
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol., 10 May 2024
Sec. Reproduction
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1397279
Currently, three crucial questions regarding the reliability of ovarian reserve measures in women with ovarian endometrioma during the reproductive age are being discussed. Firstly, the effects of endometriotic cystectomy on short and long-term ovarian reserve. Secondly, the accuracy of serum anti-Müllerian hormone (AMH) and antral follicle count (AFC) in estimating ovarian reserve in these cases. Thirdly, the impact of endometrioma itself on the ovarian reserve over time in such cases. The purpose of the present review is to critically assess available systematic reviews and meta-analyses that have explored these questions. Nine eligible reviews were found following a systematic search on PubMed.com and similarly assessed. These reviews varied considerably regarding the level of evidence, as per an identical comprehensive scoring system. Moderate to high-quality evidence demonstrates that endometriotic cystectomy, by the stripping technique, adversely affects ovarian reserve in the short and long term, up to 9-18 months post-surgery. Damage to ovarian reserve was considerable but more pronounced in bilateral cases than unilateral cases, equivalent to 39.5% and 57.0%, respectively. Repeat endometriotic cystectomy is detrimental to ovarian reserve. The impact of endometrioma diameter on ovarian reserve before or after surgery is still unclear. Moderate to high-quality evidence, relying on simultaneous assessment of both ovarian reserve measures, shows that AMH is sensitive while AFC is not in cases undergoing ovarian cystectomy. AMH should be the biomarker of choice for counseling and managing women with endometrioma in their reproductive age, especially before surgery. While there is some evidence to show that endometrioma per se may harm ovarian reserve, this evidence is not robust, and there is good-quality evidence to challenge this notion. It is necessary to conduct further targeted RCTs, systematic reviews, and meta-analyses based on solid methodological grounds to increase the level of evidence, refine quantitative estimates, investigate open questions, and decrease heterogeneity.
Ovarian reserve appraisal in women with ovarian endometrioma continues to be a challenging and debated topic in reproductive medicine. This diagnostic complexity arises from several fundamental inquiries about disease pathogenesis and patient management. These include the potential influence of endometrioma per se on ovarian reserve, the adverse effect of ovarian surgery on ovarian reserve, and the adequacy of the most established ovarian reserve measures, anti-Müllerian hormone (AMH) and antral follicle count (AFC) to accurately assess ovarian reserve in these women.
While standards for evidence-based clinical guidelines stipulate the use of systematic reviews, uncritically accepting the results of a single systematic review may have several masked risks (1). Indeed, several systematic reviews and meta-analyses exploring ovarian reserve appraisal in cases with ovarian endometrioma were published in the last decade. However, they had contrasting results, instigating confusion and potentially leading to obverse clinical management.
In the general population of reproductive age, among various tests, AFC and AMH are considered the most sensitive and reliable non-invasive methods of ovarian reserve evaluation (2, 3), with no superiority dispute between the two. Two independent systematic reviews and meta-analyses examined the impact of endometriotic cystectomy during the reproductive age, the first employing AMH and the second AFC, and they have reached contradictory conclusions. The first, by Raffi et al. in 2012, reported that surgery had a harmful effect on AMH (4), while the second, by Muzii et al. in 2014, using AFC, found that it did not affect the ovarian reserve (5). Drawing an inference on whether endometriotic cystectomy genuinely influences the ovarian reserve from these two independent systematic reviews is limited. This controversy may have resulted from different methodologies and standardizations of each systematic review containing the surgical technique, endometrioma size, laterality, previous ovarian surgery, and postoperative time interval evaluations. This controversy may also raise questions about the suitability and reliability of both AMH and AFC as ovarian reserve measures in women with endometrioma.
The purpose of the present review is to critically assess available systematic reviews and meta-analyses that have explored the impact of ovarian endometrioma and endometriotic cystectomies on ovarian reserve measures, AMH, and AFC. Looking deeper into the methodology of these systematic reviews may clarify the bias range, uncover the discrepancies between the reports, distinguish high-quality assessments, explain clinical implications, and assist in decision-making.
To reach the objective of this review, the PubMed database was searched for manuscripts that included the syntaxes (and their MeSH terms): endometriosis OR endometrioma AND ovarian reserve OR anti-Müllerian hormone OR antral follicle count. The research was restricted to systematic reviews (with or without meta-analysis) published in peer-reviewed journals for clinical studies performed in humans and English from inception until November 2, 2023. Systematic reviews that examined ovarian reserve measures, specifically AMH and AFC, in women with ovarian endometrioma before or after surgery were applicable for evaluation.
Systematic reviews that targeted women with non-endometriotic ovarian cysts, gonadal or non-gonadal malignancy, polycystic ovary syndrome, diabetes mellitus, thyroid disease, human immunodeficiency virus, autoimmune disease (such as systemic lupus erythematosus or rheumatoid arthritis), inflammatory bowel disease, and BRCA variants were excluded. Furthermore, systematic reviews that targeted females in childhood, adolescence, and peri- or postmenopausal were omitted from the evaluation. In addition, papers that evaluated women following a minimally invasive approach to managing an endometrioma, such as ablation, laser vaporization, or ethanol sclerotherapy, were excluded. Additionally, papers that aimed to evaluate salpingectomy, hysterectomy, and uterine artery embolization were omitted from the evaluation. As well, reviews that targeted acupuncture in women with endometriosis or explored the hemostatic approach following endometriotic cystectomy were excluded.
