- 1Internal Medicine Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) San Raffaele Scientific Institute, Milan, Italy
- 2Vita-Salute San Raffaele University, Milan, Italy
- 3Nephrology and Dialysis Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) San Raffaele Scientific Institute, Milan, Italy
- 4Scientific Technical Secretariat of the Ethics Committee, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) San Raffaele Scientific Institute, Milan, Italy
- 5Department of Immunology, Transplantation and Infectious Diseases, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) San Raffaele Scientific Institute, Milan, Italy
Background: Obesity and frailty are prevalent geriatric conditions that share some pathophysiological mechanisms and are associated with adverse clinical outcomes. The relationship between frailty, obesity, and polymorphism remains inadequately explored. Single nucleotide polymorphisms (SNPs) offer insights into genetic predispositions that may influence the development of both frailty and obesity.
Methods: We aimed at investigating whether SNPs associated with frailty also play a role in obesity. Data were collected from the FRASNET cross-sectional study, which included community-dwelling older individuals residing in Milan and nearby areas. Participants were recruited through random sampling. They underwent multidimensional geriatric assessments, which included the collection of blood samples for SNP analysis. Frailty was assessed using the frailty index, and body composition was evaluated using bioelectrical impedance analysis and anthropometric measures.
Results: SNPs related to frailty and linked to the renin–angiotensin system (CYP11B2 rs1799998, AGT rs5051, and AGTR1 rs2131127), apoptosis pathways (CASP8 rs6747918), growth hormone signaling (GHR rs6180), inflammation (TLR4 rs5030717, CD33 rs3865444, and FN1 rs7567647), adducin (ADD3 rs3731566), and the 9p21–23 region (rs518054) were found to be associated with various measures of obesity in community-dwelling older adults.
Conclusions: Frailty-related SNPs contribute to obesity in community-dwelling older adults. We identified a novel association between adducin SNPs and visceral fat, which has not been previously reported. Detecting genetic predispositions to obesity and frailty early could aid in identifying individuals at risk, facilitating the adoption of preventive interventions. This represents an initial step toward promoting early intervention strategies.
1 Background
The association between frailty and obesity has been documented in numerous cross-sectional and longitudinal studies (1, 2). Obesity and frailty share common pathophysiological mechanisms that collectively worsen the age-related decline in physiological reserves. Key mechanisms include inflammation, oxidative stress, activation of apoptotic pathways, and dysregulation of endocrine axes, all of which are altered in both conditions (3, 4). Abdominal obesity, in particular, significantly contributes to establishing the connection between obesity and frailty (5–9).
The increase in visceral fat leads to a simultaneous infiltration of adipose tissue by inflammatory cells that secrete pro-inflammatory cytokines. This excess of inflammatory cytokines not only exacerbates the phenomenon of “inflammaging” but also contributes to the development of various diseases, thereby increasing frailty (10–12). Hubbard et al. (7) demonstrated that individuals with elevated waist circumference had higher frailty index (FI) and frailty phenotype scores compared to those with normal waist circumference, irrespective of their body mass index (BMI). Additionally, abdominal obesity contributes to the development of metabolic dysfunction, which underpins many cardiovascular comorbidities associated with obesity and frailty. This metabolic dysfunction has also been independently linked to an increased risk of frailty progression, regardless of BMI (13).
However, it is important to note that not all obese individuals are frail, and conversely, not all frail individuals are obese. Given the global epidemics of obesity (14) and frailty in the aging world population (15), it is crucial to identify the factors that confer a genetic predisposition to both conditions. This knowledge could enable early screening and implementation of preventive strategies, potentially mitigating the onset and progression of these serious conditions before they manifest.
The relationship between frailty, obesity, and polymorphism remains inadequately explored. Single nucleotide polymorphisms (SNPs) offer insights into genetic predispositions that may influence the development of both frailty and obesity. These polymorphisms can impact diverse biological pathways involved in inflammatory responses, metabolic processes, and endocrine functions, all of which contribute to these conditions. Understanding these genetic links could illuminate why certain individuals are more susceptible to frailty and obesity, even when exposed to similar lifestyle and environmental factors.
