
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 25 June 2024
Sec. Thyroid Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1402380
This article is part of the Research Topic Recent Advances in Thermal and Nonthermal Ablative Technologies of the Thyroid View all 11 articles
Background: Radiofrequency ablation (RFA) is an alternative modality for thyroid nodules (TNs) and many studies have also confirmed its favorable efficacy and safety. The scope of RFA increases in clinical practice and the aim of our study was to evaluate the efficacy of RFA.
Methods: We conducted a prospective study to evaluate the efficacy of RFA for thyroid nodules between January 2017 and December 2022 at our institution. We assessed the change in nodal volume, volume reduction ratio (VRR), technique effective (TE) rate, complete ablation (CA) rate, and nodal regrowth rate and time after RFA.
Results: We performed RFA for 1703 patients with TNs between January 2017 and December 2022, of which a total of 970 eligible patients were enrolled in the study. The preoperative volume of TNs was 6.23 ± 8.11ml, with 821 benign and 149 malignant nodules. The post-RFA TE and adjusted TE rate were 80% and 88.8%, respectively. CA was achieved in 145 (14.9%) patients with a mean time of 18.32± 12.98 months; nodal regrowth occurred in 15 (1.5%) patients with a mean time of 29.80 ± 12.47 months. TNs volume and VRR changed significantly at years 1 and 2 after RFA and stabilized after 5 years. A serious postoperative adverse event occurred in one patient with cervical sympathetic chain injury resulting in Horner’s syndrome. A transient or permanent damage of the recurrent laryngeal nerve could not be evaluated due to the lack of postoperative laryngoscopy, and this is a significant limitation of the study.
Conclusion: The expanded RFA indications were also effective for TNs, with no significant change in long-term efficacy.
Thyroid nodules (TNs) are very common in the general population up to 68% (1) and their incidence is also gradually increasing attributed to incidental nodule findings. It includes both benign and malignant nodules, however, benign nodules are more than 90%. Most of these benign nodules are clinically asymptomatic requiring only follow-up observation (2), and treatment is indicated for those with symptoms of compression (including important structures such as the trachea, recurrent laryngeal nerve, esophagus, etc.) or aesthetic concerns. More than 90% of malignant nodules are papillary thyroid carcinomas, which are indolent tumors. Surgery is the standard treatment for thyroid nodules; however, it leads to scar formation and, there are adverse events such as recurrent laryngeal nerve, hypothyroidism, and hypoparathyroidism after surgery (3, 4). Long-term hormone replacement therapy may be required for postoperative hypothyroidism, which may have adverse effects on the bone and cardiac system (5). Moreover, thyroid nodules are becoming more common among young people; therefore, more and more patients prefer minimally invasive treatments without surgical scars. Thermal ablation has emerged as an alternative minimally invasive treatment for thyroid nodules, including radiofrequency ablation (RFA), laser ablation (LA), microwave ablation (MA), and high-frequency focused ultrasound (HIFU).
RFA is the most commonly used thermal ablation technique, and many previous studies have confirmed that RFA has no significant difference in efficacy compared with surgery (6, 7). However, the risk of adverse events such as postoperative voice changes, hypothyroidism, and hypoparathyroid function was significantly lower (6–9). Currently, the indications for RFA in multiple guidelines are benign thyroid nodules, thyroid micropapillary carcinoma, recurrent lymph nodes in the neck, those who refuse thyroid surgery, or those who are ineligible for surgery due to systemic disease (5, 10, 11). However, due to the indolent characteristics of malignant nodules and aesthetic concerns, many studies have attempted to perform RFA for low-risk papillary thyroid carcinoma (12, 13). Moreover, the RFA threshold for benign nodules has not been clearly defined, and many studies have also applied RFA to larger nodules, like Deandrea M et al. for volumes >20 ml (14). Many previous studies have evaluated RFA separately for benign and malignant nodules (4, 11–14) and our study evaluated its efficacy for both. At the same time, these studies had small sample sizes, and we conducted a 6-year continuous prospective study with a larger sample size.
