- 1Department of Emergency Medicine, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, China
- 2Department of Nutrition, The Second Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, China
- 3Department of Urology, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, China
Objective: Despite several observational studies attempting to investigate the potential association between type 1 diabetes mellitus (T1DM) and the risk of digestive cancers, the results remain controversial. The purpose of this study is to examine whether there is a causal relationship between T1DM and the risk of digestive cancers.
Methods: We conducted a Mendelian randomisation (MR) study to systematically investigate the effect of T1DM on six most prevalent types of digestive cancers (oesophageal cancer, stomach cancer, hepatocellular carcinoma, biliary tract cancer, pancreatic cancer, and colorectal cancer). A total of 1,588,872 individuals were enrolled in this analysis, with 372,756 being the highest number for oesophageal cancer and 3,835 being the lowest for pancreatic cancer. Multiple MR methods were performed to evaluate the causal association of T1DM with the risk of six site-specific cancers using genome-wide association study summary data. Sensitivity analyses were also conducted to assess the robustness of the observed associations.
Results: We selected 35 single nucleotide polymorphisms associated with T1DM as instrumental variables. Our findings indicate no significant effect of T1DM on the overall risk of oesophageal cancer (OR= 0.99992, 95% CI: 0.99979-1.00006, P= 0.2866), stomach cancer (OR=0.9298,95% CI: 0.92065-1.09466, P= 0.9298), hepatocellular carcinoma (OR= 0.99994,95% CI: 0.99987-1.00001, P= 0.1125), biliary tract cancer (OR=0.97348,95% CI: 0.8079-1.1729, P= 0.7775)), or pancreatic cancer (OR =1.01258, 95% CI: 0.96243-1.06533, P= 0.6294). However, we observed a causal association between T1DM and colorectal cancer (OR=1.000, 95% CI: 1.00045-1.0012, P<0.001), indicating that T1DM increases the risk of colorectal cancer. We also performed sensitivity analyses, which showed no heterogeneity or horizontal pleiotropy. For the reverse MR from T1DM to six digestive cancers, no significant causal relationships were identified.
Conclusions: In this MR study with a large number of digestive cancer cases, we found no evidence to support the causal role of T1DM in the risk of oesophageal cancer, stomach cancer, hepatocellular carcinoma, biliary tract cancer, or pancreatic cancer. However, we found a causal positive association between T1DM and colorectal cancer. Further large-scale prospective studies are necessary to replicate our findings.
1 Introduction
The connection between diabetes and cancer has been a subject of scientific inquiry for over a century (1). Recent reports have indicated that patients diagnosed with diabetes mellitus are at a higher risk of developing cancer by 20-25% compared to those without diabetes (2). The susceptibility to various forms of cancer seems to be elevated in individuals with both Type 1 Diabetes Mellitus (T1DM) and Type 2 Diabetes Mellitus (T2DM) (3). In addition, numerous studies have shown that a significant percentage (estimated at 8% to 18%) of cancer patients also have diabetes (4) and an increased risk of cancer-related death (5). Cancer has been noted as the second leading cause of mortality among individuals with diabetes (6). This risk is particularly associated with T2DM, which is associated with an increased risk of certain site-specific cancers (7). Similarly, previous cohort studies have reported higher cancer standardised mortality in patients with T1DM (8). However, the association between T1DM and cancer remains unclear due to a limited number of studies T1DM. Furthermore, although hyperglycaemia is common in both types of diabetes mellitus, insulin resistance and hyperinsulinemia are more pronounced in T2DM than in T1DM (9). Additionally, T2DM is less exposed to exogenously administered insulin than T1DM. Therefore, findings on the association between T2DM and the risk of cancer cannot be directly applied to T1DM due to differences in age, obesity, and underlying mechanisms between the two patient groups. These findings highlight the need for further research to better understand the relationship between T1DM and cancer.
A multi-nation study has identified a strong link between T1DM and an increased risk of common cancers. The hazard ratio for overall cancer risk in T1DM patients was 1.15 (1.11, 1.19) for men and 1.17 (1.13, 1.22) for women, surpassing that of the general population. Both sexes with T1DM exhibited a notably higher incidence of solid malignant tumor (10, 11). Oesophageal, stomach, liver, biliary tract, pancreatic, and colorectal cancers, as most prevalent digestive cancers (12, 13), are a significant health concern and impose an increasing disease burden. The risk factors for digestive cancers have been widely investigated and are shared to a great degree (14). Despite the enormous efforts made to combat digestive cancers, there is still a long way to go to reduce the disease burden of digestive cancers. Proper management and early intervention at the onset of the condition can help improve the chances of treatment success and reduce the negative impact of digestive cancers (15). Therefore, it is crucial to identify modifiable protective factors that may help alleviate the burden of the disease (16). A better understanding of the risk factors associated with digestive cancers and the adoption of preventative measures may aid in reducing the incidence of this disease and offer a better prognosis for patients (17).
Mendelian randomisation (MR) analysis has emerged as a popular method for evaluating the potential relationship between exposure factors and outcomes (18). Unlike observational studies, MR analysis is not limited by confounding and reverse causality, as it relies on genetic variations associated with exposure factors to assess their association with outcomes. This is achieved through the use of single-nucleotide polymorphisms (SNPs) as instrumental variables (IVs) to determine disease incidence (19). High-throughput genome-wide association studies (GWASs) have enabled further investigation of causal effects (20). Despite the potential benefits of MR analysis, there is still a scarcity of studies examining the potential causal relationship between T1DM and digestive cancers. Therefore, the authors of this study conducted an MR analysis utilising high-throughput GWASs to assess the potential causal associations between T1DM and digestive cancers.
2 Materials and methods
2.1 Data sources
Summary statistics of T1DM and six Digestive system tumor from the largest available genome-wide association studies (GWAS) of European ancestry and FinnGen biobank were extracted for the primary MR analysis (21, 22). Supplementary Table 1 presents a summary of various studies conducted on the association between different traits and cancer types in European populations. The studies include research on T1DM, oesophageal cancer, stomach cancer, hepatocellular carcinoma, biliary tract cancer, pancreatic cancer, and colorectal cancer. The sample sizes of these studies range from 3,835 to 377,673 individuals, and the number of cases and controls varies depending on the specific cancer type. Moreover, we assessed the causal effects, meticulously adjusting for a range of potential confounders to enhance the precision of our findings. We effectively controlled for variables such as obesity, body mass index, T2DM, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, and apolipoproteins (rs6679677, rs6909461, rs506770, rs9468618, rs689, rs10774624,rs10774624, rs8056814 and rs34536443 were eliminated). Furthermore, to delve deeper into the underlying mechanisms, we performed a mediation analysis aimed at uncovering whether these traits act as mediators in the causal relationship between T1DMand six distinct types of digestive cancers. These studies provide valuable insights into the potential genetic associations between different traits and cancer types, which could inform future research and public health efforts aimed at reducing the burden of digestive cancers. Since all the necessary data were publicly available online, no ethical approval or informed consent was required.
