
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 30 September 2024
Sec. Reproduction
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1399580
Objective: Studies have shown the adverse psychological impact of polycystic ovary syndrome (PCOS), but the state of mental health in adolescents with PCOS remains unclear. Thus, we performed a systematic review and meta-analysis to investigate the prevalence and severity of depression and anxiety, as well as potential effects on self-esteem and quality of life (QoL) in this specific population.
Methods: We systematically searched four electronic databases: PubMed, Embase, Web of Science, and the Cochrane Reviews database for articles published until 25/8/2024. We considered observational studies in which the subjects were adolescent girls with PCOS who had reported symptoms including anxiety, depression, self-esteem, and QoL. The Review Manager version 5.4 was used to analyze the available data extracted. We used the Newcastle-Ottawa Quality Assessment Scale (NOS) to evaluate the quality of selected studies. A funnel plot was utilized to assess the risk of literature bias, and a forest plot was used to represent the combined outcomes. This systematic review was previously registered in PROSPERO with the registration number CRD42022382036.
Results: We included 11 studies in the systematic review and conducted meta-analyses on 10 of them. Adolescents with PCOS reported a higher risk of depression (OR = 2.21, 95% CI: 1.23 to 4.00, p = 0.008) and a higher level of depression scores (SMD = 0.43, 95% CI: 0.16 to 0.71, p = 0.002) than controls. There were no significant differences in anxiety (OR = 1.90, 95% CI: 0.52 to 6.96, p = 0.33; SMD = 0.19, 95% CI: -0.21 to 0.59, p = 0.36), self-esteem (SMD = -0.17, 95% CI: -0.85 to 0.52, p = 0.64), and QoL (SMD = -0.15, 95% CI: -0.42 to 0.11, p = 0.26) between the two groups.
Conclusions: Our research indicated that adolescents with PCOS experienced more severe depressive symptoms than those without PCOS. This highlights the importance of evaluation and early treatment of mental health in PCOS. More clinicians should pay attention to the mental health of adolescent girls with PCOS through this study.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022382036.
Polycystic ovary syndrome (PCOS), a prevalent endocrine-metabolic disease in women of reproductive age, usually appears in adolescence (1). Its prevalence ranges from 5% to 18% in women and 3.4% to 11% in adolescent girls, depending on different diagnostic criteria (2, 3). The primary clinical characteristics encompass irregular menstrual periods, presentation of androgen excess (such as hirsutism, acne, and laboratory hyperandrogenism), and polycystic ovary morphology (PCOM) on ultrasound. According to the commonly used Rotterdam criteria, meeting at least 2 of the above criteria and excluding other diseases can be diagnosed as PCOS (4). However, the diagnosis of PCOS in adolescent girls remains challenging due to the overlap of these features with the presentation in adolescence. Based on the recent 2023 Evidence-Based Guidelines for PCOS, menstrual irregularity and clinical/biochemical hyperandrogenemia are necessary to diagnose PCOS in adolescents, and PCOM is not suggested as a diagnostic criterion for PCOS in adolescence (5).
Women with PCOS are at significant risk of a broad spectrum of complications, including infertility, obesity, insulin resistance, metabolic syndrome, diabetes, and cardiovascular disease (6). In addition to the metabolic and reproductive aspects, PCOS significantly impacts the psychological health of women affected (6). It should be noted that adolescents are vulnerable to mental health problems, given the dramatic changes in hormones, body, brain, social environment, and cognition in this special period (7). For adolescent girls with PCOS, the illness’s underlying pathophysiology and associated physical features may lead to apparent concerns about body image, reduction of self-esteem, depression, and anxiety (8–10). Depression is distinguished by a consistently low mood and a lack of interest or pleasure, with primary symptoms including appetite disorders, sleep disturbances, and concentration deficit, among others (11). In recent years, many cross-sectional studies have investigated the fact that the prevalence of depression has significantly increased in girls with PCOS. Anxiety is an emotional experience of inner nervousness and impending disaster, often manifested as excessive concern, inability to sit still, and autonomic nervous disorders, such as chest tightness, palpitations, sweating, and so on (12). Self-esteem is an individual’s subjective evaluation of self-worth, and mental disorders are related to the decline of self-esteem (13, 14). There is growing evidence that women with PCOS are prone to psychological problems. For these patients, the quality of life (QoL) can be substantially impacted in various aspects of fulfilling life and subjective well-being (15). Besides, adolescent depression and anxiety are linked to elevated odds of adult depression and anxiety disorders, thereby adding to the long-term burden of disease (16, 17). Considering elements like the greater possibility of binge eating, higher sedentary behaviors, and the use of psychiatric medications, these psychological issues are likely to contribute to weight gain and a higher risk of cardiovascular disease (18, 19). In this sense, psychological disorders in adolescence can have multiple, long-term detrimental effects on the well-being of patients.
At present, several researchers have conducted investigations on depression and anxiety in adolescent girls with PCOS. Emeksiz HC. et al. reported that female adolescents with PCOS exhibited higher levels of depression and anxiety compared to healthy controls (20). In a cross-sectional study conducted by Çoban ÖG and colleagues, the rate of psychiatric disorders, including major depressive disorder and anxiety disorder, in the PCOS group was considerably higher than that in the control subjects (21). However, Zachurzok A. et al. had a contradictory result, which showed that no association was discovered between self-esteem, anxiety, depression, and PCOS in adolescent girls (22). Collectively, the findings of studies on this topic are still inconclusive. Furthermore, to our knowledge, no meta-analysis has been performed on anxiety and depression in adolescent girls with PCOS.
