
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 29 July 2024
Sec. Cancer Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1400841
 Stephanie van der Leij1,2*†
Stephanie van der Leij1,2*† Karijn P.M. Suijkerbuijk3
Karijn P.M. Suijkerbuijk3 Medard F.M. van den Broek1,3
Medard F.M. van den Broek1,3 Gerlof D. Valk1,3
Gerlof D. Valk1,3 Jan Willem Dankbaar4
Jan Willem Dankbaar4 Hanneke M. van Santen2,5
Hanneke M. van Santen2,5Objective: Immune checkpoint inhibitors (ICIs) are revolutionary in oncology but may cause immune-related (IR) side effects, such as hypophysitis. Treatment with anti-PD-(L)1, anti-CTLA-4 or anti-CLTA-4/PD-1 may induce hypophysitis, but little is known about the differences in clinical presentation or need for different treatment. We analyzed the differences of anti-PD-(L)1, anti-CTLA-4 and anti-CTLA-4/PD-1 induced hypophysitis
Methods: retrospective analysis of 67 patients (27 anti-PD-(L)1, 6 anti-CLTA-4 and 34 anti-CTLA-4/PD-1 induced hypophysitis).
Results: The median time between starting ICIs and IR-hypophysitis was longer after anti-PD(L)-1) therapy (22 weeks versus 11 and 14 weeks after anti-CTLA-4 and anti-CTLA-4/PD-1 therapy, respectively). The majority of patients (>90%), presented with atypical complaints such as fatigue, nausea, and muscle complaints. Headache, TSH or LH/FSH deficiency were more common in anti-CTLA-4 and anti-CLTA-4/PD-1 versus anti-PD-(L)1 induced hypophysitis (83% and 58% versus 8%, 67% and 41% versus 11%, and 83% and 48% versus 7%, respectively). Pituitary abnormalities on MRI (hypophysitis or secondary empty sella syndrome) were only seen in patients receiving anti-CTLA-4 or anti-CTLA-4/PD-1 therapy. Recovery from TSH, LH/FSH and ACTH deficiency was described in 92%, 70% and 0% of patients after a mean period of 14 and 104 days, respectively, and did not differ between patients who did or did not receive high-dose steroids.
Conclusion: The clinical presentation of IR-hypophysitis varies depending on the type of ICIs. MRI abnormalities were only seen in anti-CTLA-4 or anti-CTLA-4/PD-1 induced hypophysitis. Endocrine recovery is seen for LH/FSH and TSH deficiency but not for ACTH deficiency, irrespective of the corticosteroid dose.
In recent years, the development of immune checkpoint inhibitors (ICIs) has contributed to a revolution in cancer treatment (1–4). ICIs are not only the first choice of treatment in advanced melanoma but are also widely used for several types of solid cancers. With the introduction of ICIs, a new spectrum of endocrine, immune-related adverse events (eIRAEs) are observed: immune-related (IR)-thyroiditis or hypothyroidism, IR-hypophysitis, IR-diabetes, and rarely IR-adrenal insufficiency (5–7).
Whereas primary autoimmune hypophysitis is rare - with a 1 in 9 million prevalence - IR-hypophysitis is far more common (8). The prevalence of IR-hypophysitis depends on the type of ICI: it is described in 9–10% of patients treated with anti-CTLA-4/PD-1 combination therapy, in 2–6% of patients with anti-CTLA-4 monotherapy and in 1% of patients with anti-PD(L)1 monotherapy (5, 6, 9).
Clinical symptoms of IR-hypophysitis can be vague, and its symptoms may overlap with symptoms often seen in patients with advanced cancer, which makes it harder to recognize and to diagnose in an early stage (5, 6). Clinical symptoms and the degree of hypopituitarism seem to differ between anti-PD-(L)1 induced hypophysitis and anti-CTLA-4 induced hypophysitis (9). Adrenocorticotropic hormone (ACTH) deficiency is the most common pituitary deficiency in patients with IR-hypophysitis (95–97%). In one study, the prevalence of thyroid-stimulating hormone (TSH) deficiency and luteinizing hormone (LH)/follicle-stimulating hormone (FSH) deficiency was found to be higher in anti-CTLA-4-induced hypophysitis than in anti-PD-(L)1-induced hypophysitis, 85% and 75% versus 4% and 13%, respectively (9). Recovery from TSH deficiency and LH/FSH deficiency is reported in 83%-85% of patients. However, recovery from ACTH deficiency is very rare (6, 9–11) Although recovery from TSH and LH/FSH is frequently reported, the time to recovery is unclear. It is also unclear whether combination therapy with anti-CTLA-4/PD-1 differs clinically from anti-PD-(L)1-induced hypophysitis. Unfortunately, no randomized trials on the optimal screening and management of IR-hypophysitis exist. European guidelines for screening and treatment of IR-hypophysitis differ significantly between endocrinologists and oncologists (See Supplementary Material). Although recommended treatment strategies for IR-hypophysitis due to anti-CTLA-4 or anti-PD-(L)1 are currently the same, it may be questioned whether this should be further differentiated based on its etiology. To gain a better understanding of the clinical picture of IR-hypophysitis, we aimed to describe and compare the clinical presentation, including MR imaging and course of anti-PD-(L)1, anti-CTLA-4 and anti-CTLA-4/PD-1-induced hypophysitis.
