cohort studies and 6 case-control studies. The risk of estrogen used alone was higher than when estrogen and progesterone were used together, both in cohort and case-control studies. Our research includes more articles on ERT and EPRT to reinforce this conclusion. Estrogen receptors are present on the surface of both normal ovaries and malignant ovarian tumors (61). The use of HRT in ovarian cancer carries a risk due to its estrogen element. The process of ovulation, including rupture and repair, can stimulate the oncogenesis of epithelial ovarian cells. Oral contraceptives containing both estrogen and progesterone have been shown to reduce the risk of ovarian cancer. Progesterone plays a role in counteracting the effects of estrogen in the proliferation of ovarian cells and can inhibit ovulation through negative feedback on the Hypothalamus-Pituitary-Ovary (H-P-O) axis during menstruation. However, this effect is not present after menopause (61, 62). Estrogens can act through estrogen receptors to regulate various cellular processes in ovarian cancer cells, including proliferation, epithelial-mesenchymal transition (EMT), invasiveness, differentiation, and inflammation (63), while progesterone and its receptor play an anti-tumor role in the development of ovarian cancer (71). Further research is needed to understand the concrete mechanisms of these hormones.
Previous studies have shown that both continuous and sequential hormone therapy (HT) are associated with an increased risk of ovarian cancer. However, these studies did not compare the two types of therapy (72). In our analysis, we found that continuous hormone use had a similar risk of ovarian cancer compared to sequential use. Continuous hormone use involves taking both estrogen and progesterone every day, similar to oral contraceptives. Whether this type of therapy can also protect ovarian cells from malignant transformation is still unknown. Interestingly, all the studies that differentiated EPRT users into continuous and sequential groups suggested that the former group has a lower risk of ovarian cancer. Considering that continuous hormone use with estrogen and progesterone, like oral contraceptives, has a lower cancer risk and sequential EPRT doesn’t have any major advantages, we recommend continuous EPRT treatment as the first choice for those long-time users experiencing perimenopausal syndrome, who do not prioritize their menstruation.
In addition, the overall risk of ovarian cancer did not increase for nonusers who used hormones for less than five years. In our study, we analyzed 11 case-control studies that provided data on long-term HRT use. The risk of using hormones for more than 5 years and 10 years was calculated, resulting in a summary risk of 1.13 (95%CI 0.99–1.29) and 1.37 (95%CI 1.02–1.85) respectively. Additionally, six cohort studies provided risk values for different durations. They revealed a significant risk for users who had been taking hormones for more than five years (RR=1.39, 95%CI 1.20–1.62), with an even higher risk for those exceeding 10 years of usage (RR=1.52, 95%CI 1.31–1.77). This result suggests that the risk of ovarian cancer increases as the duration of HRT use extends. Further evidence is required to support the recommendation of avoiding steroid hormone usage for more than ten years.
At last, in the analysis of histologic subgroups, we observed that serous cancer was more susceptible than other cancer types in both types of research. The cohort studies’ analysis revealed a significant increase in ovarian endometrioid cancer risk with HRT use (RR=1.44 95%CI=1.00, 2.06). For the low incidence of mucinous and clear cell carcinoma, the evidence is not very convincing.
It is necessary to evaluate the heterogeneity between-studies in meta-analysis. Moderate heterogeneity was observed in case-control studies, while cohort studies showed high heterogeneity. However, the published year and study region didn’t contribute to the heterogeneity, as determined by meta-regression analysis. The between-study heterogeneity did not significantly decrease after excluding several studies with remarkably increased or decreased RR values. And there was no significant impact on the results. Therefore, these findings can be considered reliable.
There are some advantages in our study. Firstly, we obtained more accurate and convincing results due to the sufficient data and sample size compared to previous studies. In addition, we extracted ORs and RRs from the original studies encompassing all participants to offer a comprehensive assessment of the risk associated with both all HRT users and recent HRT users. These findings suggest that modern HRT regimens are becoming safer. This can serve as a valuable reference for those considering menopausal HRT. Thirdly, for EPRT users who do not care about menstruation, the continuous pattern could be preferable to the sequential pattern. But this advantages of decreasing the ovarian cancer risk may not that prominant. Furthermore, our conclusion highlights that long-term use of HRT for more than 10 years is associated with a significantly higher risk of ovarian cancer. Moreover, the subgroup analysis revealed a strong relationship between HRT use and serous ovarian cancer, while cohort studies also indicated a higher risk of endometrioid cancer among HRT users. Finally, between-study heterogeneity and sensitivity analyses confirm the stability of our conclusions.