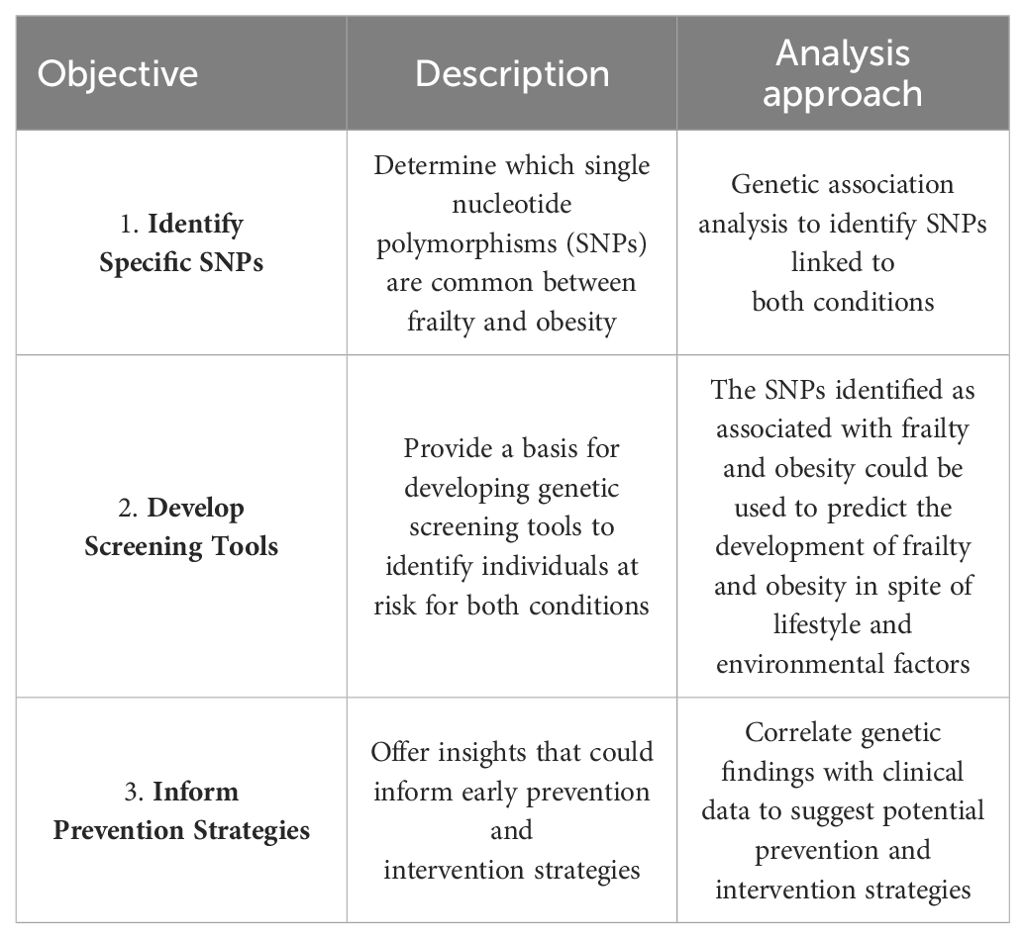
In this observational study, we leveraged data from the FRASNET study to investigate whether frailty-related SNPs also play a role in obesity. Our aims were threefold: i) to identify SNPs that are shared between frailty and obesity; ii) to lay the groundwork for developing genetic screening tools aimed at identifying individuals at risk for both conditions; and iii) to provide insights that could guide early prevention and intervention strategies to mitigate the impact of frailty and obesity in the aging population (see Table 1).
By elucidating the relationship between frailty, obesity, and genetic polymorphism, this study aims to provide valuable insights that can enhance health outcomes for the aging population.
2 Methods
2.1 Study participants
The FRASNET study was a cross-sectional multicenter observational cohort study involving healthy older volunteers (16). Initially, the project aimed to analyze genetic and biochemical factors influencing inflammaging’s effects on kidneys, muscles, cognition, and mood. Additionally, it sought to elucidate genetic and immunological mechanisms contributing to frailty development.
Approval for the study was obtained from the local review board on 9 March 2017, under Protocol No. 24/INT/2017. Healthy volunteers aged 65 years and older were recruited through random sampling methods. Recruitment efforts were conducted at San Raffaele Hospital, Residential Care Facilities for the Elderly and recreational and cultural centers.
Participants provided written informed consent before participating in the study. Enrolment occurred between 1 April 2017 and 16 October 2020, at recreational and cultural centers and retirement homes in Milan and the Monza Brianza areas, Italy. Evaluation visits were conducted at the San Raffaele Scientific Institute in Milan and Cuggiono Hospital near Milan, Italy.
2.2 Inclusion/exclusion criteria
The inclusion criteria for participants in the study were individuals aged 65 years or older, capable of walking more than 500 m without assistance, and with a life expectancy exceeding 6 months. Exclusion criteria included severe cognitive impairment [defined as Mini-Mental State Examination (MMSE) score < 18/30], inability to provide written informed consent, and severe health conditions such as uncontrolled hypertension, recent upper or lower extremity fractures, or myocardial infarction within the past year. Institutionalized participants, those lacking data necessary for calculating the FI, and participants with missing data on body composition were excluded from the present analysis. All individuals included in the study signed a written consent, and there were no cases of individuals who did not sign the consent but were willing to participate or cases that declined involvement in the study.
2.3 Procedures
Participants underwent comprehensive geriatric assessments, which included the collection of demographic and psychosocial data via a self-administered questionnaire. These assessments covered evaluations of comorbidities, pharmacological therapies, incidence of falls, and emergency department visits within the past year. Anthropometric measurements, cognitive function assessments, mood evaluations, fatigue assessments, quality of life evaluations, and assessments of physical activity were also conducted.
Body composition was determined using the Full Body Sensor Body Composition Monitor and Scale by OMRON (17). Obesity was defined as a fat mass percentage ≥42% in women and ≥30% in men (17). Furthermore, we assessed visceral fat percentage and waist circumference to evaluate abdominal obesity. Muscle performance was evaluated using the Short Physical Performance Battery (SPPB) (18), which includes subtests for standing balance, usual gait speed, and chair-stand test. Muscle strength specifically was measured using the chair-stand subtest, where values >15 s indicated reduced muscle strength and pre-sarcopenia (19).
