
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 14 June 2024
Sec. Systems Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1413690
Objectives: The relationship between adiposity and sepsis has received increasing attention. This study aims to explore the causal relationship between life course adiposity and the sepsis incidence.
Methods: Mendelian randomization (MR) method was employed in this study. Instrumental variants were obtained from genome-wide association studies for life course adiposity, including birth weight, childhood body mass index (BMI), childhood obesity, adult BMI, waist circumference, visceral adiposity, and body fat percentage. A meta-analysis of genome-wide association studies for sepsis including 10,154 cases and 454,764 controls was used in this study. MR analyses were performed using inverse variance weighted, MR Egger regression, weighted median, weighted mode, and simple mode. Instrumental variables were identified as significant single nucleotide polymorphisms at the genome-wide significance level (P < 5×10-8). The sensitivity analysis was conducted to assess the reliability of the MR estimates.
Results: Analysis using the MR analysis of inverse variance weighted method revealed that genetic predisposition to increased childhood BMI (OR = 1.29, P = 0.003), childhood obesity (OR = 1.07, P = 0.034), adult BMI (OR = 1.38, P < 0.001), adult waist circumference (OR = 1.01, P = 0.028), and adult visceral adiposity (OR = 1.53, P < 0.001) predicted a higher risk of sepsis. Sensitivity analysis did not identify any bias in the MR results.
Conclusion: The results demonstrated that adiposity in childhood and adults had causal effects on sepsis incidence. However, more well-designed studies are still needed to validate their association.
Sepsis, defined as life-threatening organ dysfunction resulting from a dysregulated host response to infection, poses a major healthcare challenge (1). There are more than 48 million reported cases of sepsis worldwide annually, accompanied by a mortality rate ranging from 22.5% to 35.0%, contributing to approximately one in five of all global deaths (2–4). And the survivors of sepsis often experience post-intensive care syndrome and chronic physical disabilities. Although infection (including bacteria, fungi, viruses, etc.) is the direct cause of sepsis, many factors such as diabetes, hypertension, neurological disorders, obesity, etc., also contribute to an increased risk of sepsis (5–7). Due to the rapid onset and progression of sepsis, primary prevention targeting the risk factors for sepsis becomes even more important.
Adiposity, characterized by being overweight or obese, has emerged as a global health problem. Current estimates suggest a global obesity prevalence of about 38%, expected to rise to 51% by 2035 (8). Adiposity, has been proven to be associated with cardiovascular diseases, diabetes, cancer, and other conditions (9–11). A survey indicates that up to 60% of patients with sepsis in the ICU are overweight or obese (12). In recent years, the impact of obesity on sepsis has increasingly drawn researchers’ interest. On the one hand, some observational studies have found that obesity is associated with an increased risk of infection, including COVID-19, severe influenza, and surgical site infections (13–15). On the other hand, other studies have found that patients with sepsis who are obese have relatively higher survival rates (16–20). Additionally, population-based studies have reported increased overall mortality due to sepsis in obese individuals (21, 22). These seemingly contradictory findings highlight the complex impact of obesity on sepsis under different pathophysiological conditions. Consequently, further research is imperative to elucidate the relationship between obesity and sepsis, offering fresh perspectives and insights for the prevention and treatment of this condition.
Currently, there are several issues with research on obesity and sepsis. Firstly, most studies on these two conditions are observational, which are often affected by potential biases, confounding factors, and reverse causality, leading to unreliable conclusions. And ethical concerns prevent the conduct of randomized controlled trials to definitively establish the impact of obesity on sepsis. Secondly, many studies currently use BMI as the indicator of obesity. However, besides BMI, the degree of adiposity can also be measured by waist circumference (WC), visceral adiposity, body fat percentage (BFP), and other indicators. Finally, but most importantly, the literature often relies on cross-sectional data at the time of onset of illness, while obesity status can change over time. The issue of adiposity transcends various life stages. Studying the relationship between adiposity during the life course and sepsis incidence helps to understand underlying mechanisms and formulate preventive measures.
Due to ethical constraints, conducting randomized controlled trials (RCTs) to study the relationship between adiposity and sepsis incidence is limited. This research employed a two-sample Mendelian randomization (MR) study and aimed to identify the causal effects of life course adiposity on sepsis incidence. The MR design method leverages publicly available datasets of risk factors and diseases obtained from comprehensive genome-wide association studies (GWAS) to assess relationship between exposures and outcomes. As an epidemiological approach, MR studies utilize single nucleotide polymorphisms (SNPs) randomly assigned during conception as an instrumental variable, offering a robust method for inferring causal relationships similar to randomized controlled trials (RCTs) (23). This approach helps mitigate the influence of confounding factors and reverse causality, providing valuable insights into the nuanced relationship between obesity indicators and sepsis risk across the life cycle.
We conducted a two-sample MR study to investigate the causal relationship between life course adiposity and sepsis. Life course adiposity was categorized into three stages: newborns, childhood, and adults. Corresponding genetic traits from published GWAS were chosen for each stage: birth weight (BW) for the newborn stage; childhood BMI and childhood obesity for the child stage; adult BMI, adult WC, adult visceral adiposity, and adult BFP for the adult stage. The overview of the study design is shown in Figure 1. The Mendelian randomization design relies on three fundamental assumptions: (1) genetic variation exhibits a strong association with the exposure of interest; (2) genetic variation remains independent of any other potential confounding variables; (3) genetic variation solely influences the outcome through its impact on the investigated exposure. As our study relied on publicly available databases and previously published studies, no additional ethics approval was required.