One-hundred fifty-seven systematic reviews were identified from the PubMed database. One-hundred and thirty-one were excluded following title and abstract reading. The remaining 26 systematic reviews were assessed for eligibility following a full manuscript inspection. Eight publications were excluded since they targeted minimally invasive methods of endometrioma management (laser and ablation) (6–8) or different hemostatic methods during conventional endometriotic cystectomy (bipolar, suture) (9–13). Another five publications targeted IVF outcomes, excessive response, or livebirth were excluded (14–18). In addition, four publications did not exclusively target women with endometrioma and were omitted from the evaluation (19–22).
Nine systematic reviews were eligible for critical evaluation and are summarized in Table 1 (4, 5, 23–29). According to the Journal Citation Reports 2022, all reviews except one (28) were published in a Journal with a well-established impact factor. Furthermore, all but one review (23) conducted a meta-analysis of the accumulated data, reaching a quantitative evaluation. In addition, eight of the nine systematic reviews targeted ovarian endometrioma, while one targeted women with endometriosis (27). Seven reviews targeted women undergoing endometriotic cystectomy (4, 5, 23, 25, 26, 28, 29), and two focused on women with endometrioma or endometriosis before surgery (24, 27). The primary outcome measure was AMH in six reviews and AFC in two (5, 27). Only one review targeted both AMH and AFC at the same time in parallel as a primary outcome measure (29).
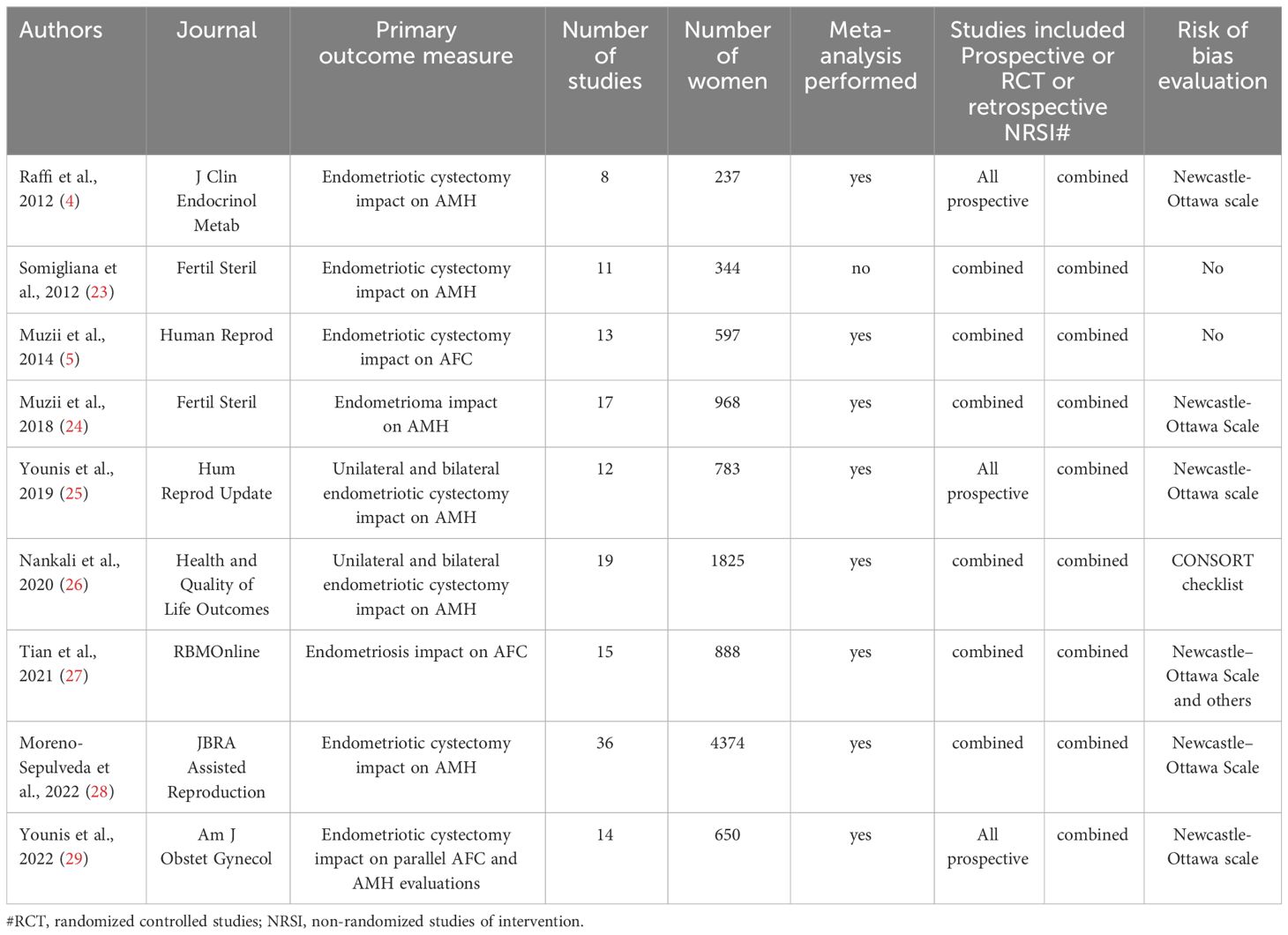
Table 1 Summary of systematic reviews exploring ovarian reserve measures (AMH and AFC) in cases with ovarian endometrioma.