The aim of our study was to conduct a large sample size, consecutive prospective study to evaluate the efficacy of RFA for thyroid nodules, both benign and malignant.
Our study was a single-center prospective study, which was approved by our Institutional Ethics Committee (No. 2022ZSY-LLK-456), and all subjects obtained informed consent prior to surgery. Patients with TNs who underwent RFA at Zhongshan Hospital of Traditional Chinese Medicine between January 2017 and December 2022 were enrolled. All thyroid nodules were evaluated for malignant risk by experienced sonographers based on the ACR TI-RADS grading system (15) prior to RFA. All patients also underwent fine-needle aspiration biopsy (FNA) of the TNs to clarify their nature prior to RFA.
The inclusion criteria for eligible patients were as follows: 1) RFA performed at our institution between January 2017 and December 2022; 2) TNs with TI-RADS grading; and 3) TNs with definitive FNA pathology results, including benign nodules and malignant nodules (papillary thyroid carcinoma). The exclusion criteria were as follows: 1) those who refused to participate in this study; 2) patients who were lost to follow-up; 3) incomplete data; and 4) those who received other RF treatments before RFA (LA, MA, HIFU).
All patients completed routine blood, biochemical tests, coagulation, thyroid function, electrocardiogram (ECG), and chest X-ray to exclude contraindications before RFA. The demographic characteristics of the patients included age, gender, and history of previous surgeries and diseases, especially thyroid surgery and treatment. All thyroid nodules were scored and risk stratified according to the ACR TI-RADS grading system.
After RFA, ultrasound(US) was performed at months 1, 3, 6, 12, and every 6 or 12 months thereafter. Thyroid function was assessed again 1 month after RFA. We followed up to assess whether there were events requiring emergency surgery or prolonged hospitalization after RFA.
All RFAs were performed by experienced surgeons and sonographers in an outpatient setting applying a bipolar RFA generator and an 18-gauge bipolar radiofrequency electrode with a 0.9 cm active tip (CelonProSurge, Olympus Surgical Technologies, Germany). The patient was placed in the supine position with full neck extension, and local anesthesia with lidocaine was applied for pain control. The RFA was performed under real-time ultrasound guidance with hydro dissection, trans-isthmic approach, and the moving shot technique (16). Appropriate length electrodes were selected for the RFA procedure based on the size and location of the TNs, and contrast-enhanced ultrasonography (CEUS) was performed before and after RFA. During the RFA procedure, we assessed the patients for changes in voice, dyspnea, and other discomforts, and discharged them after 12 hours of postoperative observation without significant discomfort.
The variables in this study were general demographic characteristics and post-RFA efficacy indicators. Demographic characteristics included age, gender, underlying disease, thyroid-related disease and their treatment history. Post-RFA indicators included TNs volume, volume reduction ratio (VRR), technical effectiveness (TE), TNs regrowth, new onset, period of stabilization of TNs after RFA and re-intervention rate.
The three-dimensional size of the TNs was measured in ultrasound and its volume was calculated: Volume equation=[length(sagittal,cm)×depth(anteroposterior, cm)×width(transverse, cm)]×0.524. Volume Reduction Rate (VRR) is an important indicator for assessing the effectiveness of RFA and is calculated as follows:VRR = [(Initial Volume - Final Volume)×100%]/Initial Volume. Technical effectiveness (TE) was a >50% reduction in TNs volume at 12 months after RFA; TNs regeneration was defined as a 50% increase in total volume over the previous minimum volume (17).
In our study, continuous variables were described by mean ± standard deviation (SD) and statistically analyzed by Students t test or Mann Whitney U test according to their distribution. Categorical variables are expressed in frequency (percentage), and statistical analysis is performed using Chi-square tests or Fisher exact tests when appropriate. We used multivariate logistic regression analysis to find the factors that affected the RFA effect, expressed by the adjusted Odds ratio (OR) and 95% confidence interval (CI). P < 0.05 was considered statistically significant. All data were analyzed using SPSS 25.0 version.