Blood samples taken were bio-banked and subsequently processed for genetic polymorphism analysis associated with frailty and obesity. Frailty was assessed using a 49-item FI based on criteria defined by Theou et al. (20). The FI encompassed deficits from multidimensional geriatric evaluations, covering i) chronic conditions (e.g., hypertension, cancer, stroke, heart diseases, dyslipidemia, diabetes, psychiatric diseases, and polypharmacy), ii) mental health [assessed using questions from the Short Form Health Survey 36 (SF-36), the Geriatric Depression Scale 15 items (GDS-15), and the Mini Mental State Examination (MMSE)], iii) self-rated health (questions from the SF-36), iv) physical performance (questions from the SF-36, reduction of gait speed, and impaired balance tasks from the SPPB), v) living behavior and social function [questions from the SF-36 and The Physical Activity Scale for the Elderly (PASE) Questionnaire], and vi) signs and symptoms (mean systolic or diastolic blood pressure elevated on three measurements and mean heart rate elevated on three measurements). Each deficit was scored as 0 for absence and 1 for presence, resulting in a score ranging from 0 to 1. Supplementary Table S1 describes the deficits included in the FI computation. A cutoff point of ≥0.25 defined “frail” individuals. Participants with over 20% missing variables were excluded from the FI computation.
In this study, we performed a targeted gene analysis focusing on SNPs known to be related with frailty (referenced in Supplementary Table S2) to explore their role in the association between frailty and obesity. Genomic DNA was extracted from peripheral whole blood using the automated Maxwell® RSC Blood DNA Kit (Promega, Madison, WI, USA). The genetic characterization of SNPs was carried out using the TaqMan OpenArray Genotyping System (Applied Biosystems, Foster City, CA, USA). This state-of-the-art genotyping platform facilitates high-throughput analysis of genetic variations with exceptional accuracy and efficiency. Custom OpenArray plates, designed specifically to accommodate multiple SNPs relevant to our study, were utilized. These plates come pre-loaded with specific assays for the SNPs of interest, enabling a streamlined and automated workflow. The system employs fluorescently labeled probes that bind specifically to DNA sequences of interest, allowing for precise detection and quantification of the target SNPs. This methodology ensures robust and reproducible genotyping results, which are critical for conducting accurate genetic association analyses in our research on frailty and obesity (21).
2.4 Statistical analyses
We employed descriptive statistics to summarize the baseline characteristics of the study population. Continuous variables were presented as mean and standard deviation (SD) for normally distributed data or as median and interquartile range (IQR) for skewed distributions. Dichotomous variables were reported as counts (N) and percentages (%). To evaluate the association between genetic polymorphisms known to be related with frailty and various measures of obesity (fat mass percentage, visceral fat percentage, waist circumference, and BMI), we conducted age- and gender-adjusted stepwise linear regression analyses. To control the false discovery rate at α = 5%, we applied the Benjamini–Hochberg procedure. This statistical method helps mitigate the risk of identifying false-positive associations when conducting multiple comparisons in genetic association studies. All statistical analyses were performed with SPSS version 25.0 (SPSS Inc., Chicago, IL, USA).
3 Results
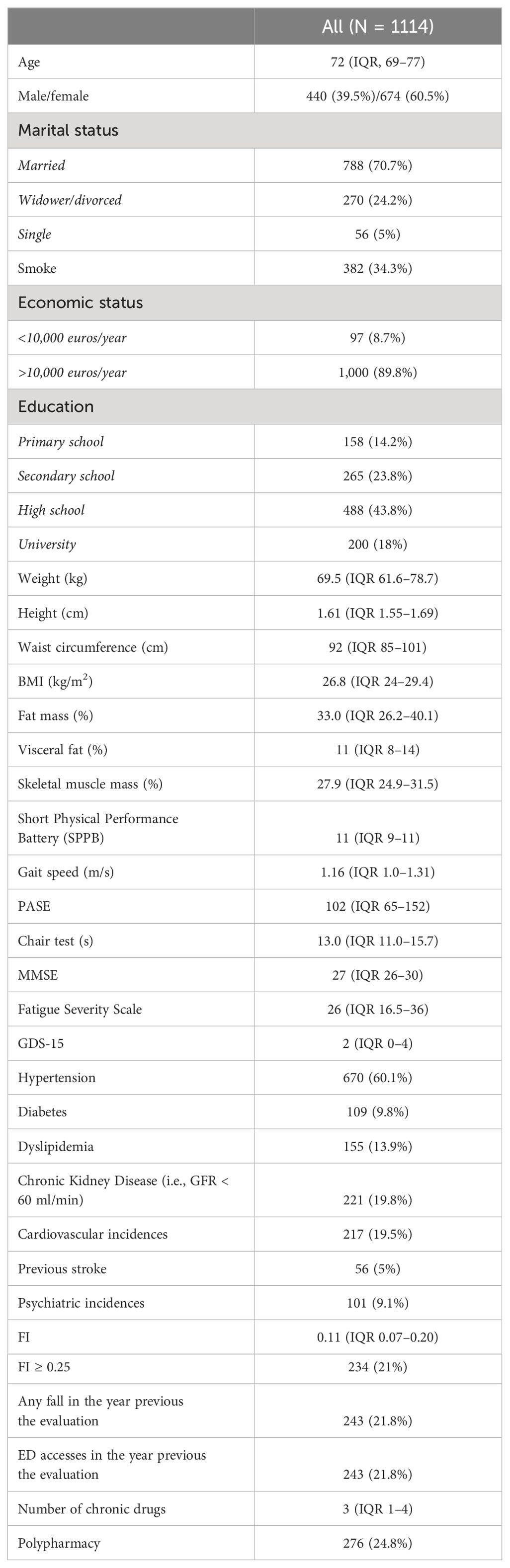
In this study, we analyzed data from 1,114 individuals previously enrolled in the FRASNET cohort to investigate whether frailty-related SNPs also contribute to obesity. Table 2 summarizes the main characteristics of the study population. The median age of participants was 72 years, with 674 (60.5%) women. A majority of the participants were married (70.7%), had a good economic status (>10,000 euro/year; 89.8%), and a high education level (43.8% high school, 18% university).