All patients with suspected IR-hypophysitis referred to the Department of Endocrinology at the University Medical Center Utrecht between 2013 and 2022 were identified (n=97). The diagnostic criteria for IR-hypophysitis were clinical signs of hypophysitis in combination with biochemical evidence of anterior pituitary hormone deficiency and/or magnetic resonance imaging (MRI) suggestive of hypophysitis (9, 12) (Figure 1). Patients with prior treatment with systemic steroids were excluded (n=30).
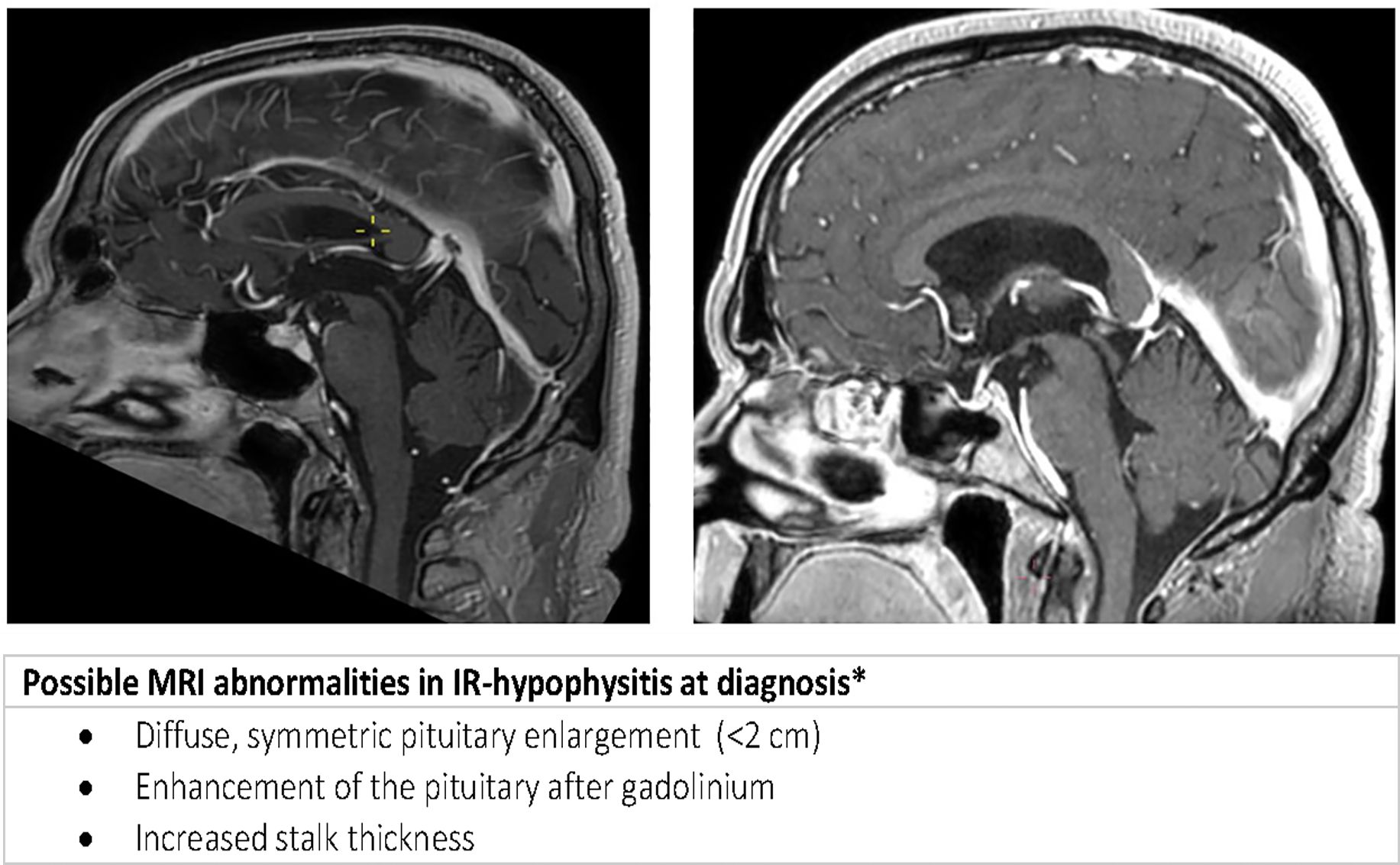
Figure 1 Sagittal MRI with radiological features of hypophysitis at presentation (left) and secondary empty sella during follow up (right).
