- 1Department of Nephrology, Dongfang Hospital of Beijing University of Chinese Medicine, Beijing, China
- 2Graduate School of Beijing University of Chinese Medicine, Beijing, China
- 3Department of Nephrology, Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital, Tianjin, China
- 4Tianjin Famous Chinese Medicine Inheritance Workshop of Mianzhi Zhang, Tianjin, China
Objective: The oxidative balance score (OBS) is a comprehensive concept that includes 20 oxidative stressors and can be used to assess individual pro-oxidant versus antioxidant exposure, and the aim of the present study was to investigate the association between OBS and the risk of diabetic kidney disease (DKD), low estimated glomerular filtration rate (low-eGFR) and albuminuria in patients with diabetes mellitus (DM).
Methods: This cross-sectional study included nationally representative consecutive National Health and Nutrition Examination Survey DM patients aged 18 years and older from 2003-2018. The continuous variable OBS was converted into categorical variables by quartiles, and weighted multiple logistic regression analyses and restricted triple spline models were used to explore the relationships. We also performed subgroup analyses and interaction tests to verify the stability of the results.
Results: A total of 5389 participants were included, representing 23.6 million non-institutionalized US residents. The results from both multivariate logistic regression analysis and restricted cubic spline models indicated that OBS and dietary OBS levels were negatively associated with the risk of DKD, low-eGFR, and albuminuria, without finding a significant correlation between lifestyle OBS and these clinical outcomes. Compared to the lowest OBS quartile group, the prevalence risk of DKD (OR = 0.61, 95% CI: 0.46-0.80), low-eGFR (OR = 0.46, 95% CI: 0.33-0.64) and albuminuria (OR = 0.68, 95% CI: 0.51-0.92) decreased by 39%, 54% and 32%, respectively, in the highest OBS quartile group. The results remained stable in subgroup analyses and no interaction between subgroups was found.
Conclusion: Higher levels of OBS and dietary OBS were associated with a lower risk of DKD, low-eGFR, and albuminuria. These findings provided preliminary evidence for the importance of adhering to an antioxidant-rich diet and lifestyle among individuals with diabetes.
1 Introduction
Diabetes Mellitus (DM) is a major disease that endangers human health. According to the International Diabetes Federation, it is estimated that the number of people affected by DM will reach 1.09 billion by 2045 (1), leading to a corresponding increase in the prevalence of diabetic kidney disease (DKD). DKD is responsible for 30% to 50% of end-stage renal disease cases worldwide (2). The burden of DKD is significant, leading to reduced quality of life, increased incapacity, premature death (3), and higher healthcare costs (4). Therefore, it is still imperative to address the urgent issues of early prevention and slowing down the progression of DKD, as well as gaining a comprehensive understanding and effectively controlling the risk factors for its development.
The pathogenesis of DKD is complex (5), and oxidative stress is one of the important triggers (6). Oxidative stress is characterized by an overproduction of reactive oxygen species (ROS) that surpasses the body’s antioxidant defense system, resulting in an imbalance in the oxidative system. Hyperglycemia can result in the formation of advanced glycation end-products and ROS. These, in turn, activate intercellular signaling pathways that lead to pro-inflammatory and pro-fibrotic gene expression, causing tissue and cellular damage (7, 8). It is critical to maintain the balance of the body’s redox system to slow the progression of DKD (9). These findings have also sparked exploration into whether the intake of antioxidants can reduce the risk of DKD. However, the results are not entirely consistent (10–12), which is associated with the presence of various antioxidant and pro-oxidant factors in diet and lifestyle. Conclusions drawn from a sole evaluation of a specific factor may be one-sided.
The oxidative balance score (OBS) is a comprehensive indicator that considers various dietary components and lifestyle factors to assess an individual’s exposure to pro-oxidants and antioxidants. A higher OBS indicates a stronger antioxidant capacity. Since oxidative stress is an important pathogenic mechanism in DKD, managing and improving oxidative balance may be a crucial strategy for the prevention and treatment of DKD. This includes consuming an adequate amount of antioxidants in the diet and maintaining a healthy lifestyle. Although the OBS has been widely used in numerous studies and a higher OBS has been associated with a lower prevalence and incidence of chronic kidney disease (CKD) (13, 14), as well as various diseases such as chronic obstructive pulmonary disease (15), kidney stones, cardiovascular disease (CVD) (16), and depression (17), research on the relationship between OBS and DKD is currently lacking. Given that individuals with diabetes are among the most susceptible populations to kidney damage, the presence of DKD complicates diabetes management, increases the risk of CVD, and mortality. The primary objective of this study is to utilize data from the National Health and Nutrition Examination Survey (NHANES) to conduct a cross-sectional analysis among the diabetic population to explore the association between OBS and DKD. The aim is to provide new insights and strategies for the prevention and treatment of DKD.
2 Materials and methods
2.1 Study population
This investigation is a nationwide cross-sectional study, utilizing data from the NHANES 2003-2018 survey cycle. NHANES is administered by the National Center for Health Statistics, a division of the Centers for Disease Control and Prevention, and has been approved by an ethics review board. The NHANES sample design incorporates a multi-year, stratified, clustered four-stage sampling approach, with data released in 2-year cycles (adjustments to the sampling methods for each cycle can be accessed via the following URL: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx#sample-design). Investigators gather comprehensive information from participants through home interviews and laboratory testing to assess the health and nutritional status of the non-institutionalized civilian population across the United States (18, 19), and all participants have provided informed consent.
Participants in this study underwent screening based on the following criteria (Figure 1): individuals were excluded if (1) <18 years; (2) Items comprising the OBS <16 (16); (3) incomplete data of urinary albumin-to-creatinine ratio (ACR); (4) incomplete data of estimated glomerular filtration rate (eGFR); (5) without DM; (6) pregnant; (7) incomplete data of covariates.
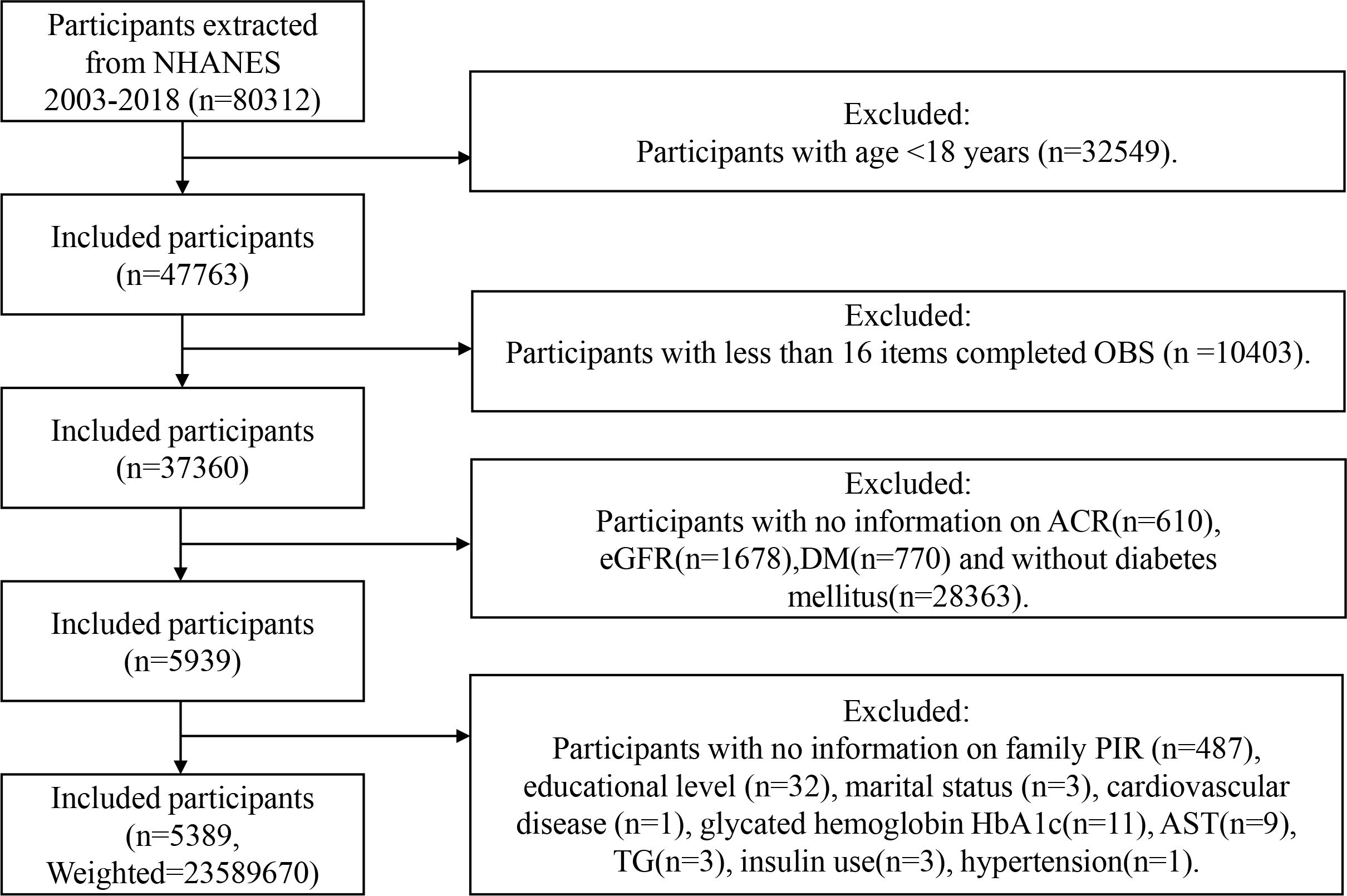
Figure 1 Flowchart of the sample selection from NHANES 2003–2018. NHANES, National Health and Nutrition Examination Survey; OBS, oxidative balance score; family PIR, family poverty income ratio; ACR, urinary albumin-to-creatinine ratio; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; AST, aspartate aminotransferase; TG, triglycerides.
