- 1Gerontology center, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, China
- 2Department of cardiac surgery, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, China
Background: Recent research has indicated a potential association between thyroid function and sarcopenia, but the specific mechanisms and a definitive causal relationship have yet to be established. Therefore, the objective of this study is to examine the potential causal connection between thyroid function and sarcopenia-related traits, including hand-grip strength, appendicular lean mass (ALM), and walking pace.
Methods: The study used a bi-directional two-sample MR design, with thyroid function examined as the exposure and sarcopenia-related traits as the outcome in the first stage, and then reversed in the second stage. The genetic instruments for thyroid function were obtained from a comprehensive meta-analysis involving 271,040 participants. Data on sarcopenia-related traits based on GWASs were collected from the UK Biobank, which includes up to 461,026 European participants. The estimates for MR were calculated using the inverse-variance weighted (IVW) method, and several sensitivity analyses were performed.
Results: After applying the Bonferroni correction for multiple testing, our MR analyses revealed no significant impact of thyroid function liability on sarcopenia-related traits. Similarly, our reverse MR analysis did not provide evidence supporting the influence of liability to sarcopenia-related traits on thyroid function. The results of the primary IVW MR analyses were largely in line with those obtained from our sensitivity MR analyses.
Conclusion: Our research findings do not suggest a link between thyroid function and sarcopenia-related traits. The associations identified in epidemiological studies may be influenced, at least in part, by shared biological mechanisms or environmental confounders.
Introduction
As the global population continues to age, the prevalence of sarcopenia is rising, leading to a decline in quality of life and increased mortality rates (1). According to the 2019 consensus update from the Asian Working Group for Sarcopenia (AWGS), the occurrence of sarcopenia in the elderly population is estimated to be between 6.8%-25.7% (2, 3). Sarcopenia is associated with an elevated risk of falls, recurrent falls, fractures, and mortality. Furthermore, it has been significantly linked to cardiometabolic disease and cognitive impairment (4, 5). Nevertheless, the precise etiology of sarcopenia remains incompletely elucidated and there are presently no efficacious pharmaceutical interventions for its management. A more comprehensive comprehension of the underlying mechanisms is imperative for advancing efforts in the prevention and treatment of this condition.
Previous studies have suggested that the pathogenesis of sarcopenia may involve various factors, including aging (6), neuromuscular dysfunction (7), mitochondrial dysfunction (8), proinflammatory cytokines (9), myocyte apoptosis (10), and genetic predisposition (11). Recent studies have indicated that thyroid disorders can result in a reduction of skeletal muscle mass and strength in mice, suggesting a correlation between both hypo- and hyperthyroidism with sarcopenia (12). In recent studies, associations have been identified between variations in normal-range thyroid function and the risk of sarcopenia in cohort studies (13). However, it remains uncertain whether these associations are causal, given that observational studies are susceptible to selection bias, residual confounding, and reverse causality. The regulation of intracellular T3 levels plays a critical role in the progression of myogenesis and contributes to the optimization of mitochondrial function in skeletal muscle (14). Several experimental studies have suggested that the supplementation of thyroid hormones in patients with hypothyroidism may potentially improve both muscle strength and cross-sectional area (15). However, the randomized controlled trials (RCTs) for these treatments have yielded conflicting results, and their limited sample sizes and/or brief follow-up durations preclude any definitive conclusions.
The studies mentioned collectively suggest a strong association between thyroid function and sarcopenia. However, it is crucial to ascertain the causality of the observed connections. MR is commonly employed to establish causality in scenarios where RCTs are not feasible or inaccessible (16). This approach leverages genetic variations as proxies to evaluate the direct influence of a factor (e.g. thyroid function) on the outcome of interest (e.g. sarcopenia) (17). The concept is grounded in the stochastic distribution of genetic variations from progenitors to descendants, akin to the randomization procedure employed in RCTs (18). Reversing the direction of causality, an observed association between the outcome under investigation and the genetically predicted exposure can provide support for a potential causal relationship, given that genetic variants have the potential to influence the outcome of interest.
During the course of this novel study, we conducted a two-sample MR to investigate the potential causal effects of variations in normal-range levels of free triiodothyronine (FT3), free thyroxine (FT4), total T3 (TT3), total T4 (TT4), thyrotropin (TSH), and FT3:FT4 ratio on sarcopenia-related traits. Additionally, reverse MR analyses were performed to assess the impact of sarcopenia-related traits on thyroid function.
Materials and methods
Two-sample Mendelian randomization
In this study, we employed a bi-directional MR study design and utilized two-Sample MR methodologies with various genome-wide association study (GWAS) summary level datasets to investigate the causal relationship and direction of causation between thyroid function (including FT3, FT4, TT3, TT4, TSH, and FT3:FT4 ratio) and sarcopenia-related traits (including hand-grip strength, ALM, and walking pace) in a European population. The study was conducted in two phases. Initially, an examination of the potential causal relationship between thyroid function and sarcopenia-related traits was undertaken. Subsequently, an assessment of the potential connection between genetic sarcopenia-related traits and thyroid function was carried out.
