- 1Department of Cardiology, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
- 2Department of Ultrasound, the Second Affiliated Hospital of Nanchang University, Nanchang, China
- 3Jiangxi Cardiovascular Research Institute, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, China
Objective: Diabetes is a significant risk factor for acute heart failure, associated with an increased risk of mortality. This study aims to analyze the prognostic significance of admission blood glucose (ABG) on 30-day mortality in Chinese patients with acute decompensated heart failure (ADHF), with or without diabetes.
Methods: This retrospective study included 1,462 participants from the JX-ADHF1 cohort established between January 2019 to December 2022. We conducted multivariate cox regression, restricted cubic spline, receiver operating characteristic curve analysis, and mediation analysis to explore the association and potential mechanistic pathways (inflammation, oxidative stress, and nutrition) between ABG and 30-day mortality in ADHF patients, with and without diabetes.
Results: During the 30-day follow-up, we recorded 20 (5.36%) deaths in diabetic subjects and 33 (3.03%) in non-diabetics. Multivariate Cox regression revealed that ABG was independently associated with 30-day mortality in ADHF patients, with a stronger association in diabetics than non-diabetics (hazard ratio: Model 1: 1.71 vs 1.16; Model 2: 1.26 vs 1.19; Model 3: 1.65 vs 1.37; Model 4: 1.76 vs 1.33). Further restricted cubic spline analysis indicated a U-shaped relationship between ABG and 30-day mortality in non-diabetic ADHF patients (P for non-linearity < 0.001), with the lowest risk at ABG levels approximately between 5-7 mmol/L. Additionally, receiver operating characteristic analysis demonstrated that ABG had a higher predictive accuracy for 30-day mortality in diabetics (area under curve = 0.8751), with an optimal threshold of 13.95mmol/L. Finally, mediation analysis indicated a significant role of inflammation in ABG-related 30-day mortality in ADHF, accounting for 11.15% and 8.77% of the effect in diabetics and non-diabetics, respectively (P-value of proportion mediate < 0.05).
Conclusion: Our study confirms that ABG is a vital indicator for assessing and predicting 30-day mortality risk in ADHF patients with diabetes. For ADHF patients, both with and without diabetes, our evidence suggests that physicians should be alert and closely monitor any changes in patient conditions when ABG exceeds 13.95 mmol/L for those with diabetes and 7.05 mmol/L for those without. Timely adjustments in therapeutic strategies, including endocrine and anti-inflammatory treatments, are advisable.
Introduction
Acute decompensated heart failure (ADHF) is one of the most common reasons for hospitalization or the need for urgent care in the elderly population, posing a significant public health issue globally (1, 2). Despite tangible advances in the discovery of new heart failure treatment methods over the past few decades, the improvement in prognosis for ADHF patients during the acute phase remains limited, as these individuals still face high risks of in-hospital mortality and readmission (2–6).
Stress hyperglycemia refers to the acute increase in blood glucose during the acute phase of an illness, typically returning to pre-attack levels upon recovery (7). Admission blood glucose (ABG) is a commonly used indicator for assessing stress hyperglycemia and has been reported in numerous studies (8–15). Overall, ABG is an important marker for assessing the prognosis of acute onset diseases and severe illnesses, both in diabetic and non-diabetic populations. ADHF, as an acute exacerbation of heart failure, shows that approximately half of the acute heart failure (AHF) patients experience elevated ABG (16, 17). However, it is noteworthy that several published studies have shown contradictory correlations between ABG and short-term adverse outcomes, such as 30-day mortality, in AHF patients (16–18). Moreover, there is currently a lack of research data on the correlation between ABG and ADHF prognosis based on the Chinese population. In this context, our aim is to further evaluate the predictive significance of ABG on 30-day mortality in ADHF patients, both with and without diabetes, through a study cohort in Jiangxi, China.
Methods
Study population and design
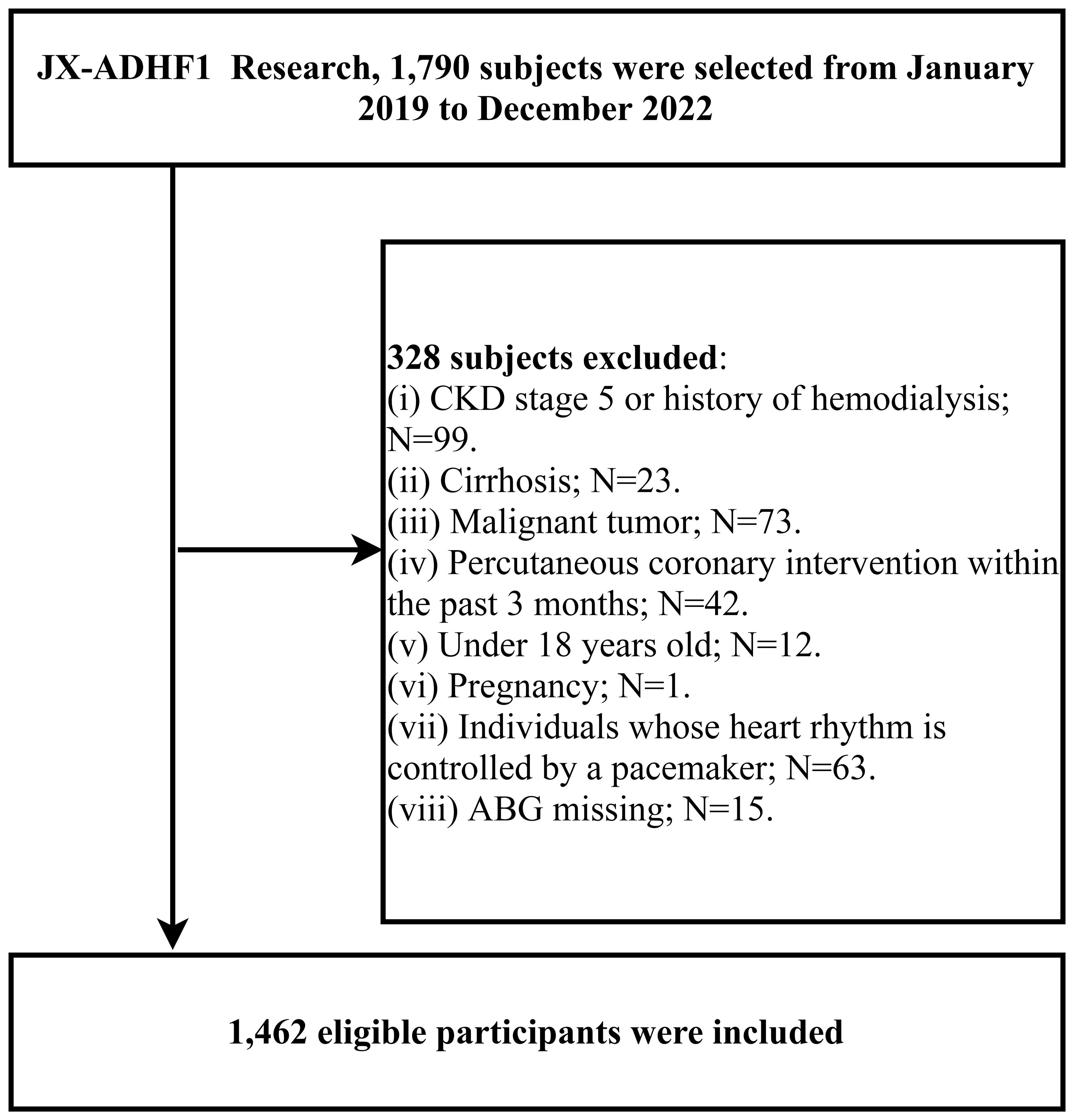
The JX-ADHF1 (Jiangxi-acute decompensated heart failure1) study is a retrospective cohort study that consecutively enrolled 1,790 patients with ADHF who were treated at Jiangxi Provincial People’s Hospital from January 2019 to December 2022. The aim was to explore significant factors affecting short-term adverse outcomes in ADHF patients, thereby facilitating improvements in ADHF treatment approaches. Exclusion criteria applied to subjects with the following baseline characteristics: (i) those with stage 5 chronic kidney disease or a history of hemodialysis (n=99), and patients with cirrhosis (n=23), considering the potential effects of other diseases on sodium and water retention (19); (ii) patients with malignant tumors, given the potential impact on life expectancy (n=73); (iii) individuals who had undergone percutaneous coronary intervention within the past three months were excluded, due to the significant role of reperfusion therapy on short-term prognosis (n=42); (iv) participants under the age of 18 (n=12); (v) pregnant participants (n=1); and (vi) individuals with pacemaker-controlled heart rhythms, as their heart rate was not expected to be under autonomic control (n=63). Additionally, for the purposes of the current study, we further excluded participants with missing information on the independent variable ABG (n=15); ultimately, the study comprised 1,462 participants. A detailed flow chart of the study population screening process was shown in Figure 1.
In this study, the diagnosis of ADHF was defined according to the 2021 ESC and 2022 ACC/AHA/HFSA Guidelines for HF (20, 21), taking into account the subjects’ clinical symptoms, signs, and laboratory findings, mainly as follows: (1) At least one sign of HF: (a) Elevated N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) or BNP; (b) pulmonary edema detected by physical examination or chest X-ray; (c) cardiac ultrasound showing structural and/or functional abnormalities of the heart. (2) Further deterioration of at least one symptom: (a) venous congestion of the body circulation; (b) dyspnea; (c) inadequate tissue perfusion.