2.2 Definition of the oxidative balance score
The OBS is based on 16 dietary nutrients and 4 lifestyle factors. The 16 dietary nutrients comprise of dietary fiber, carotenoids, riboflavin, niacin, vitamin B6, total folate, vitamin B12, vitamin C, vitamin E, calcium, magnesium, zinc, copper, selenium, total fat, and iron. The 4 lifestyle factors include physical activity, body mass index (BMI), alcohol consumption, and smoking status. The study identified pro-oxidant factors, including total fat, total iron intake, smoking status, alcohol consumption status, and BMI. The remaining factors were classified as antioxidants (20). Food type and quantity intake were obtained by two 24-hour dietary recall interviews (the first assessment was administered at the Mobile Examination Center, followed by a telephone follow-up for the second assessment. The mean value of the data from both assessments was calculated, in the event of missing data from the second interview, the results from the first interview were considered as the default.), and based on the Food Intake Analysis System of the University of Texas and the United States Department of Agriculture Survey Nutritional Database. The amount of physical activity was calculated as the total metabolic equivalent of task (MET) level of all exercises performed in a week (metabolic equivalent per physical activity × frequency of physical activity × duration). The smoking level was determined by the cotinine level. Alcohol consumption was categorized into three groups based on gender: non-drinkers, moderate drinkers (0-15 g/d for women and 0-30 g/d for men), and heavy drinkers (≥15 g/d for women and ≥30 g/d for men). Obesity scores were assigned based on weight status, obesity: BMI≥30 kg per square meter (kg/m2), overweight: 25≤BMI<30 kg/m2, and normal weight: BMI<25 kg/m2. The OBS was calculated as follows (refer to Supplementary Table 1): non-drinkers, moderate drinkers, and heavy drinkers were scored 2, 1, and 0, respectively; obesity, overweight, and normal weight were scored 0, 1, and 2, respectively. The other components were grouped in tertiles by gender, with antioxidants in groups 1 - 3 being assigned a score of 0-2 and pro-oxidant factors in groups 1-3 being assigned a score of 2-0. Taking fiber intake as an example in men, daily fiber intake of ≤12.7g is assigned 0 point, between 12.7 and 19.85g is 1 point, and >19.85g is 2 points. Each component contributes an equal weight, and the sum of the scores yields the OBS. A higher OBS indicates a stronger antioxidant profile.
2.3 Definition of DKD, low-eGFR, and albuminuria
The diagnosis of diabetes (21): the use of diabetes medication or insulin, a diagnosis by a doctor, glycated hemoglobin HbA1c ≥6.5%, fasting glucose ≥7 mmol/L, 2-h oral glucose tolerance test with blood glucose ≥11.1 mmol/L. The DKD was defined as the diabetes combined with albuminuria (ACR≥30 mg/g) and/or low-eGFR (eGFR<60 mL/min/1.73 m2) according to the KDIGO 2021 Guidelines (22). For eGFR calculation, we applied the CKD-Epidemiology Collaboration equation as follows (23): GFR = 141·min [Scr/κ,1]α × max [Scr/κ, 1] − 1.209 × 0.993 Age × 1.018 [if women] ×1.159 [if black]); κ was 0.7(women) or 0.9(men), a was −0.329 (women) or −0.411(men), and min/max indicate the minimum/maximum of Scr/κ or 1.
2.4 Covariates assessment
Based on previous research (24, 25), we included a number of covariates that may influenced the results of the study. Demographic factors included age, gender, marital status (divorced/separated/widowed, married/living with a partner, never married), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American and other), education level (less than high school, high school diploma, more than high school), the family poverty income ratio (PIR) (<1.3, 1.3-3.5, >3.5); laboratory indicators including aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), triglycerides (TG), albumin (ALB), fasting glucose, glycated hemoglobin HbA1c; Additionally, we considered a number of chronic diseases: hypertension (taking blood pressure-lowering medication, diagnosed by a physician as having a high systolic blood pressure ≥140mmhg or diastolic blood pressure ≥90mmhg) (26), hyperlipidemia (taking cholesterol-lowering drugs, total cholesterol ≥200 mg/dL, triglyceride ≥150 mg/dL, low-density lipoprotein ≥130 mg/dL, or high-density lipoprotein ≤50 mg/dL for women and ≤40 mg/dL for men) (27), CVD (coronary heart disease, congestive heart failure, heart attack, stroke, angina) and metabolic syndrome(Mets), additionally insulin use.
2.5 Statistical analyses
In light of the stratified and complex multistage sampling design employed by NHANES, we have weighted the data using the sample weight calculation method recommended by NHANES. This involved pooling data from eight cycles spanning the period from 2003 to 2018, where the weights for 16 years are calculated to be one-eighth of the weights for 2 years. This weighting approach corrects for the imbalance in the samples, allowing for a more accurate reflection of the characteristics of the overall population. Categorical variables are presented as weighted percentages, while continuous variables are described using mean ± standard deviations. The Shapiro-Wilk statistical test was employed to confirm the normal distribution of continuous variables. Variables with skewed distributions are represented by medians and quartiles. We converted the continuous variable OBS into categorical variables by quartiles (Q1: ≤P25; Q2: P25-P50; Q3: P50-P75; Q4: >P75) and assessed differences between OBS (quantile) groups using weighted t-tests or chi-square tests. Multiple logistic regression is suitable for analyzing the relationship between multiple predictor variables and a binary outcome variable, allowing for the control of other potential confounding variables. Weighted multivariate logistic regression analysis was employed to evaluate the correlation between OBS, dietary OBS, lifestyle OBS, and DKD, low-eGFR, as well as albuminuria across three models. The results were reported as odds ratios (ORs) with their 95% confidence intervals (95% CIs), p-values, and trend p-values. Collinearity among variables was assessed using the variance inflation factor (VIF). The VIF quantifies the increased variance of an estimated regression coefficient due to collinearity. A common rule of thumb is that VIF values greater than 5 indicate a problematic level of collinearity (28), which can lead to instability in regression results and weaken predictive capabilities. In Model 1, adjustments were made for gender, age, marital status, race/ethnicity, education level, and family PIR. Model 2 adjusted for hypertension, hyperlipidemia, CVD, Mets, and insulin use based on Model 1. Model 3 adjusted for all covariates. A multivariate-adjusted restricted cubic spline model was employed to more accurately capture the relationship between continuous variables and the outcome. The model established OR curves at three knot points to explore whether there exists a nonlinear dose-response association between OBS and DKD, low-eGFR, and albuminuria. To determine the potential effect moderators, subgroup analyses were performed based on subjects’ age, sex, and whether they had hypertension, hyperlipidemia, CVD, and Mets, and interaction analyses were performed to check the heterogeneity of the relationship between subgroups. A two-sided P value of <0.05 was considered a statistically significant difference. R software (R version 4.3.3) was used for all statistical analyses in this study.
3 Results
3.1 Baseline characteristics
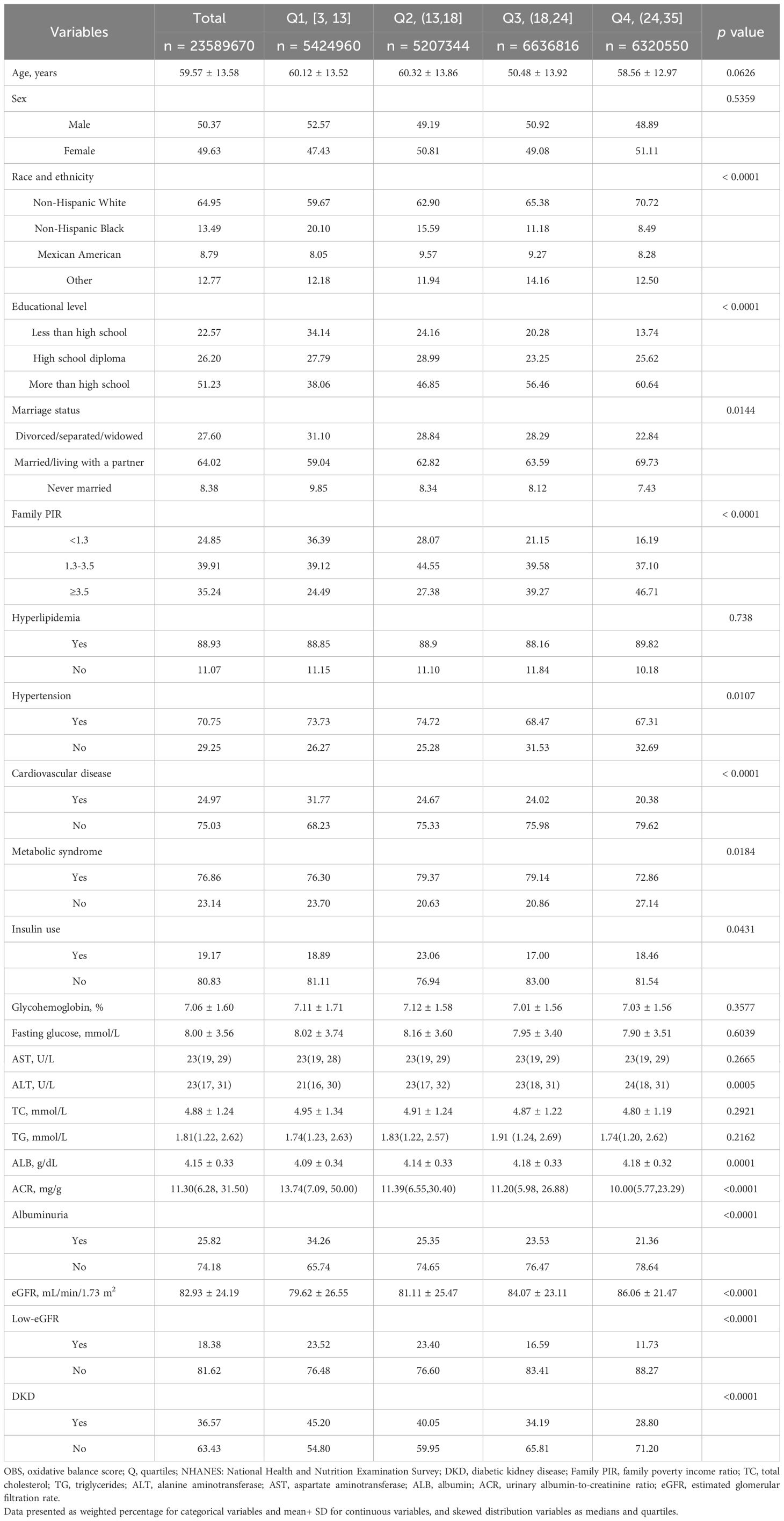
The study included 5389 participants, representing 23.6 million non-institutionalized residents of the United States. The mean age of all subjects was 59.57, with 50.37% males and 39.63% females. Among DM patients, the weighted prevalence of DKD, low-eGFR, and albuminuria was 36.57%, 18.38%, and 25.82%, respectively. Furthermore, we discovered significant differences (p < 0.05) in race, education level, marital status, family PIR, ALT, ALB, prevalence of hypertension, CVD and Mets, and insulin use among the OBS groups. Table 1 presents the baseline characteristics of the study participants, categorized by their OBS quartiles.