To critically and similarly assess in a transparent approach the preparation, conduct, and rating of the certainty of the evidence of the eligible systematic reviews, an a priori list of key entries was formulated. This comprehensive list included twenty fundamental entries applicable to the research question, the literature search methodology, the handling of included and excluded studies, and the eligibility of studies for being adequate for meta-analysis. In addition, significant points relevant to ovarian endometrioma and their impact on both ovarian reserve tests, AMH and AFC, were incorporated, including previous ovarian surgery, endometrioma diameter, and laterality. Furthermore, the design and risk of bias evaluation of studies found eligible for meta-analysis in each review were addressed. The timing and methodology of the ovarian reserve tests were also examined. Moreover, the methodology of the meta-analysis and the efforts invested to explain estimates with high heterogeneity were explored. Finally, the funding sources and conflict of interest in conducting the systematic review were assessed.
Since all meta-analyses analyzed a combination of randomized and non-randomized studies, a modification of the A Measurement Tool to Assess Systematic Reviews (AMSTAR-2) tool was incorporated into the list of crucial entries (1). Significant issues pertinent to the subject of ovarian endometrioma were also added to this list. Table 2 summarizes the performance of all nine systematic reviews found suitable in the search. Each entry in the list received a score of 2, 1, and 0, depending on whether it was implemented (and documented), partially implemented, or not in each specific review. As shown in Table 2, a wide variation between the total scores was found among the nine systematic reviews, indicating a diversity in the quality of evidence.
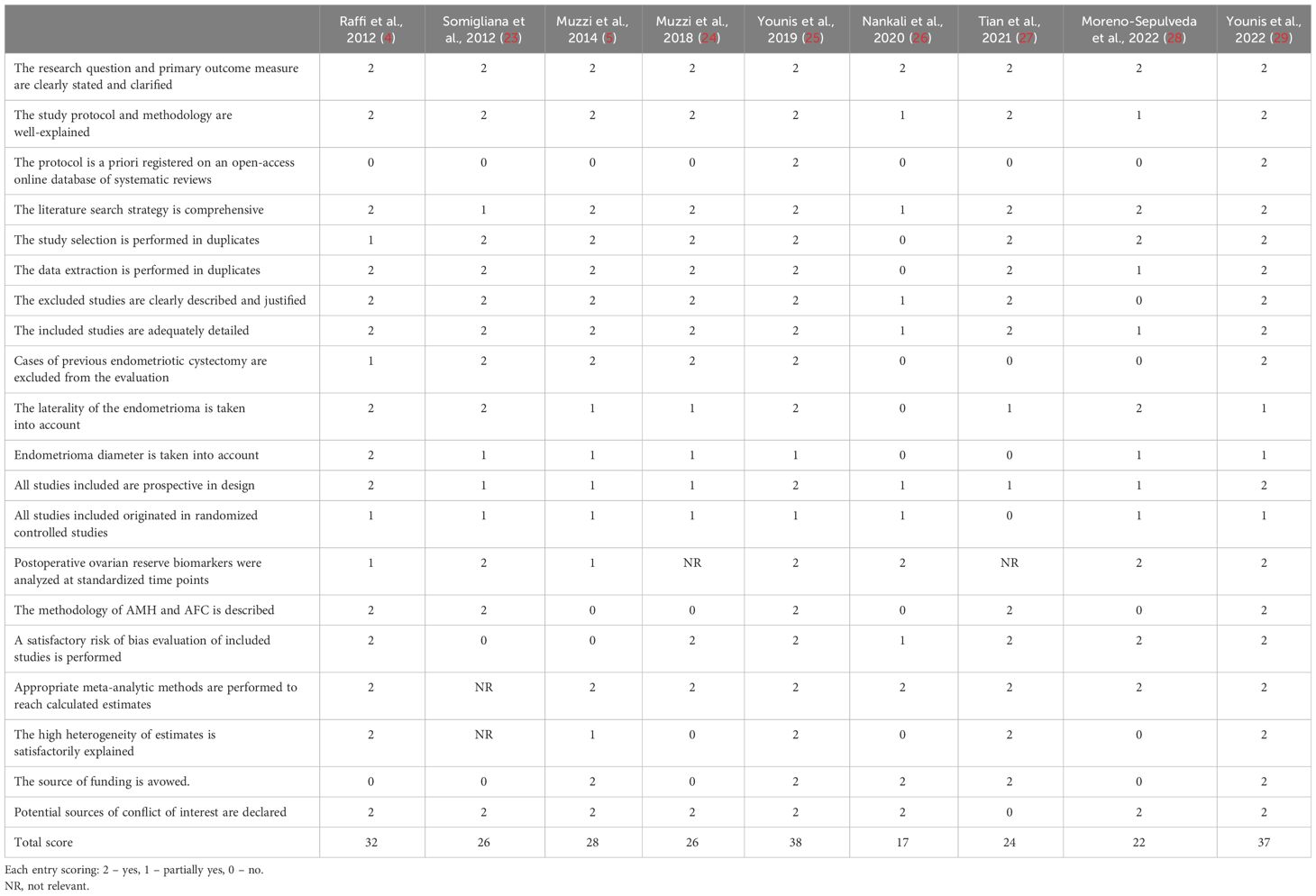
Table 2 Critical appraisal of the systematic reviews exploring the impact of endometrioma and endometriotic cystectomy on ovarian reserve measures.