Ethics and approval
The JX-ADHF1 study adhered to the ethical guidelines of the Declaration of Helsinki. The use of study data was consented to by the participants and their families. The research protocol received authorization from the Ethics Review Committee of Jiangxi Provincial People’s Hospital (IRB:2024-01). The entire research process strictly followed the STROBE reporting guidelines (Supplementary Table 1).
Data collection
Baseline information included general demographic data (gender and age), New York Heart Association (NYHA) functional classification at admission, comorbidities [hypertension, diabetes, cerebral infarction, coronary heart disease (CHD)], the most recent echocardiogram results, systolic and diastolic blood pressure (SBP and DBP), laboratory parameters from blood samples and information on medications received during hospitalization, including anti-HF treatment [diuretic, angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor inhibitors (ARB)/angiotensin receptor neprilysin inhibitors (ARNI), beta-blockers, digitalis, sodium-dependent glucose transporters 2 (SGTL-2)] and corticosteroids therapy (spirolactone, adrenaline, and glucocorticoid). The past diagnoses of hypertension/diabetes/cerebral infarction/CHD were based on patients’ self-report, ongoing medication treatment, or records in patient medical history.
Blood pressure information was the first measurement recorded after admission, taken in a quiet environment or bedside using an Omron automatic blood pressure monitor (HBP-1300).
Laboratory parameters were measured within 24 hours of admission at the Jiangxi Provincial People’s Hospital Laboratory Center, including ABG, NT-proBNP, white blood cell count (WBC), red blood cell count (RBC), hemoglobin (HGB), platelet count (PLT), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), creatinine (Cr), hemoglobin (HbA1c), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C); liver enzyme-related parameters and lipid-related parameters were determined by venous blood draw in a fasting state at admission or the next morning after admission.
Study outcome
The starting point for follow-up was defined as the time of admission, and the endpoint was 30 days post-admission. Follow-up was conducted by trained medical personnel through text messages, phone calls, and face-to-face visits in the outpatient/inpatient department. The primary endpoint of interest was death from any cause within 30 days.
Statistical analysis
In this study, we summarized the baseline characteristics of the study population according to the diabetes status of the subjects and used inverse probability of treatment weighting and unpaired chi-square tests to calculate differences between groups for continuous and categorical variables. P-values for the assessment of differences between groups were chosen from t-tests, chi-square tests, or rank-sum tests, depending on the type and distributional characteristics of the variables. The baseline information of the subjects was recorded as mean (standard deviation), median (interquartile range), or count (percentage), based on the characteristics and distribution of the variables.
We first conducted survival analysis using Kaplan-Meier and log-rank tests to assess the survival status of ADHF patients in the diabetic and non-diabetic groups. Then, we employed multivariate Cox regression models to evaluate the association between ABG and 30-day mortality in ADHF patients with and without diabetes. The adjustment of variables considered sex, age, hypertension, diabetes, cerebral infarction, CHD, NYHA classification, SBP, DBP, left ventricular ejection fraction (LVEF), WBC, RBC, Hb, PLT, ALB, AST, GGT, Cr, TG, HDL-C, LDL-C, NT-proBNP, HbA1c, etiology of ADHF, ADHF seizure characteristics, anti-heart failure treatment, and corticosteroids therapy. ALT and TC were excluded from the model due to severe collinearity with other covariates (Supplementary Table 2) (22, 23). Additionally, the Schoenfeld residuals plot of ABG over time (Supplementary Figure 1) suggested that our model met the proportional hazards assumption (P = 0.863) (24, 25). Finally, to further test the robustness of our analysis of the association between ABG and 30-day mortality in ADHF patients, we calculated an E-value based on the effect size of ABG in the final model to quantify the minimum strength of association a confounder would need with the study outcome (26).
Based on Cox regression, we continued with restricted cubic splines (RCS) to fit the dose-response relationship curve between ABG and 30-day mortality. Additionally, we conducted stratified analysis by age (stratified based on median), gender, and comorbidities, with differences between strata examined by likelihood ratio tests.
To assess the predictive value of ABG for 30-day mortality in ADHF patients in diabetic and non-diabetic populations, we constructed receiver operating characteristic (ROC) curves and calculated the area under the curve (AUC), optimal threshold, sensitivity, and specificity for the ABG of both diabetic and non-diabetic groups.
Having established the longitudinal association between ABG and 30-day mortality in ADHF patients in diabetic and non-diabetic populations, we planned to further explore the roles of inflammation (27), oxidative stress (28), and nutrition (29) in ABG-related ADHF patient 30-day mortality. Based on previous reports, we chose WBC as a marker of inflammation (30), GGT as a marker of oxidative stress (30, 31), and ALB as an indicator of nutritional status (32). We constructed mediation effect models to analyze the influence of WBC, GGT, and ALB in ABG-related ADHF patient 30-day mortality in diabetic and non-diabetic populations, quantifying mediation effects by calculating the percentage of mediation (ratio of indirect effect to total effect) and using the bootstrap sampling method (times=1000) to test the significance of mediation effects (33).
In this study, a two-sided P < 0.05 or a standardized difference value >10% was considered statistically significant. All analyses were performed using R language version 4.2.1 and Empower(R) version 2.20 statistical software.
Results
Study cohort
The current study cohort included 1,462 participants, comprising 840 males (57.46%) and 622 females (42.54%), with an average age of 68 years. We identified nine common causes of HF in these study populations, including ischemic cardiomyopathy, hypertension, non-ischemic cardiomyopathy, valvular disease, arrhythmia, acute myocarditis, congenital heart disease, pulmonary heart disease and other reasons (including pericardial disease, hypothyroidism, anemic heart disease, severe infections, rapid progression of other systemic diseases, and unknown etiologies). Of note, the primary etiologies of ADHF patients in the current cohort were ischemic and nonischemic cardiomyopathy and valvular disease (Table 1).
During the 30-day follow-up, 53 deaths (3.63%) were recorded, with a mortality rate of 5.36% (20/373) in ADHF patients with diabetes and 3.03% (33/1,089) in those without. Figure 2 illustrates the 30-day cumulative survival curves for ADHF patients with and without diabetes, indicating significantly higher mortality within 30 days for patients with diabetes compared to those without (log-rank P = 0.037).
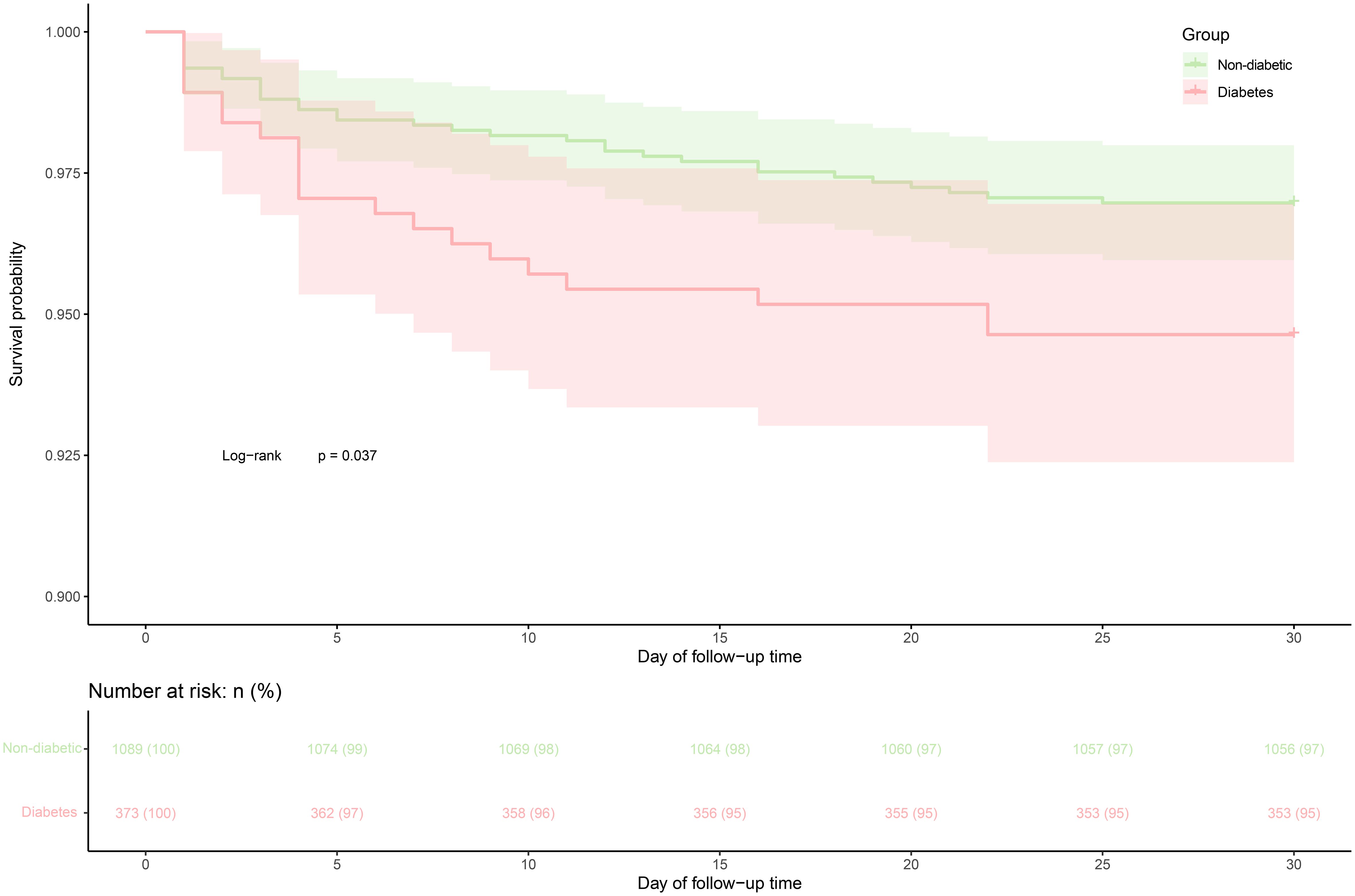
Figure 2 30-day survival curve of ADHF patients in diabetes and non-diabetic groups. ADHF, acute decompensated heart failure.