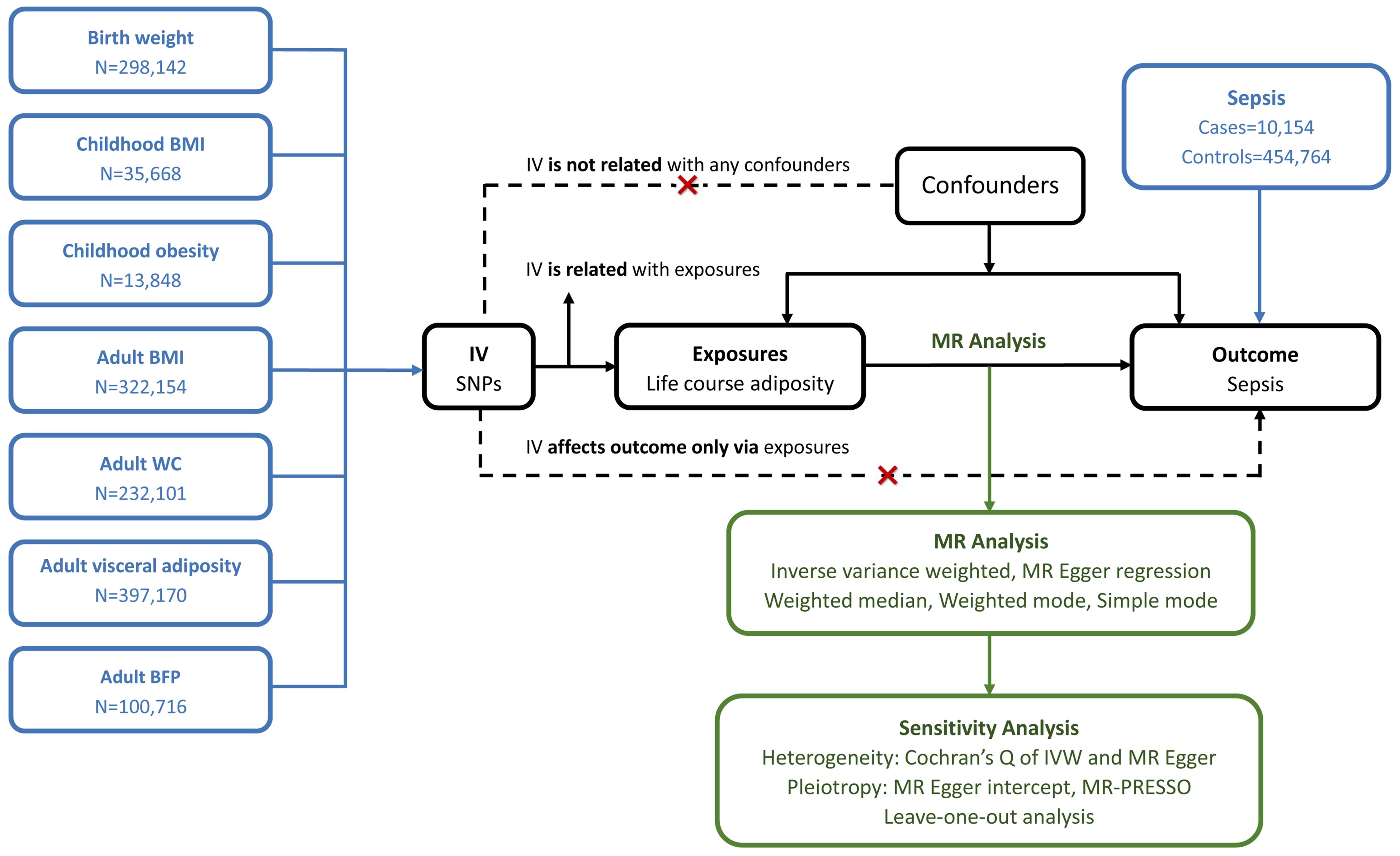
Figure 1 Principles of Mendelian randomization. BMI, body mass index; WC, waist circumference; BFP, body fat percentage; IV, inverse variance; MR, Mendelian randomization; IVW, inverse variance weighted; MR-PRESSO, Mendelian randomization pleiotropy residual sum and outline.
We conducted a search for GWASs related to these traits and identified eight recently published datasets characterized by large sample sizes and comprehensive information on genetic variants. The basic characteristics of these GWASs are described in Table 1.
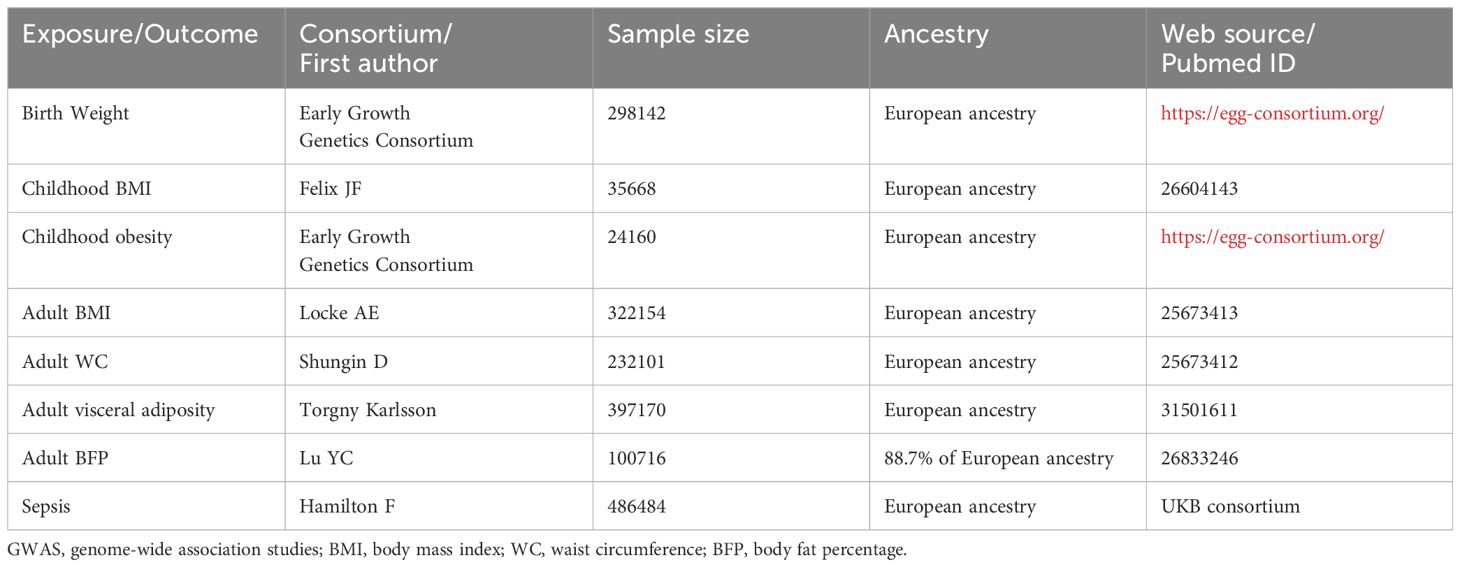
Table 1 Basic characteristics of included GWAS of birth weight, childhood BMI, childhood obesity, adult BMI, adult WC, adult visceral adiposity, adult BFP, and sepsis.
The genetic variants associated with BW were extracted from the Early Growth Genetics Consortium, which involved 298,142 individuals of European descent (24). Birth weight, defined as the weight of a neonate measured within the first hours of life, is recorded immediately after birth and before any substantial postnatal weight loss occurs.