Clinical data were obtained from the electronic patients’ files. They included sex, age, type of cancer, type of ICI, duration from start of ICIs to diagnosis of IR-hypophysitis, clinical symptoms of hypophysitis, laboratory results (sodium, glucose, ACTH, cortisol, TSH, FT4, prolactin, LH, FSH, estradiol or testosterone and insulin-like growth factor (IGF-1), imaging (MRI) and the presence of other immune related adverse events (IRAEs). The presence of IR-thyroiditis was based on the biochemical course of TSH and FT4 without the determination of thyroid peroxidase antibodies.
The following definitions for hypopituitarism were used: TSH deficiency was defined as a decreased concentration of TSH (<0.35 mIU/L) in combination with a low FT4 level (≤12 pmol/L) or a disproportionally low TSH level (0.35–7.0 mIU/L) with a decreased FT4 concentration (<10 pmol/L) (13). ACTH deficiency was defined as low random cortisol (<200 nmol/L) or a decreased morning cortisol (<250 nmol/L, without an increased ACTH concentration (4–60 ng/L), LH/FSH deficiency was defined as a low concentration of testosterone or estradiol with low or normal level of LH/FSH. Due to the absence of clinical relevance, dynamic testing of growth hormone (GH) deficiency was not available. Possible GH deficiency was defined as an IGF-1 level of ≥2 standard deviations (SD) below the sex- and age-dependent reference range. Hyponatremia was defined as a sodium concentration of <136 mmol/l. Recovery from pituitary deficiency was defined as a successful cessation of the specific hormonal suppletion, including normalization of biochemical parameters. Secondary (complete) empty sella was defined as loss of pituitary height of ≥ 33% with >50% of the sella filled with cerebrospinal fluid and pituitary thickness ≤2 mm (14).
Treatment of IR-hypophysitis was at the discretion of the physician and in accordance to the European oncology guideline (2017) (15). In this guideline high dose corticosteroids (prednisolone 0.5–1 mg/kg) were advised in case of headache and hormone replacement therapy was advised in asymptomatic patients or in case of vague symptoms (e.g. mild fatigue or anorexia) without headache.
The data were analyzed using SPSS version 25.0. Descriptive statistics were expressed as absolute numbers, percentages, median with ranges or means with standard deviation (SD) depending on data distribution. The Chi-square and Fisher exact tests were used to compare categorical data and p-values of ≤ 0.05 were considered to indicate statistical significance. Differences between continue data with a skewed distribution were examined using the Kruskal Wallis and Mann Whitney U tests.
In this study 67 patients with IR-hypophysitis were included. Baseline characteristics and the clinical presentation of IR-hypophysitis are describes in Table 1. Most patients were male (63%), the mean age at diagnosis of IR-hypophysitis was 60.4 years (SD 11.3). The median time between the start of ICI to the diagnosis of IR-hypophysitis was 17 weeks (range 2–176). The median follow-up time from IR-hypophysitis to the end of the study period was 73 weeks (range 3–100). Patients had been treated for different types of cancer: 42 (63%) for melanoma, 6 (9%) for renal cancer, 5 (7%) for lung cancer and 14 (21%) for other types of cancers. 37% of patients were treated with anti-PD-1 monotherapy (nivolumab, pembrolizumab), 3% with anti-PD-L1 monotherapy (atezolizumab), 9% with anti-CTLA-4 monotherapy (ipilimumab, tremelimumab) and 51% with anti-CTLA-4/PD-1 combination therapy (ipilimumab, nivolumab).
Clinical symptoms of IR-hypophysitis were non-specific in most cases: malaise or fatigue was the most reported symptom (93%), followed by nausea or anorexia (83%), muscle complaints such as pain or weakness (78%) and dizziness (50%). Local symptoms such as headaches and visual symptoms were present in 40% and 5% of patients, respectively.
Biochemically, ACTH deficiency was present in 97% of patients, with a median serum cortisol concentration of 40 nmol/L (reference range >130 nmol/L). Functional testing for ACTH deficiency with either a synacthen test or metyrapone test was only indicated in two patients confirming ACTH deficiency. TSH deficiency was present in 31% of patients at any point in time, LH/FSH deficiency in 36% of patients and an IGF-level of ≥ 2 SD below the reference range in 13% of patients. None of the patients developed arginine vasopressin (AVP) deficiency, formerly known as diabetes insipidus. Hyponatremia was present in 33% of patients. The presence of hyponatremia was not related to the presence of cerebral metastasis. No correlation was found between the sodium concentration and cortisol level or FT4. A typical MR image of hypophysitis (See Figure 1 and Image 1) was present at diagnosis in 38% (9 out 24) of patients.
