
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 18 December 2024
Sec. Bone Research
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1407692
 Chiao-Ling Chen1†
Chiao-Ling Chen1† Jian-Ying Wang2*†
Jian-Ying Wang2*†Background: The increasing prevalence of glucocorticoid-induced osteoporosis (GIOP) due to long-term glucocorticoid therapy underscores the need for effective treatment options. Denosumab and bisphosphonates, both key in managing GIOP, require further comparative evaluation to determine their relative efficacy and safety profiles.
Methods: We conducted a systematic review and meta-analysis, adhering to PRISMA guidelines. Our analysis included randomized controlled trials (RCTs) comparing denosumab with bisphosphonates in GIOP management. The outcomes were percent changes in bone mineral density (BMD) at various sites, bone turnovers markers (BTMs) and the incidence of adverse events.
Results: Our study comprised five RCTs with 1,043 participants. The results showed a significant mean difference in BMD percentage change from baseline at LS of 2.87% (95% CI: 1.86 to 3.87, p<0.001) and at TH of 1.39% (95% CI: 0.15 to 2.64, p=0.03). Additionally, the safety profile of denosumab was found to be comparable to bisphosphonates, with no significant increase in the incidence of adverse events or serious adverse reactions.
Conclusions: Denosumab proved more effective in enhancing BMD than bisphosphonates in GIOP, maintaining a comparable safety profile. However, the study’s limitations, including heterogeneity and the need for longer-term research, were noted.
In the U.S., statistics indicate that about 1.2% of the population uses glucocorticoids for prolonged durations, with a notably low frequency of antiosteoporotic medication use (1). Glucocorticoid-induced osteoporosis (GIOP) is a notable side effect of prolonged glucocorticoid therapy (2). This condition is characterized by a significant risk of bone loss, predominantly within the first few months of treatment (3). Studies have indicated that even low doses of prednisone or its equivalents, ranging from 2.5 to 7.5 mg daily, are associated with an increased risk of fractures (4). Therefore, GIOP requires aggressive management, especially in patients who are already at a higher risk for fractures, such as older individuals or those with a history of fragility fractures (3).
According to the 2022 guidelines of the American College of Rheumatology, it is recommended to assess fracture risk in patients aged 40 and over as soon as possible after starting a treatment with ≥2.5 mg/day of glucocorticoids (GC) for more than 3 months. This assessment should be done using the FRAX (Fracture Risk Assessment Tool) and by performing BMD testing using dual-energy x-ray absorptiometry (DXA) with vertebral fracture assessment (VFA) testing or spinal x-rays. The guidelines advise that all adult patients with medium, high, or very high fracture risk should be considered for osteoporosis therapy.
Bisphosphonates (BPs), widely used for managing osteoporosis and other bone diseases, work by inhibiting osteoclast activity, which are cells responsible for bone tissue breakdown (5). While they are a standard treatment for GIOP, oral BPs sometimes cause gastrointestinal side effects, such as esophageal irritation and ulceration, and have absorption issues (5). Although intravenous formulations can reduce gastrointestinal complications, intravenous BPs may present different side effects, including flu-like symptoms, fevers, myalgias, arthralgias, headaches, and also carry potential risks of atypical femoral fractures and osteonecrosis of the jaw (ONJ) (6). In comparison, teriparatide, another medication used for GIOP, has demonstrated better BMD improvements than BPs (7). However, its usage is generally restricted to no more than two years in a patient’s lifetime and necessitates frequent daily or weekly injections.
The challenge in treating GIOP arises from the effects of GCs on bone health. GCs increase the apoptosis of mature osteoblasts and osteocytes, leading to decreased bone formation and an impaired ability to respond to bone damage (8). They also extend the lifespan of osteoclasts by upregulating receptor activator of nuclear factor kappa-B ligand (RANKL) while suppressing osteoprotegerin (OPG) (8). Furthermore, GCs inhibit the Wnt signaling pathway—crucial for osteoblastogenesis—by increasing the expression of inhibitors like dickkopf-1 (Dkk-1) and sclerostin. This suppression results in reduced osteogenesis and poor bone regeneration (8).
