- 1Mediclinic Hospital, Abu Dhabi, United Arab Emirates
- 2Imperial College London Diabetes Center, Abu Dhabi, United Arab Emirates
- 3Warren Alpert School of Medicine, Brown University, Providence, RI, United States
- 4Cardiology, Cleveland Clinic, Abu Dhabi, United Arab Emirates
- 5Heart, Vascular and Thoracic Institute, Cleveland Clinic, Abu Dhabi, United Arab Emirates
- 6Endocrine Section, Dubai Hospital, Dubai Academic Health Corporation (DAHC), Dubai, United Arab Emirates
- 7College of Medicine, Mohammed Bin Rashid University of Medicine and Health Science (MBRU), Dubai, United Arab Emirates
- 8Cardiology Division, Mediclinic Hospitals, Al Ain, United Arab Emirates
- 9Burjeel Hospital, Abu Dhabi, United Arab Emirates
- 10Benefits Design and Strategic Purchasing Department, Healthcare, Abu Dhabi, United Arab Emirates
- 11Dubai Hospital, Dubai, United Arab Emirates
- 12Cardiology Department, Dubai Hospital, Dubai Academic Health Corporation (DAHC), Dubai, United Arab Emirates
- 13Cardiology Department, Zulekha Hospitals, Dubai, United Arab Emirates
- 14Cardiology Department, Cairo University, Cairo, Egypt
- 15The Emirates Society of Internal Medicine, Internal Medicine Rashid Hospital, Dubai Health Authority, Dubai, United Arab Emirates
- 16The Emirates Cardiac Society, Rashid Hospital, Dubai, United Arab Emirates
- 17Internal Medicine Department, Rashid Hospital, Dubai Academic Health Corporation (DAHC), Dubai, United Arab Emirates
- 18Medical Department, Dubai Medical College, Dubai, United Arab Emirates
- 19Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
Background: The combination of cardiovascular disease and diabetes is a highly prevalent condition in the United Arab Emirates. Development and dissemination of evidence-based regional recommendations for optimal screening, treatment and referrals of people with diabetes and high cardiovascular risk is an important priority.
Consensus panel: An expert panel of diabetologists, endocrinologists and cardiologists from the Emirates Cardiac Society and Emirates Diabetes and Endocrine Society as well as different entities in the UAE, discussed and reviewed evidence and also a consensus report from the American Diabetes Association to formulate contextualized recommendations that could be applied for optimal management of cardiovascular risk in people with diabetes in the UAE.
Consensus findings: The combination of heart failure and other cardiovascular risks is a highly prevalent finding among people with diabetes in the United Arab Emirates. The causal inter-relationships between diabetes and heart failure are multifactorial and regular assessments of symptoms and steps for mitigation of risk factors are an important priority. The universal definition and classification of heart failure provides a useful framework for recommending optimal screening, treatment, and referral strategies to diabetic individuals at various stages of the cardiovascular continuum. Routine measurement (at least yearly) of natriuretic peptides and high-sensitivity troponins can help identify patients requiring cardiac imaging referrals. However, recommending routine measurements of natriuretic peptides and/or high-sensitivity troponins to all diabetic individuals must balance clinical judgment and cost implications. While SGLT2i must be an important part of the standard of care, insulin, GLP1 receptor agonists and/or metformin can be useful for additional glycemic control.
Conclusion: The consensus panel hopes that the recommendations presented herein can offer guidance for optimal screening, treatment and referral of people with a concomitance of diabetes and high cardiovascular risk in the United Arab Emirates.
1 Introduction
A recent multidisciplinary in consensus statement by Pop-Busui et al. highlighted the under- appreciation of heart failure as a complication of diabetes (1). This consensus report from the American Diabetes Association, which has been endorsed by the American College of Cardiology presents several best-practice recommendations for screening, diagnosis and referral of people with a combination of diabetes and heart failure risk (1). Consensus statements differ from clinical practice guidelines and do not necessarily need grading of evidence (2). Consensus statements deal more with “what to do” kind of considerations rather than “why to do” considerations (2). Thus, very similar to the ADA consensus report, we propose this as a practical guide to the application of biomarker-based detection of complications of diabetes from a panel composed of the UAE consensus group of expert authors from multiple specialties including diabetologists, endocrinologists, cardiologists, internal medicine specialists, nephrologists, family medicine specialist and representatives of both health care regulators and insurance payors and the emirates medical association. Thus, the ADA consensus report must be viewed as a set of “what to do” recommendations derived through a consensus between a multidisciplinary group of experts.
Adapting evidence-based recommendations to the contextual specificities of a geographical region is a key aspect of evidence-based medicine (3, 4). Such Contextualization helps to ensure optimal and effective dissemination of evidence-based best practices in a region sensitive manner. The consensus statement presented herein has been synthesized by an expert multidisciplinary group of expert authors from multiple specialties including diabetologists, endocrinologists, cardiologists, internal medicine specialists, family medicine specialists, nephrologists, and representatives of both healthcare regulators and insurance payors and the Emirates Medical Association (from the Emirates Cardiac Society and Emirates Diabetes and Endocrine Society and Emirates Family Medicine Association) For optimal management (screening, diagnosis, and referral) of heart failure in people with diabetes in the Emirates region.
The Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology and Japanese Heart Failure Society have together developed a Universal Definition and Classification of Heart Failure (Figure 1). Adapted from Bozkurt B. (5). This definition reduces the emphasis of ejection fraction and focuses on the diagnosis, prevention, early-stage disease and symptomatic stages (5). Thus, it provides a useful framework for individualizing screening, diagnosis and referral-focused recommendations and best practices according to the stage of heart failure risk in people with diabetes. Overall, the recommendations provided herein is an effort to offer an expert position statement for adapting the recommendations of the ADA consensus to the specific context of the United Arab Emirates.
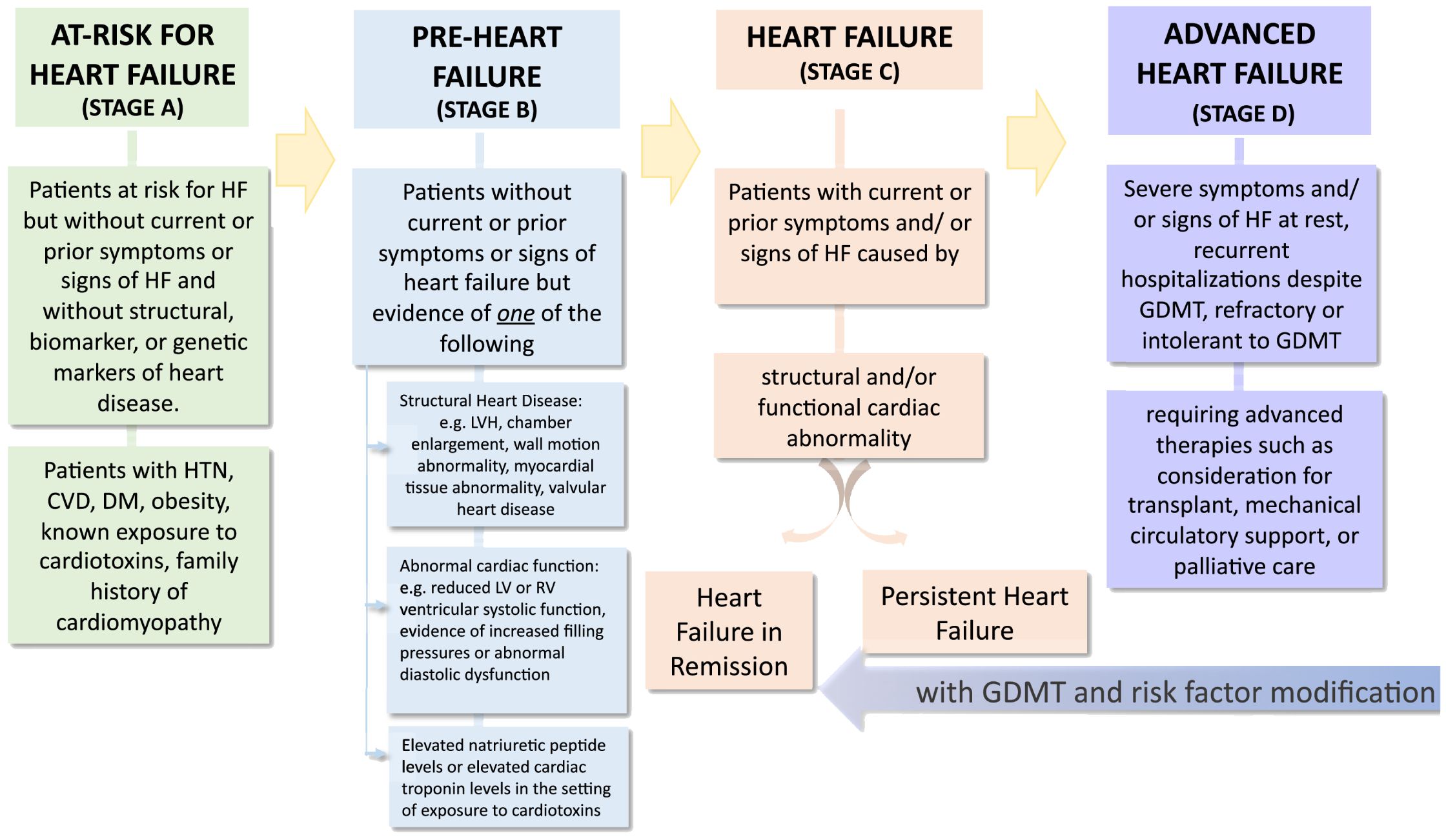
Figure 1. Reprinted with permission from / Adapted with permission from Universal Definition and Classification of Heart Failure A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure by Biykem Bozkurt, Andrew JS Coats, Hiroyuki Tsutsui, Magdy Abdelhamid, Stamatis Adamopoulos, Nancy Albert, Stefan D. Anker, John Atherton, Michael Böhm, Javed Butler, Mark H. Drazner, G. Michael Felker, Gerasimos Filippatos, Gregg C. Fonarow, Mona Fiuzat et al., licensed under 5903040821098, Elsevier.
2 Methods
An expert multidisciplinary group of cardiologists, diabetologists, and endocrinologists participated in a two-part meeting held virtually in 2022. It should be noted that the ESC guidance in 2019 recommended an algorithm for the diagnosis of HF in the primary care setting since this is a very common undiagnosed complications of Type 2 DM; however, this wasn’t widely adopted (6, 7).
In the first meeting, the multidisciplinary group reviewed the ADA consensus. Furthermore, 5 working groups were formed to review evidence for 5 separate topics. In the second meeting, the expert multidisciplinary group discussed this evidence to synthesize consensus-based recommendations for The management of cardiovascular risks in type 2 diabetes in the United Arab Emirates.
3 Consensus statements
3.1 Diabetes and conventional cardiovascular risk in the United Arab Emirates
The Association of diabetes and conventional risk factors leads to a significantly increased risk of cardiovascular events. It is recognized that if a diabetic patient has a single risk factor such as hypertension, obesity, dyslipidemia. they are considered high risk for cardiovascular events. In addition, diabetic patients with target organ damage as left ventricular hypertrophy or renal disease or albuminuria or retinopathy are at very high risk. Diabetics with multiple risk factors including any of the following: obesity, hypertension, hyperlipidemia, are at very high cardiovascular risk and this has been recognized in the guidelines from the ESC 2019. There are additional risk enhancers in patients with diabetes that are listed in the tables below (Boxes 1-3), which increases the risk of diabetic patients including an abnormal API and other biomarkers of atherosclerosis such as lipoprotein A and C-reactive protein. In the diabetic patients in the UAE additional testing has been recommended at an earlier age based on the UAE diabetes guidelines and the UAE cardiometabolic guidelines. Management of hypertension in diabetic patients is of paramount importance since it increases the risk of cardiovascular events significantly the recent American Heart Association ACC guidelines have lowered the threshold of treatment of hypertension in diabetes to encompass more treatment in a larger component of patients. It is well recognized that hypertension and coronary artery disease are major co-morbidities in diabetes and that Accelerated atherosclerosis is responsible for increased mortality and myocardial infarctions in diabetic patients. However, the less well-recognized complication is heart failure which will be expanded on in the next section (8).
Box 1. Consensus statement – Epidemiological considerations.
• The concomitant burden of diabetes and heart failure is high in the United Arab Emirates
• Steps to enrich currently available epidemiological data from the United Arab Emirates are recommended.
○ Registries and observational studies must include measures for assessing the concomitance and causal inter-relationships between diabetes and heart failure in a manner relevant to context of United Arab Emirates.
