- 1First Clinical Medical College of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 2Shandong University of Traditional Chinese Medicine Affiliated Hospital, Jinan, Shandong, China
Background: The optimal outcome of assisted reproductive technology is a successful live birth after fresh embryo transfer. However, the success pregnancy rate of fresh embryo transfer cycle in antagonist protocol is lower than that observed in other protocols. Despite the use of antagonists (GnRH-ant), the incidence of luteinizing hormone surge and elevated progesterone levels remain at approximately 5%-38%. Progesterone is widely recognized to exert adverse effects on fresh embryo transfer outcomes. This study aimed to investigate the impact of luteinizing hormone surge and progesterone levels on live birth rate following fresh embryo transfer and explore appropriate progesterone thresholds to enhance pregnancy outcomes.
Methods: This retrospective cohort study included a total of 1,177 antagonist protocol cycles with fresh embryo transfer. The patients were divided into four groups based on the presence of premature LH surge and progesterone level on trigger day>1.5ng/ml. Then, the relationship between the variables and the pregnancy outcome was analyzed and compared in each group.
Results: The transient rise of luteinizing hormone did not impact pregnancy outcomes (P=0.345; P=0.3; P=0.787), in contrast to progesterone levels on the day of hCG administration (P=0.047*; P=0.015*; P=0.021*). In cases with luteinizing hormone surge, elevated progesterone levels were correlated with higher antral follicle count (AFC), and as progesterone levels increased, a greater quantity of oocytes and embryos were obtained. However, there was no statistically significant difference in pregnancy outcomes. In cases without luteinizing hormone surge, elevated progesterone levels led to significantly poorer pregnancy outcomes. Furthermore, the curve-fitting and threshold-effect analysis revealed a notable decline in live birth rates when progesterone exceeded or equaled 1.10ng/ml (OR, 0.25; 95% CI, 0.09–0.66; P = 0.005*).
Conclusion: The GnRH-ant dosage addition should be carefully selected in flexible antagonist protocols. The presence of elevated progesterone levels may be associated with improved embryo quality when luteinizing hormone surge occurred. In the absence of a luteinizing hormone surge, progesterone levels showed a larger impact on the pregnancy outcome, and fresh embryo transfer should not be performed if the progesterone level on the day of hCG administration is higher than 1.10ng/ml.
Introduction
During in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) cycles, controlled ovarian hyperstimulation (COH) plays an essential role. The GnRH antagonist protocol employs exogenous gonadotropin (Gn) to stimulate follicle development, which competitively occupies the pituitary GnRH receptor using a gonadotropin-releasing hormone antagonist (GnRH-ant); the internal luteinizing hormone (LH) peak is thus controlled and early ovulation or luteinization of follicles is effectively inhibited. In comparison to the GnRH agonist protocol, the GnRH-ant protocol allows for timely adjustments based on follicle development. It offers enhanced efficiency, convenience, and safety by avoiding premature pituitary suppression and reducing the risk of ovarian hyper-stimulation syndrome (OHSS) (1). Over the past two decades, GnRH antagonists have replaced GnRH agonists as the preferred treatment for ART (2).
Fresh embryo transfer (ET) can reduce the time and economic burden on patients, as compared to frozen embryo transfer (FET). Patients undergoing frozen embryo transfer require additional medical care and monitoring, endometrial preparation, embryo freezing, and thawing procedures. Moreover, studies have suggested that frozen embryo transfer increases the risk of adverse pregnancy outcomes such as placenta praevia, preeclampsia, and an increased risk of leukemia due to the aforementioned procedures involved in freezing and thawing embryos (3–6). The desired outcome in assisted reproductive technology (ART) is a successful live birth through fresh embryo transfer. However, the pregnancy rate of fresh embryo transfers in patients receiving the GnRH-ant protocol is consistently lower than other protocols (7). Additionally, patients undergoing the GnRH-ant protocol are more susceptible to premature luteinizing hormone surge and elevated progesterone levels (8–11). The LH surge predominantly manifests in individuals with low and high ovarian responsiveness (12–15).
Researchers reported that LH level changes were associated with the outcome of COH. The peak LH level may be used to predict optimal oocyte yield, providing superior predictive accuracy than basal or trigger-day LH levels (16). The peak/basal LH ratio may potentially be used to predict the pregnancy outcomes in PCOS patients, and patients with a peak/basal LH ratio≥ 1 exhibiting a higher top-quality embryo (17). However, other scholars suggested that a transient premature LH surge without elevated serum progesterone may cause a detrimental effect on the embryo and pregnancy outcomes in fresh embryo transfer cycles (14, 18, 19) and on the ongoing pregnancy rate (1, 20), especially the patients of advanced age (≥37 years), and aggravated the reduced potential of embryos growth, not the number (21). However, previous studies explored patients who experienced a transient premature rise in LH and had comparable clinical pregnancy and ongoing pregnancy rates (22–24). The current research on the relationship between the LH surge and pregnancy outcomes remains controversial, but the LH surge is widely recognized to result in an elevation of P levels due to premature luteinization of granulosa cells or ovulation of small follicles. The adverse effects of elevated P levels on the trigger day have been extensively documented. These effects encompass not only impaired endometrial receptivity but also deleterious consequences on embryo quality (3, 25–28). Xu B et al. proposed that the ongoing pregnancy rate in fresh cycles was negatively correlated with serum P levels on the day of hCG administration, and different threshold concentrations of P were determined based on varying ovarian responses (29, 30). The literature suggested that patients undergoing fresh blastocyst transfer should aim for a P level of 0.8 ng/ml on the day of hCG administration, in contrast to fresh embryo transfer (31). However, the progesterone level threshold affecting the level of LH and pregnancy outcomes has not been clearly established.
In clinical practice, appropriate LH levels and progesterone levels are critical in ensuring the acquisition of mature eggs and a satisfactory pregnancy outcome. This study aimed to identify the reasons underlying the low success rate of fresh embryo transfer in the antagonist protocol and to improve the clinical pregnancy rate of fresh embryo transfer.
Materials and methods
Population and study subjects
This retrospective analysis included 1,177 infertile patients who underwent IVF/ICSI cycles and fresh embryo transfer from May 2017 to December 2022. The women were monitored until live birth after fresh embryo transfer. The study protocol was approved by the institutional human ethics committee.
The inclusion criteria were: (1) flexible antagonist protocol with fresh embryo transfer cycle, (2) basal follicle-stimulating hormone (bFSH) ≤ 15 IU/L, and (3) body mass index (BMI) <40kg/m2.
The exclusion criteria were: (1) patients with chromosomal abnormalities, reproductive malformation, and a history of recurrent spontaneous abortion; (2) patients undergoing coasting to prevent ovarian hyperstimulation syndrome; (3) incomplete information; (4) uterine pathologies that might compromise pregnancy potential; (5) patients with elevated progesterone or LH level before the addition of GnRH-ant.
The premature LH surge refers to an endogenous LH peak that occurs before follicle maturation or human chorionic gonadotrophin (hCG) injection. It was defined as either maximum LH levels exceeding two and a half times the baseline LH level on day 2 of the same menstrual cycle (13), or the absolute value > 10 IU/mL (32–34).
MLH grouping
The populations were categorized into two groups based on the presence of a transient premature maximum luteinizing hormone surge. Group MLH0 was defined as the group without transient premature luteinizing hormone surge, whereas group MLH1 included patients with transient premature luteinizing hormone surge.
P grouping
The populations were categorized into two groups based on the P level on hCG day. Patients with P ≤ 1.5 ng/ml were assigned to group P0, and those with P>1.5 ng/ml were assigned to group P1.
PMLH grouping
Furthermore, the patients were divided into four groups according to the P level on hCG day and the presence of premature LH surges. Group P1MLH1 included patients with transient premature luteinizing hormone surges and P>1.5 ng/ml; group P1MLH0 included those without transient premature luteinizing hormone surges and P>1.5 ng/ml; group P0MLH1 comprised patients with transient premature luteinizing hormone surges and P ≤ 1.5 ng/ml; group P0MLH0 consisted of individuals without transient premature luteinizing hormone surges and P ≤ 1.5 ng/ml.
Ovarian stimulation protocol
Patients received IVF/ICSI treatment according to the flexible GnRH antagonist protocol. On the second day of the menstrual cycle, recombinant human follicle-stimulating hormone 150–300 U (Gonal-F; Merck, Lyon, France; Puregon, MSD, Boulogne, France) was injected as Gn. The Gn doses were determined based on patient age, body mass index (BMI), FSH, and AFC. A daily dose of 0.25/0.125 mg of GnRH-ant (Cetrotide, Merck, Lyon, France) was administered when the leading follicle size was 14 mm or the serum E2 level reached 300pg/mL or a premature luteinizing hormone surge was recognized.
Oocyte retrieval and transfer
Oocytes were then collected by follicular aspiration under ultrasound 34–36 h after triggering with GnRH-a (Triptoreline, Decapeptyl, Ipsen, France) or recombinant hCG (rhCG, Ovitrelle, Merck, Lyon, France). IVF or ICSI fertilization was selected according to semen conditions. Eighteen hours after fertilization, embryo development was monitored daily and graded based on the number and size of blastomeres, fragmentation rate, multinucleation, and early densification. High-quality embryos were defined as embryos with seven to ten blastomeres on the third day following oocyte retrieval (35).