3.2 Association between OBS, dietary OBS, lifestyle OBS with DKD, low-eGFR and albuminuria
As shown in Supplementary Table 2, all covariates exhibit VIF values less than 5, suggesting that collinearity has a minimal impact on the results.
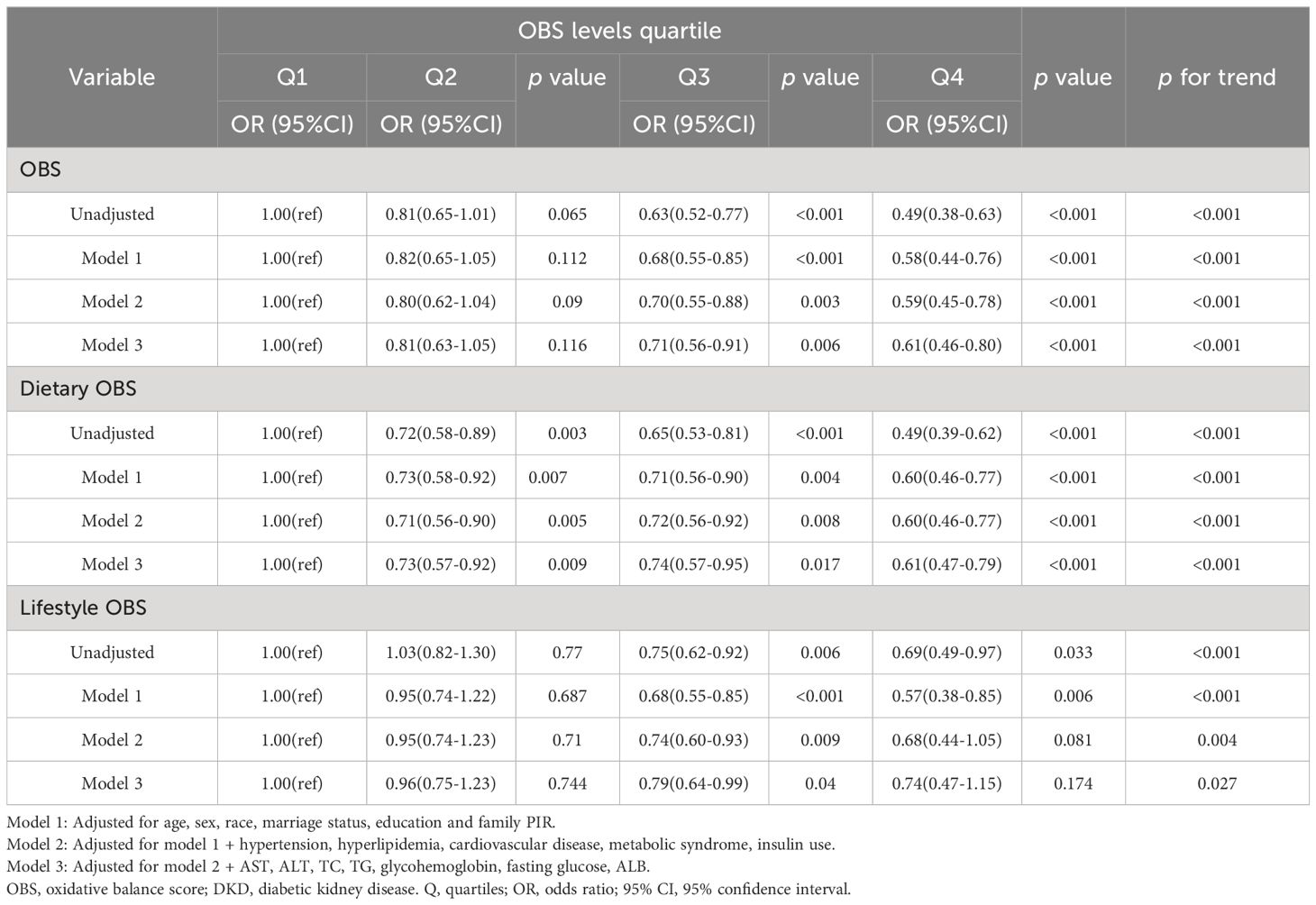
As depicted in Tables 2–4, weighted multivariate logistic regression was employed to examine the association between OBS and the risk of DKD, low-eGFR, and albuminuria. The results revealed that the risk of DKD, low-eGFR, and albuminuria decreased with increasing quartiles across all models (p for trend <0.05). In model 3, which was fully adjusted, compared to the lowest quartile of OBS, the highest quartile was associated with a 39% reduction in the risk of DKD (OR = 0.61, 95% CI: 0.46-0.80), a 54% reduction in the risk of low-eGFR (OR = 0.46, 95% CI: 0.33-0.64), and a 32% reduction in the risk of albuminuria (OR = 0.68, 95% CI: 0.51-0.92).
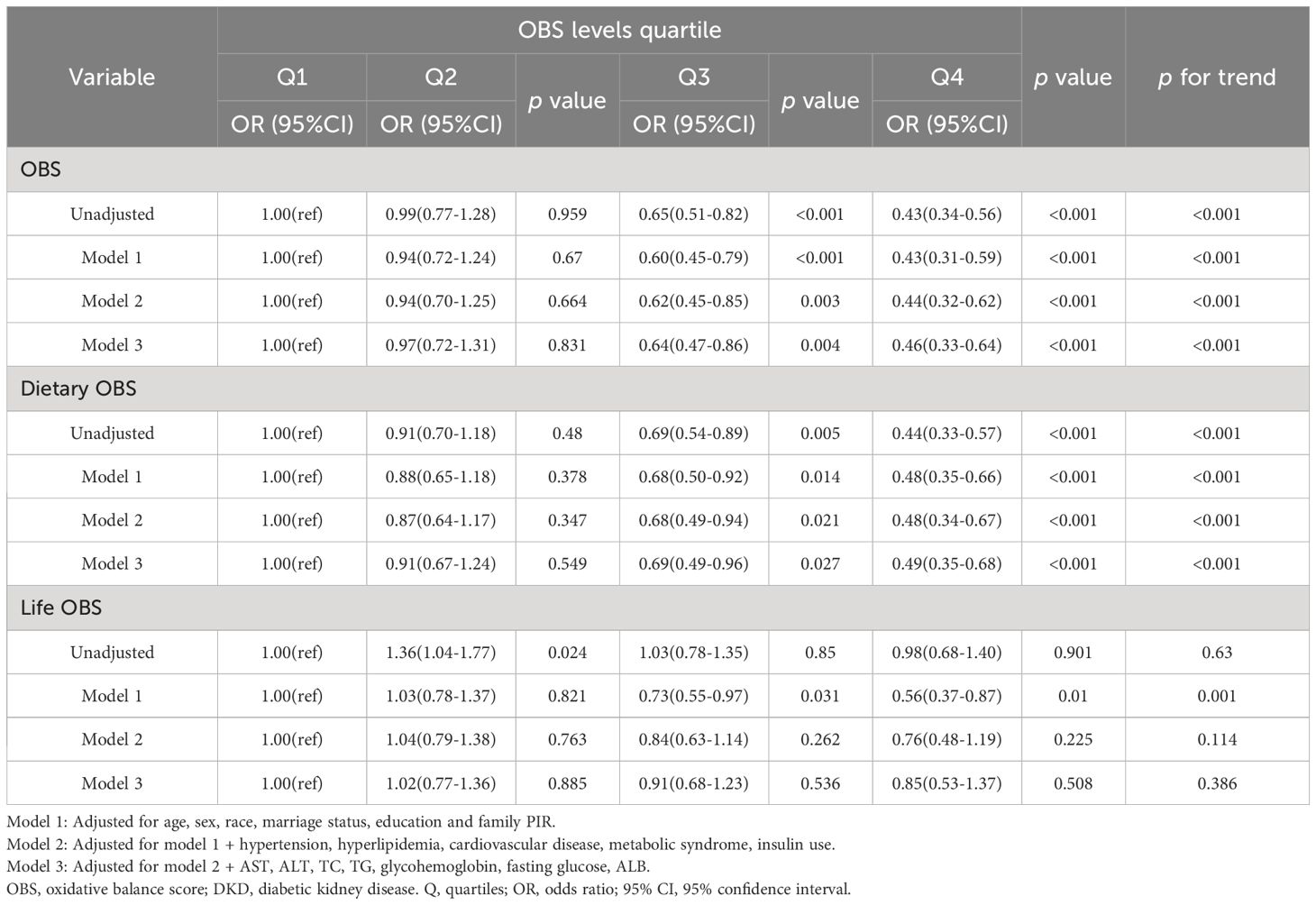
Table 3 Weighted logistic regression analysis models showing the associations between OBS and low-eGFR.
