
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol. , 25 September 2024
Sec. Cellular Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1406455
This article is part of the Research Topic Endocrine Imbalances of Mineral Ions and Vitamins in Chronic Disease Pathogenesis View all 15 articles
 Veer Patel1
Veer Patel1 Nuraly S. Akimbekov2,3
Nuraly S. Akimbekov2,3 William B. Grant4
William B. Grant4 Carolyn Dean5
Carolyn Dean5 Xiaoqian Fang6
Xiaoqian Fang6 Mohammed S. Razzaque1,7*
Mohammed S. Razzaque1,7*Neurodegenerative diseases, which are characterized by progressive neuronal loss and cognitive decline, are a significant concern for the aging population. Neuroinflammation, a shared characteristic of these diseases, is implicated in their pathogenesis. This article briefly summarizes the role of magnesium, an essential mineral involved in numerous enzymatic reactions and critical for neuronal bioactivity, in the context of neuroinflammation and cognitive decline. The potential neuroprotective effects of magnesium, including the mechanisms of neuroprotection by magnesium through maintaining neuronal ion homeostasis, reducing inflammation, and preventing excitotoxicity, are also described. Additionally, we discuss the impact of inadequate magnesium on neuroinflammation and its potential as a therapeutic agent for attenuating cognitive decline to improve neurodegenerative conditions.
As the global population ages, neurodegenerative diseases, which are characterized by an ongoing loss of neuron structure and function, are becoming increasingly public health burdens. These disorders, including dementia (along with vascular dementia), amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease, often result in cognitive decline, which severely impacts the quality of life of affected individuals. The insidious nature of these diseases and the lack of curative interventions highlight the need for novel therapeutic strategies. The global prevalence of dementia, primarily Alzheimer’s disease, is expected to double every 20 years, reaching 81.1 million by 2040 (1, 2). Similarly, Parkinson’s disease, the second most common neurodegenerative disorder, affects 2-3% of the population aged ≥65 years (3, 4). These statistics highlight the escalating public health challenge of neurodegenerative diseases.
Neuroinflammation, a common feature of neurodegenerative diseases, is recognized as a critical player in the pathogenesis of these disorders (5, 6). The inflammatory response in the brain is a double-edged sword. Whereas acute inflammation can be beneficial for neuronal repair and recovery, chronic inflammation can lead to persistent neuronal damage and eventually to neurodegeneration (7). Inflammatory processes in the brain are primarily mediated by microglia shown in Figure 1. Upon activation, microglia release proinflammatory cytokines, including interleukin 1β (IL-1β), tumor necrosis factor -α (TNF-α), IL-6, IL-18, and IL-12, reactive oxygen species (ROS), and other neurotoxic substances, including nitric oxide, glutamate, and prostaglandins, as well as enzymes such as matrix metalloproteinases (MMPs) (7). Given the critical role of neuroinflammation in neurodegeneration, modulating the inflammatory response could reduce disease progression and is likely to improve clinical outcomes.
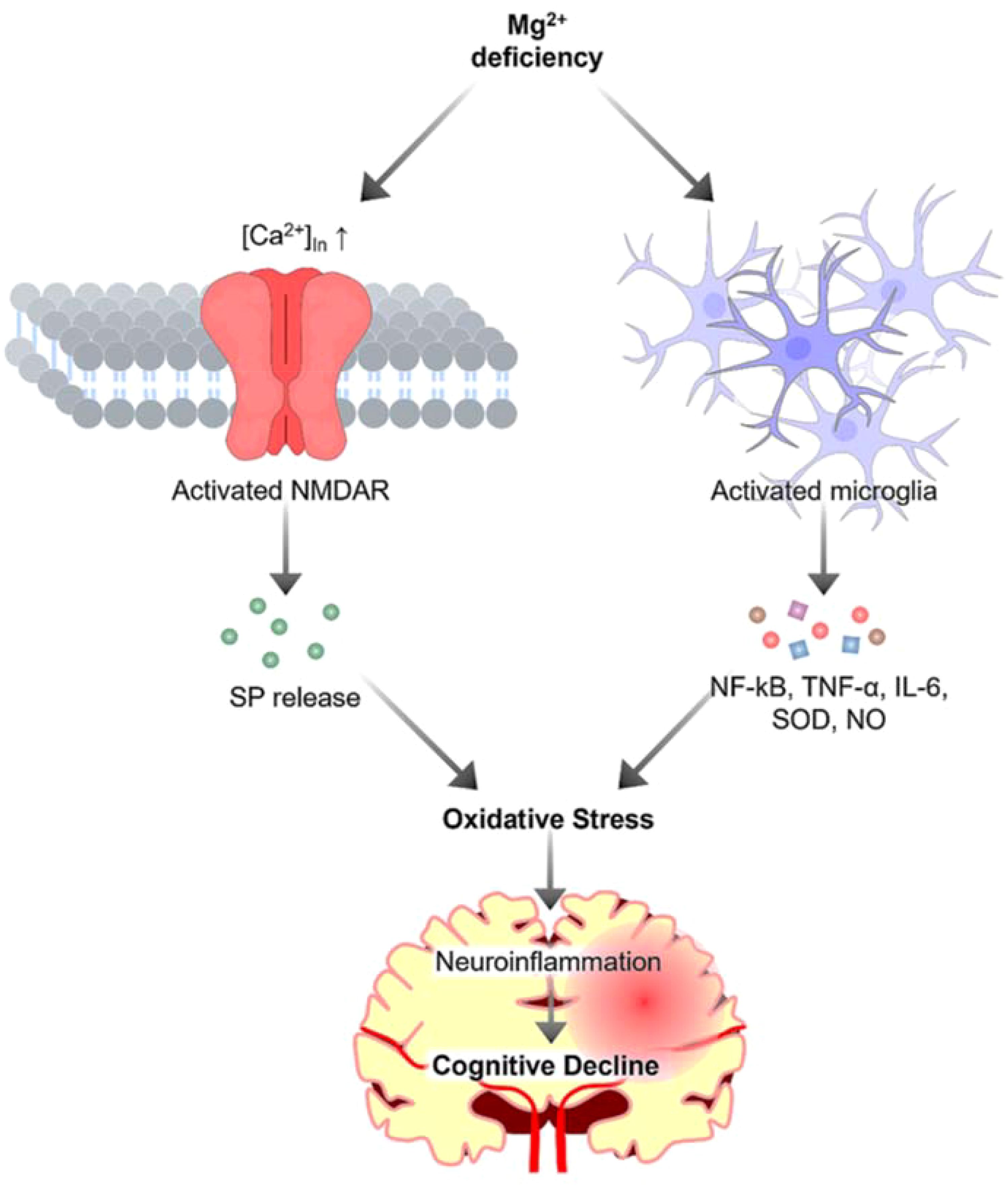
Figure 1. The role of magnesium in neuroinflammation. Magnesium deficiency activates microglia, resulting in the release of proinflammatory cytokines and toxic substances, which contribute to oxidative stress. Additionally, magnesium deficiency triggers calcium influx, inducing the release of substance P (SP), further exacerbating oxidative stress to increase neuroinflammation and ultimately contributes to cognitive decline.