Given the numerous adverse impacts of psychological problems and the contrasting conclusions regarding the mental health of adolescent girls with PCOS, it is critical to summarize available evidence in this area. Therefore, we undertook a systematic review and meta-analysis of observational studies that explored depression and anxiety of adolescent females with PCOS. This study aimed to investigate the prevalence and extent of depression and anxiety, as well as the situation of self-esteem and QoL in adolescent girls with PCOS. We anticipate that this research will be helpful for clinicians in timely identifying high-risk populations and promoting the physical and mental well-being of women with PCOS.
This meta-analysis was conducted by the PRISMA guidelines (23) (Supplementary Tables S1, S2). We systematically searched for published studies up to 25/8/2024 in the four electronic databases: PubMed, Embase, Web of Science, and the Cochrane Reviews. The search strategy used was a combination of subject headings and free words for PCOS, adolescents, and depression/anxiety (including depression, anxiety, mental disorders, mood disorders, psychological distress, mental health, and emotion). Detailed search strategies were provided in the Supplementary Materials (Supplementary Data Sheet 1). The PICOS framework for systematic review: for patients with PCOS (Participants), depression, anxiety, self-esteem, and QoL (Outcomes) for the observational group than girls without PCOS (Comparison) in observational studies (Type of studies). We also manually retrieved the references of relevant review articles for potentially eligible studies. Additionally, we looked through the relevant gray literature and dissertations and, when necessary, contacted the study authors to request additional information. The systematic review was previously registered in PROSPERO with the registration number CRD42022382036.
Studies that met the following criteria were included: (1) the study was a case-control, longitudinal cohort, or cross-sectional design with one group of subjects with PCOS and another group without PCOS; (2) the study population was between 10 and 19 years old [according to WHO (24)]; (3) the study reported the rate or level of depression/anxiety or depressive/anxiety symptoms using validated assessment tools, whether rating scales, diagnostic interviews or clinical diagnosis; and (4) the article was published in English. Studies published as reviews and abstracts were excluded owing to the lack of comprehensive information, such as detailed study protocols.
Two authors independently reviewed and checked the eligibility of studies and extracted relevant data, with any discrepancies resolved by a third author. The following general information was obtained from each included study: publication information (first author, publication year, country of publication, and quality assessment); study characteristics (study design, recruitment of participants, sample size, diagnostic criteria for PCOS, reported outcomes, and used screening tools); subjects’ information (range of age, mean age, mean body mass index, and matched variables between PCOS group and control group).
Regarding outcomes, we took depression and anxiety as the primary indices and self-esteem and QoL as the secondary index. We extracted the number of related events and the mean and standard deviation of scores as accurately as possible. Given that trait anxiety refers to consistent emotional experience and state anxiety refers to transient emotional response, we gave priority to the score of the trait anxiety subscale in the State-Trait Anxiety Inventory (25). Besides, because there was no significant cutoff value in the study of Almis H. and colleagues, we defined a trait anxiety score of less than 40 as a normal level according to the scoring rule (26).
We used the Newcastle-Ottawa Quality Assessment Scale (NOS) to evaluate the quality of selected studies, with a maximum score of 9 (27). According to the scale, 0-3 scores indicated low quality, 4-6 scores indicated moderate quality, and 7-9 scores indicated high quality. Two independent reviewers conducted the quality assessment, and a third author addressed disagreements.
All analyses were performed using Review Manager version 5.4. The prevalence of depression and anxiety was calculated using odds ratios (OR) and 95% confidence intervals (CI). The effect sizes of the mean difference in depression, anxiety, self-esteem, and QoL between PCOS subjects and controls were calculated using the standardized mean difference (SMD) and 95% CI. The I2 statistic was used to assess heterogeneity among studies. When heterogeneity was high (I2>50%), we used the random effects model to estimate pooled effect size; otherwise, we used the fixed effects model (28). Subgroup analyses were utilized to explore the reason for heterogeneity. A funnel plot was used to evaluate the risk of literature bias. P<0.05 was considered statistically significant in all analyses.
Figure 1 shows the flow diagram for study selection. Our systematic literature search identified 1,685 potentially relevant records. After removing duplicate articles and screening by title and abstract, 116 articles remained for further full-text review. 105 studies were excluded for the reasons illustrated in Figure 1, and 11 were eligible according to our predetermined inclusion criteria. However, the results in one of the studies were not described in terms of mean and standard deviation and were not included in our meta-analysis.
Table 1 summarizes the characteristics of 11 eligible studies, which comprised 545 girls with PCOS and 2,042 controls (20–22, 26, 29–35). These studies were published from 2013 to 2022, including nine cross-sectional and two cohort studies. Although six studies were performed in Turkey, there were also five studies from Lebanon, New Zealand, Italy, the United States of America (USA), and Poland, respectively. Nearly all the studies matched PCOS and control groups by age, and four matched body mass indexes (BMI). The quality assessment scores for the included studies ranged from 2 to 7, with a mean score of 4.91.