Exposures (thyroid hormone levels) datasets
The exposures of interest were normal-range levels of FT3, FT4, TT3, TT4, TSH and FT3:FT4 ratio, which were from a recent GWAS on thyroid function in the ThyroidOmics Consortium (19). We identified single nucleotide polymorphisms (SNPs) that are independently associated with normal-range thyroid hormone levels at a genome-wide significant level (p < 5×10-8), with clumping r2 = 0.001 and kb = 10,000, respectively (20). We employed the identified genetic variations as instruments to investigate the potential causal relationship between normal thyroid function and the intended outcomes.
Outcomes (sarcopenia-related traits) datasets
We chose hand-grip strength and walking speed as measures of muscle function, and ALM as an indicator of muscle mass (3). Genetic instruments for hand-grip strength (n = 461,026), ALM (n = 450,243), and walking pace (n = 459,915) were obtained from the largest GWASs conducted by the UK Biobank with participants aged 48 to 73 years at the time of enrollment (21). The UK Biobank is an expansive biomedical database and a research resource. From 2006 to 2010, more than 500,000 participants aged between 40 to 69 years were recruited into the UK Biobank. Consenting participants completed a touchscreen-based questionnaire, face-to-face verbal interviews, physical measurements, provided information on their medical history and medication use, and biological samples were also collected. Follow-up for all participants involved linkage with hospital admissions data from England, Scotland, and Wales. Genetic association studies (GWAS) on sarcopenia-related variables were undertaken in European populations using data from population-based cohorts.
Hand-grip strength was quantified using a hydraulic hand dynamometer (Jamar J00105) designed to accommodate various hand sizes. ALM was evaluated by determining the total fat-free mass in the arms and legs via bioelectrical impedance analysis (BIA). The walking pace was classified according to self-reported responses, with “slow,” “steady/average,” and “brisk” being assigned numerical codes of 0, 1, and 2, respectively.
Data on the impact of different alleles, beta coefficients (β), standard errors (SE), and p-values for the variations associated with FT3, FT4, TT3, TT4, TSH, and FT3:FT4 ratio levels were gathered from each study for MR analyses.
Statistical analyses
We employed the IVW method with multiplicative random effects as the primary approach for estimating the causal effect between exposure and outcome, which was considered to be the most reliable indicator in the absence of directional pleiotropy in the selected instrumental variables (IVs) (p for MR-Egger intercept > 0.05). The Cochran’s Q test was conducted to evaluate the presence of substantial heterogeneity among the selected independent variables. In the event that the heterogeneity is not statistically significant, it may be appropriate to utilize the fixed-effects model; however, if it is statistically significant, employing the IVW method with multiplicative random effects would be more suitable (22). In light of conducting multiple tests, we employed the Bonferroni method to adjust the level of significance by implementing a more stringent p-value threshold of 0.05/n (where n represents the number of independent hypotheses).
Sensitivity analysis
To enhance the credibility of the MR causal effect estimation, we carried out multiple sensitivity analyses.
We initially employed the MR-Egger method to detect any potential pleiotropy of IVS, and the adjusted causal effect could be derived from the estimation for MR-Egger regression slope, as previously mentioned. The methodology utilized in this study exhibits low testing efficiency, thus we exclusively employed the MR-Egger method for the identification of pleiotropic effects, as opposed to evaluating causal effects (23).
We also utilized the weighted median method as an additional approach for sensitivity analysis to assess the reliability of the MR estimates, given its demonstrated advantages over the MR-Egger method. The weighted median method has been found to possess greater capacity in detecting causal effects and lower type I error, thus ensuring robustness in generating accurate estimates even when up to 50% of the SNPs are invalid IVs (24).
We employed MR- Pleiotropy RESidual Sum and Outlier (PRESSO) as a statistical method to identify and remove potential pleiotropic IVs. This approach assumes that at least 50% of the SNPs are valid, and can detect pleiotropy by examining outliers among the selected SNPs contributing to the MR estimate. Furthermore, MR-PRESSO is capable of adjusting estimates by eliminating outliers and evaluating the significant difference in causal effect estimation before and after outlier correction. In this study, adjusted estimates obtained from MR-PRESSO were considered the primary indicator of causal effect estimation in cases where horizontal pleiotropy was present (25).
We performed all statistical analyses using the Two-sample MR package within the R software environment.