Some limitations exist in our study. Firstly, the study groups and adjusted confounders differ in each research, which may partially affect the results due to these biases. The insufficient follow-up period of some researchers would also miss some potential cases. Additionally, the therapeutic regimen of HRT has evolved over time. In the past, estrogen was commonly used alone to treat the menopausal syndrome. However, nowadays, it is preferred to prescribe a combination of estrogen and progesterone. In this meta-analysis, we prioritize selecting the data on estrogen plus progesterone hormone usage if it is described in the articles. In some studies, there is insufficient accurate data for different types of hormone use, so the summary data of OR or RR represented all hormone users. Thirdly, the relationship between HRT and histologic subtypes lacks strong evidence due to limited data. However, long-term use of HRT has consistently shown higher rates of ovarian cancer in multiple studies. At last, it is important to acknowledge that each study may have inherent biases that could influence the results. And it is also worth noting that positive results are more likely to be published, while negative findings may be overlooked in the literature search process.
In conclusion, our findings suggest that the use of HRT can increase ovarian cancer risk in certain cases. However, when we restricted the study period to the past decade, the associated risk was minimal. Considering the benefits of HRT in managing menopausal symptoms, such as preventing osteoporosis, thromboembolic disease, and climacteric disease, it has a wide range of applications. Individualized prescription of different types of HRT treatments and strict follow-up are crucial in preventing the potential side effects of tumors.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
HX: Writing – original draft, Software, Formal analysis, Data curation. LW: Writing – original draft, Data curation, Conceptualization. LS: Writing – review & editing, Resources, Investigation. SX: Writing – review & editing, Software, Resources, Funding acquisition.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This systematic review is supported by Medical Health Science and Technology Project of Zhejiang Provincial Health Commission: 2021KY883.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fathalla MF. Incessant ovulation–a factor in ovarian neoplasia? Lancet. (1971) 2:163. doi: 10.1016/S0140-6736(71)92335-X
2. Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. (1983) 71:717–21.
3. “The 2022 Hormone Therapy Position Statement of The North American Menopause Society” Advisory Panel. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. (2022) 29:767–94. doi: 10.1097/GME.0000000000002028
4. Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol. (2011) 69:163–9. doi: 10.1002/ana.22239
5. Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SAA, Pettinger M, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women’s Health Initiative randomized trial. JAMA. (2003) 290:1739–48. doi: 10.1001/jama.290.13.1739
6. Beral V, Million Women Study Collaborators, Bull D, Green J, Reeves G. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet. (2007) 369:1703–10. doi: 10.1016/S0140-6736(07)60534-0
7. Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. A prospective study of postmenopausal hormone use and ovarian cancer risk. Br J Cancer. (2007) 96:151–6. doi: 10.1038/sj.bjc.6603527
8. Moorman PG, Schildkraut JM, Calingaert B, Halabi S, Berchuck A. Menopausal hormones and risk of ovarian cancer. Am J Obstet Gynecol. (2005) 193:76–82. doi: 10.1016/j.ajog.2004.11.013
9. Lacey JV, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. (2002) 288:334–41. doi: 10.1001/jama.288.3.334