Table 3 summarizes the primary findings of the nine systematic reviews included in this appraisal. In-toto, these reviews targeted two primary questions (outcome measures) employing the best available ovarian reserve tests in reproductive age, AMH, and AFC. The first is the impact of endometriotic cystectomy, and the second is the impact of endometrioma per se on ovarian reserve biomarkers. The contradictory results of the first three systematic reviews (4, 5, 23) dealing with the impact of endometriotic cystectomy have raised a third question (outcome measure) of which of the best available ovarian reserve tests, AMH and AFC, is more reliable in this setting.
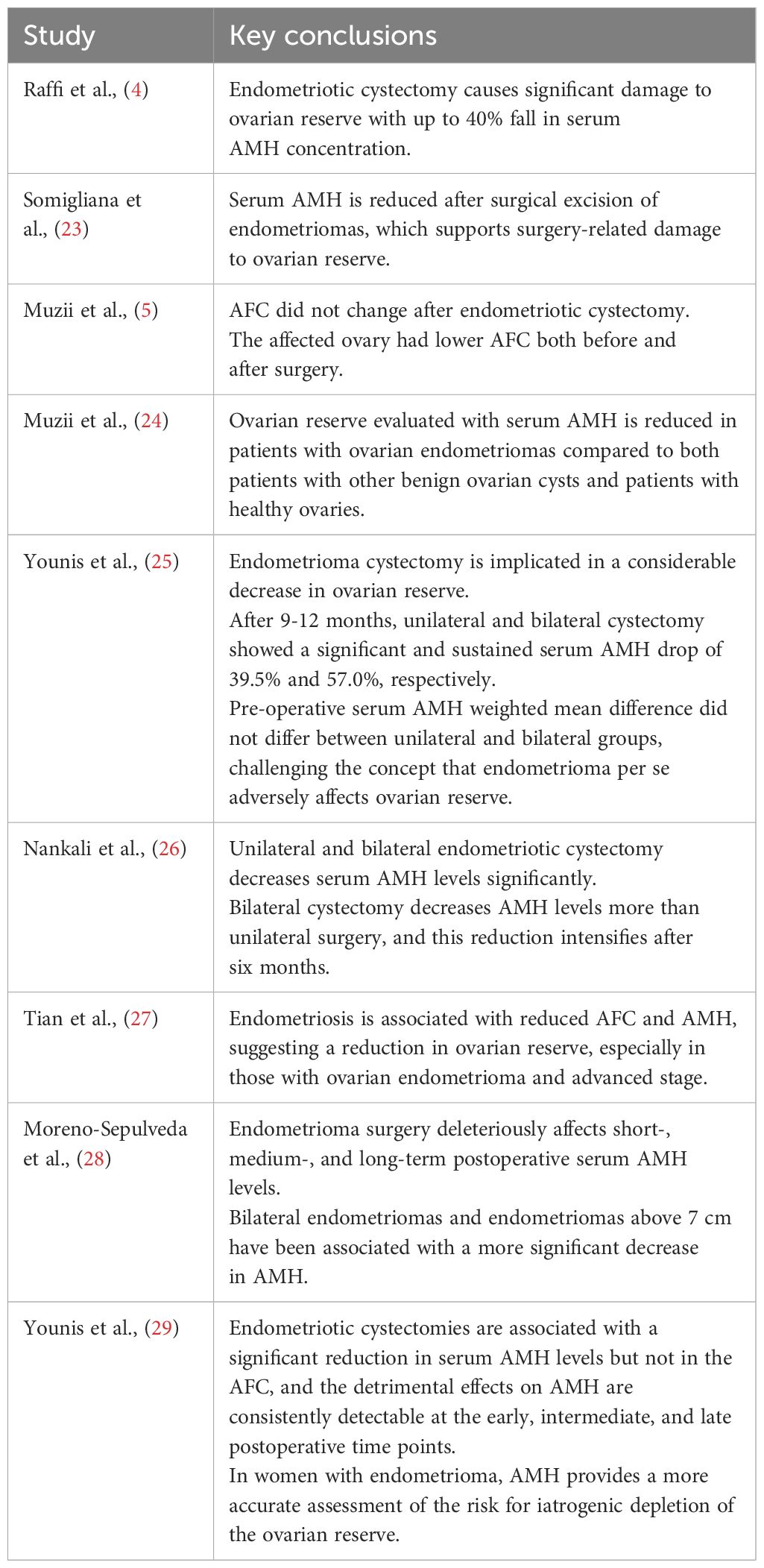
Table 3 Summary of the primary findings of the systematic reviews examining the reliability of AMH and AFC in evaluating ovarian reserve in cases with endometrioma.
The first two published systematic reviews exploring the impact of endometriotic cystectomy employing AMH showed decreased ovarian reserve following surgery (4, 23). However, a third systematic review in a similar setting employing AFC showed reassuring results with no change in ovarian reserve (5). As such, AFC in this review suggested a more specific biomarker of ovarian reserve in this setting since it controls for the laterality of the possible damage (5).
