
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 12 July 2024
Sec. Endocrinology of Aging
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1402551
This article is part of the Research Topic Hormones and Aging related diseases View all 9 articles
Objective: Observational studies have revealed a link between inflammatory bowel disease (IBD) and sarcopenia. However, it remains unclear whether this correlation between IBD and sarcopenia is causal.
Methods: The genetic instrumental variables (IVs) associated with IBD and sarcopenia-related traits were derived from publicly available genome-wide association studies. We employed a two-sample bidirectional Mendelian randomization (MR) method. we obtained genetic IVs for five phenotypes from 34,652 cases in IBD, 27,432 cases in ulcerative colitis (UC), 212356 cases in crohn’s disease (CD), 9336415 cases in low hand grip strength (LHGS), and 450243 cases in appendicular lean mass (ALM), respectively. The inverse variance weighting and other MR methods were used to explore the bidirectional causal relationship. Furthermore, we performed heterogeneity test, pleiotropy test, leave-one-out sensitivity test, and multivariate MR to evaluate the robustness of the results.
Results: The forward MR results showed that the UC (OR=0.994, 95% CI: 0.9876–0.9998, P = 0.044) and CD (OR=0.993, 95% CI: 0.988–0.998, P = 0.006) was negatively correlated with ALM. In the reverse MR analysis, we also found that LHGS was negatively correlated with the IBD (OR=0.76, 95% CI: 0.61–0.94, P = 0.012) and CD (OR=0.53, 95% CI: 0.40–0.70, P <0.001). Besides, genetically predicted higher ALM reduced IBD (OR=0.87, 95% CI: 0.79–0.95, P = 0.002), UC (OR=0.84, 95% CI: 0.76–0.93, P = 0.001), and CD (OR=0.87, 95% CI: 0.77–0.99, P = 0.029). However, the results of other MR Analyses were not statistically different.
Conclusions: We found genetically predicted UC and CD are causally associated with reduced ALM, and higher hand grip strength reduced IBD and CD risk, and higher ALM reduced IBDs risk. This MR study provides moderate evidence for a bidirectional causal relationship between IBD and sarcopenia.
According to the latest 2019 European Working Group on Sarcopenia in Older People (EWGSOP) and International Working Group on Sarcopenia (IWGS) (1), sarcopenia is a clinical syndrome characterized by the decline in the muscle mass and/or strength of the whole body, resulting in reduced physical function and quality of life, and heightened susceptibility to physical disability and mortality. It is estimated that sarcopenia affects approximately 10% to 16% of older individuals globally (2), and is linked to a range of unfavorable health consequences, including fractures, reduced function, and mortality (3). In addition to being common in the elderly, it can also develop in middle age (3) and is prevalent in certain high-risk groups, such as cancer patients (4), renal insufficiency (5), liver disease (6), and metabolic disorders (7). Therefore, identifying the causes of sarcopenia and associated risk factors is critical.
Inflammatory bowel disease (IBD) is a chronic, idiopathic inflammatory disorder affecting the gastrointestinal tract, encompassing two primary classifications: Crohn’s disease (CD) and ulcerative colitis (UC). CD has the potential to affect any segment of the gastrointestinal system, ranging from the oral cavity to the perianal region, while UC is distinguished by the presence of widespread and persistent inflammation in the colon, extending from the proximal portion of the rectum (8). The utilization of whole genome sequencing and other genetic research techniques has led to the identification of over 200 sites that are related with the risk of IBD (9, 10). Meanwhile, there has been a notable rise in the prevalence of IBDs in the newly industrialized nations of the twenty-first century (11). Although the precise origins of these events remain uncertain, it can be attributed to a multifaceted interaction between hereditary factors and environmental influences (12, 13). Several recent observational studies have examined the correlation between IBD and sarcopenia. Fatani et al. (14) found that one-fifth of IBD patients had sarcopenia, which was significantly associated with IBD treatment failure and postoperative complications. Nam et al. (15) demonstrated a significant prognostic value between sarcopenia and perianal Crohn’s disease treatment. Mulinacci et al. (16) also showed that the prevalence of sarcopenia in patients with IBD can be as high as 50%, and sarcopenia is associated with a number of adverse clinical outcomes, including higher morbidity, longer hospital stays, increased postoperative complications, and treatment failure. Considering that some patients are concerned that regular physical activity is associated with a potential increase in IBD activity (17), it is still controversial whether it is worthwhile to recommend exercise as an intervention for IBD-related diseases.
Nevertheless, it is unavoidable that observational studies would be subject to bias resulting from confounding effects and reverse causality. Hence, the potential causal connection between IBD and sarcopenia remains uncertain, highlighting the need for further research to confirm this association (18). Therefore, the aim of this study was to perform a two-sample Mendelian randomization (MR) Study design to investigate the potential bidirectional causality between genetically predicted IBD and sarcopenia by performing an updated genome-wide association studies (GWAS) meta-analysis of IBD and sarcopenia.