2.2 Selection of SNPs
This approach provides a rigorous and systematic method for selecting IVs in MR analysis, which is essential for ensuring the validity and reliability of the results. In this study, high-throughput GWASs were utilised to extract IVs for digestive cancer. The SNPs that reached a genome-wide significance level (P < 5 × 10–8) were selected as IVs (23). However, if fewer than five IVs were selected, the P value threshold for including SNPs as IVs was lowered to P < 1 × 10-5, which is a method that has been previously adopted in MR studies (24). SNPs within 10,000 kb of each other were then clumped, with a linkage disequilibrium threshold of R2 > 0.001 (25). The F-statistics of the IVs were estimated, which is an indicator of the ability of the IVs to predict the exposures. All exposures had F-statistics higher than 10, indicating that the selected IVs were strong predictors of the exposures.
2.3 Statistical analysis
The inverse-variance weighted (IVW) MR method was the primary method used in this study to ascertain the relationships between T1DM and different types of cancer risk. In addition to the IVW method, sensitivity analyses were conducted using the weighted median, MR-Egger, simple mode, and weighted mode test. The potential heterogeneity was estimated using Cochrane’s Q statistic, and the potential pleiotropy was assessed by the intercept of the MR-Egger test. Scatter plots were used to present the results of different MR methods. To assess the robustness of the results, a “leave-one-out” analysis was conducted to estimate the effect of SNPs after removing each SNP one by one. The causal effects of overall and site-specific cancer were represented using odds ratios (ORs) and 95% confidence intervals (CIs). The analyses were conducted using R software, with the “TwoSampleMR” R package employed for the analyses.
3 Results
A total of 35 SNPs associated with T1DM were chosen for analysis, as shown in Supplementary Table 2. For the reverse MR from T1DM to six digestive cancers, no significant causal relationships were identified.
3.1 Oesophageal cancer
The results of the IVW analysis indicated that there was no significant association between T1DM and oesophageal cancer (OR=0.99992, 95% CI: 0.99979-1.00006, P= 0.2866, Table 1), which was consistent with the results of the weighted median, MR-Egger, simple mode, and weighted mode analyses. The absence of directional pleiotropy was confirmed by the MR-Egger intercept test (P= 0.9037), and the heterogeneity did not reach statistical significance (P= 0.5832), as assessed by Cochran’s Q test. The forest and scatter plots, presented in Figures 1A, 2A, respectively, further support the lack of association between T1DM and oesophageal cancer. The sensitivity analysis, shown in Figure 3A, revealed that the overall estimates were not significantly influenced by any individual SNP. Additionally, the funnel plot, displayed in Figure 4A, showed no evidence of horizontal pleiotropy.
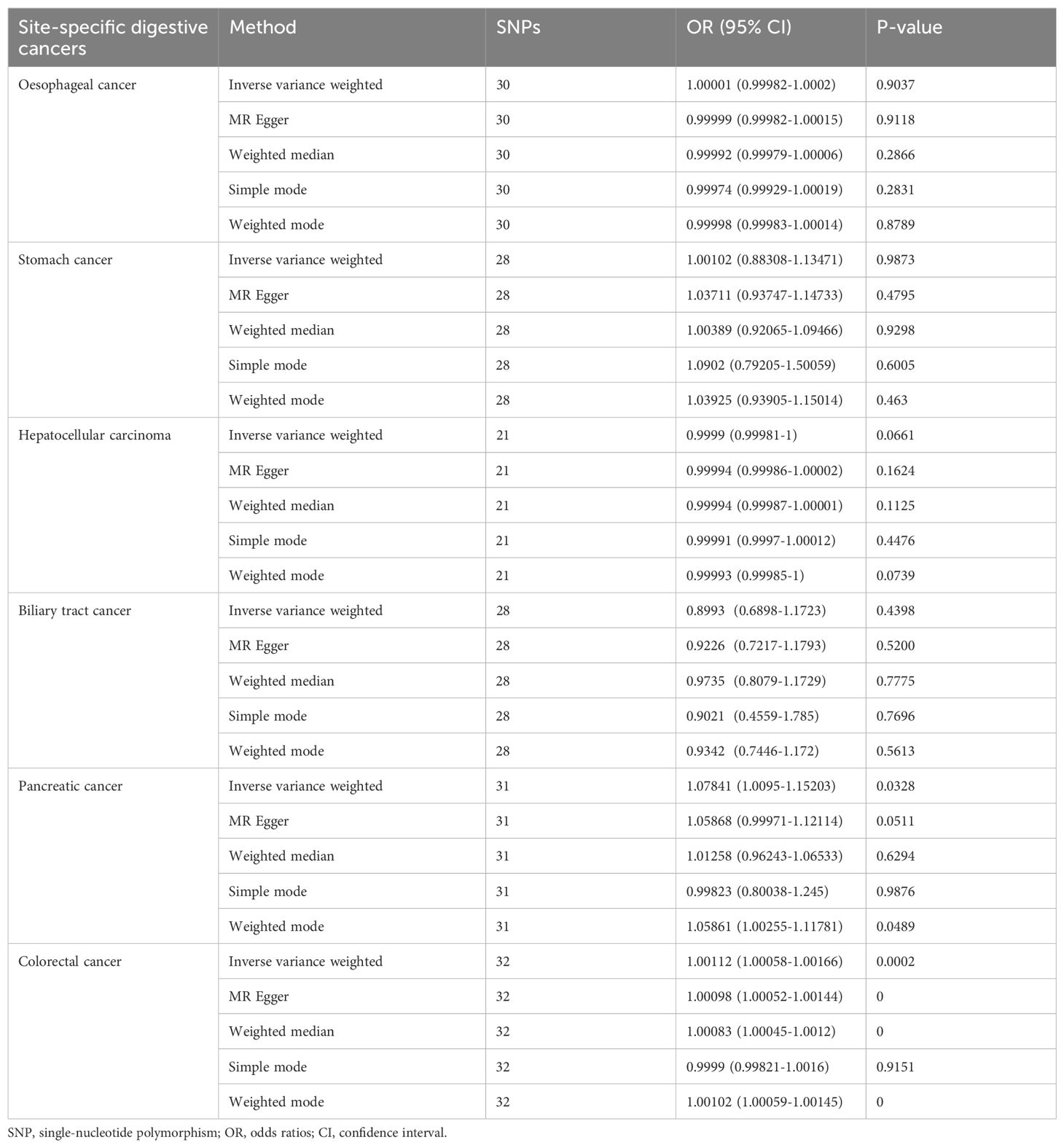
Table 1. Mendelian randomisation estimates for the effects of genetically determined T1DM on six site-specific digestive cancers.