Our group corroborated the findings of Raffi et al. (4) and Somigliana et al. (23), employing AMH as an ovarian reserve biomarker (25). Furthermore, we showed that impairment of the ovarian reserve is sustained for 9-12 months postoperatively, which is more detrimental in bilateral than unilateral cases, consistent with 39.5% and 57.0%, respectively, from baseline. This was also verified by two later published systematic reviews (26, 28).
To resolve the discrepancy between AMH and AFC performance in cases with endometrioma, our group conducted an additional systematic review and meta-analysis. In this study, both AMH and AFC were targeted for each woman concurrently (overcoming unmeasured confounders), in the same setting (overcoming surgical technique differences), and at the same three postoperative time points, namely early (1-6 weeks), intermediate (2-6 months) and late (9-18 months), to overcome time-sensitive changes (25). In this review, endometriotic cystectomies were associated with a significant reduction in serum AMH levels but not in the AFC, with the detrimental effects on AMH consistently detectable at all three time points extending to 9-18 months postoperatively. These findings clearly showed that AMH is a more sensitive biomarker of damage to the ovary than AFC and should be recommended as the biomarker of choice for women with endometrioma counseling.
The main controversial and unsettled topic that is still ongoing today is whether endometrioma per se affects ovarian reserve. In the reviews by Muzii et al., 2018 (24) and Tian et al., 2021 (27), which examined the impact of endometrioma on ovarian reserve, a decrease in AMH and AFC levels was detected compared to controls, suggesting a negative impact on ovarian reserve. However, it is essential to note that while the first review targeted women with endometrioma, the second targeted women with endometriosis. Furthermore, both reviews pooled retrospective and prospective studies together. Interestingly, previous surgery was not cited as an exclusion criterion of eligible studies in the second review (24). In addition, neither review assessed the impact on the ovarian reserve over time. A detailed methodological assessment of these two systematic reviews is summarized in Table 2.
Conversely, in a previous systematic review, pooling only prospective studies and targeting endometriotic cystectomy in unilateral versus bilateral cases in the same setting, preoperative serum AMH levels did not significantly differ between the groups (25). If endometrioma per se had a deleterious effect on the ovarian reserve, cases with bilateral compared to unilateral endometrioma should have had lower serum AMH levels at baseline before surgery. These results challenge the concept that endometrioma per se adversely affects ovarian reserve, calling for further assessment and research to gain a deeper insight into this topic.
This review is a comprehensive and critical summary of all systematic reviews that assess the performance of AMH and AFC in managing cases of endometrioma. Nine eligible reviews were analyzed after conducting a systematic search on PubMed.com. The systematic reviews varied in terms of the level of evidence, as per an identical broad scoring system. Each review was evaluated transparently, using a pre-formulated list of 20 key entries essential to the systematic review’s methodology and including hefty aspects of ovarian endometrioma characteristics related to ovarian reserve measures. This methodology helps to assess the certainty of evidence in systematic reviews, strengthens clinical practice recommendations, and assists in decision-making.
Three fundamental questions linked to the reliability of ovarian reserve measures when tackling ovarian endometrioma in the reproductive age are in discussion nowadays. The first is the impact of endometriotic cystectomy on the short and long-term of ovarian reserve. The second is the reliability of AMH and AFC in accurately estimating ovarian reserve in cases with ovarian endometrioma. The third is the impact of endometrioma per se on ovarian reserve as a function of time in such cases.
Moderate to high-quality evidence shows that endometriotic cystectomy, which is typically performed using the striping technique, is harmful to the ovarian reserve. Following the operation, serum AMH levels significantly decrease, starting in the early postoperative period and continuing until the late postoperative period, which lasts 9-18 months (29). This negative effect is more pronounced in bilateral cases than unilateral cases, equivalent to an ovarian reserve decline of 39.5% and 57.0%, respectively (25). Consequently, this decrease could have a detrimental effect on the reproductive life span. Histological studies of endometriotic cystectomy back up this conclusion and indicate that this damage is due to the unintentional removal of normal ovarian follicles near the pseudo-capsule, which is almost unavoidable even with experienced surgeons (30–32). This iatrogenic damage seems to be a composite result of the manipulation of the cortex, tearing of tissue planes, bleeding, and coagulation injury.
Furthermore, there is moderate to high-quality evidence that AMH is a much more sensitive biomarker of ovarian reserve than AFC in cases with ovarian endometrioma. Therefore, it should be the ovarian reserve biomarker of choice for women’s guidance in this setting, especially before surgery. In such cases, counseling women relying merely on AFC may be misleading for clinical practice recommendations.
The results obtained in the recent meta-analysis, assessing both AMH and AFC in parallel, in the same women, in the same setting, and at the same postoperative time points (29), corroborate basically the conclusions of the earlier systematic reviews evaluating AMH or AFC separately (4, 5, 23). It is, therefore, clear now that the discordancy between these systematic reviews on ovarian reserve findings is not the result of variances in methodology and standardization but rather the low sensitivity of AFC in detecting ovarian reserve changes in cases with ovarian endometrioma. These findings also support the notion that AFC is underestimated in cases with an intact endometrioma during the reproductive age.