We conducted a literature search using PubMed, Google Scholar, and Scopus databases. The search was performed using the keywords: “neuroinflammation”, “magnesium”, “cognitive function” and “neurodegenerative diseases”. We included peer-reviewed articles in English published between 2000 and 2023.
Neuroinflammation is partly mediated by the activation of glial cells and the release of proinflammatory mediators in the brain (8). It plays a crucial role in the pathogenesis of neurodegenerative diseases. Magnesium has been shown to modulate neuroinflammation (9, 10). It is recognized for its diverse roles in maintaining human health, specifically in modulating inflammatory signaling pathways within the neurological landscape. Magnesium plays a crucial role in over 600 enzymatic reactions in the human body (11). According to Workinger, “Magnesium is a critical mineral in the human body and is involved in ~80% of known metabolic functions” (12). The concentrations of magnesium in serum and cerebrospinal fluid (CSF) are regulated to maintain normal physiological function. Normal serum magnesium levels typically range from 0.75 to 0.95 mmol/L (13), while in CSF, they range between 0.77 and 1.17 mmol/L (14). Magnesium levels are generally higher in CSF as compared to the serum levels, perhaps due to the active transport of magnesium across the blood-brain barrier (15); the blood-brain barrier and the choroid plexus help regulate magnesium levels in the CSF. In magnesium deficiency state, CSF concentrations decline, although such reduction lags behind and is usually less pronounced than the changes noted in plasma levels of magnesium (15). Serum magnesium levels are crucial for neuromuscular function, enzyme activity, and bone structure (16). Magnesium in CSF plays a vital role in supporting various functions of the central nervous system. Decreased CSF magnesium levels correspond with reduced concentrations of extracellular brain magnesium and have been associated with epilepsy (14). Additionally, magnesium is well known for its implication in multiple neurological disorders (17). For instance, magnesium sulfate supplementation has been associated with reduced neuroinflammation in a rat model of Alzheimer’s disease (10). Studies involving animal models suggest that magnesium deficiency may trigger greater recruitment of phagocytic cells (18). These cells could lead to generation of more ROS, leading to the production of various cytokines, such as TNF-α, which are key players in the inflammatory response (18). In Alzheimer’s disease, neuroinflammation is a pathological feature exacerbated by the accumulation of amyloid-beta plaques through the activation of inflammatory proteins including IL-1, IL-6, and TNF-α. Interestingly, magnesium supplementation has been shown to reduce the levels of these proinflammatory cytokines and increase the levels of anti-inflammatory mediators in the hippocampus of a rat model of Alzheimer’s disease, suggesting modulation of an inflammatory responses (19). However, due to the complexity of the immune system in the brain, with the involvement of microglia, astrocytes, and various cytokines and chemokines, dampening inflammation alone might not be sufficient. Chronic neuroinflammation results in an adverse cascade of events, causing neuronal damage, disrupting synaptic functionality, and leading to cognitive impairment. When this inflammatory response is sustained, it results in the overproduction of proinflammatory cytokines. This hyperreactive state disrupts the delicate balance of synaptic plasticity (the ability of synapses to strengthen or weaken over time) thereby diminishing key cognitive functions like memory retention and learning (20).
Furthermore, prolonged inflammation triggers oxidative stress, wherein excess free radicals lead to neurotoxicity and cellular damage (9). This accelerates the progression of neurodegenerative processes, as observed in diseases such as Alzheimer’s disease and Parkinson’s disease, which are characterized by the accumulation of disease-specific proteins in the brain, amyloid-beta and alpha-synuclein, respectively (21). Additionally, inflammation-induced oxidative stress and resultant neuronal damage have been identified as significant contributors to cognitive decline following traumatic brain injury. These findings illustrate the detrimental link between chronic neuroinflammation and cognitive decline.