Denosumab, a human monoclonal antibody, offers a more convenient option with biannual administration. It is effective in treating osteoporosis, bone loss from other diseases, bone metastases, and giant cell tumors of the bone (5). It functions as a RANKL inhibitor, preventing bone resorption by hindering the development of osteoclasts (5). In contrast, BPs primarily work by binding to bone to inhibit osteoclasts, acting more indirectly and relying on accumulation within the bone. In a murine model of GIOP, denosumab inhibited cortical bone loss without compromising biomechanical strength (9). This demonstrates its efficacy in preserving bone integrity under conditions that typically lead to significant bone loss (9).
Based on recent studies and systematic reviews, there is a growing body of evidence regarding the use of denosumab treating GIOP (10, 11). A 2022 systematic review and meta-analysis found that denosumab outperformed BPs in increasing lumbar spine BMD at 6 and 12 months, but both treatments showed similar improvements in total hip and femoral neck BMD (11). Additionally, while denosumab more effectively suppressed bone turnover markers like serum CTx (C-terminal telopeptide) and P1NP (procollagen type 1 amino-terminal propeptide), no significant differences in side effects, infections, or fractures were noted between the two treatments (11).
Based on the current evidence, we conducted an updated meta-analysis to explore the efficacy and safety of denosumab compared to BPs for GIOP. Furthermore, our analysis will include groups that have started using glucocorticoids for less than 3 months. A key focus of our study will also be on safety concerns, particularly the risk of infections. This is especially crucial since research indicates a higher risk of infection in postmenopausal women using denosumab, a concern that is magnified in populations with prolonged use of glucocorticoids. To ensure that our updated meta-analysis is reliable and clinically relevant, we employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework (12). This approach enables a systematic evaluation of the quality of evidence, providing transparency in how conclusions are derived. The GRADE methodology was selected because it helps balance the benefits and risks of interventions, particularly in populations with complex health conditions such as GIOP. Additionally, it supports the prioritization of outcomes that matter most to patients and clinicians, such as fracture prevention and infection risk.
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13). It included randomized controlled trials (rcts) comparing the efficacy and safety of denosumab with BPs for prevention and treatment of GIOP. A comprehensive search was conducted in pubmed, Cochrane Library, and EMBASE, covering all publications until Jan 9, 2024. Search terms included “denosumab”, “bisphosphonates”, “Glucocorticoid-Induced Osteoporosis”, “Glucocorticoid”, “bone mineral density”, “Bone turnover”, “fracture” and related terms without language restrictions. Exclusion criteria were studies of less than 12 months duration or those not adhering to standard dosages. Two reviewers (C.L.C & J.Y.W) independently screened the results, resolving disagreements through discussion or a third party’s consultation. Funnel plots for publication bias assessment were used when the analysis included 10 or more studies.
Study quality was assessed using the Cochrane risk of bias (ROB) tool 2.0 (14), evaluating randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of reported results. Two independent reviewers (C.L.C & J.Y.W) conducted these assessments, with any discrepancies resolved through discussion and a third party’s consultation.
The primary outcomes for your study, as stated, will focus on the percentage changes in BMD at different sites - the lumbar spine (LS), femoral neck (FN), and total hip (TH) from baseline. Secondary outcomes encompassed risk of adverse events (AEs), including incidence of any AEs, serious AEs, infections. In addition, the occurrence of both vertebral and non-vertebral fractures was assessed.
Considering the expected variability in bisphosphonate usage, treatment duration, and patient demographics, a random-effects model was employed in the analysis. Heterogeneity was assessed using Cochran’s Q test and Higgins’s I2 statistic (15, 16). To assess the robustness of the effects in our analysis, we will employ a sensitivity analysis using the leave-one-out meta-analysis approach. Statistical analyses were performed using Review Manager Web (Revman Web) and STATA software (17, 18).
