- 1Department of Nutrition-Related Disease Prevention, Medical University of Silesia, Bytom, Poland
- 2Department of Metabolic Disease Prevention, Medical University of Silesia, Bytom, Poland
- 3Department of Cardiovascular Disease Prevention, Medical University of Silesia, Bytom, Poland
- 4Department of Nutrition and Drug Research, Institute of Public Health, Faculty of Health Science, Jagiellonian University Medical Collage, Karków, Poland
- 5Third Department of Cardiology, Silesian Centre for Heart Disease, Faculty of Medical Science in Zabrze, Medical University of Silesia, Zabrze, Poland
- 6Student Scientific Circle Affiliated of Department of Metabolic Disease Prevention, Faculty of Public Health in Bytom, Medical University of Silesia, Bytom, Poland
- 7Department of Endocrinology, District Hospital, Piekary Śląskie, Poland
Objectives: Polycystic ovary syndrome (PCOS) is a complex endocrine disorder that often coexists with cardiometabolic risk factors. Women with PCOS have a two-fold increased risk of developing type 2 diabetes and substantially elevated risk for cardiovascular disease (CVD) events later in life. PCOS patients may require more comprehensive metabolic screening to identify populations at higher risk of developing CVD and dyslipidemia. It is recommended to evaluate lipid profile, glucose tolerance and of women with PCOS every 2-3 years. Simple, short, and easy methods for the assessment of CVD risk in women with PCOS may be useful tools for implementing CVD prevention strategies by doctors or nutritionists. The aim of this study was to investigate the usefulness of anthropometric indices in the assessment of cardiometabolic risk based on lipid profile in patients with PCOS.
Material and methods: The study involved 49 of Caucasian women aged 18-39 who were diagnosed with PCOS based on the Rotterdam criteria and divided into two groups with normal lipid profile (N=14) and dyslipidemia (N=35). Biochemical parameters were tested in the morning while fasting. Anthropometric parameters such as Body Mass Index (BMI), Body Adiposity Index (BAI), Waist-to-Hip Ratio (WHR), and Waist-to-Height Ratio (WHtR) were calculated, while the Percent of Body Fat was measured using a body analyzer.
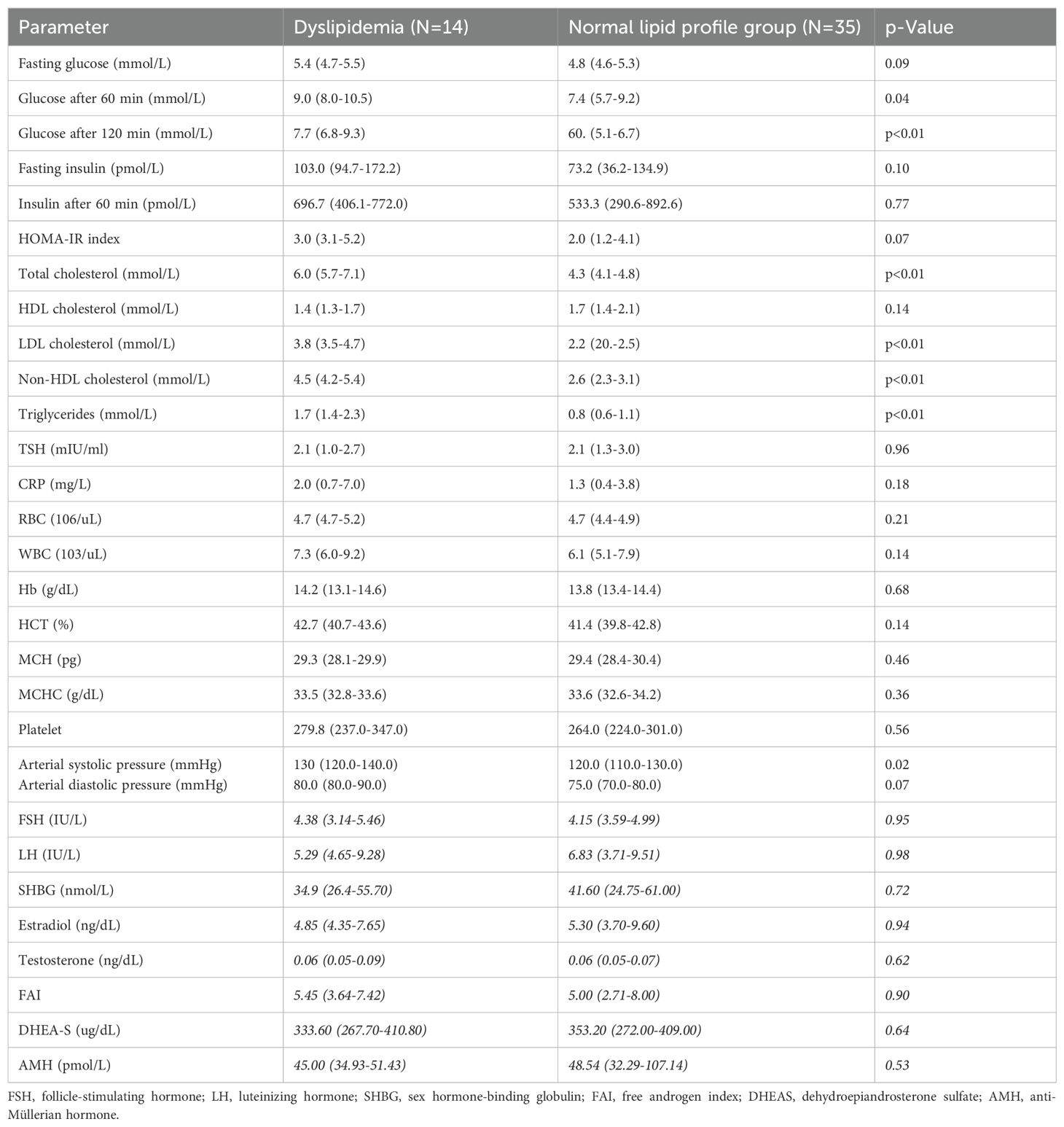
Results: The study demonstrated that women with dyslipidemia were older than the control group, 33 years (27-37) vs 24 years (21-26), p<0.01. Neither BMI nor BAI (%) correlated with total cholesterol (p=0.63 and p=0.27). Other lipid parameters, such as serum HDL cholesterol (R=-0.68, p<0.01; R=-0.58, p<0.01), LDL cholesterol (R=0.34, p=0.02; R=0.37, p=0.01), non-HDL cholesterol (R=0.40, p<0.01; R=0.42, p<0.01), and triglycerides (R=0.56, p<0.01; R=0.51, p<0.01) correlated with BMI and BAI (%). ROC analysis demonstrated a high predictive value for age in identifying dyslipidemia. ROC analysis demonstrated poor predictive value for BMI, BAI, WHR, WHtR in identifying dyslipidemia.
