- Department of Respiratory and Critical Care Medicine, Renmin Hospital of Wuhan University, Wuhan, China
Purpose: SARS-CoV-2 can invade the thyroid gland. This study was to delineate the risk of thyroid dysfunction amidst the prevalence of the Omicron variant, and to investigate the correlation between thyroid function and Coronavirus disease 2019 (COVID-19) outcomes. The study also aimed to ascertain whether thyroid dysfunction persisted during COVID-19 recovery phase.
Methods: This was a retrospective cohort study. COVID-19 patients from the Renmin Hospital of Wuhan University, China during the epidemic of Omicron variants were included, and their thyroid function were analyzed in groups.
Results: A history of thyroid disease was not associated with COVID-19 outcomes. COVID-19 can lead to a bimodal distribution of thyroid dysfunction. The severity of COVID-19 was inversely proportional to the levels of thyroid- stimulating hormone (TSH), free triiodothyronine (FT3) and free thyroxine (FT4), leading to a higher prevalence of thyroid dysfunction. Severe COVID-19 was a risk factor for euthyroid sick syndrome (ESS) (OR=22.5, 95% CI, 12.1 - 45.6). Neutrophil to lymphocyte ratio mediated the association between severe COVID-19 and ESS (mediation effect ratio = 41.3%, p < 0.001). ESS and decreased indicators of thyroid function were associated with COVID-19 mortality, while high levels of FT3 and FT4 exhibited a protective effect against death. This effect was more significant in women (p < 0.05). During the recovery period, hyperthyroidism was quite uncommon, while a small percentage of individuals (7.7%) continued to exhibit hypothyroidism.
Conclusion: COVID-19 severity was linked to thyroid dysfunction. Severe COVID-19 increased the risk of ESS, which was associated with COVID-19 mortality. Post-recovery, hyperthyroidism was rare, but some individuals continued to have hypothyroidism.
Introduction
SARS-CoV-2 is the causative agent in COVID-19 (1). The virus’s envelope contains a spike glycoprotein that interacts with the angiotensin-converting enzyme 2 (ACE2) with high specificity and affinity (2). ACE2, a transmembrane protein is found in various organs, including the endocrine system, potentially facilitating the transmission of the virus to these organs. Within the endocrine system, ACE2 is most abundantly present in the testicles, followed by the thyroid and the hypothalamus. The presence of ACE2 in the thyroid renders it a viable target for viral entry (3). Autopsy samples procured from the thyroid gland post-mortem have demonstrated that SARS-CoV-2 can directly infect the thyroid gland, the direct viral insult combined with an intense immune response may trigger or worsen thyroid conditions in predisposed individuals (4, 5).
Thyroid hormones play a pivotal role in regulating the immune system (6). Consequently, COVID-19 could potentially influence thyroid function, and in ture, the state of thyroid function could impact the prognosis of COVID-19.
Existing perspectives suggest that abnormal thyroid function can manifest during the active phase of COVID-19 and persist into the convalescence phase (7). ESS is most prevalent among COVID-19 patients (3) and has been identified as an independent risk factor for disease severity (8). Furthermore, a reduction in FT3 levels has been independently associated with all-cause mortality in patients with severe or critical COVID-19 (9). However, most of the existing research data were collected during the initial phase of the pandemic, when the SARS-CoV-2 variants of concern (VOC) were not yet widespread. Due to the limited sample size, there is a lack of comprehensive studies describing the relationship between COVID-19 and thyroid function. Furthermore, while it is known that both high and low levels of thyroid hormone can be detrimental, no previous studies have explored the non-linear relationship between these hormone levels and the outcomes of COVID-19.
From late 2022 to early 2023, China experienced a pandemic of Omicron variants. Therefore, the aim of this study is to construct a comprehensive map of COVID-19 and thyroid function in a large retrospective cohort during the Omicron variants epidemic.
Materials and methods
Subjects and study design
Our study included 1505 patients admitted to Renmin Hospital of Wuhan University in China from December 15, 2022, to January 25, 2023. All individuals had access to serum FT3, FT4, and TSH concentrations, and were diagnosed with COVID-19. Among them, 111 had pre-existing thyroid conditions, while the remaining 1394 did not. COVID-19 outcomes were compared between these two groups. The 1394 patients without thyroid disease were further categorized into four severity groups (mild, moderate, severe, critical) and two outcome groups (survival, death). Thyroid function and diagnostic categories were compared across these groups. For the longitudinal analysis of thyroid function, we examined the patient records in our study for any thyroid function tests conducted prior to their COVID-19 diagnosis (‘Before COVID-19’) and any follow-up thyroid function tests conducted after their initial hospital admission for COVID-19 (‘After COVID-19’). The study was approved by the Ethics Committee of Renmin Hospital of Wuhan University, and informed consent was exempted due to its retrospective nature.
COVID-19 diagnosis and severity
A COVID-19 diagnosis is confirmed through a real-time reverse transcriptase polymerase chain reaction (RT-PCR) test, using a nasopharyngeal swab. The severity of the disease is classified into four categories: Mild disease: Characterized by mild clinical symptoms without any evidence of pneumonia on imaging. Moderate disease: Defined by the presence of fever and respiratory symptoms, with imaging revealing signs of pneumonia. Severe disease: Diagnosed if any of the following conditions are met: respiratory rate ≥30/min, SpO2 ≤ 93% at rest, and >50% progression in 48 hours on imaging. Critical disease: Identified by the occurrence of respiratory failure necessitating mechanical ventilation, shock, or the requirement for admission to an intensive care unit (10).
Thyroid diagnostic categories
All samples were analyzed using the ADVIA 2400 Automatic Biochemical Analyzer from Siemens, Germany. The reagents for the serum free triiodothyronine (FT3), free thyroxine (FT4), and thyroid-stimulating hormone (TSH) tests were all products of Siemens. Normal ranges are as follows: TSH (0.55-4.78 mIU/L), T3 (2.3-4.2 pg/mL), and T4 (0.89-1.76 ng/dL). Overt hyperthyroidism is defined as a subnormal serum TSH with elevated FT3 and/or FT4. Subclinical hyperthyroidism is defined as a subnormal TSH with normal FT3 and FT4 (11). Overt hypothyroidism is defined as TSH above the reference range and FT3 and/or FT4 below the reference range. Subclinical hypothyroidism is defined by TSH above the reference range and both FT3, FT4 within the normal range (12). Euthyroid sick syndrome (ESS) is characterized by a decreased FT3 and/or FT4 without an increased TSH (8). Euthyroid hyperthyroxinemia/TSH-mediated hyperthyroidism are defined by TSH within or above the reference range and FT3 and/or FT4 above the normal range (11). TSH, FT3, and FT4 are all within the normal range defined as euthyroid.
Statistical analysis
Continuous variables were expressed as median (IQR) and were compared using the Mann–Whitney U test for two-group comparisons and Kruskal-Wallis test for comparisons among four groups. Categorical variables were presented as absolute values (n) or percentages (%) and were analyzed using the chi-square test. Multivariate logistic regression analyses were conducted to explore the relationships among thyroid disease and outcomes of COVID-19, the severity of COVID-19 and ESS, as well as ESS and mortality. Causal mediation analysis was conducted to examine the relationships between severe/critical COVID-19 and ESS, as well as between severe/critical COVID-19 and mortality. Restricted cubic spline (RCS) analyses were performed to investigate the nonlinear relationships between levels of TSH, FT3, and FT4, and the risk of mortality, separately for males and females. Wilcoxon paired rank sum tests and Friedman test were used for pairwise comparison of TSH, FT3, FT4 in the longitudinal data set. All statistical analyses were performed using R software version 4.3.0 (http://www.r-project.org), and double-sided P < 0.05 was defined as statistical significance.