For childhood BMI, genetic variants were extracted from a GWAS meta-analysis involving 35,668 children of European ancestry aged between 2 and 10 years (25). BMI, a numerical value derived from an individual’s height and weight, is calculated as the individual’s mass in kilograms divided by the square of their height in meters (kg/m²).
The genetic variants associated with childhood obesity were obtained from the Early Growth Genetics Consortium, involving 13,848 children aged between 2 and 18 years, with childhood obesity defined as being at or above the 95th percentile of BMI (26).
Regarding adulthood phenotypes, genetic variants for adult BMI, adult WC, adult visceral adiposity, and adult BFP were obtained from several GWAS meta-analysis. The genetic variants for adult BMI were sourced from a GWAS and Metabochip meta-analysis conducted by Locke AE et al., involving a total of 322,154 European individuals (27).
The genetic variants for adult WC were drawn from a genome-wide association meta-analyses that included 232,101 individuals (28). The WC refers to a direct anthropometric measurement representing the distance around the smallest area below the rib cage and above the iliac crest, measured at the level of the navel.
Data on visceral adiposity were obtained from a GWAS performed by Torgny Karlsson et al. with 397,170 European individuals, and visceral adiposity levels were estimated using prediction models (29).
The genetic variants for adult BFP were extracted from a genome-wide association meta-analysis involving 100,716 individuals from 56 studies, with 88.7% of the participants having European ancestry. The BFP in each study was measured either with bioimpedance analysis or dual energy X-ray absorptiometry (30).
The GWAS data of sepsis were obtained from the UKB consortium, which included 10154 sepsis cases and 454764 controls of European ancestry. Explicit sepsis is defined in our study referring to a previously published list International Classification of Disease-9 and Disease-10 codes derived by a panel of experts in critical care, infectious diseases, and sepsis epidemiology.
Our study employs following criteria for the selection of instrumental variables: (1) Significant SNPs associated with BW, childhood BMI, childhood obesity, adult BMI, adult WC, adult visceral adiposity, and adult BFP at the genome-wide significance level (P < 5×10-8) were identified as instrumental variables in our study. These SNPs were further refined by pruning at linkage disequilibrium with an r2 threshold of <0.001 within a 10Mb distance. (2) The strength of chosen instrumental variables was evaluated using F-statistics (F > 10) to mitigate potential bias arising from weak instruments. F-statistics were computed using the formula R² × (n - k - 1)/k (1 - R²), where n, k, and R² represent the sample size, the number of SNPs, and the variance explained by the instrumental variables, respectively. (3) SNPs that were disqualified from the MR analysis during the harmonization process due to being palindromic or containing incompatible alleles were systematically excluded. This meticulous step was taken to ensure the concordance of effect alleles. (4) The selected instrumental variables were verified to be non-associated with both the outcome variable and any potential confounders, ensuring the robustness of the instrument selection.
To estimate the causal effect of life course adiposity on sepsis, we utilized the aforementioned SNPs for MR analysis. Our study employed five distinct MR analysis methods: inverse variance weighted (IVW), MR Egger regression, weighted median, weighted mode, and simple mode. In the IVW meta-analysis approach, the outcome effects of instrumental variables on exposure effects are transformed into a weighted regression, with a fixed intercept at zero. This technique relies on the assumption of the absence of horizontal pleiotropy and seeks to produce unbiased estimates by minimizing the impact of confounding factors. MR Egger accounts for potential pleiotropy in estimating causal effects by utilizing an intercept term. By assigning weights to SNPs, the weighted median method provides a robust estimate even when up to 50% of the weight comes from invalid instruments. The weighted mode method demonstrates adaptability in cases where the genetic variable challenges the pleiotropy hypothesis. Similar to the weighted mode, the simple mode method may offer robustness against pleiotropy. The simple mode method identifies the mode of the distribution but assigns equal weight to each estimate. This comprehensive approach enhances the reliability of our findings.
We performed a sensitivity analysis of the MR results. The heterogeneity between instrumental variables was analyzed by Cochran’s Q test, including IVW and MR Egger. We utilized IVW as the major approach, which combined Wald ratios across the exposure -associated SNPs to get a summary estimate. MR Egger intercept and Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO) were performed to evaluate horizontal pleiotropy. In the final stage, we executed a leave-one-out analysis to meticulously assess the impact of individual SNPs on MR estimates.
The results are displayed in the form of odds ratios (ORs) and 95% confidence intervals (CIs). A Bonferroni-corrected threshold calculated as 0.007 (0.05/7 exposure phenotypes) was employed to address multiple comparisons. P-values lower than 0.007 can indicate a confirmed causal link. P-values between 0.007 and 0.05 were considered indicative proof of an underlying association. P-values greater than 0.05 implied a lack of causal relationship. All analyses were conducted using the “TwoSampleMR” package in R software (version 4.2.1).
After quality control, 128 SNPs for birth weight, 14 SNPs for childhood BMI, 14 SNPs for childhood obesity, 53 SNPs for adult BMI, 31 SNPs for adult WC, 175 SNPs for adult visceral adiposity and 7 SNPs for adult BFP were confirmed for MR analysis. All instrumental variables with their beta effects and standard errors (SE) associated with life course adiposity and sepsis were listed in Supplementary Table S1.
Five Mendelian randomization methods, including IVW, MR Egger regression, weighted median, weighted mode, and simple mode, were employed to investigate the causal effects of life course adiposity on the risk of sepsis. Analysis using the IVW method revealed that genetic predisposition to increased childhood BMI (OR = 1.29, 95% CI = 1.09–1.53, P = 0.003), childhood obesity (OR = 1.07, 95% CI = 1.00–1.13, P = 0.034), adult BMI (OR = 1.38, 95% CI = 1.17–1.64, P < 0.001), adult WC (OR= 1.01, 95% CI = 1.00–1.02, P = 0.028), and adult visceral adiposity (OR = 1.53, 95% CI = 1.37–1.71, P < 0.001) predicted a higher risk of sepsis. However, there was no evidence of causal effects for BW (OR = 0.76, 95% CI = 0.86–1.09, P = 0.584) and adult BFP (OR = 1.36, 95% CI = 0.93–1.98, P = 0.112) on sepsis. Similar direction and magnitude of causal inference between BW, childhood BMI, childhood obesity, adult BMI, adult WC, adult visceral adiposity, adult BFP and sepsis were observed using the MR-Egger, weighted median model, weighted mode, and simple mode analysis, reinforcing the reliability of our results (Figures 2, 3, Table 2).