Table 2 shows information on the baseline characteristics of the study population, and we found that the vast majority of the study population in the current cohort progressed from an acute exacerbation of chronic HF to a decompensated state, with only a small proportion of the population categorized as having new-onset ADHF. After grouping according to whether the subjects were diabetic, we also found that compared to the non-diabetic group, diabetic subjects at baseline had significantly higher proportions of hypertension, CHD, heart failure with reduced ejection fraction (LVEF < 50%), underwent SGLT-2 treatment, NYHA class IV, and higher levels of SBP, ABG, HbA1c, WBC, RBC, PLT, ALB, GGT, Cr, and TG, as well as lower levels of LVEF and HDL-C. Notably, there was a considerable difference in baseline ABG between the two groups (standardized difference value = 97%, Figure 3). Additionally, it is also important to mention that diabetic factors may lead to higher incidence of hypertension [Odds ratio (OR): 2.45, 95% confidence interval (CI): 1.91-3.14)], CHD (OR: 2.35, 95%CI: 1.82-3.02), and lower incidence of heart failure with preserved ejection fraction (OR:0.71, 95%CI: 0.56-0.93). In terms of medication, there was no significant difference between the diabetic and non-diabetic groups in the use of diuretic, ACEI/ARB/ARNI, beta-blockers, digitalis, and corticosteroids.
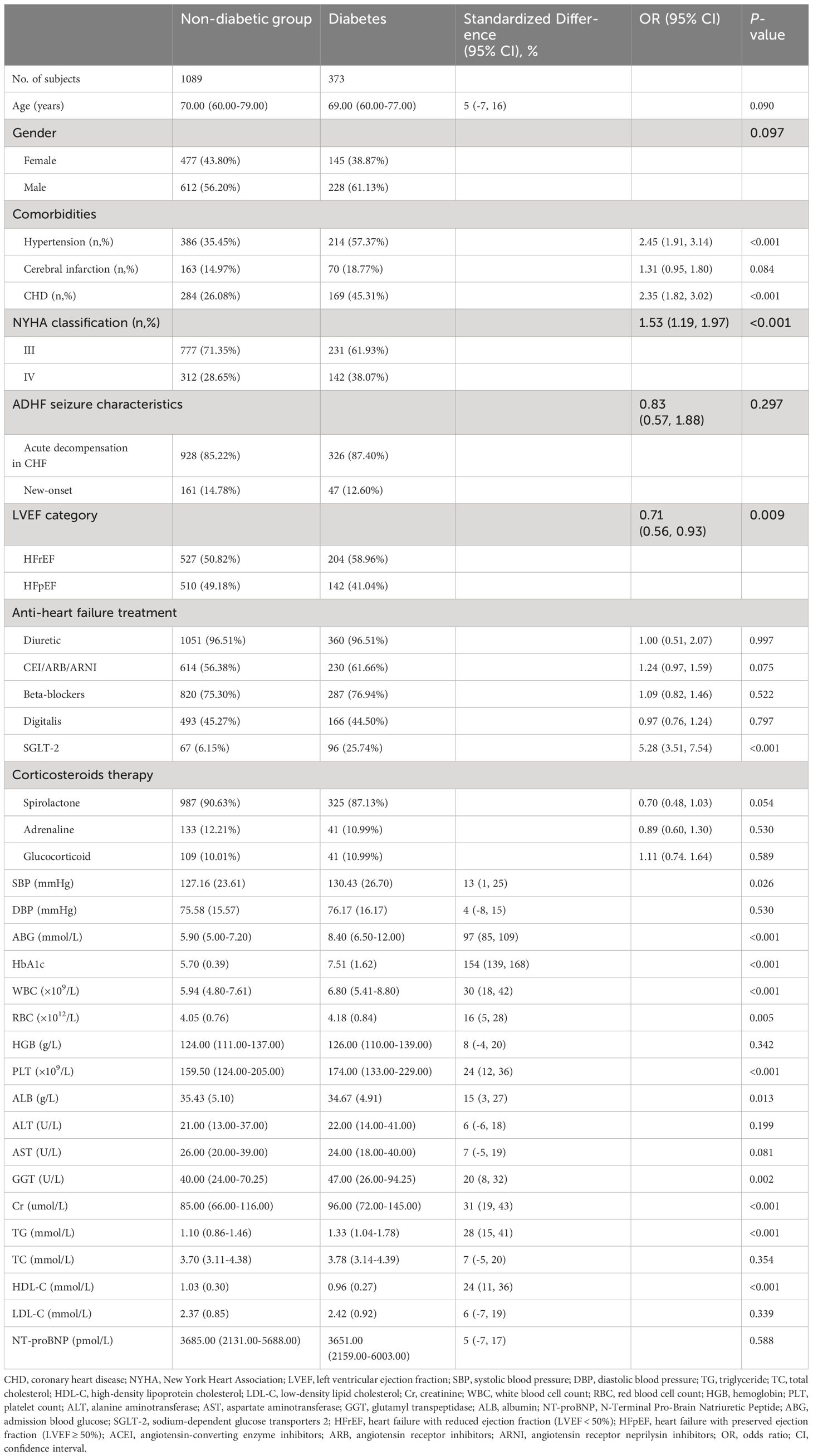
Table 2 Summarize the baseline characteristics of the study population according to whether they are complicated with diabetes or not.
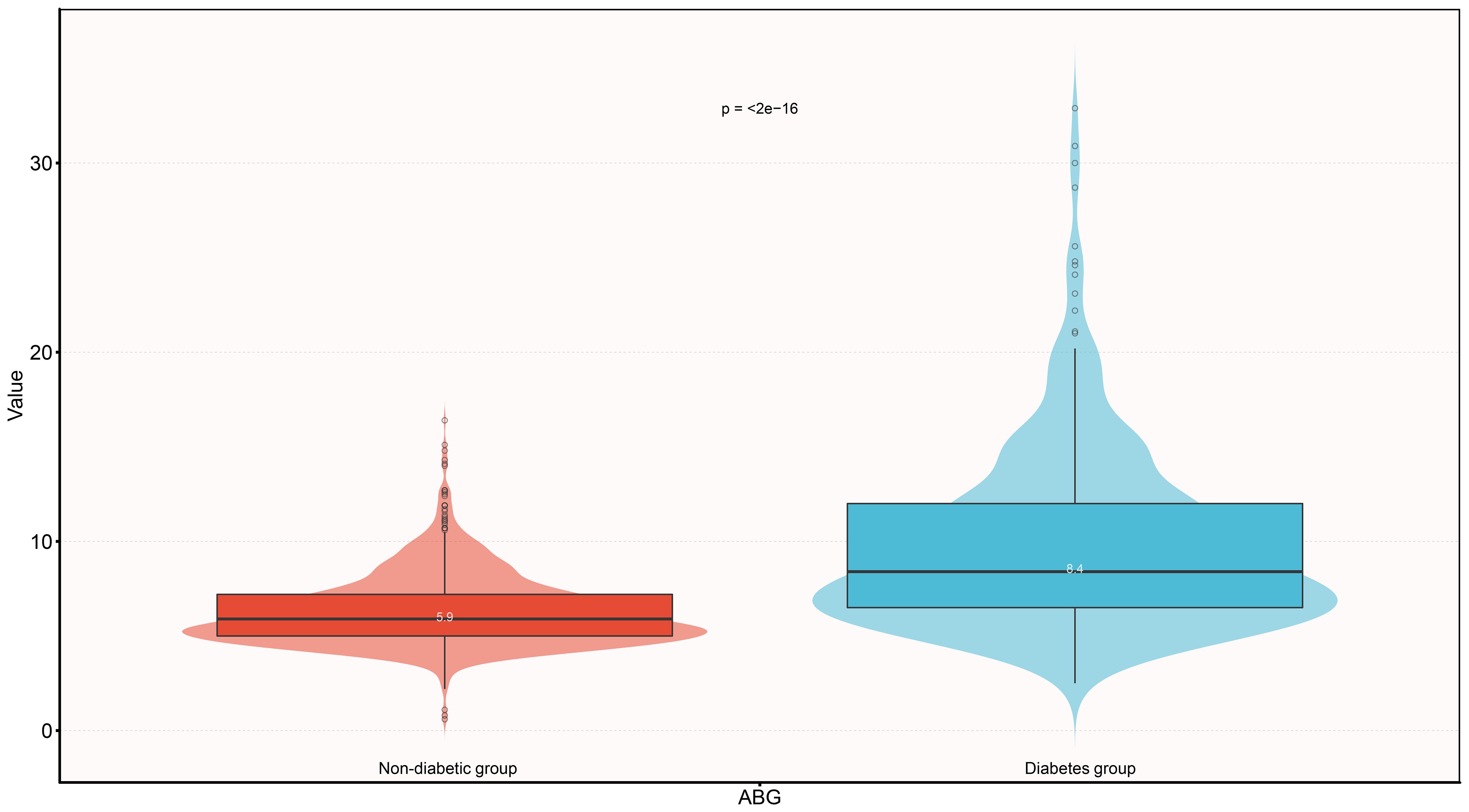
Figure 3 Violin diagram showing ABG baseline characteristics according to whether there is diabetes or not. ABG, admission blood glucose.