Results
Association between thyroid disease and COVID-19
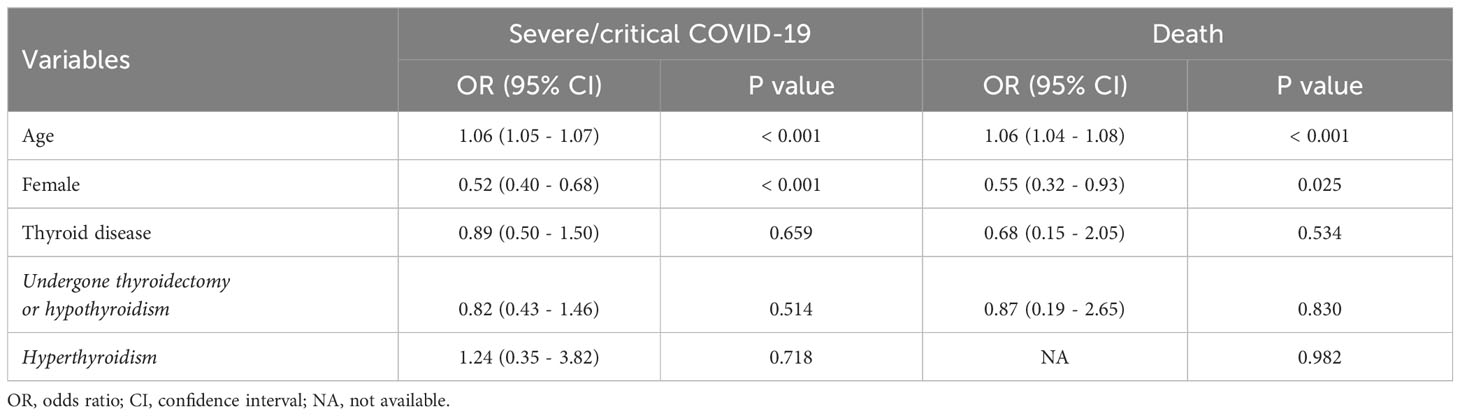
In the cohort, patients had preexisting thyroid disease had a higher proportion of women compared to the group without thyroid disease (80.2% vs 45.0%). However, no significant disparities were observed in the COVID-19 severity and mortality rates between these two groups (Table 1). A multivariate logistic regression analysis revealed that age was risk factor for severe COVID-19 and mortality, while female gender appeared to be a protective factor. A history of thyroid disease, both hyperthyroidism and thyroidectomy/hypothyroidism, did not exhibit any association with the COVID-19 outcomes (Table 2).
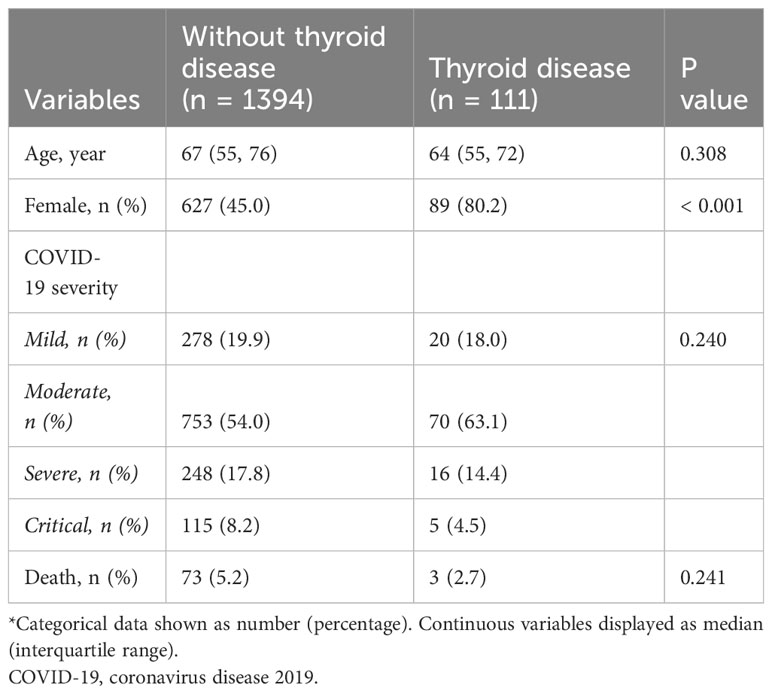
Table 1 Comparative clinical characteristics of groups without and with a history of thyroid disease*.
Association between COVID-19 severity and thyroid dysfunction
As COVID-19 severity increased, there were corresponding rises in infection indicators like white blood cell (WBC) count, neutrophil to lymphocyte ratio (NLR), C-reactive protein (CRP), serum amyloid A (SAA), and inflammatory mediators such as interleukin-6 (IL-6) and interleukin-10 (IL-10). Additionally, there was an observed dysfunction in humoral immunity, as indicated by elevated levels of immunoglobulin E (IgE). Conversely, cellular immune function tended to decrease with the severity, as evidenced by lower counts of CD3, CD4, and CD8 T cells. Furthermore, there was a noted decrease in the levels of FT3, FT4, and TSH (Table 3).