Results
Influence of genetically predicted thyroid hormone on sarcopenia-related traits
The results did not suggest any potential causal relationship between sarcopenia-related traits and exposure to thyroid hormone as a contributing factor. We have identified 6, 65, 13,162, and 13 distinct genetic variants in linkage disequilibrium (LD)-independent (r2 < 0.001 and kb = 10,000) that have reached the genome-wide significance threshold (p < 5×10−8) for FT3, FT4, TT3, TSH, and FT3:FT4 ratio levels respectively. The IVs were employed in the analysis of hand-grip strength, ALM, and walking pace. One set of IVs exhibited no significant heterogeneity (p > 0.05). The IVW fixed-effects model was utilized for those lacking heterogeneity, while the IVW random-effects model was applied to IVs demonstrating heterogeneity. We utilized a Bonferroni corrected significance threshold of 0.00333 (0.05/15) as previously mentioned. The IVW analysis revealed that there was no statistically significant causal relationship between genetically instrumented thyroid hormone and hand-grip strength, ALM, and walking pace, as the p-values did not reach the threshold for significance. The MR-Egger analysis did not reveal any evidence of directional pleiotropy for the selected IVs (Table 1). Consistent with the IVW results, both the Weighted median and MR-PRESSO estimates indicated the absence of a causal relationship between thyroid hormone and hand-grip strength (Table 2). Based on the aforementioned findings, there is no indication of a causal relationship between genetically predicted thyroid hormone levels and sarcopenia-related traits in the discovery cohort.
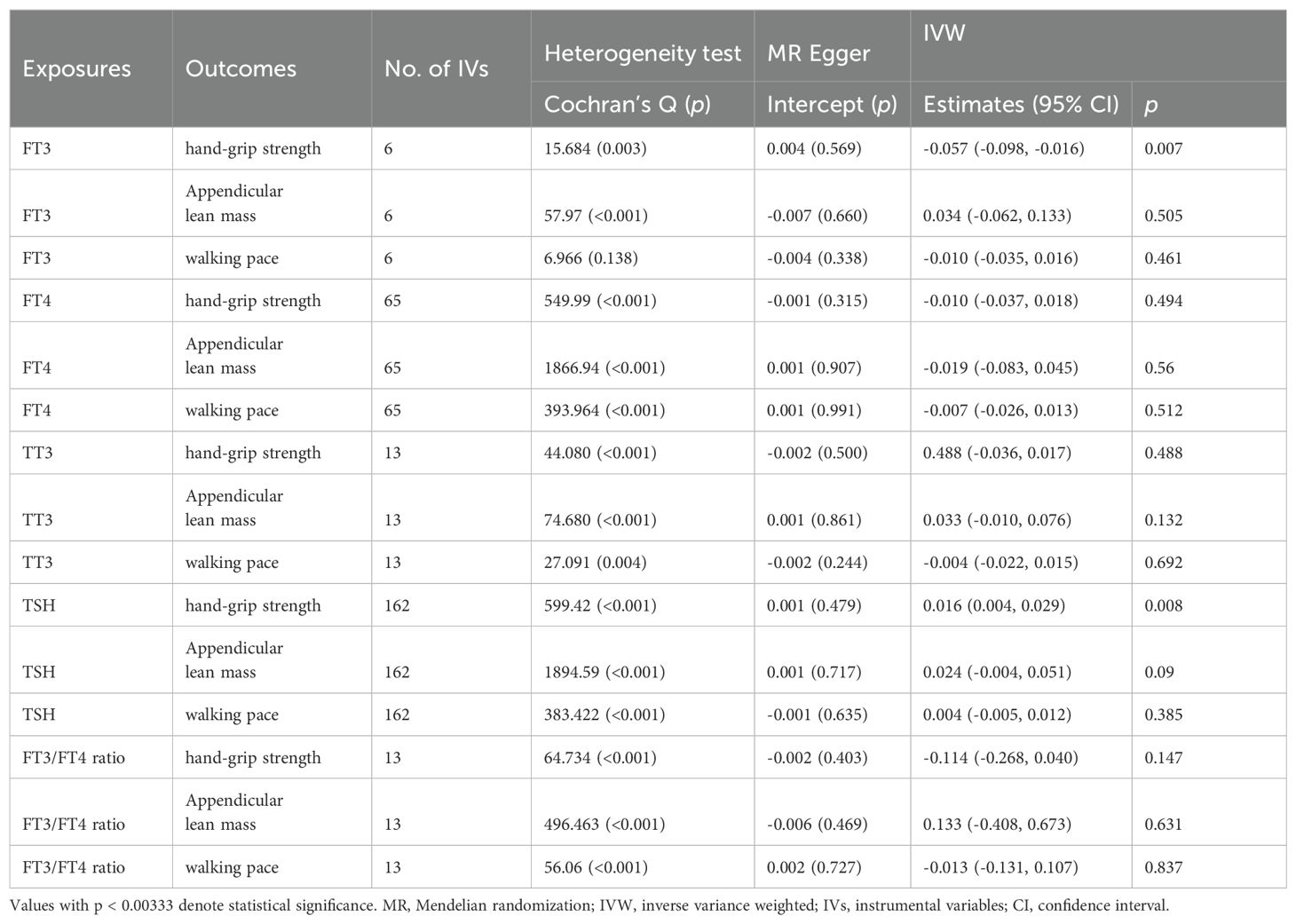
Table 1. Association of thyroid function with sarcopenia-related traits using MR-Egger and IVW analysis.