A two-sample MR analysis was used to evaluate bidirectional causation between IBD (including UC and CD subtypes) and sarcopenia-related trait, namely low hand-grip strength (LHGS) and appendicular lean mass (ALM). For this investigation, we chose phenotypic single-nucleotide polymorphisms (SNPs) as genetic instrumental variables (IVs). Meanwhile, the genetic IVs for phenotypes should satisfy the following three key assumptions (19) (Figure 1): 1. IVs should be substantially related to exposure; 2. IVs should be unaffected by any possible confounders that may influence the link between the exposure and outcome; 3. IVs should exclusively effect the outcome through exposure. In this work, ethical approval was deemed unnecessary as we used publicly accessible GWAS findings from the IIBDGC database and UK Biobank (20).
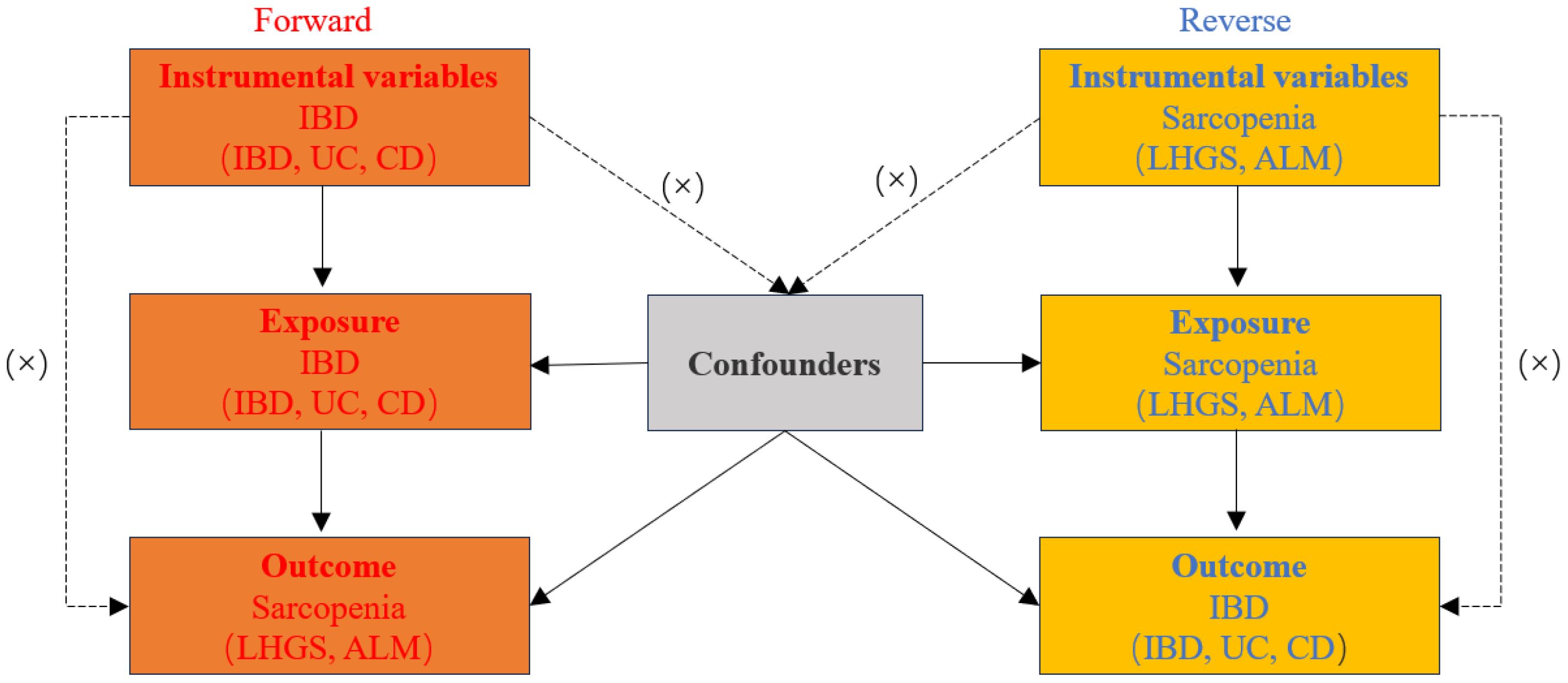
Figure 1 Diagram for three main assumptions of MR study. Lines with arrows indicate that the instrumental variables (IVs) are associated with the exposure and could only affect the outcome through the exposure. Dashed lines show that the IVs are independent of any confounding variables. IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, crohn’s disease; LHGS, low hand grip strength; ALM, appendicular lean mass.
Summary statistics for IBDs were extracted from the International Inflammatory Bowel Disease Genetics Consortium (IIBDGC). The study of IBDs involved 34,652 cases of European ancestry where the subgroup UC comprised 5,956 patients and 14,927 controls, while the CD subgroup contained 6,968 patients and 20,464 controls (10). According to European Crohn’s and Colitis Organisation, the diagnosis of CD involves a comprehensive evaluation including endoscopic, histopathological, and imaging findings to detect granulomatous inflammation and transmural lesions. The diagnosis of UC primarily relies on continuous colonic involvement and superficial inflammation, supported by histopathological findings, such as crypt architectural changes and mucosal inflammatory infiltrates (21). For IVs of sarcopenia, we selected the latest and largest available GWAS studies. The pooled data for LHGS obtained from a comprehensive meta-analysis GWAS that encompassed a substantial sample size of 256,523 individuals of European descent (22). The cut-off for LHGS was male <30 kg and female <20kg. Summary statistics for ALM were derived from 450,243 individuals in the UK Biobank study (23). Lean soft tissue mass was assessed using Dual-Energy X-ray Absorptiometry (DEXA). ALM was obtained by the sum of upper and lower limbs muscle mass. Exclusions for LHGS and ALM include pregnant women, people who are bedridden, amputees, and people who are unable to take anthropometry or body composition measurements (24).