The flow of our study was shown in Figure 1. A total of 1703 patients with TNs underwent RFA between January 2017 and December 2022. After excluding 733 ineligible patients, a total of 970 eligible patients were included in our study. The demographic characteristics of all eligible patients are shown in Table 1. There were 803 females and 167 males, with minimum and maximum ages of 5 and 78 years, and a mean age of 43.63 ± 12.36 years. Of these patients, 654 (67.4%) had multiple TNs, and, 201 (20.7%) of those with multiple nodes underwent multiple node ablation treatments during a single RFA. 18 (1.9%) patients had previous RFA and 15 (1.5%) patients were postoperative nodules of residual glands for RFA. Preoperative FNA in RFA for TNs has confirmed 821 (84.6%) as benign and 149 (15.4%) as malignant.
The post-RFA follow-up and efficacy assessments were shown in Table 2. Of the 970 patients who were eligible for consecutive follow-up, the mean follow-up time was 17.60 ± 13.66 months, with the shortest and longest follow-up times being 1 month and 66 months, respectively. Technically effective (TE) was achieved in 776 patients after RFA, and their overall TE rate was 80%. However, because some patients were followed up for less than 12 months, their adjusted TE rate was 88.8% (776/874) after excluding the 96 unsuccessful TEs in these patients. Of these 970 patients, 145 patients achieved complete ablation (CA) with a CA rate of 14.9%, and the mean time to achieve CA was 18.32 ± 12.98 months. Regrowth of TNs occurred in 15 patients with a rate of 1.5% and its mean regrowth time was 29.80 ± 12.47 months.
The changes in the volume of TNs after RFA were shown in Table 3; Figures 2A, B. The mean volume of TNs before RFA was 6.23 ± 8.11 ml. There was a gradual decrease in the volume of TNs after RFA, which was most significant at months 3 (2.60 ± 3.06 ml, p < 0.001 < 0.05) and months 12 (1.60 ± 2.30 ml, p = 0.004 < 0.05). The volume reduction rate (VRR) of TNs did not decrease at month 3 after RFA; on the contrary, an increase occurred (-70.36 ± 402.64%). The VRR gradually increased after months 3 and was most significant at postoperative 1 year (58.18 ± 76.86%, p < 0.001 < 0.05) and 2 years (68.98 ± 59.91%, p = 0.019 < 0.05). The complete ablation and re-growth rates of TNs after RFA were shown in Table 4; Figure 2C. The CR rates were 7.11%, 11.13%, 13.51%, 14.54%, 14.85%, and 14.95% at 1, 2, 3, 4, 5, and 6 years after RFA, respectively. Re-growth rates at 1, 2, 3, 4, 5, and 6 years after RFA were 0.21%, 0.52%, 1.03%, 1.44%, 1.55%, and 1.55%, respectively.
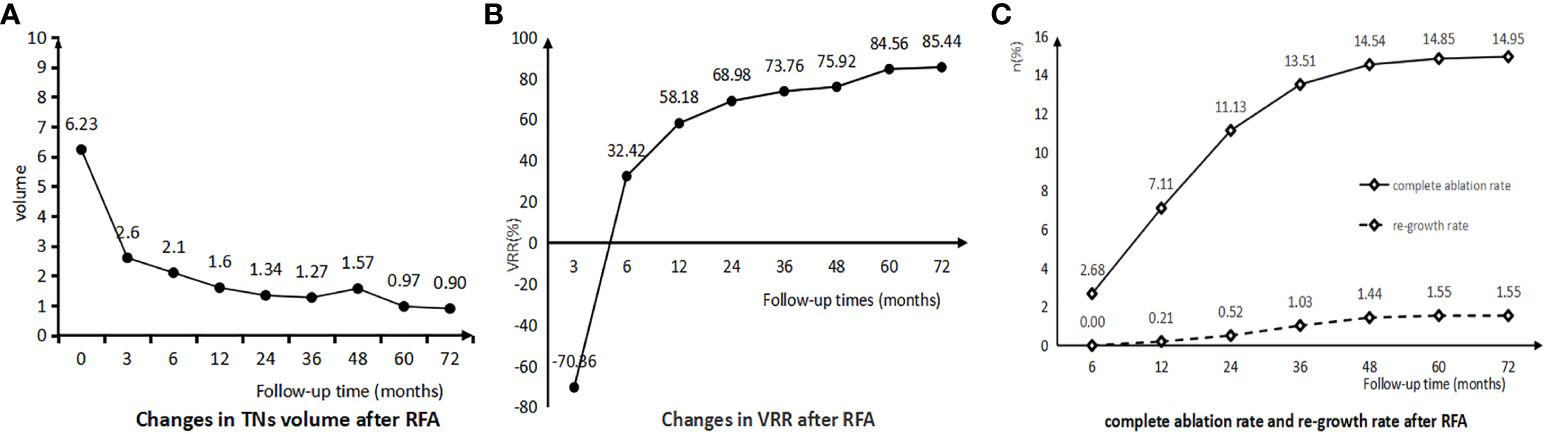
Figure 2 (A) Changes in TNs Volume after RFA. (B) Changes in TNs VRR after RFA. (C) Complete ablation rate and re-growth rate after RFA.