• The high concomitance of diabetes and heart failure in the United Arab Emirates needs implementable action for
○ Risk factor mitigation as a preventive strategy
○ Risk factor assessment for optimal referral for multidisciplinary interventions
○ Optimal screening for new-onset diabetes in people with heart failure
○ Optimal screening for undiagnosed heart failure in people with diabetes
Box 2. Consensus statement – Cardio renal Complications in diabetes.
• The diabetes cardio-renal spectrum presents an epidemiologically and pathophysiological interrelated “triple burden.”
• Studies to assess the impact of this triple burden in the United Arab Emirates is strongly recommended.
• Based on current evidence, the use of SGLT2 inhibitors are essential organ-protective and life- saving therapies in the standard of care in people with diabetes who are at the risk of cardiorenal syndromes.
Box 3. Consensus statement – Clinical risk assessment.
• Optimal clinical risk assessment is recommended in people with diabetes.
• Annual biomarker measurement (see next section) is recommended if the clinical risk factors are identified in people with diabetes.
• Referral to cardiology specialists if biomarkers identify CV disease.
• It is recommended that presence of these clinical risk factors and/or biomarkers must warrant considerations for change in treatment to drugs with established cardiovascular benefits
Box 4. Consensus statement – Biomarkers and Risk Detection.
• We recommend annual screening for all diabetic adults with natriuretic peptides.
• We also recommend measuring natriuretic peptide levels in diabetics who complain of dyspnea, fatigue, lower limb swelling, or chest pain.
• It is cost-effective, to utilize NT-proBNP and is recommended prior to echocardiography to rule out LV dysfunction in diabetic patients.
• In selected high-risk patients, there is additional prognostic information by testing C-reactive protein and high-sensitivity troponin in asymptomatic diabetic patients.
• Key symptoms that should be asked about during every visit include exertional fatigue, inability to go up one flight of stairs (NYHA functional class-II), lower limb edema, and exertional palpitation. In this situation, NT-proBNP and an ECG should be obtained with a fast-track referral to a cardiologist (99).
3.2 Epidemiology of diabetes and heart failure in the United Arab Emirates
Cardiovascular disease is the most common cause of mortality in people with diabetes and several studies have documented the high prevalence of cardiovascular risk factors in people with diabetes (9). Early identification and mitigation of these risk factors, especially the modifiable ones are essential health care priorities. In the last 50 years, UAE has witnessed significant economic growth, along with an increase in per-capita income and life expectancies (10). The lifestyle change associated with this economic growth has also led to increases in several modifiable risk factors such as a sedentary lifestyle, obesity, diabetes, hypertension and cardiovascular disease (10). A recent systematic review by Razzak et al. indicates that the prevalence of diabetes in the United Arab Emirates ranges from 0.87% to 33.3% (11). According to another report from Al Awadi et al., the prevalence of diabetes in the Emirates region was about 16.3% (12). These estimates underscore a high prevalence of diabetes. On the other hand, there is a relative paucity of heart failure data in the United Arab Emirates. However, according to a 2018 survey by AlHabeeb et al, an estimated 93,865 patients were receiving treatment for heart failure in the United Arab Emirates (13). Furthermore, the results of the Gulf CARE Registry indicate that heart failure patients from the Emirates region are about 10 years younger as compared to their age-matched Western population and about 50% of patients have diabetes (14). Overall, these data indicate that along with a high burden of diabetes, a substantial proportion of heart failure patients in the United Arab Emirates have diabetes.
Several studies have demonstrated the elevated risk of heart failure in people with diabetes. Results of the Framingham Heart Study indicate a 2-fold higher risk of heart failure in men with diabetes and a 4-fold higher increase in women with diabetes (9). A retrospective study by Nichols et al. indicates that diabetic individuals in the younger age groups may be particularly susceptible to the elevated risks of congestive heart failure (15). Shindler et al. in their report highlighted that diabetes is an independent predictor of outcomes in people with symptomatic heart failure asymptomatic left ventricular dysfunction (16).
Furthermore, the duration of diabetes and poor glycemic control are known to be risk-elevation factors for heart failure in people with diabetes (17, 18). Presence of comorbidities are also known modulators of the association between diabetes and heart failure (18, 19). Presence of macrovascular and microvascular complications of diabetes and higher levels of NT-proBNP (N-terminal pro-B-type natriuretic peptide) are known to increase the risk of incident heart failure (20–25).
Data from several studies also demonstrate the diabetogenic effects of heart failure. Paolillo et al., in their article highlighted the bidirectional association between heart failure and diabetes (26). In this study, insulin resistance was noted in about 63% of patients with severe-to-moderate heart failure (26). Heart failure also is known to aggravate the incidence of new onset diabetes. Data from the EMPHASIS-HF trial and the CHARM program indicate that the incidence of new-onset diabetes in heart failure patients could be in the range of about 21-28 per 1000 person-years, respectively (27, 28). Results of these studies indicate that factors such as elevated BMI, waist circumference, smoking along with abnormal glucose, HbA1c and systolic blood pressure aggravate the diabetogenic effects of heart failure (27, 28).
The risks of mortality, increased hospitalization, re-hospitalization and impaired quality-of-life are known to be aggravated by the bidirectional association of diabetes and heart failure places a significant burden on health outcomes and healthcare utilization (29).
Overall, available Emirates-specific data indicates that the prevalence and incidence of concomitant diabetes and heart failure is noticeably high in the United Arab Emirates (10–14). When viewed along with the data from other international studies presented above, it appears that several research and clinical recommendations need prospective implementation to attenuate the impact of diabetes and heart failure concomitance in the United Arab Emirates.
3.3 Cardio-renal complications in diabetes
A number of studies have documented the vicious epidemiological and pathophysiological inter- relationships between diabetes, heart failure, and chronic kidney disease. In a recent study, Schechter et al. note that the prevalence of cardiorenal syndrome (CRS) was 2.3% in patients with type 2 diabetes as compared to 0.4% in the entire population (N= 1,389,604; 52.2% females and 47.8% males) (30). This study further noted the prominence of type 2 diabetes among younger subjects within the diabetes-cardio-renal spectrum, which was gradually replaced by HF and eGFR < 60 mL/min/1.73m2 with increasing age (30). Ronco et al. describe five subtypes of cardiorenal syndrome, wherein type 1 and 2 cardiorenal syndrome occur because of the cardiac conditions impacting the kidneys, type 3 and 4 occur when renal conditions affect the heart. Type 5 CRS occurs when systemic conditions impact the heart and kidneys concurrently (31). Banerjee et al. in their study indicate that diabetes is strongly associated with Type 2 CRS (adjusted OR: 2.25 (CI 1.56-3.23, p < 0.001) (32). Being a systemic condition, diabetes is also known to be causally associated with type 4 and type 5 cardiorenal syndrome (32–34). Several interrelated mechanisms are known to underlie the manifestations of cardiorenal syndrome. Compensatory activation of RAAS (renin-angiotensin-aldosterone system), cardiac hypertrophy, and myocardial fibrosis along with inflammation, oxidative stress, and endothelial dysfunction are known to underlie pathophysiological mechanisms contributing to the bidirectional association between heart and kidney dysfunction (32, 33). Almost all of these pathophysiological factors also contribute to a “diabetic heart”, also known as diabetic cardiomyopathy or metabolic inflammatory cardiomyopathy.
Dei Cas et al. in their review indicate that poor outcomes in people with a concomitance of diabetes and heart failure as compared to those without diabetes could be explained by diverse metabolic and neurohormonal abnormalities (35). While the pathophysiological link between diabetes and heart failure is confounded by hypertension, microvascular dysfunction, and autonomic neuropathy, several mechanistic associations at systemic, cardiac, and cellular/molecular levels explain different aspects of myocardial dysfunction in people with diabetes (36).
It is relatively well-established that hyperglycemia and hyperinsulinemia both promote vascular smooth muscle cell proliferation and inflammation and aggravate atherosclerotic processes (37–42). The aggravating association of diabetes mellitus with atherogenic dyslipidemia and endothelial dysfunction are all known to adversely impact thrombosis, inflammation, and coronary plaque ulceration (43–45). The resulting ischemic or infarct-related consequences may underlie the heart failure and other structural heart abnormalities seen in people with diabetes. In fact, diastolic dysfunction due to left ventricular hypertrophy is seen in a majority of patients with diabetes (46–48). Levelt et al. note that cardiac steatosis may contribute to concentric remodeling and contractile dysfunction of the left ventricles in diabetes (49).
The impact of hyperglycemia on cardiac health can be explained by the effects of the former on formation of advanced glycation end products, which are implicated in collagen cross-linking, and consequent increases in myocardial fibrosis (50–52).
Furthermore, the effects of hyperglycemia on the activation of the Renin Angiotensin Aldosterone System (RAAS) and the resultant cardiac hypertrophy and exacerbation of diastolic dysfunction have been implicated in the pathological association between diabetes and heart failure (53).
Several studies have highlighted the significance of sodium–glucose cotransporter-2 (SGLT2) inhibition in attenuating the impact of cardio-renal syndrome (54, 55). While SGLT2 inhibition offers cardiovascular protection by reducing cardiac workload, blood pressure, and body weight, it offers additional protective effects on kidney by reducing albuminuria and hypoxic stress, and by restoring tubuloglomerular feedback (34).
Overall, available data indicates that diabetes and cardiorenal syndrome forms a burdensome “triple threat” with demonstrable epidemiological and pathophysiological inter-relationships at systemic and cellular levels (32, 34). While current evidence for SGLT2 inhibition is promising enough to attenuate the impact of this “triple threat”, accruing evidence from ongoing studies should refine our understanding on the place of SGLT2 inhibition in the management of diabetes and its cardio-renal complications (34).
3.4 Clinical risk assessment in T2D-clinical variables predicting heart failure
Zhou et al. in their systematic review point out that the main risk factors of heart failure in the type 2 diabetic population were age, hemoglobin A1c (HbA1c), coronary heart disease, hypertension, microalbuminuria and obesity (56). Yancy et al. in their article draw attention to the fact that several recent guidelines emphasize the need for optimal preventive and clinical management strategies for attenuating these risk factors to counter incident heart failure in people with diabetes (57). Although several models incorporating these risk factors have been developed and reported for predicting prognosis and outcomes of heart failure in people with diabetes, they lack clinical validation (58–60). Nevertheless, data from several randomized clinical trials and observational studies indicate that mitigating risk factors alleviate the burden of adverse cardiac outcomes, especially with atherosclerotic heart disease and stroke (61). However, until recently there has been a general paucity of data for specifically characterizing the impact of risk factor mitigation on incident heart failure and its outcomes. Data from the Swedish National Diabetes Register indicates that the rate of hospitalization for heart failure remains obstinately high despite optimal control of conventional risk factors (62). However, results of this registry indicate that the presence of atrial fibrillation, higher body-mass index, a low estimated glomerular filtration rate, and high glycated hemoglobin level are strong predictors for hospitalization with heart failure (62). A review of currently available evidence on the clinical risk factors underlying the concomitance of diabetes with heart failure is presented below.
A meta-analysis by Ohkuma et al. highlights that the gender disparity for heart failure among people with diabetes is skewed more towards women as compared to men (63). As per the results of this meta-analysis, the pooled multiple-adjusted relative risk for heart failure was 5.15 (95% CI 3.43, 7.74) in women and 3.47 (2.57, 4.69) in men with type 1 diabetes (63). Furthermore, the relative risk for heart failure was 1.95 (1.70, 2.22) in women and 1.74 (1.55, 1.95) in men with type 2 diabetes (63). Another study by Chadalavada et al. indicates that people with diabetes as compared to non-diabetic individuals had a twice as high risk of incident heart failure and mortality (64). Furthermore, in this study females with type 1 and type 2 diabetes had a 22% elevated risk of heart failure as compared to men (hazard ratio: 2.2 (95% CI: 1.9-2.5) vs. 1.8 (1.7-2.0) (63). Available evidence indicates that this gender-disparity could be due to a higher incidence of coronary heart disease, poor control of hypertension and glycemic control and a greater degree of endothelial dysfunction (65–69).