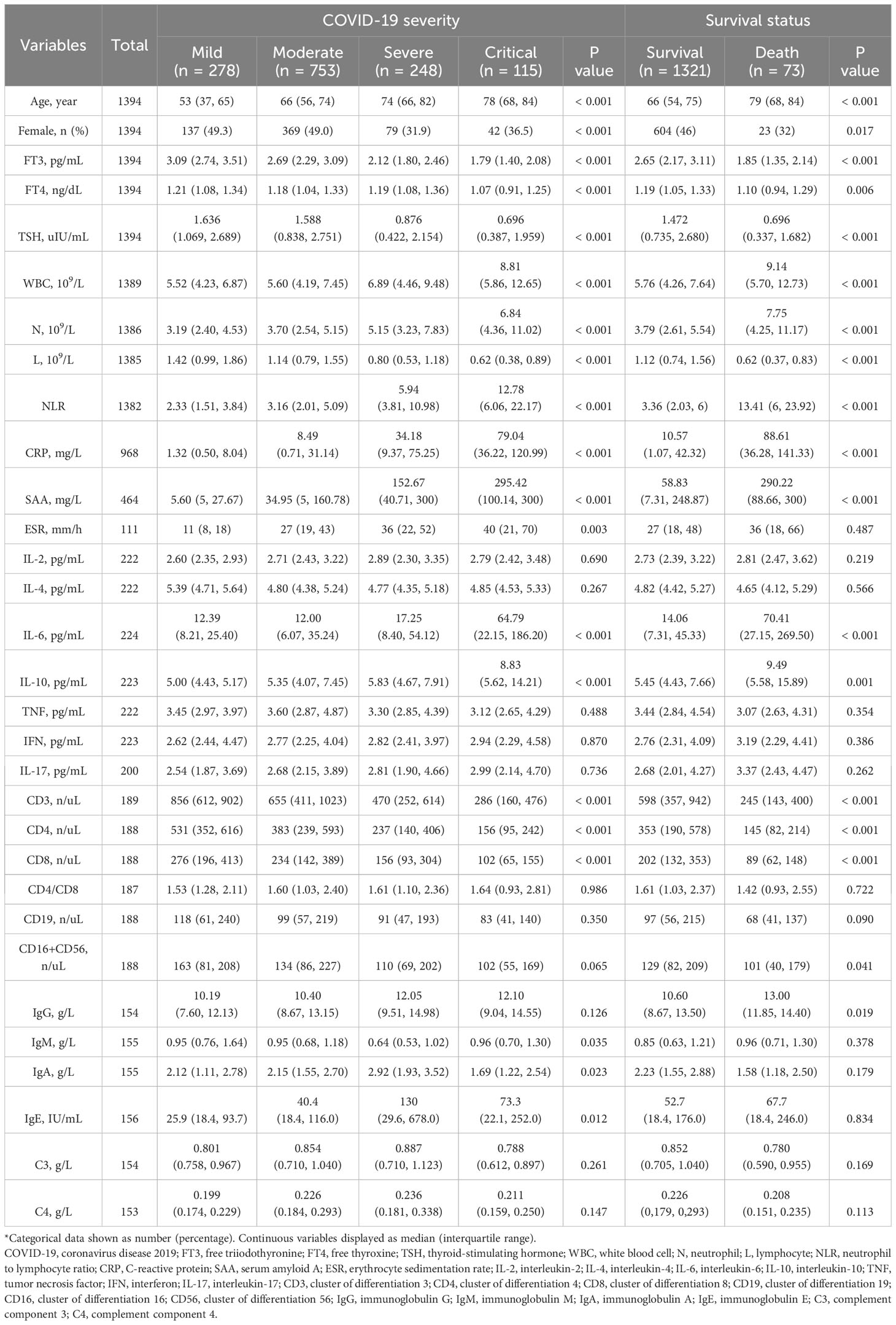
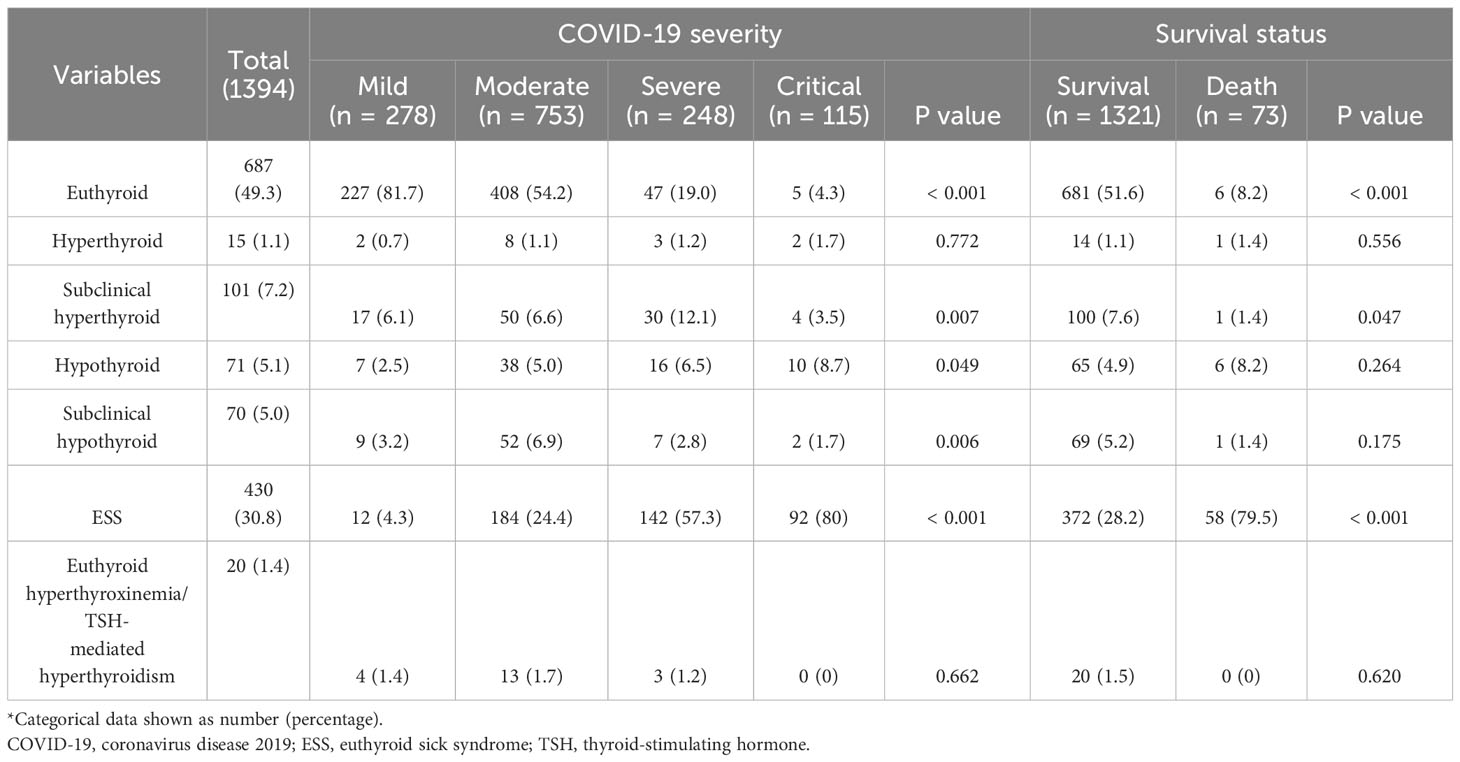
Table 3 Demographic characteristics and laboratory findings across various severity levels and outcomes of COVID-19*.
Out of the total patients, 687 (49.3%) were euthyroid and 430 (30.8%) were diagnosed with ESS. As COVID-19 severity increased, there was a corresponding increase in the proportions of patients with subclinical hyperthyroidism, hypothyroidism and ESS. Specifically, the prevalence of hypothyroidism was 2.5% in mild cases, 5.0% in moderate cases, 6.5% in severe cases, and 8.7% in critical cases (p=0.049). Similarly, the prevalence of ESS was 4.3% in mild cases, 24.4% in moderate cases, 57.3% in severe cases, and 80% in critical cases (p<.001). Interestingly, the proportions of patients with subclinical hypothyroidism decreased with COVID-19 severity. However, the proportions of hyperthyroidism and euthyroid hyperthyroxinemia/TSH-mediated hyperthyroidism were minimal and remained consistent across all severity groups (Table 4).
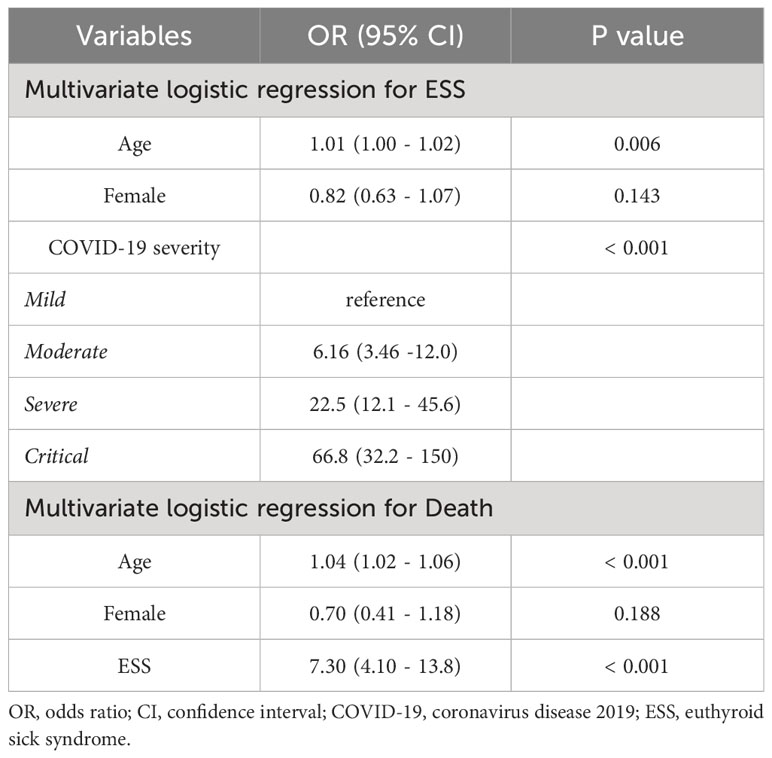
After adjusting for age and sex, the severity of COVID-19 was identified as a risk factor for ESS. Specifically, patients with severe COVID-19 were found to be 22.5 times (95% CI, 12.1 – 45.6) more likely to manifest ESS than those with mild COVID-19 (Table 5).