Thyroid nodules (TNs) are extremely common in the general population, and their incidence is increasing due to the popularity of high-resolution ultrasound and the emphasis on health issues. Many previous studies have confirmed that RFA is an optional safe and effective treatment for TNs. The majority of TNs are benign nodules and more than 90% of malignant nodules are papillary thyroid carcinomas with a biological predisposition to indolence; similarly, patients have aesthetic concerns, all of which contribute to a higher incidence of RFA. As a result, the scope of RFA has gradually expanded from benign nodules to micropapillary thyroid cancer and even to recurrent thyroid cancer and low-risk papillary thyroid cancer.
Surgery is still the mainstay of treatment for TNs, and its postoperative risk of adverse events such as hypothyroidism, hypoparathyroidism, and recurrent laryngeal nerve injury is significantly higher than that of RFA (4, 18, 19). Surgery involves removal of the thyroid gland with nodules, whereas RFA involves coagulative necrosis of the nodules into biologically inactive scar tissue by thermal ablation; therefore, TNs do not immediately decrease or disappear in apparent physical size after RFA, and may even show enlargement in the short-term. TNs volume and volume reduction rate (VRR) are important indicators for assessing the efficacy of RFA. In our study, the volume of TNs decreased from 6.23 ml preoperatively to 1.6 ml, 1.34 ml, 1.27 ml, 1.57 ml, 0.97 ml, and 0.90 ml at postoperative 1, 2, 3, 4, 5, and 6 years, respectively and its VRR was 58.18%, 68.98%, 73.76%, 75.92%, 84.56% and 85.44% at postoperative 1, 2, 3, 4, 5 and 6 years, respectively. Volume reductions in TNs were most significant at years 1 and 2 after RFA, and it stabilized after 2 years. A meta-analysis showed that benign TNs had a VRR of 75% and 87% at years 1 and 2 after RFA, respectively (4). In another meta-analysis including 24 studies of ablation of benign TNs showed a VRR of 66% and 62% at years 1 and 2 after RFA, respectively; these are consistent with our study (20). Kim MK et al. found that the VRR of malignant nodules was lower than that of benign ones at 12 months after RFA (51.4% versus 83.8%, P = 0.01 < 0.05) (21). A study of 74 patients with thyroid micropapillary carcinoma (PTMC) followed for more than 5 years found that RFA resulted in complete disappearance of the tumor lesions without local tumor progression, lymph node or distant metastasis (22). In our study, we saw complete ablation of TNs lesions in 145 (4.9%) patients, which occurred with a mean follow-up time of 18.32 ± 12.98 months.