The GRADE approach assessed the quality of evidence for each outcome, considering risk of bias, inconsistency, indirectness, imprecision, and other factors like publication bias and effect magnitude. We selected six main outcomes for evaluation, deemed essential for decision-making and focused on patient-important outcomes: percent change of BMD at LS, FN, TH, rates of any AEs, serious AEs, and any infection. The evidence was graded from very low to high and its importance categorized as critical, important, or nonimportant using GRADEpro/GDT software (12).
This systematic review did not involve direct human or animal subjects and therefore did not require ethical approval. All analyses were based on previously published data.
Our meta-analysis included 5 RCTs comprising 12 study arms with a total of 1,043 participants (19–23). A detailed flowchart of the screening process can be seen in Figure 1. The detailed characteristics of the included studies are presented in Table 1. Among these, two studies focused on populations previously treated with bisphosphonates, one was on treatment-naive individuals, and the rest did not specify or mixed populations. The age range of participants spanned from 48.0 to 68.5 years. In the treatment groups, denosumab was uniformly used at a dosage of 60 mg subcutaneously every 6 months. Among the comparator groups, two study arms involved varied bisphosphonates, two used alendronate, and two employed risedronate. The duration of the studies ranged from 12 to 24 months. The prednisolone equivalent dose ranged from 3 to 16.6 mg across the studies with treatment durations extending up to 111 months.
The risk of bias assessment results for the studies can be found in Supplementary Table S1. After the evaluation, overall, four studies were classified as ‘some concern,’ mainly due to the lack of blinding for the medication. The remaining study, which used a double-blind, double-dummy design, was assessed as ‘high risk’ due to a higher dropout rate.
The results from the random-effects model in our meta-analysis indicated significant differences in BMD changes when comparing denosumab with bisphosphonates. Specifically, denosumab demonstrated greater mean percentage changes in BMD from baseline at various sites: LS showed a mean difference of 2.87% (95% confidence interval [CI]: 1.86 to 3.87, p<0.001, I² = 84%, see Figure 2), the FN exhibited a mean difference of 1.72% (95% CI: -0.08 to 3.51, p=0.06, I² = 84%, see Figure 3), and the TH had a mean difference of 1.39% (95% CI: 0.15 to 2.64, p=0.06, I² = 93%, see Figure 4). Furthermore, the leave-one-out analysis indicated that the removal of any single study did not significantly affect the results for BMD at LS, FN, and TH.
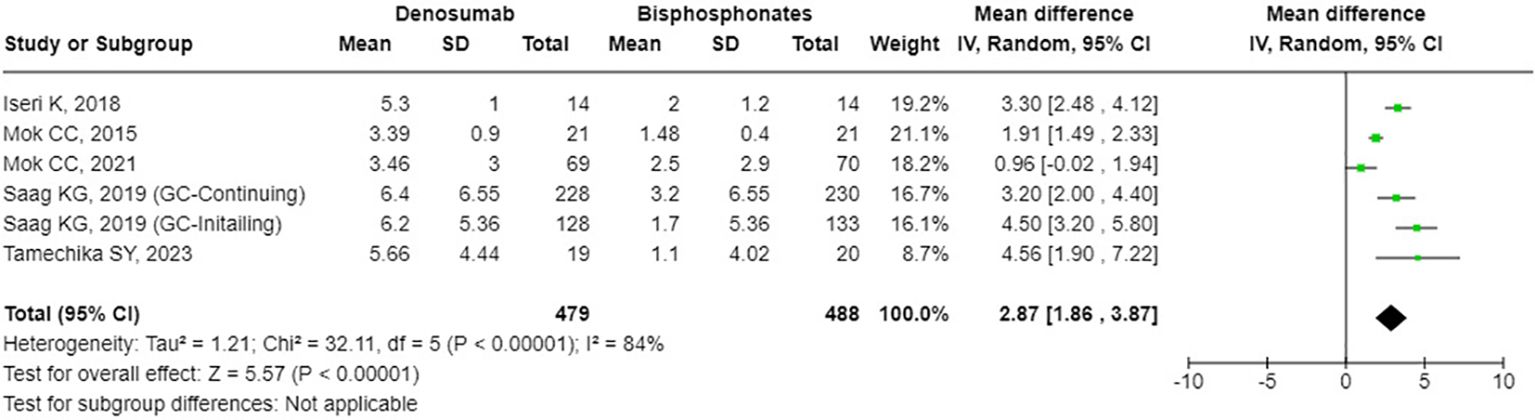
Figure 2. Forest plot of bone mineral density (BMD) percentage change from baseline at lumbar spine (LS) - denosumab vs. bisphosphonates.