The treatment strategy for IR-hypophysitis was at the treating physician’s discretion, also depending on other concurrent irAEs. Low dose corticosteroids were defined as ≤ 40 mg, medium dose defined as >40 mg and high dose defined (HD) as ≥160 mg hydrocortisone or an equivalent dosage of corticosteroids per day. Patients with monotherapy anti-CTLA-4 induced hypophysitis were more frequently treated with HD corticosteroids. Most patients (73%) were initially treated with medium dose or HD corticosteroids. The corticosteroids were tapered to replacement therapy over a period of days to weeks.
During follow-up, recovery from TSH deficiency and LH/FSH deficiency was described in 92% and 70% of patients, respectively. The dose of corticosteroids) did not affect these recovery rates. The timing of recovery from TSH deficiency and LH/FSH deficiency could only be assessed in the patients without initiation of thyroid hormone (n=6) or testosterone (n=5). The mean concentration of thyroid hormone and testosterone at diagnosis did not differ between patients with or without hormonal suppletion. Recovery from TSH deficiency in these 6 patients was seen after a mean period of 14 days (range 7–28 days), while recovery from LH/FSH deficiency was seen after a mean period of 104 days (range 47–178 days). ACTH deficiency was reassessed in 27 out of 67 patients by cessation of corticosteroids and evaluating the cortisol level in the morning. However, none of the patients recovered from ACTH deficiency. Unfortunately, reassessment of (possible) GH deficiency during follow-up was not available.
In total 72% (48 out 67) of patients experienced other immune-related adverse events before or after the diagnosis of IR-hypophysitis. The most common immune-related adverse events were primary hypothyroidism or thyroiditis (28%), dermatitis or vitiligo (25%), hepatitis (21%), pneumonitis (9%), nephritis (8%), colitis (6%) and uveitis (5%). Hepatitis was more prevalent in patients treated with anti-CTLA-4/PD-1 combination therapy.
The clinical differences between the treatment groups are outlined in Table 2.
Differences were seen both in timing and in the degree of hypophysitis; anti-PD-(L)1 induced hypophysitis was seen after a median of 21.9 weeks versus after 10.6 and 13.9 weeks in patients with and CTLA-4 and anti-CTLA-4/PD-1 induced hypophysitis. Headache was mainly present in patients with anti-CTLA-4 and anti-CTLA-4/PD-1 induced hypophysitis (83% and 58% of patients, respectively versus 8% of patients with anti-PD-(L)-1 induced hypophysitis).
Differences in pituitary insufficiency were present between the treatment groups: TSH deficiency was seen in 11%, 67% and 41% and LH/FSH deficiency was seen in 10%, 83% and 48% of patients with anti-PD-(L)1 versus, anti-CTLA-4 and anti-CTLA-4/PD-1-induced hypophysitis, respectively. We analyzed the levels of LH, FSH and testosterone in male patients because 92% of female patients were postmenopausal. In accordance to the prevalence of hormone deficiencies, the median levels of TSH, LH, FSH and testosterone were significant lower in patients treated with anti-CTLA-4 and anti-CTLA-4/PD-1-therapy No differences were seen in prevalence of ACTH deficiency, hyponatremia and IGF-1 level ≥2 SD below the reference range. Also no differences were seen in the median levels of ACTH, cortisol, sodium and IGF-SD between the different treatment groups.
MRI abnormalities at diagnosis were seen in 56% (9 out 16) patients with anti-CTLA and anti-CLTA-4/PD-1 induced hypophysitis. Secondary empty sella during follow-up was evaluated by MRI in 32 patients after a median time of 28 months (range 4 to 94). In 22% (7 out 32) of patients secondary empty sella was seen on MRI. Secondary empty sella was only seen in patients treated with anti-CTLA-4/PD-1 combination therapy. Unfortunately, only in 3 of the 7 patients with secondary empty sella a diagnostic MRI at presentation had been performed, which showed signs of hypophysitis in all 3 patients (Image 1). In 9 out of 25 patients without secondary empty sella during follow up, an MRI at initial diagnosis of IR hypophysitis was available. Four of these 9 patients had radiological features of hypophysitis at diagnosis. Radiological features of hypophysitis at initial diagnosis were thus not related to secondary empty sella (p = 0.16).