Regarding outcomes, all studies reported depression/depressive symptoms; nine studies reported anxiety/anxiety symptoms; three studies reported QoL and three studies reported self-esteem by applying multiple valid evaluation tools. The depressive symptoms were measured based on rating scales including the Beck Depression Inventory (29, 34, 35), the Child Depression Inventory (20, 26, 33), Hospital Anxiety and Depression Scale (22), the Symptom Questionnaire (31), the Center for Epidemiologic Studies-Depression (32), and the Reynolds Adolescent Depression Scale (30). The anxiety symptoms were assessed based on scales including the State-Trait Anxiety Inventory (26, 34, 35), Hospital Anxiety and Depression Scale (22), the Symptom Questionnaire (31), and the Screen for Child Anxiety Related Emotional Disorders (20, 29). In two studies, the psychiatric disorders were evaluated by the Schedule for Affective Disorders and Schizophrenia for School Age Children Present and Lifetime (21, 33).
For the prevalence of depression, data from seven studies comprising 2,084 participants was calculated in the meta-analysis (21, 22, 26, 30, 32, 33, 35) (Figure 2A). The results showed a pooled OR of 2.21 (95% CI: 1.23 to 4.00, p = 0.008) based on the random effects model, which indicated that adolescent girls with PCOS were 2.21 times more likely to have depression than subjects without PCOS. Besides, the heterogeneity of this comparison was moderate at 59% (p = 0.02).
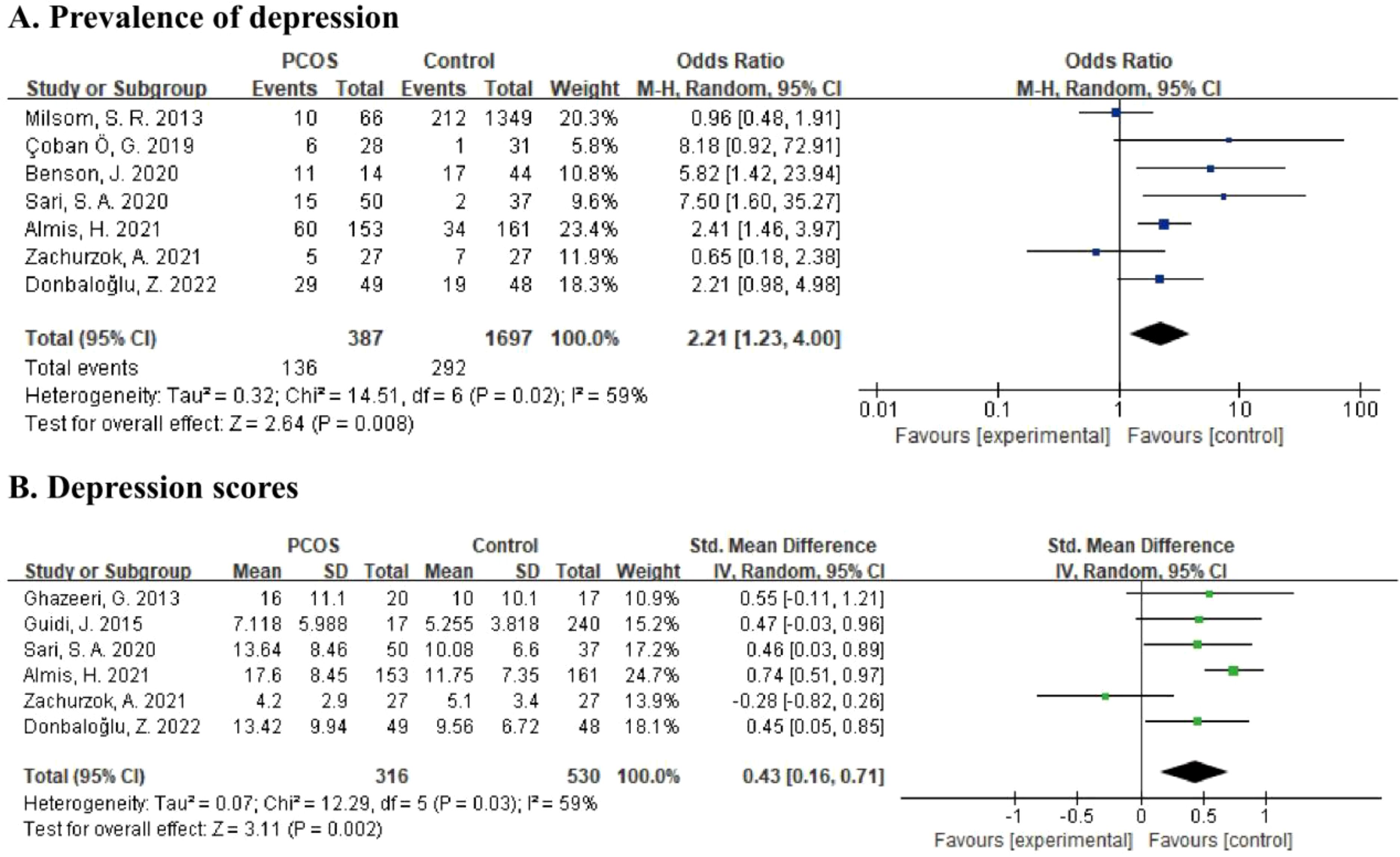
Figure 2. (A) Forest plot comparing the prevalence of depression between PCOS group and control group. (B) Forest plot of depression scores in PCOS group and control group.
For the severity of depressive symptoms, six studies reporting depression scores involving 846 subjects were included in meta-analysis (22, 26, 29, 31, 33, 35) (Figure 2B). By using the random effects model, there was a pooled SMD of 0.43 (95% CI: 0.16 to 0.71, p = 0.002) with moderate heterogeneity (I2 = 59%, p = 0.03), which showed that a higher level of depression was present in PCOS adolescents than in controls.