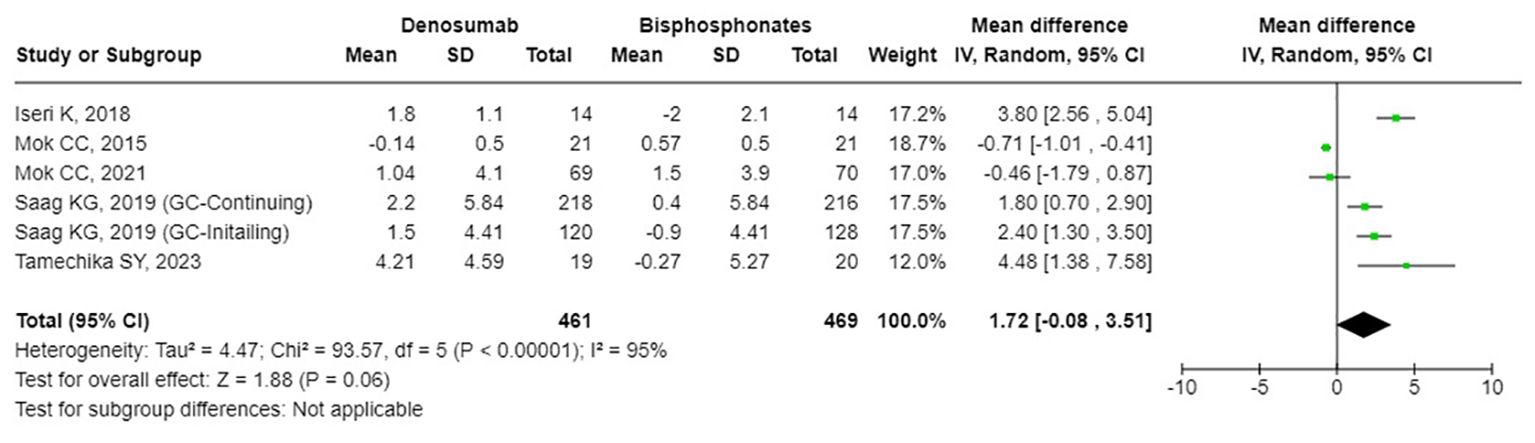
Figure 3. Forest plot of bone mineral density (BMD) percentage change from baseline at femoral neck (FN) - denosumab vs. bisphosphonates.