The GWAS data utilized in this study were obtained exclusively from the IEU OpenGWAS online database, which provides extensive genetic data (ID: ieu-a-31for IBD, ieu-a-30 for CD, ieu-a-32 for UC, ebi-a-GCST90007526 for LHGS, and ebi-a-GCST90000025 for ALM) (Table 1). The utilization of data was authorized by the ethics committee of each participating center or nation involved, and all participants provided written, informed permission (10).
In this study, we identified single-nucleotide polymorphisms (SNPs) that were strongly associated with IBD, UC, CD, LHGS and ALM, with genome-wide significance of P < 5 × 10–8. To ensure independence, IVs were subjected to a PLINK clustering process. To eliminate SNPs associated with considerable linkage disequilibrium (LD), we employed a clustering method with r2 < 0.001 and a clumping distance of 10,000 kb. We then evaluated the strength of the IVs using a F statistic greater than 10 to reduce the influence of feeble IVs on the causal analysis. The F statistic is computed using the formula outlined in the prior literature (25). To avoid potential pleiotropy, IVs associated with confounding or risk factors for sarcopenia (older age, low socioeconomic status, low physical activity, and inadequate diet) were excluded using the PhenoScanner V2 (http://www.phenoscanner.medschl.cam.ac.uk/) (3).
Lastly, SNPs excluding confounding variables were utilized for subsequent MR analysis. These IVs are described in detail in Supplementary Tables 1–5, and all confounders in sarcopenia and excluded SNPs are listed in Supplementary Table 6.
In the MR analysis, multiple statistical methodologies were combined. The primary method was the inverse variance weighted (IVW), which is expected to be stable due to its balanced pleiotropy. For supplementary and substitution analysis, weighted mode, simple mode, weighted median, and MR Egger methods were utilized concurrently. Furthermore, the MR-PRESSO (MR pleiotropy residual sum and outlier) test was performed to correct for potential confounding variables (26).
Horizontal pleiotropy occurs when IVs associated with the exposure influence the outcome via multiple factors besides the exposure. We used the MR-Egger intercept test in MR-Egger regression to determine the presence of horizontal pleiotropy. A significant intercept (P < 0.05) indicates the presence of pleiotropy, and the results should be interpreted with caution. The scatter plots are utilized to display the results of the MR-Egger intercept test. We also used Cochran’s Q statistics to investigate heterogeneity, where significant heterogeneity (P < 0.05) indicates the presence of heterogeneity among the included SNPs, and the random effects MR analysis was used as the primary analysis technique. Moreover, funnel plots were used to visualize the heterogeneity results. We use the MR-PRESSO outlier test to remove aberrant SNPs (outliers) and estimate the corrected results in order to eliminate horizontal pleiotropy. In the leave-one-out analysis, results were reanalyzed after removing one SNP at a time, and forest plots were drawn to assess the stability of the results intuitively.
The “TwoSampleMR” and “MR-PRESSO” packages of the R software (version 4.2.2) were employed for estimating causal effects and identifying outliers. Results are presented as an odds ratio (OR) with a 95% confidence interval (CI). P < 0.05 is considered to have a significant difference.
As is shown in Figure 2, the result of the IVW method in MR analysis showed that no causal association was observed between IBD, UC, and CD negatively with LHGS (all P value> 0.05).