Data from four studies, including 514 participants, was calculated in the meta-analysis21,22,26,33 (Figure 3A) for the prevalence of anxiety. According to the random effects model, the pooled OR was 1.90 (95% CI: 0.52 to 6.96, p = 0.33), and the results were highly heterogeneous (I2 = 81%, p = 0.001). For the severity of anxiety symptoms, six studies providing anxiety scores with a total of 838 subjects were included in the meta-analysis (22, 26, 29, 31, 34, 35) (Figure 3B). Using the random effects model, the pooled SMD was estimated as 0.19 (95% CI: -0.21 to 0.59, p = 0.36). Heterogeneity among studies was relatively high (I2 = 81%, p < 0.0001).
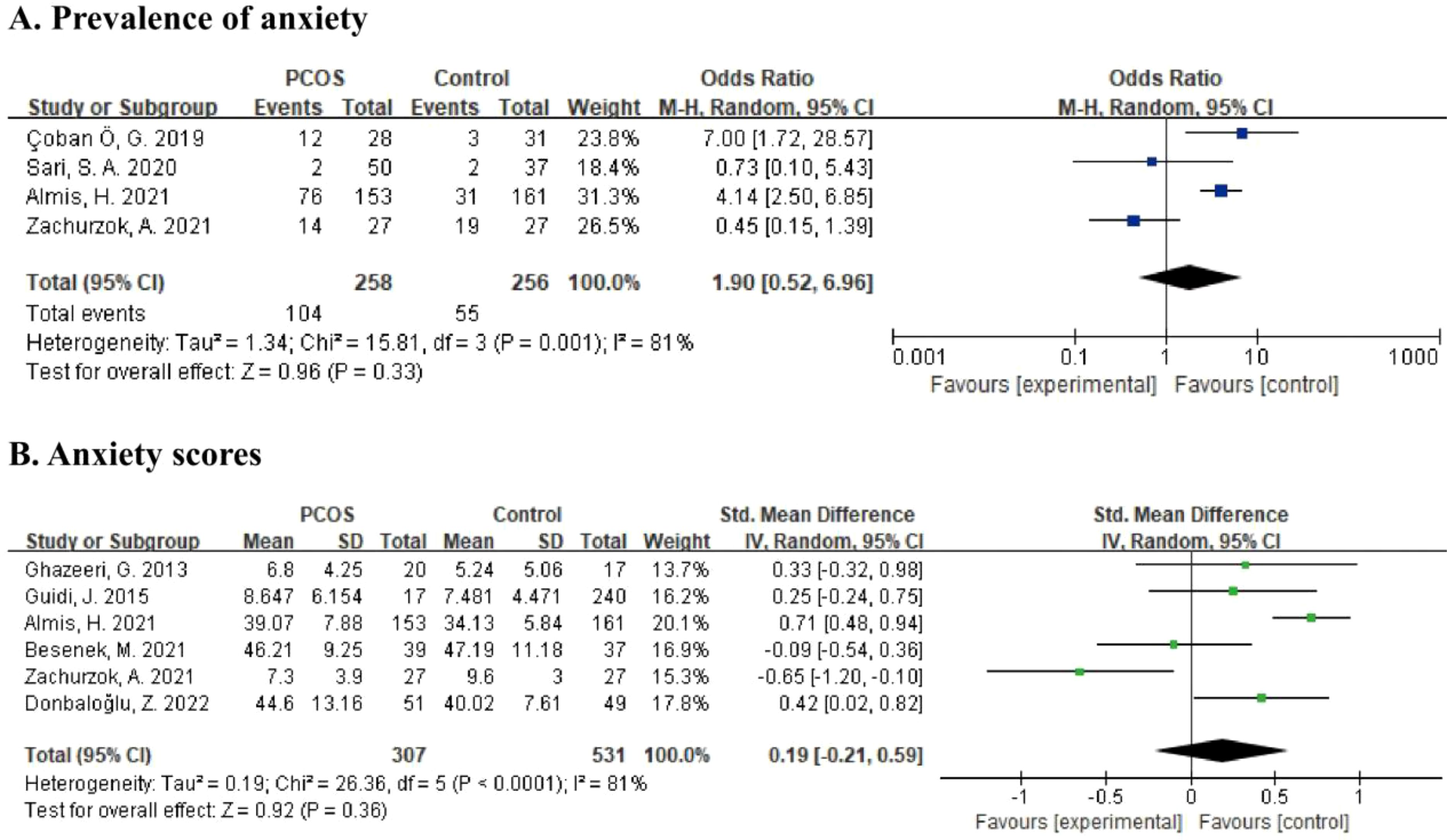
Figure 3. (A) Forest plot comparing the prevalence of anxiety between PCOS group and control group. (B) Forest plot of anxiety scores in PCOS group and control group.
Of all qualified studies, three articles examined the self-esteem status in the PCOS and control groups (21, 22, 33). Figure 4 shows no significant difference in self-esteem scores between the two groups (SMD = -0.17, 95% CI: -0.85 to 0.52, p = 0.64; I2 = 83%, p = 0.003).
Of all eligible studies, three investigated the QoL situation between the PCOS group and controls (21, 31, 35). As illustrated in Figure 5, the two groups had no significant difference in QoL scores (SMD = -0.15, 95% CI: -0.42 to 0.11, p = 0.26; I2 = 0%, p = 0.55).