Hypomagnesemia (typically below 0.61 mmol/L) can cause a wide range of disorders and has significant neurological consequences. The causes of hypomagnesemia can be related to gastrointestinal disorders, including chronic diarrhea, malabsorption syndromes (e.g., celiac disease, inflammatory bowel disease), chronic pancreatitis, and excessive vomiting. Similarly, renal disorders, including tubular dysfunction, diabetic nephropathy (leading to increased urinary magnesium loss), and the use of certain medications (e.g., diuretics, proton pump inhibitors, and some antibiotics), can cause hypomagnesemia. Alcoholism, severe burns, chronic stress, hyperaldosteronism, and prolonged parenteral fluid administration without magnesium supplementation can also lead to hypomagnesemia. Magnesium plays a key role in neural function, and its inadequacy can lead to various neurological symptoms and complications, including neuromuscular hyperexcitability, muscle twitches and cramps, tremors, and seizures. Of clinical importance, the severity of neurological symptoms often correlates with the severity of magnesium deficiency. Patients with mild hypomagnesia (below 0.61 mmol/L) may cause subtle symptoms, while severe hypomagnesia (below 0.49 mmol/L) can lead to more pronounced neurological manifestations. Severe magnesium deficiency syndromes can be associated with cognitive and mood disturbances, headaches, migraines, and neuropathy (numbness and tingling sensations, particularly in the extremities). The long-term complications of severe magnesium deficiency have also been linked to nystagmus (involuntary eye movements) and neurodegenerative diseases, possibly mediated by neuroinflammation. It is essential to maintain an optimal balance of magnesium, along with other minerals and vitamins, throughout life to support normal physiologic functions, including neurological health (22–25).
In the nervous system, magnesium is essential for maintaining neuronal ion homeostasis, modulating synaptic plasticity, and regulating neurotransmitter release (26). Kang et al. highlighted the integral role of this mineral in managing the activity of N-methyl-D-aspartate (NMDA) receptors (27). Their findings emphasize the significance of this interaction in maintaining the balance of glutamate, an excitatory neurotransmitter. If left unchecked, glutamate can potentially tip the scale toward inflammation. Kramer et al. suggested the aftereffects of magnesium deficiency (28). According to their findings, insufficient magnesium can trigger an increase in substance P, a neuropeptide that propagates inflammatory pain (28).
Other researchers have highlighted the complex interplay between magnesium and calcium within neurons (9). By restraining calcium influx into neurons, magnesium helps prevent events that could otherwise lead to intensified inflammation and neuronal injury. Whereas low levels of this mineral are associated with chronic inflammation, restoring magnesium balance has been shown to potentially counteract this condition (29). Apart from managing neurotransmitter activity, magnesium has been found to play a crucial role in modulating immune responses, particularly by interacting with nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (19). This research provides compelling evidence of the role of magnesium as an NF-κB inhibitor, a transcription factor that regulates the expression of pro-inflammatory cytokines, including TNF-α and IL-6 (19). By inhibiting NF-κB activation, magnesium can dampen the resultant proinflammatory gene expression, thereby reducing the overall inflammatory response within the brain (19). A meta-analysis by Veronese et al. revealed magnesium’s anti-inflammatory effects, marked by reductions in plasma fibrinogen and other markers, such as tartrate-resistant acid phosphatase type 5 (TRACP 5) and tumor necrosis factor-ligand superfamily member 13B (TNFSF13B) (30). Additionally, it was also noticed that ST2 protein and IL-1 levels went down. However, the study revealed no significant changes in IL-6 or total antioxidant capacity levels, indicating a selective impact of magnesium on various inflammatory markers (30). Of clinical significance, measuring circulating ionized magnesium appears to be a more accurate indicator of magnesium supplement bioavailability compared to assessing total magnesium levels in plasma (31). Although the role of magnesium in regulating neurotransmission and immune responses is well established, it also plays a crucial role in maintaining brain health by acting as an antioxidant. Research findings suggest that magnesium may contribute to neutralizing ROS to delay the progression of neurodegenerative disorders (32).
Although magnesium is not considered a component of the antioxidant defense system, research indicates that magnesium deficiency may increase oxidative stress markers. These markers encompass oxidative modification products of lipids, proteins, and DNA. Furthermore, a significant association was observed between magnesium deficiency and weakened antioxidant defense mechanisms. This relationship between magnesium deficiency and oxidative stress involves multifaceted mechanisms at both the systemic and cellular levels, including inflammation, endothelial dysfunction, mitochondrial dysfunction, and excessive fatty acid production (32). The studies suggest that magnesium may possess inherent antioxidant properties, although not as a conventional antioxidant molecule such as vitamin C or vitamin E. One notable mechanism highlighted is magnesium’s role in stabilizing the critical antioxidant enzyme superoxide dismutase (SOD) (32). SOD substantially mitigates oxidative damage by converting harmful superoxide radicals into less reactive molecular species. This stabilization of SOD by magnesium provides a unique and essential link between magnesium and the antioxidant defense system (32).
Neuroprotective agents are substances that can potentially preserve neuronal structure and function. These substances help prevent or slow the progression of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease. These agents work through various mechanisms, including reducing neuroinflammation, shielding against oxidative stress, and modulating neurotransmission (33).