The median FI score was 0.11 (IQR, 0.07–0.20), with 234 individuals (21%) classified as frail (FI ≥ 0.25). Common comorbidities included hypertension (60%), diabetes (9.1%), dyslipidemia (13.9%), chronic kidney disease (19.8%), cardiovascular events (19.5%), and previous stroke (5%). The median number of chronic medications was 3, and polypharmacy prevalence was 24.8%.
Median BMI was 26.8 kg/m2 (IQR, 24–29.4), and median waist circumference was 92 cm (IQR, 85–101). Body composition analysis revealed a median skeletal muscle mass of 27.9% (IQR, 24.9–31.5), median fat mass of 33% (IQR, 26.2–40.1), and median visceral fat of 11% (IQR, 8–14%). Median levels of physical activity (PASE score = 102), cognitive performance (MMSE score = 27), and affective status (GDS = 2) were within normal ranges.
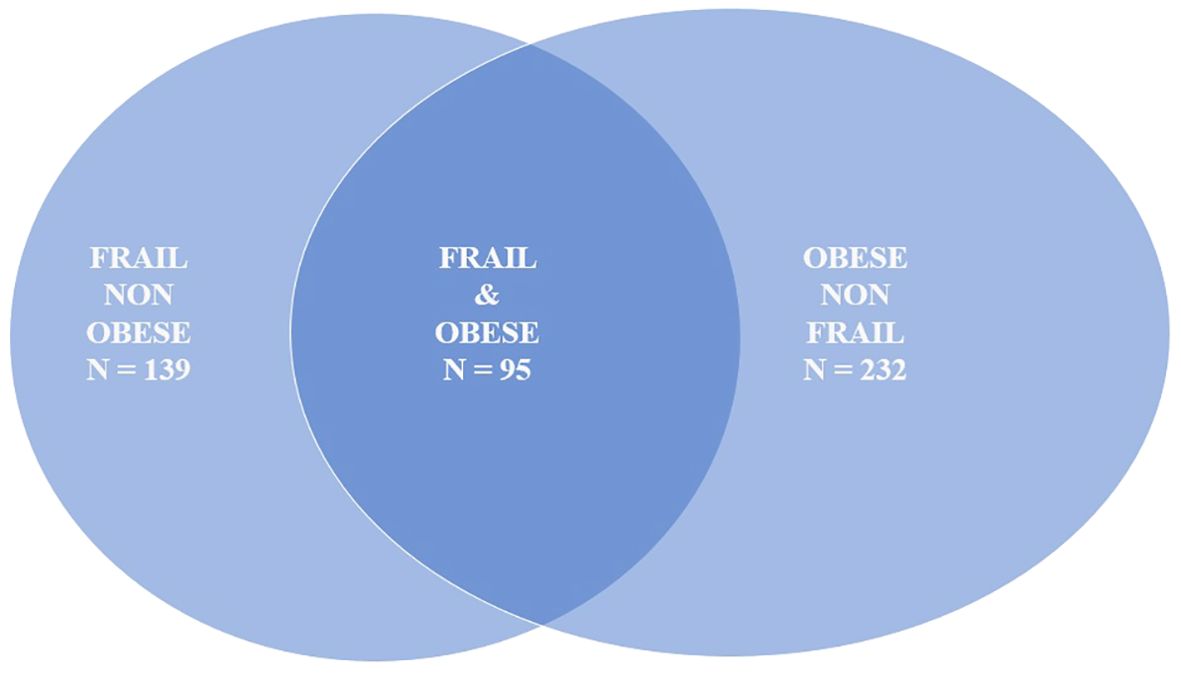
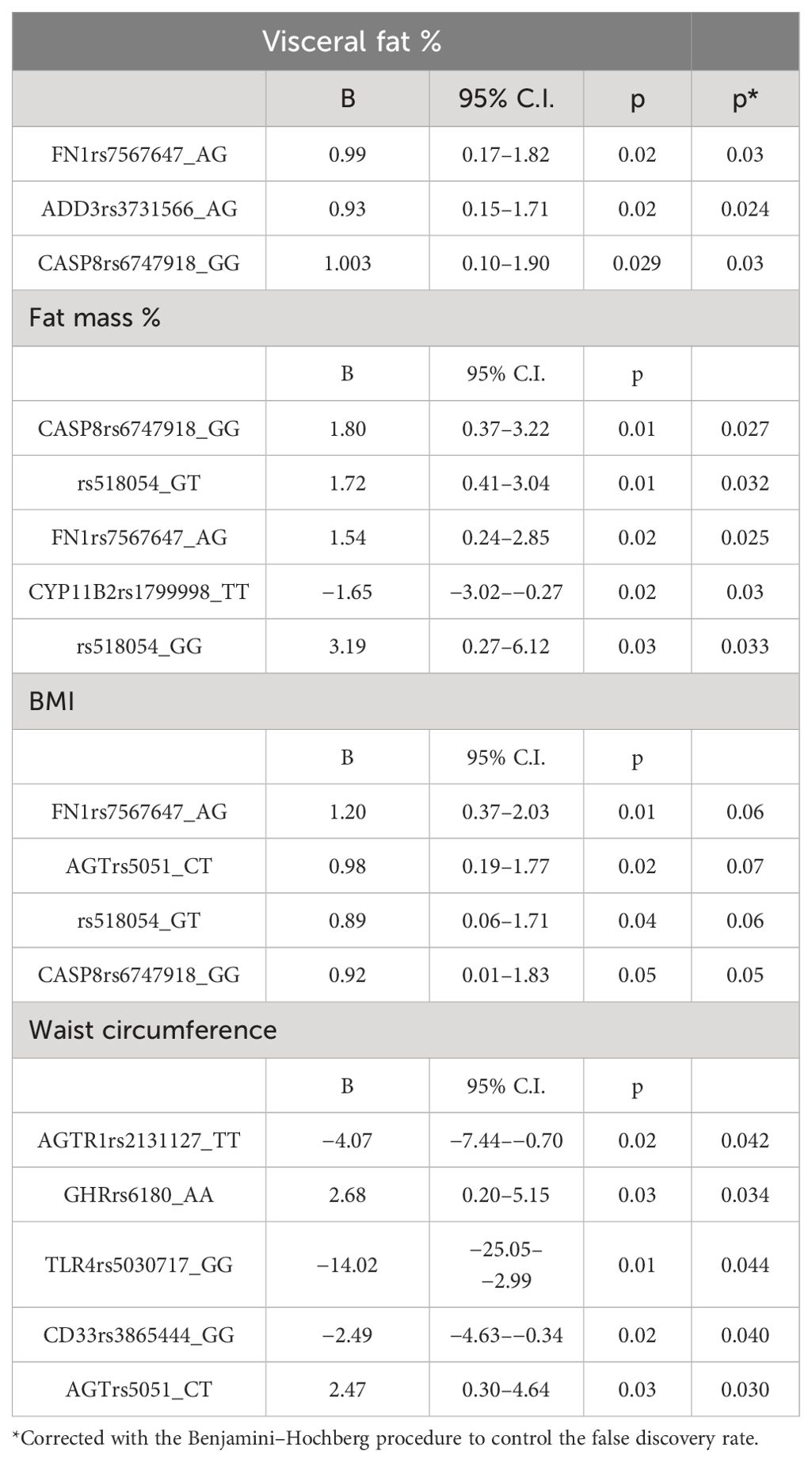
In our sample, 327 individuals (29.4%) were obese, with 95 participants classified as both obese and frail (Figure 1). Table 3 presents the associations between frailty-related SNPs and different measures of obesity. Specifically, SNPs in Fibronectin 1 (FN1 rs7567647: B 0.99; 95% CI, 0.17–1.82), Caspase 8 (CASP8 rs6747918: B 1.003; 95% CI, 0.10–1.90), and Adducin 3 (ADD3 rs3731566: B 0.93; 95% CI, 0.15–1.71) genes were associated with visceral fat. Fibronectin 1 (FN1 rs7567647: B 1.54; 95% CI, 0.24–2.85), Caspase 8 (CASP8 rs6747918: B 1.80; 95% CI, 0.37–3.22), rs518054 (GT B 1.72; 95% CI, 0.41–3.04; GG: B 3.19; 95% CI, 0.27–6.12), and Aldosterone Synthase (CYP11B2 rs1799998: B–1.65; 95% CI, −3.02 to −0.27) SNPs were associated with fat mass. Additionally, Angiotensinogen (AGT rs5051: B 2.47; 95% CI, 0.30–4.64), Angiotensin II Receptor Type 1 (AGTR1 rs2131127: B −4.07; 95% CI, −7.44 to −0.70), Toll Like receptor 4 (TLR4 rs5030717: B −14.02; 95% CI, −25.05 to −2.99), Growth Hormone Receptor (GHR rs6180: B 2.68; 95% CI, 0.20–5.15), and CD33 Molecule (CD33 rs3865444: B −2.49; 95% CI, −4.63 to −0.34) SNPs were associated with waist circumference.