In subgroup analyses by continent of origin (Supplementary Figure S1), results remained significant for depression in the Asian group, with higher prevalence and scores in PCOS versus controls. The values of I2 in the Asian group were substantially decreased, which implied that the region might be a potential source of heterogeneity. However, there was still high heterogeneity in the results of anxiety, both in the Asian and non-Asian groups. In subgroup analyses by screening tool (Supplementary Figure S2), results were still significant in the BDI (the Beck Depression Inventory) group and CDI (the Child Depression Inventory) group with minor heterogeneity, which implied that differences in screening tools might partly explain the heterogeneity. However, there was still high heterogeneity in the results of anxiety scores in both subgroups.
This is the first systematic review and meta-analysis to summarize available evidence on the situation of depression and anxiety in adolescent girls (aged 10 - 19 years old) with PCOS. In this meta-analysis, we discovered that adolescent females with PCOS had a higher risk of depression and a higher level of depression scores than controls. However, there were no significant differences in the prevalence and extent of anxiety between the two groups. In addition, self-esteem and QoL, the secondary indices in our study, were also not substantially different between the two groups.
In the current study, PCOS adolescents were 2.21 times more likely to have depression than the control group. In line with our results, the statistically significant association between PCOS and depression was also observed in some previous meta-analyses mainly targeting adults, with elevated odds of depression over two to four times in PCOS participants (36–39). Besides, we found that PCOS adolescents experienced more serious depressive symptoms than controls, which was also in accordance with previous studies (39–42). Even though our effect sizes are lower than those in all of the research mentioned above, which primarily focused on adult PCOS, it is still important to note the significant link between PCOS in adolescents and depression. Based on global estimate, approximately 280 million people are affected by depression, with its prevalence rises significantly during adolescence (43, 44). Extensive evidence has illustrated that early-life depression is linked to several adverse health outcomes, including not only suicidal tendencies, recurrence and co-occurrence of mental disorders, and increased risk of cardiovascular disease but also long-term impairments in education, employment, and social participation (16, 18, 45). As a result, our study highlighted the need to early identify and manage depression in adolescents with PCOS to foster favorable health outcomes.
The mechanisms underlying the connection between PCOS and vulnerability to depression are not fully clarified, and several potential factors have been explored (Figure 6). Women with PCOS may be distressed about the changes in physical appearance caused by obesity and hirsutism, which become a possible source of PCOS women’s depressed emotion (9, 36). Additionally, their subjective perceptions of symptoms play a more critical role than objective assessment (46). Besides, some PCOS patients’ concerns regarding infertility place a specific psychological strain on them (47). From the point of view of pathophysiology, the potential contribution of hormonal imbalance has also been investigated. Hypercortisolemia caused by chronic stress and hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis plays a crucial role in the development of depression (48). It has been shown that women with PCOS experience a more significant increase in cortisol levels after exposure to stress compared to controls (49). Persistently high cortisol levels will lead to desensitization of cortisol receptors and failure of negative feedback regulation, resulting in persistent hyperfunction of the HPA axis and a vicious cycle (50). Insulin resistance may be associated with depression in PCOS, serving as a physiologic mediator (51). In a randomized controlled trial, the increase of the homeostatic model of insulin resistance (HOMA-IR) elevated the odds of depression by 2.32 (51). One possible explanation is that insulin resistance makes the brain less adaptable, which can lead to changes in the structure or function of key brain regions to influence emotion regulation (52). In addition, in an experiment, dehydroepiandrosterone-induced PCOS mice exhibited depression-like behavior, possibly by down-regulating monoamines and/or their metabolites in brain (53). Hyperandrogenemia may be related to depression in PCOS, which needs to be further explored in human studies. Moreover, several relevant mechanisms have been discussed: inflammation (54), vitamin D insufficiency (55), and change of neurotransmitter (54, 56), which may also be involved in the development of depression in PCOS. Several studies have confirmed the link between potential factors mentioned above [such as BMI (20), hirsutism (26), acne (26), and free testosterone levels (35)] and depression in the adolescent PCOS population.
In our study, no association was observed between PCOS and anxiety in adolescent populations. Although none of the previous meta-analyses on this topic focused entirely on adolescents, the results of subgroup analyses based on age in several studies provided relevant evidence. A meta-analysis conducted by Veltman-Verhulst SM and colleagues suggested that there was no significant difference in anxiety scores within the subgroup of PCOS women who were 24 years old or younger (40). Brutocao C. et al. found that mixed adult/pediatric populations with PCOS had a 1.70-fold risk of anxiety disorder compared to controls, which was lower than in the adult group (OR= 5.22) (39). Taking our results together with previous evidence, we speculate that PCOS has not yet had a notable effect on the situation of anxiety in adolescent girls. This may be because adolescents are still in the early stage of PCOS, and the duration of symptoms is relatively short. What’s more, some symptoms of PCOS, such as irregular menstruation, are likely to be considered normal in adolescence and not enough to attract the attention of adolescents (57). Nonetheless, it should be noted that a lack of included studies constrains the existing evidence. Therefore, our conjecture needs further verification.