RFA treatment is not the removal of glands with clinically significant nodules; therefore, patients are more anxious and concerned about the recurrence of nodules after RFA. Many studies have demonstrated that incomplete ablation resulting in residual nodules at the margins is an important factor in regeneration (23, 24). It has been also noted that incomplete ablation of benign nodules may promote nodule growth to some extent (25). However, many studies indicated that the initial ablation ratio (IAR) did not correlate with nodal regrowth, but rather with VRR and the likelihood of retreatment (25–28). Performing CEUS before and after RFA for more complete ablation reduces the possibility of residual nodules; however, factors such as large nodules, restricted anatomical locations V(29), and large calcified foci (30) affect ablation efficacy, and identification of accurate borders leads to residual nodules after initial ablation. Although previous studies have indicated that the primary purpose of RFA for benign nodules is to alleviate compression symptoms rather than complete ablation (31), many studies have confirmed its favorable safety and efficacy, allowing it to treat PTMC, recurrent thyroid cancer, and even low-risk papillary thyroid cancer. Therefore, the current aims of RFA are not only to improve symptoms, but also to eliminate tumor lesions (12, 13, 22). Those puncture-proven benign nodules that do not achieve satisfactory regression or regeneration after RFA may actually be malignant. Many studies have also confirmed its and found that regenerating nodules after RFA were confirmed to be malignant during subsequent surgeries (32, 33). Therefore, for these nodules that do not significantly regress or regenerate after RFA, a puncture biopsy is recommended to exclude the possibility of malignancy and guide subsequent treatment.
In our study, we saw a significant reduction in the volume of TNs after RFA at 1 and 2 years, and its stabilization at 2 years. A meta-analysis also showed that the volume of benign nodules decreased rapidly within 12 months after ablation, with a plateau at months 12 - 36 (34). The change in VRR was consistent with the change in nodal volume, which also stabilized at 2 years. The VRR showed a negative increase in 3 months after RFA, which is consistent with previous studies; it is due to the ablation area exceeding the boundaries of the nodule (especially small nodules), and changes such as peripheral edema and inflammation affecting the definition of the boundaries in the early post-RFA period. Regrowth of the nodules predominantly started in the 2nd year after RFA and stabilized in the 4th year. Previous studies have also confirmed that regrowth occurs after 2 and 3 years (35–37). However, Sim JS et al. found that its regrowth shows a peak during the 2nd-3rd year after RFA and another peak after the 5th year (23). Valcavi R et al. found that regrowth is rare after the 4th year (38), which is consistent with our findings. These differences may be due to inconsistencies in follow-up times, leading to different definitions of minimum volume; therefore, Mauri, G et al. suggested that regrowth be defined as a 50% increase in the minimum recorded volume compared to that measured at a given follow-up time point (17).
Parameters regarding the prediction of regrowth after RFA have not been clarified (37). Yan et al. found that residual active nodule rate, initial volume, location, and vascular distribution were all independent risk factors associated with regrowth (28). Negro R et al. found that VRR at 12 months after RFA was associated with regrowth (35). An increase in the volume of the residual active nodule may be an early sign of nodule regrowth (27, 39). Many studies also indicated that margin re-expansion is an important cause of recurrence after RFA (23, 29, 40). There is currently no consensus on the timing and indications for reintervention in these regrowing nodules. Some studies have indicated that a single treatment for benign nodules treated to relieve compression symptoms or improve cosmetic problems is sufficient, even if nodule regrowth occurs (31). Kim HJ et al. demonstrated that single RFA is effective for small nodules without initial regrowth or symptomatic recurrence. However, additional treatments improved the VRR for nodules with regrowth, increased Vv, or symptomatic recurrence (41). One study found that a VRR of <66% at 1 year after RFA was a better predictor of nodal retreatment, whereas young age and large initial volume may also be associated with its retreatment (37). Another study found that an IAR >73% was a good predictor of no retreatment within 5 years after RFA (26). The study showed that the energy delivered during RFA is also a reliable predictor of retreatment (37). Therefore, avoiding residual active nodules after RFA reduces the likelihood of nodule regrowth or retreatment. Preoperative CEUS identified the targeting area and postoperative CEUS confirmed complete ablation of the targeting area. Additional RFA is indicated for patients with larger nodules, unresolved clinical problems, or regrowth or increased Vv after initial ablation. Some studies have also confirmed that subsequent RFA for large benign nodules improves VRR and efficacy. However, the optimal timing and indications for reintervention, including RFA and invasive procedures, need to be further explored.