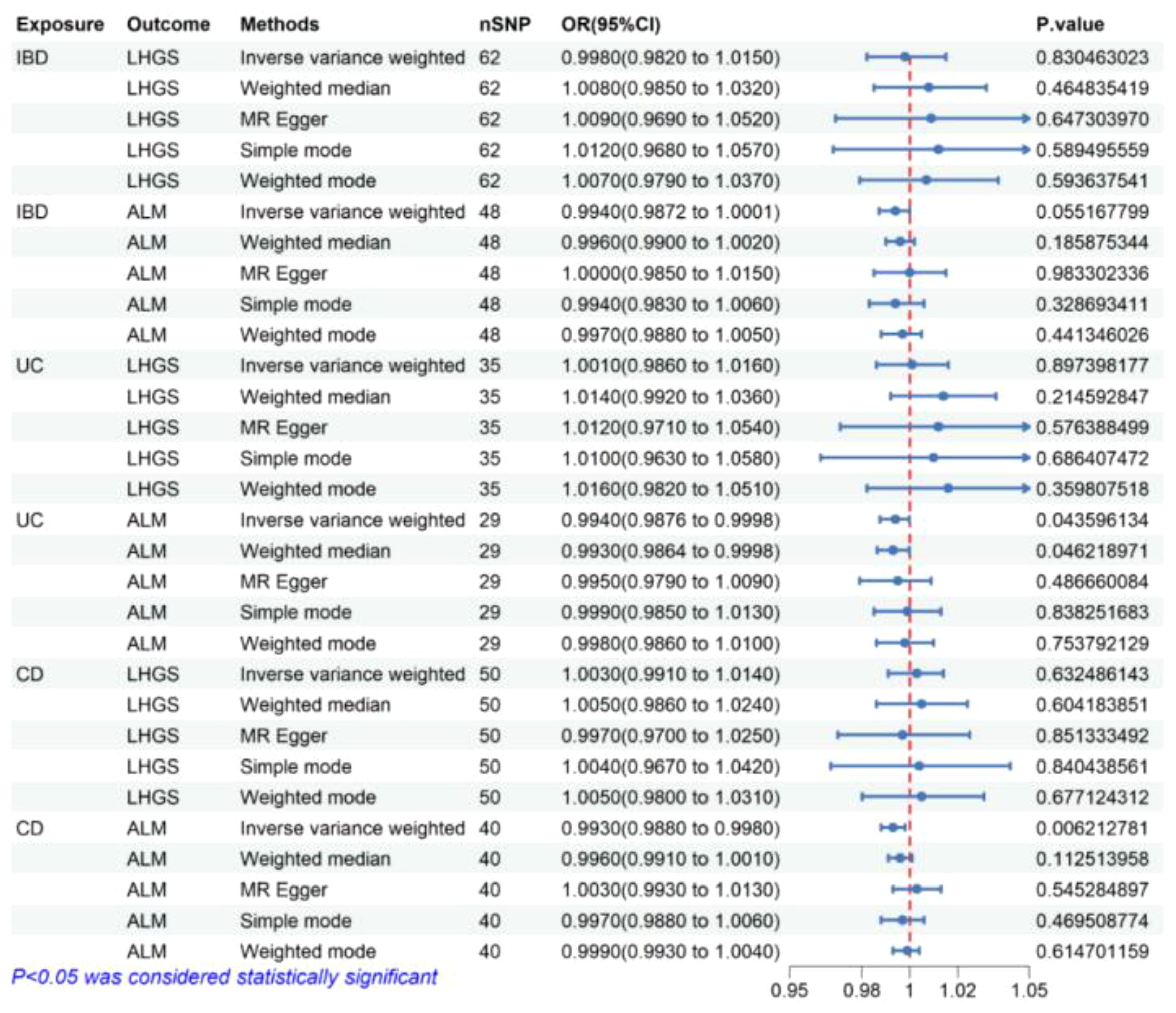
Figure 2 The risk association between IBD (including UC/CD) and sarcopenia-related traits in the validation set visualized in a forest plot. IBD, Inflammatory bowel disease; UC, ulcerative colitis; CD, crohn’s disease; LHGS, low hand-grip strength; ALM, appendicular lean mass; CI, confidence interval; MR Egger, Mendelian randomization-egger regression; nSNP, number of SNPs (instrumental variables); OR, odds ratio.
The result of the IVW method in MR analysis showed that genetically predicted UC (OR = 0.994, 95% CI 0.9876-0.9998, P = 0.044) and CD (OR = 0.993, 95% CI 0.988-0.998, P= 0.006) significantly negatively correlated with ALM. In the median weight model, causal effect of UC on ALM was consistent with the trend of the IVW model and reached statistical significance (OR = 0.993, 95% CI 0.9864-0.9998, P = 0.046), causal effect of CD on ALM showed a consistent direction but insignificant results (P> 0.05) (Figure 2).
According to the findings presented in Figure 3, the IVW method in reverse MR analysis revealed a significant negative association between the genetic predisposition to LHGS and the occurrence of IBD (OR = 0.76, 95% CI 0.61-0.94, P=0.012) as well as CD (OR = 0.76, 95% CI 0.61-0.94, P=0.012). Besides, weighted median method also showed that LHGS significantly negatively correlated with IBD (OR = 0.70, 95% CI 0.51-0.96, P=0.025) and CD (OR = 0.55, 95% CI 0.36-0.85, P=0.007).