In the present study, no substantial differences remained in the domains of self-esteem and QoL between the adolescent PCOS group and the control group. Self-esteem comes from a positive evaluation of self-worth; for teenagers, appearance is an important factor affecting self-esteem (58, 59). Although PCOS may be accompanied by some physical changes, no significant effect of the illness on adolescent girls’ self-esteem was observed in our study. In terms of QoL, only one study has conducted a review targeting adolescents with PCOS, and most of the studies in this review showed impaired QoL in PCOS subjects, which is inconsistent with our results (60). However, it is worth noting that its inclusion criteria differ from ours. Besides, our findings should be cautiously explained due to the limited evidence.
The strengths of our analysis are that it was the first to look into the prevalence and severity of depression and anxiety targeting adolescent girls with PCOS, considering that adolescence is both an early-onset stage of these disorders and a critical period of personal development. We conducted a comprehensive literature search and included studies from multiple regions worldwide, which enhanced our results’ generalizability. Several limitations of our study should be considered. Firstly, the number of studies included was relatively small, and we didn’t delve deeper into the relationship between depression, anxiety, and potential factors. Second, most subjects were Caucasian, implying that more relevant studies should be conducted in more countries. Thirdly, according to the funnel plots (Supplementary Figure S3), it was suggested that there may be some publication bias. In addition, our study still had considerable heterogeneity despite employing appropriate statistical models. Finally, most of the studies we included were cross-sectional, which made it hard to clarify causality.
In conclusion, based on the available evidence, there was a significant association between PCOS and depression among adolescent girls in our study. More large-scale, well-designed studies are needed in the future to draw a more robust conclusion and to clarify the mechanisms linking PCOS and psychological distress. Clinicians should also pay more attention to the mental health status of PCOS patients to facilitate early recognition and timely intervention.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
YL: Investigation, Formal analysis, Data curation, Writing – original draft. JZ: Writing – review & editing, Validation. XZ: Writing – review & editing, Investigation, Data curation. WL: Writing – review & editing. JG: Writing – review & editing. FC: Writing – review & editing, Conceptualization. CL: Writing – review & editing, Project administration, Funding acquisition, Conceptualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Fujian Provincial Health Science and Technology Project (No. 2022CXB016) and High-quality development Funds of The First Affiliated Hospital of Xiamen University (NO. YN81870611).
We are grateful to all the subjects for their participation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1399580/full#supplementary-material
1. Trent M, Gordon CM. Diagnosis and management of polycystic ovary syndrome in adolescents. Pediatrics. (2020) 145:S210–s218. doi: 10.1542/peds.2019-2056J
2. Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. (2022) 10:668–80. doi: 10.1016/s2213-8587(22)00163-2
3. Naz MSG, Tehrani FR, Majd HA, Ahmadi F, Ozgoli G, Fakari FR, et al. The prevalence of polycystic ovary syndrome in adolescents: A systematic review and meta-analysis. Int J Reprod biomedicine. (2019) 17:533–42. doi: 10.18502/ijrm.v17i8.4818
4. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility sterility. (2004) 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004
5. Teede HJ, Tay CT, Laven J, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertility sterility. (2023) 120:767–93. doi: 10.1016/j.fertnstert.2023.07.025
6. Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertility sterility. (2012) 97:28–38.e25. doi: 10.1016/j.fertnstert.2011.09.024
7. Blakemore SJ. Adolescence and mental health. Lancet (London England). (2019) 393:2030–1. doi: 10.1016/s0140-6736(19)31013-x
8. Farrell K, Antoni MH. Insulin resistance, obesity, inflammation, and depression in polycystic ovary syndrome: biobehavioral mechanisms and interventions. Fertility sterility. (2010) 94:1565–74. doi: 10.1016/j.fertnstert.2010.03.081
9. Alur-Gupta S, Chemerinski A, Liu C, Lipson J, Allison K, Sammel MD, et al. Body-image distress is increased in women with polycystic ovary syndrome and mediates depression and anxiety. Fertility sterility. (2019) 112:930–8.e931. doi: 10.1016/j.fertnstert.2019.06.018
10. Dowdy D. Emotional needs of teens with polycystic ovary syndrome. J Pediatr Nurs. (2012) 27:55–64. doi: 10.1016/j.pedn.2010.08.001
11. Barry MJ, Nicholson WK, Silverstein M, Chelmow D, Coker TR, Davidson KW, et al. Screening for depression and suicide risk in adults: US preventive services task force recommendation statement. Jama. (2023) 329:2057–67. doi: 10.1001/jama.2023.9297
12. Penninx BW, Pine DS, Holmes EA, Reif A. Anxiety disorders. Lancet (London England). (2021) 397:914–27. doi: 10.1016/s0140-6736(21)00359-7
13. Donnellan MB, Trzesniewski KH, Robins RW. “Self-Esteem” in The Wiley‐Blackwell Handbook of Individual Differences. (2011). pp. 718–46.