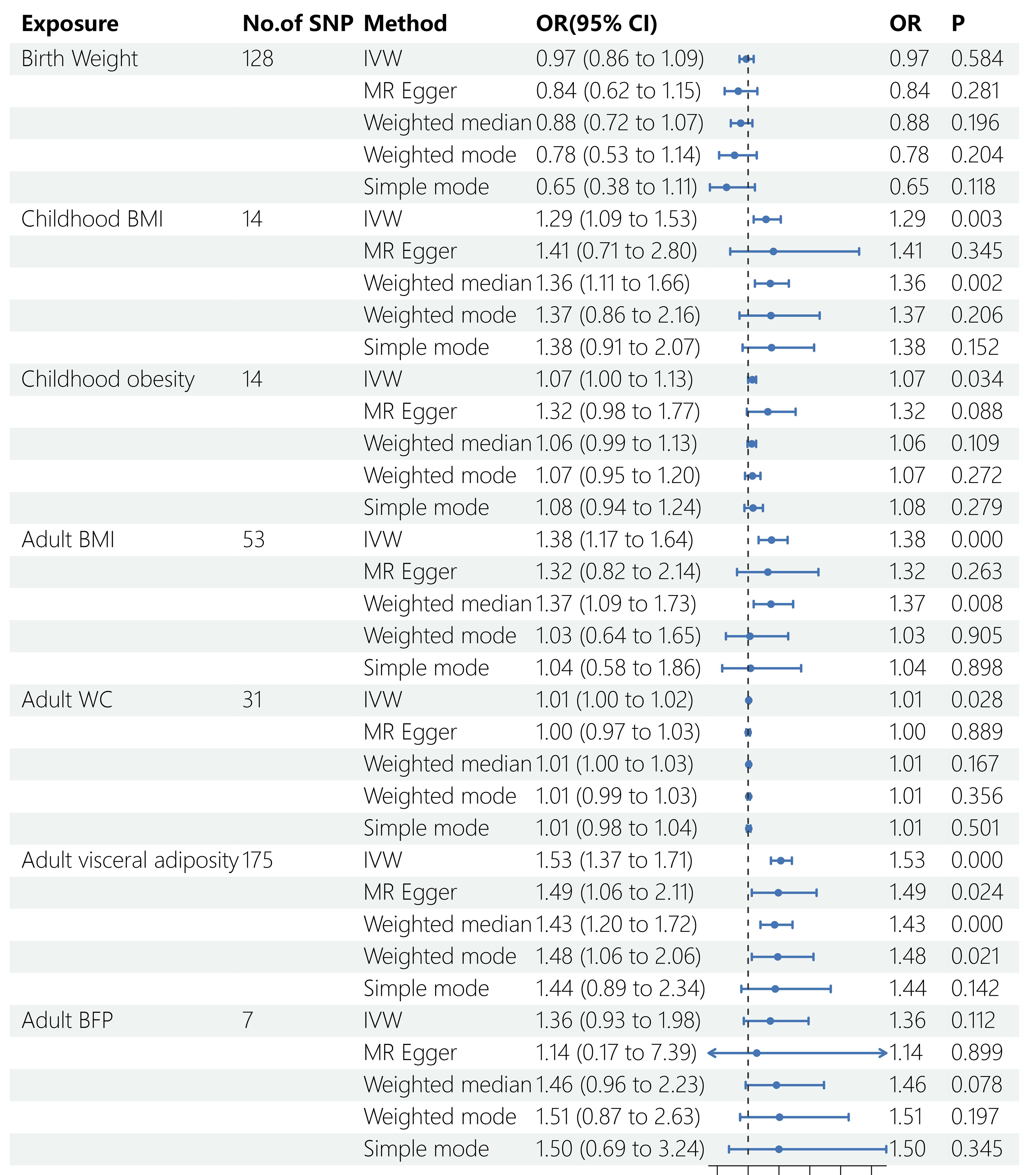
Figure 2 Results of MR analysis. SNP, single nucleotide polymorphisms; OR, odds ratio; BMI, body mass index; WC, waist circumference; BFP, body fat percentage; IVW, inverse variance weighted; MR, Mendelian randomization.
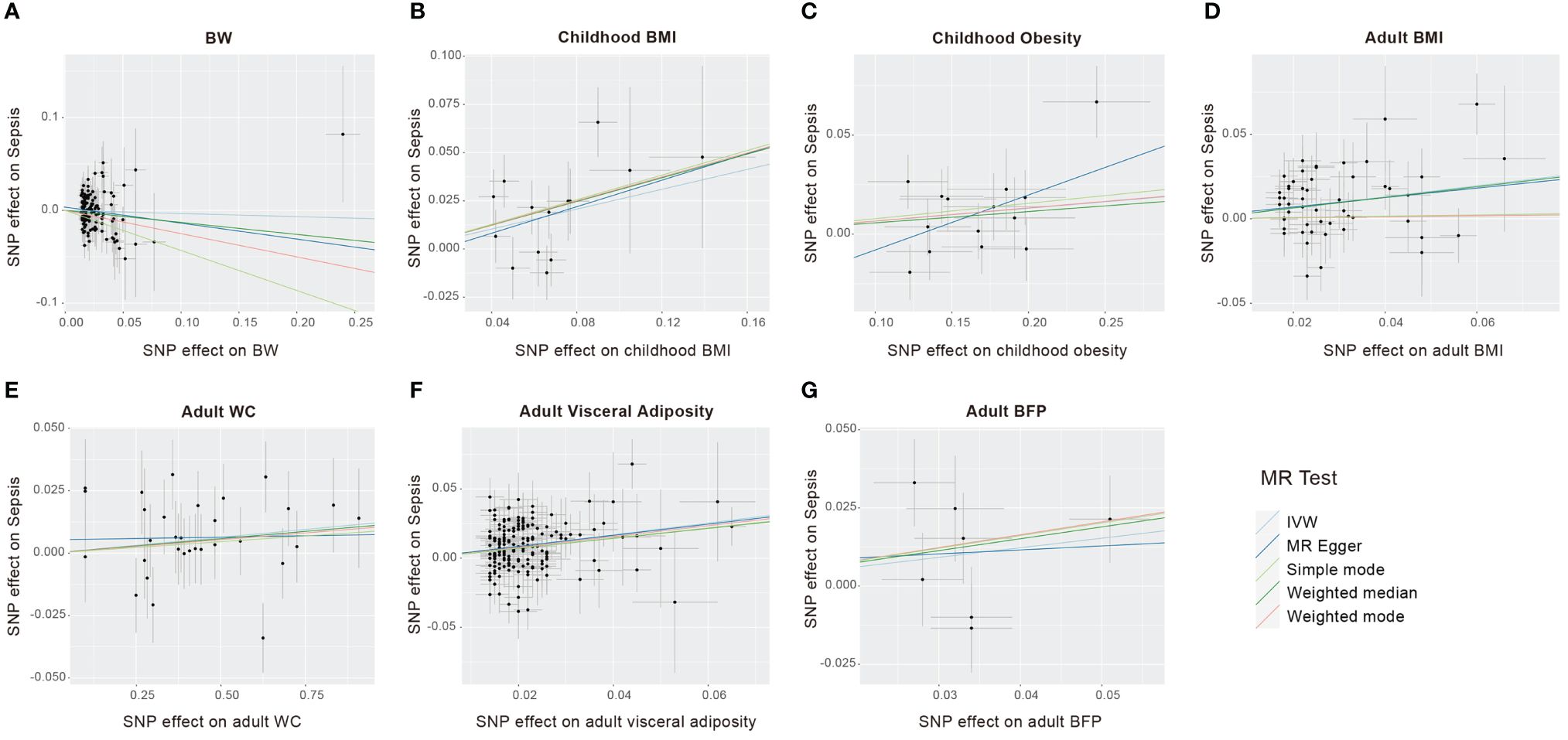
Figure 3 Scatter plots to visualize causal effect of life course adiposity [(A) BW; (B) Children BMI; (C) Children Obesity; (D) Adult BMI; (E) Adult WC; (F) Adult Visceral Adiposity; (G) Adult BFP] on the risk of sepsis.