All patients completed an IVF/ICSI cycle and then performed an ET cycle. On day 3 or day 5, one or two of the best embryos or blastocysts were selected and transferred using a soft Wallace catheter. In order to avoid multiple pregnancies and reduce the incidence of complications during pregnancy and the perinatal period, the number of embryos was selected according to the specific conditions of the patient and the embryo quality score, and one blastocyst was selected as far as possible. For luteal support in preparation for the fresh embryo transfer, 40mg of progesterone (20 mg/branch, Zhejiang Xianju Pharmaceutical Co., Ltd.) was injected daily and 30mg of oral dydrogesterone tablets (10 mg/tablet, Abbott Healthcare Products B.V.) or progesterone vaginal sustained-release gel (90 mg/dose, Crinone VR 8%, Merck, Sherano, Switzerland) were given daily. In addition, two bags of Chinese medicine, the Gushen Antai pills, were used daily.
Hormone measurement
Venous blood samples were collected on the second day of the menstrual cycle to the triggering day. Serum FSH, LH, E2, and P levels were measured by means of the automated Elecsys Immunoanalyzer (Beckmann, America).
Pregnancy outcomes measurement
The live birth rate (LBR) was the primary outcome. The secondary outcomes included biochemical pregnancy rate (BPR), clinical pregnancy rate (CPR), number of oocytes, number of fertilization, number of blastomeres, number of embryos, and number of high-quality embryos/blastomeres.
Live birth was defined as live birth per embryo transfer in a fresh cycle.
Clinical pregnancy was defined as the presence of an intrauterine gestational sac with a fetal heartbeat detected on transvaginal ultrasonography after six weeks of gestation.
Biochemical pregnancy was defined as the presence of detectable ß-hCG in the blood and the value<10mIU/mL at approximately 14 days after transplantation.
LBR = number of cycles with live birth/number of transfer cycles × 100%
BPR = number of cycles with biochemical pregnancy/number of transfer cycles × 100%
CPR = number of cycles with clinical pregnancy/number of transfer cycles × 100%
Statistical analysis
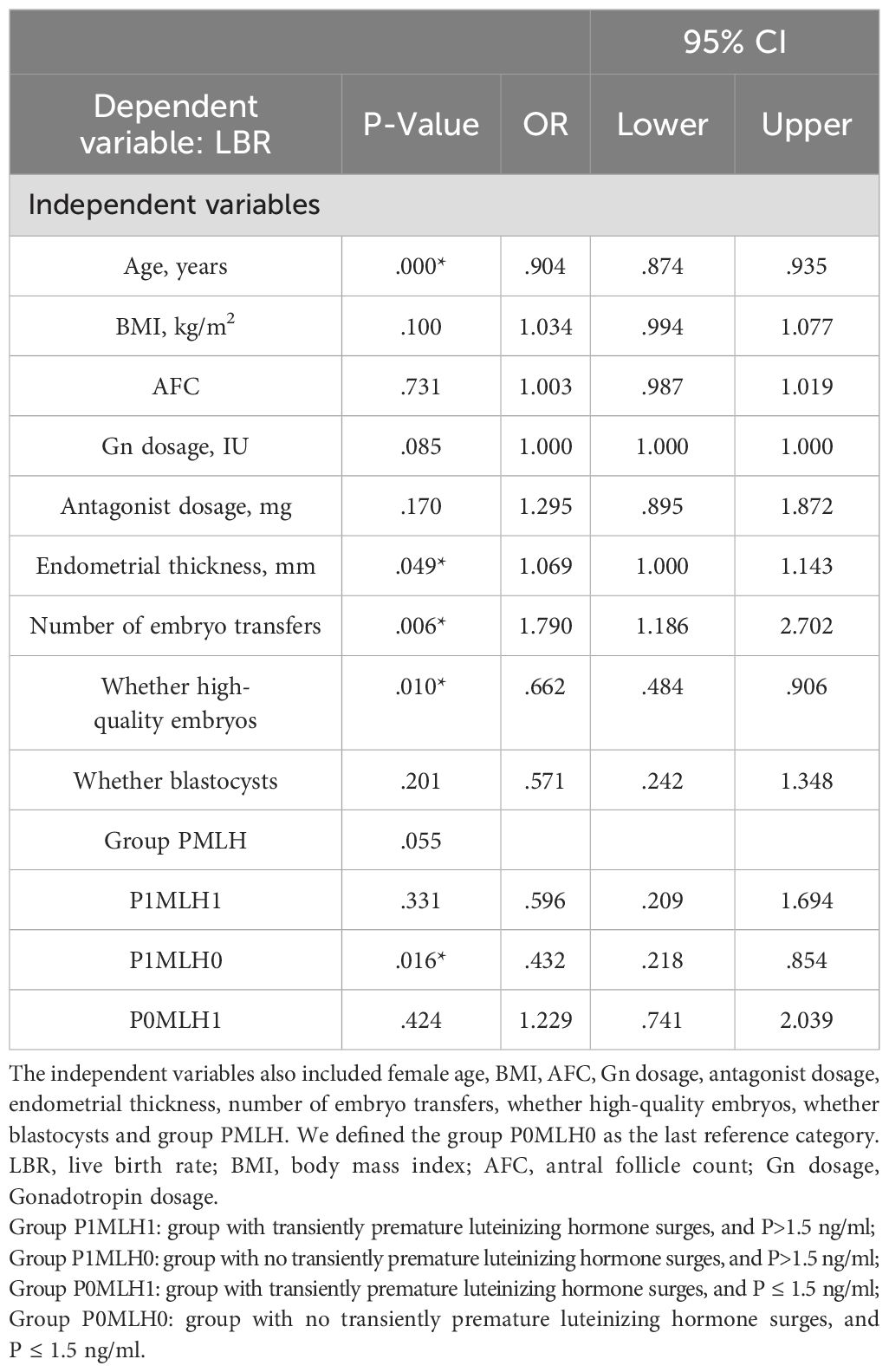
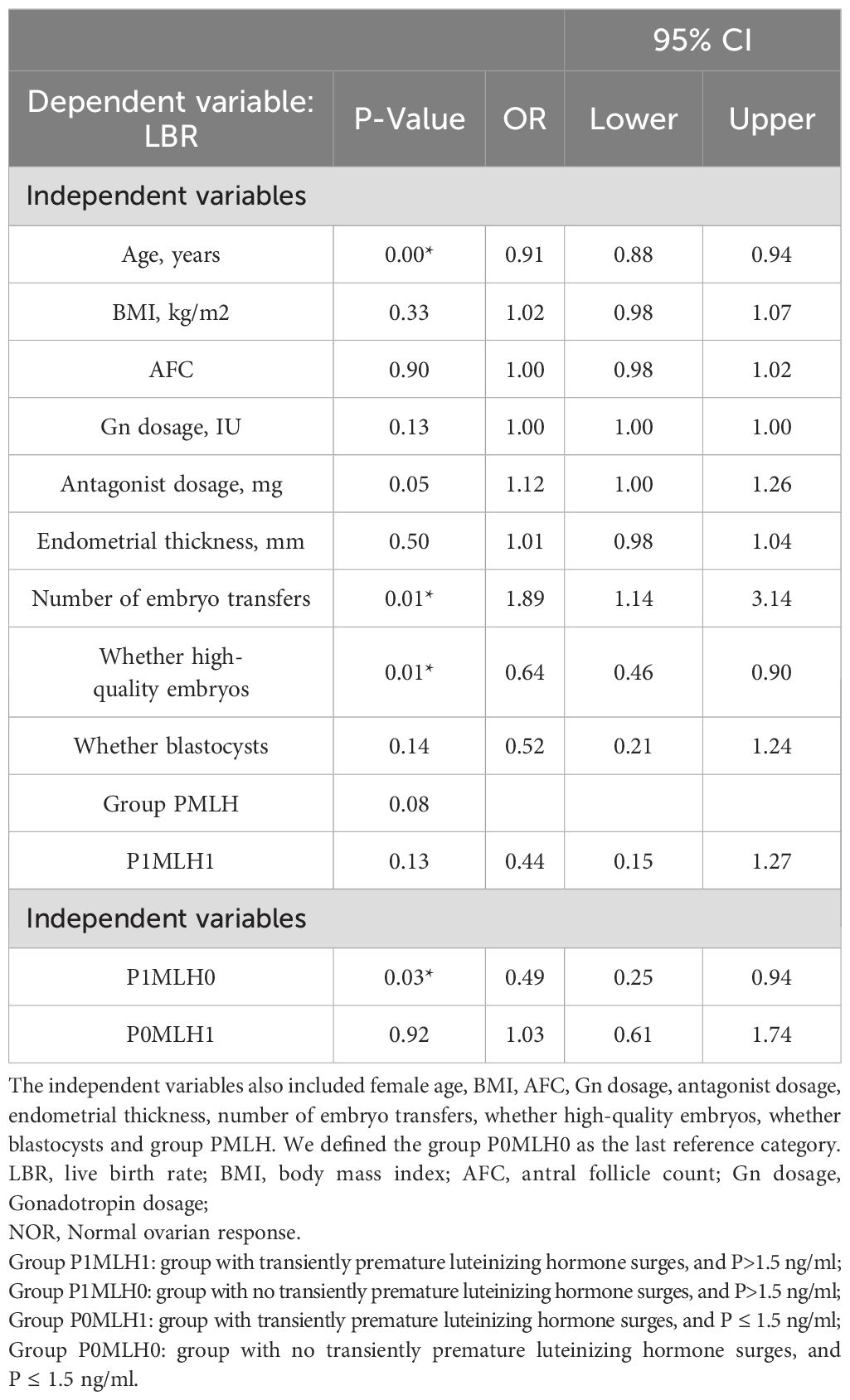
Statistical analysis was conducted using SPSS version 26 and R4.2.1. In this study, P<0.05 was considered statistically significant. The Shapiro–Wilk test was used to assess data normality. Due to skewed distributions, quantitative variables were expressed as medians (interquartile range, range between the 25th and 75th percentiles), and the Mann–Whitney U test and Kruskal-Wallis test were performed. Qualitative variables were expressed as frequencies and analyzed using the chi-square test. Moreover, correlation analysis was performed using Spearman based on the R language. Binary logistic regression analysis was performed based on the following patient characteristics: female age, BMI, AFC, Gn dosage, GnRH-ant dosage, endometrial thickness, number of embryo transfers, high-quality embryos, blastocysts, and group PMLH. The crude odds ratios (OR) and adjusted OR were calculated with 95% confidence intervals (CI). In addition, curve fitting and threshold effect analysis were performed on group MLH0. Smooth curve fitting was carried out with the gam model to identify any non-linear relationship between the P levels and LBR. A piece-wise linear regression method was used to analyze the threshold effect between the P levels and LBR.