Conclusions: Analysis of simple and rapid parameters used to assess body fat, such as BMI, BAI, WHR, and WHtR, has shown that they are poor predictors of dyslipidemia in women with PCOS. In young women with PCOS, age appears to be a more reliable predictor of dyslipidemia.
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality and morbidity worldwide, accounting for more than 31 million of deaths each year (1, 2). There are numerous risk factors of CVD, including male sex, age, excessive body mass (obesity), lipid disorders, hypertension, and diabetes (2). Polycystic ovary syndrome (PCOS) is an endocrine disorder manifested by ovulatory dysfunction, hyperandrogenism, and polycystic-appearing ovaries morphology.
Women with PCOS have a two-fold increased risk of developing type 2 diabetes (T2DM) and substantially elevated risk for CVD events later in life (3, 4). Since women with PCOS are typically within the pre-menopausal population, long-term CVD risk assessment should be given high priority (3, 4).
Excessive body fat is recognized as a metabolic disease (5). The parameter most strongly associated with cardiometabolic risk factors is visceral adipose tissue (6). Visceral adiposity is associated with dyslipidemia, hypertension, insulin resistance and may be a contributing factor in the development of CVD (6, 7). Reproductive abnormalities are more frequently related to adipose tissue and obesity and are present in 7-10% of women of reproductive age (7). A simple index of weight-for-height, known as body mass index (BMI), is commonly used to classify overweight and obesity in general population (8). Obesity may be attributed to insulin resistance (IR) leading to hyperinsulinemia which stimulates ovarian steroidogenesis (10). Chronic exposure to androgens is associated with the accumulation of visceral fat, which leads to central obesity (3, 9–11). Moreover, elevated testosterone levels and increased BMI have a substantial correlation with diabetes in women with PCOS (12). PCOS patients may require more comprehensive metabolic screening to identify populations at higher risk of developing CVD and dyslipidemia (13). It is recommended to evaluate lipid profile, glucose tolerance and of women with PCOS every 2-3 years (13). Simple, short, and easy methods for the assessment of CVD risk in women with PCOS may be useful tools for implementing CVD prevention strategies by doctors or nutritionists (13).
Objectives
The aim of this study was to investigate the usefulness of anthropometric indices in the assessment of cardiometabolic risk based on lipid profile in patients with PCOS.
Materials and methods
Study participants
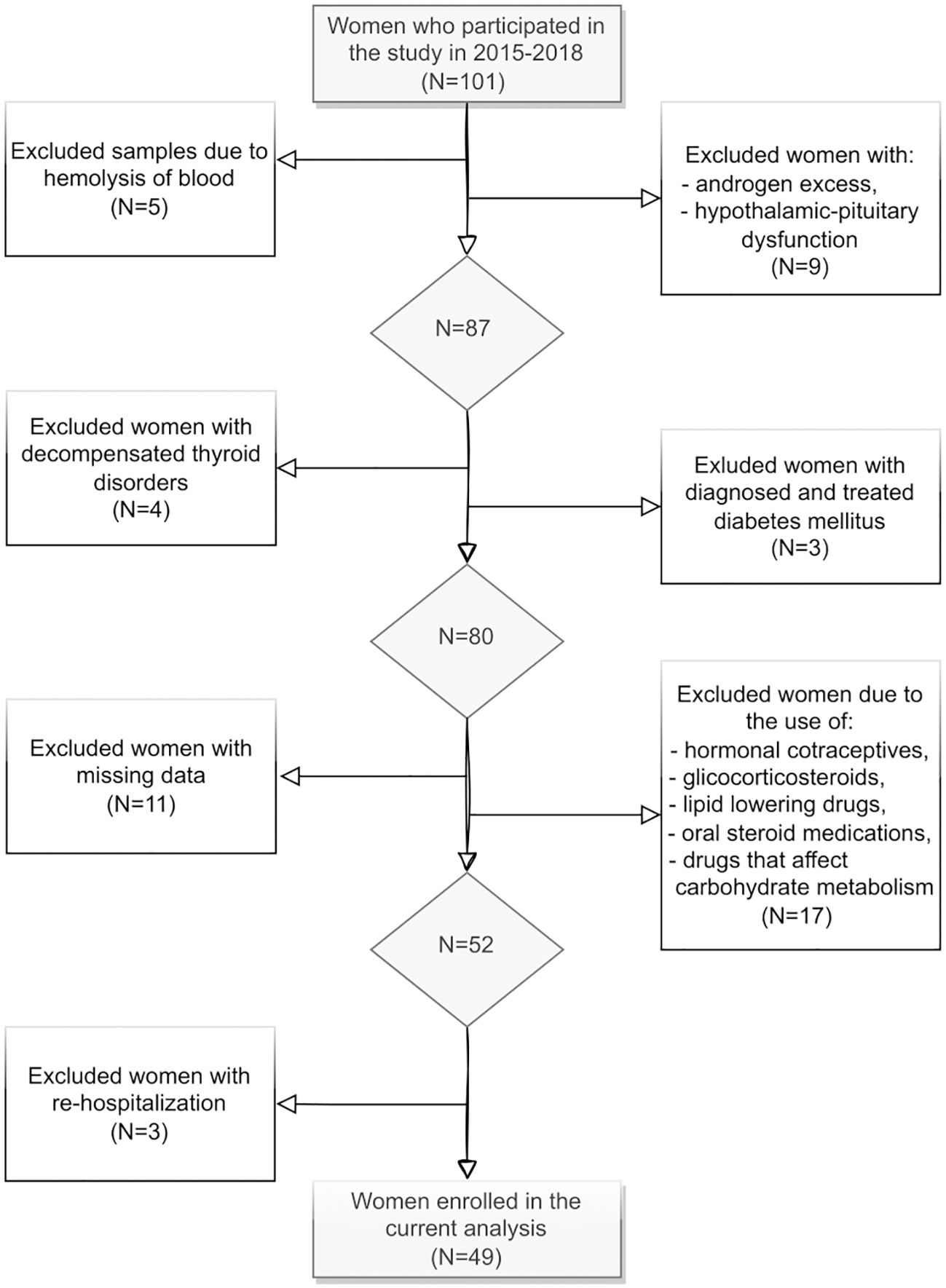
This cross-sectional study was conducted from 2015 to 2018 in the Department of Endocrinology, Piekary Medical Centre, St. Luke’s Local Hospital in Piekary Śląskie, Poland. Inclusion criteria were: PCOS diagnosis based on the 2004 Rotterdam criteria (14), age between 18–40 years and consent to participate. Exclusion criteria included: presence of a cardiac electronic implantable devices, pregnancy, lower limbs prostheses, feet dressings, the use of hormonal contraceptives, glucocorticosteroids, oral steroid medications, lipid-lowering drugs, or drugs that affect glucose metabolism, previous diagnosis and treatment of diabetes mellitus, decompensated thyroid disorders, androgen excess disorders (congenital or late-onset congenital adrenal hyperplasia, hyperprolactinemia, Cushing’s disease/syndrome, and androgen-secreting tumors, idiopathic hirsutism), depressive disorders and ongoing treatment of depression, and rehospitalization lack of patient consent to participate in the study.
