
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 02 July 2024
Sec. Thyroid Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1392247
This article is part of the Research TopicPapillary Thyroid Cancer: Prognostic Factors and Risk AssessmentView all 21 articles
A correction has been applied to this article in:
Corrigendum: Predictors of lateral lymph node metastasis and skip metastasis in patients with papillary thyroid microcarcinoma
Background: Papillary thyroid microcarcinoma (PTMC) is characterized by its favorable prognosis and potential for active surveillance (AS) as a management option. However, the presence of cervical lymph node (LN) metastasis, especially lateral LN metastasis, significantly impacts management and prognosis. Previous studies have focused on post-surgery risk factors for cervical LN metastasis. This study aims to identify predictors of lateral LN metastasis by analyzing pre-operative ultrasonographic findings alongside clinicopathological factors.
Methods: A retrospective review of medical records was conducted for patients with PTMC who underwent surgery at Chonnam National University Hwasun Hospital between 2004 and 2013. This is a case–control study that compares patients with lateral LN metastasis (N1b) to age- and sex-matched patients without LN metastasis (N0). Subgroup analysis was performed to evaluate risk factors of skip metastasis.
Results: The study included 90 patients with PTMC with lateral LN metastasis (N1b) and 268 age- and sex-matched patients without LN metastasis (N0). The mean age was 49.3 years, and female patients were dominant in both groups. Structural recurrences of 4.4% (4/90) were observed only in the N1b group. The N1b group exhibited a higher frequency of upper lobe tumor location compared to the N0 group (38.9% vs. 16.0%, p < 0.001). There was no significant difference in the locations with the presence of invasion to adjacent organs. A higher proportion of non-parallel shape was observed in the N1b group than the N0 group (80.0% vs. 66.0%, p = 0.013). There were no differences in echogenicity, sonographic feature, margin, and AP diameter of the thyroid gland between the two groups. In multivariate analysis, independent risk factors for lateral LN metastasis included extrathyroidal extension, multiplicity, upper lobe tumor location, and non-parallel shape. Skip metastasis in patients with PTMC was associated with upper lobe tumor location.
Conclusion: Detailed ultrasound examinations, evaluating tumor location, number, orientation, and the presence of ETE, are crucial in accurately predicting lateral LN metastasis especially when primary tumor was in the upper lobe to avoid missing skip metastasis. These evaluations can help guide the decision between AS and immediate surgery in patients with PTMC.
The indolent nature and excellent prognosis of papillary thyroid microcarcinoma (PTMC) have contributed to the emergence of active surveillance (AS) as a viable treatment approach for low-risk thyroid cancer (1). AS for low-risk PTMC was introduced in Japan, and the American Thyroid Association guideline also recommended AS as an applicable management strategy in selected cases of low-risk thyroid cancer (2). A Korean study revealed a good prognosis, including 1.5% recurrence rate during a median follow-up of 7.7 years and 0.1% distant metastasis rate among 8,808 patients with PTMC (3). However, physician- and patient-related barriers to accepting AS in patients with PTMC still exist, and one of the main barriers is the fear of cancer progression, even though the risk of disease progression is low. Therefore, appropriate candidate identification for AS, especially the exclusion of cases with high risks of disease progression, is important.
Many studies have evaluated the prognostic factors of PTMC, including old age, male gender, larger tumor size, extrathyroidal extension (ETE), lymph node (LN) metastasis, multifocality, BRAF mutation, and coexistence of chronic thyroiditis, with varying or inconclusive results (4–8). The majority of prognostic factors can be ascertained through postoperative pathological results, which makes their utilization challenging for patients under consideration for AS. However, all patients diagnosed with PTMC undergo ultrasonography (US) before the AS decision to identify high-risk features, such as invasion and LN metastasis. Distant metastasis in patients with PTMC was rarely observed, but it could be fatal and all patients with PTMC with distant metastasis had synchronous cervical LN metastasis at the time of the first diagnosis (3). In particular, lateral LN metastasis is a prognostic factor of recurrence in patients with PTMC (9). Most of the lateral LN metastases in patients with PTMC progress sequentially from the initial central LN metastasis to the ipsilateral lateral LN metastasis (10). However, skip metastasis with a discontinuous lymphatic spread pattern has been reported in up to 21.8% of cases and PTMC (less than 1 cm) is a risk factor of skip metastasis among patients with papillary thyroid carcinoma (PTC) (11, 12).