Our study has its own limitations as follows: 1. We followed up only for major complications of E and F classifications as defined by the Society of Interventional Radiology (SIR) (42). A serious postoperative adverse event occurred in one patient with cervical sympathetic chain injury resulting in Horner’s syndrome. A transient or permanent damage of the recurrent laryngeal nerve could not be evaluated due to the lack of postoperative laryngoscopy, and this is a significant limitation of the study. 2. Most thyroids are multiple nodules, and many patients had multiple nodules ablated during a single RFA; however, we only evaluated large nodules. Therefore, whether there is a difference between ablation of multiple nodules versus single nodules during the same RFA, and whether ablation has an effect on untreated nodules (both ipsilateral and contralateral); none of the above were involved in this study and will be explored in our subsequent studies.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
SC: Supervision, Writing – review & editing, Project administration, Methodology, Investigation, Data curation, Conceptualization. LM: Writing – review & editing, Supervision, Methodology. YZ: Investigation, Writing – review & editing, Supervision, Methodology. LH: Writing – original draft, Project administration, Formal Analysis, Data curation, Conceptualization, Writing – review & editing, Methodology, Investigation.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kant R, Davis A, Verma V. Thyroid nodules: advances in evaluation and management. Am Fam Physician. (2020) 102:298–304.
2. Durante C, Costante G, Lucisano G, Bruno R, Meringolo D, Paciaroni A, et al. The natural history of benign thyroid nodules. JAMA. (2015) 313:926–35. doi: 10.1001/jama.2015.0956
3. Weiss A, Parina RP, Tang JAB, Brumund KT, Chang DC, Bouvet M. Outcomes of thyroidectomy from a large California State database. Am J Surg. (2015) 210:1170–7. doi: 10.1016/j.amjsurg.2015.08.011
4. Trimboli P, Castellana M, Sconfenza LM, Virili C, Pescatori LC, Cesareo R, et al. Efcacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and meta-analysis. Endocrine. (2020) 67:35–43. doi: 10.1007/s12020-019-02019-3
5. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and diferentiated thyroid cancer: The American Thyroid Association Guidelines Task force on thyroid nodules and diferentiated thyroid cancer. Thyroid. (2015) 26:1–133. doi: 10.1089/thy.2015.0020
6. Kim JH, Yoo WS, Park YJ, Park DJ, Yun TJ, Choi SH, et al. Efficacy and Safety of Radiofrequency Ablation for Treatment of Locally Recurrent Thyroid Cancers Smaller than 2 cm. Radiology. (2015) 276:909–18. doi: 10.1148/radiol.15140079
7. Choi Y, Jung SL, Bae JS, Lee SH, Jung CK, Jang J, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: A single-center propensity score matching study. Int J Hyperth. (2019) 36:359–67. doi: 10.1080/02656736.2019.1571248
8. Bernardi S, Palermo A, Grasso RF, Fabris B, Stacul F, Cesareo R. Current status and challenges of US-guided radiofrequency ablation of thyroid nodules in the long term: A systematic review. Cancers (Basel). (2021) 13:27–46. doi: 10.3390/cancers13112746
9. Kim C, Lee JH, Choi YJ, Kim WB, Sung TY, Baek JH. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. (2017) 27:3128–37. doi: 10.1007/s00330-016-4690-y
10. Ahmad S, Aljammal J, Orozco I, Raashid S, Zulfiqar F, Nikravan SP, et al. Radiofrequency ablation of cervical thyroid cancer metastases-experience of endocrinology practices in the United States. J Endocr Soc. (2023) 7:bvad066. doi: 10.1210/jendso/bvad066