Concerning diabetes-related risk factors, it appears that both the level of glycemic control and duration of diabetes seem to have a significant effect on incident heart failure (70, 71). According to data from the Framingham Heart Study, each 10-year increase in the duration of diabetes is associated with a 25% increase in risk of cardiovascular events including hospitalization for heart failure (multivariable-adjusted hazard ratio: 1.25 (95% CI, 0.99– 1.57) (72). In the ARIC study, Echouffo-Tcheugui et al. report that each 5-year increase in the diabetes duration was associated with a 17% (95% CI: 11-22) relative increase in HF risk (18). Results of the UK Prospective Diabetes study demonstrated a 16% reduction in the risk of heart failure associated with a 1% reduction in glycated hemoglobin, thereby highlighting those patients with nonoptimal glycemic control have an increased risk of heart failure events (73). Results of a meta-analysis by Aune et al. indicate that individuals with diabetes are at an increased risk of developing heart failure and there is evidence of increased risk even within the pre-diabetic range of blood glucose (74).
Microalbuminuria and other microvascular complications of diabetes are known to elevate the risks of heart failure (25). Results of the RENAAL trial by Zeeuw et al. indicate that patients with high baseline albuminuria (> or =3 g/g creatinine) had a 1.92-fold (95% CI, 1.54 to 2.38) higher risk for the cardiovascular endpoint and a 2.70-fold (95% CI, 1.94 to 3.75) higher risk for heart failure compared with patients with low albuminuria (<1.5 g/g) (75). Furthermore, the Heart Outcomes Prevention Evaluation Study results indicated that any degree of albuminuria is a risk factor for CV events in individuals with or without DM (76). As per the results of this study, a urine albumin creatine ratio > 17.7 mg/g was associated with a significant increase in the relative risk of hospitalization for heart failure [3.23 (95% CI, 2.54–4.10)] (76). Furthermore, each 3.5-mg/g increase in ACR increased the risk of hospitalization for heart failure by 10.6% (95% CI, 8.4–13.0%) (76). In the EMPA-REG OUTCOME trial, the presence of microvascular disease was associated with an increased risk of heart failure-related hospitalization (hazard ratio:1.63; 95% CI, 1.06–2.49; P = 0.025) (77).
While glycemic control seems to affect the relationships between diabetes and heart failure, it appears that the choice of specific anti-hyperglycemic agents must be made carefully. Nichols et al. note that Insulin alone or in combination with sulfonylureas could increase the risk of heart failure in people with diabetes (15). Furthermore, Roumie et al. in their report indicates that sulfonylurea use, compared with metformin use, was associated with a 43% increased risk of heart failure (adjusted hazard ratio: 1.43; 95% CI, 1.30–1.57) (78). Available evidence also indicates elevated risks of heart failure with thiazolidinediones, rosiglitazone, and pioglitazone (79). Results of the Prospective Pioglitazone Clinical trial in Macrovascular Events trial demonstrated an increase in the risk of hospitalization for heart failure with pioglitazone (hazard ratio: 1.41; 95% CI, 1.10–1.80; P = 0.007) (80). Scirica et al. indicate that saxagliptin (a DPP-4 inhibitor) as compared to placebo was associated with an increased risk of hospitalization for heart failure among patients with T2D at high risk of cardiovascular outcomes (hazard ratio, 1.27; 95% CI, 1.07–1.52; P = 0.007) (81). Another study by Zannad et al. noted that alogliptin as compared to placebo demonstrated a nonsignificant increase in the risk of hospitalization for heart failure (hazard ratio, 1.19 (95% CI, 0.90–1.58) (82).
Decline in eGFR is a strong predictor of Heart failure in people with type 1 and type 2 diabetes (83). Ninomiya et al. point out that the presence of diabetes increases the rate of heart failure related hospitalization and contributes to poor survival (84). Furthermore, several cardiovascular outcome trials observed an approximately 2-fold increase in the heart failure rate in people with T2D and a reduced eGFR (85). Specifically, survival decreased from 2.8 years at an eGFR of 45–59 mL/min/1.73 m2 to 0.7 years at an eGFR of < 15
mL/min/1.73 m2 (85). Lawson et al. in their study note that in people with new onset heart failure, hospitalizations and deaths are high in patients with T2D or CKD and worst in those with both comorbidities (85).
Obrezan and Kulikov, indicate that atrial fibrillation is one of the most common concomitant diseases in patients with diabetes mellitus (DM) (86). As Obrezan and Kulikov, explain meta-analyses of multiple studies have shown that the risk of atrial fibrillation is higher for diabetic patients with impaired glucose homeostasis than for patients without diabetes. Furthermore, this study explains that patients with atrial fibrillation and diabetes were younger and had a higher frequency of arterial hypertension, chronic kidney disease, heart failure, ischemic heart disease, and stroke (87).
Additional cardiovascular risk, particularly heart failure has been reported with enlargement of epicardial adipose tissue on imaging. Several observational studies have reported that patients with type-2 diabetes have an abnormally enlarged and biologically transformed epicardial adipose tissue (EAT) compared with non-diabetic controls (88). This expanded EAT along with causing mechanical constriction of the diastolic filling is also a source of pro-inflammatory mediators capable of causing inflammation, microcirculatory dysfunction and myocardial fibrosis (88). In addition to being a cardiovascular risk factor, EAT may guide the treatment choices as drugs such as metformin, glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose cotransporter 2 inhibitors (SGLT2i), have been associated with attenuation of EAT enlargement (88).
New England Journal has published a study with the: An analysis of the Framingham study utilizing BNP in primary prevention in a community setting 3346 without heart failure, plasma natriuretic peptide levels predicted the risk of death and cardiovascular events after adjustment for traditional risk factors. The excess risk was apparent at natriuretic peptide levels well below the current thresholds used to diagnose heart failure. Examination of the relations of plasma B-type natriuretic peptide and N- terminal pro–atrial natriuretic peptide to the risk of death from any cause, a first major cardiovascular event, heart failure, atrial fibrillation, stroke or transient ischemic attack, and coronary heart disease (89).
3.5 Biomarkers and cardiovascular risk detection in type 2 diabetes
Biomarkers play a central role in the diagnosis and treatments of diabetes mellitus. Along with their diagnostic utility, biochemical parameters such as plasma glucose or HbA1C guide treatment-related decisions, as well. Over the years, accruing evidence with novel biomarkers have opened up several vistas for incremental improvements in diagnosis, prognosis and risk stratification of patients with type 2 diabetes mellitus. The American Diabetes Association recommends measuring HbA1C twice every year in diabetic individuals attaining treatment goals (90). In patients not attaining treatments goals, the American Diabetes Association recommends HbA1c testing at least once in every three months (90). Furthermore, the American Diabetes Association recommends measurement of urinary albumin at least once a year for screening of chronic kidney disease (90). It is important to note that there is a linear relationship between Urine Albumin-Creatinine Ratio and outcomes (91). There is a remarkably increased risk in mortality and cardiovascular death even in those patients with mildly elevated concentrations between 10 mg/g and 30 mg/g and care must be taken with this subset of patients, since they are often may not be identified as having chronic kidney disease risk. The utility of eGFR in predicting cardiovascular outcomes has also been relatively well-studied. The relationship between eGFR and cardiovascular death and overall mortality is “U-shaped” with the lowest risk in those patients with eGFR approximately 90–100 mL/min/m2 (92). The risk increases substantially with a lower eGFR, but also increases with eGFR >105 mL/min/m2, likely representing the hyperfiltration seen with early diabetic nephropathy (6) (Box 4).
The heterogeneity of type 2 diabetes mellitus offers several challenges for assessing cardiovascular risks. This consideration is particularly relevant when assessing risk in T2DM patients without manifest cardiovascular disease and in those with a risk of ischemic complications, which varies along with age, duration of diabetes, and comorbidities.
The findings of PEGASUS sub-study suggest that a strategy incorporating hsTn testing into a guideline-derived ASCVD risk algorithm provides enhanced risk stratification and reclassifies patients into more appropriate risk groups. This application of hsTn might be used to optimize the care of patients with ASCVD (93). Using a cut point of great plan 6ng/L showed a two-fold increase risk of cardiovascular events (94, 95). In primary prevention, the prediction of higher-risk of atherosclerotic events would guide the clinician to prescribe high-intensity statin therapy and the use of aspirin based on elevated troponin levels This effect is particularly important in diabetics as was confirmed in the zodiac study (96–98). The Utilization of biomarkers and ECG will facilitate the primary care solution and diabetologists and nephrologists to identify the higher-risk patient who would benefit from additional cardiovascular assessment, in the case of asymptomatic patient with atrial fibrillation discovered by ECG; additional therapies are indicated including anticoagulation based on CHA2DS2-VASc score AND decision on rhythm control which ultimately requires additional investigation by the cardiologist at a minimum echocardiography is essential to ensure the absence of valvular or ischemic heart disease or heart failure. The cardiologist may also despite to perform more detailed ischemic evaluation as appropriately indicated. Another scenario that would likely be encountered in clinical practice by the general physician is the elevation of NT-proBNP with the current guideline recommended cut of point of 125, this biomarker suggests not only underlying heart failure but also the presence of elevated cardiovascular risk. From this perspective, additional therapies can be recommended by the primary care physician including ACE inhibitor or ARB and particularly the addition of SGLT-2 inhibitors. Moreover, fast track referral to the cardiologist is essential to determine the type of heart failure; whether reduced ejection fraction or preserved ejection fraction and additional imaging to assess the type of LV dysfunction would be obtained. Similarly, an elevated high-sensitivity troponin would indicate the presence of high risk of atherosclerosis and predict future cardiovascular events. From the general physician standpoint, this would indicate the need of intensification of lipid- lowering therapy and addition of anti-platelet therapy as well; however, referral to the cardiologist for ischemia evaluation would be strongly recommended. Notably in the UAE, given the predominant sedentary lifestyle and increased preponderant risk factors, including family history, smoking, obesity and metabolic syndrome the likelihood of effort-inducing symptoms of angina or ischemia or heart failure is reduced due to the central lifestyle, thus specific questions for history examination should highlight the true or absence of symptoms and also exercise testing or appropriate ischemia evaluation. Additionally, a more detailed symptom evaluation in old patients is necessary, particularly in females since ethe presentation of coronary disease symptoms in females is generally atypical and symptoms of palpitation, fatigue and dizziness epigastric and other atypical chest pain may be predominant and is often accredited to long cardiac diagnosis or assumed to be non-cardiac. Thus, the utilization of more precise stratification using biomarkers for initial assessment is strongly recommended. Additional cardiovascular examinations may also be recommended in selective patients to more precisely determine the risk. Ankle Brachial index is a simple clinic-based test that can easily discriminate the presence of early stages of peripheral atherosclerosis, also referral for coronary CT calcium score is recommended in the guidelines to reclassify cardiovascular risk and recommend preventive therapy, particularly statins (8, 99).
Available data indicates that increased concentrations of natriuretic peptides in patients with type 2 diabetes are associated with increased cardiovascular risk and have a good value for predicting cardiovascular mortality and hospitalization for heart failure. Natriuretic peptides are excellent discriminators of risk and their utilization to guide the additional therapy, particularly SGLT-2 inhibitors, in order to reduce heart failure in type 2 diabetes at the earliest possible stages (96).
The ADA consensus highlights that the detection of people at high risk for HF (stage A) or those with stage B Heart failure would facilitate early interventions to avert the progression of heart failure to advanced stages (1). Furthermore, screening for heart failure in asymptomatic patients can help in replacing diabetes medications that increase the risk of heart failure with diabetes medications such as SGLT2 inhibitors, which are known to improve heart failure outcomes. While the utility of biomarkers for heart failure screening in people with diabetes was met with limited adoption, the advent of extensive and robust evidence strongly recommends the routine utilization of natriuretic peptides for detecting heart failure risks in people with diabetes. Huelsmann et al. in their study highlight that the negative predictive value of a normal value (<125 pg/mL) of NT-proBNP for short-term cardiovascular events in diabetic patients is about 98% (100).
Furthermore, the results of the PONTIAC trial demonstrate the utility of NT-proBNP in guiding referrals to cardiac clinics. In this study, hospitalization/death due to cardiac disease was reduced by 65% after two years in patients with elevated NT-proBNP (>125 pg/ml), who were referred to additional care at a cardiac outpatient clinic for the up-titration of the renin-angiotensin system antagonists and beta-blockers (101). Similar findings have been reported by the STOP-HF trial, wherein referrals to echocardiogram and cardiology clinics based on BNP testing resulted in significant reductions of LV dysfunction with or without heart failure (45%), heart failure (52%), and emergency hospitalization for major cardiovascular events (55%) (102). Overall, it appears that BNP or NT-proBNP–based screening followed by team-based care, including a cardiovascular specialist, can be useful to prevent the development of LV dysfunction or new-onset heart failure (6).
Alexandre Mebazaa et al. in the STRONG-HF trial aimed to address under-treatment in AHF patients with rapid up-titration of GDMT compared with usual standard-of-care.