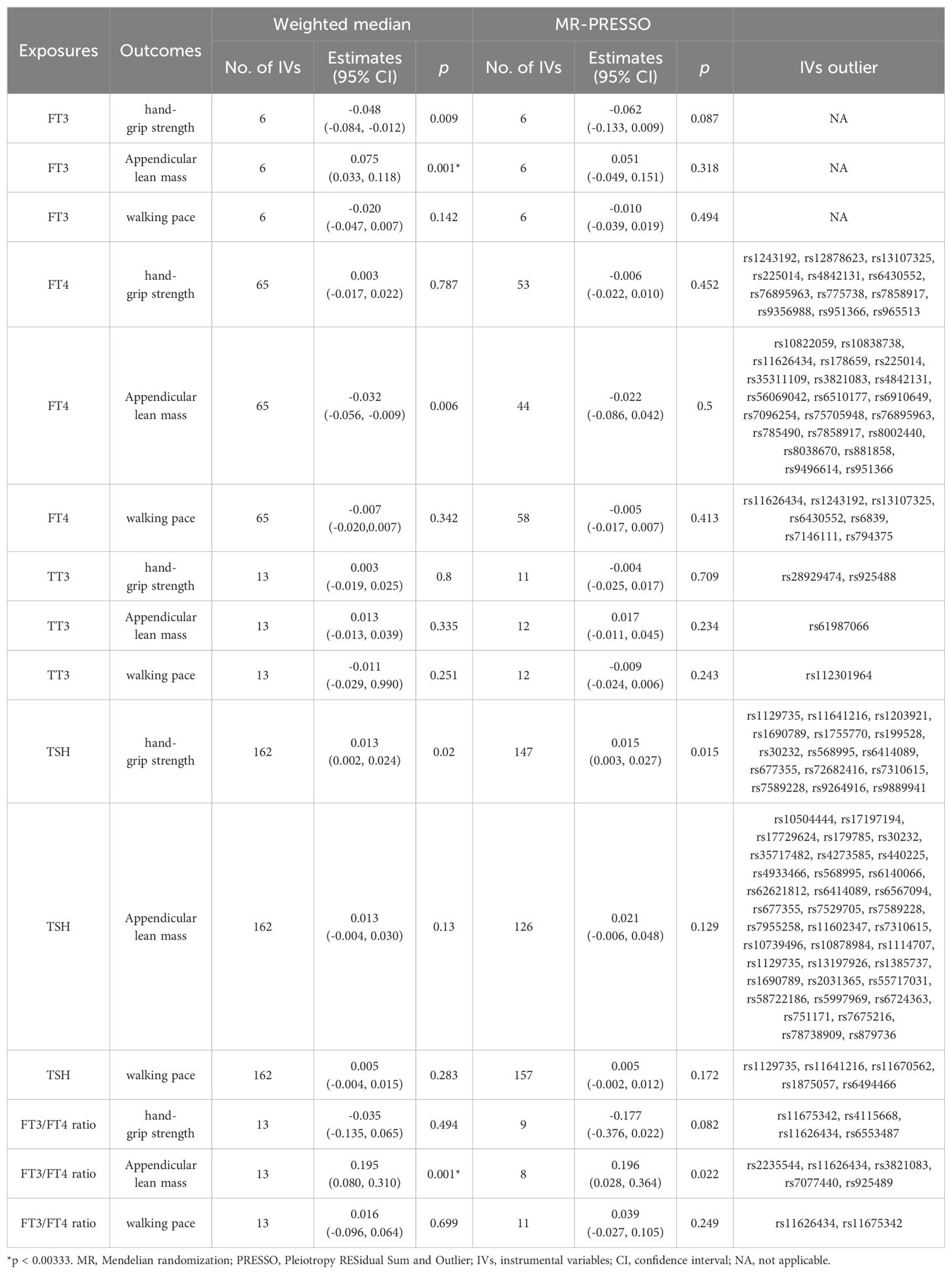
Table 2. Association of thyroid function with sarcopenia-related traits using weighted median and MRPRESSO analysis.
Influence of genetically predicted sarcopenia-related traits on thyroid hormone
The results did not demonstrate a direct association between sarcopenia-related traits and thyroid hormone. We have identified 79, 555, and 56 LD-independent (r2 < 0.001 and kb = 10,000) SNPs that have reached the genome-wide significance level (p < 5×10−8) as potential instrumental variables for hand-grip strength, ALM, and walking pace. The heterogeneity test revealed a significant diversity among the selected instrumental variables (IVs) (p < 0.05). Consequently, the MR analyses employed the IVW method with multiplicative random effects. However, the IVW analysis did not identify any significant causal relationship between genetically instrumented sarcopenia-related traits and thyroid hormone levels. The MR-Egger analysis revealed the presence of directional pleiotropy in instrumental variables for ALM and FT3/FT4 ratio (Table 3). However, upon adjustment for directional pleiotropy using MR-PRESSO, no significant causal relationship between genetically instrumented ALM and FT3/FT4 ratio was observed. The findings remained consistent when employing the Weighted median method (Table 4). Based on the aforementioned results, there is no indication of a causal association between sarcopenia-related traits and thyroid hormone levels.