11. Van Dijk SP, Coerts HI, Gunput ST, van Velsen EFS, Medici M, Moelker A, et al. Assessment of radiofrequency ablation for papillary microcarcinoma of the thyroid: A systematic review and meta-analysis. JAMA Otolaryngol Neck Surg. (2022) 148:317–25. doi: 10.1001/jamaoto.2021.4381
12. Lim HK, Cho SJ, Baek JH, Lee KD, Son CW, Son JM, et al. US-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: efficacy and safety in a large population. Korean J Radiol. (2019) 20:1653–61. doi: 10.3348/kjr.2019.0192
13. Yan L, Lan Y, Xiao J, Lin L, Jiang B, Luo Y. Long-term outcomes of radiofrequency ablation for unifocal low-risk papillary thyroid microcarcinoma: A large cohort study of 414 patients. Eur Radiol. (2021) 31:685–94. doi: 10.1007/s00330-020-07128-6
14. Deandrea M, Trimboli P, Garino F, Mormile A, Magliona G, Ramunni MJ, et al. Long-term efficacy of a single session of RFA for benign thyroid nodules: A longitudinal 5-year observational study. J Clin Endocrinol Metab. (2019) 104:3751–6. doi: 10.1210/jc.2018-02808
15. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. (2017) 14:587–95. doi: 10.1016/j.jacr.2017.01.046
16. Kim JH, Baek JH, Lim HK, Ahn HS, Baek SM, Choi YJ, et al. 2017 Thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. (2018) 19:632–55. doi: 10.3348/kjr.2018.19.4.632
17. Mauri G, Pacella CM, Papini E, Solbiati L, Goldberg SN, Ahmed M, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. (2019) 29:611–8. doi: 10.1089/thy.2018.0604
18. Che Y, Jin S, Shi C, Wang L, Zhang X, Li Y, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. (2015) 36:1321–5. doi: 10.3174/ajnr.A4276
19. Wu R, Luo Y, Tang J, Yang M, Li J, Zhang Y, et al. Ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma: A retrospective analysis of 198 patients. Int J Hyperth. (2020) 37:168–74. doi: 10.1080/02656736.2019.1708480
20. Chung SR, Suh CH, Baek JH, Park HS, Choi YJ, Lee JH. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: A systematic review and meta-analysis. Int J Hyperthermia. (2017) 33:920–30. doi: 10.1080/02656736.2017.1337936
21. Kim MK, Shin JH, Hahn SY, Kim H. Delayed cancer diagnosis in thyroid nodules initially treated as benign with radiofrequency ablation: ultrasound characteristics and predictors for cancer. Korean J Radiol. (2023) 24:903–11. doi: 10.3348/kjr.2023.0386
22. Cho SJ, Baek SM, Lim HK, Lee KD, Son JM, Baek JH. Long-term follow-up results of ultrasound-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: more than 5-year follow-up for 84 tumors. Thyroid. (2020) 30:1745–51. doi: 10.1089/thy.2020.0106
23. Sim JS, Baek JH, Lee J, Cho W, Jung SI. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J hyperthermia: Off J Eur Soc Hyperthermic Oncol North Am Hyperthermia Group. (2017) 33:905–10. doi: 10.1080/02656736.2017.1309083
24. Sung JY, Baek JH, Jung SL, Kim JH, Kim KS, Lee D, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid: Off J Am Thyroid Assoc. (2015) 25:11–27. doi: 10.1089/thy.2014.0100
25. Issa PP, Omar M, Issa CP, Buti Y, Hussein M, Aboueisha M, et al. Radiofrequency ablation of indeterminate thyroid nodules: the first north american comparative analysis. Int J Mol Sci. (2022) 23:11493. doi: 10.3390/ijms231911493
26. Bernardi S, Cavallaro M, Colombin G, Giudici F, Zuolo G, Zdjelar A, et al. Initial ablation ratio predicts volume reduction and retreatment after 5 years from radiofrequency ablation of benign thyroid nodules. Front Endocrinol. (2021) 11:582550. doi: 10.3389/fendo.2020.582550
27. Chen MH, Lin WC, Luo SD, Chiang PL, Chen YS, Chen WC, et al. Residual, regrowth, and new growth of radiofrequency ablation for benign thyroid nodules of different volumes: two-year follow-up results. Int J Hyperthermia. (2022) 39:1172–8. doi: 10.1080/02656736.2022.2112305