Rapid up-titration of GDMT under close follow-up (physical examination biomarkers including NT-proBNP) during and soon after discharge from HF hospital admission is safe, with no increase in serious adverse events versus usual care.
The STRONG-HF treatment regimen resulted in a significant 34% risk reduction of HF readmission and all-cause mortality at 180 days.
Both BNP and NT-BNP are widely available and Reimbursed by Insurance Payors in UAE. That’s why, rapid action is needed to implement the STRONG-HF management regimen into daily clinical practice.
3.6 Utilization of natriuretic peptides in patients with CKD
In those with reduced renal function, NT-proBNP can be increased because it is excreted from kidney. However, they are still valuable for cardiovascular screening for several reasons. These elevated levels of NT-proBNP in patients with CKD do not simply reflect the reduced clearance of the peptide; rather, they largely reflect a true-positive finding, identifying the presence of heart disease in these patients, while similarly indicating prognosis as well. Christopher DeFilippi et al. (https://pubmed.ncbi.nlm.nih.gov/18243865/.
Van Kimmenade et al. (https://pubmed.ncbi.nlm.nih.gov/19264247/) demonstrated that renal extraction of both BNP and NT-proBNP changed minimally across a spectrum of renal function measured down to approximately an eGFR of 30 mL · min−1 ·.
Importantly, when using NT-proBNP to evaluate a patient with dyspnea and impaired renal function, the recommended cut points of 450, 900, and 1,800 ng/L for those aged 75 years, respectively, do not require further adjustment for renal function. Thus, NT-proBNP testing remains useful for the diagnostic and prognostic evaluation of patients with CKD”.
In this >11K participants in a general population study (https://pubmed.ncbi.nlm.nih.gov/37290699/) showed that “Despite its strong inverse association with eGFR, NT-proBNP has robust associations with mortality across the full range of kidney function in the general US adult population.”
3.7 Cost effectiveness of natriuretic peptide in HF diagnosis and risk assessment in diabetic patients
The trials (Gallagher et al. paper) have proven that natriuretic peptide-based screening and targeted prevention can reduce heart failure and left ventricular dysfunction and other major cardiovascular events.
This approach is now part of North American guidelines and European Guidelines Walter et al. (103). Cost-effectiveness of NT-proBNP in Diagnosis of heart failure in high-risk patients especially Diabetic Patients on the health care system in terms of reducing the burden on the patient journey, and the healthcare system. This is very important given the UAE insurance restrictions on risk assessment and screening. Many studies have shown the budget impact and cost-effectiveness of pro-BNP and this has been universally shown to result in cost savings through reduction of medical visits, hospitalizations, and unnecessary echo-cardiographic testing. In addition, clinical trials have shown that natriuretic peptide screening and targeted preventive therapies can reduce heart failure and other major cardiovascular events. This approach is now incorporated in the ADA consensus guidelines. It is well-recognized that the earlier diagnosis using biomarkers will allow effective therapeutic preventions that prevent the incidence of heart failure in the first place and prevent progression to overt symptomatic LV dysfunction. It is also recognized the cost of acute hospitalization and inpatient care far exceeds the cost of outpatient natriuretic peptide and medical therapy (103).
The diagram below shows that biomarker-based detection has a role in the earlier molecular detection in which the cost is low if compared with the cost in a clinical event, in other words the earlier detection may have a cost-effective value (Figure 2).
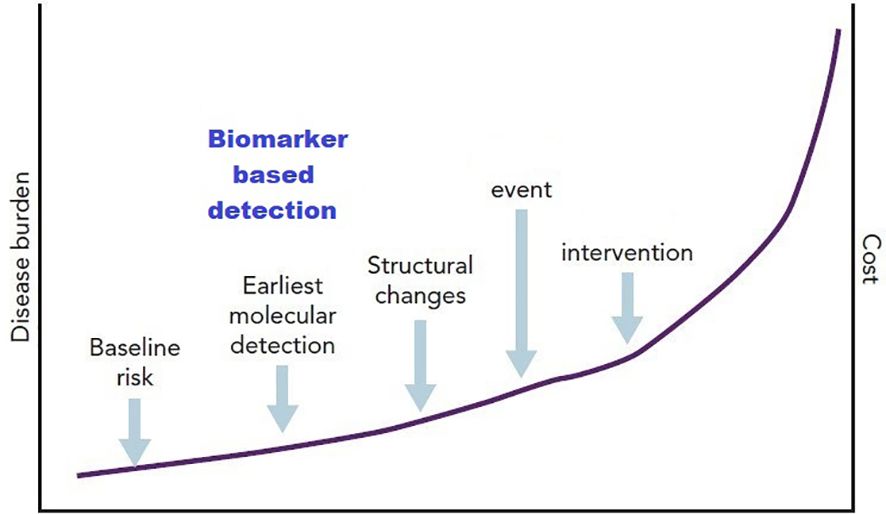
Figure 2. Diagrammatic representation of the concept of biomarkers as a component of stage B heart failure (104).
Gallagher et al., paper summarizing the 2 landmark Trials PONTIAC 1 and STOP-HF stating the use of NT-pro BNP is an effective tool in refining risk prediction for heart failure and cardiovascular disease and adding predictive power to conventional risk factors (104). A subsequent analysis of the cost-effectiveness of this approach was undertaken. The cost per quality- adjusted life year gain was €1,104 and the intervention has an 88% probability of being cost- effective at a willingness to pay threshold of €30,000 (89).
3.8 Cost-effectiveness of natriuretic peptide-based screening and collaborative care
A report from the STOP-HF (St Vincent’s Screening TO Prevent Heart Failure) study Ledwidge et al. (105), assessed the cost-effectiveness of natriuretic peptide-based screening in a sub study of 1054 participants with cardiovascular risk factors (about 18% with diabetes mellitus) found cardiovascular hospitalization savings offset increased outpatient and primary care costs. The cost per case of LVD/HF prevented was €9683 (sensitivity range –€843 to €20 210), whereas the cost per MACE prevented was €3471 (sensitivity range –€302 to €7245), in addition, the cost per QALY gain was €1104 and the intervention has an 88% probability of being cost-effective at a willingness to pay threshold of €30 000. There were 157 deaths and/or emergency hospitalizations for major adverse cardiac events (MACE) in the control group vs. 102 in the intervention group (OR 0.68; 95% CI 0.49–0.93; P = 0.01). Conclusion: Among patients with cardiovascular risk factors, natriuretic peptide-based screening and collaborative care reduced LVD, HF, and MACE, and has a high probability of being cost-effective.
A recent simulation study from Austria and Switzerland, a cost–utility model was developed to simulate the cost-effectiveness of NT-proBNP-supported screening of undetected HF with and without Diabetes as well as its long-term consequences in HF population in patients from age 60 over lifetime (103). In this study, the per-patient incremental cost–utility ratio (ICUR)/QALY of NT-proBNP vs. no screening was €3,042 and CHF 897 for HF patients in Austria and Switzerland respectively. This study concludes that screening with NT-proBNP biomarkers is a highly cost-effective or cost-saving diagnostic option for patients with HF, and a sensitivity analysis confirmed these findings.
The Irish Government in conjunction with Irish Heart Foundation conducted systemic review to assess the impact of early diagnosis if Heart Failure in the context of patient and healthcare system factors with a view to reduce the cost and frequency of heart Failure Hospitalization concluding the significant importance of early diagnosis and how it will positively impact the financial outcomes.
A survey of 372 heart failure patients in Ireland, found 60% of patients surveyed in this study received their diagnosis from a heart specialist in this hospital but with a reported 14 month waiting time to see a specialist.
Delayed diagnosis was found to have a profound impact on costs, with a 6-month delay leading to a 23% increase in emergency hospitalizations and contributing to an increased number of bed days.
This has resulted in the introduction of the Enhanced Community Care (ECC) Program, which, since 2021, has been facilitating a phased direct access to NT-proBNP blood testing for GPs for the full adult population. This will support GPs to triage patients with heart failure for referral, which as part of the Structured Chronic Disease Management Programme (CDM), will allow GPs to better prevent and manage chronic diseases, including heart failure (106). Heart failure policy and practice in Europe: Ireland (107).
Antonio Leon-Justel et al., showed that by using combined biomarkers of NT-pro BNP and high sensitivity Troponin T led to significant reduction in total hospitalization rate by 19%, and length of stay by 7 days and frequency of ED visits by 44%. The overall cost saving associated with the intervention was € 72,769 per patient (from € 201,189 to € 128,420) and €139,717.65 for the whole group over 1 year. Suggesting that greater attention should be given to this high-risk cohort to minimize the risk of hospitalization readmissions (108).
Irish Heart Foundation has published a report about the “ State of Heart ‘‘ initiative and highlighting in which the utilization of NP screening in general practice with highlighted particularly in terms of its benefit in early diagnosis of heart failure and its positive impact on patient and healthcare system. This has resulted in the introduction of the Enhanced Community Care (ECC) Program, which, since 2021, has been facilitating a phased direct access to NT-proBNP blood testing for GPs for the full adult population. This will support GPs to triage patients with heart failure for referral, which as part of the Structured Chronic Disease Management Programme (CDM), will allow GPs to better prevent and manage chronic diseases, including heart failure.
A further analysis from Leon-Justel et al. study from Spain about “Biomarkers-based personalized follow-up in chronic heart failure improves patient’s outcomes and reduces care associate cost” A cost analysis was also performed on these data. Therefore, a personalized follow-up of HF patients led to important outcome benefits and resulted in cost savings, mainly due to the reduction of patient hospitalization readmissions and a significant reduction of care-associated costs, suggesting that greater attention should be given to this high-risk cohort to minimize the risk of hospitalization readmissions (108).
4 Managing cardio-metabolic and renal risk in diabetes
Managing the cardio-renal risk in diabetes requires an integrated multi-disciplinary approach involving general practitioners, nurses, diabetes educators, endocrinologists, cardiologists and nephrologists. Proper attention to diet and physical activity is imperative. Lowering the risk of myocardial infarction, heart failure, stroke, amputation and decline in kidney functions will need control of blood pressure, blood sugar, dyslipidemia in addition to managing the heightened thrombotic risk.
4.1 Managing hypertension
Figure 3 depicts an algorithm for treatment of hypertension in people with diabetes. Adapted from Elsayed NA. (109). Several studies have demonstrated the usefulness of antihypertensive therapy in reducing atherosclerotic cardiovascular disease, heart failure, and microvascular complications in diabetes. Screening for and treating hypertension must be an integral part of the holistic treatment of diabetic patients (110, 111). The treatment of hypertension to blood pressure goals below 130/80 mm Hg is the recommended treatment goal, with drug therapies considered for people with diabetes and hypertension.