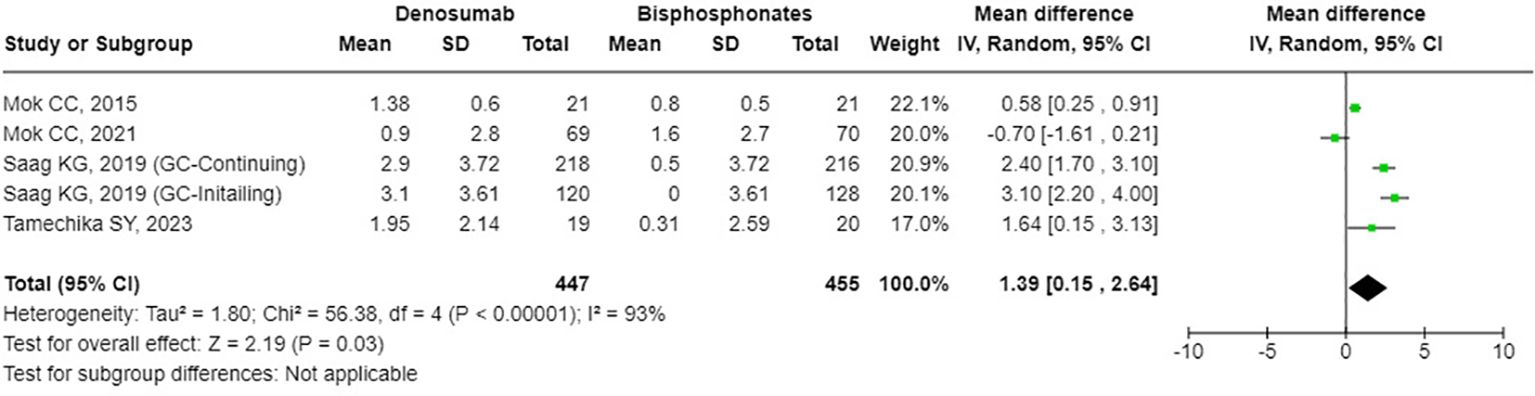
Figure 4. Forest plot of bone mineral density (BMD) percentage change from baseline at total hip (TH) - Denosumab vs. bisphosphonates.
Regarding bone turnover markers, we assessed four key indicators: P1NP, CTx, tartrate-resistant acid phosphatase 5b (TRACP-5b), and bone alkaline phosphatase (BAP). The findings showed that denosumab had a significantly higher percent change from baseline compared to BPs in P1NP, CTx, and TRACP-5b. Specifically, the mean differences were -26.93% (95% CI: -43.64 to -10.21, p=0.002, I² = 0%, see Supplementary Figure S1) for P1NP, -49.24% (95% CI: -75.97 to -22.51, p<0.001, I² = 0%, see Supplementary Figure S2) for CTx, and -26.37% (95% CI: -46.59 to -6.14, p=0.01, I² = 0%, see Supplementary Figure S3) for TRACP-5b. However, for BAP, there was no significant difference between the two treatments, with a mean difference of -11.96% (95% CI: -25.10 to 1.18, p=0.07, I² = 0%, see Supplementary Figure S4).
Regarding safety, compared to bisphosphonates, denosumab did not significantly increase the odds ratio (OR) for any AEs, which was 1.82 [95% CI: 0.75 to 4.44], p = 0.19, I² = 75%, see Figure 5). Similarly, there was no significant increase in the odds ratio for serious AEs (OR: 1.16 [95% CI: 0.32 to 4.17], p = 0.82, I² = 27%, see Figure 6). Specifically, denosumab did not show a statistically significant increase in the odds for hypocalcemia (OR: 5.80 [95% CI: 0.25 to 132.56], p = 0.27, I² not applicable, see Supplementary Figure S5), any infection (OR: 1.39 [95% CI: 0.71 to 2.74], p = 0.34, I² = 29%, see Supplementary Figure S6), or serious infections (OR: 1.36 [95% CI: 0.63 to 2.93], p = 0.43, I² = 22%, see Supplementary Figure S9). Overall, common AEs related to denosumab in the trials included mild infections, hypocalcemia, back pain, joint pain, hypertension, and gastrointestinal symptoms. Serious adverse reactions included severe rashes, symptomatic hypercalcemia, and infections requiring hospitalization, although none resulted in fetal. Additionally, one case of atypical femoral fracture was reported, and no cases of osteonecrosis of the jaw were reported. Regarding fracture incidence, denosumab showed an odds ratio of 0.73 (95% CI: 0.34 to 1.56, p = 0.42, I² = 8%, see Supplementary Figure S7) for vertebral fractures; 1.39 (95% CI: 0.70 to 2.74, p = 0.34, I² not applicable, see Supplementary Figure S8) for non-vertebral fractures.