14. Guillon MS, Crocq MA, Bailey PE. The relationship between self-esteem and psychiatric disorders in adolescents. Eur psychiatry: J Assoc Eur Psychiatrists. (2003) 18:59–62. doi: 10.1016/s0924-9338(03)00002-6
15. Olatunji BO, Cisler JM, Tolin DF. Quality of life in the anxiety disorders: a meta-analytic review. Clin Psychol Rev. (2007) 27:572–81. doi: 10.1016/j.cpr.2007.01.015
16. Johnson D, Dupuis G, Piche J, Clayborne Z, Colman I. Adult mental health outcomes of adolescent depression: A systematic review. Depression Anxiety. (2018) 35:700–16. doi: 10.1002/da.22777
17. Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. (1998) 55:56–64. doi: 10.1001/archpsyc.55.1.56
18. Goldstein BI, Korczak DJ. Links between child and adolescent psychiatric disorders and cardiovascular risk. Can J Cardiol. (2020) 36:1394–405. doi: 10.1016/j.cjca.2020.06.023
19. Chao AM, Wadden TA, Berkowitz RI. Obesity in adolescents with psychiatric disorders. Curr Psychiatry Rep. (2019) 21:3. doi: 10.1007/s11920-019-0990-7
20. Emeksiz HC, Bideci A, Nalbantoğlu B, Nalbantoğlu A, Çelik C, Yulaf Y, et al. Anxiety and depression states of adolescents with polycystic ovary syndrome. Turkish J Med Sci. (2018) 48:531–6. doi: 10.3906/sag-1708-131
21. Çoban Ö G, Tulacı Ö D, Adanır AS, Önder A. Psychiatric disorders, self-esteem, and quality of life in adolescents with polycystic ovary syndrome. J Pediatr Adolesc gynecology. (2019) 32:600–4. doi: 10.1016/j.jpag.2019.07.008
22. Zachurzok A, Pasztak-Opilka A, Gawlik AM. Depression, anxiety and self-esteem in adolescent girls with polycystic ovary syndrome. Ginekologia polska. (2021) 10.5603/GP.a2021.0042. doi: 10.5603/GP.a2021.0042
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed.). (2021) 372:n71. doi: 10.1136/bmj.n71
24. Organization WH. The second decade: Improving adolescent health and development (2001). Available at: https://www.who.int/publications/i/item/WHO_FRH_ADH_98.18_Rev.1. (Accessed January 13, 2023).
25. Endler NS, Kocovski NL. State and trait anxiety revisited. J Anxiety Disord. (2001) 15:231–45. doi: 10.1016/s0887-6185(01)00060-3
26. Almis H, Orhon F, Bolu S, Almis BH. Self-concept, depression, and anxiety levels of adolescents with polycystic ovary syndrome. J Pediatr Adolesc gynecology. (2021) 34:311–6. doi: 10.1016/j.jpag.2020.12.011
27. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses(2010). Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (Accessed January 13, 2023).
28. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Res ed.). (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
29. Ghazeeri G, Fakih A, Abbas HA, Harajly S, Awwad J. Anxiety, cognitive, and depressive assessment in adolescents with polycystic ovarian syndrome: a pilot study. J Pediatr Adolesc gynecology. (2013) 26:269–73. doi: 10.1016/j.jpag.2013.04.005
30. Milsom SR, Nair SM, Ogilvie CM, Stewart JM, Merry SN. Polycystic ovary syndrome and depression in New Zealand adolescents. J Pediatr Adolesc gynecology. (2013) 26:142–7. doi: 10.1016/j.jpag.2012.11.013
31. Guidi J, Gambineri A, Zanotti L, Fanelli F, Fava GA, Pasquali R. Psychological aspects of hyperandrogenic states in late adolescent and young women. Clin Endocrinol. (2015) 83:872–8. doi: 10.1111/cen.12783
32. Benson J, Severn C, Hudnut-Beumler J, Simon SL, Abramson N, Shomaker LB, et al. Depression in girls with obesity and polycystic ovary syndrome and/or type 2 diabetes. Can J Diabetes. (2020) 44:507–13. doi: 10.1016/j.jcjd.2020.05.015
33. Sari SA, Celik N, Uzun Cicek A. Body perception, self-esteem, and comorbid psychiatric disorders in adolescents diagnosed with polycystic ovary syndrome. J Pediatr Adolesc gynecology. (2020) 33:691–6. doi: 10.1016/j.jpag.2020.08.018
34. Besenek M, Gurlek B. Hyperandrogenism in polycystic ovary syndrome affects psychological well-being of adolescents. J obstetrics gynaecology Res. (2021) 47:137–46. doi: 10.1111/jog.14444
35. Donbaloğlu Z, Tuhan H, Çoban Ö. G, Kızılay DÖ, İsmailoğlu E, Önder A, et al. Hyperandrogenism correlates with psychological symptoms in adolescents with polycystic ovary syndrome. Clin Pediatr endocrinology: Case Rep Clin investigations: Off J Japanese Soc Pediatr Endocrinol. (2022) 31:68–76. doi: 10.1297/cpe.2022-0010
36. Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod (Oxford England). (2017) 32:1075–91. doi: 10.1093/humrep/dex044
37. Dokras A, Clifton S, Futterweit W, Wild R. Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obstetrics gynecology. (2011) 117:145–52. doi: 10.1097/AOG.0b013e318202b0a4
38. Blay SL, Aguiar JV, Passos IC. Polycystic ovary syndrome and mental disorders: a systematic review and exploratory meta-analysis. Neuropsychiatr Dis Treat. (2016) 12:2895–903. doi: 10.2147/ndt.S91700
39. Brutocao C, Zaiem F, Alsawas M, Morrow AS, Murad MH, Javed A. Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine. (2018) 62:318–25. doi: 10.1007/s12020-018-1692-3