A mediation analysis was performed to explore the potential mediating role of laboratory findings in the relationship between severe/critical COVID-19 and ESS. As depicted in Figure 1A, the NLR was found to significantly mediate this relationship, accounting for 41.3% of the effect (p <.001). However, no significant mediating effects were detected for IL-6, CD3, and IgE (all p > 0.05).
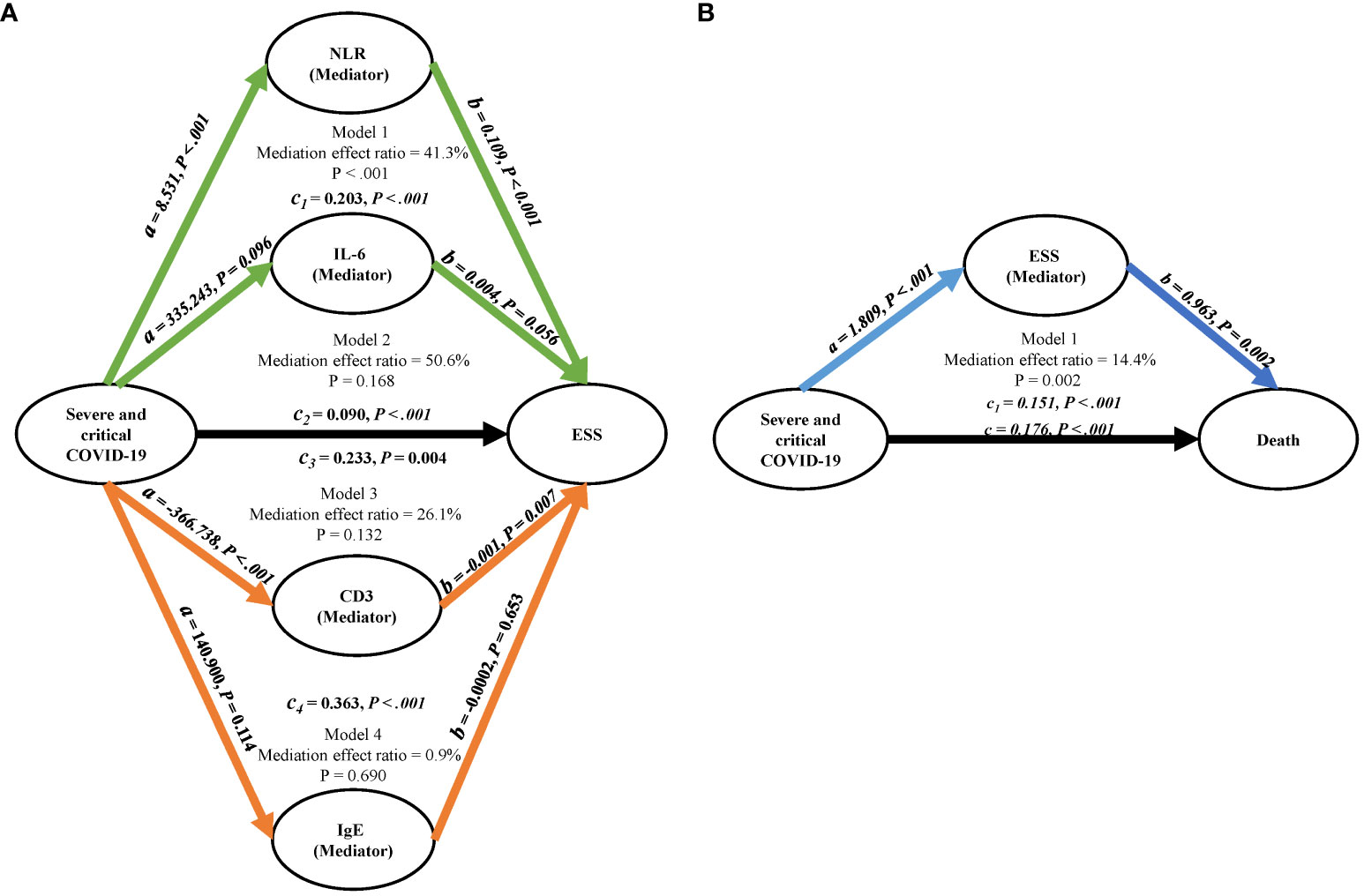
Figure 1 Mediation analysis diagrams. (A) Represents the mediation factors NLR, IL-6, CD3, and IgE in the relationship between severe/critical COVID-19 and ESS. (B) Illustrates ESS as the mediating factor in the relationship between severe/critical COVID-19 and death; Adjusted for age and gender. COVID-19, coronavirus disease 2019; NLR, neutrophil to lymphocyte ratio; CD3, cluster of differentiation 3; IgE, immunoglobulin E; ESS, euthyroid sick syndrome.
Association between thyroid dysfunction and COVID-19 mortality
As shown in the right section of Table 3, the group of COVID-19 patients who did not survive exhibited higher infection indicators and increased levels of inflammatory mediators compared to the group who survived. Furthermore, the non-survival group displayed compromised cellular immune function and diminished thyroid function. However, no significant differences were noted in the humoral immune function between the two groups, except for a mild increase in IgG levels in the non-survival group.
The group of patients who did not survive had a lower proportion of euthyroidism (8.2% vs 51.6%, p<.001) and a higher incidence of ESS (79.5% vs 28.2%, p<.001) compared to the group who survived. Interestingly, the survival group had a higher proportion of subclinical hyperthyroidism (7.6% vs 1.4%, p=0.047). No significant differences were observed in other types of thyroid dysfunction between the two groups (Table 4).
After adjusting for age and sex, ESS was identified as a risk factor for COVID-19 mortality (OR = 7.30, 95% CI, 4.10 – 13.8) (Table 5). As depicted in Figure 1B, ESS was found to significantly mediate the relationship between severe/critical COVID-19 and death, accounting for 14.4% of the effect (p = 0.002).
To investigated the relationship between thyroid function indicators and mortality due to COVID-19, RCS nonlinear correlation curves were constructed separately for male and female populations. These curves reflected the individual correlations between TSH, FT3, FT4, and COVID-19 mortality. In both male and female populations, decreased levels of TSH, FT3, and FT4 (though FT4 was not significant in women) were associated with an increased risk of death from COVID-19. Interestingly, high levels of FT3 were found to have a protective effect against death in both male and female populations, as well as high levels of FT4 in women (Figure 2).