101 women aged 18-39, with a median age of 25 years were screened. The final study sample included 49 women (Figure 1). Ultimately, the study population was divided into two groups: those with a normal lipid profile (N=14) and those with dyslipidemia (N=35).
The study follows to the principles outlined in the Declaration of Helsinki and was reviewed and approved by the local bioethics committee (KNW/0022/KB1/143/15). Data analysis consent was obtained from all participants.
Data collection
Fasting blood samples were collected in the morning. A 1 ml blood sample collected as part of routine tests was preserved for further analysis. After centrifugation, the samples were frozen and stored at -70 degrees Celsius until for further analyses. Laboratory tests used in the study were performed following the Standard Work Instructions (SWI) at KORLAB Medical Laboratories, NZOZ Rudzkie Laboratoria Analityczno-Bakteriologiczne s.c. Testosterone levels were measured using the CMIA method - chemiluminescent microparticle immunoassay on the Abbott Alinity platform. For the determination of glucose concentration, a reference enzymatic method with hexokinase was used, utilizing a Cobas Integra 400+ automated analyzer. The glycated hemoglobin was analyzed using high pressure liquid chromatography HPLC (chromatography reversed-phase cation exchange chromatography). An ADAMS A1c HA-8180 analyser was used for analysis. To determine fasting insulin, the ARCHITECT insulin assay was employed, which is a one-step immunochemical assay for the detection of human insulin in plasma or serum using chemiluminescent microparticle immunoassay (CMIA) and microparticle tracer. To determine total cholesterol concentration a Cobas Integra 400+ quantitative analyzed was used, which used the enzymatic-calorimetric method. The direct quantitative determination of cholesterol LDL-C was achieved using the MULTIGENT Direct LDL assay. This test provides a method for direct measurement of serum or plasma levels of cholesterol LDL, without the need for off-analyser pretreatment or centrifugation. An Architect c8000 analyser was used for the determination. The quantitative determination of cholesterol HDL concentration was accomplished by employing a homogeneous calorimetric enzymatic technique. A Cobas Integra 400+ analyser was used. The Cobas Integra 400+ analyser was employed to determine the concentration of using triglyceride concentration using an enzymatic-calorimetric method utilizing oxidase glycerophosphate and 4-aminophenazone.
Biochemical parameters: glucose levels, insulin levels, glycated hemoglobin A1c (HbA1c), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG) were determined and used to calculate the indices. Dyslipidemia was diagnosed based on the following criteria: total serum cholesterol > 5.00 mmol/L and/or LDL-C >3.00 mmol/L and/or serum triglycerides >1.7 mmol/L and/or HDL-C <1.2 mmol/L (15).
The anthropometric indices were calculated according to World Health Organization (WHO) norms for BMI (8) and according to the cut-offs proposed by Bergman for BAI (16).
BMI was calculated according to the following formula:
The following formula was used for the computation of BAI:
Body composition analysis was performed using the TANITA BC 420 MA analyser (TANITA, Japan) with a certificate MDD 93/42 EEC for medical devices.
PCOS diagnosis
All women in the study met the 2004 Rotterdam criteria for the diagnosis of PCOS (14), which required the presence of at least two of the three following clinical abnormalities: polycystic ovarian morphology on ultrasound exam – 12 or more follicles of 2-9 mm in diameter in each ovary and/or increased ovary volume >10 ml (17), clinical and/or biochemical hyperandrogenism (testosterone >70 ng/dl) (18), amenorrhea or oligomenorrhea. A transvaginal ultrasound was performed using a transducer with a bandwidth frequency range of 6-12 MHz, which is optimal for detailed visualization of ovarian structure in the diagnosis of polycystic ovary morphology.
Hyperandrogenism was diagnosed based on elevated androgens (testosterone and/or androstenedione) levels and/or the presence of clinical features (increased body and facial hair – hair distribution and hair growth intensity of nine different body region in Ferriman-Gallway score of >8) (19). Free androgen Index (FAI) has also been evaluated. Other causes of hyperandrogenism include congenital adrenal hyperplasia, hyperprolactinemia, Cushing’s disease, certain types of cancer and certain medications were excluded (see exclusion criteria).
Statistical analysis
Statistical analysis was performed using the STATISTICA 13 PL software (Tulsa, Oklahoma, OK, USA). The Shapiro-Wilk test was used to test the distribution. Continuous variables are expressed as means ± standard deviations (for normally distributed data) or median and interquartile range (for nonparametric data). Normally distributed data were compared using the Student’s t-test, while nonparametric data were compared using the Mann–Whitney U test. The Pearson’s correlation coefficient (normal distribution) and the Spearman rank correlation coefficient (non-normal distribution) were used to assess the relationship between variables. A P value of less than 0.05 was considered significant.