Results
Baseline characteristics
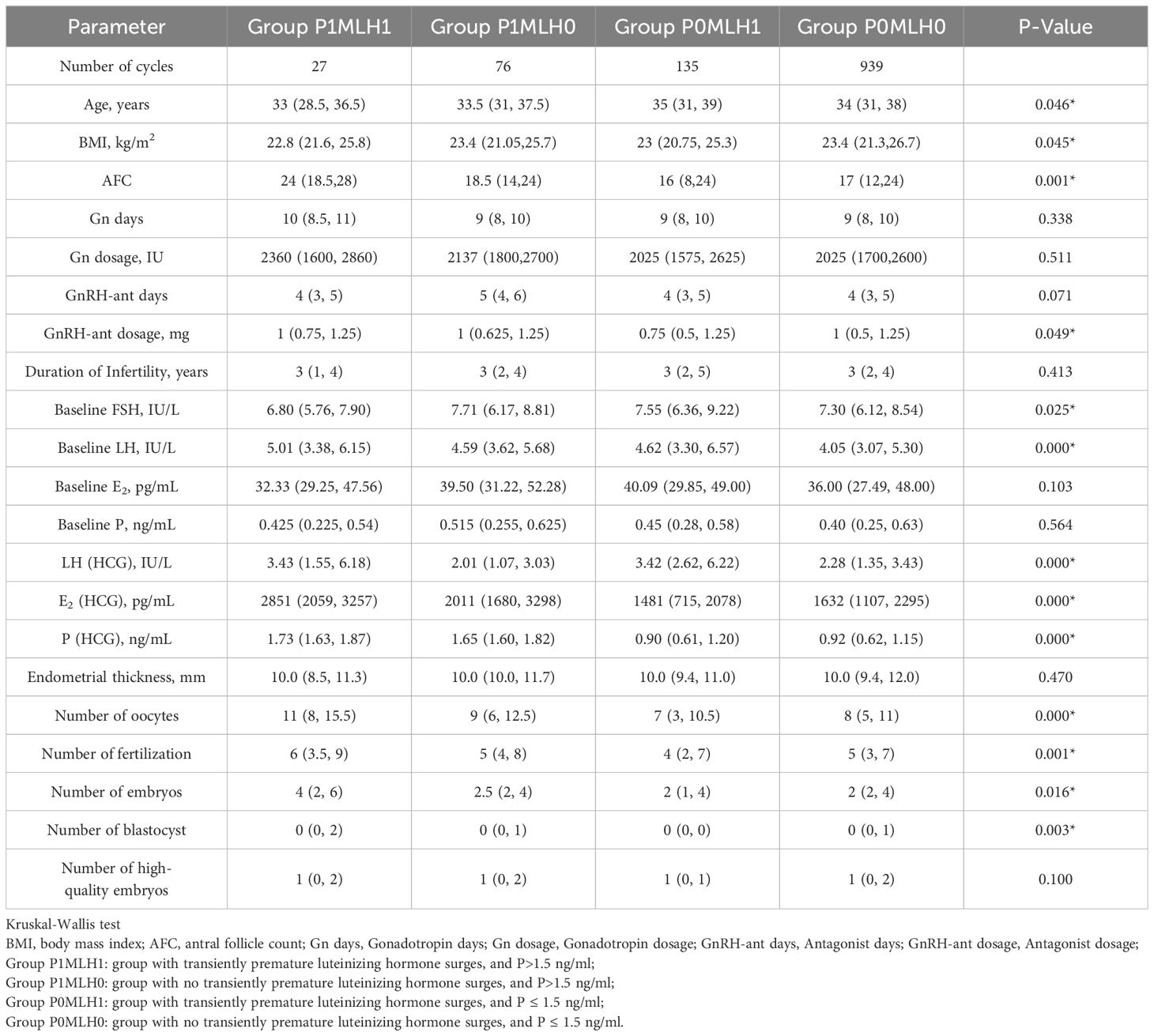
In total, 1177 patients who received fresh embryo transfer with antagonist regimens from May 2017 to December 2022 were retrospectively analyzed. As displayed in Table 1, there were 27 cases in group P1MLH1, 76 cases in group P1MLH0, 135 cases in group P0MLH1, and 939 cases in group P0MLH0. As illustrated in Table 1, the age of participants in group P0MLH1 (35(31,39)) was significantly higher than group P0MLH0 (34(31,38), P=0.049*). The BMI of P0MLH0 (23.4(21.3,26.7)) was significantly higher than group P0MLH1 (23(20.75,25.3), P=0.048*). In addition, the AFC of group P1MLH1 24(18.5,28) was significantly higher than group P0MLH1 (16(8,24), P=0.001*) and group P0MLH0 (17(12,24), P=0.011*). The GnRH-ant dosage of group P1MLH0 (1(0.625,1.25)) was the highest (P=0.049*). The baseline FSH of group P0MLH1 (7.55(6.36,9.22)) was the highest (P=0.025*). The baseline LH of group P1MLH1 (5.01(3.38,6.15)) was the highest (P=0.025*). Furthermore, group P1MLH1 showed a significantly higher number of oocytes (11(8,15.5)) than group P0MLH1 (7(3,10.5), P=0.000*) and group P0MLH0 (8(5,11), P=0.007*). Oocyte numbers of group P1MLH0 (9(6,12.5)) was also higher than group P0MLH1 (7(3,10.5), P=0.001*) and group P0MLH0 (8(5,11), P=0.044*). The fertilization numbers of P1MLH1 (6(3.5,9), P=0.025*) and group P1MLH0 (5(4,8), P=0.004*) were higher than group P0MLH1 (4(2,7)). Embryo numbers of group P1MLH1(4(2,6)) were higher than group P0MLH1 (2(1,4), P=0.026*). The blastocyst number of group P1MLH1 (0(0,2)) was the highest (P=0.003*).