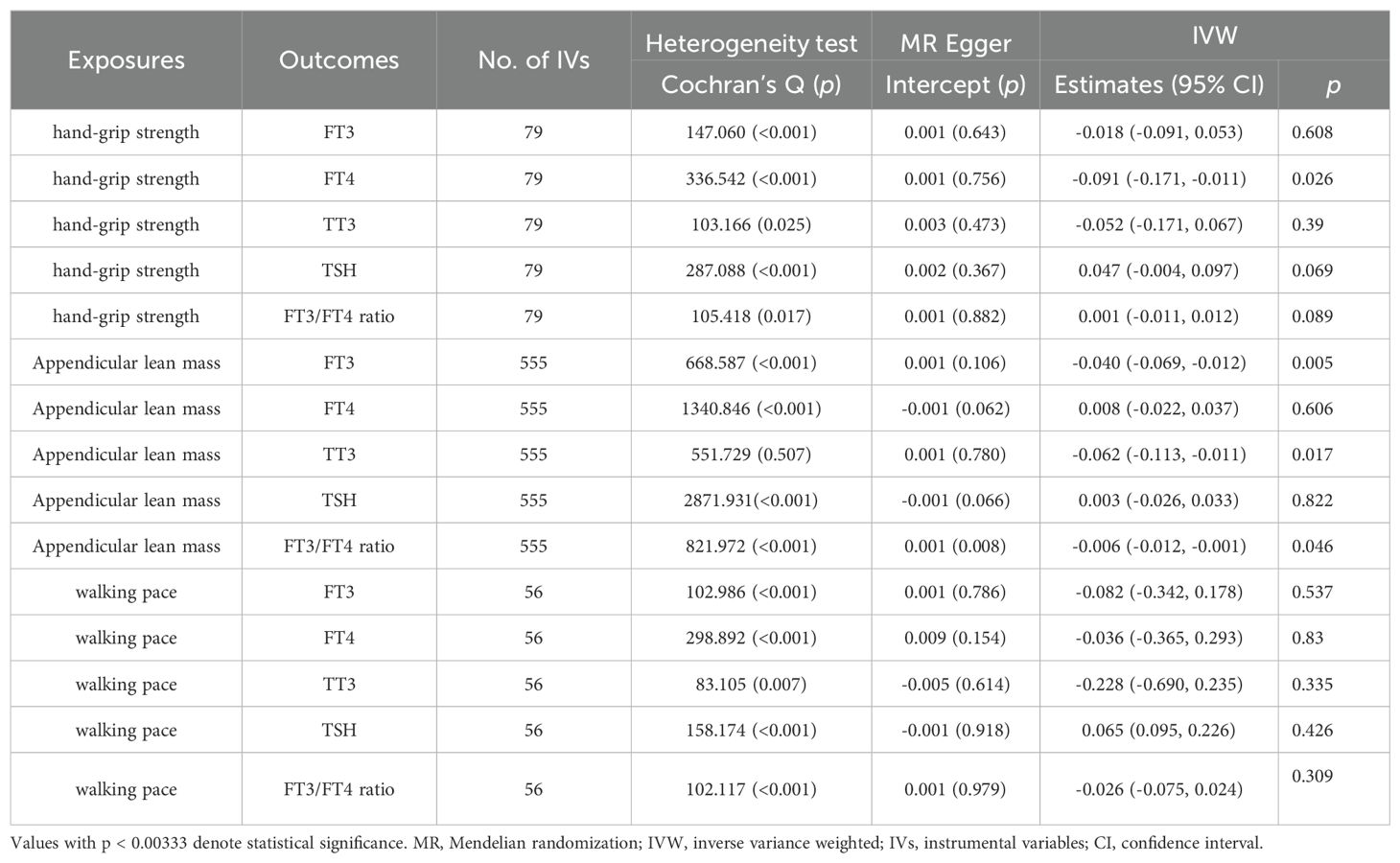
Table 3. Association of sarcopenia-related traits with thyroid function using MR-Egger and IVW analysis.