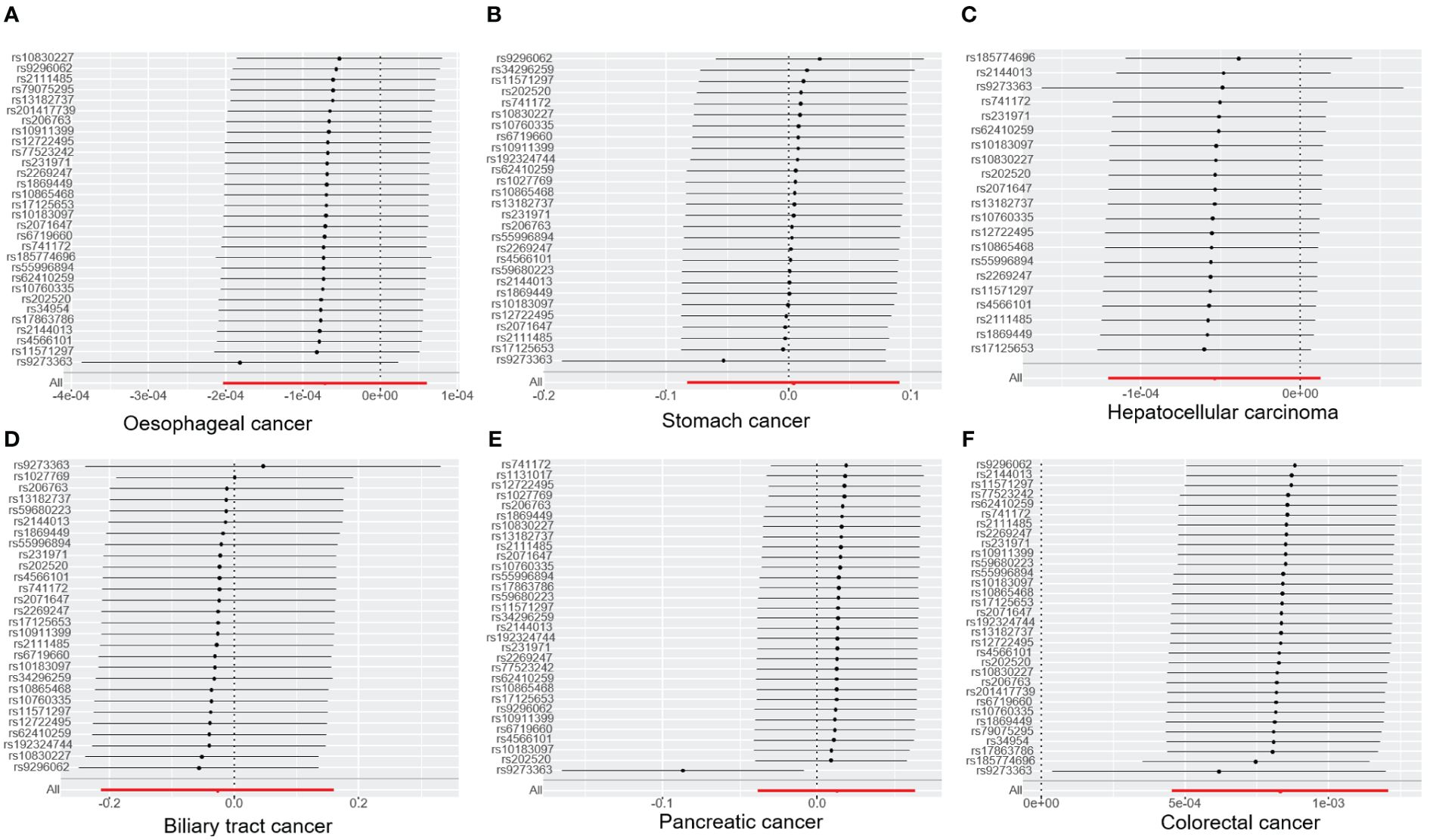
Figure 1. The forest plot illustrates the association between T1DM and digestive cancers, where each black dot represents an SNP. (A) oesophageal cancer. (B) stomach cancer. (C) hepatocellular carcinoma. (D) biliary tract cancer. (E) pancreatic cancer. (F) colorectal cancer. T1DM, type 1 diabetes mellitus; SNP, single-nucleotide polymorphism.
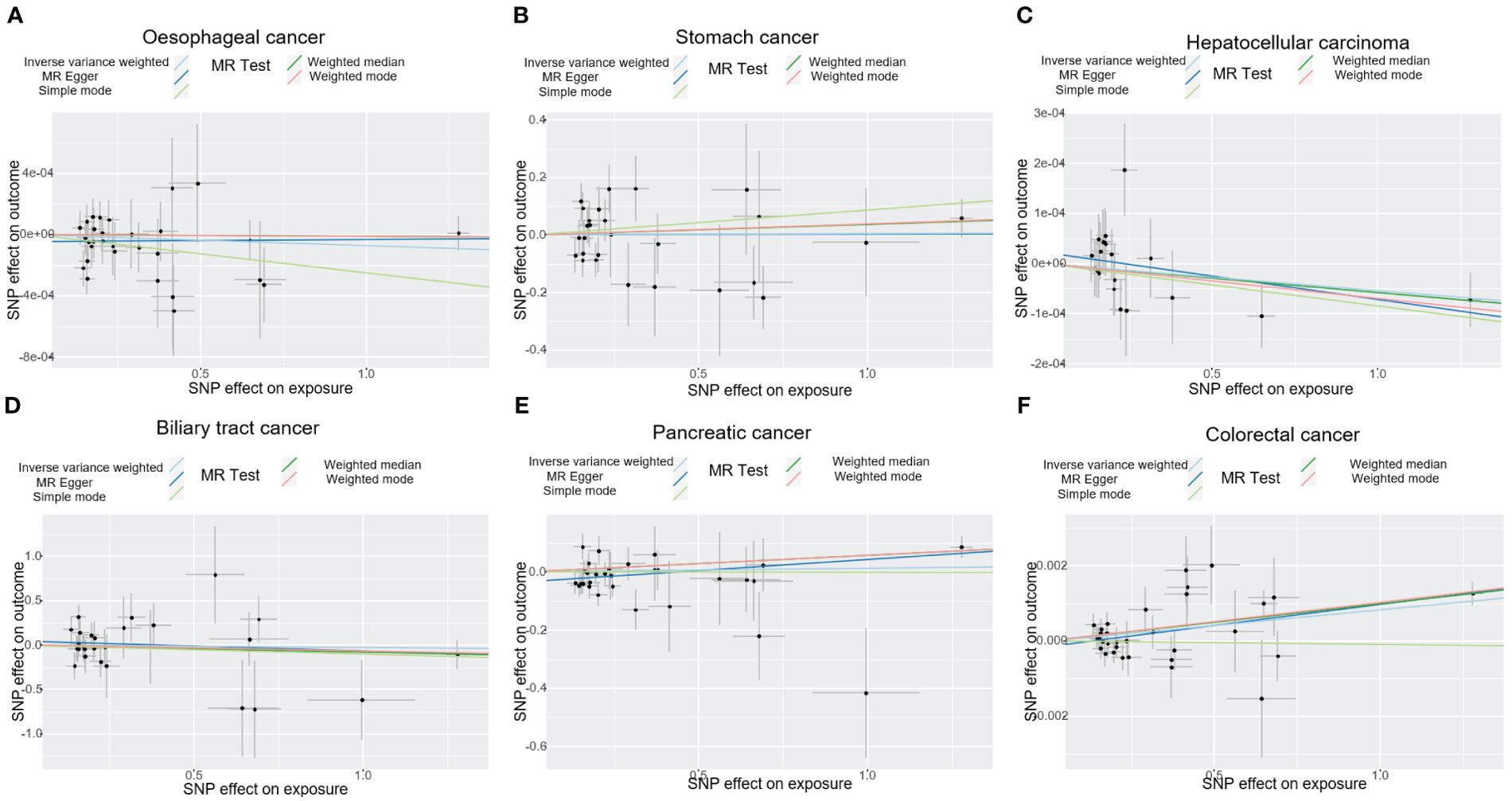
Figure 2. The plot displays the estimated causal effect of T1DM and the risk of digestive cancers based on various methods. The black dots in the graph signify SNPs with an estimated effect on T1DM and digestive cancers risk, while the slopes of the lines represent the causal-effect. (A) oesophageal cancer. (B) stomach cancer. (C) hepatocellular carcinoma. (D) biliary tract cancer. (E) pancreatic cancer. (F) colorectal cancer. T1DM, type 1 diabetes mellitus; SNP, single-nucleotide polymorphism.