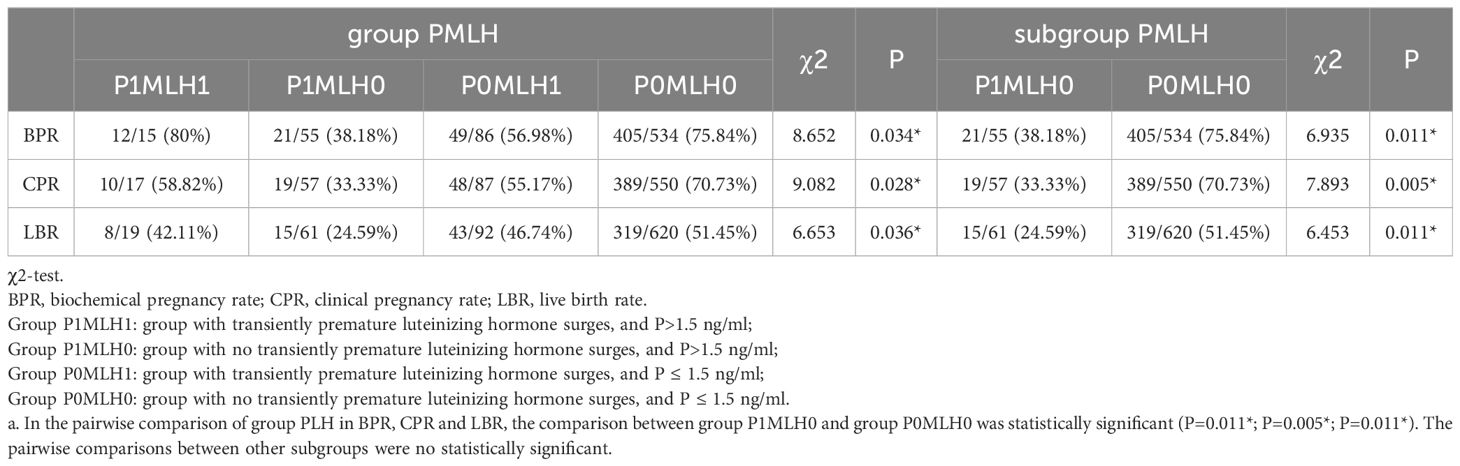
Pregnancy outcome between group PMLH and subgroup comparisons
The relationship between each group PMLH and pregnancy outcomes was analyzed and compared. As displayed in Table 2, BPR (P=0.034*), CPR (P=0.028*), and LBR (P=0.036*) were significantly different across the PMLH subgroups. In the pairwise comparison of group PMLH, the comparison between group P1MLH0 and group P0MLH0 showed a statistically significant difference (BPR: P=0.011*; CPR: P=0.005*; LBR: P=0.011*). The pairwise comparisons between other subgroups were no statistically significant.
Correlation analysis
Figure 1 displays the correlation analysis results between the indicators. A significant negative association was found between the maximum LH level and BMI (R=-0.13, P=0.000*), GnRH-ant days (R=-0.07, P=0.018*), GnRH-ant dosage (R=-0.11, P=0.000*), and the outcome of COH, including number of fertilization (NOF) (R=-0.08, P=0.005*), number of embryos (NOE) (R=-0.07, P=0.023*), number of high-quality embryo (NOH) (R=-0.07, P=0.012*). In contrast, a significant positive association was observed between the maximum LH level and female age (R=0.08, P=0.004*). In addition, the same trend was observed between the maximum LH and P levels on the hCG day, indicating a positive correlation (R=0.08, P=0.005*). The P level exhibited a negative correlation with BMI (R=-0.1, P=0.000*) and age (R=-0.11, P=0.000*), while demonstrating a positive association with AFC (R=0.18, P=0.000*) and COH outcome, including number of oocytes (NOC) (R=0.3, P=0.000*), NOF (R=0.23, P=0.000*), NOE (R=0.15, P=0.000*), number of blastocyst (NOB) (R=0.11, P=0.000*), NOH (R=0.09, P=0.002*).
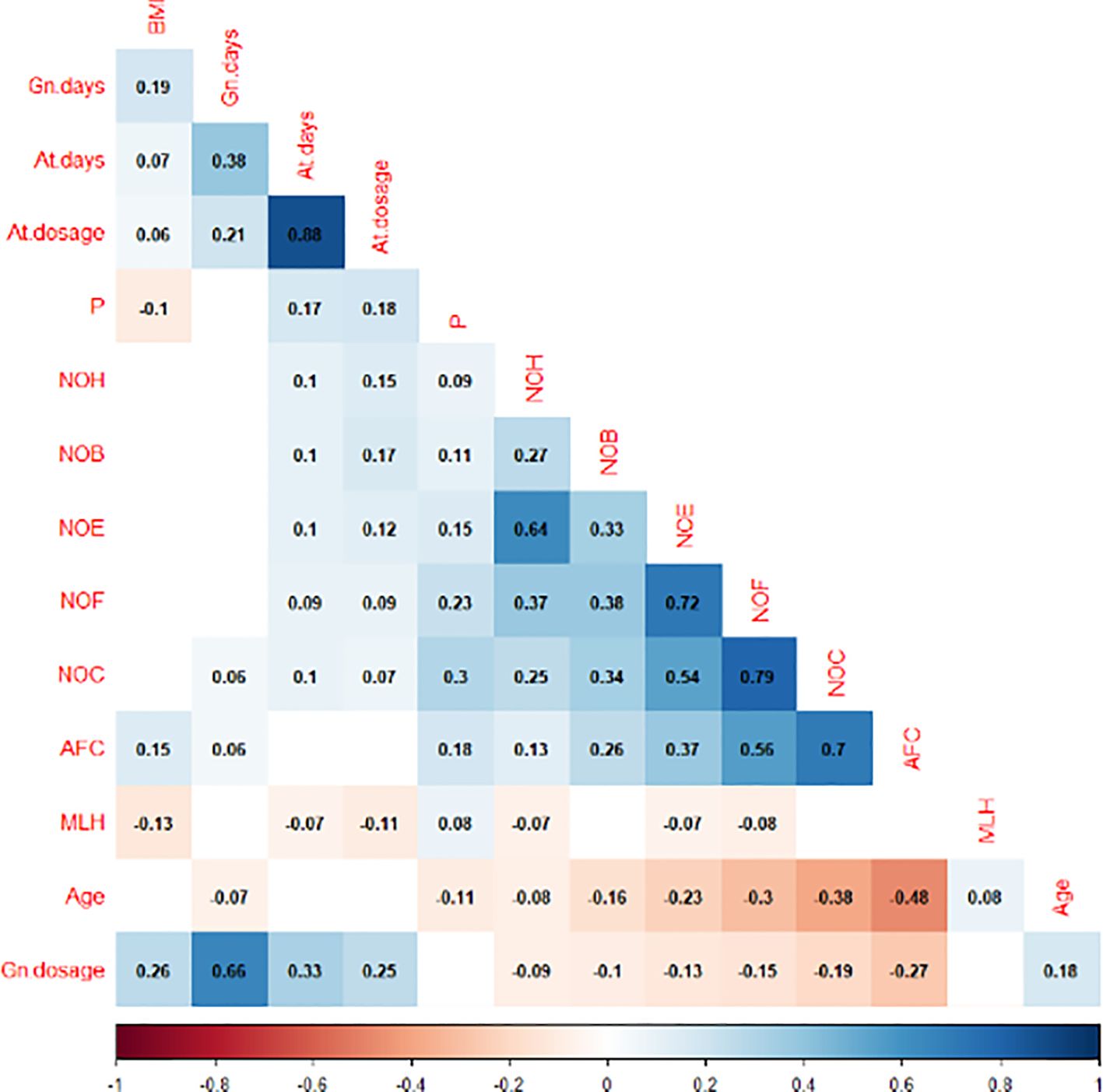
Figure 1. Correlation analysis between the maximum level of LH, P on the HCG day and other indicators.