Many preclinical and clinical studies have suggested the potential of magnesium as a neuroprotective agent (Figure 2). Magnesium is present both intracellularly and extracellularly, with its intracellular presence in compartments such as the nuclei, mitochondria, and endoplasmic reticulum being crucial for central nervous system functions, including synaptic connectivity (34). Intracellular magnesium can modify synaptic properties, influencing various neuronal processes. For instance, recent research by Liu’s group reported that presynaptic intracellular magnesium plays a crucial role in mediating the transition between two synaptic configurations: one involved in information encoding and learning, and the other in information storage and memorization (35). Research has demonstrated that magnesium can enhance cognitive function and synaptic plasticity in animal models of Alzheimer’s disease, offering optimism for addressing cognitive decline (10). Additionally, a study conducted on a rat model of Alzheimer’s disease demonstrated that magnesium sulfate supplementation improved cognitive function, synaptic plasticity, and dendritic spine morphology (10). Moreover, intracellular magnesium levels have been shown to correlate with Parkinson’s disease activity. In 1-methyl-4-phenylpyridinium (MPP+) model of Parkinson’s disease, the application of MPP+ induced an increase in intracellular magnesium concentration, which inhibited cellular ROS production, maintained ATP generation, and preserved cell viability, thereby protecting neurons from MPP+ toxicity (36). In demyelination rat models, a mutation in the mitochondrial magnesium uptake gene disrupted magnesium homeostasis in oligodendrocytes, affecting ATP production and leading to axonal demyelination (37). Besides supporting myelin formation, intracellular magnesium also enhanced oligodendrocytes’ tolerance against cellular stress, increasing resistance to a hypoxic-ischemic injury (38). Although preclinical studies suggest that magnesium has potential neuroprotective effects, translating these findings to humans presents numerous challenges. Differences in metabolism, blood−brain barrier permeability, and magnesium bioavailability between humans and animal models may affect its efficacy in clinical settings. Additionally, the optimal dosage, duration of treatment, and form of magnesium (e.g., magnesium sulfate, magnesium citrate, etc.) that are both effective and safe for humans require rigorous clinical trials. A gap exists between demonstrating neuroprotection under controlled laboratory conditions and achieving measurable, meaningful outcomes in diverse human populations with varying stages of neurological conditions. Supplementation with magnesium sulfate increased brain magnesium contents and attenuated memory deficits induced by intracerebroventricular administration of streptozocin (ICV-STZ). Furthermore, magnesium reduces tau hyperphosphorylation, a hallmark of Alzheimer’s disease, and modulates the PI3K/Akt signaling pathway (10). Additionally, magnesium supplementation has been associated with improved neurological outcomes in models of acute brain injury, demonstrating its relevance in central nervous system injuries (39). Moreover, in an experimental setting involving a rat model of sciatic nerve injury, a diet rich in magnesium was found to stimulate neurological function recovery and enhance nerve regeneration, revealing its potential in the treatment of peripheral nerve disorders (39). The neuroprotective effects of magnesium are believed to stem from its capacity to regulate neuronal calcium homeostasis, thus reducing excitotoxicity, and its ability to modulate neuroinflammatory processes (10). The mechanisms by which magnesium exerts its effects (e.g., calcium homeostasis regulation, reduction in excitotoxicity, anti-inflammatory actions) suggest that its neuroprotective properties could be applicable to a wide range of neurological conditions. However, this also raises questions about specificity and targeted therapy. For instance, although reducing tau hyperphosphorylation is promising for treating Alzheimer’s disease, it is unclear how these mechanisms interact in the presence of other neurodegenerative disorders or comorbidities. The multifunctional nature of magnesium might mean that its efficacy could vary greatly depending on the specific pathological context. Additionally, magnesium appears to influence nitric oxide production; nitric oxide is a molecule critical for regulating cerebral blood flow and neuronal damage.
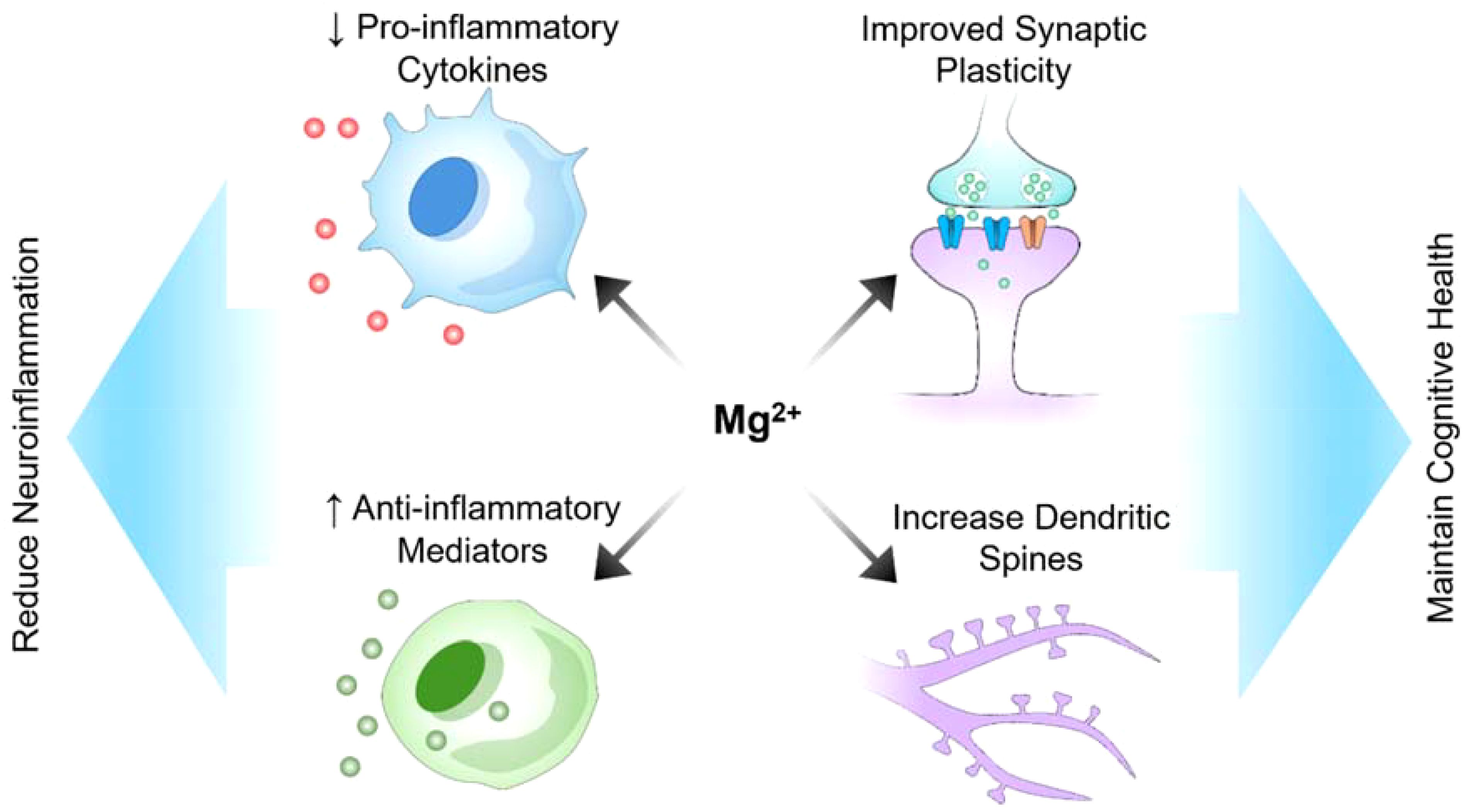
Figure 2. Potential role of magnesium in reducing neuroinflammation and maintaining cognitive health.