We conducted a sensitivity analysis to identify potential biases in our analysis. The F-statistics for all selected SNPs were consistently larger than 10, suggesting the absence of weak instrumental variable bias (Supplementary Table S1). Cochran’s Q test applied to both the MR-Egger and IVW approaches revealed no significant heterogeneity among the selected SNPs (Table 3). The results of the funnel chart also support this conclusion (Figure 4). All P-values for the MR Egger intercept and MR-PRESSO global test exceeded 0.05, indicating the absence of directional horizontal pleiotropy (Table 3). The findings highlight the robustness of the identified causal effects from life course adiposity to sepsis, as the results remained consistent in direction. Additionally, leave-one-out analysis and single SNP analysis did not reveal any notable abnormalities (Supplementary Figure S1). These results collectively underscore the reliability and robustness of our study.
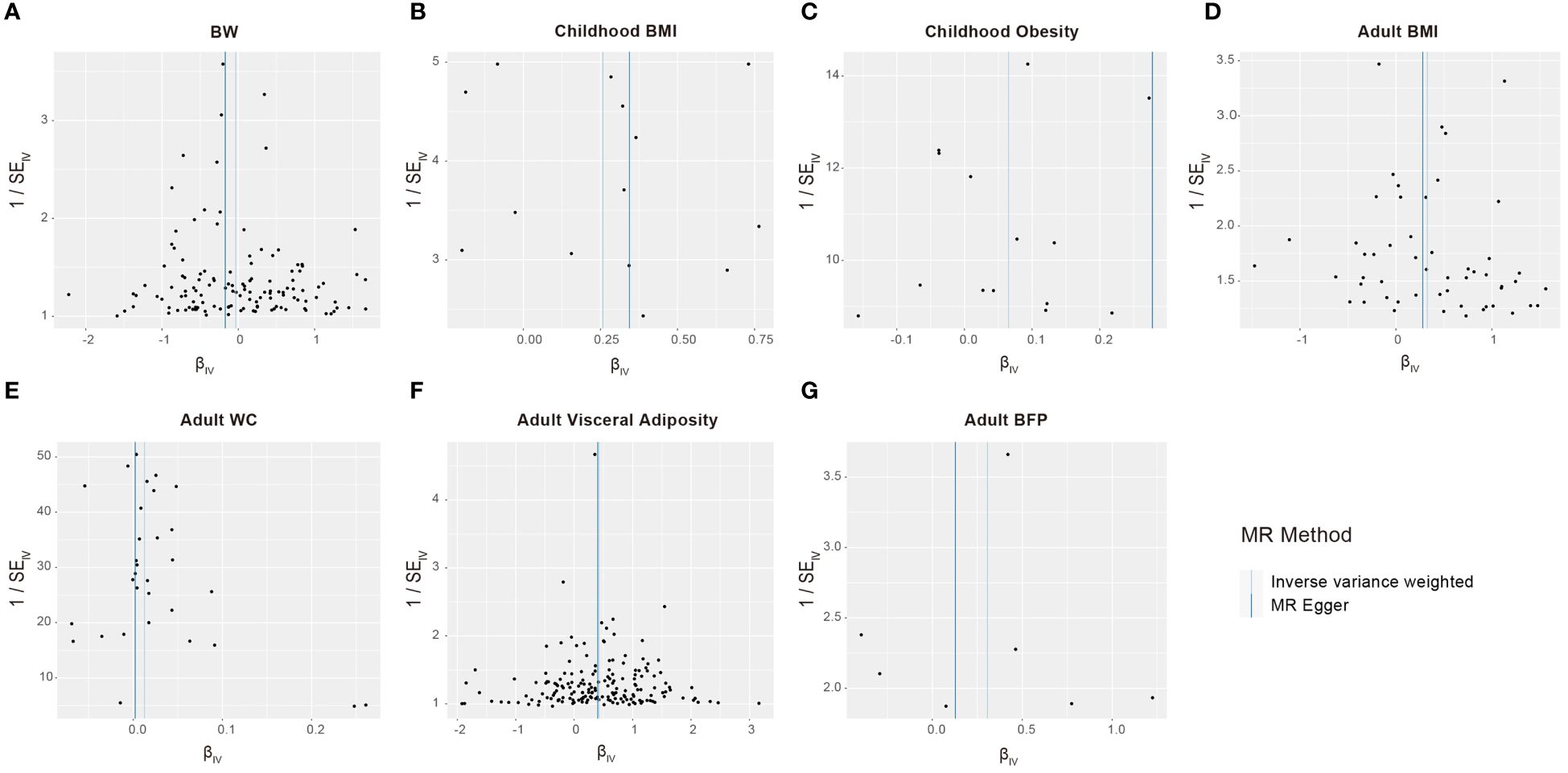
Figure 4 Funnel plots to visualize overall heterogeneity of Mendelian randomization estimates for the effect of life course adiposity [(A) BW; (B) Children BMI; (C) Children Obesity; (D) Adult BMI; (E) Adult WC; (F) Adult Visceral Adiposity; (G) Adult BFP] on the risk of sepsis.