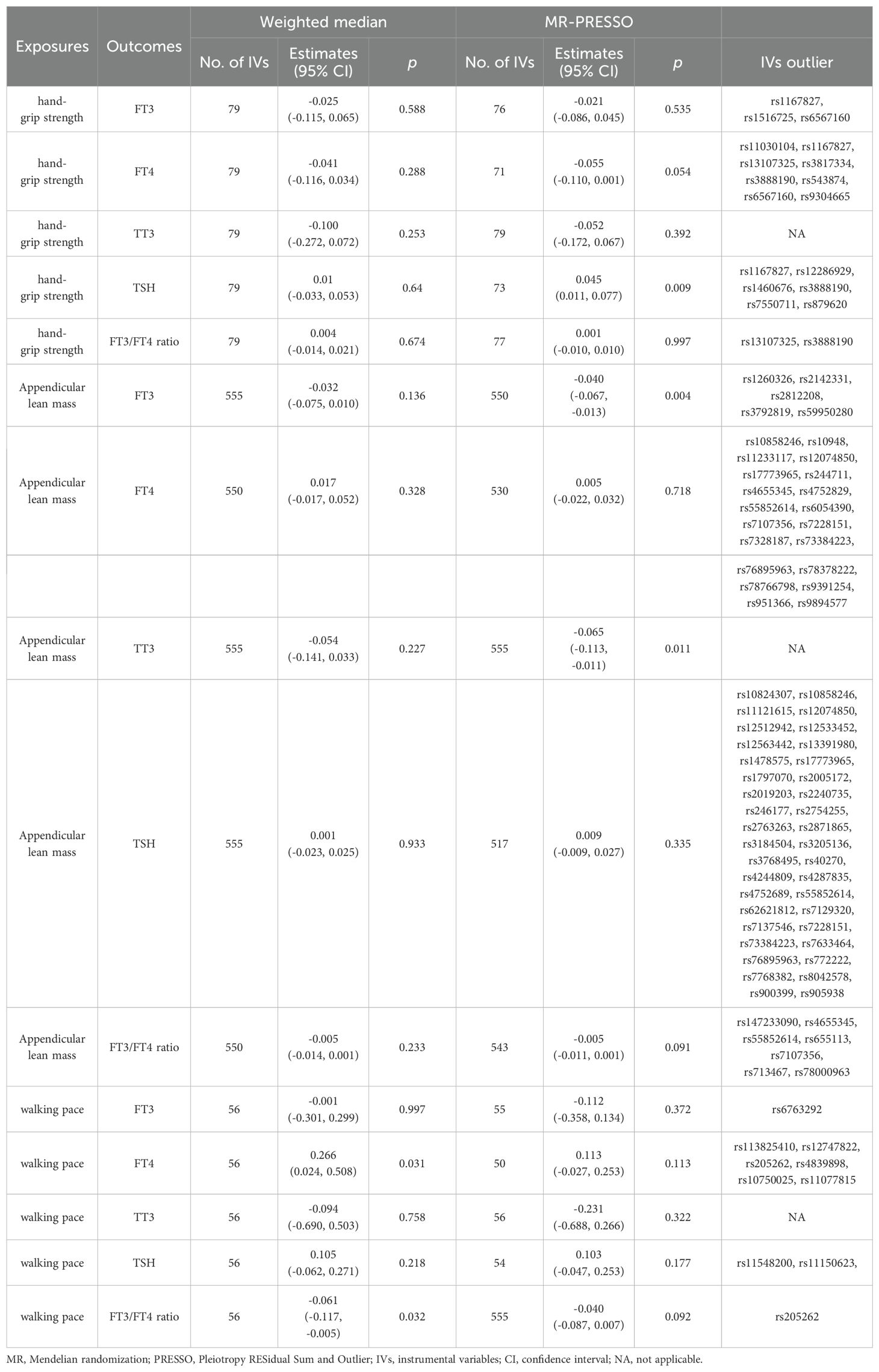
Table 4. Association of sarcopenia-related traits with thyroid function using weighted median and MRPRESSO analysis.
Discussion
In this study, we performed a bi-directional two-sample MR analysis to investigate the causal relationships between susceptibility to various thyroid hormone levels and sarcopenia-related traits. Using this approach, we did not observe any significant correlation between susceptibility to thyroid hormone and hand-grip strength, ALM, and walking pace. This suggests that the reported associations in epidemiological studies may be confounded by unmeasured variables or common genetic factors. We performed multiple sensitivity analyses to differentiate between a true negative result and potential lack of validity in the MR studies, ensuring that the MR assumptions were satisfied. Our consistent findings across various methods provide us with confidence in the robustness of our MR analyses, indicating no substantial causal effects of the exposures on the outcomes.
Understanding the correlation between thyroid hormone and sarcopenia is essential for the management and prevention of these conditions, given their substantial implications for human health (26). A study conducted by Sheng et al. revealed evidence supporting a correlation between lower FT3 levels within the normal range and an elevated risk of sarcopenia in elderly individuals (27). In addition, Li et al. investigated TT3, FT3, TT4, FT4, TSH, rT3, TBG levels and their association with hand-grip strength and gait speed. The findings indicated a positive relationship between FT3, TT3 and hand-grip strength as well as gait speed. However, after adjusting for various factors including age, gender, BMI, physical activity level, FRIAL scores, ALT and sCr levels; it was observed that TT3 rather than FT3 exhibited a significant association with sarcopenia and hand-grip strength (28). The results do not align with the findings of Sheng et al’s study. One potential explanation for these inconsistencies is that the participants in each study were at different life stages. Age can exert a significant influence on thyroid hormone levels, particularly FT3, as well as on the levels of deiodinase and TBG, which directly impact FT3 levels (6, 29). The FT3:FT4 ratio serves as an indicator of the conversion rate from FT4 to FT3. As suggested by Hata S et al., assessments of the FT3/FT4 ratios could potentially serve as significant indicators for muscle mass and strength in male individuals, thus highlighting its potential relevance in clinical research (30). The study presents findings that are inconsistent with the results of our research. Despite the abundance of epidemiological studies, including those mentioned previously, examining the association between thyroid function and sarcopenia, there remains ongoing debate regarding this relationship primarily due to potential selection bias, residual confounding, and reverse causality in observational studies, which complicates the ability to establish causal conclusions. Our analysis, utilizing two samples, yielded a more robust and potentially broadly applicable assessment of the causal relationship between thyroid hormone and sarcopenia.