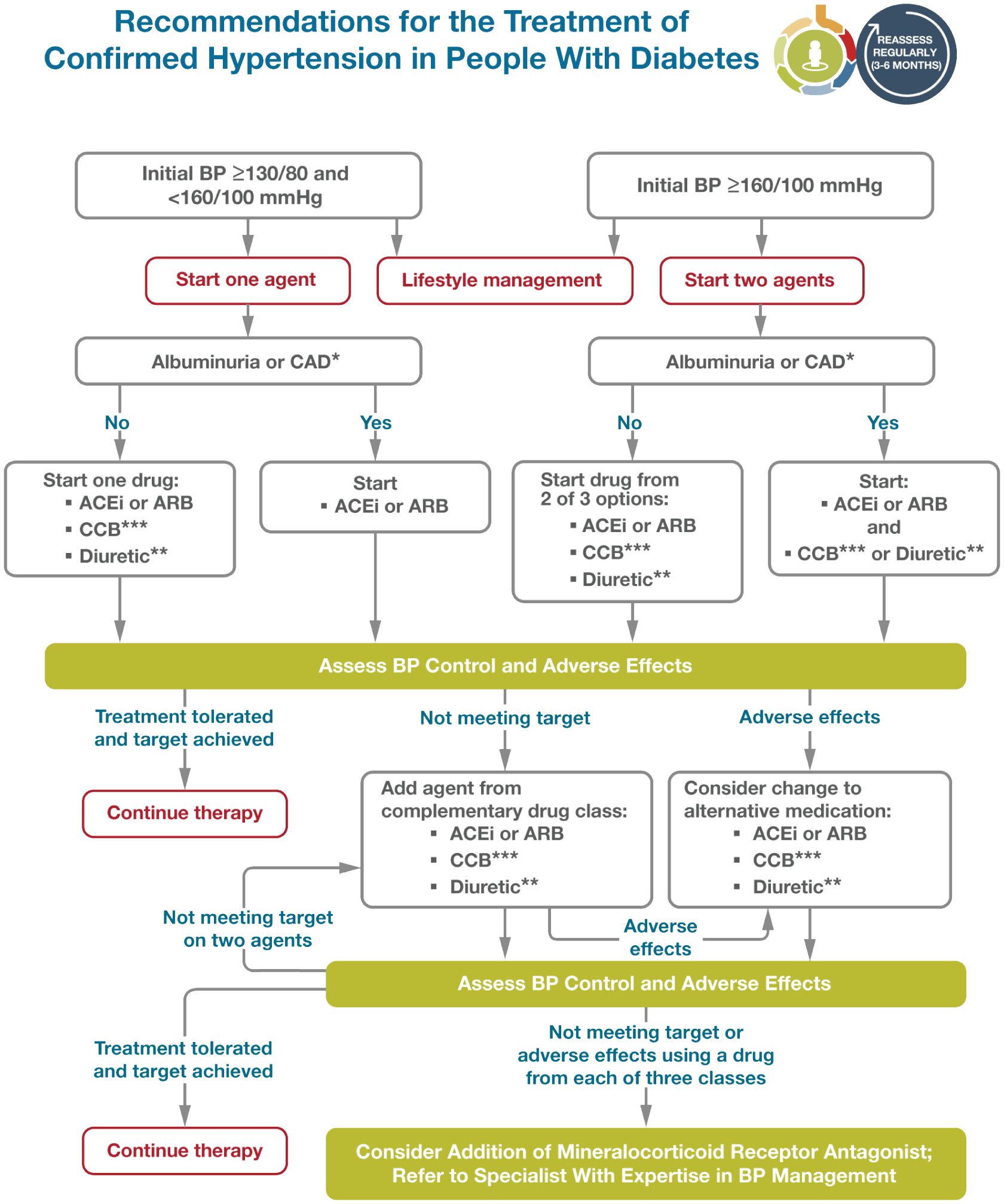
Figure 3. Reprinted with permission from / Adapted with permission from 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes— 2024 by American Diabetes Association Professional Practice Committee; ElSayed, Nuha A., licensed under 5903060036974, American Diabetes Association.
According to the recent update of the ADA 2023 standard of care has changed the recommendation on blood pressure treatment goals in individuals with diabetes, this was revised to target a blood pressure of <130/80 mmHg. In particular, the recently reported results of the STEP (Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients) trial were added. In addition, the guideline was updated to consider pharmacological treatment in people with diabetes and a confirmed blood pressure ≥130/80 (98).
4.2 Managing hyperglycemia
Lifestyle modifications are key adjuncts to diabetes therapy and should be emphasized at all stages in the management of patients with diabetes mellitus (112). Adapted from Elsayed NA. (113) (Figure 4).
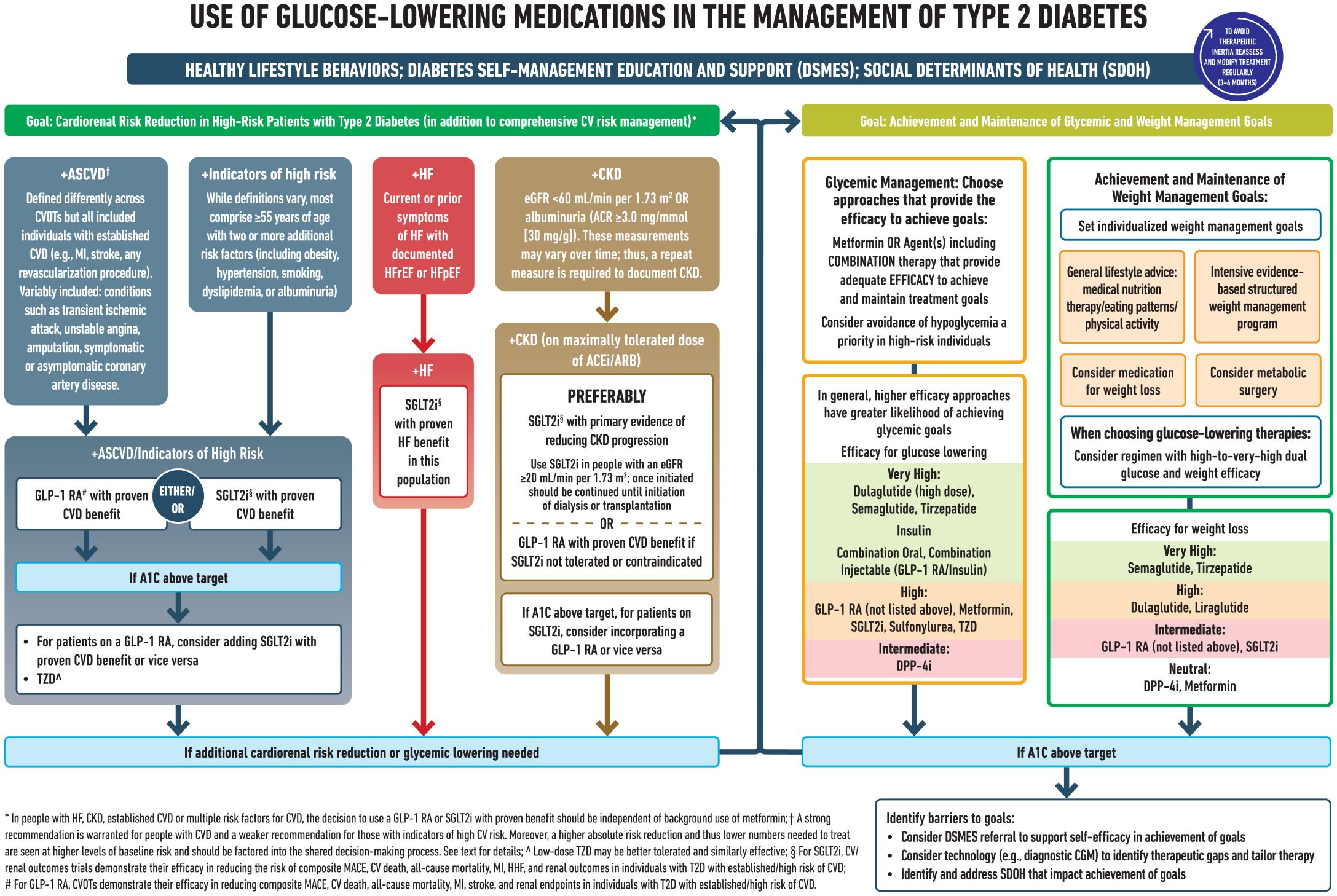
Figure 4. Reprinted with permission from / Adapted with permission from 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes— 2023 by ElSayed, Nuha A.; Aleppo, Grazia, licensed under 5903051181630, American Diabetes Association.
Furthermore, results of several recent cardiovascular outcome trials (CVOTs) indicate that SGLT2i and GLP1RA, along with lower glucose levels also afford clinically meaningful cardio-renal benefits (114, 115). The current guidelines heavily prioritize the early initiation and utilization of cardio-protective and renal-protective diabetes medications particularly SGLT2 and GLP1 irrespective of HBA1C (even if HBA1C is controlled) in particular, this would apply to patients who are recognized to have early stage heart failure during screening with natriuretic peptides.
Piopioglitazone is another agent with cardiovascular benefits, especially in stroke patients; however must not be used in patients with heart failure, i.e natriuretic peptide screening may identify patient’s ineligible for Pioglitazone (81, 116). In addition, saxagliptin is a black box warning for heart failure and should only be used with appropriate heart failure-related caution again natriuretic peptides can help the clinician identify patients contra-indicated with saxagliptin (117, 118). Once the cardiovascular, CKD, or HF conditions have been addressed, anti-hyperglycemic regimens aimed at glucose reduction should be implemented to meet glycemic goals for the individual patient (119).
Individualized goals for HbA1c and other glucose measures should be considered. As stipulated in recommendations from AACE and ADA, HbA1c goal between 6.5% and 7.0% is appropriate for most patients. Younger, healthier patients at lower cardiovascular risk may benefit from HbA1c goals closer to normal (<6.0%), whereas higher A1C goals (~7.5% or higher) may be appropriate for older patients with more complex diseases complicated by multiple comorbidities.
Combination therapy with agents having complementary mechanisms of action must be considered to reduce clinical inertia in patients whose HbA1c is >1- 2% above their individualized goal, even if such patients have newly diagnosed T2D. Incretin classes (GLP1-RAs and dipeptidyl peptidase 4 (DPP4) inhibitors should not be combined with each other. Although insulin is associated with weight gain and the risk of hypoglycemia, it should not be withheld from patients who cannot meet their glucose goals using other agents, and insulin should be used in any patient exhibiting symptoms of uncontrolled diabetes.
4.3 Managing dyslipidemia
It is advisable to perform a lipid profile at the time of diagnosis or initial evaluation of diabetes and at least every 5 years thereafter in patients under the age of 40 years. A lipid panel should also be obtained immediately before initiating statin therapy and 4–12 weeks after. Further testing should be done after dose changes and periodically on an individual basis to screen for adherence.
Atherosclerotic cardiovascular disease risk is variable among individuals with diabetes and is influenced by traditional risk factors and risk modifiers specific to diabetes (117). A clinician’s global assessment of risk can help identify the benefits of an increase in statin therapy. Risk-enhancing factors that should be taken into consideration in patients with dyslipidemia and diabetes are listed in Box 5. Adapted from Byrne RA. (107) (Figure 5).
Box 5. Diabetes-specific risk enhancing factors.
• Long duration (≥10 years for T2DM, ≥20 years for T1DM)
•Albuminuria ≥30 mcg of albumin/mg creatinine
• eGFR <60 mL/min/1.73 m3
• Retinopathy
• Neuropathy
○ ABI<0.9
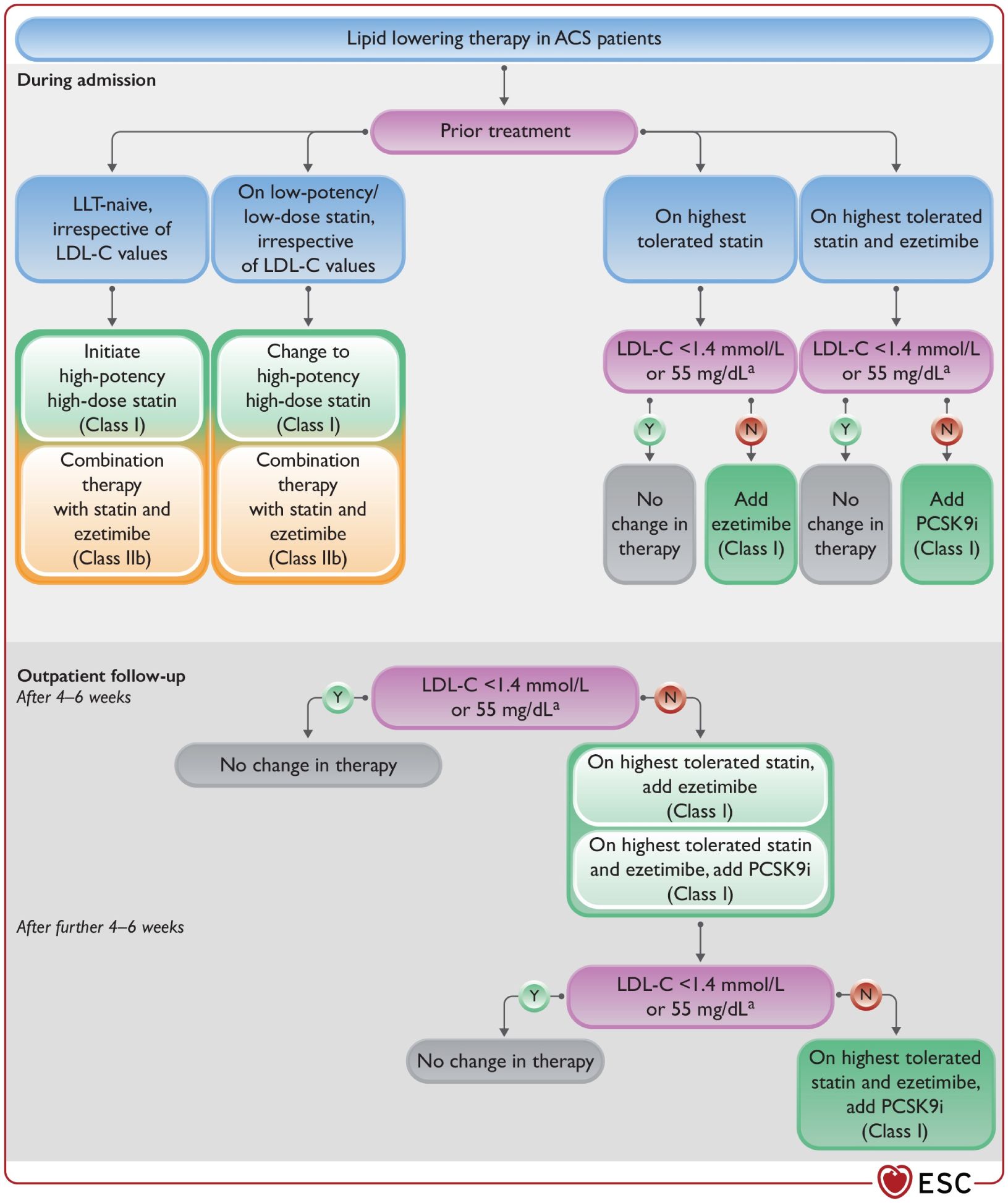
Figure 5. Lipid-lowering therapy in ACS patients. ACS, acute coronary syndrome; LDL-C, low- density lipoprotein (107).