Four essential and relevant concerns to endometrioma should be considered when evaluating the reliability of ovarian reserve measures in this setting during reproductive age. These concerns include previous ovarian surgery, the diameter of the endometrioma, its laterality, and the postoperative timing of ovarian reserve evaluation. All of these concerns were incorporated into the list of 20 key entries formulated a priori to examine the level of evidence of each of the systematic reviews in this critical contemporary review.
It has been found that both recurrent endometriotic cystectomy and bilateral endometriotic cystectomy can have a severe damaging impact on ovarian reserve. Previous studies have shown that there is a significant risk of premature ovarian insufficiency, either early or late, in such situations (33–35). Furthermore, it is essential to consider the timing of ovarian reserve appraisal following surgery, as natural changes can occur over time. For example, in women in their 30s, the natural decline in AMH is about 5% per year (36). However, the impact of surgery can exceed this natural decline by a considerable margin equivalent to 5-10 years (25). In addition, the diameter of the endometrioma may also significantly affect the damage to the ovarian reserve caused by endometriotic cystectomy. Recent research suggests that the threshold to distinguish between endometriomas that may or may not interfere with the ovarian response is 4 cm in diameter (37). Conversely, other investigators have reported that in women with no previous history of ovarian surgery, serum AMH levels were increased in cases with large endometriomas (38, 39). Due to the significance of AMH level association with endometrioma diameter and its related broader implications, prospective well-conducted studies with repeat measures of endometrioma diameter and AMH concentrations are vitally required.
A third fundamental question, which is still debated and not yet settled, is whether endometrioma per se may adversely affect ovarian reserve in the reproductive age. In the in-vitro setting, endometrioma was shown to instigate intra-ovarian inflammatory reactions attributed to the high concentration of free iron, reactive oxygen species, proteolytic enzymes, and inflammatory molecules. Macrophages, cytokines, and vasoactive substances mediate these reactions, which may damage adjacent ovarian cortical tissue. Various molecular, histological, and morphological evidence supports such mechanisms. However, the evidence is far from being conclusive (40).
In the clinical setting, two previous systematic reviews have explored this question, the first employing AMH and the second AFC, showing that an intact endometrioma may harm ovarian reserve (24, 27). However, several methodological concerns were raised in the results section and Table 2. Furthermore, no changes were found in serum AMH levels at baseline comparing unilateral and bilateral endometrioma cases, challenging the concept that endometrioma per se might have a negative impact on ovarian reserve (29). Further targeted randomized, well-controlled studies and adequately designed systematic reviews and meta-analyses are needed to explore this question adequately.
We acknowledge that our search for systematic reviews relied on one search engine, PubMed.com. For common clinical questions, PubMed.com is a reliable engine that can narrow the search and provides the highest-quality, most relevant, and most readable hits. Cochrane, considered the gold standard for clinical systematic reviews, recommends searching PubMed.com as one of the most commended databases.
Moderate to high-quality evidence shows that endometriotic cystectomy is detrimental to ovarian reserve. Ovarian reserve damage is more pronounced in bilateral than unilateral cases and in women with previous ovarian surgery. Furthermore, moderate to high-quality evidence demonstrates that AMH is significantly sensitive to surgical impact on ovarian reserve, while AFC is not. Serum AMH should be the ovarian reserve biomarker for counseling women with endometrioma during the reproductive age, especially before surgery. Although there is some evidence that endometrioma per se may harm ovarian reserve, it is not robust. Furthermore, there is good evidence to challenge this notion. The impact of endometrioma diameter on ovarian reserve before or following surgery is still unclear. Further targeted RCTs, systematic reviews, and meta-analyses based on solid methodological grounds are essential to investigate open questions, increase the level of evidence, refine quantitative estimates, and decrease heterogeneity.