Sepsis is a significant disease that leads to health and economic losses. With the worrying rise in the prevalence of obesity in recent years, there has been growing attention on the relationship between obesity and sepsis. Presently, there exists controversy surrounding this relationship. While obesity may increase the incidence of sepsis, it also appears to be a protective factor against mortality in sepsis patients, a phenomenon known as the obesity paradox (16, 31). The underlying mechanisms of this paradox are not fully understood and may involve factors such as metabolic reserves and immune responses. Additionally, methodological limitations such as study heterogeneity, selection bias, and insufficient adjustment for confounding variables may also contribute to this phenomenon. Higher-level evidence is needed to elucidate the relationship between obesity and sepsis. This study investigates the impact of obesity throughout the life course on the incidence of sepsis. Due to ethical issues preventing the conduct of RCTs, we employed MR analysis, a method similar to RCTs.
To our knowledge, this study was the first MR analysis to meticulously evaluate the potential causal association between life course adiposity and sepsis incidence. The outcomes of our MR analysis unveiled significant associations, indicating that genetically predicted higher childhood BMI and childhood obesity, as well as elevated adult BMI, adult WC, and adult visceral adiposity, are linked to an increased risk of sepsis. Notably, we have done for the first time to discover the association between childhood adiposity and increased risk of future sepsis.
Observational studies exploring the correlation between BW and susceptibility to non-neonatal sepsis are notably scarce. BW, a valuable indicator of fetal intrauterine growth, has been linked to the onset of conditions such as insulin resistance, metabolic syndrome. During fetal intrauterine growth and development, the influence of early embryonic imprinted genes and epigenetic changes may extend to subsequent developmental stages, potentially shaping lifelong health events. However, our analysis revealed that genetically predicted BW is not significantly associated with the risk of sepsis. Several factors may contribute to this lack of association. BW is influenced by a myriad of factors, including gestational age, the number of fetuses, and maternal health, among others. Momoko et al. found that only a fraction, approximately 15%, of the variance in birth weight can be attributed to fetal genetic variation (32). Additionally, it is conceivable that both high and low birth weight could be linked to an increased risk of sepsis. The two variables may exhibit a “U-shaped” relationship. Thus, the intricate relationship between birth weight and the subsequent development of sepsis necessitates further exploration.
The prevalence of obesity among children has seen a significant rise (33). To our knowledge, this is the first study investigating the association between obesity in childhood and subsequent sepsis incidence. Our study revealed a positive genetic association between obesity in childhood and sepsis incidence. Firstly, Simmonds et al. have suggested that the likelihood of obesity in adulthood is five times higher in obese children compared to non-obese children (34). Our research also revealed an association between adult obesity and sepsis. Therefore, the correlation between childhood obesity and sepsis may be mediated by adult obesity. Secondly, previous research has firmly established childhood obesity as a risk factor for some health conditions, encompassing diabetes, essential hypertension, and Alzheimer’s Disease (35, 36). These conditions are recognized risk factors for sepsis and have been associated with elevated mortality rates in sepsis cases (1). It is probably that obesity in childhood heightens susceptibility to sepsis through the manifestation of these associated health issues. Thirdly, obesity in childhood may also increase the risk of sepsis through the gut microbiome-immune axis. Research has demonstrated an association between alterations in the gut microbiota and increased susceptibility to sepsis (37), with obesity causing shifts in the composition of the intestinal bacterial community (38). An expanding body of evidence suggests that the gut microbiota plays a pivotal role in immune system maturation and disease prevention throughout infancy, childhood, and adulthood. During early life, the body’s T cell pool expanse alongside the proliferation of human intestinal flora steadily. Intestinal dendritic cells in infancy consume colonizing bacteria in the gut, stimulating the development of bacteria-specific T cells (39). Gut dysbiosis can also lead to changes in hematopoietic stem cells in the bone marrow and alter the differentiation of progenitor cells, particularly in the context of obesity (40). The immune system, shaped by the early-life intestinal microbiota in obesity individuals, may be more susceptible to perturbation by pathogens and develop sepsis. In summary, prioritizing weight management in children may help prevent sepsis. With obesity increasingly affecting more children, maintaining a healthy weight during childhood becomes even more crucial.
In line with the findings of Hu et al., our study revealed a positive association between genetically predicted adult BMI and sepsis (41). Actually, BMI as an indicator of overall obesity cannot precisely capture the distribution and content of fat. The location of fat distribution is associated with susceptibility to specific diseases. For instance, individuals with abdominal obesity, as assessed by WC or visceral adiposity, are more prone to type 2 diabetes and adverse cardiovascular events (42). An 8-year longitudinal cohort study involving 30,239 subjects indicated that WC was a superior predictor of future sepsis risk compared to BMI (43). Although a higher BMI correlates with increased WC, visceral fat, and BFP, variations in the three indicates also exist among individuals with a normal BMI. Therefore, we investigated the relationship between WC, visceral adiposity, BFP, and susceptibility to sepsis, respectively. Our results suggested that genetically predicted WC and visceral adiposity were associated with an increased risk of sepsis, whereas genetically predicted adult BFP did not. There are some explanations for the causal relationship between adult adiposity and sepsis. First, obese individuals are more prone to develop skin folds and excessive sweating, which favor microbial proliferation and impede wound healing. Second, the susceptibility to infections associated with obesity may also be attributed to high blood sugar, hyperinsulinemia, and leptinemia, which lead to a weakening of both innate and adaptive immune responses (44). Third, adipose tissue has the capability to induce inflammation through its direct secretion of pro-inflammatory mediators and elevated levels of adipokines (45). Excessive fat accumulation can become a significant source of cytokine production in the body following an infection. Furthermore, the adipose tissue within the body can be categorized into visceral fat and subcutaneous fat. Research has demonstrated that, when compared to subcutaneous fat, visceral fat is more susceptible to lipolysis, leading to an elevation in free fatty acids, and an increasing the risk of metabolic syndrome. Visceral fat is also more prolific in the secretion of pro-inflammatory cytokines (46). This observation may partially explain the association between an elevated risk of sepsis and factors such as WC and visceral adipose, while BFP does not demonstrate a significant relationship. In conclusion, these results underscore the importance of not only monitoring BMI but also controlling WC and visceral fat content to combat susceptibility to sepsis.
This study boasts several notable strengths. First and foremost, we ventured to comprehensively explore the causal link between obesity and sepsis throughout the entire life cycle for the first time, utilizing GWAS data from newborn infants, children, and adults. This might be the first study confirm the association between obesity in childhood and subsequent sepsis. Second, the utilization of Mendelian randomization design mitigates biases, confounding factors, and reverse causality effects, rendering the conclusions more reliable. Last, the inclusion of a large sample size of GWAS data in this study substantially enhances statistical efficiency, allowing for a more robust result.