Results
Table 1 shows baseline and clinical characteristics. The study demonstrated that women with dyslipidemia were older than the control group, 33 years (27-37) vs 24 years (21-26), p<0.01. No significant differences in body weight, waist circumference, hip circumference, WHR, waist-to-height ratio (WHtR), or BMI were found between both groups. However, women with dyslipidemia had a higher percentage of body fat than women with normal lipid profile (40.6% vs. 35.1%, p=0.04). Similar results were observed with BAI, where higher values of BAI (35.6%) were found in the dyslipidemia group compared to the normal lipid profile group (32.0%) (p=0.02).
Women with dyslipidemia had higher glucose levels after 60 min and 120 minutes (p=0.04 and p=0.01, respectively) in the oral glucose tolerance test. There were no significant differences in FSH, LH, SHBG, estradiol, testosterone, FAI, DHEA-S, or AMH between the dyslipidemia group and the normal lipid profile group (Table 2).
Table 3 presents correlations between lipid profile parameters and anthropometric indices (BMI, BAI, and percentage of body fat). BMI and BAI did not correlate with total cholesterol levels (p=0.63; p=0.27 respectively). However, serum HDL cholesterol (respectively p<0.01; p<0.01), LDL cholesterol (respectively p=0.02 and p=0.01), non-HDL cholesterol (respectively p<0.01; p<0.01) and triglycerides (respectively p<0.01; p<0.01) correlated with BMI, BAI, and percentage of body fat.
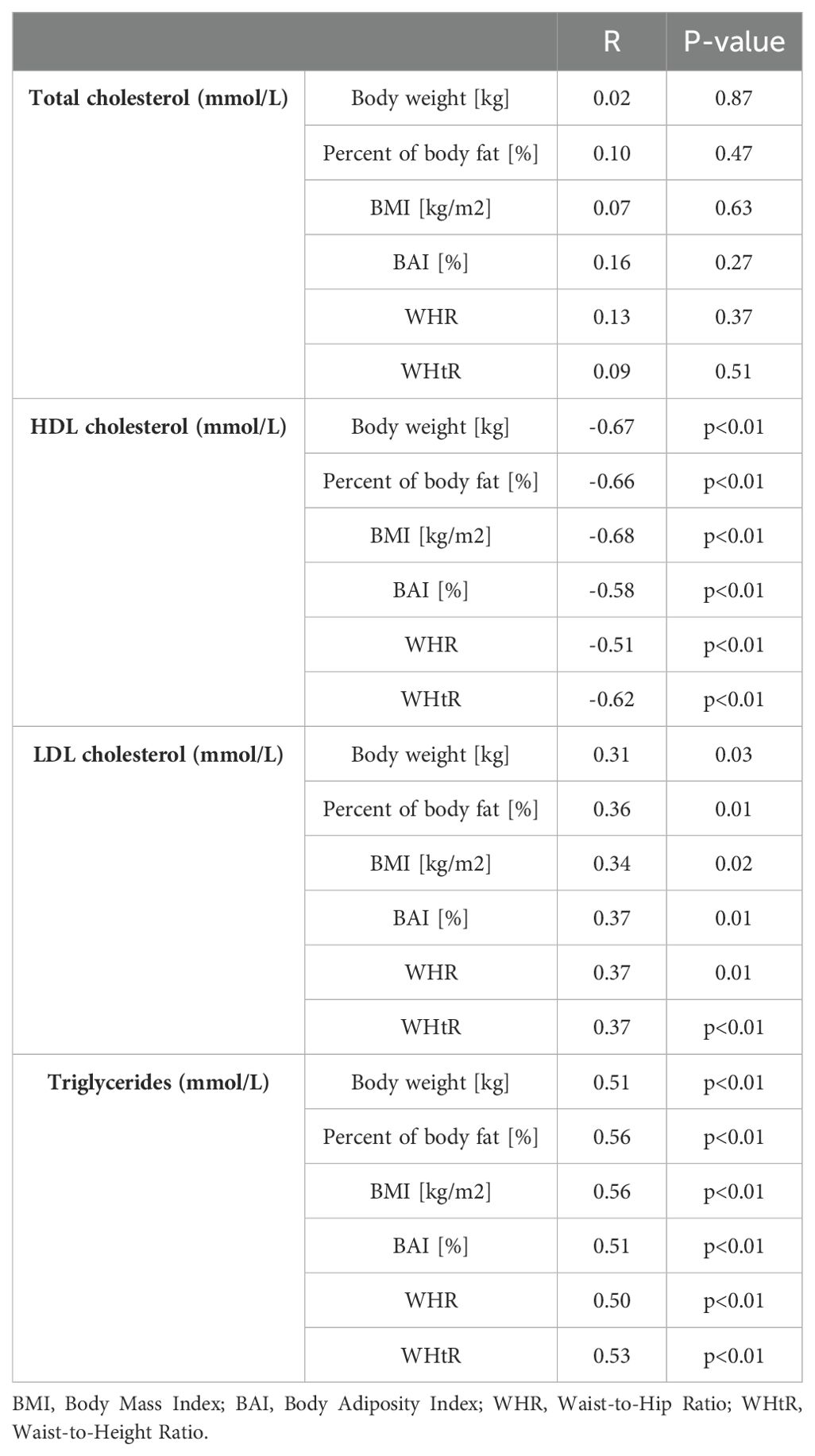
Table 3. Correlation between lipid profile parameters and anthropometric indices and percentage of body fat among study group.
ROC analysis demonstrated a high predictive value for age in identifying dyslipidemia. ROC analysis demonstrated poor predictive value for BMI, BAI, WHR, WHtR in identifying dyslipidemia (Table 4, Figures 2, 3).