Hence, the primary objective of this study is to assess potential risk factors associated with the development of lateral LN metastasis in patients with PTMC. This will be achieved by analyzing US findings in conjunction with clinicopathological factors among patients with lateral LN metastasis (N1b) and comparing them to age- and sex-matched patients without LN metastasis (N0). Additionally, we analyzed risk factors for skip metastasis in patients with PTMC with lateral LN metastasis.
This study is a retrospective, age- and sex-matched case–control cohort study where we reviewed medical records of patients diagnosed with PTMC who underwent surgery at Chonnam National University Hwasun Hospital between 2004 and 2013. The eligibility criteria included patients with PTMC with lateral LN metastasis (N1b) at the first surgery, resulting in the identification of 95 patients. Propensity score matching (PSM) analysis was used to match patients with PTMC with lateral LN metastasis (N1b) and those without LN metastasis (N0) based on age and sex as a confounding factor, with a matching ratio of 1:3. We utilized the “Matchit” package in R software (version 3.3.3) for PSM analysis, and 285 patients were enrolled as controls. Among the 95 patients with lateral LN metastasis, we excluded three patients who had no preoperative US and poor US quality, as well as two patients with insufficient follow-up periods of less than 6 months. In the control group without LN metastasis, we excluded 6 patients with no preoperative US and poor US quality, and 11 patients with insufficient follow-up periods of less than 6 months. Finally, we included and analyzed 90 patients with lateral LN metastasis (N1b) and 268 patients without LN metastasis (N0) to identify risk factors for lateral LN metastasis in patients with PTMC (Figure 1).
Informed consent was waived due to the retrospective nature of the study, and the Institutional Review Board of Chonnam National University Hwasun Hospital (No. CNUHH-2020–271) approved this study.
To assess risk factors for lateral LN metastasis, we collected and analyzed baseline clinical characteristics and pathologic results. ETE included both minimal and gross invasion in pathologic results. Skip metastasis and stepwise metastasis were defined as lateral LN metastasis without central LN metastasis and lateral LN metastasis with central LN metastasis, respectively. Thyroid-stimulating hormone (TSH) levels were measured using Elecsys and Cobase analyzer kits (Roche Diagnostics, GmbH, Mannheim, Germany), with laboratory reference ranges of 0.4–4.48 mIU/L. At the last follow-up point, structural recurrence was defined as evidence of structural or functional disease, regardless of serum thyroglobulin (Tg) level or anti-Tg antibody (2).
Preoperatively, thyroid US was performed to assess the thyroid gland and neck. Gray-scale static US images of the thyroid and nodules were acquired using either the Logiq9 system (GE Medical System, Milwaukee, WI, USA) or ACUSON Antares system (Siemens Medical Solutions, Malvern, PA, USA) with a linear high-frequency probe (5–13 MHz). Two expert endocrinologists (K.C.H. and K.H.K.) conducted the US imaging. Following a training session with randomly selected US images, consensus criteria for the US images were defined, and one endocrinologist (P.J.Y.) reviewed the images. In cases where multiple suspicious nodules were present, the dominant nodule was determined, based on its aggressive nature as determined by the pathologic result, or if aggressiveness was not discernible, the largest nodule was selected as the dominant nodule.