Our study has some limitations. Firstly, MR analysis operates on the premise of a linear relationship between exposure and outcome, which restricts our ability to assess non-linear associations using summary-level data. Underweight can also be a risk factor of sepsis (47). However, we were unable to analyze the relationship between underweight and sepsis. Due to data constraints, stratified analyses based on exposure factors were not feasible. Secondly, it remains uncertain whether our findings can be generalized to other racial and ethnic groups, although limiting our study to European populations helps mitigate population structure bias. Lastly, while sensitivity analysis confirms the robustness of the MR results, further validation is warranted through additional high-quality, population-based observational studies.
In summary, our MR study has uncovered a noteworthy connection between childhood BMI, childhood obesity, adult BMI, adult WC, and adult visceral adiposity, and an increased incidence of sepsis. It’s important to recognize that, the issue of childhood obesity requires further attention. Additionally, even in individuals classified as ‘non-obese’ based on a normal BMI, increased WC or greater visceral fat should also be a cause for concern. Obesity and excess fat are modifiable risk factors that can be addressed through lifestyle interventions. It is crucial to educate the public about obesity and the various forms of fat accumulation.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethical approval was not required for the studies involving humans because this study was based on public databases and published studies, no additional ethics approval was needed. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because this study was based on public databases and published studies, no additional informed consent was needed.
ZC: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Formal analysis, Data curation, Conceptualization. JL: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Formal analysis, Data curation, Conceptualization. WT: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Formal analysis, Data curation, Conceptualization. TL: Writing – review & editing, Supervision, Resources, Methodology, Conceptualization. CZ: Writing – review & editing, Supervision, Resources, Methodology, Conceptualization. JM: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization. GL: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key Research and Development Program of China (2021YFC2701800, 2021YFC2701805), Shanghai Municipal Health System Key Supporting Discipline Project (2023ZDFC0103) and Zhejiang Medical Health Science and Technology Project (2023YK1117).
The authors are grateful to the Early Growth Genetics Consortium, UKB consortium, and all investigators who made the GWAS summary data publicly available.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1413690/full#supplementary-material
Supplementary Figure 1 | MR leave-one-out sensitivity analysis for the effect of life course adiposity on sepsis.
MR, Mendelian randomization; BMI, body mass index; WC, waist circumference; BFP, body fat percentage; GWAS, genome-wide association studies; SNPs, single nucleotide polymorphisms; RCTs, randomized controlled trials; BW, birth weight; MR-PRESSO, Mendelian randomization pleiotropy residual sum and outlier; IVW, inverse variance weighted; ORs, odds ratios; Cis, confidence intervals; SE, standard error.
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
3. Liu YC, Yao Y, Yu MM, Gao YL, Qi AL, Jiang TY, et al. Frequency and mortality of sepsis and septic shock in China: a systematic review and meta-analysis. BMC Infect Dis. (2022) 22:564. doi: 10.1186/s12879-022-07543-8
4. Fleischmann-Struzek C, Mellhammar L, Rose N, Cassini A, Rudd KE, Schlattmann P, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. (2020) 46:1552–62. doi: 10.1007/s00134-020-06151-x
5. Ma Z, Jiang Z, Li H, Lu A, Wu S, Lu H, et al. Prevalence, early predictors, and outcomes of sepsis in neurocritical illnesses: a prospective cohort study. Am J Infect Control. (2024). S0196–6553(24)00056–7. doi: 10.1016/j.ajic.2024.01.017
6. Ahlberg CD, Wallam S, Tirba LA, Itumba SN, Gorman L, Galiatsatos P. Linking Sepsis with chronic arterial hypertension, diabetes mellitus, and socioeconomic factors in the United States: A scoping review. J Crit Care. (2023) 77:154324. doi: 10.1016/j.jcrc.2023.154324
7. Ayalon I, Bodilly L, Kaplan J. The impact of obesity on critical illnesses. Shock. (2021) 56:691–700. doi: 10.1097/SHK.0000000000001821
8. Koliaki C, Dalamaga M, Liatis S. Update on the obesity epidemic: after the sudden rise, is the upward trajectory beginning to flatten? Curr Obes Rep. (2023) 12:514–27. doi: 10.1007/s13679-023-00527-y
9. Powell-Wiley TM, Poirier P, Burke LE, Despres JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation. (2021) 143:e984–e1010. doi: 10.1161/CIR.0000000000000973
10. Piché ME, Tchernof A, Despres JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. (2020) 126:1477–500. doi: 10.1161/CIRCRESAHA.120.316101
11. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories A systematic review and meta-analysis. Jama J Am Med Assoc. (2013) 309:71–82. doi: 10.1001/jama.2012.113905
12. Robinson MK, Mogensen KM, Casey JD, McKane CK, Moromizato T, Rawn JD, et al. The relationship among obesity, nutritional status, and mortality in the critically ill. Crit Care Med. (2015) 43:87–100. doi: 10.1097/CCM.0000000000000602
13. Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. (2020) 71:896–7. doi: 10.1093/cid/ciaa415
14. Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis. (2011) 52:301–12. doi: 10.1093/cid/ciq152
15. Meijs AP, Koek MBG, Vos MC, Geerlings SE, Vogely HC, de Greeff SC. The effect of body mass index on the risk of surgical site infection. Infect Control Hosp Epidemiol. (2019) 40:991–6. doi: 10.1017/ice.2019.165
16. Yeo HJ, Kim TH, Jang JH, Jeon K, Oh DK, Park MH, et al. Obesity paradox and functional outcomes in sepsis: A multicenter prospective study. Crit Care Med. (2023) 51:742–52. doi: 10.1097/CCM.0000000000005801
17. Pepper DJ, Sun J, Welsh J, Cui X, Suffredini AF, Eichacker PQ. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: a systematic review and meta-analysis. Crit Care. (2016) 20:181. doi: 10.1186/s13054-016-1360-z