Association between ABG and 30-day mortality in ADHF Patients with and without diabetes
Five progressively adjusted Cox regression models were constructed to assess the relationship between ABG and 30-day mortality in ADHF patients with and without diabetes (Table 3). In the unadjusted model, ABG was positively associated with 30-day mortality, with hazard ratios (HRs) of 1.36 in non-diabetic ADHF patients and 1.20 in diabetic ones. In the first adjusted model (Model 1), considering the potential impact of age, gender, NT-proBNP, routine blood parameters (WBC, RBC, Hb, PLT), and lipid parameters (TG, HDL-C, LDL-C), ABG remained positively associated with 30-day mortality in both groups. The results of the second model (Model 2), which further adjusted for Alb, AST, GGT, Cr, LVEF, were similar to Model 1. Model 3 was further adjusted for NYHA classification, SBP, DBP, hypertension, cerebral infarction and CHD on the basis of Model 2, and the results showed that ABG remained positively correlated with 30-day mortality in patients with ADHF in both diabetic and non-diabetic groups. The fifth model (Model 4), our final model, included all non-collinear covariates except TC and ALT, showing that with every 1 mmol/L increase in ABG, the 30-day mortality risk increased by 76% [HR 1.76, 95% CI: 1.02, 3.04] in diabetic ADHF patients and by 33% (HR 1.33, 95% CI: 1.06, 1.66) in non-diabetic ones. The relative 30-day mortality risk was higher in diabetic ADHF patients compared to non-diabetics (HR: 1.76 vs 1.33). Based on Model 4 results, we further calculated the E-values for the association between ABG and 30-day mortality in ADHF patients with and without diabetes, with point estimates of 2.92 and 1.99, respectively.
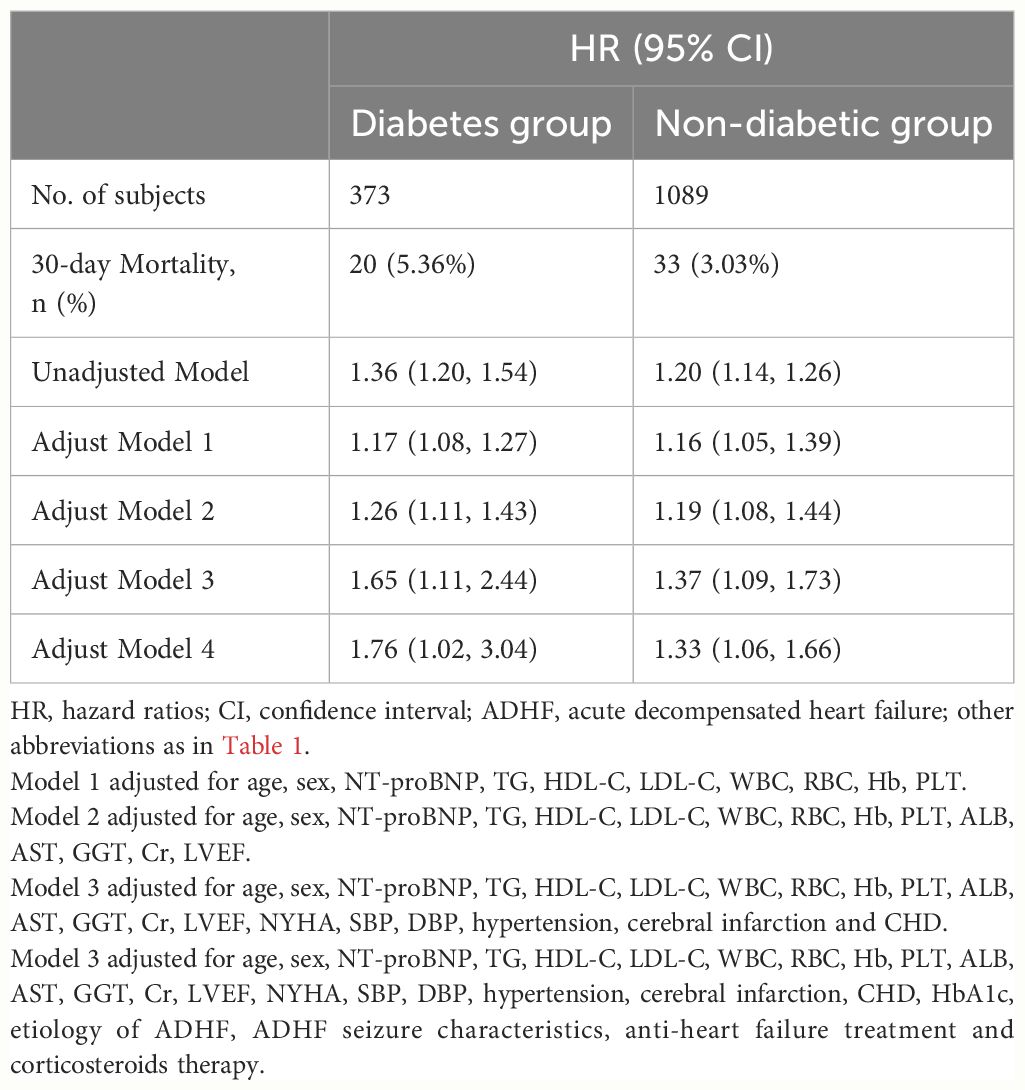
Table 3 Multivariable Cox regression analysis of the association between ABG and 30-day mortality in patients with ADHF.
Dose-response relationship
Using RCS, we further analyzed the dose-response relationship between ABG and 30-day mortality in ADHF patients, grouped according to diabetes diagnosis, with variable adjustments following the Model 4 scheme from Table 3. As depicted in Figure 4, a U-shaped association between ABG and 30-day mortality was observed in non-diabetic ADHF patients (P for non-linearity < 0.001), with the lowest risk of mortality around 5-7 mmol/L ABG. In diabetic ADHF patients, a linear relationship was noted (P for non-linearity = 0.744), showing a linear increase in mortality with rising ABG levels (Figure 4).
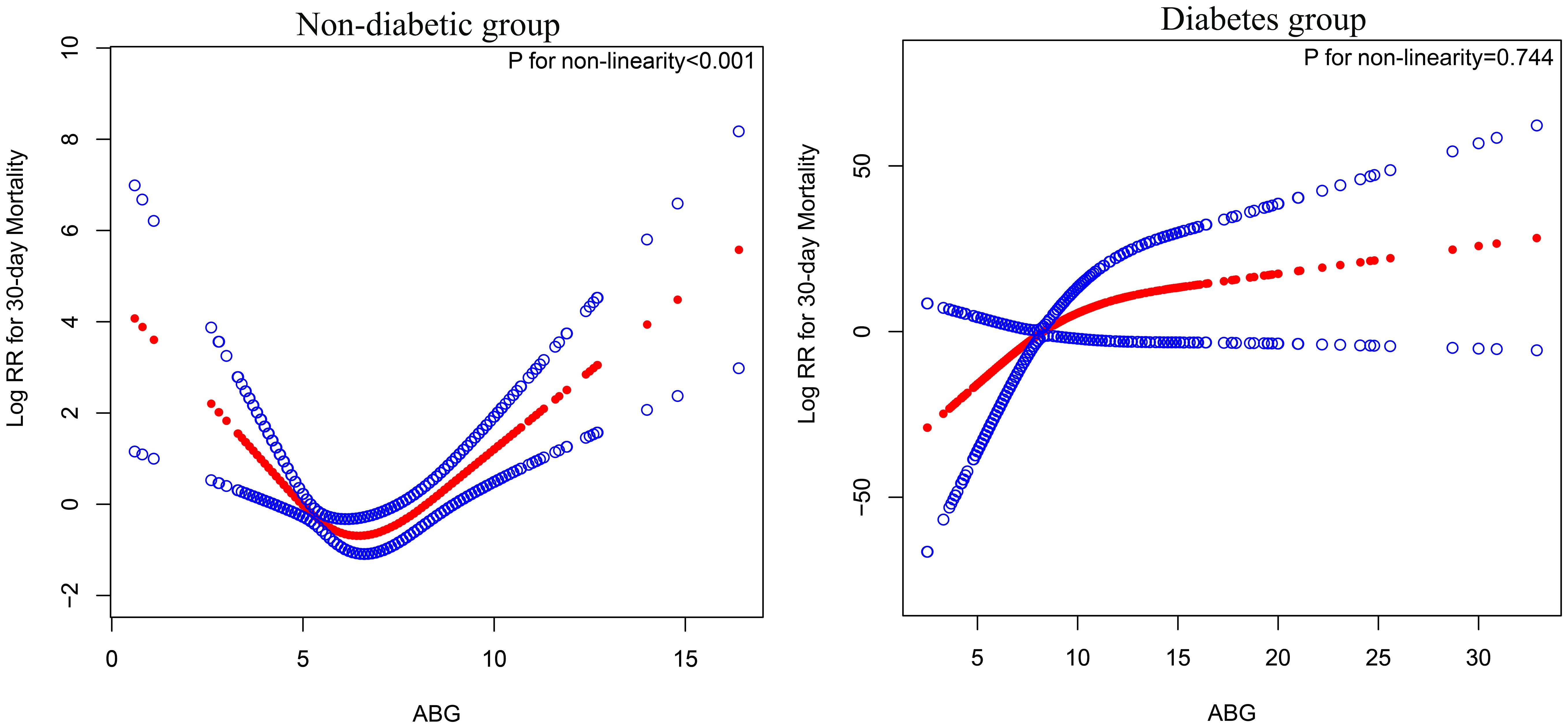
Figure 4 Fitting the dose-response relationship between ABG and 30-day mortality in ADHF patients with 4 knots restricted cubic spline. ABG, admission blood glucose; ADHF, acute decompensated heart failure. Adjusted for age, gender, NT-proBNP, TG, HDL-C, LDL-C, WBC, RBC, Hb, PLT, ALB, AST, GGT, Cr, LVEF, NYHA, SBP, DBP, hypertension, cerebral infarction and CHD.