10. Lacey JV, Brinton LA, Leitzmann MF, Mouw T, Hollenbeck A, Schatzkin A, et al. Menopausal hormone therapy and ovarian cancer risk in the National Institutes of Health-AARP Diet and Health Study Cohort. J Natl Cancer Inst. (2006) 98:1397–405. doi: 10.1093/jnci/djj375
11. Rossing MA, Cushing-Haugen KL, Wicklund KG, Doherty JA, Weiss NS. Menopausal hormone therapy and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. (2007) 16:2548–56. doi: 10.1158/1055-9965.EPI-07-0550
12. Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertil Steril. (2004) 82:186–95. doi: 10.1016/j.fertnstert.2004.03.013
13. Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. (2010) 95:s1–s66. doi: 10.1210/jc.2009-2509
14. Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. IV. The pathogenesis of epithelial ovarian cancer. Collaborative Ovarian Cancer Group. Am J Epidemiol. (1992) 136:1212–20. doi: 10.1093/oxfordjournals.aje.a116429
15. Garg PP, Kerlikowske K, Subak L, Grady D. Hormone replacement therapy and the risk of epithelial ovarian carcinoma: a meta-analysis. Obstet Gynecol. (1998) 92:472–9. doi: 10.1016/S0029-7844(98)00139-2
16. Coughlin SS, Giustozzi A, Smith SJ, Lee NC. A meta-analysis of estrogen replacement therapy and risk of epithelial ovarian cancer. J Clin Epidemiol. (2000) 53:367–75. doi: 10.1016/S0895-4356(99)00179-1
17. Adami HO, Persson I, Hoover R, Schairer C, Bergkvist L. Risk of cancer in women receiving hormone replacement therapy. Int J Cancer. (1989) 44:833–9. doi: 10.1002/ijc.2910440515
18. Rodriguez C, Patel AV, Calle EE, Jacob EJ, Thun MJ. Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. JAMA. (2001) 285:1460–5. doi: 10.1001/jama.285.11.1460
19. Bakken K, Alsaker E, Eggen AE, Lund E. Hormone replacement therapy and incidence of hormone-dependent cancers in the Norwegian Women and Cancer study. Int J Cancer. (2004) 112:130–4. doi: 10.1002/ijc.20389
20. Folsom AR, Anderson JP, Ross JA. Estrogen replacement therapy and ovarian cancer. Epidemiology. (2004) 15:100–4. doi: 10.1097/01.ede.0000091606.31903.8e
21. Kumle M, Weiderpass E, Braaten T, Adami H-O, Lund E, Norwegian-Swedish Women’s Lifestyle and Health Cohort Study. Risk for invasive and borderline epithelial ovarian neoplasias following use of hormonal contraceptives: the Norwegian-Swedish Women’s Lifestyle and Health Cohort Study. Br J Cancer. (2004) 90:1386–91. doi: 10.1038/sj.bjc.6601715
22. Kiani F, Knutsen S, Singh P, Ursin G, Fraser G. Dietary risk factors for ovarian cancer: the Adventist Health Study (United States). Cancer Causes Control. (2006) 17:137–46. doi: 10.1007/s10552-005-5383-z
23. Mørch LS, Løkkegaard E, Andreasen AH, Krüger-Kjaer S, Lidegaard O. Hormone therapy and ovarian cancer. JAMA. (2009) 302:298–305. doi: 10.1001/jama.2009.1052
24. Braem MGM, Onland-Moret NC, van den Brandt PA, Goldbohm RA, Peeters PHM, Kruitwagen RFPM, et al. Reproductive and hormonal factors in association with ovarian cancer in the Netherlands cohort study. Am J Epidemiol. (2010) 172:1181–9. doi: 10.1093/aje/kwq264
25. Trabert B, Wentzensen N, Yang HP, Sherman ME, Hollenbeck A, Danforth KN, et al. Ovarian cancer and menopausal hormone therapy in the NIH-AARP diet and health study. Br J Cancer. (2012) 107:1181–7. doi: 10.1038/bjc.2012.397
26. Yang HP, Trabert B, Murphy MA, Sherman ME, Sampson JN, Brinton LA, et al. Ovarian cancer risk factors by histologic subtypes in the NIH-AARP Diet and Health Study. Int J Cancer. (2012) 131:938–48. doi: 10.1002/ijc.26469
27. Li K, Hüsing A, Fortner RT, Tjønneland A, Hansen L, Dossus L, et al. An epidemiologic risk prediction model for ovarian cancer in Europe: the EPIC study. Br J Cancer. (2015) 112:1257–65. doi: 10.1038/bjc.2015.22