Differences in correlation indicators
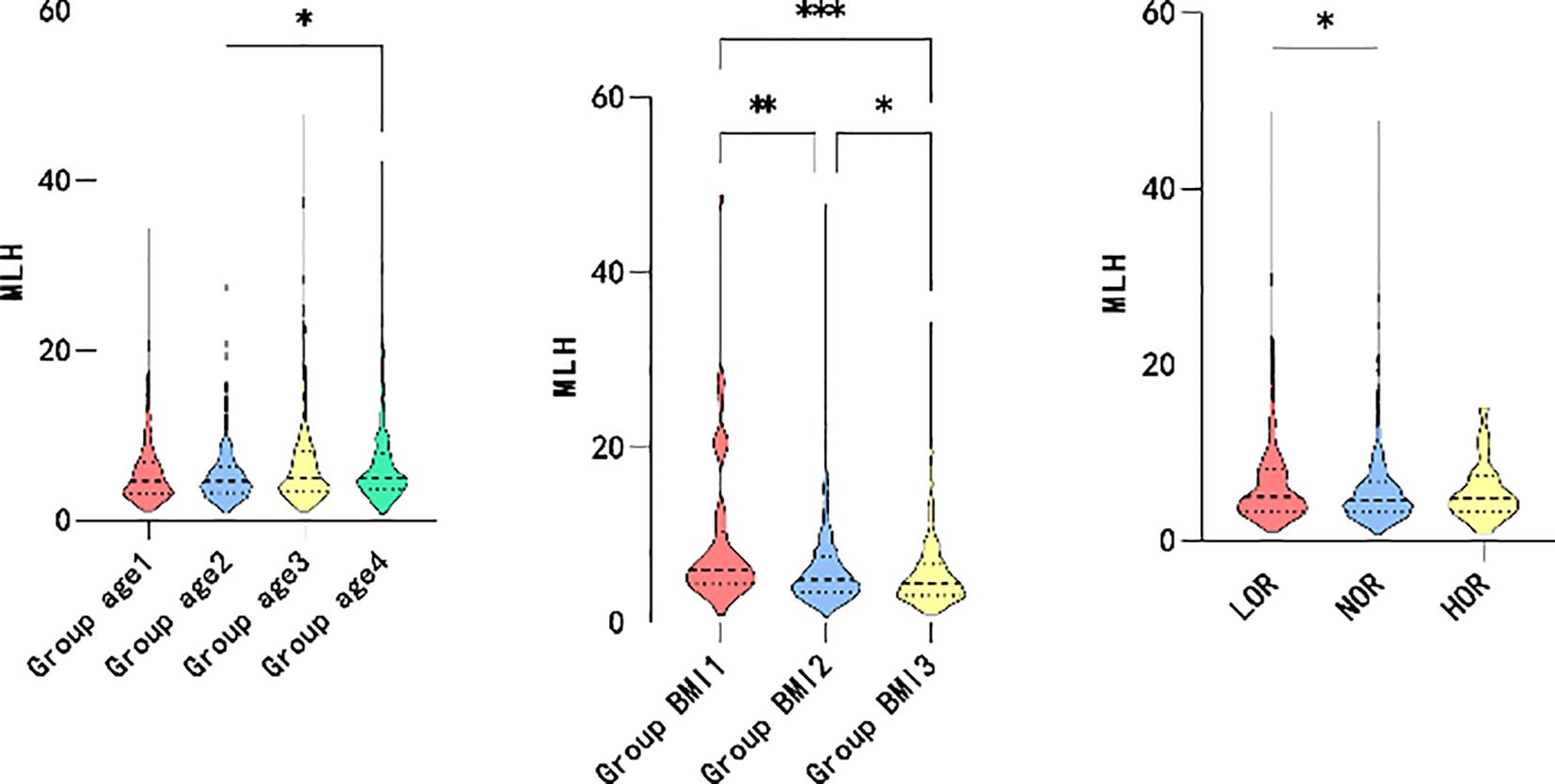
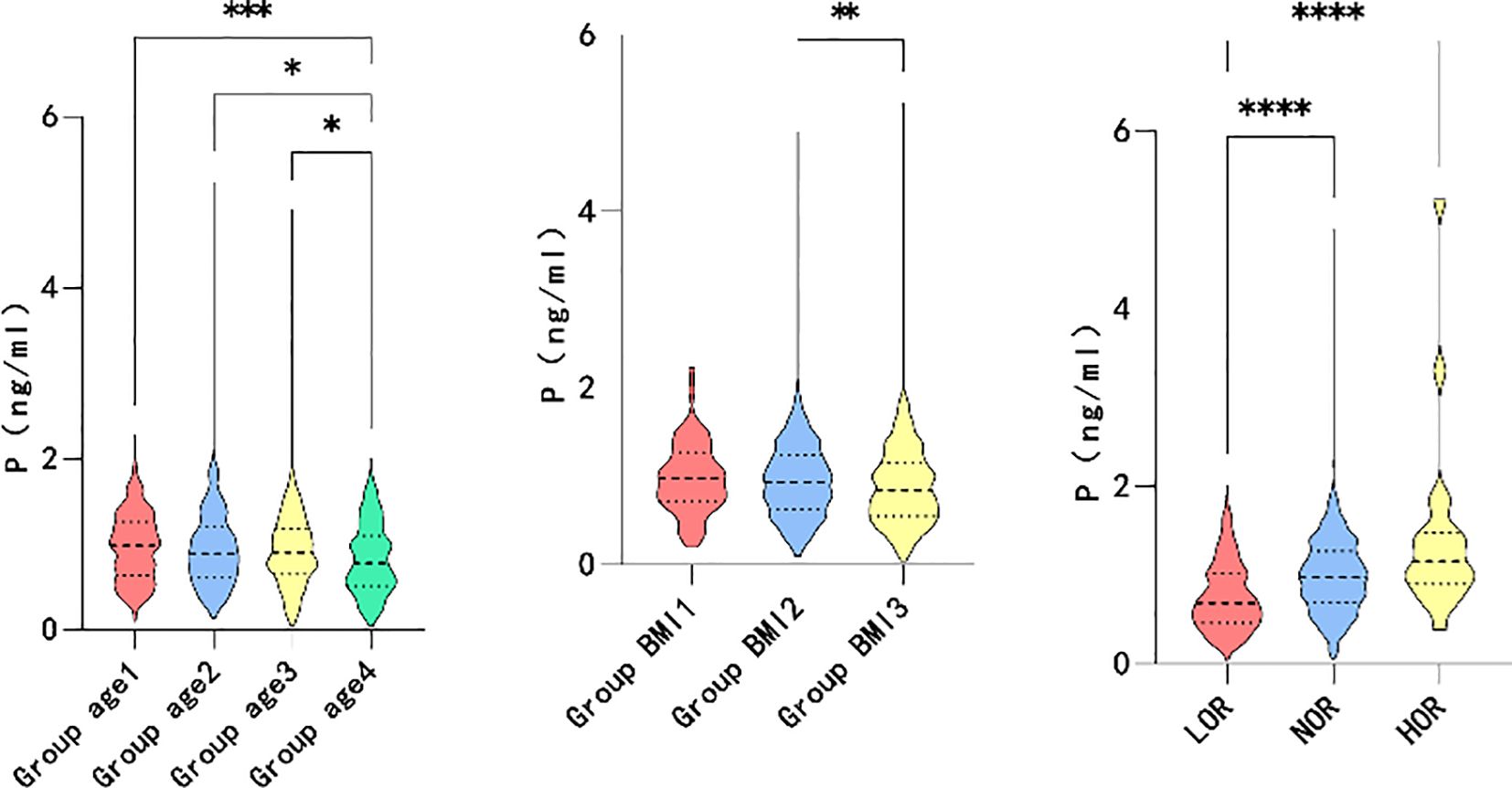
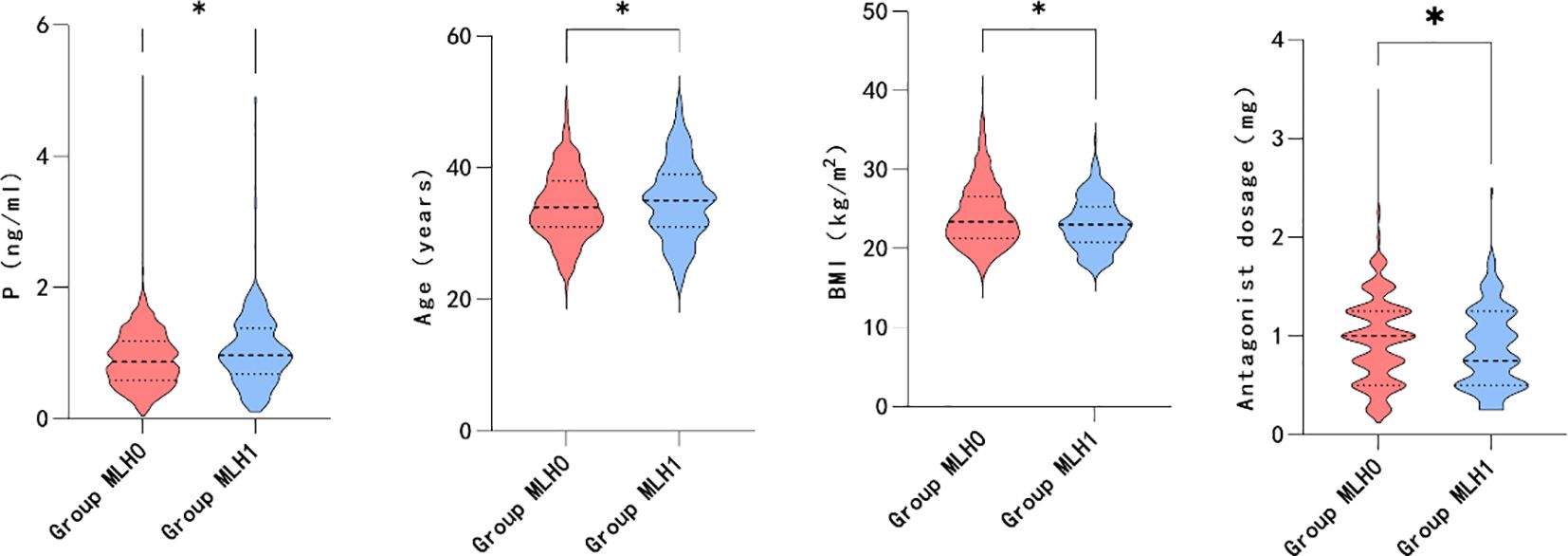
Based on the correlation analysis results, the patients were further subgrouped by age and BMI. The participants were divided into quartiles of age ≤ 30, 30<age ≤ 34, 34<age ≤ 38, and 38<age. Moreover, the patients were subgrouped by BMI into BMI<18.5, 18.5 ≤BMI<24, and 24≤BMI based on guidelines for the prevention and management of overweight and obesity in the adult Chinese population (2004). High and low ovarian responses were defined as >18 oocytes retrieved and <6 oocytes retrieved, respectively. As shown in Figure 2, higher maximum LH levels were associated with older age (P=0.006*), lower BMI (P=0.000*), and decreased ovarian response (P=0.046*). However, elevated P levels on hCG day were associated with younger individuals (P=0.001*), participants with lower BMI (P=0.006*), and higher ovarian response (P=0.000*), as illustrated in Figure 3.