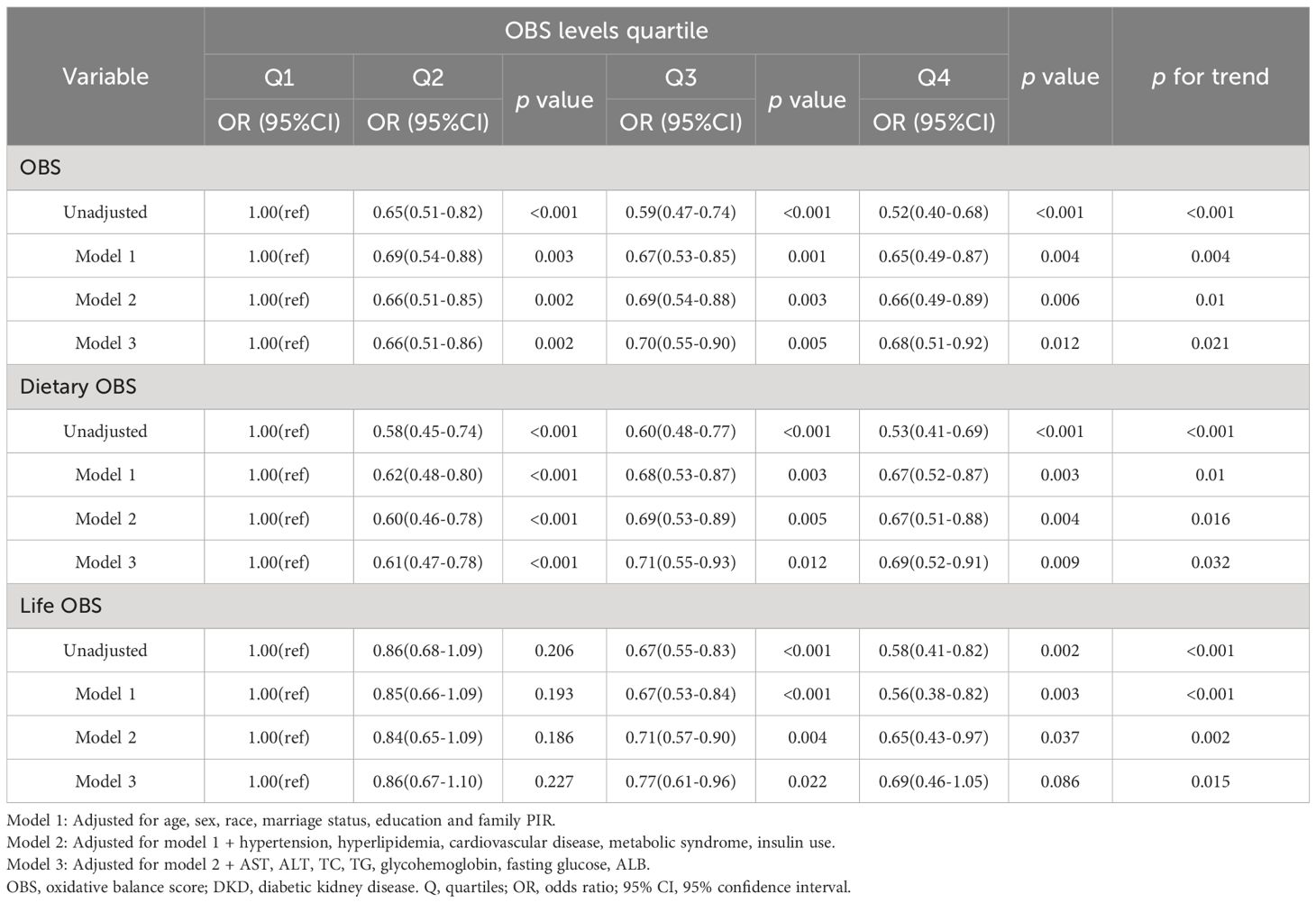
Table 4 Weighted logistic regression analysis models showing the associations between OBS and albuminuria.
Moreover, weighted multivariate logistic regression was also used to investigate the impact of dietary OBS and lifestyle OBS on the risk of DKD, low-eGFR, and albuminuria. The results indicated that both dietary OBS and lifestyle OBS were considered protective factors, although there was no statistically significant difference between the highest and lowest quartiles of lifestyle OBS.
3.3 Analysis of restricted cubic spline regression
Based on the results of the multivariate regression analysis, restricted cubic spline regression was employed to examine the relationship between OBS, dietary OBS, and the outcomes. After adjusting for all covariates, it was found that OBS was linearly associated with DKD, low-eGFR, and albuminuria. Figures 2A–C respectively display the trend of OR values for DKD, low-eGFR, and albuminuria decreasing with increasing OBS. Notably, although dietary OBS exhibited linear associations with DKD and low-eGFR (Figures 2D, E), it showed a non-linear association with albuminuria (p for non-linearity = 0.021, p for overall = 0.004). Specifically, Figure 2F illustrates that as dietary OBS increased, the risk of albuminuria initially decreased and then increased.
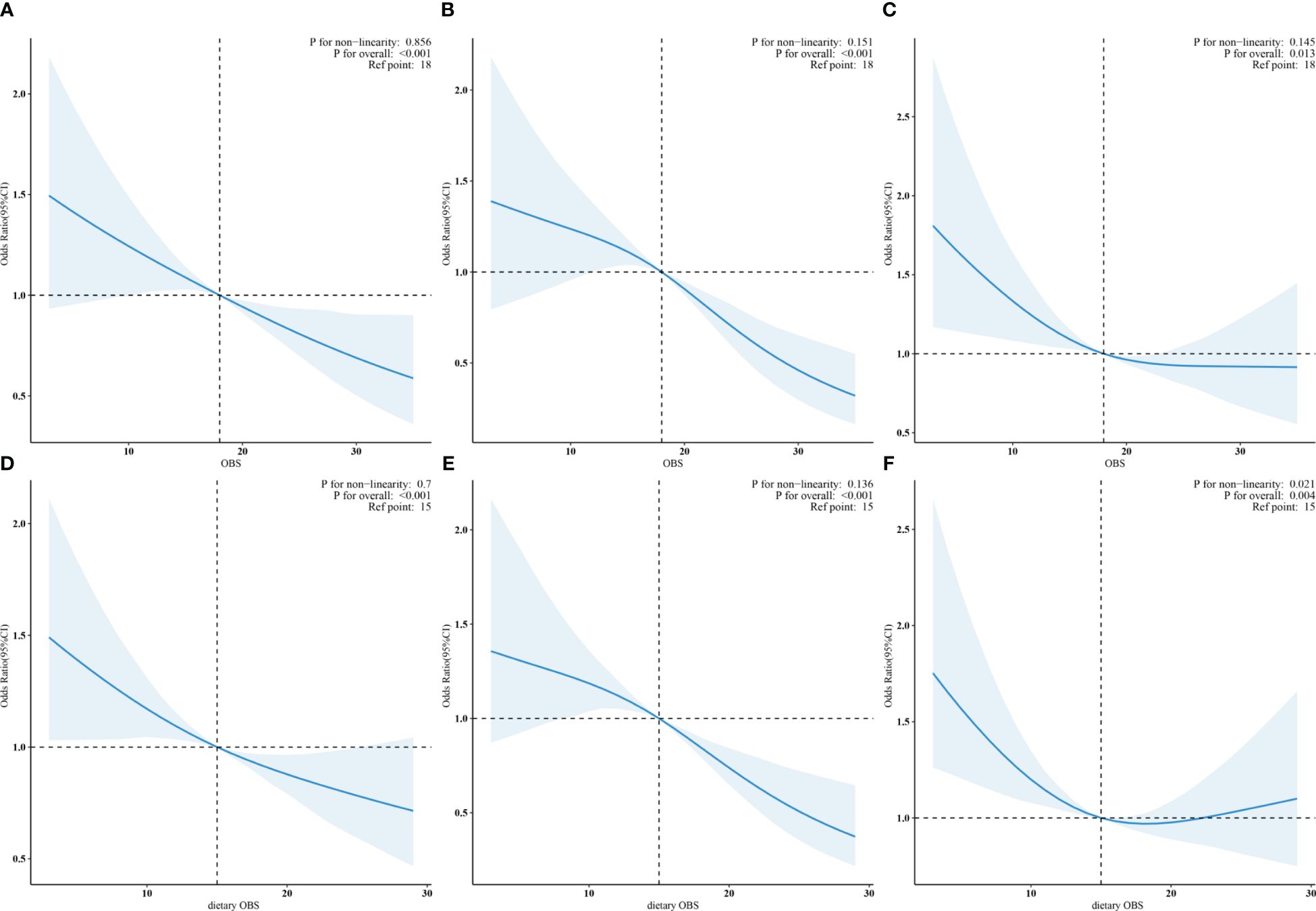
Figure 2 (A–C): Linear associations of OBS with DKD, low-eGFR, and albuminuria. (D, E): Linear trends in dietary OBS related to DKD and low-eGFR. (F): Non-linear relation of dietary OBS to albuminuria.