28. Perri T, Lifshitz D, Sadetzki S, Oberman B, Meirow D, Ben-Baruch G, et al. Fertility treatments and invasive epithelial ovarian cancer risk in Jewish Israeli BRCA1 or BRCA2 mutation carriers. Fertil Steril. (2015) 103:1305–12. doi: 10.1016/j.fertnstert.2015.02.011
29. Urban N, Hawley S, Janes H, Karlan BY, Berg CD, Drescher CW, et al. Identifying post-menopausal women at elevated risk for epithelial ovarian cancer. Gynecol Oncol. (2015) 139:253–60. doi: 10.1016/j.ygyno.2015.08.024
30. Bethea TN, Palmer JR, Adams-Campbell LL, Rosenberg L. A prospective study of reproductive factors and exogenous hormone use in relation to ovarian cancer risk among Black women. Cancer Causes Control. (2017) 28:385–91. doi: 10.1007/s10552-016-0840-4
31. Simin J, Tamimi R, Lagergren J, Adami H-O, Brusselaers N. Menopausal hormone therapy and cancer risk: An overestimated risk? Eur J Cancer. (2017) 84:60–8. doi: 10.1016/j.ejca.2017.07.012
32. Simin J, Tamimi RM, Callens S, Engstrand L, Brusselaers N. Menopausal hormone therapy treatment options and ovarian cancer risk: A Swedish prospective population-based matched-cohort study. Int J Cancer. (2020) 147:33–44. doi: 10.1002/ijc.32706
33. Hildreth NG, Kelsey JL, LiVolsi VA, Fischer DB, Holford TR, Mostow ED, et al. An epidemiologic study of epithelial carcinoma of the ovary. Am J Epidemiol. (1981) 114:398–405. doi: 10.1093/oxfordjournals.aje.a113207
34. Weiss NS, Lyon JL, Krishnamurthy S, Dietert SE, Liff JM, Daling JR. Noncontraceptive estrogen use and the occurrence of ovarian cancer. J Natl Cancer Inst. (1982) 68:95–8.
35. Cramer DW, Hutchison GB, Welch WR, Scully RE, Ryan KJ. Determinants of ovarian cancer risk. I. Reproductive experiences and family history. J Natl Cancer Inst. (1983) 71:711–6.
36. Tzonou A, Day NE, Trichopoulos D, Walker A, Saliaraki M, Papapostolou M, et al. The epidemiology of ovarian cancer in Greece: a case-control study. Eur J Cancer Clin Oncol. (1984) 20:1045–52. doi: 10.1016/0277-5379(84)90107-X
37. Hartge P, Hoover R, McGowan L, Lesher L, Norris HJ. Menopause and ovarian cancer. Am J Epidemiol. (1988) 127:990–8. doi: 10.1093/oxfordjournals.aje.a114902
38. Booth M, Beral V, Smith P. Risk factors for ovarian cancer: a case-control study. Br J Cancer. (1989) 60:592–8. doi: 10.1038/bjc.1989.320
39. Kaufman DW, Kelly JP, Welch WR, Rosenberg L, Stolley PD, Warshauer ME, et al. Noncontraceptive estrogen use and epithelial ovarian cancer. Am J Epidemiol. (1989) 130:1142–51. doi: 10.1093/oxfordjournals.aje.a115441
40. Polychronopoulou A, Tzonou A, Hsieh CC, Kaprinis G, Rebelakos A, Toupadaki N, et al. Reproductive variables, tobacco, ethanol, coffee and somatometry as risk factors for ovarian cancer. Int J Cancer. (1993) 55:402–7. doi: 10.1002/ijc.2910550312
41. Parazzini F, La Vecchia C, Negri E, Villa A. Estrogen replacement therapy and ovarian cancer risk. Int J Cancer. (1994) 57:135–6. doi: 10.1002/ijc.2910570124
42. Risch HA. Estrogen replacement therapy and risk of epithelial ovarian cancer. Gynecol Oncol. (1996) 63:254–7. doi: 10.1006/gyno.1996.0315
43. Hempling RE, Wong C, Piver MS, Natarajan N, Mettlin CJ. Hormone replacement therapy as a risk factor for epithelial ovarian cancer: results of a case-control study. Obstet Gynecol. (1997) 89:1012–6. doi: 10.1016/S0029-7844(97)00118-X
44. Purdie DM, Bain CJ, Siskind V, Russell P, Hacker NF, Ward BG, et al. Hormone replacement therapy and risk of epithelial ovarian cancer. Br J Cancer. (1999) 81:559–63. doi: 10.1038/sj.bjc.6690731
45. Salazar-Martinez E, Lazcano-Ponce EC, Gonzalez Lira-Lira G, Escudero-De los Rios P, Salmeron-Castro J, Hernandez-Avila M. Reproductive factors of ovarian and endometrial cancer risk in a high fertility population in Mexico. Cancer Res. (1999) 59:3658–62.