40. Veltman-Verhulst SM, Boivin J, Eijkemans MJ, Fauser BJ. Emotional distress is a common risk in women with polycystic ovary syndrome: a systematic review and meta-analysis of 28 studies. Hum Reprod Update. (2012) 18:638–51. doi: 10.1093/humupd/dms029
41. Barry JA, Kuczmierczyk AR, Hardiman PJ. Anxiety and depression in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod (Oxford England). (2011) 26:2442–51. doi: 10.1093/humrep/der197
42. Yin X, Ji Y, Chan CLW, Chan CHY. The mental health of women with polycystic ovary syndrome: a systematic review and meta-analysis. Arch women's Ment Health. (2021) 24:11–27. doi: 10.1007/s00737-020-01043-x
43. GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. (2022) 9:137–50. doi: 10.1016/s2215-0366(21)00395-3
44. Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. (2022) 27:281–95. doi: 10.1038/s41380-021-01161-7
45. Clayborne ZM, Varin M, Colman I. Systematic review and meta-analysis: adolescent depression and long-term psychosocial outcomes. J Am Acad Child Adolesc Psychiatry. (2019) 58:72–9. doi: 10.1016/j.jaac.2018.07.896
46. Pasch L, He SY, Huddleston H, Cedars MI, Beshay A, Zane LT, et al. Clinician vs self-ratings of hirsutism in patients with polycystic ovarian syndrome: associations with quality of life and depression. JAMA Dermatol. (2016) 152:783–8. doi: 10.1001/jamadermatol.2016.0358
47. Tan S, Hahn S, Benson S, Janssen OE, Dietz T, Kimmig R, et al. Psychological implications of infertility in women with polycystic ovary syndrome. Hum Reprod (Oxford England). (2008) 23:2064–71. doi: 10.1093/humrep/den227
48. Belmaker RH, Agam G. Major depressive disorder. New Engl J Med. (2008) 358:55–68. doi: 10.1056/NEJMra073096
49. Benson S, Arck PC, Tan S, Hahn S, Mann K, Rifaie N, et al. Disturbed stress responses in women with polycystic ovary syndrome. Psychoneuroendocrinology. (2009) 34:727–35. doi: 10.1016/j.psyneuen.2008.12.001
50. Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:722–9. doi: 10.1016/j.pnpbp.2010.04.011
51. Greenwood EA, Pasch LA, Cedars MI, Legro RS, Eisenberg E, Huddleston HG, et al. Insulin resistance is associated with depression risk in polycystic ovary syndrome. Fertility sterility. (2018) 110:27–34. doi: 10.1016/j.fertnstert.2018.03.009
52. Rasgon NL, McEwen BS. Insulin resistance-a missing link no more. Mol Psychiatry. (2016) 21:1648–52. doi: 10.1038/mp.2016.162
53. Yu Q, Hao S, Wang H, Song X, Shen Q, Kang J. Depression-like behavior in a dehydroepiandrosterone-induced mouse model of polycystic ovary syndrome. Biol Reprod. (2016) 95:79. doi: 10.1095/biolreprod.116.142117
54. Aydogan Kirmizi D, Baser E, Onat T, Demir Caltekin M, Yalvac ES, Kara M, et al. Sexual function and depression in polycystic ovary syndrome: Is it associated with inflammation and neuromodulators? Neuropeptides. (2020) 84:102099. doi: 10.1016/j.npep.2020.102099
55. Naqvi SH, Moore A, Bevilacqua K, Lathief S, Williams J, Naqvi N, et al. Predictors of depression in women with polycystic ovary syndrome. Arch women's Ment Health. (2015) 18:95–101. doi: 10.1007/s00737-014-0458-z
56. Chaudhari N, Dawalbhakta M, Nampoothiri L. GnRH dysregulation in polycystic ovarian syndrome (PCOS) is a manifestation of an altered neurotransmitter profile. Reprod Biol endocrinology: RB&E. (2018) 16:37. doi: 10.1186/s12958-018-0354-x
57. Ibáñez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, et al. An international consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Hormone Res paediatrics. (2017) 88:371–95. doi: 10.1159/000479371
58. Biro FM, Striegel-Moore RH, Franko DL, Padgett J, Bean JA. Self-esteem in adolescent females. J Adolesc health: Off Publ Soc Adolesc Med. (2006) 39:501–7. doi: 10.1016/j.jadohealth.2006.03.010
59. van den Berg PA, Mond J, Eisenberg M, Ackard D, Neumark-Sztainer D. The link between body dissatisfaction and self-esteem in adolescents: similarities across gender, age, weight status, race/ethnicity, and socioeconomic status. J Adolesc health: Off Publ Soc Adolesc Med. (2010) 47:290–6. doi: 10.1016/j.jadohealth.2010.02.004
Keywords: polycystic ovary syndrome, adolescent, depression, anxiety, self-esteem
Citation: Li Y, Zhang J, Zheng X, Lu W, Guo J, Chen F and Liu C (2024) Depression, anxiety and self-esteem in adolescent girls with polycystic ovary syndrome: a systematic review and meta-analysis. Front. Endocrinol. 15:1399580. doi: 10.3389/fendo.2024.1399580
Received: 12 March 2024; Accepted: 10 September 2024;
Published: 30 September 2024.
Edited by:
Spyridon N. Karras, Aristotle University of Thessaloniki, GreeceReviewed by:
Konstantinos Michalakis, Athens State University, United StatesCopyright © 2024 Li, Zhang, Zheng, Lu, Guo, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changqin Liu, bGl1Y2hhbmdxaW5AeG11LmVkdS5jbg==; Fuhong Chen, MTEzNDAwNzY1M0BxcS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.