3.4 Analysis of subgroup
In the subgroup analysis, we observed inconsistent associations. In the CVD and Mets subgroups, OBS was negatively correlated with DKD. However, in other subgroups, the correlation between OBS and DKD was only observed in individuals aged ≥60 years, males, those with hyperlipidemia or hypertension (Figure 3). A negative correlation between OBS and low-eGFR was present in all subgroups (Figure 4). The correlation between OBS and albuminuria was confined to subgroups of individuals aged ≥60 years, males, and those with hyperlipidemia, hypertension, CVD, or Mets (Figure 5). No interaction was found among the subgroups.
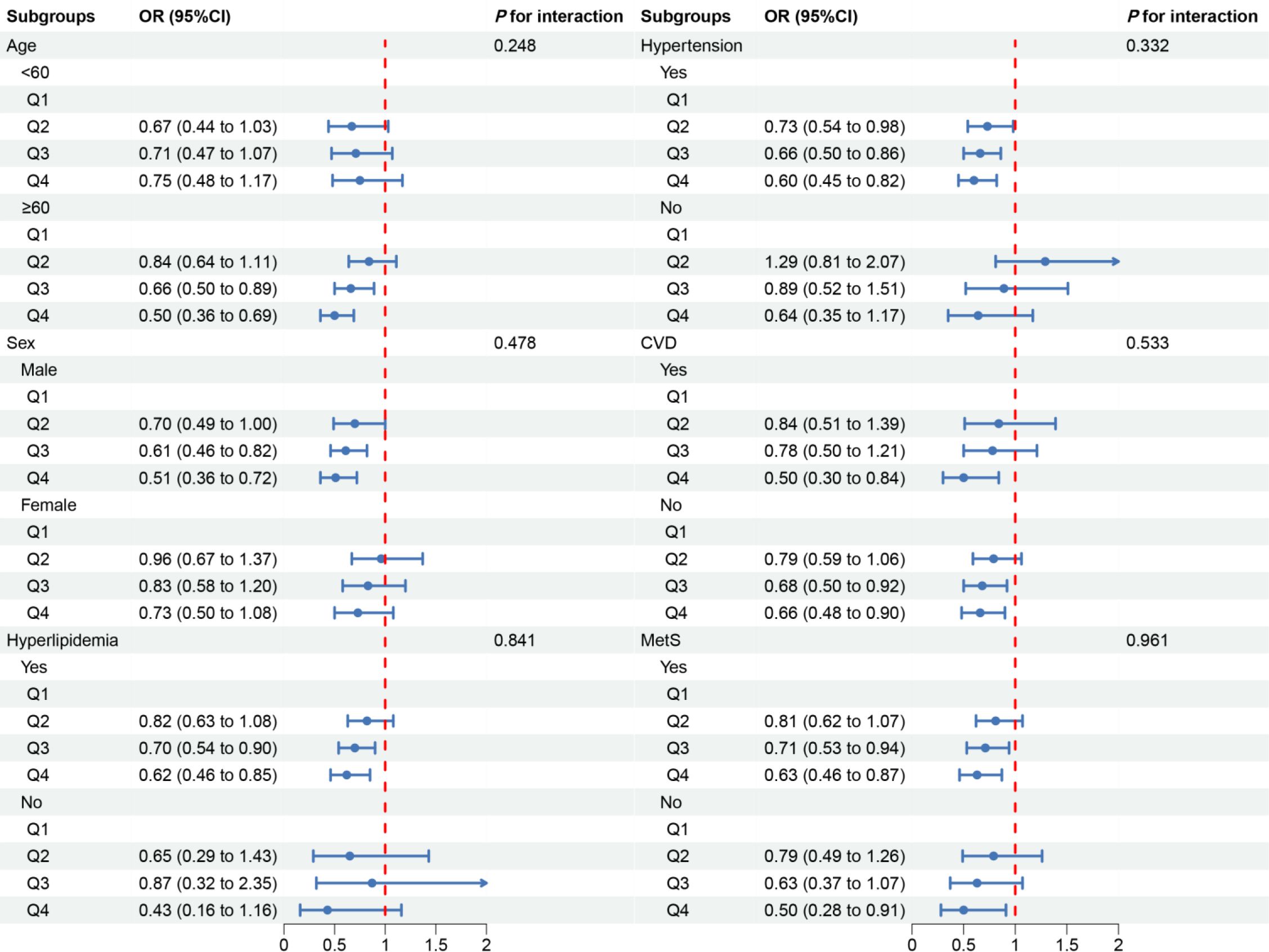
Figure 3 Associations between OBS and DKD in different subgroups. OR, odds ratio; 95% CI, 95% confidence interval; CVD, cardiovascular disease; Mets, metabolic syndrome. Except for the stratification component itself, each stratification factor was adjusted for all covariates.
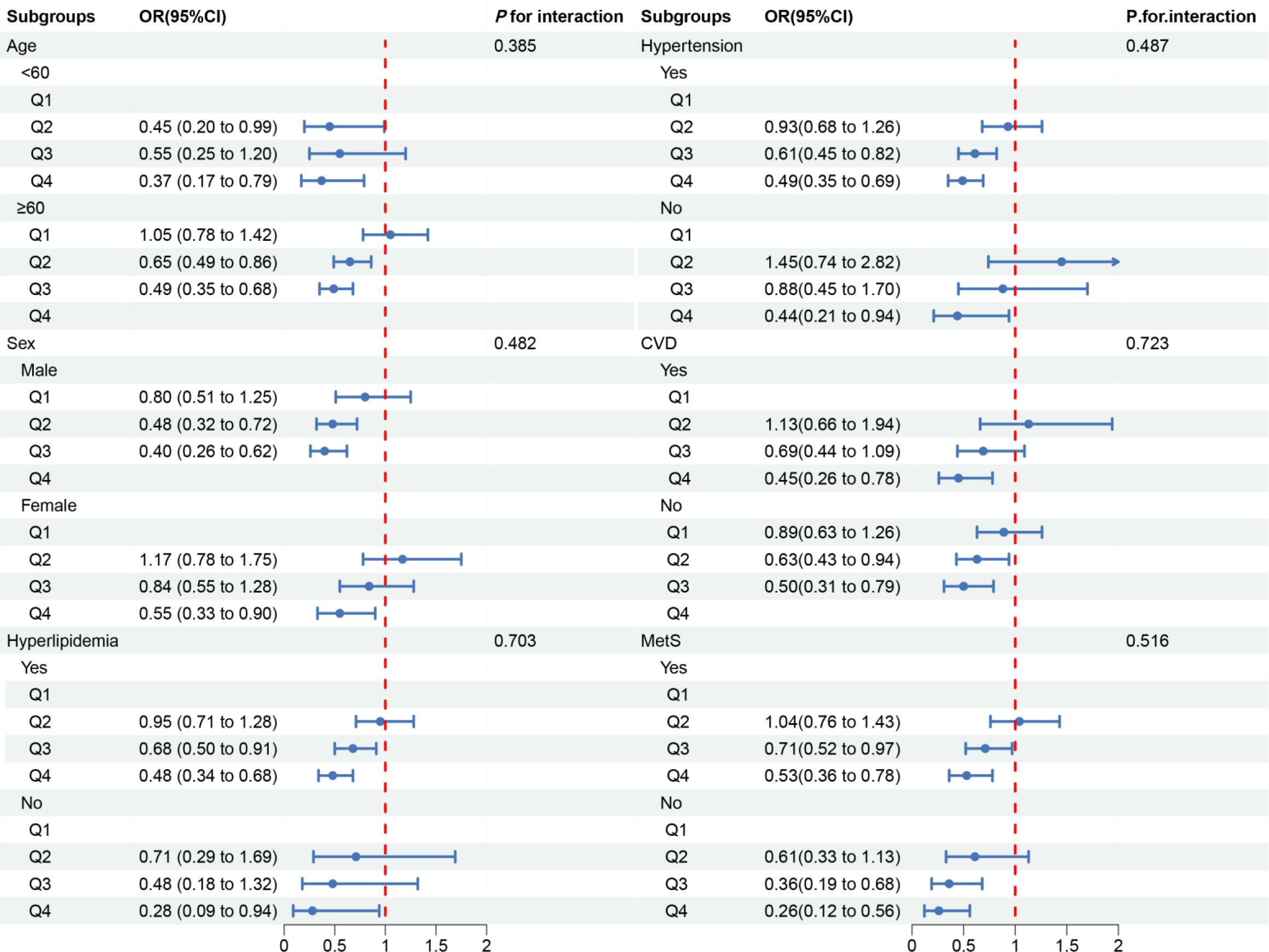
Figure 4 Associations between OBS and low-eGFR in different subgroups. OR, odds ratio; 95% CI, 95% confidence interval; CVD, cardiovascular disease; Mets, metabolic syndrome. Except for the stratification component itself, each stratification factor was adjusted for all covariates.