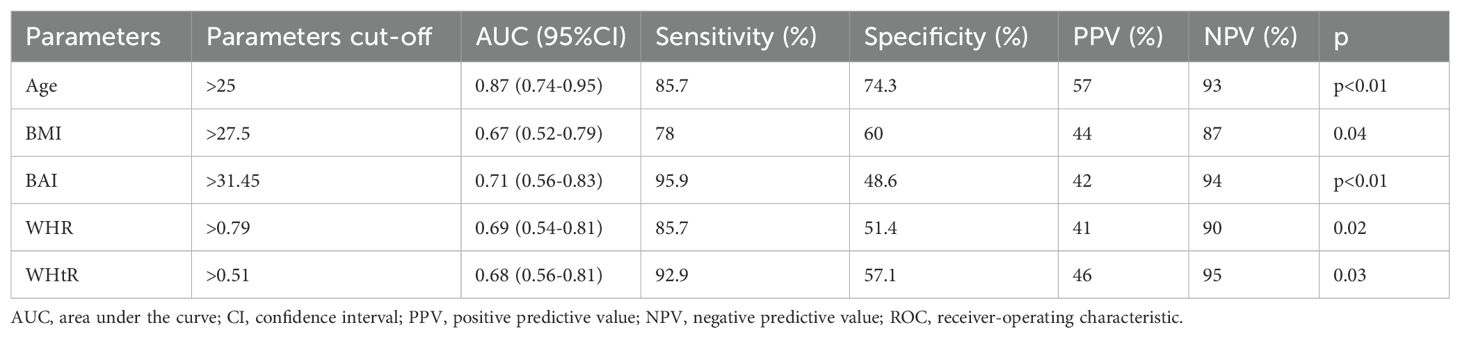
Table 4. Receiver-operating characteristic (ROC) curves identifying the threshold for existing dyslipidemia for BMI, BAI, WHR, and WHtR.
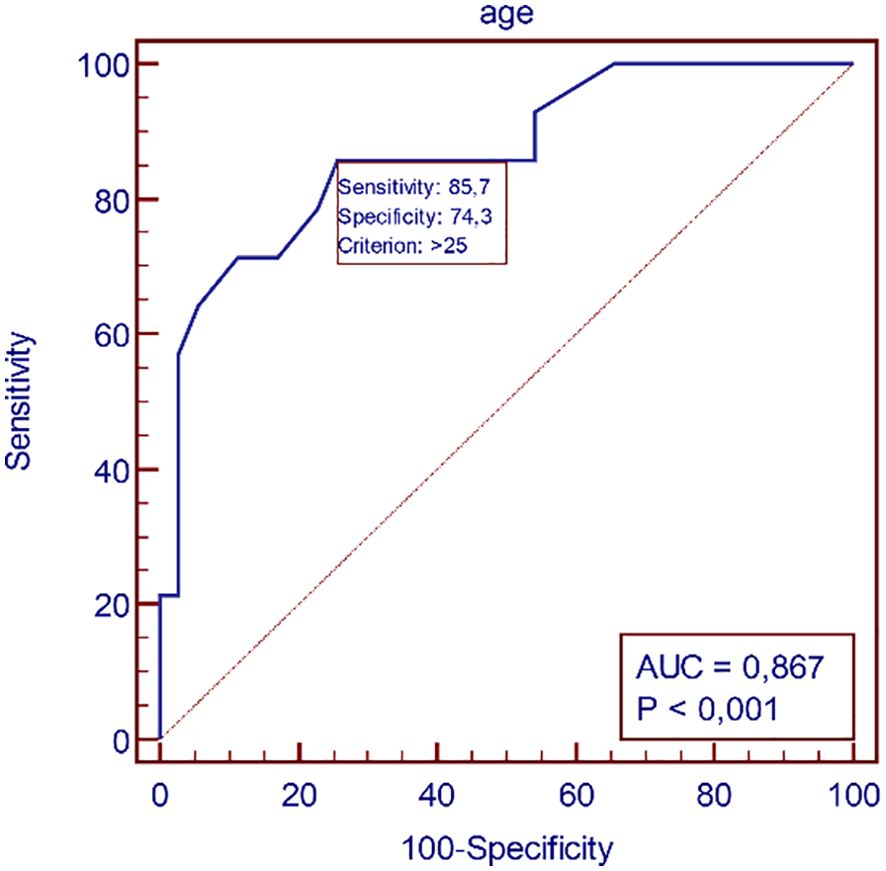
Figure 2. Receiver-operating characteristic (ROC) curves identifying the threshold for dyslipidemia for age.
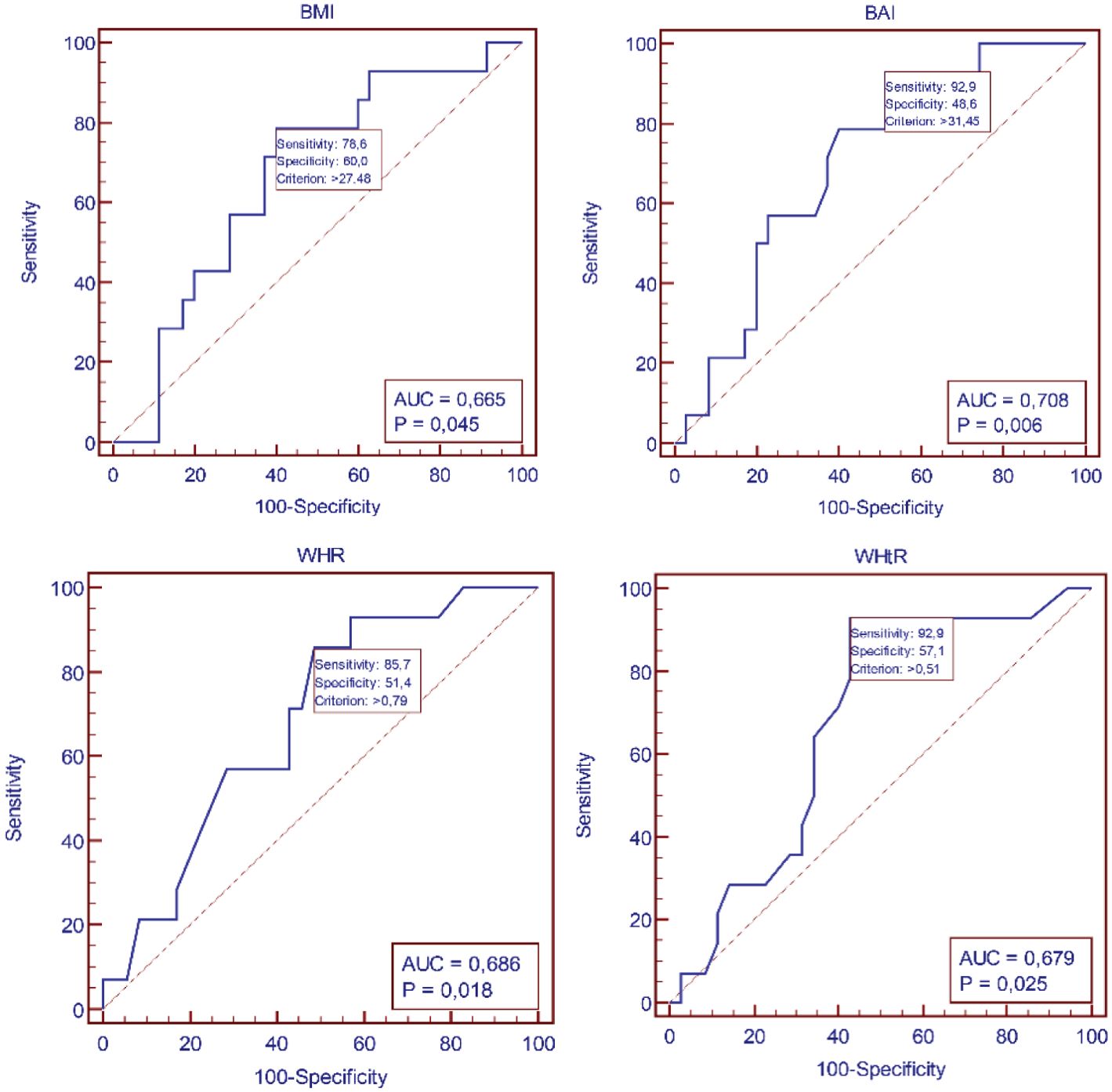
Figure 3. Receiver-operating characteristic (ROC) curves identifying the threshold for dyslipidemia for BMI, BAI, WHR, and WHtR.