Between 2002 and 2008, several randomized clinical trials explored the potential of magnesium sulfate for neuroprotection in preterm births and its effects on cerebral palsy (40). Although these studies did not consistently achieve statistical significance for their primary outcomes, they indicated that magnesium sulfate exposure significantly reduced the likelihood of cerebral palsy in preterm infants. A similar clinical study by Temkin et al., 2007 involving 499 participants aimed to test whether magnesium treatment favorably affects outcomes in head-injured patients (41). The results show that participants who were randomly assigned to the lower dose magnesium group performed significantly worse than those in the placebo group. Therefore, there was greater mortality with the magnesium dose than with the placebo. These findings highlight a discrepancy between preclinical expectations and clinical observations, suggesting that the magnesium infusions given to patients within 8 hours of traumatic brain injury did not have a neuroprotective effect on traumatic brain injury (41). However, other studies have claimed that intravenous magnesium infusion and hyperbaric oxygen therapy could reduce the clinical symptoms of brain injury (42–44). Therefore, additional pre-clinical and clinical research is needed to provide stronger scientific validation.
Another study investigated the combined effects of magnesium supplementation and treadmill exercise on memory deficits in aged rats (45); combined approach led to improved memory function in the aged rats. In the context of central nervous system injury, a comprehensive review highlighted the significant decrease in blood and brain (free) magnesium concentrations following both direct and indirect neurotrauma (46). A decrease in magnesium was associated with neurological deficits and oxidative stress, emphasizing the importance of magnesium homeostasis in central nervous system injury. The administration of magnesium salts, such as magnesium sulfate and magnesium chloride, increased brain (free) magnesium concentrations and improved functional outcomes (46).
Research has demonstrated that magnesium supplementation can effectively increase extracellular magnesium levels, particularly in the serum, which may help inhibit the aggregation of calciprotein particles and reduce vascular calcifications, helping manage conditions such as chronic kidney disease (47). However, the effects on intracellular magnesium levels are more complex and require a long-term, consistent approach to supplementation. This slow adjustment is necessary because of the body’s regulatory mechanisms, which ensure that cellular functions remain stable and effective.
In neurological disorders such as Alzheimer’s disease and Parkinson’s disease, the neurodegenerative process has occurred for many years, potentially reducing the responsiveness of these disorders to the benefits of magnesium. Magnesium impacts calcium regulation and neurotransmitter functions, which are implicated in the pathophysiology of these diseases. In Parkinson’s disease, abnormal magnesium levels are linked to transporter dysfunctions, suggesting that supplementation could stabilize these transport mechanisms and potentially slow disease progression (48).
Conversely, in acute neurological conditions such as stroke or traumatic brain injury, rapid onset and progression do not allow magnesium levels to be corrected in a timeframe that influences immediate outcomes. In these cases, emergency treatments focus on restoring blood flow or reducing inflammation rather than correcting metabolic imbalances. The slow cellular uptake and regulatory effects of magnesium are less practical here because the therapeutic window is very narrow, and the rapid physiological changes postinjury require immediate interventions that go beyond magnesium supplementation. Therefore, while chronic neurological disorders could benefit from sustained magnesium research owing to their slow progression, acute disorders would receive minimal benefit from such research. This is due to need for immediate and aggressive treatment in acute conditions, where the timing and rapid action are critical.
Magnesium supplementation varies significantly in form and administration, each tailored for specific clinical scenarios. Oral magnesium, available in forms such as oxide, citrate, and glycinate, is commonly used for long-term management of conditions such as cardiovascular health and migraine prophylaxis. These forms are preferred for their high bioavailability and ease of administration, making them ideal for ongoing, nonemergency supplementation. Conversely, intravenous magnesium, primarily known as magnesium sulfate, is used in emergency settings where rapid correction of magnesium levels is critical. This form is used in acute medical conditions such as severe asthma, eclampsia, or life-2threatening arrhythmias. Direct administration into the bloodstream provides an immediate therapeutic effect, which is crucial in life-saving interventions. Topical magnesium, often in the form of oils or gels, is used for local applications such as muscle soreness and cramps. While it offers the advantages of bypassing the gastrointestinal system and avoiding some side effects associated with oral forms, its systemic absorption and overall efficacy are less documented.