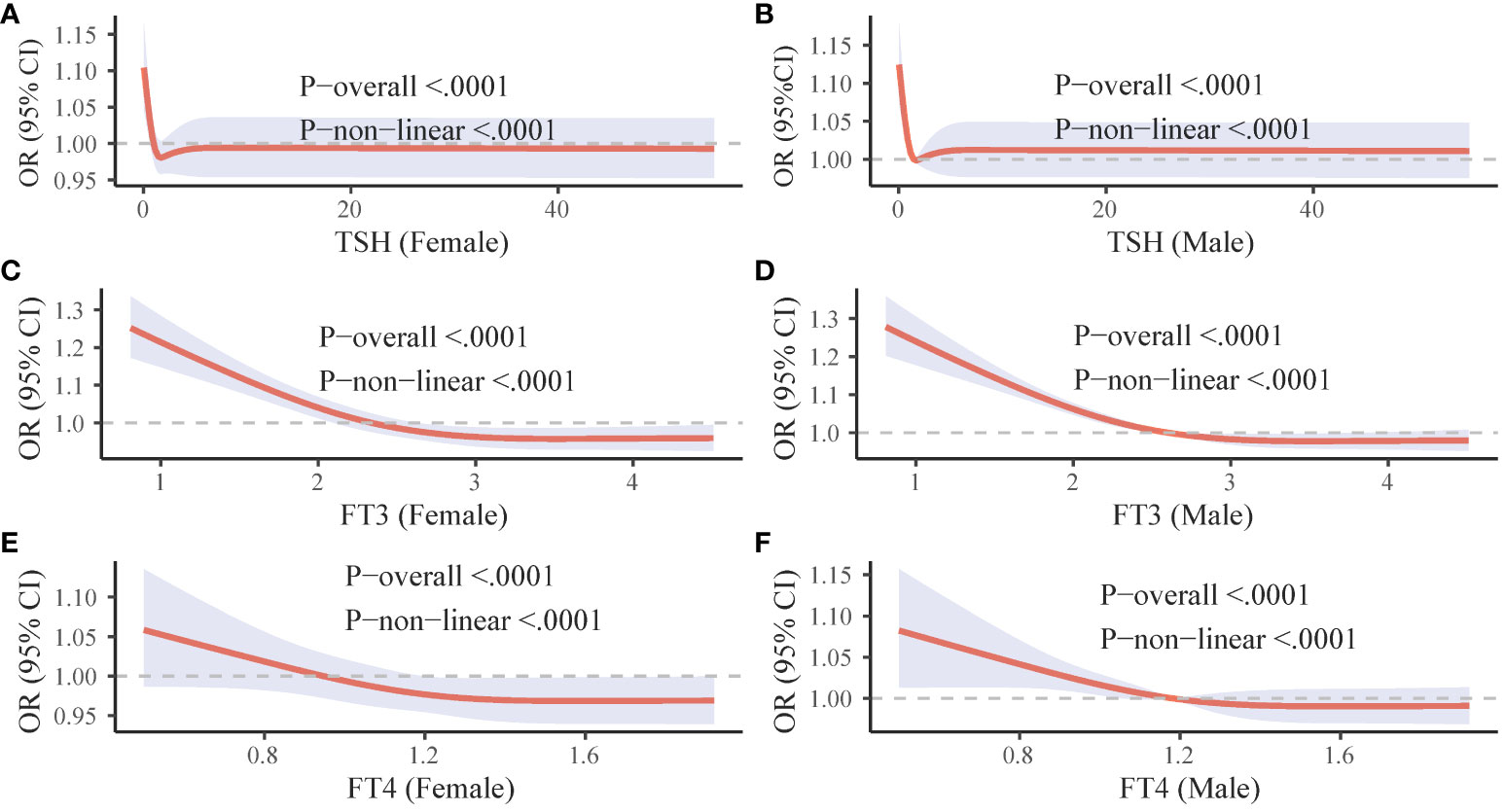
Figure 2 Gender-specific RCS curves for thyroid hormone levels and COVID-19 mortality. The figure is divided into six panels. (A, C, and E (left column) illustrate the association between TSH, FT3, and FT4 levels respectively, and COVID-19 mortality in females. (B, D, and F (right column) depict the corresponding associations in males. OR, odds ratio; CI, confidence interval; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone.
Thyroid function across different time points
Of 1394 patients without prior thyroid disease, thyroid function tests were available for 411 patients before COVID-19 and for 234 survivors during their convalescence. As depicted in the left section (A, C, E) of Figure 3, both TSH and FT3 levels at the time of COVID-19 admission were significantly lower than their respective baseline values, while FT4 levels were significantly higher (all p <0.05). Conversely, the right section (B, D, F) of Figure 3 shows that during the recovery period, both TSH and FT3 levels were significantly higher than those at admission, while FT4 levels were significantly lower (all p <0.05).
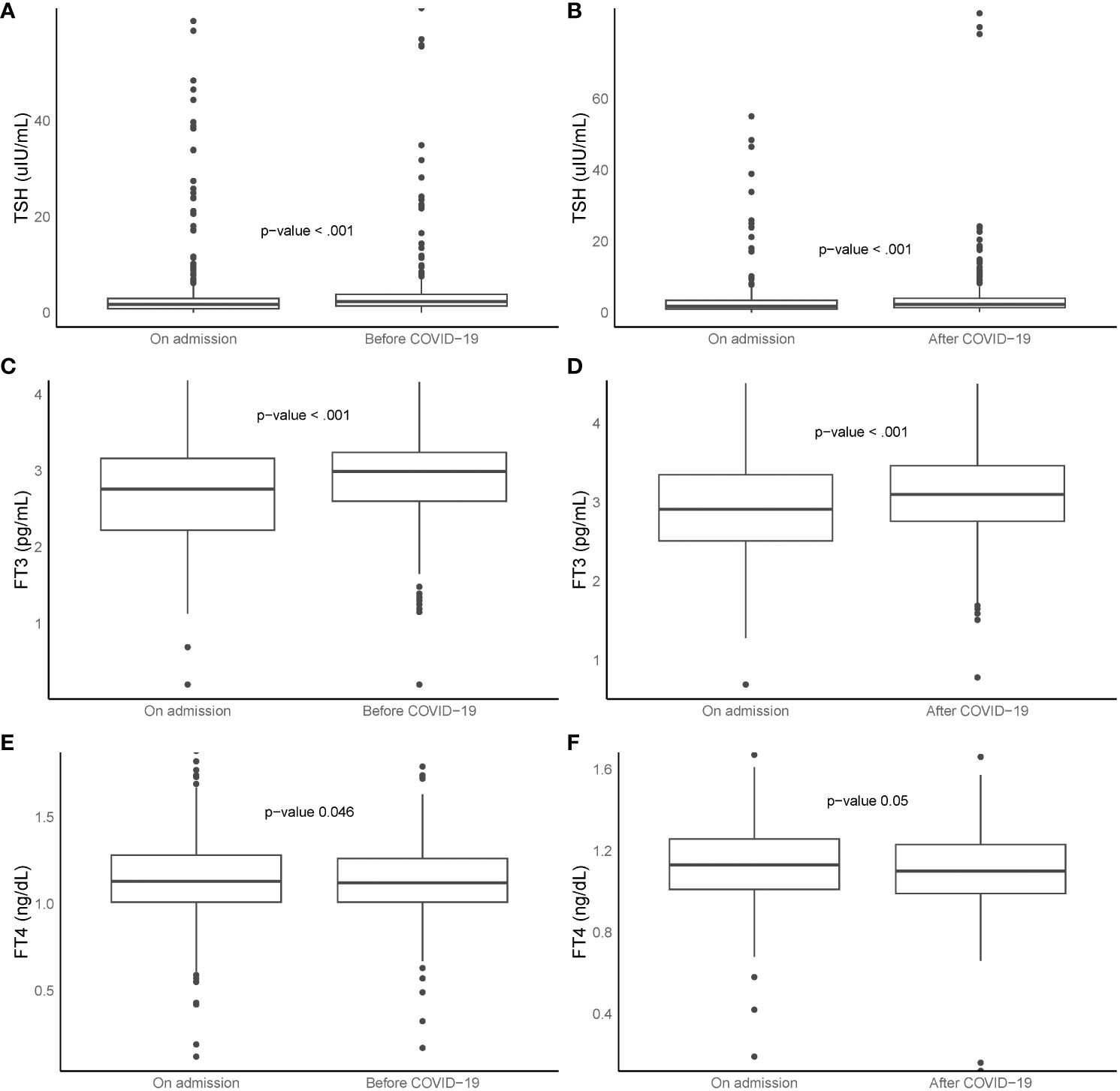
Figure 3 Box plots represent TSH, FT3, and FT4 levels at ‘before COVID-19’, ‘on admission’, and ‘after COVID-19’. Left section (A, C, E, n = 411): comparison between ‘before COVID-19’ and ‘on admission’. Right section (B, D, F, n = 234): comparison between ‘on admission’ and ‘after COVID-19’. Boxes indicate 25th and 75th percentiles, whiskers indicate 5th and 95th percentiles, and line in box indicates median. Wilcoxon paired rank sum tests used for comparisons. FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone; COVID-19, coronavirus disease 2019.