The regulation of skeletal muscle function is governed by a complex system involving signaling from the neuroendocrine system (31). Research has indicated a correlation between thyroid hormone and the initiation of sarcopenia. These findings suggest that the development of sarcopenia may be impacted by dysfunction in the thyroid hormone system (26, 27, 32). The changes observed in skeletal muscle during the aging process bear resemblance to the modifications associated with a decrease in thyroid hormone signaling (33, 34). It remains unclear whether variations in thyroid hormone levels are a consequence of sarcopenia or if they play a role in its progression. While no direct genetic link between thyroid hormone and sarcopenia-related traits was identified in this study, further research is needed to fully understand the association between thyroid hormone and sarcopenia.
Our study has several limitations. Since it was conducted in Europe, the generalizability of our findings to other populations may be limited. Additionally, old age and an individual’s specific health condition are profoundly intertwined with thyroid hormone and development of sarcopenia. Regrettably, we were precluded from investigating these variables in greater depth owing to the unavailability of individual-level data. Finally, although evidence from our study did not support a causal association thyroid hormone and sarcopenia-related traits, we could not completely rule out the possibility that the effect size might be relatively small, although the sample size of our study was large enough to detect this difference, however we did not perform subgroup analysis according to the age and sex in the present research.
Conclusions
Our research results showed that there were no causal relationships between thyroid hormone and sarcopenia-related traits at the genetic level. However, that does not rule out the possibility that they are related on a level other than genetic. Deeper and more extensive research is needed to investigate these possible links.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Ethics Committee of the People’s Hospital of Xinjiang Uygur Autonomous Region (approval no. KY20240312074). It was carried out according to the standards of the Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
RX: Data curation, Writing – original draft. YL: Data curation, Writing – review & editing. HX: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to extend our heartfelt appreciation to all the ThyroidOmics Consortium and the UK Biobank for generously providing us with the GWAS summary data for thyroid hormone and sarcopenia-related traits.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
2. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012
3. Gao K, Cao LF, Ma WZ, Gao YJ, Luo MS, Zhu J, et al. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: Findings from the China health and retirement longitudinal study. EClinicalMedicine. (2022) 44:101264. doi: 10.1016/j.eclinm.2021.101264
4. Liu X, Wang Y, Wang Z, Li L, Yang H, Liu J, et al. Association between sarcopenia-related traits and cardiovascular diseases: a bi-directional Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1237971. doi: 10.3389/fendo.2023.1237971
5. Liu YH, Ma LL, Hu LK, Cui L, Li YL, Chen N, et al. The joint effects of sarcopenia and cardiometabolic risk factors on declined cognitive function: Evidence from a 7-year cohort study. J Affect Disord. (2024) 344:644–52. doi: 10.1016/j.jad.2023.10.056
6. Luo M, Sun M, Wang T, Zhang S, Song X, Liu X, et al. Gut microbiota and type 1 diabetes: a two-sample bidirectional Mendelian randomization study. Front Cell Infect Microbiol. (2023) 13:1163898. doi: 10.3389/fcimb.2023.1163898
7. Arnold WD, Clark BC. Neuromuscular junction transmission failure in aging and sarcopenia: The nexus of the neurological and muscular systems. Ageing Res Rev. (2023) 89:101966. doi: 10.1016/j.arr.2023.101966
8. Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, et al. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol. (2013) 45:2288–301. doi: 10.1016/j.biocel.2013.06.024
9. Bagheri A, Hashemi R, Soltani S, Heshmat R, Dorosty Motlagh A, Larijani B, et al. The relationship between food-based pro-inflammatory diet and sarcopenia: findings from a cross-sectional study in Iranian elderly people. Front Med (Lausanne). (2021) 8:649907. doi: 10.3389/fmed.2021.649907
10. Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. (2015) 22:100–6. doi: 10.1016/j.coph.2015.04.003
11. Aslam MA, Ma EB, Huh JY. Pathophysiology of sarcopenia: Genetic factors and their interplay with environmental factors. Metabolism. (2023) 149:155711. doi: 10.1016/j.metabol.2023.155711