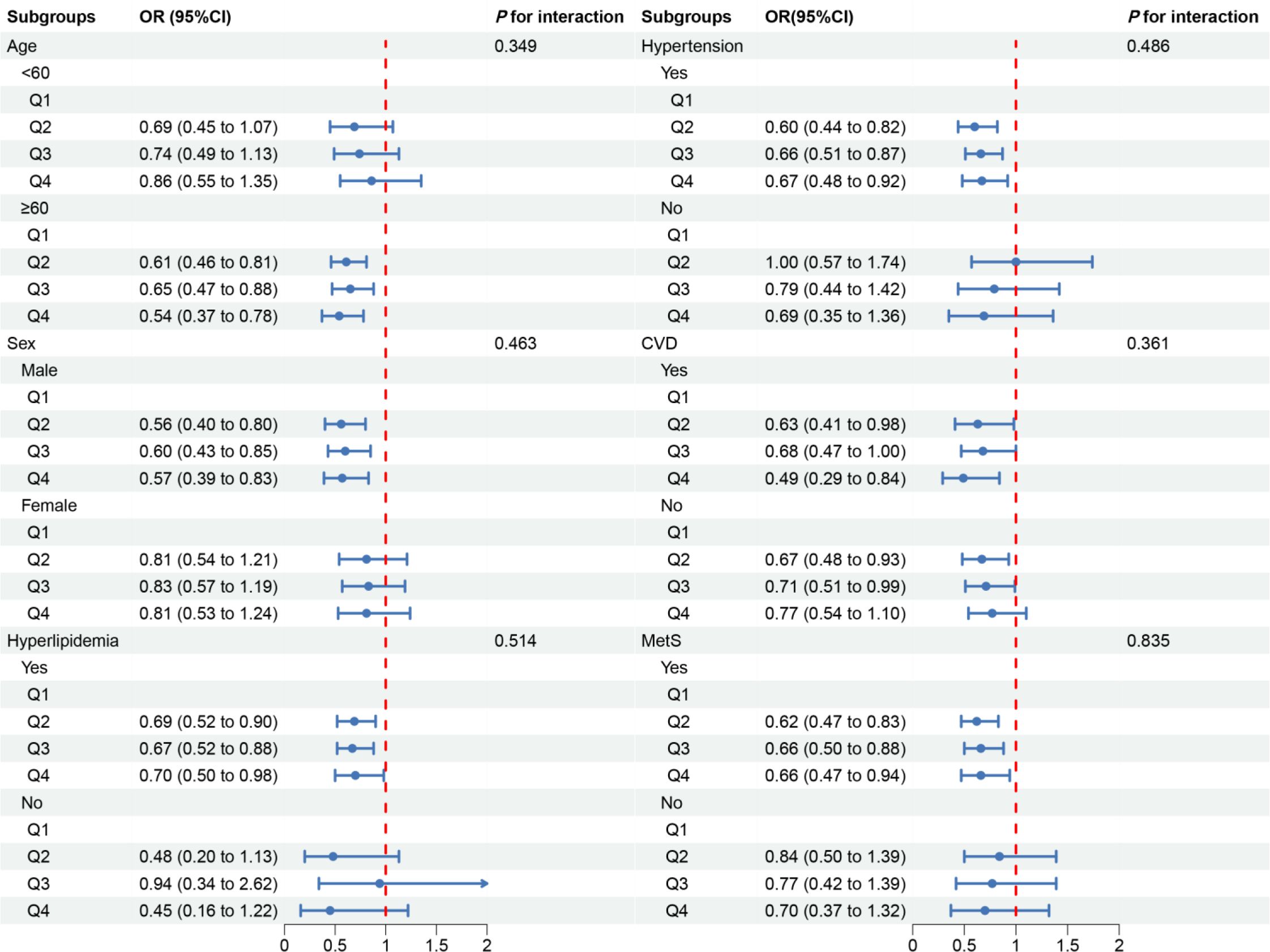
Figure 5 Associations between OBS and albuminuria in different subgroups. OR, odds ratio; 95% CI, 95% confidence interval; CVD, cardiovascular disease; Mets, metabolic syndrome. Except for the stratification component itself, each stratification factor was adjusted for all covariates.
4 Discussion
We conducted a cross-sectional analysis of 5,389 participants from the NHANES, representing an estimated 23.6 million noninstitutionalized residents of the United States. To the best of our knowledge, this is the first study to explore the relationship between OBS and DKD. The results showed that OBS, as well as dietary OBS, was negatively associated with the risk of DKD, low-eGFR, and albuminuria, but no significant correlation was found between lifestyle OBS and these clinical outcomes. In the subgroup analysis, OBS was associated with DKD in both the CVD and Mets subgroups, in other subgroups, the association was observed only in individuals aged ≥60 years, males, those with hyperlipidemia or hypertension. Collectively, our study provides preliminary evidence for the relationship between OBS and DKD and offers new insights for future clinical and basic research.
In this study, we investigated the relationship between 20 oxidative stressors and DKD. Most of these oxidative stressors have been shown to prevent the development of DKD by modulating redox homeostasis. A cross-sectional study based on the MHANES database similarly found that maintaining an adequate antioxidant diet, as reflected in higher composite dietary antioxidant index (CDAI) levels, may lower the risk of DKD and mortality in diabetic individuals (29). This further supports our findings, yet differently, the OBS incorporates a more comprehensive range of antioxidant dietary factors while also considering lifestyle factors, which may provide a broader perspective for the comprehensive management of diabetic patients. Another study assessing the correlation between OBS, CDAI, and diabetic retinopathy (DR) found a negative association between OBS and DR after adjusting for potential confounders, but no such association was found in CDAI (30). It is well-known that both DR and DKD are microvascular complications of diabetes, with a shared pathogenesis involving endothelial dysfunction, and oxidative stress being one of the key pathological pathways (31). Additionally, some studies have focused on the impact of single factors on DKD, with varying results. In terms of dietary factors, an animal experiment (32) have suggested that crocin (water-soluble carotenoids) may reduce proteinuria levels by its antioxidant properties, enhancing the host’s antioxidant defense system, inhibiting inflammation and fibrosis. Ozcelik et al. (33, 34) found that zinc sulfate supplementation in diabetic rats can reduce kidney damage by activating metallothionein, which interact with Zn and iron to reduce ROS. A study (35) has demonstrated that vitamin C and E supplementation, as well as a combination of magnesium, zinc, and vitamins C and E, can decrease urinary albumin excretion levels and improve glomerular function in type 2 diabetes patients. Selenium supplementation for 12 weeks in patients with DKD has been shown to be beneficial for plasma glutathione peroxidase (GPx) and serum insulin levels (36). Folic acid, betaine, vitamins B6 and B12 have also been reported to delay the development of T2 DM by methylating degraded homocysteine (37). However, a meta-analysis suggests that neither vitamin B alone nor in combination improved renal function or blood pressure in diabetic patients. Due to the limited number and lower quality of included studies, these findings require further confirmation (38). In terms of lifestyle, exercise training has been shown to alleviate oxidative stress and inflammation in type 2 diabetic rats (39). Moreover, a lack of physical activity is more likely to cause obesity, exacerbating kidney damage. Smoking can lead to insulin resistance, induce advanced glycation end products, and increase kidney vascular permeability. Excessive alcohol consumption leads to the production of large amounts of ROS and reduces the antioxidant activity of GPx (40). Most of the above studies focusing on the association of a single factor with DKD are consistent with our results considering multiple factors together, suggesting that OBS may improve DKD by modulating oxidative homeostasis.
In the present study, restricted cubic spline regression showed that the OR of albuminuria showed a trend of decreasing followed by a slight increase with increasing dietary OBS, but overall the risk of albuminuria was still significantly lower in the higher dietary OBS group compared with the lower dietary OBS group, which may be related to the threshold effect of some antioxidants and interactions between antioxidants (41), beyond which antioxidants may exhibit pro-oxidant or depleting antioxidant properties. For example, the antioxidant properties of carotenoids depend on their interactions with vitamins E and C (42), and carotenoids may lose their antioxidant properties at high concentrations or high partial pressures of oxygen. Copper may also be pro-oxidant in certain environments, and vitamin E may play an inhibitory role in copper-dependent low density lipoprotein oxidation. In addition, lifestyle factors such as adequate physical activity, smoking cessation, alcohol restriction and weight control have beneficial antioxidant effects in a variety of diseases, including DKD. However, no direct relationship between lifestyle OBS and DKD, low-eGFR and albuminuria was found in the present study, which may be due to the insufficient degree of lifestyle variability in the study population or the possibility that lifestyle factors may influence DKD by interacting with other variables. Studies have reported clear benefits of an antioxidant lifestyle in reducing blood pressure and improving insulin resistance (43, 44). A cohort study conducted in China (45) explored the relationship between the triglyceride-glucose (TyG) index, a biomarker associated with insulin resistance, and the risk of CKD in hypertensive patients with abnormal glucose metabolism. The study found that a higher TyG index was associated with an increased risk of CKD, suggesting that an antioxidant lifestyle may indirectly influence the progression of DKD by affecting metabolic parameters such as the TyG index (46–48). For this reason, the present study also prefers to use the overall OBS to systematically assess the redox status of the organism and to avoid considering the effect of a single factor while ignoring the complex correlations and interactions between antioxidants.