18. Pepper DJ, Demirkale CY, Sun JF, Rhee C, Fram D, Eichacker P, et al. Does obesity protect against death in sepsis? A retrospective cohort study of 55,038 adult patients. Crit Care Med. (2019) 47:643–50. doi: 10.1097/CCM.0000000000003692
19. Trivedi V, Bavishi C, Jean R. Impact of obesity on sepsis mortality: A systematic review. J Crit Care. (2015) 30:518–24. doi: 10.1016/j.jcrc.2014.12.007
20. Wang S, Liu X, Chen Q, Liu C, Huang C, Fang X. The role of increased body mass index in outcomes of sepsis: a systematic review and meta-analysis. BMC Anesthesiol. (2017) 17:118. doi: 10.1186/s12871-017-0405-4
21. Paulsen J, Askim A, Mohus RM, Mehl A, Dewan A, Solligard E, et al. Associations of obesity and lifestyle with the risk and mortality of bloodstream infection in a general population: a 15-year follow-up of 64 027 individuals in the HUNT Study. Int J Epidemiol. (2017) 46:1573–81. doi: 10.1093/ije/dyx091
22. Twig G, Geva N, Levine H, Derazne E, Goldberger N, Haklai Z, et al. Body mass index and infectious disease mortality in midlife in a cohort of 2.3 million adolescents. Int J Obes. (2018) 42:801–7. doi: 10.1038/ijo.2017.263
23. Gupta V, Walia GK, Sachdeva MP. ‘Mendelian randomization’: an approach for exploring causal relations in epidemiology. Public Health. (2017) 145:113–9. doi: 10.1016/j.puhe.2016.12.033
24. Warrington NM, Beaumont RN, Horikoshi M, Day FR, Helgeland O, Laurin C, et al. Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat Genet. (2019) 51:804–14. doi: 10.1038/s41588-019-0403-1
25. Felix JF, Bradfield JP, Monnereau C, van der Valk RJ, Stergiakouli E, Chesi A, et al. Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum Mol Genet. (2016) 25:389–403. doi: 10.1093/hmg/ddv472
26. Bradfield JP, Taal HR, Timpson NJ, Scherag A, Lecoeur C, Warrington NM, et al. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet. (2012) 44:526–31. doi: 10.1038/ng.2247
27. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Felix R, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. (2015) 518:197–206. doi: 10.1038/nature14177
28. Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Magi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. (2015) 518:187–96. doi: 10.1038/nature14132
29. Karlsson T, Rask-Andersen M, Pan G, Höglund J, Wadelius C, Ek WE, et al. Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat Med. (2019) 25:1390–5. doi: 10.1038/s41591-019-0563-7
30. Lu YC, Day FR, Gustafsson S, Buchkovich ML, Na JB, Bataille V, et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat Commun. (2016) 7:10495. doi: 10.1038/ncomms10495
31. Jagan N, Morrow LE, Walters RW, Plambeck RW, Wallen TJ, Patel TM, et al. Sepsis and the obesity paradox: size matters in more than one way. Crit Care Med. (2020) 48:e776–e82. doi: 10.1097/CCM.0000000000004459
32. Horikoshi M, Beaumont RN, Day FR, Warrington NM, Kooijman MN, Fernandez-Tajes J, et al. Genome-wide associations for birth weight and correlations with adult disease. Nature. (2016) 538:248–52. doi: 10.1038/nature19806
33. Rafei A, Elliott MR, Jones RE, Riosmena F, Cunningham SA, Mehta NK. Obesity incidence in U.S. Children and young adults: A pooled analysis. Am J Prev Med. (2022) 63:51–9. doi: 10.1016/j.amepre.2021.12.021
34. Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev. (2016) 17:95–107. doi: 10.1111/obr.12334
35. Valaiyapathi B, Gower B, Ashraf AP. Pathophysiology of type 2 diabetes in children and adolescents. Curr Diabetes Rev. (2020) 16:220–9. doi: 10.2174/1573399814666180608074510
36. Li X, Tian Y, Yang YX, Ma YH, Shen XN, Chen SD, et al. Life course adiposity and Alzheimer’s disease: A Mendelian randomization study. J Alzheimers Dis. (2021) 82:503–12. doi: 10.3233/JAD-210345
37. Adelman MW, Woodworth MH, Langelier C, Busch LM, Kempker JA, Kraft CS, et al. The gut microbiome’s role in the development, maintenance, and outcomes of sepsis. Crit Care. (2020) 24:278. doi: 10.1186/s13054-020-02989-1
38. Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. (2017) 23:859–68. doi: 10.1038/nm.4358
39. Zegarra-Ruiz DF, Kim DV, Norwood K, Kim M, Wu WH, Saldana-Morales FB, et al. Thymic development of gut-microbiota-specific T cells. Nature. (2021) 594:413–7. doi: 10.1038/s41586-021-03531-1
40. Luo Y, Chen GL, Hannemann N, Ipseiz N, Kronke G, Bauerle T, et al. Microbiota from obese mice regulate hematopoietic stem cell differentiation by altering the bone niche. Cell Metab. (2015) 22:886–94. doi: 10.1016/j.cmet.2015.08.020
41. Hu J, Gan Q, Zhou D, Xia X, Xiang W, Xiao R, et al. Evaluating the risk of sepsis attributing to obesity: a two-sample Mendelian randomization study. Postgrad Med J. (2023) 99:1266–71. doi: 10.1093/postmj/qgad072
42. Smith U. Abdominal obesity: a marker of ectopic fat accumulation. J Clin Invest. (2015) 125:1790–2. doi: 10.1172/JCI81507
43. Wang HE, Griffin R, Judd S, Shapiro NI, Safford MM. Obesity and risk of sepsis: a population-based cohort study. Obes (Silver Spring). (2013) 21:E762–9. doi: 10.1002/oby.20468
44. Muscogiuri G, Pugliese G, Laudisio D, Castellucci B, Barrea L, Savastano S, et al. The impact of obesity on immune response to infection: Plausible mechanisms and outcomes. Obes Rev. (2021) 22:e13216. doi: 10.1111/obr.13216
45. Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. (2005) 174:3137–42. doi: 10.4049/jimmunol.174.6.3137
46. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. (2010) 11:11–8. doi: 10.1111/j.1467-789X.2009.00623.x
Keywords: obesity paradox, pediatric obesity, body mass index, obesity, abdominal, waist circumference, fat body
Citation: Cheng Z, Li J, Tong W, Liu T, Zhang C, Ma J and Lu G (2024) Exploring the relationship between life course adiposity and sepsis: insights from a two-sample Mendelian randomization analysis. Front. Endocrinol. 15:1413690. doi: 10.3389/fendo.2024.1413690
Received: 07 April 2024; Accepted: 03 June 2024;
Published: 14 June 2024.
Edited by:
Irene Karampela, National and Kapodistrian University of Athens, GreeceReviewed by:
Marlene Starr, University of Kentucky, United StatesCopyright © 2024 Cheng, Li, Tong, Liu, Zhang, Ma and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoping Lu, MTM3ODg5MDQxNTBAMTYzLmNvbQ==; Jian Ma, bWppYW5lckAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.