The relationship between magnesium intake and cognitive function is a promising research area. A study from the National Health and Nutrition Examination Survey (NHANES) 2011 to 2014 investigated the associations of vitamin D status and magnesium with cognitive status in older adults (49). The study found that higher serum 25-hydroxyvitamin D [25(OH)D] levels, linked with magnesium metabolism, were associated with reduced risk of declining cognitive function. Specifically, an inverse association of higher serum 25(OH)D levels with cognitive function was observed primarily among participants with a daily total magnesium intake of <254 mg or ≤375 mg. Essential roles of magnesium in the activation of vitamin D has been explained in various research publications (49–53). The associations between serum 25(OH)D and risk of mortality may be modified by the intake level of magnesium (49). Nevertheless, some studies reportered that there were no clear associations for cognitive function with overall magnesium intake (54). Although not directly focused on magnesium, research has highlighted the potential cognitive benefits of other dietary components. For instance, a study conducted in Qatar revealed that habitual consumption of nuts (almonds, cashews, Brazil nuts, and walnuts), which are rich in magnesium, is positively associated with cognitive function, especially among older adults (55).
Furthermore, a multicenter study of hemodialysis patients revealed a U-shaped association between serum magnesium levels and mild cognitive impairment. Both lower and higher serum magnesium levels were observed to increase the risk of mild cognitive impairment in this specific population. The optimal range of magnesium levels for the lowest risk of mild cognitive impairment was identified as 1.12–1.24 mmol/L (56). This discrepancy suggests that while the current reference range for serum magnesium (0.75–0.95 mmol/L) may be adequate for typical physiological functions, higher levels would be necessary for optimal cognitive health. This indicates that standard ranges might not fully address the specific needs of the brain and neurological health. Therefore, maintaining serum magnesium levels at the higher end of the range could provide potential neuroprotective benefits. The empirical data from specialized populations like hemodialysis patients delineate magnesium’s potential as a cognitive lifeline. The observed associations between magnesium levels and cognitive outcomes highlight the significance of this mineral and raise questions about optimal intake levels for cognitive preservation.
Magnesium glycinate, known for its high bioavailability, ensures that magnesium is efficiently absorbed into the bloodstream and, consequently, available to the body and brain (57). Although direct studies on the impact of magnesium glycinate on cognitive function are limited, its role in enhancing sleep quality and reducing anxiety could indirectly support cognitive health by promoting restorative sleep and lowering stress levels, both of which are beneficial for cognitive performance and neuroprotection (58). Magnesium L-threonate has been specifically studied for its unique ability to increase magnesium concentrations in the brain, thus directly influencing cognitive functions. Rats supplemented with magnesium L-threonate showed a significant increase in synaptic density in regions of the brain associated with memory and learning, translating to a 15% improvement in maze navigation tasks compared to controls. This study demonstrated that this form of magnesium could reverse certain aspects of brain aging and improve synaptic density, suggesting that magnesium has promising implications for delaying and treating cognitive decline associated with aging and neurodegenerative diseases (59).
The existing body of research underscores the need for more rigorous, long-term clinical trials to provide conclusive evidence. A study by Nosheny et al. emphasized the role of dyadic cognitive reports and subjective cognitive decline in early Alzheimer’s disease research and trials (60). Although this study did not focus on magnesium directly, it highlighted the importance of long-term monitoring and the complexities in data interpretation, suggesting that similar rigorous methodologies should be applied to studies on magnesium. Furthermore, research by Planche et al. on brain atrophy subtypes during aging indicated that certain atrophy patterns might predict long-term cognitive decline and future Alzheimer’s disease (61).
The role of magnesium in cognitive health and neuroprotection is both compelling and complex, a testament to the sophisticated nature of the nervous system and its interplay with essential nutrients. Research has revealed that magnesium is a critical player in maintaining and regulating neurobiological behaviors. In fact, its ability to mediate inflammatory signaling pathways and inhibit the activation of NF-κB provides a basis for its potent anti-inflammatory effects. By reducing oxidative burden and inflammation (two phenomena significantly contributing to cognitive decline), magnesium helps to preserve neuronal integrity. Epidemiological and clinical research consistently stresses the importance of adequate magnesium levels for improving cognitive health. Studies have shown a direct correlation between magnesium intake and cognitive function in healthy individuals. Although existing studies have laid a substantial foundation, they also highlight the need for further in-depth research, including more comprehensive, long-term clinical trials to determine the therapeutic potency of magnesium in improving cognitive health to provide safe and compassionate patient care (62), to reduce the burden of neurodegenerative diseases.
MR: Conceptualization, Supervision, Writing – review & editing. VP: Writing – original draft. NA: Visualization, Writing – review & editing. WG: Writing – review & editing. CD: Writing – review & editing. XF: Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors thank Dr. Peace Uwambaye for providing useful suggestions. Additional information has been collected from online sources, including ChatGPT and Google Scholar. VP is a former student of Master of Medical Science at the Lake Erie College of Osteopathic Medicine (LECOM), Erie (USA).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. (2022) 7:e105–25.
2. Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: A delphi consensus study. Lancet. (2005) 366:2112–7.
3. Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. (2017) 3:17013.
4. Willis AW, Roberts E, Beck JC, Fiske B, Ross W, Savica R, et al. Incidence of parkinson disease in North America. NPJ Parkinsons Dis. (2022) 8:170.
5. Risen SJ, Boland SW, Sharma S, Weisman GM, Shirley PM, Latham AS, et al. Targeting neuroinflammation by pharmacologic downregulation of inflammatory pathways is neuroprotective in protein misfolding disorders. ACS Chem Neurosci. (2024) 5:1533–47.
6. Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease–a double-edged sword. Neuron. (2002) 35:419–32.
7. Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. (2007) 8:57–69.