In this cohort of patients with IR-hypophysitis, we could confirm clinically relevant differences between anti-PD-(L)1 versus anti-CTLA-4 mono- or combination therapy induced hypophysitis. The time to develop IR-hypophysitis seems to be shorter after anti-CTLA-4 mono- or combination therapy when compared to anti-PD-(L)1 monotherapy (median 10.6 and 13.9 versus 21.9 weeks, respectively), as was shown previously for other irAEs (16). Headache was more frequent in patients treated with anti-CTLA-4 mono- or combination therapy. MRI abnormalities (signs of hypophysitis or empty sella syndrome) were only seen in patients treated with anti-CTLA-4 mono- or combination therapy. Last, anti-CTLA-4 mono- or combination therapy induced more severe hypopituitarism with, in addition to ACTH deficiency, also TSH and LH/FSH deficiencies. Recovery from TSH and LH/FSH deficiency occurred in the majority of patients. ACTH deficiency, however, seems to be permanent.
Our study provides new data on the course and severity of anti-PD-(L)1 monotherapy versus anti-CTLA-4- but also anti-CTLA-4/PD-1 combination therapy induced hypophysitis. Most studies have described IR-hypophysitis caused by either anti-CLTA-4 or PD-(L)1 monotherapy (9, 10, 17–19). In a 10-year assessment by Di Dalmazi et al. differences between IR-hypophysitis caused by anti-CTLA-4 versus anti-PD-(L)1 monotherapy are described, but the number of patients with IR-hypophysitis caused by anti-CTLA-4/PD-1 combination therapy was too small to analyze, which was the reason for us to perform this study (9). A recent study performed by Jessel et al. described the differences between anti-CTLA-4/PD-1 induced hypophysitis (n = 53) versus anti-PD-(L)1 induced hypophysitis (n = 13) (19). Our study results are in accordance with their results, with shorter latency time in patients with anti-CTLA-4/PD-1 therapy, higher prevalence of headache in anti-CTLA-4/PD-1-induced hypophysitis, and more frequent TSH deficiency and LH/FSH deficiency in anti-CTLA-4/PD-1 induced hypophysitis compared to anti-PD-(L)1 monotherapy induced hypophysitis. However, in contrast to our findings, with no MRI abnormalities in patients with anti-PD(L1) induced hypophysitis, Jessel et al. found MRI abnormalities in 1 (out of 5) patient with anti-PD-(L)1-induced hypophysitis.
In the study of Dalmazi et al. differences between monotherapy with anti-PD-1 and monotherapy with anti-CTA-4 were analyzed (9). Comparable to our study pituitary deficiencies were seen more frequently after anti-CTLA-4 versus anti-PD-(L)1 therapy. The same authors found a higher prevalence of hyponatremia in anti-PD-(L1) versus anti-CTLA-4 induced hypophysitis (62% versus 39%, respectively). The authors hypothesized that this difference could be related to more severe ACTH deficiency in anti-PD-(L)1-induced hypophysitis. However, we did not find a difference in the median sodium level or the prevalence of hyponatremia between PD-(L)1 versus CTLA-4 mono- or combination therapy induced hypophysiits. Moreover, no correlation was found between the cortisol and sodium level, which does not support the hypothesis of Dalmazi et al. (9).
In our cohort, none of the patients developed AVP-deficiency and this seems very rare in IR-hypophysitis (10, 17, 18, 20), although Dalmazi et al. reported AVP deficiency in 2–3% of patients (9). In our opinion, AVP deficiency should raise the suspicion for pituitary metastasis and is an indication for imaging.
In our study, recovery from TSH and LH/FSH deficiency was found in the far majority of tested patients (92% and 70%, respectively). Nguyen et al. (21) described 62 patients with IR-hypophysitis and reported a recovery rate of TSH, LH/FSH and ACTH deficiency of respectively 24%, 58% and 0% (using comparable definitions for TSH deficiency and recovery time). The difference in recovery rate of TSH deficiency between the studies might be explained by a more severe TSH deficiency in patients with anti-CTLA-4 induced hypophysitis; 57 of 62 patients in the study of Nguyen were treated with anti-CTLA-4 therapy (21). Currently, no guidelines exist regarding re-evaluation of TSH or LH/FSH deficiency. Reevaluation of pituitary deficiencies is dependent on the preference of the physician as well as the patient’s clinical condition and prognosis, which may explain the wide range in recovery rates of TSH and LH/FSH deficiency reported in literature. Recovery from ACTH deficiency was not seen in our cohort, which is in line with previous reports (10, 17–21).