Furthermore, the association between transient premature LH surge and the above subgroups was explored. The statistically significant indicators are shown in Figures 4, 5. The patients with transiently premature LH surges had older age (P=0.045*), lower BMI (P=0.014*), lower GnRH-ant dosage (P=0.020*), and higher P levels (P=0.011*) on the hCG day. However, the increased P levels were mainly related to patients’ AFC (P=0.004*). Higher P levels were associated with a greater number of oocytes (P=0.000*), fertilization (P=0.002*), and number of embryos (P=0.046*).
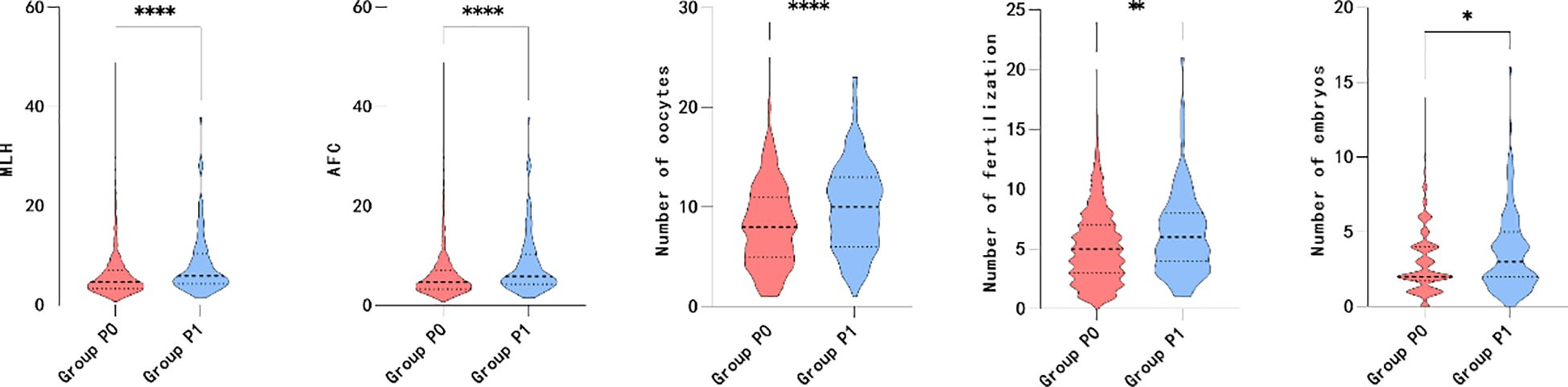
Figure 5. Maximum LH, AFC, number of oocytes, number of fertilization and number of embryos between group P.
Effects of group PMLH on LBR
Binary logistic regression analysis revealed that group P1MLH0 (OR =0.432 [0.218–0.854]; P =0.016*) had opposite influences on the live birth rate compared with group P0MLH0, as indicated in Table 3. The same results were obtained in normal ovarian response (OR =0.49 [0.25–0.94]; P =0.03*), as indicated in Table 4.
Curve fitting between P and LBR with no transiently premature LH surges
Figure 6 shows the fitted curves after adjustment for confounders between the P level on the hCG day and the live birth rate without transient premature LH surge. The results yielded a parabolic reverse-U curve as the P level increased. The curve initially increased before declining at the highest P level on the hCG day.
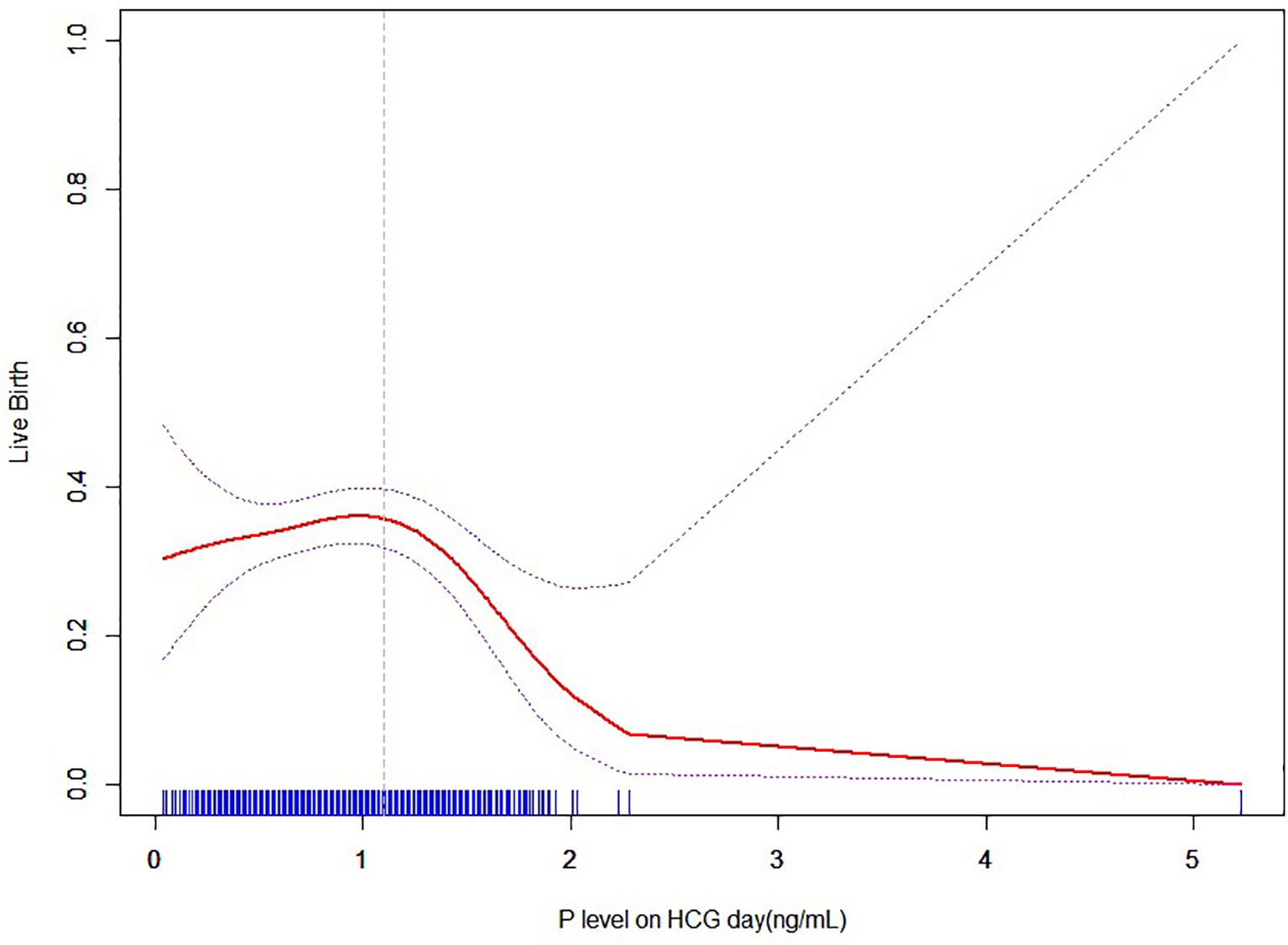
Figure 6. Nonlinear association between P level and live birth rate with no transiently premature LH surges.
Threshold effect analysis of P and LBR with no transient premature LH surge
The results of the threshold effect analysis of the P levels and LBR are presented in Table 5. The live birth rate showed a significant decrease when the P level was ≥1.1ng/ml.
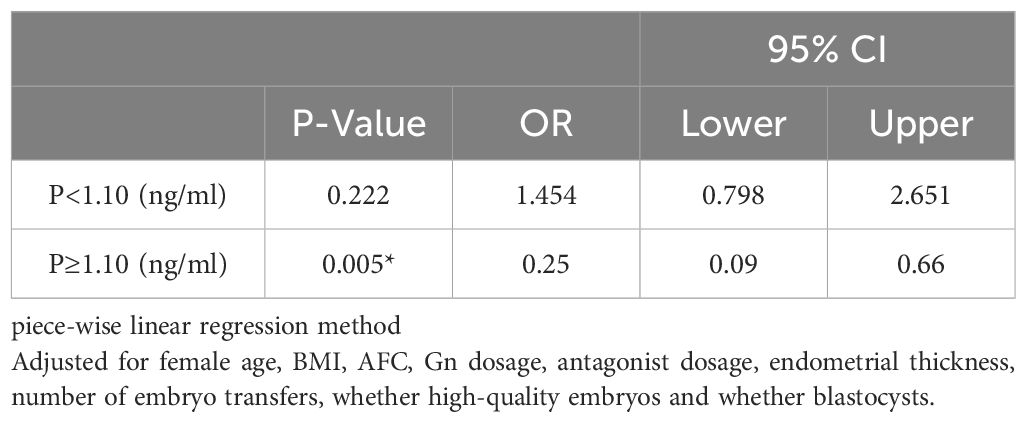
Table 5. Threshold effect analysis between P level and live birth rate with no transiently premature LH surges.