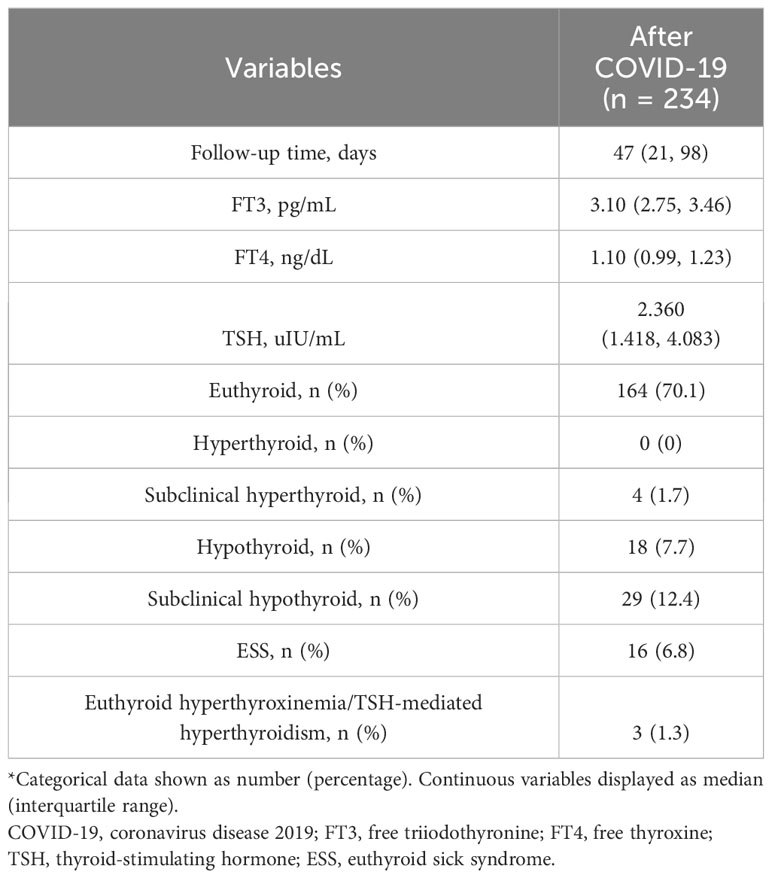
During the recovery phase from COVID-19, with a median follow-up of 47 days (ranging from 21 to 98 days), the majority of survivors (70.1%) exhibited euthyroidism. Hyperthyroxinemia and subclinical hyperthyroidism were very rare, with no cases of overt hyperthyroidism observed. However, a small proportion of patients still exhibited ESS (6.8%), hypothyroidism (7.7%), and subclinical hypothyroidism (12.4%) (Table 6).
A subset of 164 patients with complete sets of T3, T4, and TSH measurements taken at three distinct time points: before, during, and after COVID-19. These trends were visually represented in Figure 4. The data showed that both FT3 and TSH levels initially decreased upon admission, but eventually returned to their baseline levels during the recovery phase. In contrast, FT4 levels remained relatively stable, with no significant change observed (p = 0.639).
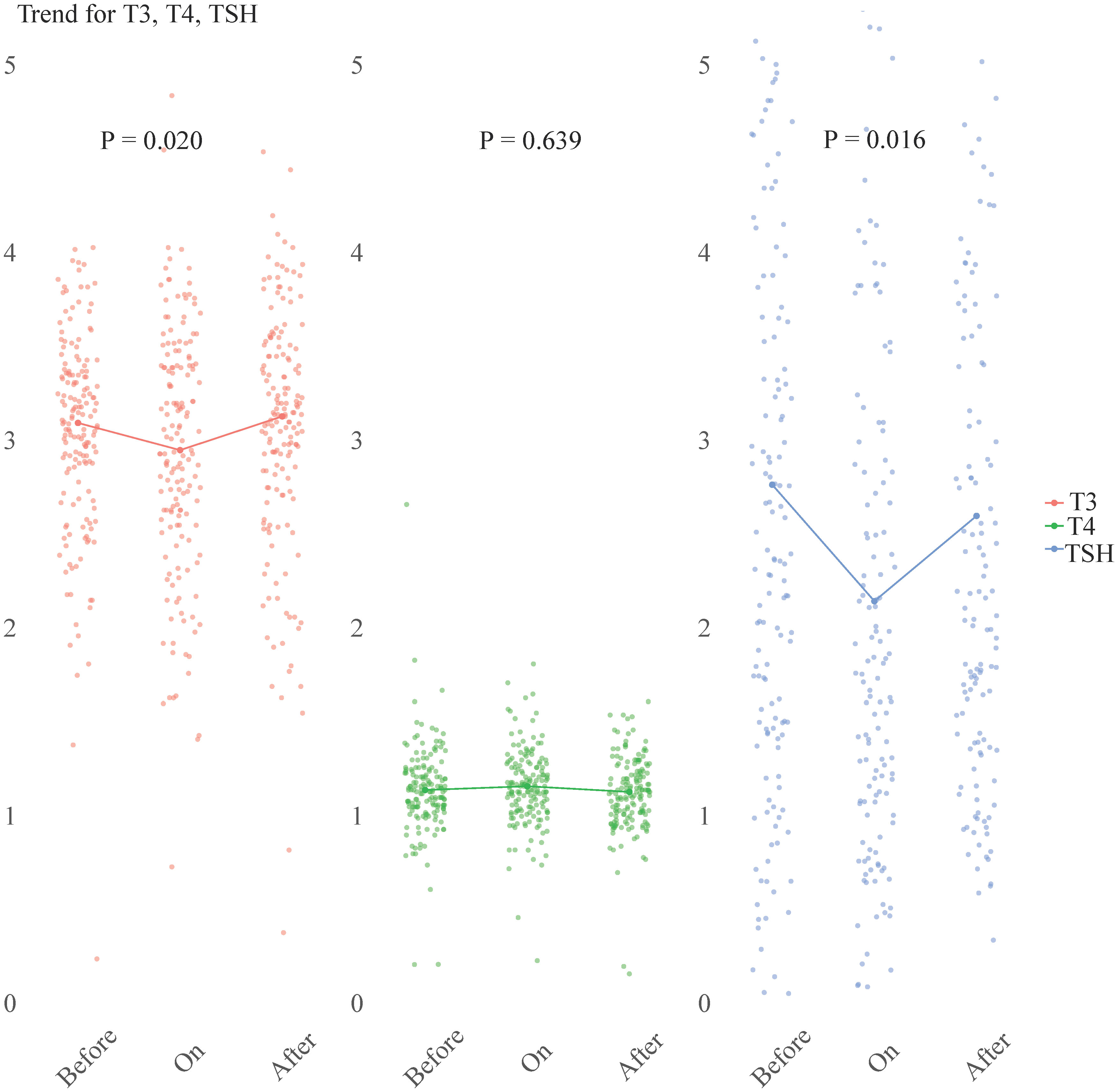
Figure 4 Longitudinal study of TSH, FT3, and FT4 levels at three distinct time points: ‘before COVID-19’, ‘on admission’, and ‘after COVID-19’. The scatter plots (n = 164) represent the individual hormone levels at each time point. Within each section, a line connects the median values of T3, T4, and TSH, providing a visual representation of the central tendency. Statistical analysis was performed using the Friedman test. FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone.
Discussion
In this study, we found that a prior history of thyroid disease, encompassing both hyperthyroidism and thyroidectomy/hypothyroidism, did not impact the prognosis of COVID-19 during the Omicron variant outbreak. A previous retrospective cohort study involving 3703 COVID-19 patients revealed that 6.8% had pre-existing hypothyroidism. This condition was not associated with an increased risk of hospitalization or mortality (13). But, a recent study has identified the absence of a history of hyperthyroidism as a protective factor against COVID-19 mortality (14). It’s crucial to underscore that in our cohort, among those with a history of thyroid disease, a mere 16 individuals (14.4%) had hyperthyroidism, while the majority 95 individuals (85.6%) had undergone thyroidectomy or were hypothyroidism (not reflected in the tables). Therefore, the conclusions of this study align with those of previous research.