JY: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. HT: Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
2. Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. (2020) 114:1151–7. doi: 10.1016/j.fertnstert.2020.09.134
3. Nelson SM. Biomarkers of ovarian response: current and future applications. Fertil Steril. (2013) 99:963–9. doi: 10.1016/j.fertnstert.2012.11.051
4. Raffi F, Metwally M, Amer S. The impact of excision of ovarian endometrioma on ovarian reserve: a systematic review and meta-analysis. J Clin Endocrinol Metab. (2012) 97:3146–54. doi: 10.1210/jc.2012-1558
5. Muzii L, Di Tucci C, Di Feliciantonio M, Marchetti C, Perniola G, Panici PB. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: a systematic review and meta-analysis. Hum Reprod. (2014) 29:2190–8. doi: 10.1093/humrep/deu199
6. Giannini A, Tebache L, Noti G, Cosimi G, Nisolle M, Simoncini T. Impact on ovarian reserve and fertility using carbon dioxide laser for endometriosis treatment: a systematic review. Gynecol Endocrinol. (2022) 38:617–22. doi: 10.1080/09513590.2022.2087218
7. Zhang Y, Zhang S, Zhao Z, Wang C, Xu S, Wang F. Impact of cystectomy versus ablation for endometrioma on ovarian reserve: a systematic review and meta-analysis. Fertil Steril. (2022) 118:1172–82. doi: 10.1016/j.fertnstert.2022.08.860
8. Adamyan L, Kasyan V, Pivazyan L, Isaeva S, Avetisyan J. Laser vaporization compared with other surgical techniques in women with ovarian endometrioma: a systematic review and meta-analysis. Arch Gynecol Obstet. (2023) 308:413–25. doi: 10.1007/s00404-022-06799-4
9. Ata B, Turkgeldi E, Seyhan A, Urman B. Effect of hemostatic method on ovarian reserve following laparoscopic endometrioma excision; comparison of suture, hemostatic sealant, and bipolar desiccation. A systematic review and meta-analysis. J Minim Invasive Gynecol. (2015) 22:363–72. doi: 10.1016/j.jmig.2014.12.168
10. Pergialiotis V, Prodromidou A, Frountzas M, Bitos K, Perrea D, Doumouchtsis SK. The effect of bipolar electrocoagulation during ovarian cystectomy on ovarian reserve: a systematic review. Am J Obstet Gynecol. (2015) 213:620–8. doi: 10.1016/j.ajog.2015.04.006
11. Peters A, Rindos NB, Lee T. Hemostasis during ovarian cystectomy: systematic review of the impact of suturing versus surgical energy on ovarian function. J Minim Invasive Gynecol. (2017) 24:235–46. doi: 10.1016/j.jmig.2016.12.009
12. Deckers P, Ribeiro SC, Simões RDS, Miyahara CBDF, Baracat EC. Systematic review and meta-analysis of the effect of bipolar electrocoagulation during laparoscopic ovarian endometrioma stripping on ovarian reserve. Int J Gynaecol Obstet. (2018) 140:11–7. doi: 10.1002/ijgo.12338
13. Baracat CMF, Abdalla-Ribeiro HSA, Araujo RSDC, Bernando WM, Ribeiro PA. The impact on ovarian reserve of different hemostasis methods in laparoscopic cystectomy: A systematic review and meta-analysis. Rev Bras Ginecol Obstet. (2019) 41:400–8. doi: 10.1055/s-0039-1692697
14. Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. (2006) 12:685–718. doi: 10.1093/humupd/dml034
15. Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. (2009) 91:705–14. doi: 10.1016/j.fertnstert.2007.12.013
16. Broer SL, Dólleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. (2011) 17:46–54. doi: 10.1093/humupd/dmq034
17. Gao X, Zhang Y, Xu X, Lu S, Yan L. Effects of ovarian endometrioma aspiration on in vitro fertilization-intracytoplasmic sperm injection and embryo transfer outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet. (2022) 306:17–28. doi: 10.1007/s00404-021-06278-2
18. Li NJ, Yao QY, Yuan XQ, Huang Y, Li YF. Anti-müllerian hormone as a predictor for live birth among women undergoing IVF/ICSI in different age groups: an update of systematic review and meta-analysis. Arch Gynecol Obstet. (2023) 308:43–61. doi: 10.1007/s00404-022-06683-1
19. Iwase A, Nakamura T, Nakahara T, Goto M, Kikkawa F. Assessment of ovarian reserve using anti-Müllerian hormone levels in benign gynecologic conditions and surgical interventions: a systematic narrative review. Reprod Biol Endocrinol. (2014) 12:125. doi: 10.1186/1477-7827-12-125
20. Mohamed AA, Al-Hussaini TK, Fathalla MM, El Shamy TT, Abdelaal II, Amer SA. The impact of excision of benign non-endometriotic ovarian cysts on ovarian reserve: a systematic review. Am J Obstet Gynecol. (2016) 215:169–76. doi: 10.1016/j.ajog.2016.03.045
21. Tsiampa E, Spartalis E, Tsourouflis G, Dimitroulis D, Nikiteas N. Impact on ovarian reserve after minimally invasive single-port laparoscopic ovarian cystectomy in patients with benign ovarian cysts: A systematic review and meta-analysis. Int J Clin Pract. (2021) 75:e14875. doi: 10.1111/ijcp.14875
22. Liu Y, Pan Z, Wu Y, Song J, Chen J. Comparison of anti-Müllerian hormone and antral follicle count in the prediction of ovarian response: a systematic review and meta-analysis. J Ovarian Res. (2023) 16:117. doi: 10.1186/s13048-023-01202-5
23. Somigliana E, Berlanda N, Benaglia L, Viganò P, Vercellini P, Fedele L. Surgical excision of endometriomas and ovarian reserve: a systematic review on serum antimüllerian hormone level modifications. Fertil Steril. (2012) 98:1531–8. doi: 10.1016/j.fertnstert.2012.08.009