The exact mechanism underlying IR-hypophysitis is still largely unknown. Interestingly, ACTH deficiency is prominent in IR-hypophysitis. However, in other pituitary disorders including acquired forms after radiation treatment, the secretion of GH and TSH are most vulnerable and are frequently affected before ACTH or LH/FSH deficiency develops (22, 23). Because anti-PD-(L)1 induced hypophysitis primarily causes ACTH deficiency and anti-CTLA-4 therapy is associated with more pronounced hypopituitarism with involvement of plural pituitary axes, it is hypothesized that the pathophysiological mechanism of IR-hypophysitis differs between the two. The checkpoint CTLA-4 is not only expressed on T-cells but also on pituitary cells. The degree of CTLA-4 expression in the adenohypophyses varies greatly between individuals, possibly explaining why some patients develop IR-hypophysitis and others do not. Complement deposition as well as infiltration of lymphocytes was seen in the pituitary tissue of mice injected with ipilimumab and in an autopsy report of a patient with IR-hypophysitis (24, 25). CTLA-4 is an IgG1 monoclonal antibody which can bind and activate the complement cascade, leading to a type 2 hypersensitivity reaction. It is postulated that this type-2 reaction triggers the adaptive immune system, leading to a type-4 hypersensitivity reaction. This type-4 hypersensitivity reaction is more classical for autoimmune disorders. Although pituitary antibodies were detected by indirect immunofluorescence in one study, the exact pituitary antigens are not yet identified (26).
Much less is known about the pathophysiological mechanism of anti-PD-(L)1-induced hypophysitis. Nivolumab, an IgG4 monoclonal antibody, cannot activate the complement cascade. Maybe the pathogenesis of anti-PD-(L)1-induced hypophysitis resembles IgG-4 related hypophysitis. Because of the predominance of ACTH deficiency in anti-PD-(L)1-induced hypophysitis, it has also been postulated that ACTH secreting cells express the highest levels of PD-1 (7, 9). In the first autopsy study of a patient with anti-PD-1-induced hypophysitis, lymphocyte infiltrates were found in the anterior lobe of the pituitary and the number of ACTH cells was reduced (27). Another study analyzed pituitary antibodies in patients with IR-hypophysitis and found anti-corticotroph antibodies In 10% (2 out 20) of patients (28). Interestingly, these two patients also exhibited ectopic ACTH expression in the tumor. Ectopic ACTH expression is reported previously in various cancers. In the patients without ectopic ACTH expression in the tumor no anti-corticotroph antibodies have been detected. It was hypothesized that ACTH expression in tumors may evoke autoreactive T cells with specific injury to the corticotroph cells, possibly explaining the predominance of ACTH deficiency in IR-hypophysitis.
Interestingly, the current guidelines for treatment of IR-hypophysitis differ significantly between endocrinologists and oncologists (Supplementary Material) (5, 6). The guideline of the European Society of Endocrinology is restrictive in corticosteroid use and the need for MRI or visual field examination in comparison to the guideline of The European Society for Medical Oncology. Because of the clinical differences such as headache and MRI abnormalities in patients with anti-PD-(L)1 monotherapy versus anti-CTLA-4 (mono- or combination) therapy, it may be questioned whether the treatment strategy should be similar (9, 19). Percik et al. suggested to classify separate forms of hypophysitis: IR-hypophysitis should be reserved to describe the symptomatic phase of hypophysitis with headache and imaging suggestive of hypophysitis while isolated ACTH deficiency should be used in PD-(L)1 induced hypophysitis without local symptoms and without other pituitary dysfunction or radiological evidence of hypophysitis. IR-hypopituitarism can be used to describe the long-term deficiencies of at least two pituitary hormones. This new nomenclature can facilitate different treatment strategies (29). Our results underscore the use of this new nomenclature.
Corticosteroids are the cornerstone in the treatment of IR-hypophysitis. However, there is still debate upon the optimal dose of corticosteroids. Endocrine irAEs are associated with an improved overall and progression-free survival, probably because eIRAEs are a marker of immune response and thus of anticancer effects in patients treated with ICIs (30–32). HD corticosteroids do not seem to affect the recovery rate of hypopituitarism (1–19–21). Importantly, the use of HD glucocorticoids in patients with anti-CTLA-4-induced hypophysitis was reported to affect overall survival negatively (10). Unfortunately, we could not analyze the effect of corticosteroids dosage on survival because of the heterogeneity of our cohort with patients treated adjuvant or in palliative setting. In our opinion, because of the potential negative impact of HD corticosteroids on overall survival and the lack of expected benefits, in accordance with the ESE guideline (6) (Table 3), HD corticosteroids should be used in case of IR-hypophysitis plus chiasm compression or severe headache (after exclusion of pituitary metastasis). In case of adrenal crisis, treatment with intravenous or intramuscular hydrocortisone is indicated. Suppletion of thyroid hormones or sex hormones should be initiated on biochemical and clinical grounds. If biochemical aberrations are small without clinical signs of hormonal insufficiency, monitoring for spontaneous recovery could be an alternative strategy. If thyroid hormones or sex hormones are started, and the clinical condition and prognosis of the patient are positive, reevaluation of TSH and LH/FSH deficiency may be considered after 3 months. Reassessment of ACTH deficiency is not recommended, given the virtually absent recovery from ACTH deficiency in the literature (9, 10, 17–20).