In subgroup analyses, OBS was associated with DKD in both the CVD and Mets subgroups, in other subgroups, the association was present only in individuals aged 60 years or older, males and those with hyperlipidemia or hypertension. A cross-sectional study suggests that OBS is inversely associated with accelerated phenotypic ageing (49). Oxidative damage is considered to contribute to the progression of ageing and fundamental components of pathological pathways, which are thought to drive various age-related diseases (50), such as DKD. Hypertension, hyperlipidemia, and type 2 diabetes are common comorbidities. Compared to non-diabetic individuals, diabetic patients have twice the incidence of hypertension (51), and the specific pathogenesis is related to oxidative stress. Hyperglycemia, hypertension, and hyperlipidemia can all lead to increased vascular ROS generation, and oxidative stress activation promotes post-translational oxidation of proteins, mitochondrial dysfunction, etc., thereby causing cellular damage and vascular dysfunction (52), and leading to kidney injury. Furthermore, it has been reported that gender differences may also be one of the key factors affecting the progression of DKD (53). In animal models, estrogens can counteract renal fibrosis and apoptosis (54), while testosterone promotes inflammatory, apoptotic, and fibrotic processes (55), which may explain the significant association between OBS and DKD in male populations. However, these findings differ from human-based studies, which indicate that oral contraceptives and estrogen replacement therapy are associated with an increased risk of microalbuminuria and declining renal function (56, 57). Therefore, the different mechanisms of gender differences in DKD warrant further exploration. Furthermore, considering the possibility of multiple comparisons and type I errors, the results of subgroup analyses still need to be validated in future studies.
This study has several strengths. Firstly, it is based on the NHANES database, which employs a multistage complex probability sampling design and weighted adjustments, enhancing the representativeness and reliability of the study results—a crucial factor for improving the generalizability of our findings within the American context. Secondly, our study provides preliminary evidence of the correlation between OBS and DKD, with information on OBS being obtained solely through questionnaires and incorporating the comprehensive impact of 20 oxidative stress factors, which enables OBS to have the potential as a tool for diabetes risk stratification. This integration offers new insights for the development of personalized and more effective preventive strategies. Furthermore, our research paves the way for future longitudinal study designs and the exploration of the mechanisms by which OBS affects DKD. Confirmation and expansion of these findings will assist in the formulation or adjustment of public health policies, such as recommending specific antioxidant dietary and lifestyle practices for diabetes patients, which will contribute to the broader goal of reducing the global burden of DKD.
This study, despite employing weighted multivariate logistic regression models for adjustment and validating the stability of results through subgroup analyses, still has certain limitations. Firstly, the inherent nature of cross-sectional study restricts the establishment of a causal relationship between OBS and DKD. Acknowledging this limitation, we recommend that future research on the relationship between OBS and DKD should employ a longitudinal design. This would enable researchers to determine whether OBS precedes the development of DKD, thereby providing more compelling evidence for a potential causal relationship. Secondly, despite the stratified and multistage sampling methods used in NHANES aiming to produce a nationally representative sample, there may still be some potential selection biases, such as non-response bias, self-selection bias, and three-stage sampling bias. These biases could result in the study findings differing from the true situation in the overall population. Although we used the weighted methods recommended by NHANES guidelines to analyze the data, which may have corrected this bias to some extent, caution should still be exercised when interpreting the results. Additionally, the OBS data in this study were collected through a 24-hour food recall questionnaire, a convenient method but potentially subject to recall bias and selection bias. In future studies, we will work towards identifying relevant objective biological markers to reduce this bias. Thirdly, the study sample was limited to the US population, hence the generalizability of the results to other racial backgrounds requires further validation in diverse racial populations.
5 Conclusion
Our study findings indicated that higher levels of OBS and dietary OBS were associated with a reduced risk of DKD, low-eGFR, and albuminuria. These results provided preliminary evidence for the importance of adhering to an antioxidant-rich diet and lifestyle among diabetic patients and highlighted the potential utility in formulating feasible dietary and lifestyle recommendations for them. Consequently, the integration of OBS into clinical practice may represent an innovative strategy to mitigate the burden of DKD in diabetic patients. However, further large-scale prospective studies are necessary to validate and expand upon our observations.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: The data employed in this study can be accessed through the NHANES website: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CL: Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. JY: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing. HL: Conceptualization, Methodology, Visualization, Writing – review & editing. YD: Formal analysis, Software, Visualization, Writing – review & editing. PH: Conceptualization, Methodology, Validation, Visualization, Writing – review & editing. JZ: Validation, Writing – review & editing. MZ: Data curation, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Project of Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital (No. 403245).
Acknowledgments
The author thanks the staff and the participants of the NHANES study for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1412823/full#supplementary-material
Supplementary Table 1 | Components of the oxidative balance score. OBS, oxidative balance score; A, antioxidant; P, prooxidant; RE, retinol equivalent; ATE, alpha-tocopherol equivalent; MET, metabolic equivalent.
Supplementary Table 2 | The collinearity assessment outcomes. Family PIR, family poverty income ratio; TC, total cholesterol; TG, triglycerides; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin.
References
1. Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besancon S, et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2020) 162:108072. doi: 10.1016/j.diabres.2020.108072
2. Ruiz-Ortega M, Rodrigues-Diez RR, Lavoz C, Rayego-Mateos S. Special issue "Diabetic nephropathy: diagnosis, prevention and treatment. J Clin Med. (2020) 9:813. doi: 10.3390/jcm9030813
3. Wyld M, Morton RL, Aouad L, Magliano D, Polkinghorne KR, Chadban S. The impact of comorbid chronic kidney disease and diabetes on health-related quality-of-life: a 12-year community cohort study. Nephrol Dial Transplant. (2021) 36:1048–56. doi: 10.1093/ndt/gfaa031
4. Nichols GA, Ustyugova A, Deruaz-Luyet A, O'Keeffe-Rosetti M, Brodovicz KG. Health Care Costs by Type of Expenditure across eGFR Stages among Patients with and without Diabetes, Cardiovascular Disease, and Heart Failure. J Am Soc Nephrol. (2020) 31:1594–601. doi: 10.1681/ASN.2019121308
5. Vallon V, Komers R. Pathophysiology of the diabetic kidney. Compr Physiol. (2011) 1:1175–232. doi: 10.1002/cphy.c100049
6. Tuttle KR, Agarwal R, Alpers CE, Bakris GL, Brosius FC, Kolkhof P, et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. (2022) 102:248–60. doi: 10.1016/j.kint.2022.05.012
7. Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. Jama. (2002) 288:2579–88. doi: 10.1001/jama.288.20.2579
8. Pichler R, Afkarian M, Dieter BP, Tuttle KR. Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am J Physiol Renal Physiol. (2017) 312:F716–31. doi: 10.1152/ajprenal.00314.2016
9. Su S, Ma Z, Wu H, Xu Z, Yi H. Oxidative stress as a culprit in diabetic kidney disease. Life Sci. (2023) 322:121661. doi: 10.1016/j.lfs.2023.121661
10. Rapa SF, Di Iorio BR, Campiglia P, Heidland A, Marzocco S. Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int J Mol Sci. (2019) 21:263. doi: 10.3390/ijms21010263
11. Zhong Q, Piao Y, Yin S, Zhang K. Association of serum lycopene concentrations with all-cause and cardiovascular mortality among individuals with chronic kidney disease: a cohort study. Front Nutr. (2022) 9:1048884. doi: 10.3389/fnut.2022.1048884
12. Hodkova M, Dusilova-Sulkova S, Kalousova M, Soukupova J, Zima T, Mikova D, et al. Influence of oral vitamin E therapy on micro-inflammation and cardiovascular disease markers in chronic hemodialysis patients. Ren Fail. (2006) 28:395–9. doi: 10.1080/08860220600683698
13. Son DH, Lee HS, Seol SY, Lee YJ, Lee JH. Association between the oxidative balance score and incident chronic kidney disease in adults. Antioxidants (Basel). (2023) 12:335. doi: 10.3390/antiox12020335
14. Ilori TO, Sun RY, Kong SY, Gutierrez OM, Ojo AO, Judd SE, et al. Oxidative balance score and chronic kidney disease. Am J Nephrol. (2015) 42:320–7. doi: 10.1159/000441623
15. Liu Z, Zeng H, Zhang H. Association of the oxidation balance score with the prevalence of chronic obstructive pulmonary disease from the NHANES 2007-2012: a large-scale cross-sectional study. Heart Lung. (2024) 65:84–92. doi: 10.1016/j.hrtlng.2024.02.005
16. Chen X, Wang C, Dong Z, Luo H, Ye C, Li L, et al. Interplay of sleep patterns and oxidative balance score on total cardiovascular disease risk: Insights from the National Health and Nutrition Examination Survey 2005-2018. J Glob Health. (2023) 13:4170. doi: 10.7189/jogh.14.04170
17. Liu X, Liu X, Wang Y, Zeng B, Zhu B, Dai F. Association between depression and oxidative balance score: National Health and Nutrition Examination Survey (NHANES) 2005-2018. J Affect Disord. (2023) 337:57–65. doi: 10.1016/j.jad.2023.05.071
18. Archer E, Pavela G, Lavie CJ. The inadmissibility of what we eat in America and NHANES dietary data in nutrition and obesity research and the scientific formulation of national dietary guidelines. Mayo Clin Proc. (2015) 90:911–26. doi: 10.1016/j.mayocp.2015.04.009
19. Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat. (2013) 2:1–24.