Discussion
This study examined the maximum levels of luteinizing hormone (MLH) during controlled ovarian hyperstimulation (COH) and the P level on the hCG day. Patients with lower BMI, older age, lower ovarian response, and lower GnRH-ant dosage were more likely to develop LH surge. Although the LH surge could lead to a slight reduction in the number of embryos, there was no apparent correlation observed between the occurrence of LH surge and pregnancy outcomes with a fresh embryo transfer cycle. This finding aligns with part of the literature that suggests LH surge does not impact pregnancy outcomes.
The increase in P level on hCG day is also associated with the patient’s age, BMI, and ovarian response. Similar to LH levels, higher BMI was associated with a lower likelihood of progesterone elevation, which may be attributed to the increased Gn requirement for inducing rises in both progesterone and LH levels in patients with higher body weights. In contrast to the LH level, a higher incidence of elevated P levels was observed with younger age, higher number of AFC, and higher ovarian response. However, significantly poorer pregnancy outcomes were observed in patients with P levels exceeding 1.5ng/ml, which was consistent with previous literature. In our center, a P level on the day of hCG of more than 1.5 ng/ml was used as the threshold to decide whether to perform fresh embryo transfer. This threshold was also confirmed in the present study, demonstrating a clear decrease in the LBR.
Interestingly, while the LH surge was not associated with pregnancy outcomes, its occurrence was significantly associated with increased P levels on the hCG day. Moreover, it was worth considering that the presence of elevated progesterone levels may contribute to adverse pregnancy outcomes, while elevated progesterone levels were positively correlated with an increased number of oocytes retrieved, fertilized, and embryos. In addition, LH surge was more commonly observed in older patients and patients with diminished ovarian response. Conversely, a higher P level was typically found in younger patients and individuals with a higher ovarian response and higher AFC. Therefore, the patients were categorized into four groups based on the presence of the LH surge and P > 1.5ng/ml in order to investigate the impact of the LH surge and progesterone levels on live birth. Through pairwise combinations, no LH surge and P>1.5 ng/ml in the subgroup P1MLH0, resulting in a significantly lower live birth rate than group P0MLH0.
Furthermore, the patients were analyzed based on the PMLH grouping. The patients in group P1MLH0 had the highest GnRH-ant dosage and GnRH-ant days. The lowest pregnancy rate was observed in group P1MLH0, which might be related to the high dosage of GnRH-ant used, the elevated progesterone levels, and the occurrence of an occult LH surge. Poorer pregnancy outcomes were observed as the dosage of GnRH-ant increased, with exerting a detrimental impact on the receptivity of the endometrium and the development of embryos (36–38). However, the total GnRH-ant dosage was associated with the occurrence of LH surge. The appropriate range of GnRH-ant dosage for different patients lacks definitive studies at present, and further research is required.
When P ≤1.5ng/ml, the occurrence of LH surge (Group P0MLH1) was associated with advanced age, lower BMI, and lower GnRH-ant dosage. The numbers of embryos were the lowest in Group P0MLH1. However, after inter-group correction, no statistically significant differences were observed in IVF/ICSI outcomes and pregnancy outcomes among individuals experiencing an LH surge compared to those who did not. Conversely, when P>1.5 ng/ml, there was no notable distinction between the groups with or without LH surge.
In cases with an LH surge, elevated P levels did not have a detrimental impact on the pregnancy outcome of fresh embryo transfer, and positive correlation was observed between elevated P levels and improved outcomes in terms of numbers of oocytes, fertilization, embryos and blastocysts. This phenomenon could be attributed to enhanced embryo quality counteracting the potential negative impact of elevated progesterone levels on endometrial receptivity. In cases without LH surge, patients with P > 1.5 ng/ml (Group P1MLH0) had highest GnRH-ant dosage (P=0.049*), and lower oocyte numbers, fertilization numbers and embryos than group P1MLH1. Therefore, the poorest pregnancy outcome in the group P1MLH0 is more likely attributed to the receptivity of the endometrium or insufficient high-quality embryos to counterbalance adverse endometrial factors.
Our study may contribute to the establishment of an appropriate threshold for fresh embryo transfer without LH surge. Luteinizing hormone and progesterone levels were analyzed to investigate their effects on pregnancy outcomes. Our findings suggest that higher GnRH-ant dosages have a detrimental impact on both embryo quality and the pregnancy outcome after fresh embryo transfers. Additionally, when an LH surge occurs, increased levels of progesterone are associated with improved embryo outcomes; however, the pregnancy rate after fresh embryo transfer showed no significant improvement due to the negative effect of progesterone on endometrial receptivity. Conversely, in cases without LH surge, higher levels of progesterone led to lower live birth rates. Therefore, we recommend cryopreserving all embryos when progesterone levels exceeded or equaled1.10 ng/ml in order to mitigate the adverse effects of elevated progesterone and GnRH-ant dosage on live birth rates. Nevertheless, the retrospective nature of our study imposed certain limitations, necessitating further prospective studies to validate our findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This retrospective study was registered with the Reproductive Ethics Committees of the Affiliated Hospital of Shandong University of TCM (ref approval no. SDTCM202311031) on October 31, 2023. The name of the IRB is the Reproductive Ethics Committees of the Affiliated Hospital of Shandong University of traditional Chinese medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
C-XW: Writing – original draft. J-WZ: Writing – review & editing. SX: Writing – original draft. FL: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We want to thank all the participants in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
hCG, human chronic gonadotropin; IVF-ET, in vitro fertilization-embryo transfer; ART, assisted reproductive technology; BPR, biochemical pregnancy rate; CPR, clinical pregnancy rate; LBR, live birth rate; E2, estradiol; MLH, maximum LH level during Gn days; AFC, antral follicle count; BMI, body mass index; Gn dosage, Gonadotropin dosage; Gn days, Gonadotropin days; GnRH-ant/At. dosage, Antagonist dosage; GnRH-ant/At. days, Antagonist days; NOC, Number of oocytes; NOF, Number of fertilization; NOE, Number of embryos; NOB, Number of blastocyst; NOH, Number of high-quality embryo; LOR, Low ovarian response; NOR, Normal ovarian response; HOR, High ovarian response; Group MLH0, group with no transiently premature luteinizing hormone surges; Group MLH1, group with transiently premature luteinizing hormone surges; Group P0, group with P ≤ 1.5 ng/ml; group P1, P>1.5 ng/ml; Group P1MLH1, group with transiently premature luteinizing hormone surges, and P>1.5 ng/ml; Group P1MLH0, group with no transiently premature luteinizing hormone surges, and P>1.5 ng/ml; Group P0MLH1, group with transiently premature luteinizing hormone surges, and P ≤ 1.5 ng/ml; Group P0MLH0, group with no transiently premature luteinizing hormone surges, and P ≤ 1.5 ng/ml.