Subgroup analysis
Exploratory subgroup analyses were conducted to investigate the differences in the association between ABG and 30-day mortality in ADHF patients with and without diabetes across various populations, stratified by age, gender, and comorbidities. Across multiple subgroups, we did not observe any significant interactions (Table 4; all P-interaction > 0.05), and these findings suggested that the current research results were relatively stable.
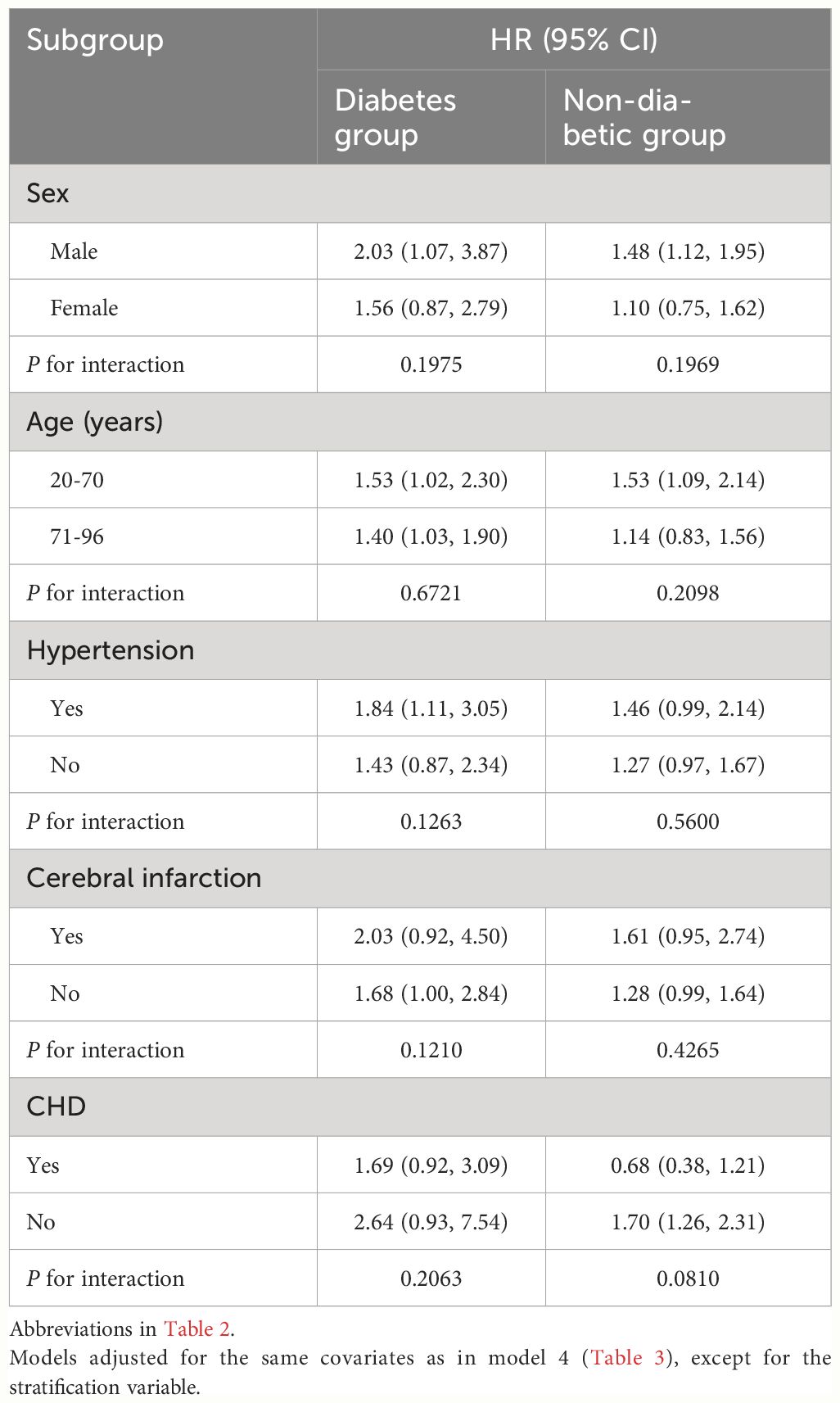
Table 4 Stratified analysis showed the relationship between ABG and 30-day mortality in patients with ADHF in different age, sex, NYHA class, LVEF and whether combined with hypertension/Cerebral infarction/CHD.
ROC analysis
The accuracy of ABG in predicting 30-day mortality in ADHF patients with and without diabetes was further assessed using ROC analysis. As shown in Table 5, the AUC for ABG in predicting 30-day mortality was 0.7124 in non-diabetic ADHF patients, with an optimal threshold of 7.05 mmol/L. In diabetic ADHF patients, the AUC was 0.8751 with a predictive threshold of 13.95 mmol/L. Compared to the non-diabetic group, ABG demonstrated much higher accuracy in predicting 30-day mortality in diabetic ADHF patients, with a significantly higher predictive threshold.
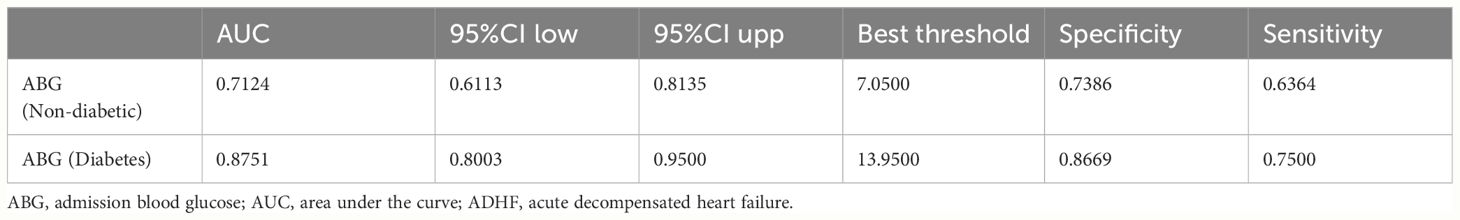
Table 5 ROC analysis of the predictive value of ABG for 30-day mortality in ADHF patients with or without diabetes.
Mediation analysis
Following the establishment of a longitudinal association between ABG and 30-day mortality in ADHF patients, mediation models were used to analyze the effects of WBC, GGT, and ALB in mediating this relationship in patients with and without diabetes. Mediation analysis results (Table 6) revealed that among the three pathways, inflammation, oxidative stress, and nutrition, only inflammation significantly mediated the ABG-related 30-day mortality (P-value of proportion mediated < 0.05). Specifically, inflammation accounted for approximately 11.15% of the mediation effect on the ABG-related 30-day mortality in diabetic ADHF patients, and about 8.77% of the effect in non-diabetic ADHF patients.