In our GRADE assessment (detailed in Supplementary Table S2), specific limitations were identified that impacted the certainty of evidence for our outcomes. For the BMD Percentage Change at LS, FN, and TH, the risk of bias due to lack of blinding and dropout rates was a serious concern. High heterogeneity also influenced the certainty of evidence, alongside the small number of studies. When assessing the OR for any AEs, serious AEs, and any infection, similar issues were encountered. Consequently, the evidence was graded from very low to moderate, underscoring the need for careful interpretation in clinical decision-making for GIOP treatment.
Our meta-analysis offers critical insights into the efficacy and safety of denosumab in comparison to bisphosphonates, particularly in the context of pr GC therapy and long-term GC treatment. This analysis is especially pertinent given the diversity of patient groups, including those with prior bisphosphonate treatment, treatment-naive individuals. The marked improvement in BMD at the LS and TH with denosumab underscores its potential superiority over bisphosphonates, demonstrating notable effectiveness for both newly initiated and long-term GC users. This is of significant interest for patients initiating GC therapy, where early intervention is crucial for preventing GIOP. Although changes in BMD at the FN was less pronounced, they align with the trend favoring denosumab. Complementing our findings, the study by Geusens P et al. (24), although not included in our analysis, reveals similar trends in patients initiating GC therapy or on long-term GC therapy. Their study employed high-resolution peripheral quantitative computed tomography scans and found that denosumab was superior to risedronate in preventing failure load loss at the distal radius and tibia (24).
In terms of bone turnover markers, notably P1NP, CTx, and TRACP-5b, denosumab demonstrated a significant reduction compared to bisphosphonates. These findings are indicative of denosumab’s potent antiresorptive properties, essential in the early stages of GC therapy where bone turnover may be rapidly affected. In addition, changes BAP levels with denosumab versus BPs were not significant, contrasting with findings in postmenopausal women studies (25, 26). This warrants further investigation to understand these differing responses.
Regarding safety profiles, some studies have indicated that denosumab may increase the risk of infections (20, 27, 28). In populations undergoing long-term immunosuppressive therapy, the risk of infection is always a significant concern. Our study demonstrates that, in the GIOP population, denosumab has a similar safety profile to bisphosphonates, without increasing the risks of infection or fractures.
While our study confirms the efficacy and safety of denosumab, there are several limitations. Firstly, inherent methodological constraints, particularly non-uniform blinding protocols across included studies, necessitate a guarded interpretation of our outcomes. This lack of blinding introduces a potential for systematic bias, which is a common limitation in clinical trials, as noted in the literature. Secondly, the analysis revealed significant heterogeneity among the included studies, which may be attributed to variations in methodological designs. Differences in the types of BPs used, study durations, and patient demographics, such as comorbidities and the duration of GCs use, likely contributed to the observed inconsistencies. Although our leave-one-out sensitivity analysis underscored the stability of our results, the limited and selective pool of studies, particularly those of larger scale such as Saag KG et al., may skew the meta-analytical outcomes (19). Thirdly, the potential for publication bias remains a concern. The small number of included studies inherently limits the scope of our analysis and precludes comprehensive subgroup evaluations. This limitation constrains the extent to which our findings can be generalized across diverse clinical scenarios.