4 Discussion
In this observational study, we identified associations between frailty-related SNPs and various measures of obesity (visceral fat, fat mass percentage, and waist circumference) among community-dwelling older adults. Specifically, SNPs related to the renin–angiotensin system (RAS) (CYP11B2 rs1799998, AGT rs5051, and AGTR1 rs2131127), apoptosis pathways and cellular stress management (CASP8 rs6747918 and CD33 rs3865444), inflammation (TLR4 rs5030717 and FN1 rs7567647), growth hormone signaling (GHR rs6180), adducin (ADD3 rs3731566), and the 9p21–23 region (rs518054) showed significant associations.
Both obesity and frailty are complex, polygenic conditions that often exhibit clinical overlap (1, 2) and share underlying pathophysiological mechanisms (3). Our study contributes to understanding a shared genetic predisposition that promotes the development of both conditions. Importantly, we have identified, for the first time, an association between the adducin (ADD3 rs3731566) SNP and the rs518054 SNP in the 9p21–23 region with obesity.
The SNPs identified as associated with both frailty and obesity hold potential as biomarkers for predicting the onset of these conditions, independent of lifestyle and environmental influences. Integrating these genetic markers into screening tools could effectively identify individuals with a heightened genetic susceptibility to frailty and obesity from an early age. This proactive identification could facilitate targeted prevention strategies and personalized interventions aimed at reducing the impact of these complex health issues.