46. Tavani A, Ricci E, La Vecchia C, Surace M, Benzi G, Parazzini F, et al. Influence of menstrual and reproductive factors on ovarian cancer risk in women with and without family history of breast or ovarian cancer. Int J Epidemiol. (2000) 29:799–802. doi: 10.1093/ije/29.5.799
47. Chiaffarino F, Pelucchi C, Parazzini F, Negri E, Franceschi S, Talamini R, et al. Reproductive and hormonal factors and ovarian cancer. Ann Oncol. (2001) 12:337–41. doi: 10.1023/A:1011128408146
48. Bosetti C, Negri E, Franceschi S, Trichopoulos D, Beral V, La Vecchia C. Relationship between postmenopausal hormone replacement therapy and ovarian cancer. JAMA. (2001) 285:3089. doi: 10.1001/jama.285.24.3089
49. Modugno F, Ness RB, Wheeler JE. Reproductive risk factors for epithelial ovarian cancer according to histologic type and invasiveness. Ann Epidemiol. (2001) 11:568–74. doi: 10.1016/S1047-2797(01)00213-7
50. Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, et al. Risk factors for invasive epithelial ovarian cancer: results from a Swedish case-control study. Am J Epidemiol. (2002) 156:363–73. doi: 10.1093/aje/kwf048
51. Sit ASY, Modugno F, Weissfeld JL, Berga SL, Ness RB. Hormone replacement therapy formulations and risk of epithelial ovarian carcinoma. Gynecol Oncol. (2002) 86:118–23. doi: 10.1006/gyno.2002.6746
52. Tung K-H, Goodman MT, Wu AH, McDuffie K, Wilkens LR, Kolonel LN, et al. Reproductive factors and epithelial ovarian cancer risk by histologic type: a multiethnic case-control study. Am J Epidemiol. (2003) 158:629–38. doi: 10.1093/aje/kwg177
53. Glud E, Kjaer SK, Thomsen BL, Høgdall C, Christensen L, Høgdall E, et al. Hormone therapy and the impact of estrogen intake on the risk of ovarian cancer. Arch Intern Med. (2004) 164:2253–9. doi: 10.1001/archinte.164.20.2253
54. Mills PK, Riordan DG, Cress RD, Goldsmith DF. Hormone replacement therapy and invasive and borderline epithelial ovarian cancer risk. Cancer Detect Prev. (2005) 29:124–32. doi: 10.1016/j.cdp.2004.11.002
55. Kotsopoulos J, Lubinski J, Neuhausen SL, Lynch HT, Rosen B, Ainsworth P, et al. Hormone replacement therapy and the risk of ovarian cancer in BRCA1 and BRCA2 mutation carriers. Gynecol Oncol. (2006) 100:83–8. doi: 10.1016/j.ygyno.2005.07.110
56. Schneider C, Jick SS, Meier CR. Risk of gynecological cancers in users of estradiol/dydrogesterone or other HRT preparations. Climacteric. (2009) 12:514–24. doi: 10.3109/13697130903075352
57. Koskela-Niska V, Pukkala E, Lyytinen H, Ylikorkala O, Dyba T. Effect of various forms of postmenopausal hormone therapy on the risk of ovarian cancer–a population-based case control study from Finland. Int J Cancer. (2013) 133:1680–8. doi: 10.1002/ijc.28167
58. Pasalich M, Su D, Binns CW, Lee AH. Reproductive factors for ovarian cancer in southern Chinese women. J Gynecol Oncol. (2013) 24:135–40. doi: 10.3802/jgo.2013.24.2.135
59. Rasmussen ELK, Hannibal CG, Dehlendorff C, Baandrup L, Junge J, Vang R, et al. Parity, infertility, oral contraceptives, and hormone replacement therapy and the risk of ovarian serous borderline tumors: A nationwide case-control study. Gynecol Oncol. (2017) 144:571–6. doi: 10.1016/j.ygyno.2017.01.002