Some limitations must be considered when interpreting our results. The retrospective nature of this study and the small number of patients with monotherapy CTLA-4 indicate the necessity to confirm our results in larger cohorts. Furthermore, we could not calculate the incidence of IR-hypophysitis due to the lack of information on the total number of patients treated with ICIs in this period. Unfortunately, the timing of recovery from TSH and LH/FSH deficiency was challenging to assess because of its dependency on the physician’s evaluation strategy. Despite these limitations, we were able to evaluate the long-term follow-up of a large cohort of patients with IR-hypophysitis and to provide new data on the clinical differences between anti-PD-(L)1 and anti-CTLA-4 (mono and combination) therapy induced hypophysitis.
IR-hypophysitis is a common eIRAE of treatment with ICIs. Anti-PD-(L)1 induced hypophysitis differs from anti-CTLA-4 mono- or combination induced hypophysitis with regard to clinical symptoms, MRI abnormalities and the degree of hypopituitarism, which is more pronounced after anti-CTLA-4 mono- or combination therapy. Although ACTH deficiency seems to be permanent, recovery from LH/FSH and TSH deficiency may be expected in the majority of patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Medische ethische toetsings commissie UMC Utrecht. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
SL: Formal Analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. KS: Writing – review & editing. MD: Writing – review & editing. GV: Writing – review & editing. JD: Investigation, Writing – review & editing. HS: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
KS has consulting/advisory relationships with Bristol-Myers Squibb, Merck Sharp and Dome, Abbvie, Pierre Fabre Novartis, Sairopa, received honoraria from Novartis, Roche, Merck Sharp and Dome and received research funding from TigaTx, Bristol Myers Squibb and Philips. HS has received research funding from Pfizer Quality Improvement Grant and travel plus accommodation costs for an international meeting from Rhythm Pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1400841/full#supplementary-material
1. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med. (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
2. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. New Engl J Med. (2015) 372:2018–28. doi: 10.1056/NEJMoa1501824
3. Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. (2019) 393:2404–15. doi: 10.1016/S0140-6736(19)30723-8
4. Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discovery. (2022) 21:509–28. doi: 10.1038/s41573-021-00345-8
5. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up ☆. Ann Oncol. (2022) 33:1217–38. doi: 10.1016/j.annonc.2022.10.001
6. Husebye ES, Castinetti F, Criseno S, Curigliano G, Decallonne B, Fleseriu M, et al. Endocrine-related adverse conditions in patients receiving immune checkpoint inhibition: an ESE clinical practice guideline. Eur J Endocrinol. (2022) 187:G1–G21. doi: 10.1530/EJE-22-0689
7. Wright JJ, Powers AC, Johnson DB. Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. (2021) 17:389–99. doi: 10.1038/s41574-021-00484-3
8. Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. Autoimmune hypophysitis. Endocr Rev. (2005) 26:599–614. doi: 10.1210/er.2004-0011
9. Di Dalmazi G, Ippolito S, Lupi I, Caturegli P. Hypophysitis induced by immune checkpoint inhibitors: a 10-year assessment. Expert Rev Endocrinol Metab. (2019) 14:381–98. doi: 10.1080/17446651.2019.1701434
10. Albarel F, Gaudy C, Castinetti F, Carré T, Morange I, Conte-Devolx B, et al. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol. (2015) 172:195–204. doi: 10.1530/EJE-14-0845
11. Albarel F, Castinetti F, Brue T. Management of endocrine disease immune check point inhibitors-induced hypophysitis. Eur J Endocrinol. (2019) 181:R107–18. doi: 10.1530/EJE-19-0169
12. Mekki A, Dercle L, Lichtenstein P, Nasser G, Marabelle A, Champiat S, et al. Machine learning defined diagnostic criteria for differentiating pituitary metastasis from autoimmune hypophysitis in patients undergoing immune checkpoint blockade therapy. Eur J Cancer. (2019) 119:44–56. doi: 10.1016/j.ejca.2019.06.020
13. Alexopoulou O, Beguin C, De Nayer P, Maiter D. Clinical and Hormonal Characteristics of Central Hypothyroidism at Diagnosis and during Follow-up in Adult Patients. Eur J Endocrinol. (2014) 150(1):1–8. doi: 10.1530/eje.0.1500001
14. Lupi I, Zhang J, Gutenberg A, Landek-Salgado M, Tzou S, Mori S, et al. From pituitary expansion to empty sella: Disease progression in a mouse model of autoimmune hypophysitis. Endocrinology. (2011) 152:4190–8. doi: 10.1210/en.2011-1004
15. Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv119–42. doi: 10.1093/annonc/mdx225
16. Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, et al. Ipilimumab-induced hypophysitis: A detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. (2014) 99:4078–85. doi: 10.1210/jc.2014-2306
17. Jessel S, Weiss SA, Austin M, Austin W, Mahajan A, Etts K, et al. Immune checkpoint inhibitor-induced hypophysitis and patterns of loss of pituitary function. Front Oncol. (2022) 12:836859. doi: 10.3389/fonc.2022.836859
18. Min L, Hodi FS, Giobbie-Hurder A, Ott PA, Luke JJ, Donahue H, et al. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: A retrospective cohort study. Clin Cancer Res. (2015) 21:749–55. doi: 10.1158/1078-0432.CCR-14-2353
19. Nguyen H, Shah K, Waguespack SG, Hu MI, Habra MA, Cabanillas ME, et al. Immune checkpoint inhibitor related hypophysitis: Diagnostic criteria and recovery patterns. Endocr Relat Cancer. (2021) 28:419–31. doi: 10.1530/ERC-20-0513
20. Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R, et al. Hormonal replacement in hypopituitarism in adults: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2016) 101:3888–921. doi: 10.1210/jc.2016-2118. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD.