24. Muzii L, Di Tucci C, Di Feliciantonio M, Galati G, Di Donato V, Musella A, et al. Antimüllerian hormone is reduced in the presence of ovarian endometriomas: a systematic review and meta-analysis. Fertil Steril. (2018) 110:932–940.e1. doi: 10.1016/j.fertnstert.2018.06.025
25. Younis JS, Shapso N, Fleming R, Ben-Shlomo I, Izhaki I. Impact of unilateral versus bilateral ovarian endometriotic cystectomy on ovarian reserve: a systematic review and meta-analysis. Hum Reprod Update. (2019) 25:375–91. doi: 10.1093/humupd/dmy049
26. Nankali A, Kazeminia M, Jamshidi PK, Shohaimi S, Salari N, Mohammadi M, et al. The effect of unilateral and bilateral laparoscopic surgery for endometriosis on Anti-Mullerian Hormone (AMH) level after 3 and 6 months: a systematic review and meta-analysis. Health Qual Life Outcomes. (2020) 18:314. doi: 10.1186/s12955-020-01561-3
27. Tian Z, Zhang Y, Zhang C, Wang Y, Zhu HL. Antral follicle count is reduced in the presence of endometriosis: a systematic review and meta-analysis. Reprod BioMed Online. (2021) 42:237–47. doi: 10.1016/j.rbmo.2020.09.014
28. Moreno-Sepulveda J, Romeral C, Niño G, Pérez-Benavente A. The effect of laparoscopic endometrioma surgery on anti-müllerian hormone: A systematic review of the literature and meta-analysis. JBRA Assist Reprod. (2022) 26:88–104. doi: 10.5935/1518-0557.20210060
29. Younis JS, Shapso N, Ben-Sira Y, Nelson SM, Izhaki I. Endometrioma surgery-a systematic review and meta-analysis of the effect on antral follicle count and anti-Müllerian hormone. Am J Obstet Gynecol. (2022) 226:33–51.e7. doi: 10.1016/j.ajog.2021.06.102
30. Alborzi S, Foroughinia L, Kumar PV, Asadi N, Alborzi S. A comparison of histopathologic findings of ovarian tissue inadvertently excised with endometrioma and other kinds of benign ovarian cyst in patients undergoing laparoscopy versus laparotomy. Fertil Steril. (2009) 92:2004–7. doi: 10.1016/j.fertnstert.2008.09.014
31. Roman H, Tarta O, Pura I, Opris I, Bourdel N, Marpeau L, et al. Direct proportional relationship between endometrioma size and ovarian parenchyma inadvertently removed during cystectomy, and its implication on the management of enlarged endometriomas. Hum Reprod. (2010) 25:1428–32. doi: 10.1093/humrep/deq069
32. Muzii L, Marana R, Angioli R, Bianchi A, Cucinella G, Vignali M, et al. Histologic analysis of specimens from laparoscopic endometrioma excision performed by different surgeons: does the surgeon matter? Fertil Steril. (2011) 95:2116–9. doi: 10.1016/j.fertnstert.2011.02.034
33. Busacca M, Riparini J, Somigliana E, Oggioni G, Izzo S, Vignali M, et al. Postsurgical ovarian failure after laparoscopic excision of bilateral endometriomas. Am J Obstet Gynecol. (2006) 195:421–5. doi: 10.1016/j.ajog.2006.03.064
34. Coccia ME, Rizzello F, Mariani G, Bulletti C, Palagiano A, Scarselli G. Ovarian surgery for bilateral endometriomas influences age at menopause. Hum Reprod. (2011) 26:3000–7. doi: 10.1093/humrep/der286
35. Takae S, Kawamura K, Sato Y, Nishijima C, Yoshioka N, Sugishita Y, et al. Analysis of late-onset ovarian insufficiency after ovarian surgery: retrospective study with 75 patients of post-surgical ovarian insufficiency. PloS One. (2014) 9:e98174. doi: 10.1371/journal.pone.0098174
36. Nelson SM, Iliodromiti S, Fleming R, Anderson R, McConnachie A, Messow CM. Reference range for the antimullerian hormone Generation II assay: a population study of 10,984 women, with comparison to the established Diagnostics Systems Laboratory nomogram. Fertil Steril. (2014) 101:523–9. doi: 10.1016/j.fertnstert.2013.10.021
37. Somigliana E, Palomino MC, Castiglioni M, Mensi L, Benaglia L, Vercellini P, et al. The impact of endometrioma size on ovarian responsiveness. Reprod BioMed Online. (2020) 41:343–8. doi: 10.1016/j.rbmo.2020.03.003
38. Marcellin L, Santulli P, Bourdon M, Comte C, Maignien C, Just PA, et al. Serum antimüllerian hormone concentration increases with ovarian endometrioma size. Fertil Steril. (2019) 111:944–952.e1. doi: 10.1016/j.fertnstert.2019.01.013
39. Roman H, Chanavaz-Lacheray I, Mircea O, Berby B, Dehan L, Braund S, et al. Large ovarian endometriomas are associated with high pre-operative anti-Müllerian hormone concentrations. Reprod BioMed Online. (2021) 42:158–64. doi: 10.1016/j.rbmo.2020.09.008
40. Sanchez AM, Viganò P, Somigliana E, Panina-Bordignon P, Vercellini P, Candiani M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update. (2014) 20:217–30. doi: 10.1093/humupd/dmt053
Keywords: endometrioma, endometriotic cystectomy, ovarian reserve, Anti-Müllerian hormone, antral follicle count, meta-analysis
Citation: Younis JS and Taylor HS (2024) The impact of ovarian endometrioma and endometriotic cystectomy on anti-Müllerian hormone, and antral follicle count: a contemporary critical appraisal of systematic reviews. Front. Endocrinol. 15:1397279. doi: 10.3389/fendo.2024.1397279
Received: 07 March 2024; Accepted: 24 April 2024;
Published: 10 May 2024.
Edited by:
Marc Yeste, University of Girona, SpainReviewed by:
Enes Taylan, Wayne State University, United StatesCopyright © 2024 Younis and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johnny S. Younis, anlvdW5pc0Bwb3JpYS5oZWFsdGguZ292Lmls
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.