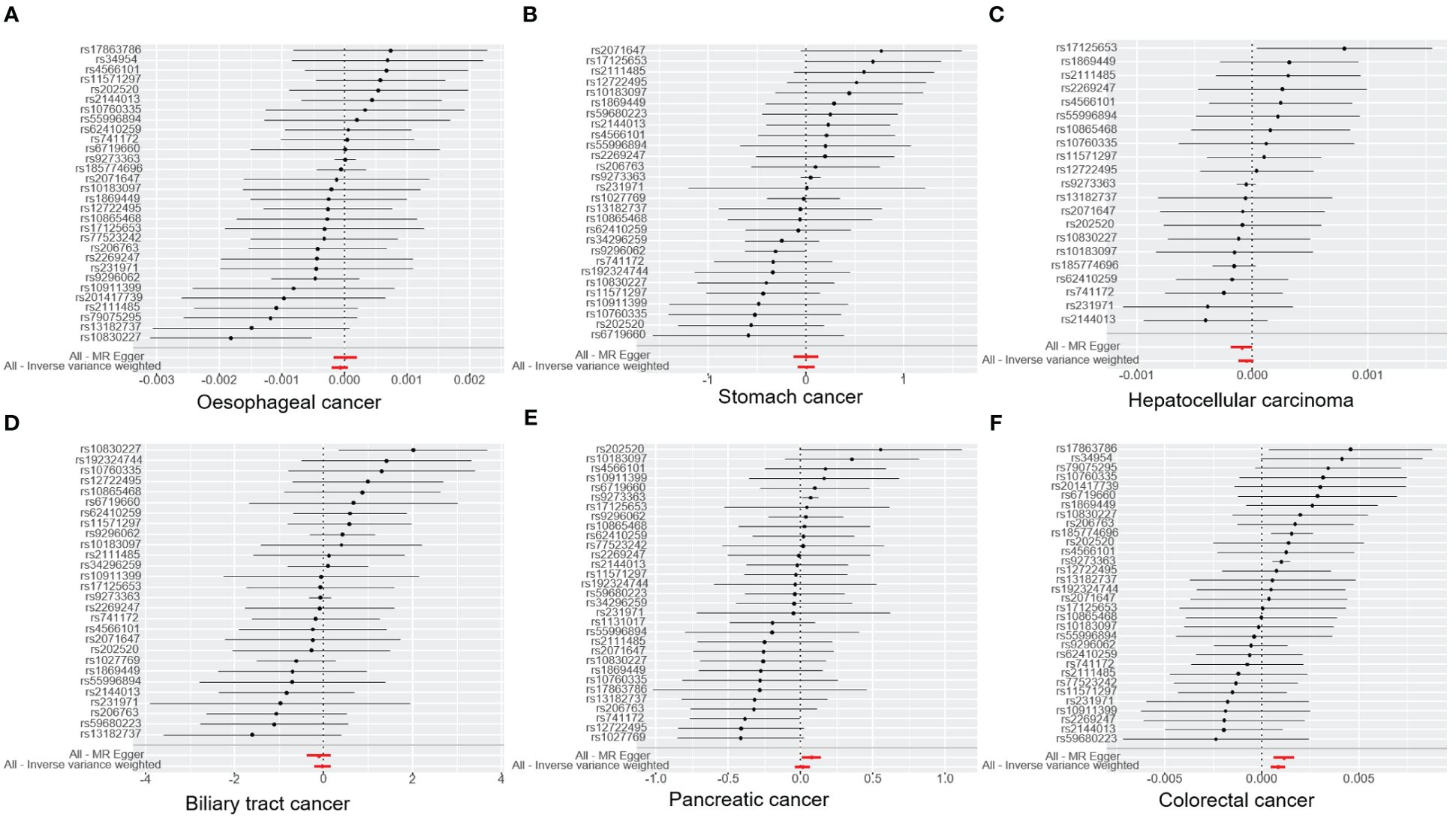
Figure 3. The graph illustrates the association of T1DM with digestive cancers risk and estimates and confidence intervals when a particular SNP is removed. (A) oesophageal cancer. (B) stomach cancer. (C) hepatocellular carcinoma. (D) biliary tract cancer. (E) pancreatic cancer. (F) colorectal cancer. T1DM, type 1 diabetes mellitus; SNP, single-nucleotide polymorphism.