20. Zhang W, Peng SF, Chen L, Chen HM, Cheng XE, Tang YH. Association between the oxidative balance score and telomere length from the national health and nutrition examination survey 1999-2002. Oxid Med Cell Longev. (2022) 2022:1345071. doi: 10.1155/2022/1345071
21. Ma R, Xie C, Wang S, Xiao X. Retinol intake is associated with the risk of chronic kidney disease in individuals with type 2 diabetes mellitus: results from NHANES. Sci Rep. (2023) 13:11567. doi: 10.1038/s41598-023-38582-z
22. Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100:S1–276. doi: 10.1016/j.kint.2021.05.021
23. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AR, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
24. Li X, Wang L, Liu M, Zhou H, Xu H. Association between neutrophil-to-lymphocyte ratio and diabetic kidney disease in type 2 diabetes mellitus patients: a cross-sectional study. Front Endocrinol (Lausanne). (2023) 14:1285509. doi: 10.3389/fendo.2023.1285509
25. Guo W, Song Y, Sun Y, Du H, Cai Y, You Q, et al. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011-2018. Front Endocrinol (Lausanne). (2022) 13:1071465. doi: 10.3389/fendo.2022.1071465
26. Xiao Y, Xiao Z. Association between serum klotho and kidney stones in US middle-aged and older individuals with diabetes mellitus: results from 2007 to 2016 national health and nutrition survey. Am J Nephrol. (2023) 54:224–33. doi: 10.1159/000531045
27. Liu H, Wang D, Wu F, Dong Z, Yu S. Association between inflammatory potential of diet and self-reported severe headache or migraine: a cross-sectional study of the National Health and Nutrition Examination Survey. Nutrition. (2023) 113:112098. doi: 10.1016/j.nut.2023.112098
28. Dang X, Yang R, Jing Q, Niu Y, Li H, Zhang J, et al. Association between high or low-quality carbohydrate with depressive symptoms and socioeconomic-dietary factors model based on XGboost algorithm: From NHANES 2007-2018. J Affect Disord. (2024) 351:507–17. doi: 10.1016/j.jad.2024.01.220
29. Zhang J, Chen Y, Zou L, Jin L, Yang B, Shu Y, et al. Dose-response relationship between dietary antioxidant intake and diabetic kidney disease in the US adults with diabetes. Acta Diabetol. (2023) 60:1365–75. doi: 10.1007/s00592-023-02125-9
30. Qiao Q, Liu X, Xue W, Chen L, Hou X. Analysis of the association between high antioxidant diet and lifestyle habits and diabetic retinopathy based on NHANES cross-sectional study. Sci Rep. (2024) 14:11868. doi: 10.1038/s41598-024-62707-7
31. Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes. (2017) 9:434–49. doi: 10.1111/1753-0407.12521
32. Abou-Hany HO, Atef H, Said E, Elkashef HA, Salem HA. Crocin mediated amelioration of oxidative burden and inflammatory cascade suppresses diabetic nephropathy progression in diabetic rats. Chem Biol Interact. (2018) 284:90–100. doi: 10.1016/j.cbi.2018.02.001
33. Ozcelik D, Naziroglu M, Tuncdemir M, Celik O, Ozturk M, Flores-Arce MF. Zinc supplementation attenuates metallothionein and oxidative stress changes in kidney of streptozotocin-induced diabetic rats. Biol Trace Elem Res. (2012) 150:342–9. doi: 10.1007/s12011-012-9508-4
34. Cai L. Metallothionein as an adaptive protein prevents diabetes and its toxicity. Nonlinearity Biol Toxicol Med. (2004) 2:89–103. doi: 10.1080/15401420490464367
35. Farvid MS, Jalali M, Siassi F, Hosseini M. Comparison of the effects of vitamins and/or mineral supplementation on glomerular and tubular dysfunction in type 2 diabetes. Diabetes Care. (2005) 28:2458–64. doi: 10.2337/diacare.28.10.2458
36. Bahmani F, Kia M, Soleimani A, Asemi Z, Esmaillzadeh A. Effect of selenium supplementation on glycemic control and lipid profiles in patients with diabetic nephropathy. Biol Trace Elem Res. (2016) 172:282–9. doi: 10.1007/s12011-015-0600-4
37. Zhu J, Saikia G, Zhang X, Shen X, Kahe K. One-carbon metabolism nutrients, genetic variation, and diabetes mellitus. Diabetes Metab J. (2024) 48:170–83. doi: 10.4093/dmj.2023.0272
38. Raval AD, Thakker D, Rangoonwala AN, Gor D, Walia R. Vitamin B and its derivatives for diabetic kidney disease. Cochrane Database Syst Rev. (2015) 1:CD009403. doi: 10.1002/14651858.CD009403.pub2
39. Saberi S, Askaripour M, Khaksari M, Amin RM, Abbas BM, Akhbari M, et al. Exercise training improves diabetic renal injury by reducing fetuin-a, oxidative stress and inflammation in type 2 diabetic rats. Heliyon. (2024) 10:e27749. doi: 10.1016/j.heliyon.2024.e27749
40. Ojeda ML, Nogales F, Del CGM, Carreras O. Binge drinking during the adolescence period causes oxidative damage-induced cardiometabolic disorders: a possible ameliorative approach with selenium supplementation. Life Sci. (2022) 301:120618. doi: 10.1016/j.lfs.2022.120618
41. Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins. Ann N Y Acad Sci. (1992) 669:7–20. doi: 10.1111/j.1749-6632.1992.tb17085.x
42. Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. (2001) 385:20–7. doi: 10.1006/abbi.2000.2149
43. Valenzuela PL, Carrera-Bastos P, Galvez BG, Ruiz-Hurtado G, Ordovas JM, Ruilope LM, et al. Lifestyle interventions for the prevention and treatment of hypertension. Nat Rev Cardiol. (2021) 18:251–75. doi: 10.1038/s41569-020-00437-9
44. Papakonstantinou E, Oikonomou C, Nychas G, Dimitriadis GD. Effects of diet, lifestyle, chrononutrition and alternative dietary interventions on postprandial glycemia and insulin resistance. Nutrients. (2022) 14:823. doi: 10.3390/nu14040823
45. Zhu Q, Chen Y, Cai X, Cai L, Hong J, Luo Q, et al. The non-linear relationship between triglyceride-glucose index and risk of chronic kidney disease in hypertensive patients with abnormal glucose metabolism: a cohort study. Front Med (Lausanne). (2022) 9:1018083. doi: 10.3389/fmed.2022.1018083
46. Lu J, Li Z, Yang Y, Wei F. Chronic exercise improves renal at(1) and ETB receptor functions via modulating GRK4 expression in obese Zucker rats. Clin Exp Hypertens. (2024) 46:2323532. doi: 10.1080/10641963.2024.2323532
47. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. (2004) 114:1752–61. doi: 10.1172/JCI21625
48. Lin L, Tan W, Pan X, Tian E, Wu Z, Yang J. Metabolic syndrome-related kidney injury: a review and update. Front Endocrinol (Lausanne). (2022) 13:904001. doi: 10.3389/fendo.2022.904001
49. Wu D, Shen Y, Qu C, Huang P, Geng X, Zhang J, et al. Association between dietary and behavioral-based oxidative balance score and phenotypic age acceleration: a cross-sectional study of Americans. Epidemiol Health. (2024) 46:e2024023. doi: 10.4178/epih.e2024023
50. Mozzini C, Pagani M. Oxidative stress in chronic and age-related diseases. Antioxidants (Basel). (2022) 11:521. doi: 10.3390/antiox11030521
51. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. (2018) 34:575–84. doi: 10.1016/j.cjca.2017.12.005
52. Adeshara KA, Diwan AG, Tupe RS. Diabetes and complications: cellular signaling pathways, current understanding and targeted therapies. Curr Drug Targets. (2016) 17:1309–28. doi: 10.2174/1389450117666151209124007
53. Giandalia A, Giuffrida AE, Gembillo G, Cucinotta D, Squadrito G, Santoro D, et al. Gender differences in diabetic kidney disease: focus on hormonal, genetic and clinical factors. Int J Mol Sci. (2021) 22:5808. doi: 10.3390/ijms22115808
54. Stringer KD, Komers R, Osman SA, Oyama TT, Lindsley JN, Anderson S. Gender hormones and the progression of experimental polycystic kidney disease. Kidney Int. (2005) 68:1729–39. doi: 10.1111/j.1523-1755.2005.00589.x
55. Metcalfe PD, Leslie JA, Campbell MT, Meldrum DR, Hile KL, Meldrum KK. Testosterone exacerbates obstructive renal injury by stimulating TNF-alpha production and increasing proapoptotic and profibrotic signaling. Am J Physiol Endocrinol Metab. (2008) 294:E435–43. doi: 10.1152/ajpendo.00704.2006
56. Ahmed SB, Culleton BF, Tonelli M, Klarenbach SW, Macrae JM, Zhang J, et al. Oral estrogen therapy in postmenopausal women is associated with loss of kidney function. Kidney Int. (2008) 74:370–6. doi: 10.1038/ki.2008.205
Keywords: diabetic kidney disease, oxidative balance score, type 2 diabetes mellitus, NHANES, cross-sectional study
Citation: Liu C, Yang J, Li H, Deng Y, He P, Zhang J and Zhang M (2024) Association between oxidative balance score and diabetic kidney disease, low estimated glomerular filtration rate and albuminuria in type 2 diabetes mellitus patients: a cross-sectional study. Front. Endocrinol. 15:1412823. doi: 10.3389/fendo.2024.1412823
Received: 05 April 2024; Accepted: 16 July 2024;
Published: 31 July 2024.
Edited by:
Federico Biscetti, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Wencai Liu, Shanghai Jiao Tong University, ChinaXintian Cai, People’s Hospital of Xinjiang Uygur Autonomous Region, China
Copyright © 2024 Liu, Yang, Li, Deng, He, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mianzhi Zhang, emhhbmdtaWFuemhpQHZpcC5zaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Cong Liu
Cong Liu Jiju Yang
Jiju Yang Hongdian Li1
Hongdian Li1