The RAS, in addition to its traditional role in regulating electrolyte balance and cardiovascular function, can also be locally synthesized in various tissues (22). Local activation of the RAS has been associated with sarcopenia, a significant factor in physical frailty (22). Furthermore, RAS activity at the mitochondrial level enhances oxidative stress (22, 23), leading to compromised mitochondrial function and integrity. This reduction in ATP production contributes to decreased energy levels, which is a critical component of frailty. Moreover, excessive reactive oxygen species (ROS) from mitochondrial dysfunction can affect hypothalamic neurons involved in regulating appetite (24), potentially influencing obesity.
Apoptosis and impaired cellular stress management are critical biological mechanisms underlying frailty (25). Obesity, similarly, has been associated with a pro-apoptotic phenotype in both animal and human studies (26). Specifically, caspases, which play pivotal roles in the later stages of apoptosis at the mitochondrial level (27), have been shown to be activated in adipocytes of obese mice and humans (27). In our study, we observed an association between the CASP8 SNP and measures of fat mass and visceral fat. CD33, involved in cellular stress management and repair of ROS-induced cell damage (28), showed a negative association with waist circumference in our analysis, suggesting an increased vulnerability to ROS damage among obese individuals.
Inflammation, recognized as one of the six “hallmarks of aging” (29), plays a pivotal role in driving frailty (25) and is closely intertwined with obesity and its metabolic complications. White adipocytes, particularly those found in visceral fat, express monocyte chemoattractant protein (MCP)-1 (30), which attracts monocytes from the bloodstream. These monocytes differentiate into macrophages within adipose tissue, where they secrete pro-inflammatory cytokines (31). Toll-like receptors (TLRs), part of the innate immune system, typically recognize pathogen-associated molecular patterns (PAMPs) rich in lipids (32). However, they can also bind to saturated fatty acids consumed in excess and fibronectin (33), initiating an inflammatory signaling cascade. Previous research has indicated a correlation between TLR4 expression and BMI (34). Our study revealed associations between a TLR4 SNP and visceral fat and between a FN1 SNP and both overall and visceral fat accumulation.
The role of the GH axis in determining frailty and obesity remains a subject of debate. Physiologically, the production of GH and IGF-1 declines with age, contributing to changes in body composition associated with aging (35). Obese individuals typically exhibit lower GH pulsatile secretion compared to those with normal weight (36), and diminished levels of GH and IGF-1 have been linked to an elevated risk of chronic conditions such as diabetes and heart disease (37). Studies by Mohamad et al. demonstrated reduced IGF-1 levels in frail men compared to robust controls (38). Interestingly, lower IGF-1 levels have been associated with increased survival probability in some contexts (39, 40), and mutations in the GH receptor that reduce its activity have been linked to protection against several age-related diseases (41). In our study, we observed an association between the GHR rs6180 AA SNP and increased waist circumference.
Adducin is a membrane skeleton protein crucial for signal transduction in various cellular physiological processes. Genetic variations in adducin can influence these functions, impacting multiple pathogenic pathways. Previous research has associated adducin polymorphisms with conditions such as hypertension, cancer, and biliary atresia (42). However, to our knowledge, no prior study has demonstrated an association between adducin SNPs and visceral fat.
The 9p21–23 region is recognized as a risk locus for various diseases, and its rs518054 SNP has previously been associated with frailty (43). Our study provides novel evidence demonstrating that the rs518054 SNP in this region is also associated with obesity.
Our study has the merit of illustrating the association between various genetic polymorphisms linked to frailty and different measures of obesity in community-dwelling older adults. These findings suggest a common genetic predisposition that influences both conditions. However, some limitations should be mentioned: the regional nature of the study, which may impair the generalizability of our findings, and the cross-sectional design, which prevents us from inferring any cause-effect relationships between the frailty SNPs and obesity. Future research should focus on longitudinal studies and include diverse populations to validate and expand upon these findings.
5 Conclusions
By identifying SNPs associated with both frailty and obesity, we have enhanced the knowledge of the genetic determinants underlying these conditions. This may help explain why these prevalent geriatric conditions do not always coincide. Furthermore, early identification of genetic risk factors for obesity and frailty could facilitate the timely implementation of preventive interventions, potentially mitigating the onset of these conditions and their associated adverse outcomes.
Data availability statement
The original contributions presented in the study are publicly available. The data in the San Raffaele Open Research Data Repository, V1 can be found here: 10.17632/wd77gnrmxw.1, and the SNP data can be found here: https://www.ncbi.nlm.nih.gov/SNP/snp_viewBatch.cgi?sbid=1063657.
Ethics statement
The studies involving humans were approved by Ospedale San Raffaele Comitato Etico. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SD: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. LC: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. LZ: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. EB: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. CM: Conceptualization, Data curation, Investigation, Validation, Writing – original draft, Writing – review & editing. MS: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. RL: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. MR: Data curation, Investigation, Writing – original draft, Writing – review & editing. SS: Data curation, Investigation, Writing – original draft, Writing – review & editing. ES: Data curation, Investigation, Writing – original draft, Writing – review & editing. MM: Data curation, Investigation, Writing – original draft, Writing – review & editing. GV: Data curation, Investigation, Writing – original draft, Writing – review & editing. PM: Resources, Supervision, Validation, Visualization, Writing – review & editing, Data curation, Funding acquisition, Project administration. AM: Funding acquisition, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. CL: Data curation, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PR: Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Fondazione CARIPLO, Milano grant to PM (grant number 2016–0980). The costs of the publication will be sustained by the Age-it project.