Discussion
We aimed to assess cardiometabolic risk in women with PCOS using lipid profiles and anthropometric measures. According to the authors, despite the relatively young age of women with PCOS they are at an elevated risk for CVD. This underscores the importance of early proactive monitoring and management of CVD risk, incorporating both pharmacological and non-pharmacological strategies. However, the findings, seem to be conflicting. Interestingly, a 32-year follow-up study of the Gothenburg PCOS cohort, where participants reached a mean age of over 80 years and age-matched individuals of similar BMI, did not show an increased risk of CVD mortality or morbidity (20). These results shed new light on CVD risk in relation to confounding lipid deregulations in women with PCOS. The CVD risk of women with PCOS might be affected by factors other than the ones measured in our study. For instance, the authors of the Gothenburg study highlighted that deceased woman with PCOS had higher WHR parameters. Therefore, additional anthropometric measures might provide further insights into the CVD risk in PCOS. In contrast, a study conducted by Atanasova et al. indicated that WHtR and lipid accumulation product (LAP) are accurate indicators for predicting incident CVD in women with PCOS. In this study, the mean WHtR value in women with PCOS was significantly higher than in the control group (7). This suggests that WHtR could be a relevant anthropometric measure for assessing cardiometabolic risk in women with PCOS. Our study contributes to the existing data on metabolic risk assessment in women with PCOS. However, the discrepancies between our findings and those of other studies underscore the complexity of CVD risk factors in PCOS. The potential of additional anthropometric indices, such as WHR and WHtR, to better understand underlying mechanism of CVD risk.
Lipid metabolism constitutes one of the components of CVD risk. Additionally, the influence of glucose metabolism on CVD risk appraisal merits consideration. The authors of the study conducted an analysis of more complex anthropometric indices: Visceral Adiposity Index (VAI), Body Roundness Index (BRI), LAP, as well as atherogenic indices: Atherogenic Index of Plasma (AIP), Atherogenic Coefficient (AC), Castelli’s risk index-I, Castelli’s risk index-II, METS-IR, TG/HDL-C ratio, TyG index, TyG-BMI index, TyG-WC index in the study group. However, due to inherent inclusion of select lipid parameters within these indices, their explicit evaluation was precluded in the present study. Nonetheless, these indices demonstrated significant correlations with glucose and insulin parameters within the cohort of patients diagnosed with PCOS (21).
Among the Iranian cohort of 176 women diagnosed with PCOS and hyperandrogenism (A & B phenotypes) based on the diagnostic criteria of NIH, Rotterdam and AE-PCOS, had significantly higher WHtR and elevated TG levels compared to the healthy group (22). This highlights the association of hyperandrogenism with adverse cardiometabolic outcomes in women with PCOS.
A retrospective cohort study conducted in 8.000 young women with PCOS (mean age 28 years) revealed that these women had a 63% higher incidence of coronary artery disease (CAD) than healthy controls during 6-year of follow-up (23). Interestingly, the study found that a single factor of PCOS, such as infertility, irregular menstruation, or obesity, was not independently associated with CAD risk in this group. Instead, the authors emphasized that comorbidities such as hyperlipidemia, hypertension or diabetes might interact with PCOS in escalating CAD risk (23). This suggests that a comprehensive approach to managing cardiometabolic risk factors is essential in women with PCOS to mitigate the risk of CAD.
It has been demonstrated that higher levels of C-reactive protein (CRP) in obese women with PCOS are associated with susceptibility to dyslipidemia and diminish endothelial function in these individuals (24, 25). Considering that elevated CRP levels are known to by a major risk factor of CVD (24), it is crucial to understand its implications in women with PCOS. Lipid abnormalities among women with PCOS are commonly manifested as elevated levels of LDL-C, VLDL-C, TG, and lowered HDL-C levels. A meta-analysis of 30 studies that took into consideration BMI demonstrated that women with PCOS had higher LDL-C levels by 9.2 mg/dL, higher non-HDL-C levels by 16.3 mg/dL, and higher TG levels by 26.4 mg/dL compared to women without PCOS (26). Our study provides evidence of significant differences in lipid profiles in women with PCOS. This underscores the importance of close monitoring of lipid profiles in women with PCOS, especially in those with excess BMI or/and BAI. Furthermore, potential usefulness of BAI in predicting dysregulation of glucose metabolism has been hypothesized (27). Recognized cut-off values for BAI and BMI could serve as quick and non-expensive markers of cardiometabolic risk among women with PCOS.
Studies report heterogeneity in both CVD risk and in the clinical manifestation of PCOS associated with the PCOS diagnosis, which suggests that PCOS types appear to play a significant role in the occurrence of CVD risk (22, 28, 29).