12. Szlejf C, Suemoto CK, Janovsky C, Barreto SM, Diniz M, Lotufo PA, et al. Thyroid function and sarcopenia: results from the ELSA-Brasil study. J Am Geriatr Soc. (2020) 68:1545–53. doi: 10.1111/jgs.16416
13. Sun J, Huang J, Lu W. TT3, a more practical indicator for evaluating the relationship between sarcopenia and thyroid hormone in the euthyroid elderly compared with FT3 [Letter]. Clin Interv Aging. (2023) 18:1361–2. doi: 10.2147/CIA.S434298
14. Bloise FF, Cordeiro A, Ortiga-Carvalho TM. Role of thyroid hormone in skeletal muscle physiology. J Endocrinol. (2018) 236:R57–57R68. doi: 10.1530/JOE-16-0611
15. Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid. (2006) 16:375–80. doi: 10.1089/thy.2006.16.375
16. Boef AG, Dekkers OM, le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol. (2015) 44:496–511. doi: 10.1093/ije/dyv071
17. Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: A review. Res Synth Methods. (2019) 10:486–96. doi: 10.1002/jrsm.1346
18. Spiga F, Gibson M, Dawson S, Tilling K, Davey Smith G, Munafò MR, et al. Tools for assessing quality and risk of bias in Mendelian randomization studies: a systematic review. Int J Epidemiol. (2023) 52:227–49. doi: 10.1093/ije/dyac149
19. Sterenborg R, Steinbrenner I, Li Y, Bujnis MN, Naito T, Marouli E, et al. Multi-trait analysis characterizes the genetics of thyroid function and identifies causal associations with clinical implications. Nat Commun. (2024) 15:888. doi: 10.1038/s41467-024-44701-9
20. Clarke L, Zheng-Bradley X, Smith R, Kulesha E, Xiao C, Toneva I, et al. The 1000 Genomes Project: data management and community access. Nat Methods. (2012) 9:459–62. doi: 10.1038/nmeth.1974
21. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PloS Med. (2015) 12:e1001779. doi: 10.1371/journal.pmed.1001779
22. Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. (2016) 35:1880–906. doi: 10.1002/sim.6835
23. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
24. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
25. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
26. Bloise FF, Oliveira TS, Cordeiro A, Ortiga-Carvalho TM. Thyroid hormones play role in sarcopenia and myopathies. Front Physiol. (2018) 9:560. doi: 10.3389/fphys.2018.00560
27. Sheng Y, Ma D, Zhou Q, Wang L, Sun M, Wang S, et al. Association of thyroid function with sarcopenia in elderly Chinese euthyroid subjects. Aging Clin Exp Res. (2019) 31:1113–20. doi: 10.1007/s40520-018-1057-z
28. Chen J, Wei L, Zhu X, Xu W, Zou Y, Qi X, et al. TT3, a more practical indicator for evaluating the relationship between sarcopenia and thyroid hormone in the euthyroid elderly compared with FT3. Clin Interv Aging. (2023) 18:1285–93. doi: 10.2147/CIA.S420558
29. Mammen J. Thyroid and aging. Endocrinol Metab Clin North Am. (2023) 52:229–43. doi: 10.1016/j.ecl.2022.10.008
30. Hata S, Okada H, Minamida M, Hironaka J, Hasegawa Y, Kondo Y, et al. Associations between thyroid hormones and appendicular skeletal muscle index, and hand grip strength in people with diabetes: The KAMOGAWA-A study. Diabetes Res Clin Pract. (2024) 209:111573. doi: 10.1016/j.diabres.2024.111573
31. Piancone F, La Rosa F, Marventano I, Hernis A, Miglioli R, Trecate F, et al. Modulation of neuroendocrine and immunological biomarkers following rehabilitation in sarcopenic patients. Cells. (2022) 11:2477. doi: 10.3390/cells11162477
32. Netzer S, Chocano-Bedoya P, Feller M, Janett-Pellegri C, Wildisen L, Büchi AE, et al. The effect of thyroid hormone therapy on muscle function, strength and mass in older adults with subclinical hypothyroidism-an ancillary study within two randomized placebo controlled trials. Age Ageing. (2023) 52:afac326. doi: 10.1093/ageing/afac326
33. Chen X, Zheng X, Ding Z, Su Y, Wang S, Cui B, et al. Relationship of gender and age on thyroid hormone parameters in a large Chinese population. Arch Endocrinol Metab. (2020) 64:52–8. doi: 10.20945/2359-3997000000179
Keywords: Mendelian randomization, thyroid function, sarcopenia, hand-grip strength, appendicular lean mass, walking pace
Citation: Xu R, Li Y-Y and Xu H (2024) Mendelian randomization analysis reveals no causal relationship between thyroid function and sarcopenia-related traits. Front. Endocrinol. 15:1406165. doi: 10.3389/fendo.2024.1406165
Received: 18 June 2024; Accepted: 19 August 2024;
Published: 13 September 2024.
Edited by:
Roberto Cesareo, Hospital Santa Maria Goretti, ItalyReviewed by:
Takuma Inagawa, National Center of Neurology and Psychiatry (Japan), JapanDa Zhou, Capital Medical University, China
Copyright © 2024 Xu, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Xu, eW9zaWVzb21vZGdAaG90bWFpbC5jb20=
 Rui Xu
Rui Xu Yan-Yan Li
Yan-Yan Li Hong Xu
Hong Xu