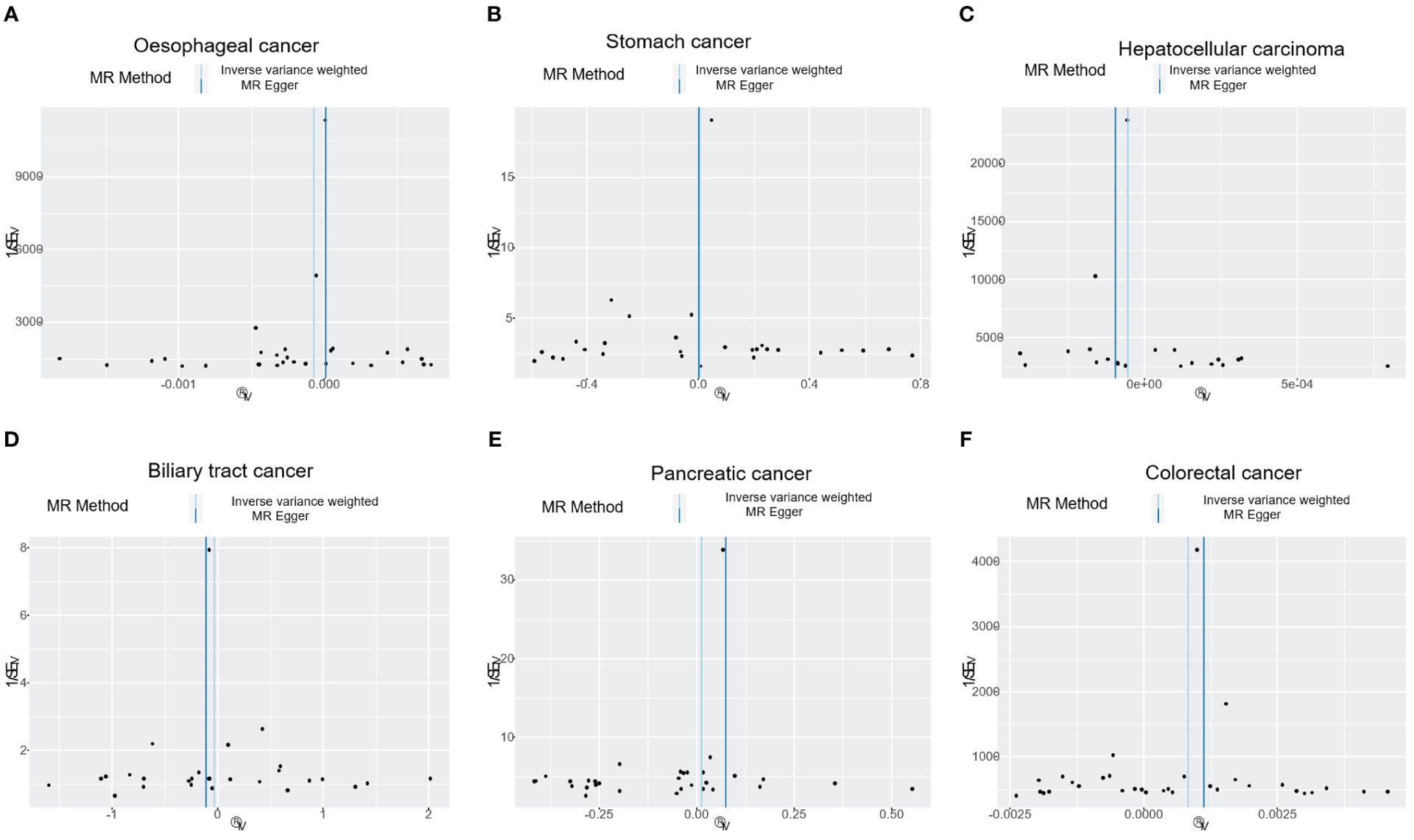
Figure 4. The funnel plot shows the correlation between T1DM and digestive cancers risk, with each black dot representing an SNP. (A) oesophageal cancer. (B) stomach cancer. (C) hepatocellular carcinoma. (D) biliary tract cancer. (E) pancreatic cancer. (F) colorectal cancer. T1DM, type 1 diabetes mellitus; SNP, single-nucleotide polymorphism.
3.2 Stomach cancer
The results of our study consistently showed no causal associations between T1DM and stomach cancer, with an OR of 0.9298 (95% CI: 0.92065-1.09466, P= 0.9298), as presented in Table 1. The weighted median, MR-Egger, simple mode, and weighted mode analyses produced consistent estimates, and no evidence of directional pleiotropy was detected (P= 0.9873). Additionally, the heterogeneity was not statistically significant (P= 0.5832). The forest and scatter plots, displayed in Figures 1B, 2B, respectively, further support the lack of association between T1DM and stomach cancer. The sensitivity analysis, presented in Figure 3B, revealed that no individual SNP caused the MR estimates to deviate. Furthermore, the funnel plot, illustrated in Figure 4B, showed no indication of horizontal pleiotropy.
3.3 Hepatocellular carcinoma
Our study consistently found no evidence of causal associations between T1DM and hepatocellular carcinoma, with an OR of 0.99994 (95% CI: 0.99987-1.00001, P= 0.1125), as presented in Table 1. The weighted median, MR-Egger, simple mode, and weighted mode analyses produced consistent estimates, and no evidence of directional pleiotropy was detected (P= 0.602). Additionally, the heterogeneity was not statistically significant (P= 0.7815). The forest and scatter plots, displayed in Figures 1C, 2C, respectively, further support the lack of association between T1DM and hepatocellular carcinoma. The sensitivity analysis, presented in Figure 3C, revealed that no individual SNP caused the MR estimates to deviate. Furthermore, the funnel plot, illustrated in Figure 4C, showed no indication of horizontal pleiotropy.
3.4 Biliary tract cancer
Our findings consistently revealed no evidence of causal associations between T1DM and biliary tract cancer, with an OR of 0.97348 (95% CI: 0.8079-1.1729, P= 0.7775), as presented in Table 1. The weighted median, MR-Egger, simple mode, and weighted mode analyses produced consistent estimates, and no evidence of directional pleiotropy was detected (P= 0.2575). Additionally, the heterogeneity was not statistically significant (P= 0.7815). The forest and scatter plots, displayed in Figures 1D, 2D, respectively, further support the lack of association between T1DM and biliary tract cancer. The sensitivity analysis, presented in Figure 3D, revealed that no individual SNP caused the MR estimates to deviate. Furthermore, the funnel plot, illustrated in Figure 4D, showed no indication of horizontal pleiotropy.
3.5 Pancreatic cancer
Our study consistently found no evidence of causal associations between T1DM and pancreatic cancer, with an OR of 1.01258 (95% CI: 0.96243-1.06533, P= 0.6294), as presented in Table 1. The weighted median, MR-Egger, simple mode, and weighted mode analyses produced consistent estimates, and no evidence of directional pleiotropy was detected (P>0.05). Additionally, the heterogeneity was not statistically significant (P=0.4699). The forest and scatter plots, displayed in Figures 1E, 2E, respectively, further support the lack of association between T1DM and pancreatic cancer. The sensitivity analysis, presented in Figure 3E, revealed that no individual SNP caused the MR estimates to deviate. Furthermore, the funnel plot, illustrated in Figure 4E, showed no indication of horizontal pleiotropy.
3.6 Colorectal cancer
Our findings revealed a causal association between T1DM and colorectal cancer, with an OR of 1.000 (95% CI: 1.00045-1.0012, P<0.001), as displayed in Table 1. The weighted median, MR-Egger and weighted mode analyses produced consistent estimates except for simple mode, and directional pleiotropy was detected (P= 0.0134). Additionally, the heterogeneity was not statistically significant (P= 0.4699). Figures 1F, 2F depict the forest and scatter plots, respectively, regarding the relationship between T1DM and colorectal cancer, indicating similar findings. As demonstrated in Figure 3F, the sensitivity analysis revealed that no individual SNP caused the MR estimates to deviate. Furthermore, Figure 4F illustrating the funnel plot, demonstrated no indication of horizontal pleiotropy.
4 Discussion
Our study utilised MR methods based on GWAS summary datasets to screen for possible causal associations between T1DM and six site-specific digestive cancers (the most prevalent digestive cancer types (12). We found that T1DM was causally associated with an increased risk of colorectal cancer. However, we did not observe any causal effect of T1DM on oesophageal cancer, stomach cancer, hepatocellular carcinoma, biliary tract cancer, or pancreatic cancer.