4.4 Managing the thrombotic risk
The benefit of using aspirin in primary prevention remains controversial (120, 121). Data from randomized controlled trials of aspirin in diabetics failed to show a significant reduction in overall ASCVD end points (122, 123). A meta- analysis by Baigent et al., showed that aspirin reduces the risk of serious vascular events by 12% (relative risk 0.88 [95% CI 0.82–0.94]) (124). In this study, the most significant reduction was for nonfatal MI, with little effect on cardiovascular mortality (relative risk 0.95 [95% CI 0.78–1.15]) or total stroke (124). Furthermore, results of the ASCEND (A Study of Cardiovascular Events in Diabetes) trial indicates that aspirin was associated with major bleeding (125). Aspirin appears to have a modest effect on ischemic vascular events, with the absolute decrease in events depending on the underlying ASCVD risk (126).
Pregnant individuals with type 1 or type 2 diabetes should be prescribed low-dose aspirin 100–150 mg/day starting at 12 to 16 weeks of gestation to lower the risk of preeclampsia. Use aspirin therapy (75–162 mg/day) as a secondary prevention strategy in those with diabetes and a history of atherosclerotic cardiovascular disease. Combination therapy with aspirin plus low-dose rivaroxaban should be considered for individuals with stable coronary and/or peripheral artery disease and low bleeding risk to prevent major adverse limb and cardiovascular events. Aspirin is not recommended for those at low risk of ASCVD (such as men and women aged <50 years with diabetes with no other major ASCVD risk factors) as the low benefit is likely to be outweighed by the risks of bleeding.
Clinical judgment should be used for those at intermediate risk (younger patients with one or more risk factors or older patients with no risk factors) until further research is available (96, 98).
Aspirin is recommended for primary prevention in men and women aged ≥ 50 years with diabetes and at least one additional major risk factor such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease/albuminuria and who are not at increased risk of bleeding (127–129).
Furthermore, use of noninvasive imaging techniques such as coronary calcium scoring may potentially help further tailor aspirin therapy, particularly in those at low risk. For patients above the age of 70 years (with or without diabetes), the risk of bleeding with aspirin outweighs it’s benefit and is not recommended (123, 125, 130).
Further details on the management of ischemic heart and vascular diseases, including the selection of antianginal agents, indications for revascularization, rehabilitation protocols, and the specific management of heart failure, coronary artery disease, and peripheral vascular disease, are beyond the scope of this document. For comprehensive guidance on these topics, readers are directed to the relevant society guidelines on the management of these conditions.
4.5 Managing cardio-renal risk in diabetes
The management of diabetes requires a comprehensive approach that addresses both macrovascular and microvascular complications to reduce the overall burden of the disease. It is imperative to implement strategies that effectively mitigate these complications, as they are significant contributors to morbidity and mortality in diabetic patients. The selection of anti-diabetic agents should be made with careful consideration of the patient’s cardiovascular risk profile. In particular, the use of SGLT2 inhibitors and long-acting GLP1 receptor agonists (GLP1-RAs) is strongly recommended for individuals with an elevated risk of atherosclerotic cardiovascular disease (ASCVD), chronic kidney disease (CKD), and/or heart failure. These agents have demonstrated significant benefits in reducing cardiovascular events and slowing the progression of kidney disease in high-risk populations.
Furthermore, for primary prevention of cardiovascular events, the use of aspirin is advised in men and women aged 50 years or older who have diabetes and at least one additional major risk factor, such as a family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease/albuminuria, provided they are not at an increased risk of bleeding. This recommendation underscores the importance of individualized risk assessment in the management of diabetic patients.
In addition to these pharmacologic interventions, the use of renin-angiotensin-aldosterone system (RAAS) blockers, particularly angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin II receptor blockers (ARBs), is indicated in diabetic patients to reduce cardiovascular and renal risks. The recent inclusion of finerenone, a non-steroidal mineralocorticoid receptor antagonist, further enhances the therapeutic options available for preventing cardiorenal complications in diabetes mellitus. By incorporating these strategies, healthcare providers can more effectively manage the complex interplay between diabetes and cardiovascular disease, ultimately improving patient outcomes.
These recommendations are summarized in Box 6.
Box 6. Consensus statement – Managing Cardio-renal Risk in Diabetes.
• Strategies to mitigate both macrovascular and microvascular complications of diabetes are recommended.
• Choice of anti-diabetic agents must be made in a way so as to reduce the burden of cardiovascular risk.
• SGLT2 inhibitors and long-acting GLP1-RAs are recommended for people with elevated risk of atherosclerotic cardiovascular disease, chronic kidney disease and/or heart failure.
• Aspirin is recommended for primary prevention in men and women aged ≥ 50 years with diabetes and at least one additional major risk factor such as family history of premature ASCVD, hypertension, dyslipidemia, smoking, or chronic kidney disease/albuminuria and who are not at increased risk of bleeding.
• RAAS blockers ACE/ARB are indicated in particular.
○ Finerenone is also recommended for prevention of cardiorenal risk in DM.
5 Conclusion
The combination of diabetes and cardiovascular disease, particularly undiagnosed heart failure persists as a major burden in United Arab Emirates. Several modifiable and non-modifiable risk factors such as advanced age, long-standing duration of diabetes, poor glycemic control, CKD and common albuminuria with presence of co-morbidities are predictors of heart failure. In people with these risk factors, it is very important to recommend routine measurement (at least yearly) of natriuretic peptides before referring to imaging tests and in selected patients high-sensitivity troponins can also be performed. This approach will identify patients with early stages of cardiovascular disease and heart failure and guide treatment early with cardio-protective therapy.
With respect to treatments, standards of care must include SGLT2 inhibitors combined with RAAS blockers for heart failure stages B, C and D and GLP-1 receptor agonist with metformin for atherosclerotic cardiovascular disease or peripheral artery disease. While insulin DPP-4 and/or metformin can be useful for additional glycemic control and thiazolidinediones must be avoided in heart failure stages B, C and D.
Author contributions
HS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. WA: Conceptualization, Resources, Supervision, Writing – review & editing. FA: Formal analysis, Investigation, Resources, Validation, Writing – original draft. AS: Conceptualization, Supervision, Visualization, Writing – review & editing. AZ: Formal analysis, Methodology, Writing – original draft. AB: Investigation, Validation, Writing – original draft. AG: Investigation, Methodology, Writing – original draft. FR: Investigation, Methodology, Validation, Writing – original draft. HZ: Investigation, Writing – original draft. HH: Formal analysis, Writing – original draft, Writing – review & editing. JB: Data curation, Investigation, Writing – original draft. JT: Methodology, Writing – original draft. KH: Investigation, Writing – original draft. MF: Investigation, Writing – original draft. MH: Methodology, Writing – original draft. JJ: Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We gratefully acknowledge the generous support of Roche Diagnostics Middle East FZCO, whose sponsorship has made the publication of this consensus paper possible, advancing collaborative efforts to improve patient care and medical outcomes. As well, we acknowledge Dr. Noha Ghanem, ICOM Group’s Medical Education, Research, and Publications Department director, for managing the whole process of communications and the medical writing of this consensus.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pop-Busui R, Januzzi JL, Bruemmer D, Butalia S, Butalia S, Green JB, et al. Heart failure: an underappreciated complication of diabetes. A consensus report of the american diabetes association. Diabetes. Care. (2022) 45:1670–90. doi: 10.2337/dci22-0014
2. Djulbegovic B, Guyatt G. Evidence vs consensus in clinical practice guidelines. Jama. (2019) 322:725–6. doi: 10.1001/jama.2019.9751
3. Weiner SJ. From research evidence to context: the challenge of individualising care. Equine. Vet J. (2006) 38:195–6. doi: 10.2746/042516406776866426
4. Harrison MB, Légaré F, Graham ID, Fervers B. Adapting clinical practice guidelines to local context and assessing barriers to their use. Cmaj. (2010) 182:E78–84. doi: 10.1503/cmaj.081232
5. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: A report of the heart failure society of America, heart failure association of the european society of cardiology, Japanese heart failure society and writing committee of the universal definition of heart failure. J Card Fail. (2021) 1:1–5. doi: 10.1016/j.cardfail.2021.01.022
6. Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, Rosano GM, Davis BR, Ridao M, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: A systematic review with network meta-analyses. PloS Med. (2016) 13:e1001971. doi: 10.1371/journal.pmed.1001971
7. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. (2019) 21:715–31. doi: 10.1002/ejhf.1494
8. Hassanein M, Sabbour H, Al Awadi F, Abusnana S, Afandi B, Al Kaabi J, et al. Cardiometabolic guidelines: cardiovascular risk assessment and management in patients with dysglycemia. Dubai Diabetes Endocrinol J. (2023) 29:67–88. doi: 10.1159/000531107
9. Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. Jama. (1979) 241:2035–8. doi: 10.1001/jama.241.19.2035
10. Sulaiman N, Elbadawi S, Hussein A, Abusnana S, Madani A, Mairghani M, et al. Prevalence of overweight and obesity in United Arab Emirates Expatriates: the UAE National Diabetes and Lifestyle Study. Diabetol Metab Syndr. (2017) 9:88. doi: 10.1186/s13098-017-0287-0
11. Razzak HA, Harbi A, Shelpai W, Qawas A. Epidemiology of diabetes mellitus in the United Arab Emirates. Curr Diabetes. Rev. (2018) 14:542–9. doi: 10.2174/1573399813666170920152913
12. Al Awadi F, Hassanein M, Hussain HY, Mohammed H, Ibrahim G, Khater A, et al. Prevalence of diabetes and associated health risk factors among adults in Dubai, United Arab Emirates: results from Dubai household survey 2019. Dubai. Diabetes. Endocrinol J. (2021) 26:164–73.
13. AlHabeeb W, Akhras K, AlGhalayini K, Al-Mudaiheem H, Ibrahim B, Lawand S, et al. Understanding heart failure burden in Middle East countries: economic impact in Egypt, Saudi Arabia and United Arab Emirates. Value. Health. (2018) 21:S123.