Fourthly, while our study included research with a maximum duration of 24 months, it’s important to consider the long-term implications of denosumab therapy. Research in postmenopausal women has demonstrated the efficacy and safety of denosumab over a 10-year period. This highlights a need for extended-duration studies to explore the long-term therapeutic outcomes and safety in patients with GIOP, including fracture risk. Moreover, the issue of discontinuation is crucial. As shown in the study by Saag KG et al., the focus was on rheumatoid arthritis patients receiving glucocorticoid treatment (29). The research found that upon discontinuation of denosumab, bone turnover markers and bone mineral density returned to baseline levels within a year (29). This emphasizes the need for ongoing bone health management in this specific patient group after stopping denosumab therapy. Lastly, although not directly addressed in this study, recent systematic reviews on cost-effectiveness analyses have indicated that most studies suggested denosumab is a cost-effective or superior option compared to oral bisphosphonates. This aspect is particularly important for future research in GIOP populations, especially regarding long-term use.
In conclusion, our findings suggest denosumab may be a more effective option than BPs for patients on long-term glucocorticoid therapy, with a comparable safety profile. However, given the limitations observed, further studies, particularly larger and more diverse clinical trials, are necessary to fully understand denosumab’s role in this patient population. Future research should also focus on the long-term implications of denosumab use, including strategies for safe discontinuation and cost-effectiveness analysis.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
C-LC: Writing – original draft, Writing – review & editing. J-YW: Software, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1407692/full#supplementary-material
1. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: A general population perspective. Arthritis Care Res (Hoboken). (2013) 65:294–8. doi: 10.1002/acr.21796
2. Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. (2018) 379:2547–56. doi: 10.1056/NEJMcp1800214
3. Yamaguchi Y, Morita T, Kumanogoh A. The therapeutic efficacy of denosumab for the loss of bone mineral density in glucocorticoid-induced osteoporosis: A meta-analysis. Rheumatol Adv Pract. (2020) 4:rkaa008. doi: 10.1093/rap/rkaa008
4. Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. (2000) 15:993–1000. doi: 10.1359/jbmr.2000.15.6.993
5. Compston JE. Extensive expertise in endocrinology: advances in the management of glucocorticoid-induced osteoporosis. Eur J Endocrinol. (2023) 188:R46–r55. doi: 10.1093/ejendo/lvad029
6. Ganesan K, Goyal A, Roane D. Bisphosphonate. In: StatPearls. StatPearls Publishing, Treasure Island, FL. (2023). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK470248/.
7. Dong B, Zhou Y, Wang J, Li C, Fu Z, Huang Y, et al. Comparison of bisphosphonates versus teriparatide in therapy of the glucocorticoid-induced osteoporosis (Giop): A meta-analysis of randomized controlled trials. Horm Metab Res. (2023) 55:236–44. doi: 10.1055/a-2015-1747
8. Messina OD, Vidal LF, Wilman MV, Bultink IEM, Raterman HG, Lems W. Management of glucocorticoid-induced osteoporosis. Aging Clin Exp Res. (2021) 33:793–804. doi: 10.1007/s40520-021-01823-0
9. Hofbauer LC, Zeitz U, Schoppet M, Skalicky M, Schüler C, Stolina M, et al. Prevention of glucocorticoid-induced bone loss in mice by inhibition of RANKL. Arthritis Rheum. (2009) 60:1427–37. doi: 10.1002/art.24445
10. Saag KG, Wagman RB, Geusens P, Adachi JD, Messina OD, Emkey R, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: A multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. (2018) 6:445–54. doi: 10.1016/s2213-8587(18)30075-5
11. Jiang L, Dong J, Wei J, Liu L. Comparison of denosumab and oral bisphosphonates for the treatment of glucocorticoid-induced osteoporosis: A systematic review and meta-analysis. BMC Musculoskelet Disord. (2022) 23:1027. doi: 10.1186/s12891-022-05997-0
12. Evidence Prime - Gradepro Gdt. Gradepro Guideline Development Tool. Mcmaster University (2015). Available online at: https://Gradepro.Org (accessed January 2024).