Previous observational epidemiological studies, including case-control and cohort studies, have reported inconsistent findings regarding the association between T1DM and cancer risk (10, 26). However, these studies have several limitations. Firstly, there is a possibility of misclassifying T2DM as T1DM, which could lead to an overestimation of the association between T1DM and cancer risk (11). Additionally, the criteria for defining T1DM varied across the studies. For example, Hassan et al. only mentioned insulin-dependent or non-insulin-dependent diabetes mellitus without specifying how they defined them (27), while Valent et al. defined T1DM as insulin-treated diabetes (28). Hsu et al. used the International Classification of Disease ninth version, Clinical Modification (ICD-9-CM) codes to define T1DM (29). Furthermore, most studies defined patients with T1DM as those who were 30 years old or younger, or those who were diagnosed with diabetes before the age of 30 or 45 years (11). Several studies have yielded inconsistent findings, with some early research failing to reveal significant associations between T1DM and certain types of cancer (30). For instance, large UK cohort studies indicated no increased risk or mortality from urinary bladder cancer in T1DM or T2DM patients (31, 32). However, a Netherlands Cohort Study and a Swedish study suggested a positive association between T2DM, and possibly T1DM, with the risk of invasive bladder cancer (33). Some studies show no significant link between T1DM and breast cancer risk in women, and UK and US cohort studies do not report a general increase in all-cause cancer mortality among T1DM patients; however, there are observed variations in cancer risk related to country and the duration of T1DM (34, 35). Upon examining the causal link, we identified variability among subjects. Further analysis with ebi-a-GCST90014023 (22) data did not confirm consistent findings (Supplementary Table 3). Caution is advised when interpreting Mendelian study outcomes, as results from different datasets can diverge or contradict each other. Finally, confounding factors such as tobacco consumption, alcohol intake, obesity, physical activity, family history of cancer, and socioeconomic status were not adjusted for in most of the included studies, which may have affected the association between T1DM and cancer risk. Therefore, conducting new research that excludes confounding factors and is based on clear definitions and patient classifications for T1DM can help identify the specific role of T1DM in the prevention and development of digestive cancers.
Likewise, our findings of the study suggest that T1DM is not a significant risk factor for oesophageal cancer, stomach cancer, hepatocellular carcinoma, biliary tract cancer, or pancreatic cancer in European populations. A cohort consisted of 23,473 UK patients with insulin-treated diabetes were followed for an average of 30 years for cancer incidence and mortality compared with general population rates showed that patients with T1DM had significantly raised risks only for ovarian and vulval cancers, with the greatest risk when diabetes was diagnosed at ages 10-14 (36). Currently, there is a relative lack of research on the potential association between T1DM and oesophagus cancer, biliary tract cancer, or pancreatic cancer. Further research is needed to fully understand the potential relationships between T1DM and these types of cancers, particularly in other populations and geographic regions.
Some previous studies have suggested that T1DM may be associated with an increased risk of stomach cancer. Zendehdel K et al. used a population-based cohort in Sweden to investigate the association between T1DM and stomach cancer risk and showed that patients with T1DM had a significantly increased risk of stomach cancer compared to the general population (37). However, these results should be interpreted with caution due to chance findings, misclassification, and several potential confounding factors. Some scholars attempted to explain this association from the perspectives of several possible mechanisms. Firstly, the long-term use of insulin to treat diabetic patients has been linked to an increase in body weight and abdominal fat deposit, which are both associated with an increased risk of stomach cancer according to a meta-analysis of cohort studies (38). Additionally, the increased risk of stomach cancer among patients with T1DM may be linked to a high prevalence of helicobacter pylori infection in those patients or a high incidence of pernicious anaemia, which is closely related to a high risk of stomach cancer because parietal cell antibodies are more frequent in patients with T1DM compared to the general population (39, 40).
The causal effects of T1DM on the risk of liver cancer remain controversial. While our study found no significant association between T1DM and liver cancer, some previous studies have suggested that T1DM may be associated with an increased risk of liver cancer (41). Possible biological mechanisms for this increased risk include alterations in hepatocellular activity, possibly mitosis related to metabolic changes in patients with diabetes, and steatohepatitis related to obesity and fibrotic confirm whether T1DM can promote liver cancer in humans and to identify the mechanisms by which T1DM exerts its effects (42).
T1DM was associated with an increased risk of colorectal cancer in our study. However, the OR value is close to 1, which suggests that T1DM may be just one of many causes of colorectal cancer. Nevertheless, since diabetes is a modifiable risk factor, its impact can be managed through interventions in daily life. Research on the relevant mechanisms conducted by Bellier J et al. aimed to investigate the link between methylglyoxal (MGO), a by-product of glycolysis, and resistance to cetuximab anti-epidermal growth factor receptor (anti-EGFR) antibodies in colorectal cancer (43). The results showed that MGO promotes tumor growth and metastasis and induces AKT activation through phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin 2 (mTORC2) and Hsp27 regulation, suggesting that MGO is a potential target to tackle EGFR-targeted therapy resistance in colorectal cancer. Yamagishi S et al. discussed the potential molecular link between diabetes and colorectal cancer, proposing several ways to test the hypothesis that advanced glycation end products (AGE) could explain the molecular link between diabetes and colorectal cancer (44). Oxidative stress stands out as a crucial mediator in the intricate interplay between cancer and diabetes. Reactive oxygen species (ROS), which are byproducts of this stress, are capable of modulating gene expression and pivotal pathways that are fundamental to the genesis of cancer (45). These ROS also have a hand in modulating cell proliferation and apoptosis by activating NF-κB pathways, which are frequently hyperactive in various cancers, notably colorectal (46). Furthermore, hyperinsulinemia has been associated with an increased risk of diverse cancers, encompassing the endometrium, ovary, breast, colon, pancreas, and kidney (47). The involvement of insulin and its receptor in cancer development is underscored by the fact that elevated insulin levels can augment IGF-1 production (48), a factor linked to an increased risk of specific cancers. Both IGF-1 and IGF-2 have demonstrated the ability to stimulate cancer cell proliferation and metastasis (49). The activation of the PI3K/Akt/mTOR signalling pathway by insulin and IGFs is recognised for its role in propelling cancer progression (50).
Our study has several notable strengths. Firstly, we utilised a random grouping of participants based on genotype, similar to the procedure of a randomised controlled trial, which allowed us to examine causal relationships. Secondly, we employed a MR study design, which avoids confounding biases and reverse causation commonly observed in traditional observational studies, enabling us to analyse a putative causal association between T1DM and digestive cancer. However, some limitations should be acknowledged. Firstly, genetic liability may only account for a limited proportion of the variability across individuals. Secondly, our data source primarily comprised European populations, making it challenging to generalise our results to other populations worldwide. Thirdly, the potential biological mechanism between T1DM and colorectal cancer should be further investigated with using next generation sequencing (NGS) data, such as proteomics, transcriptomics, etc. Despite these limitations, our study provides valuable insights into the causal relationship between T1DM and digestive cancer risk, which may be useful for clinicians and researchers in developing preventive strategies and interventions to mitigate the impact of this disease.
In conclusion, our study found an association between T1DM and an increased risk of colorectal cancer. However, we did not find clear evidence for a causal role of T1DM in the risk of oesophageal cancer, stomach cancer, hepatocellular carcinoma, biliary tract cancer, or pancreatic cancer. This suggests that previous associations may be confounded by potential biases or due to reverse causation.