References
1. Ragni G, Vegetti W, Riccaboni A, Engl B, Brigante C, Crosignani PG. Comparison of GnRH agonists and antagonists in assisted reproduction cycles of patients at high risk of ovarian hyperstimulation syndrome. Hum Reprod. (2005) 20:2421–5. doi: 10.1093/humrep/dei074
2. Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, Kolibianakis E, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI†. Hum Reprod Open. (2020) 2020. doi: 10.1093/hropen/hoaa
3. Luke B, Brown MB, Wantman E, Schymura MJ, Browne ML, Fisher SC, et al. The risks of birth defects and childhood cancer with conception by assisted reproductive technology. Hum Reprod. (2022) 37:2672–89. doi: 10.1093/humrep/deac196
4. Bourdon M, Alwohaibi A, Maignien C, Marcellin L, Chargui A, Pocate Cheriet K, et al. IVF/ICSI outcomes after a freeze-all strategy: an observational cohort study. Reprod Sci. (2023) 30:2283–91. doi: 10.1007/s43032-023-01173-4
5. Hargreave M, Jensen A, Hansen MK, Dehlendorff C, Winther JF, Schmiegelow K, et al. Association between fertility treatment and cancer risk in children. Jama. (2019) 322. doi: 10.1001/jama.2019.18037
6. Hargreave M. Fertility treatment and childhood cancer risk. JAMA Network Open. (2022) 5. doi: 10.1001/jamanetworkopen.2022.30162
7. Papamentzelopoulou M, Stavros S, Mavrogianni D, Kalantzis C, Loutradis D, Drakakis P. Meta-analysis of GnRH-antagonists versus GnRH-agonists in poor responder protocols. Arch Gynecology Obstetrics. (2021) 304:547–57. doi: 10.1007/s00404-020-05954-z
8. Dovey S, McIntyre K, Jacobson D, Catov J, Wakim A. Is a premature rise in luteinizing hormone in the absence of increased progesterone levels detrimental to pregnancy outcome in GnRH antagonist in vitro fertilization cycles. Fertil Steril. (2011) 96:585–9. doi: 10.1016/j.fertnstert.2011.06.042
9. Bosch E, Valencia I, Escudero E, Crespo J, Simón C, Remohí J, et al. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril. (2003) 80:1444–9. doi: 10.1016/j.fertnstert.2003.07.002
10. Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. (2010) 25:2092–100. doi: 10.1093/humrep/deq125
11. Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. (2017) 23:560–79. doi: 10.1093/humupd/dmx017
12. Kao T-C, Hsieh Y-C, Yang I-J, Wu M-Y, Chen M-J, Yang J-H, et al. Progestin-primed ovarian stimulation versus GnRH antagonist protocol in poor responders: Risk of premature LH surge and outcome of oocyte retrieval. J Formosan Med Assoc. (2023) 122:29–35. doi: 10.1016/j.jfma.2022.08.023
13. Reichman DE, Zakarin L, Chao K, Meyer L, Davis OK, Rosenwaks Z. Diminished ovarian reserve is the predominant risk factor for gonadotropin-releasing hormone antagonist failure resulting in breakthrough luteinizing hormone surges in in vitro fertilization cycles. Fertil Steril. (2014) 102:99–102. doi: 10.1016/j.fertnstert.2014.04.010
14. Geng Y, Lai Q, Xun Y, Jin L. The effect of premature luteinizing hormone increases among high ovarian responders undergoing a gonadotropin-releasing hormone antagonist ovarian stimulation protocol. Int J Gynecology Obstetrics. (2018) 142:97–103. doi: 10.1002/ijgo.12485
15. Kochhar P, Ghosh P. Diminished ovarian reserve predisposes to premature luteinizing hormone surges in gonadotropin-releasing hormone antagonist cycles in In vitro fertilization. J Hum Reprod Sci. (2020) 13. doi: 10.4103/jhrs.JHRS_133_19
16. Lee WH-Y, Lin K-T, Hsieh Y-C, Kao T-C, Huang T-C, Chao K-H, et al. The value of LH maximum level in predicting optimal oocyte yield following GnRH agonist trigger. Front Endocrinol. (2023) 14. doi: 10.3389/fendo.2023.1216584
17. Wang J, Ding J, Qu B, Zhang Y, Zhou Q. Does serum LH level influence IVF outcomes in women with PCOS undergoing gnRH-antagonist stimulation: A novel indicator. J Clin Med. (2022) 11. doi: 10.3390/jcm11164670
18. Zhang D, Zhang D, Sun Z, Deng C, Yu Q, Zhen J. The effect of a transient premature luteinizing hormone surge without elevated serum progesterone on in vitro fertilization outcomes in a gonadotropin-releasing hormone antagonist flexible protocol. Gynecological Endocrinol. (2019) 36:550–3. doi: 10.1080/09513590.2019.1683730
19. Bansal S, Singh N, Gupta P, Malhotra N, Mahendru R. Does basal luteinizing hormone help predict the fate of in vitro fertilization? JBRA Assisted Reprod. (2016) 20. doi: 10.5935/1518-0557.20160016
20. Zhou J-S, Chen J-H, Tang F-F, Ou J-P, Tao X, Cai L-H. The effect of luteinizing hormone changes in GnRH antagonist protocol on the outcome of controlled ovarian hyperstimulation and embryo transfer. BMC Pregnancy Childbirth. (2023) 23. doi: 10.1186/s12884-023-05916-8
21. Gao F, Wang Y, Wu D, Fu M, Zhang Q, Ren Y, et al. A premature rise of luteinizing hormone is associated with a reduced cumulative live birth rate in patients ≥37 years old undergoing gnRH antagonist in vitro fertilization cycles. Front Endocrinol. (2021) 12. doi: 10.3389/fendo.2021.722655
22. Kummer NE, Weitzman VN, Benadiva CA, Schmidt DW, Engmann LL, Nulsen JC. In vitro fertilization outcomes in patients experiencing a premature rise in luteinizing hormone during a gonadotropin-releasing hormone antagonist cycle. Fertility Sterility. (2011) 95:2592–4. doi: 10.1016/j.fertnstert.2010.12.046
23. Zhang Y, Xu Y, Yu J, Wang X, Xue Q, Shang J, et al. A premature luteinizing hormone surge without elevated progesterone levels has no adverse effect on cumulative live birth rate in patient undergoing a flexible GnRH antagonist protocol: a retrospective study. J Ovarian Res. (2023) 16. doi: 10.1186/s13048-023-01219-w
24. Huang Q, Nong Y, Zhang X, Huang L, Tang T, Huang J, et al. Effects of increasing serum luteinizing hormone levels during early phase of the gonadotropin-releasing hormone antagonist protocol on clinical outcomes of the in vitro fertilization cycle. Gynecological Endocrinol. (2021) 38:135–9. doi: 10.1080/09513590.2021.1955341
25. Kalakota NR, George LC, Morelli SS, Douglas NC, Babwah AV. Towards an improved understanding of the effects of elevated progesterone levels on human endometrial receptivity and oocyte/embryo quality during assisted reproductive technologies. Cells. (2022) 11. doi: 10.3390/cells11091405
26. Bourgain C, Devroey P. The endometrium in stimulated cycles for IVF. Hum Reprod Update. (2003) 9:515–22. doi: 10.1093/humupd/dmg045
27. Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update. (2013) 19:433–57. doi: 10.1093/humupd/dmt014
28. Zhang J, Du M, Wu Y, Wei Z, Guan Y. Effect of serum progesterone levels on hCG trigger day on pregnancy outcomes in GnRH antagonist cycles. Front Endocrinol. (2022) 13. doi: 10.3389/fendo.2022.982830
29. Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J, et al. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril. (2012) 97:1321–1327.e4. doi: 10.1016/j.fertnstert.2012.03.014
30. Wu Z, Dong Y, Ma Y, Li Y, Li L, Lin N, et al. Progesterone elevation on the day of hCG trigger has detrimental effect on live birth rate in low and intermediate ovarian responders, but not in high responders. Sci Rep. (2019) 9. doi: 10.1038/s41598-019-41499-1
31. Xu J, Zhang C, Wang S, Zhang S. Impact of progesterone concentration on human chorionic gonadotropin trigger day on clinical outcomes with one top-quality cleavage-stage embryo or blastocyst transfer in fresh in vitro fertilization cycles. Front Endocrinol. (2023) 14. doi: 10.3389/fendo.2023.1085287
32. Gordon UD, Harrison RF, Fawzy M, Hennelly B, Gordon AC. A randomized prospective assessor-blind evaluation of luteinizing hormone dosage and in vitro fertilization outcome. Fertil Steril. (2001) 75:324–31. doi: 10.1016/S0015-0282(00)01701-5
33. Lambalk CB, Leader A, Olivennes F, Fluker MR, Andersen AN, Ingerslev J, et al. Treatment with the GnRH antagonist ganirelix prevents premature LH rises and luteinization in stimulated intrauterine insemination: results of a double-blind, placebo-controlled, multicentre trial*. Hum Reprod. (2006) 21:632–9. doi: 10.1093/humrep/dei386
34. Kolibianakis EM, Venetis CA, Kalogeropoulou L, Papanikolaou E, Tarlatzis BC. Fixed versus flexible gonadotropin-releasing hormone antagonist administration in in vitro fertilization: a randomized controlled trial. Fertil Steril. (2011) 95:558–62. doi: 10.1016/j.fertnstert.2010.05.052
35. Scott RT, Hofmann GE, Veeck LL, Jones HW, Muasher SJ. Embryo quality and pregnancy rates in patients attempting pregnancy through in vitro fertilization. Fertil Steril. (1991) 55:426–8. doi: 10.1016/S0015-0282(16)54141-7
36. Feng L, Fan R, Jiang A, Jiang J, Wang Q, Sun Y, et al. The effect of flexible low-dose GnRH antagonist on pregnancy outcome in the fresh embryo transfer cycle of IVF-ET: a randomized controlled trial. Reprod Biol Endocrinol. (2022) 20. doi: 10.1186/s12958-022-00927-0
37. Chen Q, Fan Y, Zhou X, Yan Z, Kuang Y, Zhang A, et al. GnRH antagonist alters the migration of endometrial epithelial cells by reducing CKB. Reproduction. (2020) 159:733–43. doi: 10.1530/REP-19-0578
Keywords: luteinizing hormone surge, progesterone, flexible antagonist protocol, fresh cycle, live birth rate
Citation: Wei C-X, Zhang J-W, Xiang S and Lian F (2024) Reproductive outcomes in fresh transfer cycles and antagonists with premature luteinizing and/or progesterone surge: a single center retrospective cohort study. Front. Endocrinol. 15:1411106. doi: 10.3389/fendo.2024.1411106
Received: 02 April 2024; Accepted: 29 August 2024;
Published: 24 September 2024.
Edited by:
Yuting Fan, Boston IVF, United StatesReviewed by:
Fenghua Liu, Guangdong Province Women and Children Hospital, ChinaElkin Muñoz, IVI Madrid - Clínica de Reproducción Asistida y Fertilidad (IVIRMA), Spain
Copyright © 2024 Wei, Zhang, Xiang and Lian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Lian, NzEwMDA5MDRAc2R1dGNtLmVkdS5jbg==
 Chun-Xiao Wei
Chun-Xiao Wei Jian-Wei Zhang1
Jian-Wei Zhang1