60. Liu Y, Ma L, Yang X, Bie J, Li D, Sun C, et al. Menopausal hormone replacement therapy and the risk of ovarian cancer: A meta-analysis. Front Endocrinol (Lausanne). (2019) 10:801. doi: 10.3389/fendo.2019.00801
61. Lau KM, Mok SC, Ho SM. Expression of human estrogen receptor-alpha and -beta, progesterone receptor, and androgen receptor mRNA in normal and Malignant ovarian epithelial cells. Proc Natl Acad Sci U.S.A. (1999) 96:5722–7. doi: 10.1073/pnas.96.10.5722
62. Mukherjee K, Syed V, Ho S-M. Estrogen-induced loss of progesterone receptor expression in normal and Malignant ovarian surface epithelial cells. Oncogene. (2005) 24:4388–400. doi: 10.1038/sj.onc.1208623
63. Kozieł MJ, Piastowska-Ciesielska AW. Estrogens, estrogen receptors and tumor microenvironment in ovarian cancer. Int J Mol Sci. (2023) 24:14673. doi: 10.3390/ijms241914673
64. Collaborative Group On Epidemiological Studies Of Ovarian Cancer, Beral V, Gaitskell K, Hermon C, Moser K, Reeves G, et al. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet. (2015) 385:1835–42. doi: 10.1016/S0140-6736(14)61687-1
65. Baber RJ, Panay N, Fenton A, IMS Writing Group. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric. (2016) 19:109–50. doi: 10.3109/13697137.2015.1129166
66. Davis SR, Taylor S, Hemachandra C, Magraith K, Ebeling PR, Jane F, et al. The 2023 practitioner’s toolkit for managing menopause. Climacteric. (2023) 26:517–36. doi: 10.1080/13697137.2023.2258783
67. Rees M, Angioli R, Coleman RL, Glasspool R, Plotti F, Simoncini T, et al. European Menopause and Andropause Society (EMAS) and International Gynecologic Cancer Society (IGCS) position statement on managing the menopause after gynecological cancer: focus on menopausal symptoms and osteoporosis. Maturitas. (2020) 134:56–61. doi: 10.1016/j.maturitas.2020.01.005
68. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SAA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. (2004) 291:1701–12. doi: 10.1001/jama.291.14.1701
69. Zhou B, Sun Q, Cong R, Gu H, Tang N, Yang L, et al. Hormone replacement therapy and ovarian cancer risk: a meta-analysis. Gynecol Oncol. (2008) 108:641–51. doi: 10.1016/j.ygyno.2007.12.003
70. Pearce CL, Chung K, Pike MC, Wu AH. Increased ovarian cancer risk associated with menopausal estrogen therapy is reduced by adding a progestin. Cancer. (2009) 115:531–9. doi: 10.1002/cncr.23956
71. Borella F, Fucina S, Mangherini L, Cosma S, Carosso AR, Cusato J, et al. Hormone receptors and epithelial ovarian cancer: recent advances in biology and treatment options. Biomedicines. (2023) 11:2157. doi: 10.3390/biomedicines11082157
Keywords: ovarian cancer, cancer risk, hormone replacement therapy, systematic review, meta-analysis
Citation: Xiang H, Wang L, Sun L and Xu S (2024) The risk of ovarian cancer in hormone replacement therapy users: a systematic review and meta-analysis. Front. Endocrinol. 15:1414968. doi: 10.3389/fendo.2024.1414968
Received: 09 April 2024; Accepted: 28 June 2024;
Published: 17 July 2024.
Edited by:
Maria Magdalena Montt-Guevara, University of Pisa, ItalyReviewed by:
Ahmet Fatih Durmusoglu, Istanbul Medipol University, TürkiyeCopyright © 2024 Xiang, Wang, Sun and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Song Xu, d2h4dXNvbmdAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.