Acknowledgments
We acknowledge co-funding from Next Generation EU, in the context of the National Recovery and Resilience Plan, Investment PE8 – Project Age-It: “Ageing Well in an Ageing Society”. This resource was co-financed by the Next Generation EU [DM 1557 11.10.2022]. The views and opinions expressed are only those of the authors and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MC declared a past co-authorship with the author PR to the handling editor.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views and opinions expressed are only those of the authors and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1412160/full#supplementary-material
References
1. Feng Z, Lugtenberg M, Franse C, Fang X, Hu S, Jin C, et al. Risk factors and protective factors associated with incident or increase of frailty among communitydwelling older adults: A systematic review of longitudinal studies. PloS One. (2017) 12:e0178383. doi: 10.1371/journal.pone.0178383
2. Landré B, Czernichow S, Goldberg M, Zins M, Ankri J, Herr M. Association between life-course obesity and frailty in older adults: findings in the GAZEL cohort. Obesity. (2020) 28:388–96. doi: 10.1002/oby.22682
3. Silveira EA, Mendonça CR, Delpino FM, Elias Souza GV, Pereira de Souza Rosa L, de Oliveira C, et al. Sedentary behavior, physical inactivity, abdominal obesity and obesity in adults and older adults: A systematic review and meta-analysis. Clin Nutr ESPEN. (2022) 50:63–73. doi: 10.1016/j.clnesp.2022.06.001
4. Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology. (2011) 57:66–75. doi: 10.1159/000279755
5. Afonso C, Sousa-Santos AR, Santos A, Borges N, Padrão P, Moreira P, et al. Frailty status is related to general and abdominal obesity in older adults. Nutr Res. (2021) 85:21–30. doi: 10.1016/j.nutres.2020.10.009
6. García-Esquinas E, García-García FJ, León-Muñoz LM, Carnicero JA, Guallar-Castillón P, Harmand MGC, et al. Obesity, fat distribution, and risk of frailty in twoPopulation-based cohorts of older adults in Spain. Obesity. (2015) 23:847–55. doi: 10.1002/oby.21013
7. Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. (2010) 65:377–81. doi: 10.1093/gerona/glp186
8. Crow RS, Lohman MC, Titus AJ, Cook SB, Bruce ML, Mackenzie TA, et al. Association of obesity and frailty in older adults: NHANES 1999-2004. J Nutr Health Aging. (2019) 23:138–44. doi: 10.1007/s12603-018-1138-x
9. Yuan L, Chang M, Wang J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: a systematic review and meta-analysis. Age Ageing. (2021) 50:1118–28. doi: 10.1093/ageing/afab039
10. Franceschi C, Judith Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. (2014) 69 Suppl 1:S4–9. doi: 10.1093/gerona/glu057
11. Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the cardiovascular health study. Arch Intern Med. (2002) 162:2333. doi: 10.1001/archinte.162.20.2333
12. Song X, Zhang W, Hallensleben C, Versluis A, van der Kleij R, Jiang Z, et al. Associations between obesity and multidimensional frailty in older chinese people with hypertension. Clin Interv Aging. (2020) 15:811–20. doi: 10.2147/CIA.S234815
13. He D, Qiu Y, Yan M, Zhou T, Cheng Z, Li J, et al. Associations of metabolic heterogeneity of obesity with frailty progression: Results from two prospective cohorts. J Cachexia Sarcopenia Muscle. (2023) 14:632–41. doi: 10.1002/jcsm.13169
14. Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. (2019) 7:231–40. doi: 10.1016/S2213-8587(19)30026-9
15. Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet. (2016) 387:2145–54. doi: 10.1016/S0140-6736(15)00516-4
16. Brioni E, Magnaghi C, Villa G, Giannetta N, Manara DF, Magni G, et al. The FRASNET study: identification of clinical and social factors of renal failure in an elderly population. G Ital Nefrol. (2022) 39:2022–vol3.
17. Available online at: https://omronhealthcare.com/wp-content/uploads/hbf-510w-instruction-manual.pdf.
18. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. (1995) 332:556–61. doi: 10.1056/NEJM199503023320902
19. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
20. Theou O, Haviva C, Wallace L, Searle SD, Rockwood K. How to construct a frailty index from an existing dataset in 10 steps. Age Ageing. (2023) 52:afad221. doi: 10.1093/ageing/afad221
21. Available online at: https://tools.thermofisher.com/content/sfs/manuals/MAN0014351_OpenArray_Genotyping_Experiments_QR.pdf (Accessed 2 July 2019).