PCOS with hyperandrogenism and ovulatory dysfunction has been proposed to pose severe risk factors for T2DM and to increase the risk of CVD in women (20, 28, 29). On the other hand, Iranian women with PCOS who had only ovulatory dysfunction and polycystic ovulatory morphology had similar cardiometabolic characteristics to healthy women (22). These findings suggest that women with PCOS who have only ovulatory dysfunction and no other features might have a lower cardiometabolic risk. The presence of hyperandrogenism and anovulation in PCOS types could lead to hyperandrogenism and hormonal imbalances, promoting IR and dyslipidemia. On the other hand, types of PCOS that are primarily defined by ovulatory dysfunction without prominent hyperandrogenism appear to have a lower risk of CVD. They are usually associated with a lower BMI and reduced abdominal obesity along with a lower prevalence of MetS and dyslipidemia (28, 29). These observations suggest that classification of PCOS may have implications for CVD risk stratification and management. Women with reproductive and hyperandrogenic PCOS clinical scenario may benefit from more vigilant monitoring and targeted interventions to address their higher cardiometabolic risk. In contrast, those with non-typical PCOS types may require a different approach, focusing on ovulatory dysfunction management and maintaining healthy lifestyle to minimize the risk.
A 17-year follow-up study of 195.675 women with PCOS who underwent deliveries compared to 71.240.633 women without PCOS reported that a diagnosis of PCOS during delivery was associated with increased cost and length of hospitalization (p<0.01). In addition, it was linked to an elevated risk of preeclampsia/eclampsia, peripartum cardiomyopathy, obesity, diabetes, and dyslipidemia (3).
We found the association of increased dyslipidemia in older women with PCOS (average 33.5 years old) compared to those with normal lipid profile (average 24 years old). This suggest that age may indeed play a role in the development of dyslipidemia in women with PCOS. As women with PCOS age, they may experience hormonal changes and metabolic changes that could contribute to an increased risk of dyslipidemia and cardiovascular complications. In addition to age, other features of PCOS have been proposed as contributing factors to diabetogenic and cardiometabolic factors. Hyperandrogenemia, low sex hormone binding globulin levels, and obesity are all known to be associated with PCOS and have been linked to an increased risk of developing diabetes and cardiovascular diseases (30). These factors may create a complex interplay that aggravates cardiometabolic risk in women with PCOS.
Recent advances in medical treatment have provided promising options for managing cardiometabolic risk in women with PCOS. Sodium glucose cotransport-2 inhibitors (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptors agonists have shown efficacy in improving diabetes management and cardiovascular outcomes (3, 31). These medications offer potential benefits for women with PCOS who may be at an increased risk of developing diabetes and CVD due to their underlying hormonal and metabolic imbalance. Overall, our study underscores the importance of early cardiometabolic risk assessment in women with PCOS, considering their increased risk to cardiovascular complications. Improving patient outcomes require targeted intervention that address the specific cardiometabolic risk factors associated with PCOS. In addition to promoting a healthy lifestyle, integrating the latest evidence-based pharmacological therapies such as SGLT-2 inhibitors and GLP-1 receptor agonist into clinical practice may improve outcomes and enhance the overall management of PCOS-related CVD. However, further research is warranted to fully elucidate the long-term benefits, safety, and potential risks of these medications in women with PCOS.
Conclusions
Analysis of simple and rapid parameters used to assess body fat, such as BMI, BAI, WHR, and WHtR, has shown that they are poor predictors of dyslipidemia in women with PCOS. In young women with PCOS, age appears to be a more reliable predictor of dyslipidemia.
Limitations
The conclusion drawn from this study should be interpreted in the context of its limitations. Relatively small number of participants, which may have affected the statistical power and rendered some differences statistically insignificant. However, other a substantial proportion of studies on women with PCOS are also relatively small.
Despite the limitations, our study underscores the significance of prioritizing cardiometabolic risk assessment among young women with PCOS, particularly in those with excessive body mass and abdominal visceral fat.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the bioethics board at the Medical University of Silesia KNW/0022/KB1/143/15). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MJ: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. JN: Conceptualization, Writing – original draft, Writing – review & editing. PJ: Formal analysis, Software, Writing – original draft. BH: Formal analysis, Project administration, Supervision, Validation, Writing – review & editing. JB: Data curation, Writing – review & editing. BZ-S: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Medical University of Silesia grant PCN-1-100/K/2/Z.
Acknowledgments
Authors greatly appreciate the dedicated faculty and staff who contributed to the completion of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ali S, Alam R, Ahsan H, Khan S. Role of adipokines (omentin and visfatin) in coronary artery disease. Nutr Metab Cardiovasc Dis. (2023) 33:483–93. doi: 10.1016/j.numecd.2022.11.023
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
2. Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Trigliceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. (2022) 21(1):68. doi: 10.1186/s12933-022-01511-x
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
3. Zahid S, Zia Khan M, Gowda S, Faza NN, Honigberg MC, Vaught AJ, et al. Trends, predictors, and outcomes of cardiovascular complications associated with polycystic ovary syndrome during delivery hospitalizations: A national inpatient sample analysis (2002-2019). J Am Heart Assoc. (2022) 11(16):e025839. doi: 10.1161/JAHA.121.025839
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
4. Stamm E, Marques-Vidal P, Gonzalez Rodriguez EG, Vollenweider P, Hans D, Lamy O. Association of adiposity evaluated by anthropometric, BIA, and DXA measures with cardiometabolic risk factors in nonobese postmenopausal women: the CoLaus/OsteoLaus cohort. Menopause. (2022) 29(4):450–9. doi: 10.1097/GME.0000000000001930
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
5. Zwierzchowska A, Celebańska D, Rosołek B, Gawlik K. Is body mass index (BMI) or body adiposity index (BAI) a better indicator to estimate body fat and selected cardiometabolic risk factors in adults with intellectual disabilities? BMC Cardiovasc Disord. (2021) 21:119. doi: 10.1186/s12872-021-01931-9
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
6. Neeland IJ, Poirier P, Després JP. Cardiovascular and metabolic heterogeneity of obesity. Circulation. (2018) 137:1391–406. doi: 10.1161/CIRCULATIONAHA.117.029617
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
7. Atanasova A, Panova ivanova D. Lipid accumulation product (LAP), anthropometric and metabolic parameters associated with hormonal profile in women with PCOS. Atherosclerosis. (2018) 275:e222. doi: 10.1055/s-0032-1333261
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
8. World Health Organization. Obesity and overweight Fact sheet (2020). Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (Accessed 1 Jun 2020).