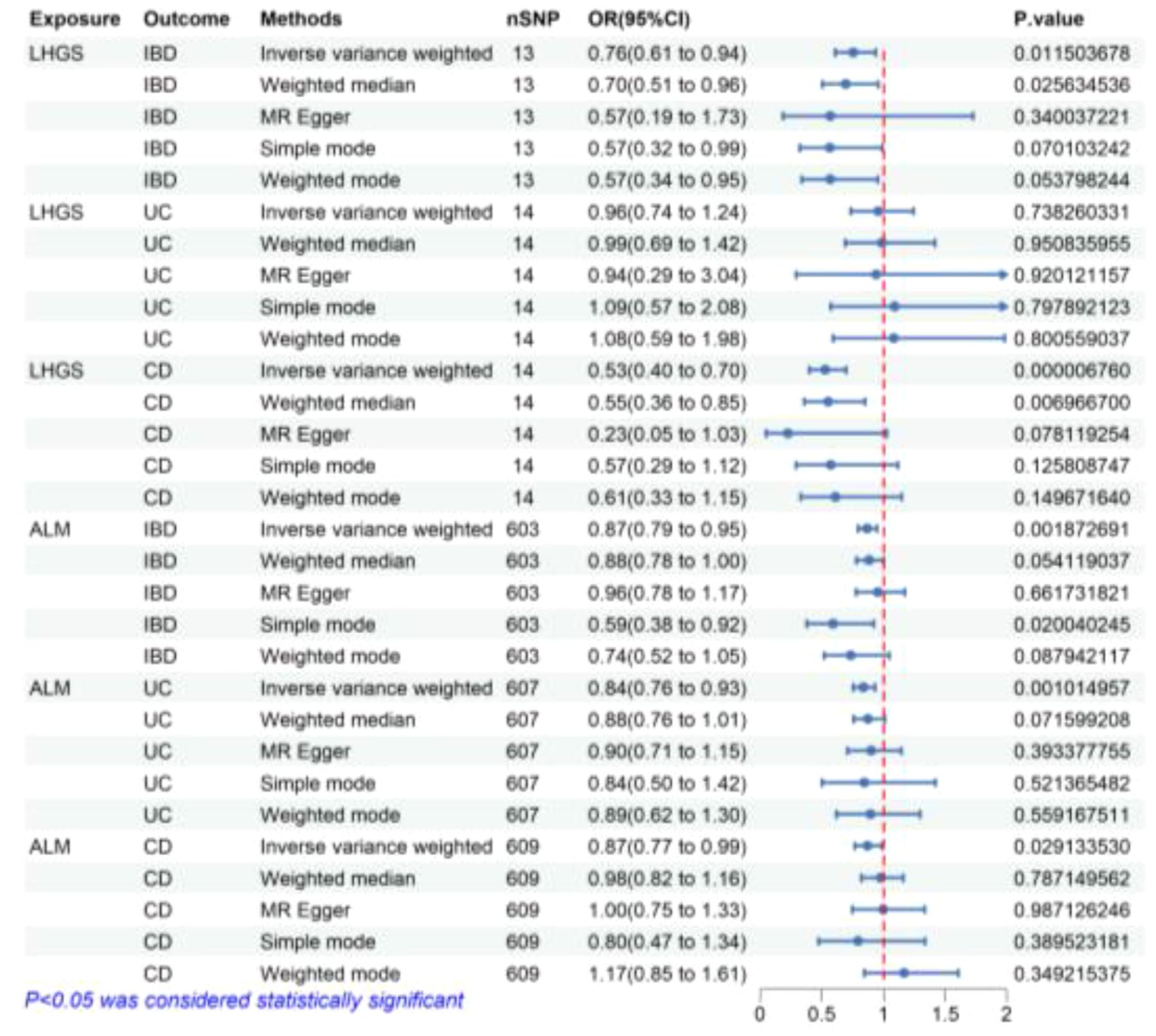
Figure 3 The risk association between sarcopenia-related traits and IBD (including UC/CD) in the validation set visualized in a forest plot. LHGS, low hand-grip strength; ALM, appendicular lean mass; IBD, Inflammatory bowel disease; UC, ulcerative colitis; CD, crohn’s disease; CI, confidence interval; MR Egger, Mendelian randomization-egger regression; nSNP, number of SNPs (instrumental variables); OR, odds ratio.
Similarly, the IVW method yielded findings that indicate a negative connection between ALM and IBD and IBD subtypes, including IBD (OR = 0.87, 95% CI 0.79-0.95, P=0.002), UC (OR = 0.84, 95% CI 0.76-0.93, P=0.001) and CD (OR = 0.87, 95% CI 0.77-0.99, P=0.029). Meanwhile, simple mode method also showed that ALM significantly negatively correlated with IBD (OR = 0.59, 95% CI 0.38-0.92, P=0.020) and CD (OR = 0.55, 95% CI 0.36-0.85, P=0.007). The MR estimates from remaining different methods of assessing the causal effect between ALM and IBD related diseases did not indicate a directly causal effect.
Following a sequence of sensitivity analyses, it was determined that the IVW results were stable after implementing the MR-PRESSO correction and weighted median method (Figures 2, 3). Cochrane’s Q and Q-derived p values were computed in order to evaluate the heterogeneity of our estimates. Although significant heterogeneity was detected in some of our results (Supplementary Figure 1), the importance of the IVW estimates remains after adjustment using a random effects model. Furthermore, in order to identify possible potential pleiotropic effects, we employed the p-value associated with the MR-Egger intercept. It is worth noting that only the causal impact of CD on ALM exhibits potential pleiotropy (P=0.029) (Supplementary Figure 2). Furthermore, we used the weighted mode, the simple mode, the MR-Egger, and the weighted median to evaluate the effects of genetically predicted exposures on outcomes, and the results were found to be relatively robust. In addition, the leave-one-out analysis revealed that the majority of results did not exhibit any significant alteration in the correlation when individual variation was removed (Supplementary Figure 3).