22. Ramalingam L, Menikdiwela K, LeMieux M, Dufour JM, Kaur G, Kalupahana N, et al. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim Biophys Acta Mol Basis Dis. (2017) 1863:1106–14. doi: 10.1016/j.bbadis.2016.07.019
23. Cosarderelioglu C, Nidadavolu LS, George CJ, Oh ES, Bennett DA, Walston JD, et al. Brain renin–angiotensin system at the intersect of physical and cognitive frailty. Front Neurosci. (2020) 14:586314. doi: 10.3389/fnins.2020.586314
24. Horvath TL, Andrews ZB, Diano S. Fuel utilization by hypothalamic neurons: Roles for ROS. Trends Endocrinol Metab. (2009) 20:78–87. doi: 10.1016/j.tem.2008.10.003
25. Cardoso AL, Fernandes A, Aguilar-Pimentel JA, de Angelis MH, Guedes JR, Brito MA, et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. (2018) 47:214–77. doi: 10.1016/j.arr.2018.07.004
26. Glasstetter LM, Oderinde TS, Mirchandani M, Rajagopalan KS, Barsom SH, Thaler R, et al. Obesity and dyslipidemia are associated with partially reversible modifications to DNA hydroxymethylation of apoptosis- and senescence-related genes in swine adipose-derived mesenchymal stem/stromal cells. Stem Cell Res Ther. (2023) 14:143. doi: 10.1186/s13287-023-03372-x
27. Kantari C, Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta. (2011) 1813:558–63. doi: 10.1016/j.bbamcr.2011.01.026
28. Guzmán-Beltrán S, Pedraza-Chaverri J, Gonzalez-Reyes S, Hernández-Sánchez F, Juarez-Figueroa UE, Gonzalez Y, et al. Nordihydroguaiaretic acid attenuates the oxidative stress-induced decrease of CD33 expression in human monocytes. Oxid Med Cell Longev. (2013) 2013:375893. doi: 10.1155/2013/375893
29. Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. (2018) 14:576–90. doi: 10.1038/s41574-018-0059-4
30. Wu Y, Wu C, Shi T, Cai Q, Wang T, Xiong Y, et al. FAP expression in adipose tissue macrophages promotes obesity and metabolic inflammation. Proc Natl Acad Sci U.S.A. (2023) 120:e2303075120. doi: 10.1073/pnas.2303075120
31. Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. (2021) 320:C375–C39. doi: 10.1152/ajpcell.00379.2020
32. Frank MG, Fleshner M, Maier SF. Exploring the immunogenic properties of SARS-CoV-2 structural proteins: PAMP:TLR signaling in the mediation of the neuroinflammatory and neurologic sequelae of COVID-19. Brain Behav Immun. (2023) 111:259–69. doi: 10.1016/j.bbi.2023.04.009
33. Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. (2001) 276:16683–9. doi: 10.1074/jbc.M011695200
34. Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. (2010) 33:861–8. doi: 10.2337/dc09-1799
35. Bartke A. Growth hormone and aging: updated review. World J Mens Health. (2019) 37:19–30. doi: 10.5534/wjmh.180018
36. Hjelholt A, Høgild M, Bak AM, Arlien-Søborg MC, Bæk A, Jessen N, et al. Growth hormone and obesity. Endocrinol Metab Clin N Am. (2020) 49:239–50. doi: 10.1016/j.ecl.2020.02.009
37. Ashpole NM, Sanders JE, Hodges EL, Yan H, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp Gerontol. (2015) 68:76–81. doi: 10.1016/j.exger.2014.10.002
38. Mohamad M, Khater MS. Evaluation of insulin like growth factor-1 (IGF-1) level and its impact on muscle and bone mineral density in frail elderly male. Arch Gerontol Geriatr. (2015) 60:124–7. doi: 10.1016/j.archger.2014.08.011
39. Ben-Avraham D, Govindaraju DR, Budagov T, Fradin D, Durda P, Liu B, et al. The GH receptor exon 3 deletion is a marker of male-specific exceptional longevity associated with increased GH sensitivity and taller stature. Sci Adv. (2017) 3:e1602025. doi: 10.1126/sciadv.1602025
40. Milman S, Atzmon G, Huffman DM, Wan J, Crandall JP, Cohen P, et al. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. (2014) 13:769–71. doi: 10.1111/acel.12213
41. Laron Z. The GH-IGF1 axis and longevity. The paradigm of IGF1 deficiency. Hormones (Athens). (2008) 7:24–7. doi: 10.14310/horm.2002.1149
42. Kiang KMY, Leung GKK. A review on adducin from functional to pathological mechanisms: future direction in cancer. BioMed Res Int. (2018) 2018:3465929. doi: 10.1155/2018/3465929
Keywords: obesity, frailty, SNP, predisposition, older people
Citation: Damanti S, Citterio L, Zagato L, Brioni E, Magnaghi C, Simonini M, De Lorenzo R, Ruggiero M, Santoro S, Senini E, Messina M, Vitali G, Manunta P, Manfredi AA, Lanzani C and Rovere Querini P (2024) DNA polymorphisms in inflammatory and endocrine signals linked to frailty are also associated with obesity: data from the FRASNET cohort. Front. Endocrinol. 15:1412160. doi: 10.3389/fendo.2024.1412160
Received: 04 April 2024; Accepted: 28 June 2024;
Published: 11 October 2024.
Edited by:
Florencia Maria Barbé-Tuana, Pontifical Catholic University of Rio Grande do Sul, BrazilReviewed by:
Maria Conte, University of Bologna, ItalyAna Catarina Rocha, Universidade do Porto, Portugal
IGP Suka Aryana, Udayana University, Indonesia
Copyright © 2024 Damanti, Citterio, Zagato, Brioni, Magnaghi, Simonini, De Lorenzo, Ruggiero, Santoro, Senini, Messina, Vitali, Manunta, Manfredi, Lanzani and Rovere Querini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah Damanti, ZGFtYW50aS5zYXJhaEBoc3IuaXQ=
 Sarah Damanti
Sarah Damanti Lorena Citterio
Lorena Citterio Laura Zagato
Laura Zagato Elena Brioni
Elena Brioni Cristiano Magnaghi4
Cristiano Magnaghi4 Rebecca De Lorenzo
Rebecca De Lorenzo Paolo Manunta
Paolo Manunta Angelo Andrea Manfredi
Angelo Andrea Manfredi Chiara Lanzani
Chiara Lanzani Patrizia Rovere Querini
Patrizia Rovere Querini