21. van Iersel L, Mulder RL, Denzer C, Cohen LE, Spoudeas HA, Meacham LR, et al. Hypothalamic-pituitary and other endocrine surveillance among childhood cancer survivors. Endocr Rev. (2022) 43:794–823. doi: 10.1210/endrev/bnab040
22. Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Am J Pathol. (2016) 186(12):3225–35. doi: 10.1016/j.ajpath.2016.08.020
23. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. (2014) 6:230–45. doi: 10.1126/scitranslmed.3008002
24. Kobayashi T, Iwama S, Sugiyama D, Yasuda Y, Okuji T, Ito M, et al. Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2021-002493
25. Okabe N, Kobayashi T, Furuse J, Fujiwara M, Kamma H. An autopsy case study of lymphocytic hypophysitis induced by nivolumab treatment for esophageal Malignant melanoma. Pathol Int. (2021) 71:831–6. doi: 10.1111/pin.13161
26. Kanie K, Iguchi G, Bando H, Urai S, Shichi H, Fujita Y, et al. Mechanistic insights into immune checkpoint inhibitor-related hypophysitis: a form of paraneoplastic syndrome. Cancer Immunol Immunother. (2021) 70:3669–77. doi: 10.1007/s00262-021-02955-y
27. Percik R, Criseno S, Adam S, Young K, Morganstein DL. Diagnostic criteria and proposed management of immune-related endocrinopathies following immune checkpoint inhibitor therapy for cancer. Endocr Connect. (2023) 12:e220513. doi: 10.1530/EC-22-0513
28. Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. (2020) 18. doi: 10.1186/s12916-020-01549-2
29. Paschou SA, Liontos M, Eleftherakis-Papaiakovou E, Stefanaki K, Markellos C, Koutsoukos K, et al. Oncological patients with endocrine complications after immunotherapy with checkpoint inhibitors present longer progression-free and overall survival. Front Oncol. (2022) 12:847917. doi: 10.3389/fonc.2022.847917
30. Johnson J, Goldner W, Abdallah D, Qiu F, Ganti AK, Kotwal A. Hypophysitis and secondary adrenal insufficiency from immune checkpoint inhibitors: diagnostic challenges and link with survival. J Natl Compr Canc Netw. (2023) 21:281–7. doi: 10.6004/jnccn.2022.7098
31. Tang SQ, Tang LL, Mao YP, Li WF, Chen L, Zhang Y, et al. The pattern of time to onset and resolution of immune-related adverse events caused by immune checkpoint inhibitors in cancer: A pooled analysis of 23 clinical trials and 8,436 patients. Cancer Res Treat. (2021) 53(2):339–54. doi: 10.4143/crt.2020.790
Keywords: IR-hypophysitis, treatment corticosteroids, immune checkpoint inhibitors, empty sella, immune therapy toxicity
Citation: van der Leij S, Suijkerbuijk KPM, van den Broek MFM, Valk GD, Dankbaar JW and van Santen HM (2024) Differences in checkpoint-inhibitor-induced hypophysitis: mono- versus combination therapy induced hypophysitis. Front. Endocrinol. 15:1400841. doi: 10.3389/fendo.2024.1400841
Received: 14 March 2024; Accepted: 17 June 2024;
Published: 29 July 2024.
Edited by:
Yutaka Takahashi, Nara Medical University, JapanReviewed by:
Hiraku Kameda, Cedars Sinai Medical Center, United StatesCopyright © 2024 van der Leij, Suijkerbuijk, van den Broek, Valk, Dankbaar and van Santen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie van der Leij, cy5tLnZhbmRlcmxlaWpAdW1jdXRyZWNodC5ubA==
†ORCID: Stephanie van der Leij, orcid.org/0000-0002-7679-0382
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.