We underscored that COVID-19 severity was inversely related to the plasma levels of TSH, FT3, and FT4, thereby increasing the risk of hypothyroidism and ESS. A meta-analysis encompassing 565 samples from 6 studies revealed that the severe COVID-19 group exhibited significantly lower TSH and FT3 levels compared to the mild group. However, no significant differences were observed in FT4 levels between the groups (15). The variations in FT4 levels observed in our study can be attributed to a more detailed classification of COVID-19 severity. Notably, only the decrease in FT4 levels in critically ill patients reached statistical significance. A recent meta-analysis found that among COVID-19 patients, ESS was the most prevalent thyroid disease with a pooled prevalence of 26%, followed by thyrotoxicosis and hypothyroidism, with pooled prevalences of 10% and 3%, respectively (3). In contrast, our study observed a different pattern. ESS had an incidence of 30.8%, and hypothyroidism was more prevalent than in the meta-analysis. The rate of hyperthyroidism was notably lower at 1.1%. This discrepancy in the prevalence of hyperthyroidism and hypothyroidism may be a characteristic of the Omicron variant infection. Further exploration revealed that COVID-19 severity independently contributes to the risk of ESS. Interestingly, the impact of severe COVID-19 on ESS appeared to be partially mediated through the NLR (mediation effect ratio = 41.3%). Studies have shown that neutrophils exhibited continuous basal hyperactivation in the peripheral circulating blood of COVID-19 patients, which was associated with vascular endothelial injury (16). Neutrophils played a crucial role in the progression of COVID-19 through various mechanisms, including cytokine storms, tissue injury, and thrombotic events (17). In infected thyroid tissues, infiltrates of innate immune cells (macrophages and polymorphonuclear neutrophils) were prevalent (5). In fact, a high NLR was associated with severe COVID-19 and poor prognosis (18). In the context of SARS-CoV-2 infection, it’s currently understood that the immune system may become hyperactive, potentially leading to the development and progression of autoimmune thyroid diseases. This phenomenon might be attributed to abnormal responses of T-cell subtypes, the presence of autoantibodies, and an overproduction of inflammatory cytokines, specifically IL-6, IFN-γ, and TNF-α (19). Our study did observe an increase in the level of inflammatory factors, depletion of cellular immunity, and disorder of humoral immunity with the severity of COVID-19. However, these findings may be limited by the number of patients tested. The roles of IL-6, CD3, and IgE as mediating factors of severe COVID-19 and ESS were not found to be significant.
Although a history of thyroid disease was not found to be associated with COVID-19 outcomes, this study discovered a correlation between thyroid dysfunction resulting from COVID-19 and an increased risk of mortality. Our findings confirmed that ESS was a risk factor for death from COVID-19, and that the high mortality rate of severe COVID-19 could be partially attributed to ESS, with a mediation effect ratio of 14.4%. ESS was typically associated with the severity of the disease and a deteriorating prognosis in critical illnesses. Consistent with previous studies, ESS was associated with severe disease and death from COVID-19, and was considered an early and reliable indicator of poor prognosis for COVID-19 (8, 20). Our study further demonstrated through the RCS curve that across all populations, low TSH and low FT3 increased the risk of death from COVID-19, while high FT3 appeared to have a protective effect. Many previous studies have also observed that low FT3 was more common in patients who died from COVID-19. Low FT3 was associated with excessive inflammation, coagulation, and disorders of the fibrinolytic system, making low FT3 status a risk factor for death (20–23). FT3 was considered to prevent early tissue hypoxia during sepsis, potentially reducing secondary organ failure (24). An ongoing randomized placebo-controlled clinical trial (NCT04348513) aims to investigate whether the administration of T3 (liothyronine, 0.8 g/kg i.v.) to ICU-admitted COVID-19 patients reduces their need for cardiorespiratory support (25). This suggests that our nonlinear model provided a more comprehensive description of the relationship between thyroid hormones and COVID-19 mortality. It’s important to note that the effects of FT3 and FT4 may differ between genders, and the protective effects of FT3 and FT4 may be more pronounced in women. This nuanced understanding of the role of thyroid hormones in COVID-19 outcomes underscores the complexity of this disease and the need for further research.
In paired analysis, we observed that the thyroid function in COVID-19 patients typically decreased upon admission and subsequently returned to baseline during the recovery phase. This observation aligned with previous research, which found that in follow-up studies of COVID-19 survivors, 82.4% (42 out of 51) of abnormal thyroid function tests observed during acute phase of COVID-19 resolved over a span of 6 months (26). Additionally, after a period of 3 or 4 months, COVID-19 patients who underwent pulmonary rehabilitation exhibited an increase in FT3 values (27). Moreover, the thyroid structure also demonstrated spontaneous recovery over time. with 85.7% (6 of 7) patients showing resolved features of thyroiditis after 4 months (28). However, it’s crucial to remain cognizant of the fact that some individuals may continue to experience hypothyroidism.
Our study presents distinct advantages. The substantial sample size of the cohort we included was ample to unveil, for the first time, a comprehensive association between COVID-19 and thyroid function during the Omicron pandemic. However, our research also has its limitations. Firstly, our study lacks data on thyroid autoantibodies and thyroid ultrasound, and dose not address the development of autoimmune conditions post COVID-19. Secondly, this study did not collect information on the underlying disease status and treatment status of COVID-19 patients, with only age and gender employed as confounding factors for analysis and exclusion. It has been demonstrated that obesity is strongly associated with COVID-19 severity and poor outcome, yet our study lacks data on body mass index. Lastly, our study is not prospective and thus cannot establish a definitive causal relationship between COVID-19 and thyroid function.
Conclusion
Our study delineates a reciprocal relationship between COVID-19 and thyroid function during the Omicron variant pandemic. COVID-19 can detrimentally impact thyroid function, leading to a bimodal distribution of thyroid dysfunction. Inflammation mediates the effects of COVID-19 on ESS, and ESS partially mediates mortality from severe COVID-19. Decreased thyroid function increases the risk of death from COVID-19, while elevated FT3 and FT4 levels appear to confer a protective effect, especially in women. Additionally, there remains a risk of hypothyroidism during the recovery phase from COVID-19. These findings underscore the importance of monitoring thyroid function in COVID-19 patients and highlight the potential therapeutic implications of managing thyroid hormone levels during both treatment and recovery phases.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Renmin Hospital of Wuhan University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because in accordance with standard research protocols, retrospective studies are typically exempt from the requirement of obtaining written informed consent. This is due to the fact that these studies utilize existing data and do not involve direct interaction with the participants or alterations to their behavior.
Author contributions
QZ: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. ZZ: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. XL: Writing – review & editing, Resources, Formal analysis. YW: Writing – review & editing, Resources, Formal analysis. HC: Writing – original draft, Formal analysis, Data curation, Conceptualization. YYH: Writing – original draft, Formal analysis, Data curation, Conceptualization. SZ: Writing – original draft, Formal analysis, Data curation, Conceptualization. JZ: Writing – review & editing, Formal analysis. YH: Writing – review & editing, Formal analysis. BZ: Writing – review & editing, Formal analysis. KH: Writing – review & editing, Supervision, Methodology.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (82270101), and the Science and technology key project on novel coronavirus pneumonia of Hubei province, China (2020FCA002).