Data availability statement
The data presented in the study are deposited in the GWAS repository (https://gwas.mrcieu.ac.uk/).
Author contributions
JZ: Formal Analysis, Writing – review & editing. WL: Formal Analysis, Writing – original draft. LC: Data curation, Writing – review & editing. ML: Data curation, Writing – review & editing. WD: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Health Research Project of Hunan Provincial Health Commission (grant number: W20243162).
Acknowledgments
The authors acknowledge the efforts of the consortia in providing high-quality GWAS resources for researchers.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1407329/full#supplementary-material
References
1. Greenwood M, Wood F. The relation between the cancer and diabetes death-rates. J Hyg. (1914) 14:83–118. doi: 10.1017/S0022172400005702
2. Iliodromiti S, McLaren J, Ghouri N, Miller MR, Dahlqvist Leinhard O, Linge J, et al. Liver, visceral and subcutaneous fat in men and women of South Asian and white European descent: a systematic review and meta-analysis of new and published data. Diabetologia. (2023) 66:44–56. doi: 10.1007/s00125-022-05803-5
3. Cignarelli A, Genchi VA, Caruso I, Natalicchio A, Perrini S, Laviola L, et al. Diabetes and cancer: Pathophysiological fundamentals of a ‘dangerous affair.’. Diabetes Res Clin Pract. (2018) 143:378–88. doi: 10.1016/j.diabres.2018.04.002
4. Suh S, Kim K-W. Diabetes and cancer: cancer should be screened in routine diabetes assessment. Diabetes Metab J. (2019) 43:733. doi: 10.4093/dmj.2019.0177
5. Chen Y, Wu F, Saito E, Lin Y, Song M, Luu HN, et al. Association between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia. (2017) 60:1022–32. doi: 10.1007/s00125-017-4229-z
6. Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N, et al. Estimated life expectancy in a scottish cohort with type 1 diabetes, 2008-2010. JAMA. (2015) 313:37. doi: 10.1001/jama.2014.16425
7. Zeng H, Yuan C, Morze J, Fu R, Wang K, Wang L, et al. New onset of type 2 diabetes after colorectal cancer diagnosis: Results from three prospective US cohort studies, systematic review, and meta-analysis. eBioMedicine. (2022) 86:104345. doi: 10.1016/j.ebiom.2022.104345
8. Dawson SI, Willis J, Florkowski CM, Scott RS. Cause-specific mortality in insulin-treated diabetic patients: A 20-year follow-up. Diabetes Res Clin Pract. (2008) 80:16–23. doi: 10.1016/j.diabres.2007.10.034
9. Sinclair A, Saeedi P, Kaundal A, Karuranga S, Malanda B, Williams R. Diabetes and global ageing among 65–99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2020) 162:108078. doi: 10.1016/j.diabres.2020.108078
10. on behalf of the Diabetes and Cancer Research Consortium, Carstensen B, Read SH, Friis S, Sund R, Keskimäki I, et al. Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia. (2016) 59:980–8. doi: 10.1007/s00125-016-3884-9
11. Sona MF, Myung S-K, Park K, Jargalsaikhan G. Type 1 diabetes mellitus and risk of cancer: a meta-analysis of observational studies. Japanese J Clin Oncol. (2018) 48:426–33. doi: 10.1093/jjco/hyy047
12. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-A Cancer J For Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
13. Ma Z-R, Lin K-Q, Guo H, Yang K-Y, Cao M, Song X, et al. Fatal, non-fatal burden of cancer in the elderly in China, 2005–2016: a nationwide registry-based study. BMC Public Health. (2023) 23:877. doi: 10.1186/s12889-023-15686-9
14. Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology (2020) 159:335–49.e15. doi: 10.1053/j.gastro.2020.02.068
15. Islami F, Chen W, Yu XQ, Lortet-Tieulent J, Zheng R, Flanders WD, et al. Cancer deaths and cases attributable to lifestyle factors and infections in China, 2013. Ann Oncol. (2017) 28:2567–74. doi: 10.1093/annonc/mdx342
16. Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clin Gastroenterol Hepatol. (2019) 17:275–89. doi: 10.1016/j.cgh.2018.07.012
17. Gaesser GA. Whole grains, refined grains, and cancer risk: A systematic review of meta-analyses of observational studies. Nutrients. (2020) 12:3756. doi: 10.3390/nu12123756
18. Woolf B, Di Cara N, Moreno-Stokoe C, Skrivankova V, Drax K, Higgins JPT, et al. Investigating the transparency of reporting in two-sample summary data Mendelian randomization studies using the MR-Base platform. Int J Epidemiol. (2022) 51:1943–56. doi: 10.1093/ije/dyac074
19. Yang M, Wan X, Zheng H, Xu K, Xie J, Yu H, et al. No evidence of a genetic causal relationship between ankylosing spondylitis and gut microbiota: A two-sample mendelian randomization study. Nutrients. (2023) 15:1057. doi: 10.3390/nu15041057
20. Darci-Maher N, Alvarez M, Arasu UT, Selvarajan I, Lee SHT, Pan DZ, et al. Cross-tissue omics analysis discovers ten adipose genes encoding secreted proteins in obesity-related non-alcoholic fatty liver disease. eBioMedicine. (2023) 92:104620. doi: 10.1016/j.ebiom.2023.104620
21. Forgetta V, Manousaki D, Istomine R, Ross S, Tessier M-C, Marchand L, et al. Rare genetic variants of large effect influence risk of type 1 diabetes. Diabetes. (2020) 69:784–95. doi: 10.2337/db19-0831
22. Liu Z, Wang H, Yang Z, Lu Y, Zou C. Causal associations between type 1 diabetes mellitus and cardiovascular diseases: a Mendelian randomization study. Cardiovasc Diabetol. (2023) 22:236. doi: 10.1186/s12933-023-01974-6
23. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype–phenotype associations. Bioinformatics. (2019) 35:4851–3. doi: 10.1093/bioinformatics/btz469
24. Wang L, Xie Z, Li G, Li G, Liang J. Two-sample Mendelian randomization analysis investigates causal associations between gut microbiota and attention deficit hyperactivity disorder. Front Microbiol. (2023) 14:1144851. doi: 10.3389/fmicb.2023.1144851
25. Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. (2015) 31:3555–7. doi: 10.1093/bioinformatics/btv402
26. Hemminki K, Försti A, Sundquist K, Li X. Cancer of unknown primary is associated with diabetes. Eur J Cancer Prev. (2016) 25:246–51. doi: 10.1097/CEJ.0000000000000165