IBD is a chronic condition affecting the digestive system, characterized by immune-mediated inflammation of unknown origin. It is frequently accompanied by extraintestinal symptoms, including peripheral arthritis, oral aphthous ulcers, episcleritis, and erythema nodosum (27). The incidence of IBD is steadily growing, which will result in a larger population being susceptible to problems associated with the condition in the coming decade (28). To mitigate this risk and advance care, there exists a strong requirement for enhanced risk stratification instruments that can detect potentially modifiable characteristics and provide guidance on the appropriate timing and selection of treatment options. However, there are currently limited risk stratification tools for IBD, mainly because currently identified risks do not include indicators that reflect periods of persistent inflammation (29). Individuals with IBD frequently experience compromised nutritional status, which is evident by disturbances in energy or nutrient consumption. This impairment encompasses several forms of malnutrition, such as protein-energy malnutrition, disease-associated malnutrition, deficits in micronutrients, and sarcopenia (28).
Sarcopenia, a medical disorder characterized by the decline in muscular strength and mass, can be attributed to the presence of inflammation. Sarcopenia, commonly linked to the aging process, has been shown to start at an earlier stage in life in recent study. Furthermore, this condition has been found to have a significant correlation with heightened amounts of inflammatory cytokines circulating inside the body (1). Clinical researches have also shown a higher prevalence of sarcopenia in patients with inflammation (30). In addition, over the past few years, there has been increasing evidence that sarcopenia is strongly associated with adverse clinical outcomes, including abdominal surgery (31, 32). Nardone et al. (33) demonstrated that the implementation of suitable interventions for sarcopenia is crucial, alongside achieving clinical remission, in order to enhance clinical outcomes among patients diagnosed with IBD.
Nevertheless, data to assess both IBD as a risk stratification tool for sarcopenia and sarcopenia as a risk stratification tool for IBD are heterogeneous and scarce, which also leads to an unclear understanding of the interaction between IBD and sarcopenia (34). The current study by Campbell et al. found that 24% of patients with CD were identified as sarcopenia (35). Fatani et al. (14) also found that the overall prevalence of sarcopenia in IBD was noted to be 42%, with a higher prevalence in CD (57%). These prevalence rates were significantly higher as compared with patients without IBD. However, Lee et al. (36) demonstrated that sarcopenia was not statistically significant in the prognosis of CD. But unfortunately, given the retrospective nature of prior studies, observational studies or meta-analyses don’t have enough evidence to prove a causal relationship between IBD and sarcopenia (37).
In this study, we employed the largest available GWAS data from the IIBDGC large-scale and a sarcopenia meta-analysis to examine the potential bidirectional causal relationship between IBDs and sarcopenia-related traits susceptibility. According to our forward MR Analysis, both UC and CD were causally related with reduced ALM (OR=0.99, 95% CI: 0.99 - 1.00; OR=0.99, 95% CI: 0.99 - 1.00, respectively). Notable, reverse MR analysis also showed a causal association between LHGS and IBD (OR=0.76, 95% CI: 0.61- 0.94) or CD (OR=0.53, 95%CI: 0.40 - 0.70). Meantime, the increase in ALM was causally related with a reduced risk of IBD (OR=0.87, 95%CI: 0.79-0.95) and IBD subtype (OR=0.84, 95%CI: 0.76 - 0.93 for UC; OR=0.87, 95% CI: 0.77-0.99 for CD).
The primary finding of our MR study was a significant causal association between sarcopenia and IBDs. This result is similar to previous findings that sarcopenia is substantially associated with an increased risk of adverse outcomes for IBD such as treatment failure and postoperative complications (38–40). Although only UC and CD were causally associated with decreased ALM, it was not associated with decreased grip strength. However, it is important to note that persistent chronic inflammation and malnutrition collectively contribute to the susceptibility of individuals with IBD to the onset of sarcopenia (28). This also highlights the potential of UC and CD as risk stratification tools for sarcopenia.
As a study using SNP as a tool to explore the causal relationship between IBDs and sarcopenia. Our study offers several advantages. First, we showed that IBD was found to have a significant negatively effect on ALM and not causally associated with decreased grip strength. Second, the MR Study is regarded as a natural randomized controlled trial study, and its findings exhibit a higher level of robustness compared to earlier observational studies. Furthermore, we performed some sensitivity analyses to assess the robustness of the results. Finally, the research was constrained to a European demographic in order to mitigate any biases in the selection of the population, while also excluding confounding factors such as age, physical activity, socioeconomic status, and poor diet.
Despite the progress made in enhancing our present comprehension of the correlation between IBD and sarcopenia, the study still has some shortcomings. Firstly, although the fact that the F statistic suggests that the biases due to feeble IVs can be disregarded, the pathway from exposure to outcome is extremely complex, and some results are heterogeneous, so caution should be exercised when interpreting these results. Secondly, we only included LHGS and ALM in sarcopenia-related traits, and other important indicators such as walking speed were not included. Thirdly, all GWAS data in this study came primarily from European populations. While such measures can be effective in reducing bias from different populations, the study’s conclusions may not be directly transferable to other ethnic groups. Fourthly, the causal relationship analyzed by MR is derived from the genetic level, and the body contains a variety of complex biological pathways, so the potential causal relationship is not a definite causal relationship. Finally, the statistical efficacy of MR Analysis is relatively weak due to the limited sample size and number of SNP of IBD, and a larger and updated IBD database may be needed to confirm causality.