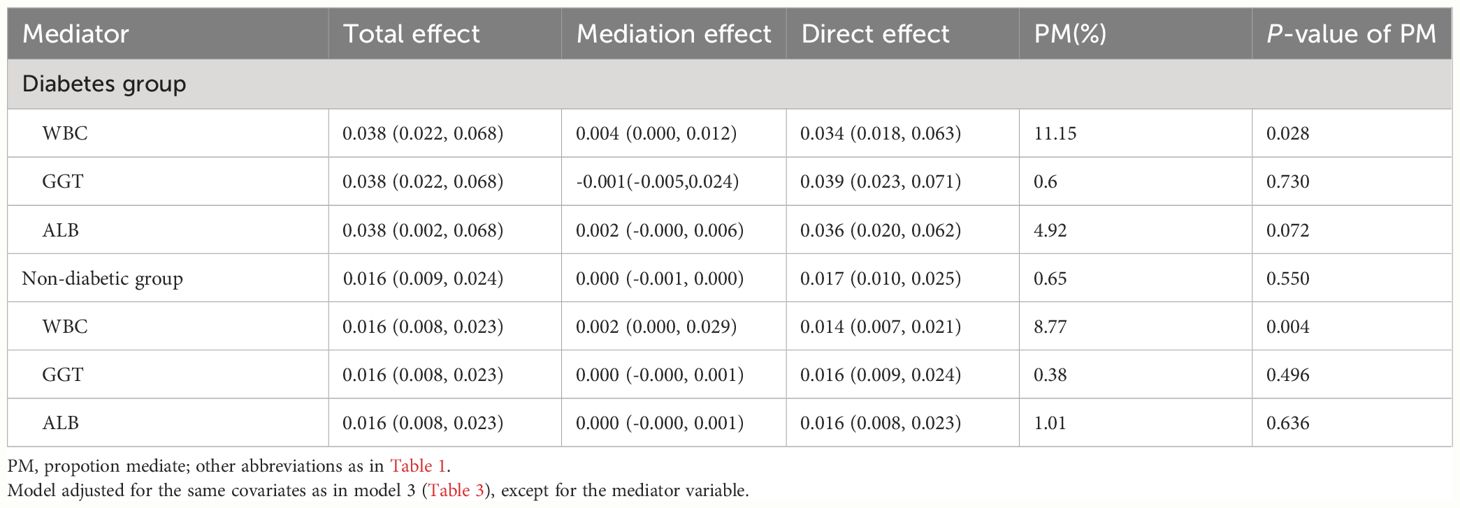
Table 6 Mediated analysis was performed to explore the roles of inflammation, oxidative stress and nutritional pathways in the association between ABG and the 30-day mortality rate in ADHF patients.
Discussion
In this study, using the JX-ADHF1 study cohort, we discovered that ABG was an independent predictor of 30-day mortality in Chinese patients with ADHF. ABG was particularly applicable for assessing 30-day mortality risk and predicting death events in ADHF patients with diabetes, more so than in those without.
Elevated blood glucose levels are very common in patients presenting with acute symptoms (7, 16, 17), and this stress response adversely impacts various medical conditions, including AHF (16), acute coronary syndromes (9, 34), acute ischemic stroke (35), acute pancreatitis (36), sepsis (13, 37), and various critical illnesses (8, 38). In the context of AHF, the relationship between ABG and 30-day mortality remains controversial, with contradictory findings in completed studies. For instance, studies by Professor Mebazaa A and Professor Sud M in independent European and American cohorts indicated that ABG was independently related to 30-day mortality in AHF patients (17, 18). Conversely, research by Professor Kosiborod M and Professor Cox ZL in independent American elderly cohorts suggested no correlation between ABG and 30-day mortality in AHF patients (16, 39). The studies by Professors Mebazaa and Sud included a broader range of ages and samples from multiple countries in Europe and America, potentially offering greater representativeness in their findings. In our current study, based on the adult ADHF cohort from Jiangxi, China, our evidence supported a correlation between ABG and 30-day mortality in ADHF patients, with no significant differences observed in the age subgroup analysis between the elderly and other age groups. Furthermore, we assessed this association in diabetic and non-diabetic populations, finding that, after adjusting for confounding factors, ADHF patients with diabetes had a relatively higher 30-day mortality risk compared to those without (HR: 1.65 vs 1.37). Notably, the unadjusted model indicated a higher correlation between ABG and 30-day mortality in non-diabetic ADHF patients, suggesting that in patients with a known diagnosis of diabetes, hyperglycemia might receive better monitoring and treatment from the onset of hospital admission.
The predictive value of ABG for 30-day mortality in AHF patients has been reported in previous studies, such as that by Professor Mebazaa A (17), but there remains a lack of sufficient evidence to clearly establish ABG’s utility in the short-term prognosis of ADHF patients. Retrospective studies have provided evidence of ABG’s predictive value for short-term adverse outcomes in other diseases. As summarized in Supplementary Table 3, in the context of severe trauma, studies from Germany and Switzerland have shown ABG’s utility in predicting in-hospital shock (AUC=0.62) and in-hospital mortality (AUC=0.788), respectively (40, 41). Furthermore, in predicting in-hospital major adverse events for acute and critical illnesses, AUC values for myocardial infarction (42), spontaneous intracerebral hemorrhage (43), acute stroke (44), and intensive care unit admissions (45) hover around 0.7. These findings suggest that ABG is a valuable predictor of short-term adverse outcomes in critical illnesses; however, it is important to note the need for stratification by diabetes, given the general disparity in ABG levels between diabetic and non-diabetic patients (7, 16, 17). In our study, to clarify ABG’s predictive value for 30-day mortality in ADHF patients, we conducted ROC analysis after stratifying by diabetes status. Our results showed that ABG’s AUC for predicting 30-day mortality was 0.8751 in diabetic ADHF patients and 0.7124 in non-diabetics, indicating higher accuracy in diabetic patients.
After establishing ABG’s predictive value and risk assessment capability for 30-day mortality in ADHF patients, it is crucial to estimate clinically applicable threshold values. In a previous study by Mebazaa A, the ABG threshold for estimating 30-day mortality in non-diabetic ADHF patients was found to be 7 mmol/L, and 10 mmol/L in diabetics (17). In our study, through RCS, we evaluated the dose-response relationship curve between ABG and 30-day mortality in ADHF patients, revealing a U-shaped association in non-diabetics, with the lowest risk around 5-7 mmol/L. We observed a linear positive relationship in diabetics without a significant threshold or saturation effect point. ROC analysis further identified predictive thresholds for 30-day mortality, which were 7.05 mmol/L for non-diabetics and 13.95 mmol/L for diabetics, aligning with Mebazaa A’s findings and suggesting higher thresholds in diabetic patients (17). Additionally, considering ABG’s predictive thresholds in other diseases (Supplementary Table 3) (40–46), we recommend closer monitoring and intensified endocrine treatment for critical illness patients with ABG exceeding 10 mmol/L (7, 47–49). For ADHF patients in China, those with diabetes showing ABG levels above 13.95 mmol/l and those without diabetes exceeding 7.05 mmol/l should be closely monitored for potential short-term severe adverse outcomes. Strengthening surveillance and optimizing glycemic management strategies are advised in these scenarios.
Our findings broadly confirmed that ABG can serve as a risk biomarker for 30-day mortality in ADHF patients. However, it cannot be excluded that stress hyperglycemia acts as a mediator to produce adverse effects by mediating other important pathways, because stress hyperglycemia occurs through highly complex interactions of regulatory hormones such as cytokines, cortisol, growth hormone, and catecholamines (50–52). The exact mechanisms of interaction between ABG and adverse outcomes remain unclear; however, stress hyperglycemia exacerbating inflammation, cytokine, and oxidative stress responses, potentially forming a vicious cycle leading to further hyperglycemia, should be noted (53–55). This cycle might be a key factor in the poor short-term prognosis of high-ABG ADHF patients. From a cardiac perspective, high glucose levels directly promote myocardial cell calcium metabolic disorder, increased NF-κB levels, and upregulation of matrix metalloproteinases, leading to apoptosis and progressive remodeling (17, 56). High glucose levels also might cause abnormal increases in circulating free fatty acid concentrations, raising the risk of arrhythmias (57). Additionally, our study assessed the mediating roles of inflammation (27, 53, 54), oxidative stress (28, 53, 54), and nutritional pathways (29). We found that only inflammation significantly mediated ABG-related 30-day mortality in ADHF patients, accounting for 11.15% in diabetics and 8.77% in non-diabetics. This highlights the critical assessment value of inflammation in acute exacerbation diseases, where inflammation not only independently affects adverse outcomes in ADHF patients but also indirectly exacerbates these outcomes through ABG (58).
The short-term adverse events in patients with ADHF have always been among the most challenging issues in clinical settings, as hospitalization for ADHF itself signifies a poor prognosis (1, 2). In the present study, we validated the prognostic value of a simple yet effective marker, the ABG, for predicting short-term adverse events in patients with ADHF. It is important to mention that in addition to the high predictive power of ABG for poor prognosis in patients with ADHF, the measurement of this index is quite simple and is routinely performed in both outpatient and inpatient clinical settings. Hence, ABG emerges as a promising tool for risk stratification and prognostic assessment in ADHF patients, offering valuable additional information to clinicians. It is crucial to emphasize that, although this study demonstrated the value of ABG in predicting the short-term mortality of ADHF patients, ABG should not be used in isolation in the clinical treatment decision-making process. It should always be integrated with the clinical judgment of professional physicians, adopting a comprehensive approach that primarily guides the use of validated risk assessment tools in the clinical decision-making process, guided by the personal professional knowledge of clinicians. The rational and correct application of risk assessment tools will aid clinicians in identifying the most appropriate management pathways, thereby promoting effective and safe clinical decision-making (59, 60). Furthermore, as glucose markers in patients may change during treatment, close monitoring and frequent reassessment are essential for subsequent clinical decisions to ensure that the patient’s condition is moving in the right direction (61). It should also be noted that the application of ABG in prognostic evaluation and risk stratification should be accompanied by efforts to identify the triggers of ADHF episodes, as recognizing these triggers occupies a central place in the management and disposition of ADHF. Clinical practitioners should always consider and fully address all factors that could potentially worsen the patient’s condition. Finally, in conjunction with the findings of the current study, for future research we suggest focusing on stress-related parameters in acute exacerbation diseases and testing the joint role of combined ABG and inflammatory factors.