8. Yang QQ, Zhou JW. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia. (2019) 67:1017–35.
9. Lingam I, Robertson NJ. Magnesium as a neuroprotective agent: A review of its use in the fetus, term infant with neonatal encephalopathy, and the adult stroke patient. Dev Neurosci. (2018) 40:1–12.
10. Xu Z-P, Li L, Bao J, Wang Z-H, Zeng J, Liu E-J, Li X-G, et al. Magnesium protects cognitive functions and synaptic plasticity in streptozotocin-induced sporadic alzheimer’s model. PLoS One. (2014) 9:e108645.
11. Gerry K. Schwalfenberg SJG. The importance of magnesium in clinical healthcare. Scientifica. (2017) 2017:4179326.
12. Workinger JL, Doyle RP, Bortz J. Challenges in the diagnosis of magnesium status. Nutrients. (2018) 10:1202.
13. Elin RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res. (2010) 23:S194–198.
14. Haensch CA. Cerebrospinal fluid magnesium level in different neurological disorders. Neurosci Med. (2010) 1:60–3.
15. Morris ME. Brain and CSF magnesium concentrations during magnesium deficit in animals and humans: neurological symptoms. Magnes Res. (1992) 5:303–13.
16. Fiorentini D, Cappadone C, Farruggia G, Prata C. Magnesium: biochemistry, nutrition, detection, and social impact of diseases linked to its deficiency. Nutrients. (2021) 13:1136.
17. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. (2018) 10:730.
18. Patrycja L, Wojciech N, Edmond R, Yves R, Andrzej M. Phagocyte priming by low magnesium status: input to the enhanced inflammatory and oxidative stress responses. Magnesium Res. (2010) 23:1–4.
19. Sugimoto J, Romani AM, Valentin-Torres AM, Luciano AA, Ramirez Kitchen CM, Funderburg N, et al. Magnesium decreases inflammatory cytokine production: A novel innate immunomodulatory mechanism. Am Assoc Immunologists. (2012) 188:6338–46.
20. Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. (2008) 33:18–41.
21. Irwin DJ, Lee VMY, Trojanowski JQ. Parkinson's disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. (2013) 14:626–36.
22. Akimbekov NS, Coban SO, Atfi A, Razzaque MS. The role of magnesium in pancreatic beta-cell function and homeostasis. Front Nutr. (2024) 11. doi: 10.3389/fnut.2024.1458700
23. Ahmad R, Shaju R, Atfi A, Razzaque MS. Zinc and diabetes: A connection between micronutrient and metabolism. Cells. (2024) 13:1359.
24. Anne Marie U, Murererehe J, Rehman M, Chittilla M, Uwambaye P, Razzaque MS. Oral manifestations of iron imbalance. Front Nutr. (2023) 10:1272902.
25. Acquaviva J, Abdelhady HG, Razzaque MS. Phosphate dysregulation and neurocognitive sequelae. Adv Exp Med Biol. (2022) 1362:151–60.
26. Maier JAM LL, Fedele G, Cazzaniga A, Mazur A. Magnesium and the brain: A focus on neuroinflammation and neurodegeneration. Int J Mol Sci. (2023) 24:233. Magnesium.
27. Kang SW, Choi S-K, Park E, Chae S-J, Choi S, Jin Joo H, et al. Neuroprotective effects of magnesium-sulfate on ischemic injury mediated by modulating the release of glutamate and reduced of hyperreperfusion. Brain Res. (2011) 1371:121–8.
28. Kramer JH, Mak IT, Chmielinska JJ, Spurney CF, Phillips TM, Weglicki WB. Experimental hypomagnesemia induces neurogenic inflammation and cardiac dysfunction. Hearts. (2020) 1:99–116.
29. Kostov K HL. Role of magnesium deficiency in promoting atherosclerosis, endothelial dysfunction, and arterial stiffening as risk factors for hypertension. Int J Mol Sci. (2018) 19:1724.
30. Veronese N, Pizzol D, Smith L, Dominguez LJ, Barbagallo M. Effect of magnesium supplementation on inflammatory parameters: A meta-analysis of randomized controlled trials. Nutrients. (2022) 14:679.
31. Zhan J, Wallace TC, Butts SJ, Cao S, Ansu V, Spence LA, et al. Circulating ionized magnesium as a measure of supplement bioavailability: results from a pilot study for randomized clinical trial. Nutrients. (2020) 12:1245.
32. Zheltova AA, Kharitonova MV, Iezhitsa IN, Spasov AA. Magnesium deficiency and oxidative stress: an update. BioMedicine. (2016) 6:20.
33. Karvandi MS, Sheikhzadeh Hesari F, Aref AR, Mahdavi M. The neuroprotective effects of targeting key factors of neuronal cell death in neurodegenerative diseases: The role of ER stress, oxidative stress, and neuroinflammation. Front Cell Neurosci. (2023) 17:1105247.
34. Romani A. Regulation of magnesium homeostasis and transport in mammalian cells. Arch Biochem Biophys. (2007) 458:90–102.
35. Zhou H, Bi GQ, Liu G. Intracellular magnesium optimizes transmission efficiency and plasticity of hippocampal synapses by reconfiguring their connectivity. Nat Commun. (2024) 15:3406.
36. Shindo Y, Yamanaka R, Suzuki K, Hotta K, Oka K. Intracellular magnesium level determines cell viability in the MPP(+) model of Parkinson's disease. Biochim Biophys Acta. (2015) 1853:3182–91.
37. Kuramoto T, Kuwamura M, Tokuda S, Izawa T, Nakane Y, Kitada K, et al. A mutation in the gene encoding mitochondrial Mg²+ channel MRS2 results in demyelination in the rat. PLoS Genet. (2011) 7:e1001262.