In conclusion, this study suggests that UC and CD disease are causally associated with reduced ALM. In turn, higher hand grip strength reduced the risk of IBD and CD, and higher ALM was associated with a reduced risk of IBD (including UC and CD subtypes). These findings suggest that intervention with sarcopenia may help prevent adverse outcomes in patients with IBD.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
All datasets provided in GWAS have been approved by the relevant ethics committees. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
ZS: Writing – original draft. GL: Writing – review & editing, Data curation, Conceptualization. JX: Writing – review & editing, Project administration, Data curation. XZ: Writing – review & editing, Supervision. HW: Writing – review & editing, Project administration. GW: Writing – original draft, Visualization. JJ: Writing – original draft, Visualization, Funding acquisition.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Science and Technology Plan Project of Jiangxi Provincial Administration of Traditional Chinese Medicine (2022B778).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1402551/full#supplementary-material
1. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:601. doi: 10.1093/ageing/afz046
2. Yuan S, Larsson SC. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism. (2023) 144:155533. doi: 10.1016/j.metabol.2023.155533
3. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
4. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. (2016) 57:58–67. doi: 10.1016/j.ejca.2015.12.030
5. Shu X, Lin T, Wang H, Zhao Y, Jiang T, Peng X, et al. Diagnosis, prevalence, and mortality of sarcopenia in dialysis patients: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:145–58. doi: 10.1002/jcsm.12890
6. Tantai X, Liu Y, Yeo YH, Praktiknjo M, Mauro E, Hamaguchi Y, et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol. (2022) 76:588–99. doi: 10.1016/j.jhep.2021.11.006
7. Feng L, Gao Q, Hu K, Wu M, Wang Z, Chen F, et al. Prevalence and risk factors of sarcopenia in patients with diabetes: A meta-analysis. J Clin Endocrinol Metab. (2022) 107:1470–83. doi: 10.1210/clinem/dgab884
8. Podolsky DK. Inflammatory bowel disease. N Engl J Med. (2002) 347:417–29. doi: 10.1056/NEJMra020831
9. Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. (2012) 491:119–24. doi: 10.1038/nature11582
10. Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. (2015) 47:979–86. doi: 10.1038/ng.3359
11. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. (2017) 390:2769–78. doi: 10.1016/S0140-6736(17)32448-0
12. Yao Z, Jiang F, Luo H, Zhou J, Shi W, Xu S, et al. Causal effects of blood lipid traits on inflammatory bowel diseases: A mendelian randomization study. Metabolites. (2023) 13. doi: 10.3390/metabo13060730
13. Jakobsen C, Cleynen I, Andersen PS, Vermeire S, Munkholm P, Paerregaard A, et al. Genetic susceptibility and genotype-phenotype association in 588 Danish children with inflammatory bowel disease. J Crohns Colitis. (2014) 8:678–85. doi: 10.1016/j.crohns.2013.12.010
14. Fatani H, Olaru A, Stevenson R, Alharazi W, Jafer A, Atherton P, et al. Systematic review of sarcopenia in inflammatory bowel disease. Clin Nutr. (2023) 42:1276–91. doi: 10.1016/j.clnu.2023.05.002
15. Nam K, Lee JY, Ko Y, Kim KW, Lee HS, Hong SW, et al. Impact of sarcopenia on clinical course of inflammatory bowel disease in Korea. Dig Dis Sci. (2023) 68:2165–79. doi: 10.1007/s10620-023-07838-z
16. Mulinacci G, Pirola L, Gandola D, Ippolito D, Viganò C, Laffusa A, et al. Ultrasound muscle assessment for sarcopenia detection in inflammatory bowel disease: A prospective study. United Eur Gastroenterol J. (2024) 12:562–73. doi: 10.1002/ueg2.12566
17. Gravina AG, Pellegrino R, Durante T, Palladino G, D'Onofrio R, Mammone S, et al. Inflammatory bowel diseases patients suffer from significant low levels and barriers to physical activity: The "BE-FIT-IBD" study. World J Gastroenterol. (2023) 29:5668–82. doi: 10.3748/wjg.v29.i41.5668
18. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070
19. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Bmj. (2018) 362:k601. doi: 10.1136/bmj.k601
20. Zou M, Zhang W, Shen L, Xu Y, Zhu Y. Causal association between inflammatory bowel disease and herpes virus infections: a two-sample bidirectional Mendelian randomization study. Front Immunol. (2023) 14:1203707. doi: 10.3389/fimmu.2023.1203707
21. Zhang L, Shen P, Ge W, Liao W, Luo Q, Li C, et al. Mediating role of chiro-inositol metabolites on the effects of HLA-DR-expressing CD14 + monocytes in inflammatory bowel disease. BMC Gastroenterol. (2024) 24:200. doi: 10.1186/s12876-024-03271-2