9. Sam S. Obesity and polycystic ovary syndrome. Obes Manage. (2007) 3:69–73. doi: 10.1089/obe.2007.0019
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
10. Lim SS, Davies MJm Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. (2012) 18:618–37. doi: 10.1093/humupd/dms030
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
11. Rachon Dm Teede H. Ovarian function and obesity-interrelationship, impact on women’s reproductive lifespan and treatment options. Mol Cell Endocrinol. (2010) 316:172–9. doi: 10.1016/j.mce.2009.09.026
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
12. Zhu T, Cui J, Goodarzi MO. Polycystic ovary syndrome and risk of type 2 diabetes, coronary heart disease, and stroke. Diabetes. (2021) 70:627–37. doi: 10.2337/db20-0800
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
13. Guo F, Gong Z, Fernando T, Zhang L, Zhu X, Shi Y. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: A retrospective study in Chinese population. Front Endocrinol (Lausanne). (2021) 13:892125. doi: 10.3389/fendo.2022.892125
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
14. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. (2004) 19:41–7. doi: 10.1093/humrep/deh098
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
15. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. (2021) 42:3227–337. doi: 10.1093/eurheartj/ehab484
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
16. Li F, Yao L, Wu H, Cao S. Analysis on endocrine and metabolic features of different phenotypes of polycystic ovary syndrome patients. Pak J Pharm Sci. (2016) 29:1735–8.
17. Bergman RN, Stefanovski D, Buchanan TA, Sumner AE, Reynolds JC, Sebring NG, et al. A better index of body adiposity. Obes (Silver Spring). (2011) 19(5):1083–9. doi: 10.1038/oby.2011.38
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
18. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. International PCOS network, recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reproduct. (2023) 38(9):1655–79. doi: 10.1016/j.fertnstert.2018.05.004
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
19. Soares-Jr JM, Sá MFS, Baracat EC. New criteria for the clinical diagnosis of hyperandrogenism in polycystic ovarian syndrome and the risk of overdiagnosis. Rev Bras Ginecol Obstet. (2019) 41:361–2. doi: 10.1055/s-0039-1693530
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
20. Forslund M, Schmidt J, Brännström M, Landin-Wilhelmsen K, Dahlgren E. Morbidity and mortality in PCOS: A prospective follow-up to a mean age above 80 years. Eur J Obstet Gynecol Reprod Biol. (2022) 271:195–203. doi: 10.1016/j.ejogrb.2022.02.020
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
21. Jabczyk M, Nowak J, Jagielski P, Hudzik B, Kulik-Kupka K, Włodarczyk A, et al. Metabolic deregulations in patients with polycystic ovary syndrome. Metabolites. (2023) 13:302. doi: 10.3390/metabo13020302
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
22. Farhadi-Azar M, Behboudi-Gandevani S, Rahmati M, Mahboobifard F, Khalili Pouya E, Ramezani Tehrani F, et al. The prevalence of polycystic ovary syndrome, its phenotypes and cardio-metabolic features in a community sample of Iranian population: tehran lipid and glucose study. Front Endocrinol (Lausanne). (2022) 13:825528. doi: 10.3389/fendo.2022.825528
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
23. Ding DC, Tsai IJ, Wang JH, Lin SZ, Sung FC. Coronary artery disease risk in young women with polycystic ovary syndrome. Oncotarget. (2018) 9(9):8756–64. doi: 10.18632/oncotarget.23985
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
24. Gnanadass SA, Divakar Prabhu Y, Gopalakrishnan AV. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): an update. Arch Gynecol Obstet. (2021) 303:631–43. doi: 10.1007/s00404-020-05951-2
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
25. Fathi FH. C-reactive protein and adiposity in women with polycystic ovary syndrome. Tikrit J Pure Sci. (2018) 23:47–51. doi: 10.25130/tjps.v23i7.695
26. Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. (2011) 95:1073–9 e1-11. doi: 10.1016/j.fertnstert.2010.12.027
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
27. Jabłonowska-Lietz B, Wrzosek M, Włodarczyk M, Nowicka G. New indexes pf body fat distribution, visceral adiposity index, body adiposity index, waist-to-height ratio, and metabolic disturbances in the obese. Kardiol Pol. (2017) 75:1185–91. doi: 10.5603/KP.a2017.0149
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
28. Osibogun O, Ogunmoroti O, Muchos ED. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. (2020) 30:399–404. doi: 10.1016/j.tcm.2019.08.010
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
29. Rizzo M, Berneis K, Hersberger M, Pepe I, Di Fede G, Rini GB, et al. Milder forms of atherogenic dyslipidemia on ovulatory versus anovulatory polycystic ovary syndrome phenotype. Hym Reprod. (2009) 24(9):2286–92. doi: 10.1093/humrep/dep121
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
30. Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab. (2006) 91(4):1357–63. doi: 10.1210/jc.2005-2430
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
31. Han Y, Li Y, He B. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reprod BioMed Online. (2019) 39:332–42. doi: 10.1016/j.rbmo.2019.04.017
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Keywords: polycystic ovary syndrome, lipid disorders, cardiometabolic risk factors, anthropometry, metabolic profile
Citation: Jabczyk M, Nowak J, Jagielski P, Hudzik B, Borszcz J and Zubelewicz-Szkodzińska B (2024) Interplay between lipid profile and anthropometric measures as indicators of cardiometabolic risk in women with polycystic ovary syndrome. Front. Endocrinol. 15:1398017. doi: 10.3389/fendo.2024.1398017
Received: 08 March 2024; Accepted: 07 October 2024;
Published: 20 November 2024.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Iwona Sidorkiewicz, Medical University of Bialystok, PolandHarpal Singh Randeva, University Hospitals Coventry and Warwickshire NHS Trust, United Kingdom
Copyright © 2024 Jabczyk, Nowak, Jagielski, Hudzik, Borszcz and Zubelewicz-Szkodzińska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marzena Jabczyk, ZDIwMDk5M0AzNjUuc3VtLmVkdS5wbA==; Justyna Nowak, anVzdHluYS5ub3dha0BzdW0uZWR1LnBs
 Marzena Jabczyk
Marzena Jabczyk Justyna Nowak
Justyna Nowak Paweł Jagielski
Paweł Jagielski Bartosz Hudzik
Bartosz Hudzik Jakub Borszcz6
Jakub Borszcz6