38. Itoh K, Maki T, Shindo A, Egawa N, Liang AC, Itoh N, et al. Magnesium sulfate protects oligodendrocyte lineage cells in a rat cell-culture model of hypoxic-ischemic injury. Neurosci Res. (2016) 106:66–9.
39. Pan HC, Sheu ML, Su HL, Chen YJ, Chen CJ, Yang DY, et al. Magnesium supplement promotes sciatic nerve regeneration and down-regulates inflammatory response. Magnes Res. (2011) 24:54–70.
40. Jameson RA, Bernstein HB. Magnesium sulfate and novel therapies to promote neuroprotection. Clinics Perinatology. (2019) 46:187–201.
41. Temkin NR, Anderson GD, Winn HR, Ellenbogen RG, Britz GW, Schuster J, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. (2007) 6:29–38.
42. Gonzales-Portillo B, Lippert T, Nguyen H, Lee JY, Borlongan CV. Hyperbaric oxygen therapy: A new look on treating stroke and traumatic brain injury. Brain Circ. (2019) 5:101–5.
44. Ruiz E, Brunette DD, Robinson EP, Tomlinson MJ, Lange J, Wieland MJ, et al. Cerebral resuscitation after cardiac arrest using hetastarch hemodilution, hyperbaric oxygenation and magnesium ion. Resuscitation. (1986) 14:213–23.
45. El-Domiaty HF, El-Roghy ES, Salem HR. Combination of magnesium supplementation with treadmill exercise improves memory deficit in aged rats by enhancing hippocampal neurogenesis and plasticity: a functional and histological study. Appl Physiology Nutrition Metab. (2022) 47:296–308.
46. Vink R, Cernak I. Regulation of intracellular free magnesium in central nervous system injury. FBL. (2000) 5:656–65.
47. Silaghi CN, Ilyes T, Van Ballegooijen AJ, Craciun AM. Calciprotein particles and serum calcification propensity: hallmarks of vascular calcifications in patients with chronic kidney disease. J Clin Med. (2020) 9:1287.
48. Ray S. The neuroprotective potential of magnesium in Parkinson's disease. Magnes Res. (2024) 36:69–81.
49. Deng X, Song Y, Manson JE, Signorello LB, Zhang SM, Shrubsole MJ, et al. Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. (2013) 11:187.
50. Erem S, Atfi A, Razzaque MS. Anabolic effects of vitamin D and magnesium in aging bone. J Steroid Biochem Mol Biol. (2019) 193:105400.
52. Uwitonze AM, Rahman S, Ojeh N, Grant WB, Kaur H, Haq A, et al. Oral manifestations of magnesium and vitamin D inadequacy. J Steroid Biochem Mol Biol. (2020) 200:105636.
53. Uwitonze AM, Razzaque MS. Role of Magnesium in vitamin D activation and function. J Am Osteopath Assoc. (2018) 118:181–9.
54. Peeri NC, Egan KM, Chai W, Tao MH. Association of magnesium intake and vitamin D status with cognitive function in older adults: an analysis of US National Health and Nutrition Examination Survey (NHANES) 2011 to 2014. Eur J Nutr. (2021) 60:465–74.
55. Nafea H AO, Qaddourah S, Abdulwahab Z, Moawad J, Shi Z. Higher habitual nuts consumption is associated with better cognitive function among Qatari adults. Nutrients. (2021) 12:3580.
56. Yang Y, Long Y, Yuan J, Zha Y. U-shaped association of serum magnesium with mild cognitive impairment among hemodialysis patients: a multicenter study. Ren Fail. (2023) 45:2231084.
57. Cinar V. The effects of magnesium supplementation on thyroid hormones of sedentars and Tae-Kwon-Do sportsperson at resting and exhaustion. Neuro Endocrinol Lett. (2007) 28:708–12.
58. Nielsen FH, Johnson LK, Zeng H. Magnesium supplementation improves indicators of low magnesium status and inflammatory stress in adults older than 51 years with poor quality sleep. Magnes Res. (2010) 23:158–68.
59. Slutsky I, Abumaria N, Wu L-J, Huang C, Zhang L, Li B, et al. Enhancement of learning and memory by elevating brain magnesium. Neuron. (2010) 65:165–77.
60. Nosheny RL, Amariglio R, Sikkes SAM, Van Hulle C, Bicalho MAC, Dowling NM, et al. The role of dyadic cognitive report and subjective cognitive decline in early ADRD clinical research and trials: Current knowledge, gaps, and recommendations. Alzheimers Dement (N Y). (2022) 8:e12357.
61. Planche V, Coupé P, Helmer C, Le Goff M, Amieva H, Tison F, et al. Evolution of brain atrophy subtypes during aging predicts long-term cognitive decline and future Alzheimer's clinical syndrome. Neurobiol Aging. (2019) 79:22–9.
Keywords: magnesium, neuroinflammation, neurodegenerative disease, cognitive decline, neuroprotection
Citation: Patel V, Akimbekov NS, Grant WB, Dean C, Fang X and Razzaque MS (2024) Neuroprotective effects of magnesium: implications for neuroinflammation and cognitive decline. Front. Endocrinol. 15:1406455. doi: 10.3389/fendo.2024.1406455
Received: 25 March 2024; Accepted: 28 August 2024;
Published: 25 September 2024.
Edited by:
Arthur David Conigrave, The University of Sydney, AustraliaReviewed by:
Enikö Kallay, Medical University of Vienna, AustriaCopyright © 2024 Patel, Akimbekov, Grant, Dean, Fang and Razzaque. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed S. Razzaque, bW9oYW1tZWQucmF6emFxdWVAdXRyZ3YuZWR1; bXNyLm5hZ2FzYWtpQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.