27. Hassan MM, Hwang L-Y, Hatten CJ, Swaim M, Li D, Abbruzzese JL, et al. Risk factors for hepatocellular carcinoma: Synergism of alcohol with viral hepatitis and diabetes mellitus: Risk factors for hepatocellular carcinoma: Synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. (2002) 36:1206–13. doi: 10.1053/jhep.2002.36780
28. Valent F. Diabetes mellitus and cancer of the digestive organs: An Italian population-based cohort study. J Diabetes its Complications. (2015) 29:1056–61. doi: 10.1016/j.jdiacomp.2015.07.017
29. Hsu P-C, Lin W-H, Kuo T-H, Lee H-M, Kuo C, Li C-Y. A population-based cohort study of all-cause and site-specific cancer incidence among patients with type 1 diabetes mellitus in Taiwan. J Epidemiol. (2015) 25:567–73. doi: 10.2188/jea.JE20140197
30. Green A, Jensen OM. Frequency of cancer among insulin-treated diabetic patients in Denmark. Diabetologia. (1985) 28:128–30. doi: 10.1007/BF00273858
31. Swerdlow AJ, Laing SP, Qiao Z, Slater SD, Burden AC, Botha JL, et al. Cancer incidence and mortality in patients with insulin-treated diabetes: a UK cohort study. Br J Cancer. (2005) 92:2070–5. doi: 10.1038/sj.bjc.6602611
32. Goossens ME, Zeegers MP, Bazelier MT, De Bruin ML, Buntinx F, De Vries F. Risk of bladder cancer in patients with diabetes: a retrospective cohort study. BMJ Open. (2015) 5:e007470. doi: 10.1136/bmjopen-2014-007470
33. Van Den Brandt PA. Diabetes and the risk of bladder cancer subtypes in men and women: results from the Netherlands Cohort Study. Eur J Epidemiol. (2024) 39:379–91. doi: 10.1007/s10654-024-01100-0
34. Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992–1999. Diabetologia. (2006) 49:660–6. doi: 10.1007/s00125-005-0120-4
35. Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes. (2010) 59:3216–22. doi: 10.2337/db10-0862
36. Swerdlow AJ, Jones ME, Slater SD, Burden ACF, Botha JL, Waugh NR, et al. Cancer incidence and mortality in 23 000 patients with type 1 diabetes in the UK: Long-term follow-up. Intl J Cancer. (2023) 153:512–23. doi: 10.1002/ijc.34548
37. Zendehdel K. Cancer incidence in patients with type 1 diabetes mellitus: A population-based cohort study in Sweden. CancerSpectrum Knowledge Environ. (2003) 95:1797–800. doi: 10.1093/jnci/djg105
38. Yang P, Zhou Y, Chen B, Wan H-W, Jia G-Q, Bai H-L, et al. Overweight, obesity and gastric cancer risk: Results from a meta-analysis of cohort studies. Eur J Cancer. (2009) 45:2867–73. doi: 10.1016/j.ejca.2009.04.019
39. De Block CE, De Leeuw IH, Van Gaal LF. High prevalence of manifestations of gastric autoimmunity in parietal cell antibody- positive type 1 (Insulin-dependent) diabetic patients. The Belgian Diabetes Registry. J Clin Endocrinol Metab. (1999) 84:4062–7. doi: 10.1210/jc.84.11.4062
40. Demir AM, Berberoğlu Ateş B, Hızal G, Yaman A, Tuna Kırsaçlıoğlu C, Oğuz AS, et al. Autoimmune atrophic gastritis: The role of Helicobacter pylori infection in children. Helicobacter. (2020) 25:e12716. doi: 10.1111/hel.12716
41. Portincasa P, Bonfrate L, Wang DQ-H, Frühbeck G, Garruti G, Di Ciaula A. Novel insights into the pathogenic impact of diabetes on the gastrointestinal tract. Eur J Clin Invest. (2022) 52:e13846. doi: 10.1111/eci.13846
42. Zhang X, Ha S, Lau HC-H, Yu J. Excess body weight: Novel insights into its roles in obesity comorbidities. Semin Cancer Biol. (2023) 92:16–27. doi: 10.1016/j.semcancer.2023.03.008
43. Bellier J, Nokin M-J, Caprasse M, Tiamiou A, Blomme A, Scheijen JL, et al. Methylglyoxal scavengers resensitize KRAS-mutated colorectal tumors to cetuximab. Cell Rep. (2020) 30:1400–1416.e6. doi: 10.1016/j.celrep.2020.01.012
44. Yamagishi S, Nakamura K, Inoue H, Kikuchi S, Takeuchi M. Possible participation of advanced glycation end products in the pathogenesis of colorectal cancer in diabetic patients. Med Hypotheses. (2005) 64:1208–10. doi: 10.1016/j.mehy.2005.01.015
45. Aggeli I-K, Theofilatos D, Beis I, Gaitanaki C. Insulin-induced oxidative stress up-regulates heme oxygenase-1 via diverse signaling cascades in the C2 skeletal myoblast cell line. Endocrinology. (2011) 152:1274–83. doi: 10.1210/en.2010-1319
46. Fenton JI, Birmingham JM. Adipokine regulation of colon cancer: Adiponectin attenuates interleukin-6-induced colon carcinoma cell proliferation via STAT-3. Mol Carcinogenesis. (2010) 49:700–9. doi: 10.1002/mc.20644
47. Friberg E, Mantzoros CS, Wolk A. Diabetes and risk of endometrial cancer: A population-based prospective cohort study. Cancer Epidemiology Biomarkers Prev. (2007) 16:276–80. doi: 10.1158/1055-9965.EPI-06-0751
48. Chen W, Wang S, Tian T, Bai J, Hu Z, Xu Y, et al. Phenotypes and genotypes of insulin-like growth factor 1, IGF-binding protein-3 and cancer risk: evidence from 96 studies. Eur J Hum Genet. (2009) 17:1668–75. doi: 10.1038/ejhg.2009.86
49. Sakatani T, Kaneda A, Iacobuzio-Donahue CA, Carter MG, De Boom Witzel S, Okano H, et al. Loss of imprinting of igf2 alters intestinal maturation and tumorigenesis in mice. Science. (2005) 307:1976–8. doi: 10.1126/science.1108080
Keywords: diabetes mellitus, oesophageal cancer, stomach cancer, hepatocellular carcinoma, biliary tract cancer, pancreatic cancer, colorectal cancer, Mendelian randomisation
Citation: Zhao J, Li W, Chen L, Li M and Deng W (2024) Casual effects of type 1 diabetes mellitus on site-specific digestive cancers: a Mendelian randomisation analysis. Front. Endocrinol. 15:1407329. doi: 10.3389/fendo.2024.1407329
Received: 26 March 2024; Accepted: 17 July 2024;
Published: 05 September 2024.
Edited by:
Thiago Quinaglia A. C. Silva, Massachusetts General Hospital, United StatesReviewed by:
Joaquim Barreto, State University of Campinas, BrazilFengyun Wu, Characteristic Medical Center of Chinese People’s Armed Police Force, China
Han Yang, Hospital of Chengdu University of Traditional Chinese Medicine, China
Xiang Xiao, Hospital of Chengdu University of Traditional Chinese Medicine, China, in collaboration with reviewer HY
Copyright © 2024 Zhao, Li, Chen, Li and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiming Deng, ZGVuZ3dlaW1AbWFpbDIuc3lzdS5lZHUuY24=
†These authors have contributed equally to this work
 Jinli Zhao1†
Jinli Zhao1† Weiming Deng
Weiming Deng