22. Jones G, Trajanoska K, Santanasto AJ, Stringa N, Kuo CL, Atkins JL, et al. Genome-wide meta-analysis of muscle weakness identifies 15 susceptibility loci in older men and women. Nat Commun. (2021) 12:654. doi: 10.1038/s41467-021-20918-w
23. Pei YF, Liu YZ, Yang XL, Zhang H, Feng GJ, Wei XT, et al. The genetic architecture of appendicular lean mass characterized by association analysis in the UK Biobank study. Commun Biol. (2020) 3:608. doi: 10.1038/s42003-020-01334-0
24. Nascimento GM, Longo GZ, Valmorbida A, Ferreira FG, Trindade E. Prevalence of body composition phenotypes and their associations with glycemic, lipidic, and inflammatory biomarkers: a population-based study. Cad Saude Publica. (2024) 40:e00109823. doi: 10.1590/0102-311xen109823
25. Zhao J, Li K, Liao X, Zhao Q. Causal associations between inflammatory bowel disease and primary biliary cholangitis: a two-sample bidirectional Mendelian randomization study. Sci Rep. (2023) 13:10950. doi: 10.1038/s41598-023-35785-2
26. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
27. Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal manifestations of inflammatory bowel disease: Current concepts, treatment, and implications for disease management. Gastroenterology. (2021) 161:1118–32. doi: 10.1053/j.gastro.2021.07.042
28. Massironi S, Viganò C, Palermo A, Pirola L, Mulinacci G, Allocca M, et al. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol Hepatol. (2023) 8:579–90. doi: 10.1016/S2468-1253(23)00011-0
29. McMahon KR, Allen KD, Afzali A, Husain S. Predicting post-operative complications in crohn's disease: an appraisal of clinical scoring systems and the NSQIP surgical risk calculator. J Gastrointest Surg. (2020) 24:88–97. doi: 10.1007/s11605-019-04348-0
30. An HJ, Tizaoui K, Terrazzino S, Cargnin S, Lee KH, Nam SW, et al. Sarcopenia in autoimmune and rheumatic diseases: A comprehensive review. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21165678
31. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. (2014) 69:547–58. doi: 10.1093/gerona/glu010
32. Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: A meta-analysis. Ann Surg. (2018) 268:58–69. doi: 10.1097/SLA.0000000000002679
33. Nardone OM, Armuzzi A. Sarcopenia: A new route on the map for risk stratification in inflammatory bowel disease. Dig Liver Dis. (2023) 55:829–30. doi: 10.1016/j.dld.2023.04.014
34. Faye AS, Dodson JA, Shaukat A. Sarcopenia as a risk prediction tool in inflammatory bowel disease. Inflammation Bowel Dis. (2022) 28:1932–3. doi: 10.1093/ibd/izac069
35. Campbell JP, Teigen L, Manski S, Blumhof B, Guglielmo FF, Shivashankar R, et al. Sarcopenia is more prevalent among inflammatory bowel disease patients undergoing surgery and predicts progression to surgery among medically treated patients. Inflammation Bowel Dis. (2022) 28:1844–50. doi: 10.1093/ibd/izac013
36. Lee CH, Yoon H, Oh DJ, Lee JM, Choi YJ, Shin CM, et al. The prevalence of sarcopenia and its effect on prognosis in patients with Crohn's disease. Intest Res. (2020) 18:79–84. doi: 10.5217/ir.2019.00107
37. Liu Y, Xu H, Zhao Z, Dong Y, Wang X, Niu J. No evidence for a causal link between Helicobacter pylori infection and nonalcoholic fatty liver disease: A bidirectional Mendelian randomization study. Front Microbiol. (2022) 13:1018322. doi: 10.3389/fmicb.2022.1018322
38. Ge X, Jiang L, Yu W, Wu Y, Liu W, Qi W, et al. The importance of sarcopenia as a prognostic predictor of the clinical course in acute severe ulcerative colitis patients. Dig Liver Dis. (2021) 53:965–71. doi: 10.1016/j.dld.2021.03.031
39. Ge X, Xia J, Wu Y, Ye L, Liu W, Qi W, et al. Sarcopenia assessed by computed tomography is associated with colectomy in patients with acute severe ulcerative colitis. Eur J Clin Nutr. (2022) 76:410–8. doi: 10.1038/s41430-021-00953-y
Keywords: inflammatory bowel disease, ulcerative colitis, Crohn’s disease, sarcopenia-related traits, Mendelian randomization
Citation: Sun Z, Liu G, Xu J, Zhang X, Wei H, Wu G and Jiang J (2024) The relationship between inflammatory bowel disease and sarcopenia-related traits: a bidirectional two-sample mendelian randomization study. Front. Endocrinol. 15:1402551. doi: 10.3389/fendo.2024.1402551
Received: 17 March 2024; Accepted: 01 July 2024;
Published: 12 July 2024.
Edited by:
Rossella Cannarella, Cleveland Clinic, United StatesReviewed by:
Raffaele Pellegrino, University of Campania Luigi Vanvitelli, ItalyCopyright © 2024 Sun, Liu, Xu, Zhang, Wei, Wu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Jiang, b3J0aG9wZWRpY3MwOTE1QDE2My5jb20=; Guobao Wu, NTcyNTkyNjcyQHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.