Acknowledgments
The authors thank all participants included in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382(8):727–33. doi: 10.1056/NEJMoa2001017
2. Bakhshandeh B, Jahanafrooz Z, Abbasi A, Goli MB, Sadeghi M, Mottaqi MS, et al. Mutations in SARS-CoV-2; Consequences in structure, function, and pathogenicity of the virus. Microb Pathog. (2021) 154:104831. doi: 10.1016/j.micpath.2021.104831
3. Ashrafi S, Hatami H, Bidhendi-Yarandi R, Panahi MH. The prevalence of thyroid disorders in COVID-19 patients: a systematic review and meta-analysis. BMC Endocr Disord. (2024) 24(1):5. doi: 10.1186/s12902-023-01534-9
4. Macedo S, Pestana A, Santos L, Neves C, Guimarães S, Duarte-Neto A, et al. Detection of SARS-CoV-2 infection in thyroid follicular cells from a COVID-19 autopsy series. Eur Thyroid J. (2022) 11(4):e220074. doi: 10.1530/etj-22-0074
5. Poma AM, Basolo A, Bonuccelli D, Proietti A, Macerola E, Ugolini C, et al. Activation of type I and type II interferon signaling in SARS-coV-2-positive thyroid tissue of patients dying from COVID-19. Thyroid. (2021) 31(12):1766–75. doi: 10.1089/thy.2021.0345
6. De Vito P, Incerpi S, Pedersen JZ, Luly P, Davis FB, Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. (2011) 21(8):879–90. doi: 10.1089/thy.2010.0429
7. Rossetti CL, Cazarin J, Hecht F, Beltrão FEL, Ferreira ACF, Fortunato RS, et al. COVID-19 and thyroid function: What do we know so far? Front Endocrinol (Lausanne). (2022) 13:1041676. doi: 10.3389/fendo.2022.1041676
8. Zou R, Wu C, Zhang S, Wang G, Zhang Q, Yu B, et al. Euthyroid sick syndrome in patients with COVID-19. Front Endocrinol (Lausanne). (2020) 11:566439. doi: 10.3389/fendo.2020.566439
9. Gao W, Guo W, Guo Y, Shi M, Dong G, Wang G, et al. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinol Invest. (2021) 44(5):1031–40. doi: 10.1007/s40618-020-01460-w
10. Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY, et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab. (2021) 106(2):e926–35. doi: 10.1210/clinem/dgaa813
11. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. (2016) 26(10):1343–421. doi: 10.1089/thy.2016.0229
12. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. (2017) 390(10101):1550–62. doi: 10.1016/s0140-6736(17)30703-1
13. van Gerwen M, Alsen M, Little C, Barlow J, Naymagon L, Tremblay D, et al. Outcomes of patients with hypothyroidism and COVID-19: A retrospective cohort study. Front Endocrinol (Lausanne). (2020) 11:565. doi: 10.3389/fendo.2020.00565
14. Carvalho VP, Pontes JPJ, Neto DRB, Borges CER, Campos GRL, Ribeiro HLS, et al. Mortality and associated factors in patients with COVID-19: cross-sectional study. Vaccines (Basel). (2022) 11(1):71. doi: 10.3390/vaccines11010071
15. Wei J, Zhang F. Effects of SARS-CoV-2 infection on hypothyroidism and subclinical hypothyroidism: a meta-analysis. Front Endocrinol (Lausanne). (2023) 14:1291774. doi: 10.3389/fendo.2023.1291774
16. Loyer C, Lapostolle A, Urbina T, Elabbadi A, Lavillegrand JR, Chaigneau T, et al. Impairment of neutrophil functions and homeostasis in COVID-19 patients: association with disease severity. Crit Care. (2022) 26(1):155. doi: 10.1186/s13054-022-04002-3
17. Li J, Zhang K, Zhang Y, Gu Z, Huang C. Neutrophils in COVID-19: recent insights and advances. Virol J. (2023) 20(1):169. doi: 10.1186/s12985-023-02116-w
18. Higaki A, Okayama H, Homma Y, Sano T, Kitai T, Yonetsu T, et al. Predictive value of neutrophil-to-lymphocyte ratio for the fatality of COVID-19 patients complicated with cardiovascular diseases and/or risk factors. Sci Rep. (2022) 12(1):13606. doi: 10.1038/s41598-022-17567-4
19. Mohammadi B, Dua K, Saghafi M, Singh SK, Heydarifard Z, Zandi M, et al. COVID-19-induced autoimmune thyroiditis: Exploring molecular mechanisms. J Med Virol. (2023) 95(8):e29001. doi: 10.1002/jmv.29001
20. Sparano C, Zago E, Morettini A, Nozzoli C, Yannas D, Adornato V, et al. Euthyroid sick syndrome as an early surrogate marker of poor outcome in mild SARS-CoV-2 disease. J Endocrinol Invest. (2022) 45(4):837–47. doi: 10.1007/s40618-021-01714-1
21. Lang S, Liu Y, Qu X, Lu R, Fu W, Zhang W, et al. Association between thyroid function and prognosis of COVID-19: A retrospective observational study. Endocr Res. (2021) 46(4):170–7. doi: 10.1080/07435800.2021.1924770
22. Colonnello E, Criniti A, Lorusso E, Curreli M, Santulli M, Angeloni A, et al. Thyroid hormones and platelet activation in COVID-19 patients. J Endocrinol Invest. (2023) 46(2):261–9. doi: 10.1007/s40618-022-01896-2
23. Deng J, Zhang S, Peng F, Zhang Q, Li Y, Zhong Y. The association between FT3 with the outcome and inflammation/coagulopathy/fibrinolysis of COVID-19. Front Endocrinol (Lausanne). (2022) 13:877010. doi: 10.3389/fendo.2022.877010
24. Lourbopoulos AI, Mourouzis IS, Trikas AG, Tseti IK, Pantos CI. Effects of thyroid hormone on tissue hypoxia: relevance to sepsis therapy. J Clin Med. (2021) 10(24):5855. doi: 10.3390/jcm10245855
25. Pantos C, Kostopanagiotou G, Armaganidis A, Trikas A, Tseti I, Mourouzis I. Triiodothyronine for the treatment of critically ill patients with COVID-19 infection: A structured summary of a study protocol for a randomised controlled trial. Trials. (2020) 21(1):573. doi: 10.1186/s13063-020-04474-0
26. Lui DTW, Tsoi KH, Lee CH, Cheung CYY, Fong CHY, Lee ACH, et al. A prospective follow-up on thyroid function, thyroid autoimmunity and long COVID among 250 COVID-19 survivors. Endocrine. (2023) 80(2):380–91. doi: 10.1007/s12020-022-03281-8
27. Croce L, Zampogna E, Coperchini F, Costa P, Pignatti P, Visca D, et al. Thyroid hormones modifications among COVID-19 patients undergoing pulmonary rehabilitation. Front Endocrinol (Lausanne). (2023) 14:1192561. doi: 10.3389/fendo.2023.1192561
Keywords: COVID-19, thyroid dysfunction, hypothyroidism, hyperthyroidism, Omicron variant, euthyroid sick syndrome
Citation: Zhang Q, Zhang Z, Liu X, Wang Y, Chen H, Hao Y, Zha S, Zhang J, He Y, Zhou B and Hu K (2024) Thyroid dysfunction in the wake of Omicron: understanding its role in COVID-19 severity and mortality. Front. Endocrinol. 15:1412320. doi: 10.3389/fendo.2024.1412320
Received: 04 April 2024; Accepted: 03 July 2024;
Published: 16 July 2024.
Edited by:
Jeff M. P. Holly, University of Bristol, United KingdomReviewed by:
Tarik A. A. A. Elhadd, Hamad Medical Corporation, QatarAnello Marcello Poma, University of Pisa, Italy
Copyright © 2024 Zhang, Zhang, Liu, Wang, Chen, Hao, Zha, Zhang, He, Zhou and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke Hu, aHVrZS1ybWhvc3BpdGFsQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
‡ORCID: Ke Hu, orcid.org/0000-0001-9862-7239
 Qingfeng Zhang†
Qingfeng Zhang† Yixuan Wang
Yixuan Wang Yueying Hao
Yueying Hao Ke Hu
Ke Hu