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
14. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (2021). Chichester, UK: Wiley-Blackwell.
15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
16. Cochran WG. The combination of estimates from different experiments. Biometrics. (1954) 10:101–29. doi: 10.2307/3001666
19. Saag KG, Pannacciulli N, Geusens P, Adachi JD, Messina OD, Morales-Torres J, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: final results of a twenty-four-month randomized, double-blind, double-dummy trial. Arthritis Rheumatol. (2019) 71:1174–84. doi: 10.1002/art.40874
20. Mok CC, Ho LY, Ma KM. Switching of oral bisphosphonates to denosumab in chronic glucocorticoid users: A 12-month randomized controlled trial. Bone. (2015) 75:222–8. doi: 10.1016/j.bone.2015.03.002
21. Mok CC, Ho LY, Leung SMT, Cheung HN, Chen SPL, Ma KM. Denosumab versus alendronate in long-term glucocorticoid users: A 12-month randomized controlled trial. Bone. (2021) 146:115902. doi: 10.1016/j.bone.2021.115902
22. Tamechika SY, Ohmura SI, Maeda S, Naniwa T. Efficacy of denosumab on bisphosphonate-treated osteoporosis and osteopenia in systemic rheumatic disease patients receiving glucocorticoids. J Bone Miner Metab. (2023) 41:203–11. doi: 10.1007/s00774-022-01393-9
23. Iseri K, Iyoda M, Watanabe M, Matsumoto K, Sanada D, Inoue T, et al. The effects of denosumab and alendronate on glucocorticoid-induced osteoporosis in patients with glomerular disease: A randomized, controlled trial. PloS One. (2018) 13:e0193846. doi: 10.1371/journal.pone.0193846
24. Geusens P, Bevers MS, van Rietbergen B, Messina OD, Lespessailles E, Oliveri B, et al. Effect of denosumab compared with risedronate on bone strength in patients initiating or continuing glucocorticoid treatment. J Bone Miner Res. (2022) 37:1136–46. doi: 10.1002/jbmr.4551
25. Kong SH, Kim JH, Kim SW, Jeong AJ, Lee SH, Ye SK, et al. Effect of denosumab on the change of osteoclast precursors compared to zoledronate treatment in postmenopausal women with osteoporosis. J Bone Metab. (2022) 29:93–101. doi: 10.11005/jbm.2022.29.2.93
26. Nakamura Y, Suzuki T, Kato H. Serum bone alkaline phosphatase is a useful marker to evaluate lumbar bone mineral density in Japanese postmenopausal osteoporotic women during denosumab treatment. Ther Clin Risk Manag. (2017) 13:1343–8. doi: 10.2147/tcrm.S142828
27. Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, et al. Comparison of denosumab and bisphosphonates in patients with osteoporosis: A meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. (2019) 104:1753–65. doi: 10.1210/jc.2018-02236
28. Watts NB, Roux C, Modlin JF, Brown JP, Daniels A, Jackson S, et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: coincidence or causal association? Osteoporos Int. (2012) 23:327–37. doi: 10.1007/s00198-011-1755-2
Keywords: denosumab, bisphosphonates, glucocorticoid-induced osteoporosis, bone mineral density, meta-analysis
Citation: Chen C-L and Wang J-Y (2024) Superiority of denosumab over bisphosphonates in preventing and treating glucocorticoid-induced osteoporosis: a systematic review and meta-analysis with GRADE quality assessment. Front. Endocrinol. 15:1407692. doi: 10.3389/fendo.2024.1407692
Received: 27 March 2024; Accepted: 02 December 2024;
Published: 18 December 2024.
Edited by:
Jonathan H. Tobias, University of Bristol, United KingdomReviewed by:
Dina Keumala Sari, Universitas Sumatera Utara, IndonesiaCopyright © 2024 Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Ying Wang, Y2hhcmxpbjM1MzUxMUBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.