14. Hassan M. Gulf CARE: heart failure in the middle east. Glob Cardiol Sci Pract. (2015) 2015:34. doi: 10.5339/gcsp.2015.34
15. Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes. Care. (2004) 27:1879–84. doi: 10.2337/diacare.27.8.1879
16. Shindler DM, Kostis JB, Yusuf S, Quinones MA, Pitt B, Stewart D, et al. Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) Trials and Registry. Am J Cardiol. (1996) 77:1017–20. doi: 10.1016/s0002-9149(97)89163-1
17. Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. (2001) 103:2668–73. doi: 10.1161/01.cir.103.22.2668
18. Echouffo-Tcheugui JB, Zhang S, Florido R, Hamo C, Pankow JS, Michos ED, et al. Duration of diabetes and incident heart failure: the ARIC (Atherosclerosis risk in communities) study. JACC Heart. Fail. (2021) 9:594–603. doi: 10.1016/j.jchf.2021.06.005
19. Boriani G. The impact of diabetes and comorbidities on the outcome of heart failure patients treated with cardiac resynchronization therapy: implications for patient management. Circ Arrhythm. Electrophysiol. (2016) 9:e004463. doi: 10.1161/circep.116.004463
20. Laakso M. Heart in diabetes: a microvascular disease. Diabetes Care. (2011) 34 Suppl 2:S145–149. doi: 10.2337/dc11-s209
21. Stratmann B, Tschoepe D. Heart in diabetes: not only a macrovascular disease. Diabetes Care. (2011) 34 Suppl 2:S138–144. doi: 10.2337/dc11-s208
22. Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. (2019) 140:e294–324. doi: 10.1161/cir.0000000000000691
23. Fringu FI, Sitar-Taut AV, Caloian B, Zdrenghea D, Comsa D, Gusetu G, et al. The role of NT PRO-BNP in the evaluation of diabetic patients with heart failure. Acta Endocrinol. (2020) 16:183–91. doi: 10.4183/aeb.2020.183
24. Malachias MVB, Jhund PS, Claggett BL, Wijkman MO, Bentley-Lewis R, Chaturvedi N, et al. NT-proBNP by itself predicts death and cardiovascular events in high-risk patients with type 2 diabetes mellitus. J Am Heart Assoc. (2020) 9:e017462. doi: 10.1161/jaha.120.017462
25. Kaze AD, Santhanam P, Erqou S, Ahima RS, Bertoni A, Echouffo-Tcheugui JB. Microvascular disease and incident heart failure among individuals with type 2 diabetes mellitus. J Am Heart. Assoc. (2021) 10:e018998. doi: 10.1161/jaha.120.018998
26. Paolillo S, Rengo G, Pellegrino T, Formisano R, Pagano G, Gargiulo P, et al. Insulin resistance is associated with impaired cardiac sympathetic innervation in patients with heart failure. Eur Heart J Cardiovasc Imaging. (2015) 16:1148–53. doi: 10.1093/ehjci/jev061
27. Preiss D, Zetterstrand S, McMurray JJ, Ostergren J, Michelson EL, Granger CB, et al. Predictors of development of diabetes in patients with chronic heart failure in the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) program. Diabetes. Care. (2009) 32:915–20. doi: 10.2337/dc08-1709
28. Preiss D, van Veldhuisen DJ, Sattar N, Krum H, Swedberg K, Shi H, et al. Eplerenone and new-onset diabetes in patients with mild heart failure: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Eur J Heart Fail. (2012) 14:909–15. doi: 10.1093/eurjhf/hfs067
29. Campbell P, Krim S, Ventura H. The bi-directional impact of two chronic illnesses: heart failure and diabetes - A review of the epidemiology and outcomes. Card Fail Rev. (2015) 1:8–10. doi: 10.15420/cfr.2015.01.01.8
30. Schechter M, Melzer Cohen C, Yanuv I, Rozenberg A, Chodick G, Bodegård J, et al. Epidemiology of the diabetes-cardio-renal spectrum: a cross-sectional report of 1.4 million adults. Cardiovasc Diabetol. (2022) 21:104. doi: 10.1186/s12933-022-01521-9
31. Ronco C. Cardiorenal syndromes: definition and classification. Contrib. Nephrol. (2010) 164:33–38. doi: 10.1159/000313718
32. Banerjee S, Panas R. Diabetes and cardiorenal syndrome: Understanding the “Triple Threat. Hellenic. J Cardiol. (2017) 58:342–7. doi: 10.1016/j.hjc.2017.01.003
33. Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: A scientific statement from the american heart association. Circulation. (2019) 139:e840–78. doi: 10.1161/cir.0000000000000664
34. Kalra S, Aydin H, Sahay M, Ghosh S, Ruder S, Tiwaskar M, et al. Cardiorenal syndrome in type 2 diabetes mellitus - rational use of sodium-glucose cotransporter-2 inhibitors. Eur Endocrinol. (2020) 16:113–21. doi: 10.17925/ee.2020.16.2.113
35. Dei Cas A, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart. Fail. (2015) 3:136–45. doi: 10.1016/j.jchf.2014.08.004
36. Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. (2018) 71:339–51. doi: 10.1016/j.jacc.2017.11.019
37. Semenkovich CF. Insulin resistance and atherosclerosis. J Clin Invest. (2006) 116:1813–22. doi: 10.1172/jci29024
38. Kanter JE, Averill MM, Leboeuf RC, Bornfeldt KE. Diabetes-accelerated atherosclerosis and inflammation. Circ Res. (2008) 103:e116–117. doi: 10.1161/circresaha.108.182642
39. Razani B, Chakravarthy MV, Semenkovich CF. Insulin resistance and atherosclerosis. Endocrinol Metab Clin North Am. (2008) 37:603-621, viii. doi: 10.1016/j.ecl.2008.05.001
40. Xi G, Shen X, Wai C, White MF, Clemmons DR. Hyperglycemia induces vascular smooth muscle cell dedifferentiation by suppressing insulin receptor substrate-1-mediated p53/KLF4 complex stabilization. J Biol Chem. (2019) 294:2407–21. doi: 10.1074/jbc.RA118.005398
41. Chandel S, Sathis A, Dhar M, Giri H, Nathan AA, Samawar SKR, et al. Hyperinsulinemia promotes endothelial inflammation via increased expression and release of Angiopoietin-2. Atherosclerosis. (2020) 307:1–10. doi: 10.1016/j.atherosclerosis.2020.06.016
42. Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. (2020) 21:1835. doi: 10.3390/ijms21051835
43. Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk. Manage. (2007) 3:853–76.
44. Hamilton SJ, Watts GF. Endothelial dysfunction in diabetes: pathogenesis, significance, and treatment. Rev Diabet. Stud. (2013) 10:133–56. doi: 10.1900/rds.2013.10.133
45. Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation. (2016) 133:2459–502. doi: 10.1161/circulationaha.116.022194
46. von Bibra H, St John Sutton M. Diastolic dysfunction in diabetes and the metabolic syndrome: promising potential for diagnosis and prognosis. Diabetologia. (2010) 53:1033–45. doi: 10.1007/s00125-010-1682-3
47. Araz M, Bayrac A, Ciftci H. The impact of diabetes on left ventricular diastolic function in patients with arterial hypertension. North. Clin Istanb. (2015) 2:177–81. doi: 10.14744/nci.2015.55477
48. Mohan M, Dihoum A, Mordi IR, Choy AM, Rena G, Lang CC. Left ventricular hypertrophy in diabetic cardiomyopathy: A target for intervention. Front Cardiovasc Med. (2021) 8:746382. doi: 10.3389/fcvm.2021.746382
49. Levelt E, Mahmod M, Piechnik SK, Ariga R, Francis JM, Rodgers CT, et al. Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes. (2016) 65:44–52. doi: 10.2337/db15-0627
50. Hori O, Yan SD, Ogawa S, Kuwabara K, Matsumoto M, Stern D, et al. The receptor for advanced glycation end-products has a central role in mediating the effects of advanced glycation end-products on the development of vascular disease in diabetes mellitus. Nephrol. Dial. Transplant. (1996) 11 Suppl 5:13–6. doi: 10.1093/ndt/11.supp5.13
51. Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. (1999) 84:489–97. doi: 10.1161/01.res.84.5.489
52. Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. (2004) 63:582–592. doi: 10.1016/j.cardiores.2004.05.001
53. Waddingham MT, Edgley AJ, Tsuchimochi H, Kelly DJ, Shirai M, Pearson JT. Contractile apparatus dysfunction early in the pathophysiology of diabetic cardiomyopathy. World. J Diabetes. (2015) 6:943–60. doi: 10.4239/wjd.v6.i7.943
54. Cice G, Calo L, Monzo L. Sodium-glucose co-transporter 2 inhibitors for the treatment of cardio-renal syndrome. Eur Heart. J Suppl. (2022) 24:I68–i71. doi: 10.1093/eurheartjsupp/suac101
55. Salvatore T, Galiero R. An overview of the cardiorenal protective mechanisms of SGLT2 inhibitors. Int J Mol Sci. (2022) 23:3651. doi: 10.3390/ijms23073651
56. Zhou L, Deng W, Zhou L, Fang P, He D, Zhang W, et al. Prevalence, incidence and risk factors of chronic heart failure in the type 2 diabetic population: systematic review. Curr Diabetes. Rev. (2009) 5:171–84. doi: 10.2174/157339909788920938
57. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of america. Circulation. (2017) 136:e137–e161. doi: 10.1161/cir.0000000000000509
58. Yang H, Negishi K, Otahal P, Marwick TH. Clinical prediction of incident heart failure risk: a systematic review and meta-analysis. Open Heart. (2015) 2:e000222. doi: 10.1136/openhrt-2014-000222
59. Segar MW, Vaduganathan M, Patel KV, McGuire DK, Butler J, Fonarow GC, et al. Machine learning to predict the risk of incident heart failure hospitalization among patients with diabetes: the WATCH-DM risk score. Diabetes. Care. (2019) 42:2298–306. doi: 10.2337/dc19-0587
60. Williams BA, Geba D, Cordova JM, Shetty SS. A risk prediction model for heart failure hospitalization in type 2 diabetes mellitus. Clin Cardiol. (2020) 43:275–83. doi: 10.1002/clc.23298
61. Joseph JJ, Deedwania P, Acharya T, Aguilar D, Bhatt DL, Chyun DA, et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: A scientific statement from the american heart association. Circulation. (2022) 145:e722–59. doi: 10.1161/cir.0000000000001040
62. Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson AM, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2018) 379:633–44. doi: 10.1056/NEJMoa1800256
63. Ohkuma T, Komorita Y, Peters SAE, Woodward M. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia. (2019) 62:1550–60. doi: 10.1007/s00125-019-4926-x
64. Chadalavada S, Jensen MT, Aung N, Cooper J, Lekadir K, Munroe PB, et al. Women with diabetes are at increased relative risk of heart failure compared to men: insights from UK biobank. Front Cardiovasc Med. (2021) 8:658726. doi: 10.3389/fcvm.2021.658726
65. Barrett-Connor E, Giardina EG, Gitt AK, Gudat U, Steinberg HO, Tschoepe D. Women and heart disease: the role of diabetes and hyperglycemia. Arch Intern Med. (2004) 164:934–942. doi: 10.1001/archinte.164.9.934
66. Nilsson PM, Theobald H, Journath G, Fritz T. Gender differences in risk factor control and treatment profile in diabetes: a study in 229 swedish primary health care centres. Scand J Prim. Health Care. (2004) 22:27–31. doi: 10.1080/02813430310003264
67. Duggirala MK, Cuddihy RM, Cuddihy MT, Nyman MA, Naessens JM, Pankratz VS. Women with diabetes have poorer control of blood pressure than men. J Womens Health (Larchmt). (2005) 14:418–23. doi: 10.1089/jwh.2005.14.418
68. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. Bmj. (2006) 332:73–8. doi: 10.1136/bmj.38678.389583.7C
69. Bertram MY, Vos T. Quantifying the duration of pre-diabetes. Aust N Z J Public Health. (2010) 34:311–4. doi: 10.1111/j.1753-6405.2010.00532.x
70. Pfister R, Cairns R, Erdmann E, Schneider CA. A clinical risk score for heart failure in patients with type 2 diabetes and macrovascular disease: an analysis of the PROactive study. Int J Cardiol. (2013) 162:112–6. doi: 10.1016/j.ijcard.2011.05.056
71. Škrtić M, Cherney DZ. Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol. Hypertens. (2015) 24:96–103. doi: 10.1097/mnh.0000000000000084
72. Fox CS, Sullivan L, D’Agostino RB, Wilson PW. The significant effect of diabetes duration on coronary heart disease mortality: the Framingham Heart Study. Diabetes. Care. (2004) 27:704–8. doi: 10.2337/diacare.27.3.704
73. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj. (2000) 321:405–12. doi: 10.1136/bmj.321.7258.405
74. Aune D, Schlesinger S, Neuenschwander M, Feng T, Janszky I, Norat T, et al. Diabetes mellitus, blood glucose and the risk of heart failure: A systematic review and meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. (2018) 28:1081–91. doi: 10.1016/j.numecd.2018.07.005
75. de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. (2004) 110:921–7. doi: 10.1161/01.cir.0000139860.33974.28
76. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. Jama. (2001) 286:421–6. doi: 10.1001/jama.286.4.421
77. Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation. (2019) 139:1384–95. doi: 10.1161/circulationaha.118.037778
78. Roumie CL, Min JY, D’Agostino McGowan L, Presley C, Grijalva CG, Hackstadt AJ, et al. Comparative safety of sulfonylurea and metformin monotherapy on the risk of heart failure: A cohort study. J Am Heart. Assoc. (2017) 6:e005379. doi: 10.1161/jaha.116.005379
79. Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. (2007) 370:1129–36. doi: 10.1016/s0140-6736(07)61514-1
80. Charbonnel B, Dormandy J, Erdmann E, Massi-Benedetti M, Skene A. The prospective pioglitazone clinical trial in macrovascular events (PROactive): can pioglitazone reduce cardiovascular events in diabetes? Study design and baseline characteristics of 5238 patients. Diabetes. Care. (2004) 27:1647–53. doi: 10.2337/diacare.27.7.1647
81. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. (2013) 369:1317–26. doi: 10.1056/NEJMoa1307684
82. Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. (2015) 385:2067–76. doi: 10.1016/s0140-6736(14)62225-x
83. Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. (2009) 20:1813–21. doi: 10.1681/asn.2008121270
84. Sacre JW, Magliano DJ, Shaw JE. Heart failure hospitalisation relative to major atherosclerotic events in type 2 diabetes with versus without chronic kidney disease: A meta-analysis of cardiovascular outcomes trials. Diabetes. Metab. (2021) 47:101249. doi: 10.1016/j.diabet.2021.101249
85. Lawson CA, Seidu S, Zaccardi F, McCann G, Kadam UT, Davies MJ, et al. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UK over twenty years. EClinicalMedicine. (2021) 32:100739. doi: 10.1016/j.eclinm.2021.100739
86. Obrezan AG, Kulikov NV. Atrial fibrillation and diabetes mellitus: the control of thromboembolic risk. Kardiologiia. (2020) 60:108–14. doi: 10.18087/cardio.2020.7.n1146
87. Salvatore T, Galiero R, Di Martino A, Albanese G, Colantuoni S, Medicamento G, et al. Dysregulated epicardial adipose tissue as a risk factor and potential therapeutic target of heart failure with preserved ejection fraction in diabetes. Biomolecules. (2022) 12:176. doi: 10.3390/biom12020176
88. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. (2002) 48:436–72.
89. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. (2004) 350:655–63. doi: 10.1056/NEJMoa031994
90. Mann JF, Yi QL, Gerstein HC. Albuminuria as a predictor of cardiovascular and renal outcomes in people with known atherosclerotic cardiovascular disease. Kidney. Int. (2004) Suppl:S59–62. doi: 10.1111/j.1523-1755.2004.09215.x
91. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. (2010) 375:2073–81. doi: 10.1016/s0140-6736(10)60674-5
92. Huelsmann M, Neuhold S, Strunk G, Moertl D, Berger R, Prager R, et al. NT- proBNP has a high negative predictive value to rule-out short-term cardiovascular events in patients with diabetes mellitus. Eur Heart. J. (2008) 29:2259–64. doi: 10.1093/eurheartj/ehn334
93. Farmakis D, Mueller C, Apple FS. High-sensitivity cardiac troponin assays for cardiovascular risk stratification in the general population. Eur Heart. J. (2020) 41:4050–6. doi: 10.1093/eurheartj/ehaa083
94. Ford I, Shah AS, Zhang R, McAllister DA, Strachan FE, Caslake M, et al. High- sensitivity cardiac troponin, statin therapy, and risk of coronary heart disease. J Am Coll Cardiol. (2016) 68:2719–28. doi: 10.1016/j.jacc.2016.10.020
95. Bhatt DL, Eikelboom JW, Connolly SJ, Steg PG, Anand SS, Verma S, et al. Role of combination antiplatelet and anticoagulation therapy in diabetes mellitus and cardiovascular disease: insights from the COMPASS trial. Circulation. (2020) 141:1841–54. doi: 10.1161/circulationaha.120.046448
96. Hendriks SH, van Dijk PR, van Hateren KJ, van Pelt JL, Groenier KH, Bilo HJ, et al. High-sensitive troponin T is associated with all-cause and cardiovascular mortality in stable outpatients with type 2 diabetes (ZODIAC-37). Am Heart J. (2016) 174:43–50. doi: 10.1016/j.ahj.2015.12.015
97. Fonseca C, Bettencourt P, Brito D, Febra H, Pereira Á, Genovez V, et al. NT-proBNP for heart failure diagnosis in Primary Care: Costs or savings? A budget impact study. Rev Port Cardiol. (2022) 41:183–93. doi: 10.1016/j.repc.2021.03.009
98. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. Cardiovascular disease and risk management: standards of care in diabetes-2023. Diabetes Care. (2023) 46:S158–90.
99. Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes: Developed by the task force on the management of cardiovascular disease in patients with diabetes of the European Society of Cardiology (ESC). Eur Heart J. (2023) 44:4043–140.
100. Huelsmann M, Neuhold S, Resl M, Strunk G, Brath H, Francesconi C, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. (2013) 62:1365–72. doi: 10.1016/j.jacc.2013.05.069
101. Ledwidge M, Gallagher J, Conlon C, Tallon E, O’Connell E, Dawkins I, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. Jama. (2013) 310:66–74. doi: 10.1001/jama.2013.7588
102. McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: A meta-analysis. JAMA Cardiol. (2021) 6:148–58. doi: 10.1001/jamacardio.2020.4511
103. Walter E, Arrigo M, Allerstorfer S, Marty P, Hülsmann M. Cost-effectiveness of NT-proBNP-supported screening of chronic heart failure in patients with or without type 2 diabetes in Austria and Switzerland. J Med Econ. (2023) 26:1287–300. doi: 10.1080/13696998.2023.2264722
104. Gallagher J, Watson C, Campbell P, Ledwidge M, McDonald K. Natriuretic peptide-based screening and prevention of heart failure. Card Fail Rev. (2017) 3:83–5. doi: 10.15420/cfr.2017:20:1
105. Ledwidge MT, O’Connell E, Gallagher J, Tilson L, James S, Voon V, et al. Cost- effectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP- HF (St Vincent’s Screening TO Prevent Heart Failure) study. Eur J Heart Fail. (2015) 17:672–9. doi: 10.1002/ejhf.286
106. The Heart Failure Policy Network. NT-proBNP FAQ for GPs. (2020). Retrieved from https://www.mater.ie/docs/healthcare-professionals/gp-referrals/ntprobnp-faq-for-gps.pdf.
107. Byrne RA, Rossello X, Coughlan J, Barbato E, Berry C, Chieffo A, et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. (2023) 44:3720–826.
108. Leon-Justel A, Morgado Garcia-Polavieja JI, Alvarez-Rios AI, Caro Fernandez FJ, Merino PAP, Galvez Rios E, et al. Biomarkers-based personalized follow-up in chronic heart failure improves patient’s outcomes and reduces care associate cost. Health Qual. Life Outcomes. (2021) 19:142. doi: 10.1186/s12955-021-01779-9
109. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. Chronic kidney disease and risk management: standards of care in diabetes-2023. Diabetes Care. (2023) 46:S191–s202. doi: 10.2337/dc23-S011
110. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. Jama. (2015) 313:603–15. doi: 10.1001/jama.2014.18574
111. de Boer IH, Bangalore S. Diabetes and hypertension: A position statement by the american diabetes association. Diabetes Care. (2017) 40:1273–84. doi: 10.2337/dci17-0026
112. Nesti L, Tricò D, Mengozzi A, Natali A. Rethinking pioglitazone as a cardioprotective agent: a new perspective on an overlooked drug. Cardiovasc Diabetol. (2021) 20:109. doi: 10.1186/s12933-021-01294-7
113. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. (2023) 46:S140–s157. doi: 10.2337/dc23-S009
114. Sheahan, Wahlberg, Gilbert. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. (2020) 96:156–61. doi: 10.1136/postgradmedj-2019-137186
115. Sheng Z, Cao J-Y, Pang Y-C, Xu H-C, Chen J-W, Yuan J-H. Effects of lifestyle modification and anti-diabetic medicine on prediabetes progress: A systematic review and meta-analysis. Journal of Diabetes & Metabolic Syndrome: Clinical Research & Reviews (2019) 10:455. doi: 10.3389/fendo.2019.00455
116. Liao H-W, Saver JL, Wu YL, Chen T-H, Lee M, Ovbiagele B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. BMJ Open. (2017) 7:e013927. doi: 10.1136/bmjopen-2016-013927
117. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. (2012) 380:581–90. doi: 10.1016/s0140-6736(12)60367-5
118. Handelsman Y, Anderson JE, Bakris GL, Ballantyne CM, Beckman JA, Bhatt DL, et al. DCRM Multispecialty Practice Recommendations for the management of diabetes, cardiorenal, and metabolic diseases. J Diabetes Complications. (2022) 36:108101. doi: 10.1016/j.jdiacomp.2021.108101
119. Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American association of clinical endocrinologists and American college of endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endocr. Pract. (2020) 26:107–39. doi: 10.4158/cs-2019-0472
120. Zhang C, Sun A, Zhang P, Wu C, Zhang S, Fu M, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: A meta-analysis. Diabetes Res Clin Pract. (2010) 87:211–8. doi: 10.1016/j.diabres.2009.09.029
121. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. (2012) 33:1635–701. doi: 10.1093/eurheartj/ehs092
122. De Berardis G, Sacco M, Strippoli GF, Pellegrini F, Graziano G, Tognoni G, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. Bmj. (2009) 339:b4531. doi: 10.1136/bmj.b4531
123. Bowman L, Mafham M, Stevens W, Haynes R, Aung T, Chen F, et al. ASCEND: A Study of Cardiovascular Events iN Diabetes: Characteristics of a randomized trial of aspirin and of omega-3 fatty acid supplementation in 15,480 people with diabetes. Am Heart J. (2018) 198:135–44. doi: 10.1016/j.ahj.2017.12.006
124. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. (2005) 366:1267–78. doi: 10.1016/s0140-6736(05)67394-1
125. Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: a cost-utility analysis. Ann Intern Med. (2006) 144:326–36. doi: 10.7326/0003-4819-144-5-200603070-00007
126. Huxley RR, Peters SA, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2015) 3:198–206. doi: 10.1016/s2213-8587(14)70248-7
127. Kalyani RR, Lazo M, Ouyang P, Turkbey E, Chevalier K, Brancati F, et al. Sex differences in diabetes and risk of incident coronary artery disease in healthy young and middle-aged adults. Diabetes Care. (2014) 37:830–8. doi: 10.2337/dc13-1755
128. Miedema MD, Duprez DA, Misialek JR, Blaha MJ, Nasir K, Silverman MG, et al. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual. Outcomes. (2014) 7:453–60. doi: 10.1161/circoutcomes.113.000690
129. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. (2014) 57:1542–51. doi: 10.1007/s00125-014-3260-6
Keywords: diabetes, heart failure, cardiovascular risks, biomarkers, UAE
Citation: Sabbour H, Almahmeed W, Alawadi F, Shehab A, Al Zubaidi A, Bashier A, Ghulam AR, Rashid F, Zaky H, Heshmat Kassemn H, Adi JB, Tahir J, Hafidh K, Farghali M, Hassanien M and Januzzi J (2025) Emirates consensus recommendations on cardiovascular risk management in type 2 diabetes. Front. Endocrinol. 15:1395630. doi: 10.3389/fendo.2024.1395630
Received: 04 March 2024; Accepted: 24 September 2024;
Published: 06 January 2025.
Edited by:
Andrea Salzano, University of Leicester, United KingdomReviewed by:
Ichiro Sakuma, Hokko Memorial Hospital, JapanBasil Nwaneri Okeahialam, University of Jos, Nigeria
Copyright © 2025 Sabbour, Almahmeed, Alawadi, Shehab, Al Zubaidi, Bashier, Ghulam, Rashid, Zaky, Heshmat Kassemn, Adi, Tahir, Hafidh, Farghali, Hassanien and Januzzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hani Sabbour, bWVkaWNhbHB1YmxpY2F0aW9uNDYxQGdtYWlsLmNvbQ==
†ORCID: Fatheya Alawadi, orcid.org/0000-0001-8911-866X
Abdullah Shehab, orcid.org/0000-0001-9419-8051
Abdul Rauf Ghulam, orcid.org/0009-0008-3653-7914
Hosam Zaky, orcid.org/0000-0001-6674-7121
Hussien Heshmat Kassem, orcid.org/0000-0003-2142-8877
Khadija Hafidh, orcid.org/0000-0002-3632-4336
James Januzzi, orcid.org/0000-0002-8338-1798
 Hani Sabbour
Hani Sabbour Wael Almahmeed
Wael Almahmeed Fatheya Alawadi6,7†
Fatheya Alawadi6,7† Alaaeldin Bashier
Alaaeldin Bashier Fauzia Rashid
Fauzia Rashid Hussien Heshmat Kassemn
Hussien Heshmat Kassemn Juwairia Tahir
Juwairia Tahir Mohamed Hassanien
Mohamed Hassanien