Strengths and limitations
The main strength of this study lies in its novel revelation of the relationship between ABG and short-term adverse outcomes in Chinese ADHF patients, with comprehensive analysis stratified by diabetes status. This study is the first to identify a U-shaped association between ABG and 30-day mortality in non-diabetic ADHF patients and to determine the ABG threshold for ADHF patients with and without diabetes. Additionally, it’s the first to identify the significant role of the inflammation pathway in ABG-related 30-day mortality in ADHF patients through mediation analysis. Overall, our study fills a gap in the understanding of the relationship between ABG and adverse outcomes in ADHF patients in the Chinese population and significantly expands the understanding of stress hyperglycemia in predicting adverse outcomes, particularly with regard to diabetes stratification.
However, there are limitations to this study: (i) The lack of continuous blood glucose monitoring data during the hospitalization of ADHF patients, which might provide more advantageous risk stratification information compared to ABG. (ii) Another limitation, due to the retrospective and non-interventional nature of the study, is the inability to obtain blood glucose information of the subjects at the end of the 30-day follow-up. (iii) Our study population mainly comes from Jiangxi, China, and caution is advised when other ethnic groups or other regions in China consider our findings. (iv) Due to the retrospective design, we couldn’t fully account for all disease-related measurement information; hence, despite significant efforts to control confounding factors, some residual confounding is inevitable (62). As a supplementary explanatory approach, we further calculated the E-value based on Model 3 results (26), suggesting relative stability of our findings as the high estimate HRs of 2.92 and 1.99 for 30-day mortality are unlikely to be achieved by most confounders. (v) Due to the retrospective design and non-interventional principle, we couldn’t quantitatively analyze the impact of endocrine and anti-inflammatory treatments on the outcomes in ADHF patients after admission. (vi) Finally, it is also important to mention that retrospective designs rely on pre-existing data are more susceptible to a variety of biases, including selection bias and information bias, which limits the scope and depth of the study.
Conclusion
Our study confirms ABG as an independent predictor of 30-day mortality in ADHF patients. Compared to non-diabetic subjects, ABG may be more suitable for assessing 30-day mortality risk and predicting death events in ADHF patients with diabetes. For ADHF patients with/without diabetes, our evidence suggests that when ABG exceeds 13.95/7.05 mmol/l, respectively, physicians should be alert and closely monitor any changes in patient conditions. Prompt adjustments to existing treatment plans, particularly in endocrine therapy and anti-inflammatory treatment, are recommended.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics review committee of Jiangxi Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JH: Formal analysis, Investigation, Validation, Writing – original draft. HY: Formal analysis, Investigation, Validation, Writing – original draft. MY: Formal analysis, Investigation, Software, Validation, Writing – original draft. CY: Investigation, Writing – review & editing. JQ: Investigation, Writing – review & editing. GX: Data curation, Investigation, Software, Writing – review & editing. GS: Data curation, Investigation, Writing – review & editing. MK: Formal analysis, Investigation, Software, Validation, Writing – original draft. YZ: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Jiangxi Province (20232BAB216004 and 20224ACB206001), the National Natural Science Foundation of China (82160095) and the Jiangxi Province Traditional Chinese Medicine Scienceand Technology Plan Project (2023B1218).
Acknowledgments
We would like to thank Jiangxi Provincial People’s Hospital for its strong support to the research project and the members of the JX-ADHF1 research team for their great efforts in the data collection process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1403452/full#supplementary-material
References
1. Lala A, Hamo CE, Bozkurt B, Fiuzat M, Blumer V, Bukhoff D, et al. Standardized definitions for evaluation of acute decompensated heart failure therapies: HF-ARC expert panel paper. JACC Heart Fail. (2024) 12:1–15. doi: 10.1016/j.jchf.2023.09.030
2. Lepage S. Acute decompensated heart failure. Can J Cardiol. (2008) 24 Suppl B:6B–8B. doi: 10.1016/S0828-282X(08)71022-5
3. Popa IP, Haba MŞC, Mărănducă MA, Tănase DM, Şerban DN, Şerban LI, et al. Modern approaches for the treatment of heart failure: recent advances and future perspectives. Pharmaceutics. (2022) 14:1964. doi: 10.3390/pharmaceutics14091964
4. Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure–associated hospitalizations in the United States. J Am Coll Cardiol. (2013) 61:1259–67. doi: 10.1016/j.jacc.2012.12.038
5. Lagu T, Pekow PS, Shieh MS, Stefan M, Pack QR, Kashef MA, et al. Validation and comparison of seven mortality prediction models for hospitalized patients with acute decompensated heart failure. Circ Heart Fail. (2016) 9:e002912. doi: 10.1161/CIRCHEARTFAILURE.115.002912
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. (2009) 360:1418–28. doi: 10.1056/NEJMsa0803563
7. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. (2009) 373:1798–807. doi: 10.1016/S0140-6736(09)60553-5
8. Liu S, Zhang B, You J, Chen L, Yuan H, Zhang S. Elevated fasting blood glucose at admission is associated with poor outcomes in patients with COVID-19. Diabetes Metab. (2021) 47:101189. doi: 10.1016/j.diabet.2020.08.004
9. Bauters C, Ennezat PV, Tricot O, Lauwerier B, Lallemant R, Saadouni H, et al. Stress hyperglycaemia is an independent predictor of left ventricular remodelling after first anterior myocardial infarction in non-diabetic patients. Eur Heart J. (2007) 28:546–52. doi: 10.1093/eurheartj/ehl546
10. Norris T, Razieh C, Yates T, Zaccardi F, Gillies CL, Chudasama YV, et al. Admission blood glucose level and its association with cardiovascular and renal complications in patients hospitalized with COVID-19. Diabetes Care. (2022) 45:1132–40. doi: 10.2337/dc21-1709
11. Fogelholm R, Murros K, Rissanen A, Avikainen S. Admission blood glucose and short term survival in primary intracerebral haemorrhage: a population based study. J Neurol Neurosurg Psychiatry. (2005) 76:349–53. doi: 10.1136/jnnp.2003.034819
12. Timóteo AT, Papoila AL, Rio P, Miranda F, Ferreira ML, Ferreira RC. Prognostic impact of admission blood glucose for all-cause mortality in patients with acute coronary syndromes: added value on top of GRACE risk score. Eur Heart J Acute Cardiovasc Care. (2014) 3:257–63. doi: 10.1177/2048872614528858
13. Marik PE, Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. (2004) 30:748–56. doi: 10.1007/s00134-004-2167-y
14. Kataja A, Tarvasmäki T, Lassus J, Cardoso J, Mebazaa A, Køber L, et al. The association of admission blood glucose level with the clinical picture and prognosis in cardiogenic shock - Results from the CardShock Study. Int J Cardiol. (2017) 226:48–52. doi: 10.1016/j.ijcard.2016.10.033
15. Cui K, Fu R, Yang J, Xu H, Yin D, Song W, et al. Admission blood glucose and 2-year mortality after acute myocardial infarction in patients with different glucose metabolism status: A prospective, nationwide, and multicenter registry. Front Endocrinol (Lausanne). (2022) 13:898384. doi: 10.3389/fendo.2022.898384
16. Kosiborod M, Inzucchi SE, Spertus JA, Wang Y, Masoudi FA, Havranek EP, et al. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation. (2009) 119:1899–907. doi: 10.1161/CIRCULATIONAHA.108.821843
17. Mebazaa A, Gayat E, Lassus J, Meas T, Mueller C, Maggioni A, et al. Association between elevated blood glucose and outcome in acute heart failure: results from an international observational cohort. J Am Coll Cardiol. (2013) 61:820–9. doi: 10.1016/j.jacc.2012.11.054
18. Sud M, Wang X, Austin PC, Lipscombe LL, Newton GE, Tu JV, et al. Presentation blood glucose and death, hospitalization, and future diabetes risk in patients with acute heart failure syndromes. Eur Heart J. (2015) 36:924–31. doi: 10.1093/eurheartj/ehu462
19. Chrysohoou C, Mantzouranis E, Dimitroglou Y, Mavroudis A, Tsioufis K. Fluid and salt balance and the role of nutrition in heart failure. Nutrients. (2022) 14:1386. doi: 10.3390/nu14071386
20. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
21. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. AHA/ACC/HFSA guideline for the management of heart failure: A report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79:e263–421. doi: 10.1016/j.jacc.2021.12.012
22. Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. (2019) 72:558–69. doi: 10.4097/kja.19087