28. Yan L, Luo Y, Xie F, Zhang M, Xiao J. Residual vital ratio: predicting regrowth after radiofrequency ablation for benign thyroid nodules. Int J Hyperthermia. (2020) 37:1139 48. doi: 10.1080/02656736.2020.1825835
29. Yan L, Zhang M, Li X, Li Y, Luo Y. A nomogram to predict regrowth after ultrasound-guided radiofrequency ablation for benign thyroid nodules. Front Endocrinol. (2022) 12:774228. doi: 10.3389/fendo.2021.774228
30. Park KW, Shin JH, Han BK, Ko EY, Chung JH. Inoperable symptomatic recurrent thyroid cancers: preliminary result of radiofrequency ablation. Ann Surg Oncol. (2011) 18:2564 8. doi: 10.1245/s10434-011-1619-1
31. Deandrea M, Limone P, Basso E, Mormile A, Ragazzoni F, Gamarra E, et al. US-Guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol. (2008) 34:784–91. doi: 10.1016/j.ultrasmedbio.2007.10.018
32. Bernardi S, Dobrinja C, Fabris B, Bazzocchi G, Sabata N, Ulcigrai V, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. (2014) 2014:934595. doi: 10.1155/2014/934595
33. Oddo S, Spina B, Vellone VG, Giusti M. A case of thyroid cancer on the track of the radiofrequency electrode 30 months after percutaneous ablation. J Endocrinol Invest. (2017) 40:101–102. doi: 10.1007/s40618-016-0527-4
34. Cho SJ, Baek JH, Chung SR, Choi YJ, Lee JH. Long-term results of thermal ablation of benign thyroid nodules: A systematic review and meta-analysis. Endocrinol Metab. (2020) 35:339–50. doi: 10.3803/EnM.2020.35.2.339
35. Negro R, Greco G, Deandrea M, Rucco M, Trimboli P. Twelve-month volume reduction ratio predicts regrowth and time to regrowth in thyroid nodules submitted to laser ablation: A 5-year follow-up retrospective study. Korean J Radiol. (2020) 21:764 72. doi: 10.3348/kjr.2019.0798
36. Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. (2013) 23:1044 9. doi: 10.1007/s00330-012-2671-3
37. Bernardi S, Giudici F, Cesareo R, Antonelli G, Cavallaro M, Deandrea M, et al. Five-year results of radiofrequency and laser ablation of benign thyroid nodules: A multicenter study from the Italian minimally invasive treatments of the thyroid group. Thyroid. (2020) 30:1759–70. doi: 10.1089/thy.2020.0202
38. Valcavi R, Riganti F, Bertani A, Formisano D, Pacella CM. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. (2010) 20:1253–61. doi: 10.1089/thy.2010.0189
39. Baek JH, Ha EJ, Choi YJ, Sung JY, Kim JK, Shong YK. Radiofrequency versus ethanol ablation for treating predominantly cystic thyroid nodules: A randomized clinical trial. Korean J Radiol. (2015) 16:1332–40. doi: 10.3348/kjr.2015.16.6.1332
40. Baek JH, Moon WJ, Kim YS, Lee JH, Lee D. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J surgery. (2009) 33:1971–7. doi: 10.1007/s00268-009-0130-3
41. Kim HJ, Baek JH, Cho W, Sim JS. Long-term follow-up of the radiofrequency ablation of benign thyroid nodules: the value of additional treatment. Ultrasonography. (2022) 41:661–9. doi: 10.14366/usg.21231
Keywords: radiofrequency ablation (RFA), volume reduction ratio (VRR), technique effective, regrowth, efficacy
Citation: Chuanke S, Ming L, Zhideng Y and Huan L (2024) A 6-year single-center prospective follow-up study of the efficacy of radiofrequency ablation for thyroid nodules. Front. Endocrinol. 15:1402380. doi: 10.3389/fendo.2024.1402380
Received: 17 March 2024; Accepted: 10 June 2024;
Published: 25 June 2024.
Edited by:
Pia Pace-Asciak, University of Toronto, CanadaReviewed by:
Kyriakos Vamvakidis, Henry Dunant Hospital, GreeceCopyright © 2024 Chuanke, Ming, Zhideng and Huan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liu Huan, Y211bGl1aHVhbkBvdXRsb29rLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.