23. Tariqujjaman M, Hasan MM, Mahfuz M, Hossain M, Ahmed T. Association between mother's education and infant and young child feeding practices in South Asia. Nutrients (2022) 14(7):1514. doi: 10.3390/nu14071514
24. Schoenfeld D. Partial residuals for the proportional hazards regression-model. Biometrika. (1982) 69:239–41. doi: 10.1093/biomet/69.1.239
25. Wax Y. Collinearity diagnosis for a relative risk regression analysis: an application to assessment of diet-cancer relationship in epidemiological studies. Stat Med. (1992) 11:1273–87. doi: 10.1002/sim.4780111003
26. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. (2017) 167:268–74. doi: 10.7326/M16-2607
27. Reina-Couto M, Pereira-Terra P, Quelhas-Santos J, Silva-Pereira C, Albino-Teixeira A, Sousa T. Inflammation in human heart failure: major mediators and therapeutic targets. Front Physiol. (2021) 12:746494. doi: 10.3389/fphys.2021.746494
28. Ceriello A. Acute hyperglycaemia and oxidative stress generation. Diabetes Med. (1997) 14 Suppl 3:S45–9. doi: 10.1002/(sici)1096-9136(199708)14:3+3.3.co;2-i
29. Driggin E, Cohen LP, Gallagher D, Karmally W, Maddox T, Hummel SL, et al. Nutrition assessment and dietary interventions in heart failure: JACC review topic of the week. J Am Coll Cardiol. (2022) 79:1623–35. doi: 10.1016/j.jacc.2022.02.025
30. Huang Q, Wan J, Nan W, Li S, He B, Peng Z. Association between manganese exposure in heavy metals mixtures and the prevalence of sarcopenia in US adults from NHANES 2011-2018. J Hazard Mater. (2024) 464:133005. doi: 10.1016/j.jhazmat.2023.133005
31. Lee DH, Blomhoff R, Jacobs DR Jr. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. (2004) 38:535–9. doi: 10.1080/10715760410001694026
32. Andrassy RJ, Durr ED. Albumin: use in nutrition and support. Nutr Clin Pract. (1988) 3:226–9. doi: 10.1177/0115426588003006226
33. Imai K, Keele L, Tingley D, Yamamoto T. Causal mediation analysis using R. Lecture Notes Stat. (2010) 196:129–54. doi: 10.1007/978-1-4419-1764-5_8
34. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. (2000) 355:773–8. doi: 10.1016/S0140-6736(99)08415-9
35. Ngiam JN, Cheong CWS, Leow AST, Wei YT, Thet JKX, Lee IYS, et al. Stress hyperglycaemia is associated with poor functional outcomes in patients with acute ischaemic stroke after intravenous thrombolysis. QJM. (2022) 115:7–11. doi: 10.1093/qjmed/hcaa253
36. Yang X, Shi N, Yao L, He W, Zhu P, Li S, et al. Impact of admission and early persistent stress hyperglycaemia on clinical outcomes in acute pancreatitis. Front Endocrinol (Lausanne). (2022) 13:998499. doi: 10.3389/fendo.2022.998499
37. Wei X, Min Y, Yu J, Wang Q, Wang H, Li S, et al. Admission blood glucose is associated with the 30-days mortality in septic patients: A retrospective cohort study. Front Med (Lausanne). (2021) 8:757061. doi: 10.3389/fmed.2021.757061
38. Lin S, He W, Zeng M. Association of diabetes and admission blood glucose levels with short-term outcomes in patients with critical illnesses. J Inflamm Res. (2020) 13:1151–66. doi: 10.2147/JIR.S287510
39. Cox ZL, Lai P, Lewis CM, Lindenfeld J. Change in admission blood glucose from chronic glycemic status in acute heart failure hospitalization and 30-day outcomes: A retrospective analysis. Int J Cardiol. (2020) 299:180–5. doi: 10.1016/j.ijcard.2019.07.069
40. Winkelmann M, Butz AL, Clausen JD, Blossey RD, Zeckey C, Weber-Spickschen S, et al. Admission blood glucose as a predictor of shock and mortality in multiply injured patients. SICOT J. (2019) 5:17. doi: 10.1051/sicotj/2019015
41. Kreutziger J, Wenzel V, Kurz A, Constantinescu MA. Admission blood glucose is an independent predictive factor for hospital mortality in polytraumatised patients. Intensive Care Med. (2009) 35:1234–9. doi: 10.1007/s00134-009-1446-z
42. Sanjuan R, Blasco ML, Martinez-Maicas H, Carbonell N, Miñana G, Nuñez J, et al. Acute myocardial infarction: high risk ventricular tachyarrhythmias and admission glucose level in patients with and without diabetes mellitus. Curr Diabetes Rev. (2011) 7:126–34. doi: 10.2174/157339911794940675
43. Kim Y, Han MH, Kim CH, Kim JM, Cheong JH, Ryu JI. Increased short-term mortality in patients with spontaneous intracerebral hemorrhage and its association with admission glucose levels and leukocytosis. World Neurosurg. (2017) 98:503–11. doi: 10.1016/j.wneu.2016.11.087
44. Nardi K, Milia P, Eusebi P, Paciaroni M, Caso V, Agnelli G. Predictive value of admission blood glucose level on short-term mortality in acute cerebral ischemia. J Diabetes Complications. (2012) 26:70–6. doi: 10.1016/j.jdiacomp.2012.03.001
45. Subramanian K, Radha D, Narayanan N, Natarajaboopathi R, Reddy KS, Shanagonda D, et al. Admission blood glucose level as a predictor of outcome in intensive care patients: A cross-sectional study. Cureus. (2022) 14:e32801. doi: 10.7759/cureus.32801
46. Alexiou GA, Lianos G, Fotakopoulos G, Michos E, Pachatouridis D, Voulgaris S. Admission glucose and coagulopathy occurrence in patients with traumatic brain injury. Brain Inj. (2014) 28:438–41. doi: 10.3109/02699052.2014.888769
47. van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. (2001) 345:1359–67. doi: 10.1056/NEJMoa011300
48. Kitabchi AE, Freire AX, Umpierrez GE. Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients. Metabolism. (2008) 57:116–20. doi: 10.1016/j.metabol.2007.08.014
49. Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. (2007) 13 Suppl 1:1–68. doi: 10.4158/EP.13.S1.1
50. Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, et al. Glucose metabolism and catecholamines. Crit Care Med. (2007) 35:S508–18. doi: 10.1097/01.CCM.0000278047.06965.20
51. Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond). (1999) 96:513–23. doi: 10.1042/cs0960513
52. Ishihara M, Kagawa E, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, et al. Impact of admission hyperglycemia and diabetes mellitus on short- and long-term mortality after acute myocardial infarction in the coronary intervention era. Am J Cardiol. (2007) 99:1674–9. doi: 10.1016/j.amjcard.2007.01.044
53. Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. (2004) 53:2079–86. doi: 10.2337/diabetes.53.8.2079
54. Yu WK, Li WQ, Li N, Li JS. Influence of acute hyperglycemia in human sepsis on inflammatory cytokine and counterregulatory hormone concentrations. World J Gastroenterol. (2003) 9:1824–7. doi: 10.3748/wjg.v9.i8.1824
55. Ling PR, Smith RJ, Bistrian BR. Hyperglycemia enhances the cytokine production and oxidative responses to a low but not high dose of endotoxin in rats. Crit Care Med. (2005) 33:1084–9. doi: 10.1097/01.CCM.0000163225.88827.63
56. Uemura S, Matsushita H, Li W, Glassford AJ, Asagami T, Lee KH, et al. Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ Res. (2001) 88:1291–8. doi: 10.1161/hh1201.092042
57. Coronel R, Wilms-Schopman FJ, Den Ruijter HM, Belterman CN, Schumacher CA, Opthof T, et al. Dietary n-3 fatty acids promote arrhythmias during acute regional myocardial ischemia in isolated pig hearts. Cardiovasc Res. (2007) 73:386–94. doi: 10.1016/j.cardiores.2006.10.006
58. Inan D, Erdogan A, Pay L, Genc D, Demırtola AI, Yıldız U, et al. The prognostic impact of inflammation in patients with decompensated acute heart failure, as assessed using the pan-immune inflammation value (PIV). Scand J Clin Lab Invest. (2023) 83:371–8. doi: 10.1080/00365513.2023.2233890
59. Adler ED, Voors AA, Klein L, Macheret F, Braun OO, Urey MA, et al. Improving risk prediction in heart failure using machine learning. Eur J Heart Fail. (2020) 22:139–47. doi: 10.1002/ejhf.1628
60. Kwon JM, Kim KH, Jeon KH, Lee SE, Lee HY, Cho HJ, et al. Artificial intelligence algorithm for predicting mortality of patients with acute heart failure. PloS One. (2019) 14:e0219302. doi: 10.1371/journal.pone.0219302
61. Peacock WF, Fonarow GC, Ander DS, Collins SP, Gheorghiade M, Kirk JD, et al. Society of Chest Pain Centers recommendations for the evaluation and management of the observation stay acute heart failure patient-parts 1-6. Acute Card Care. (2009) 11:3–42. doi: 10.1080/02652040802688690
Keywords: admission blood glucose, acute decompensated heart failure, diabetes, heart failure, poor prognoses
Citation: Hu J, Yang H, Yu M, Yu C, Qiu J, Xie G, Sheng G, Kuang M and Zou Y (2024) Admission blood glucose and 30-day mortality in patients with acute decompensated heart failure: prognostic significance in individuals with and without diabetes. Front. Endocrinol. 15:1403452. doi: 10.3389/fendo.2024.1403452
Received: 19 March 2024; Accepted: 24 June 2024;
Published: 05 July 2024.
Edited by:
Amirmohammad Khalaji, Tehran University of Medical Sciences, IranReviewed by:
Amir Hossein Behnoush, Tehran University of Medical Sciences, IranPietro Scicchitano, ASLBari - Azienda Sanitaria Localedella provincia di Bari (ASL BA), Italy
Copyright © 2024 Hu, Yang, Yu, Yu, Qiu, Xie, Sheng, Kuang and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Zou, anh5eHl6eUAxNjMuY29t
 Jing Hu1
Jing Hu1 Changhui Yu
Changhui Yu Jiajun Qiu
Jiajun Qiu Guobo Xie
Guobo Xie Guotai Sheng
Guotai Sheng Maobin Kuang
Maobin Kuang Yang Zou
Yang Zou