Each nodule was evaluated using at least two US images, including the transverse and longitudinal planes. Tumor location was classified into upper, mid, and lower categories (13). For the assessment of invasion, tumor location 2 was categorized into various groups, including intra-thyroidal, adjacent to the anterolateral capsule, adjacent to the post-capsule, adjacent to the trachea, gross ETE to the strap muscle, gross ETE to the recurrent laryngeal nerve (RLN), or gross ETE to the trachea (14). The trachea invasion risk stratification (tumor location 3) was divided into three categories based on the angles between the tumor and the trachea: acute angle, right angle, or obtuse angle (15). Nodule composition was categorized as solid, partially cystic, partially solid, or cystic based on the ACR TI-RADS (American College of Radiology Thyroid Imaging, Reporting, and Data System) (16, 17). Nodular echogenicity was classified as marked hypoechoic, mild hypoechoic, isoechoic, or hyperechoic compared to the echogenicity of anterior neck muscles and normal thyroid parenchyma as reference (18). Nodular margin was divided into three categories: smooth, irregular, or ill-defined (16). Nodular orientation was categorized as either parallel or non-parallel (taller than wide shape). Finally, calcifications were divided into four categories: no calcification, punctate echogenic foci, macrocalcification, or rim (peripheral) calcification (16).
To assess the presence of diffuse thyroid disease (DTD), US findings of the thyroid were evaluated, including echogenicity (normal versus decreased), echotexture (fine or coarse/micronodular), margin (smooth, microlobulated, or macrolobulated), and the anteroposterior (AP) diameter of the thyroid gland (considering 1–2 cm as the normal reference, decreased, or increased) (19, 20). The vascularity of the thyroid was not analyzed in this study due to the lack of available data.
Among patients with lateral LN metastasis, preoperative US findings of suspicious LNs were collected. The LNs were evaluated based on size, cystic change, calcifications, and echogenicity (particularly abnormal hyperechogenicity) (21).
The data are presented as mean ± standard deviation or as n (%). Continuous variables were analyzed using Student’s t-test, while categorical variables were analyzed using the Chi-square test and Mann–Whitney test. Kaplan-Meier analysis with the log-rank test was used to compare recurrence-free survival between the lateral LN metastasis group and the no-LN metastasis group. Clinical response data were dichotomized based on the presence of structural recurrence. Binary logistic regression models were employed to evaluate predictive risk factors for lateral LN metastasis in patients with PTMC. All statistical analyses were conducted using SPSS Statistics, version 28 (IBM, Armonk, NY), and a p-value <0.05 was considered statistically significant.
The study included a total of 90 patients with PTMC with lateral LN metastasis (N1b) and 268 patients without LN metastasis (N0) (Table 1). The mean age in both groups was 49.3 years (48.8 years in N1b group and 49.4 years in N0 group, respectively). All patients in the N1b group underwent total thyroidectomy with central neck dissection and therapeutic lateral cervical neck dissection. Sixty-one patients (67.8%) in the N1b group were underwent therapeutic central neck dissection due to central LN metastasis (N1a).
Except for one patient who opted not to receive RAI therapy due to personal preference, RAI therapy was performed in the N1b group. In the N0 group, 59.0% underwent total thyroidectomy and 23.3% received RAI therapy. Classic PTC was the most common subtype in both groups, and there was no significant difference in PTC subtypes between the two groups.
The N1b group showed a larger tumor size (0.7 ± 0.4 cm vs. 0.6 ± 0.3 cm, p = 0.009) and more aggressive pathological features, including bilaterality (26.7% vs. 8.6%, p < 0.001), multiplicity (44.4% vs. 14.2%, p < 0.001), and ETE (25.6% vs. 4.5%, p < 0.001) compared to the N0 group. There were no significant differences in capsular invasion, lymphovascular invasion, strap muscle invasion, or concurrent chronic thyroiditis [Hashimoto’s thyroiditis (HT)] between the two groups. The N1b group had ipsilateral LN metastasis, except for two cases with contralateral LN metastasis originating from the primary tumor.
Only one case of metachronous distant metastasis at 82 months after the first surgery was observed in the N1b group. Patients in the N0 group had better structural recurrence-free survival compared to those in the N1b group (p = 0.002) (Figure 2). Structural recurrence was observed in 4.4% (4/90) of the N1b group. The N1b group had a longer follow-up duration than the N0 group (112.4 ± 41.5 months vs. 94.6 ± 48.5 months, p < 0.001).
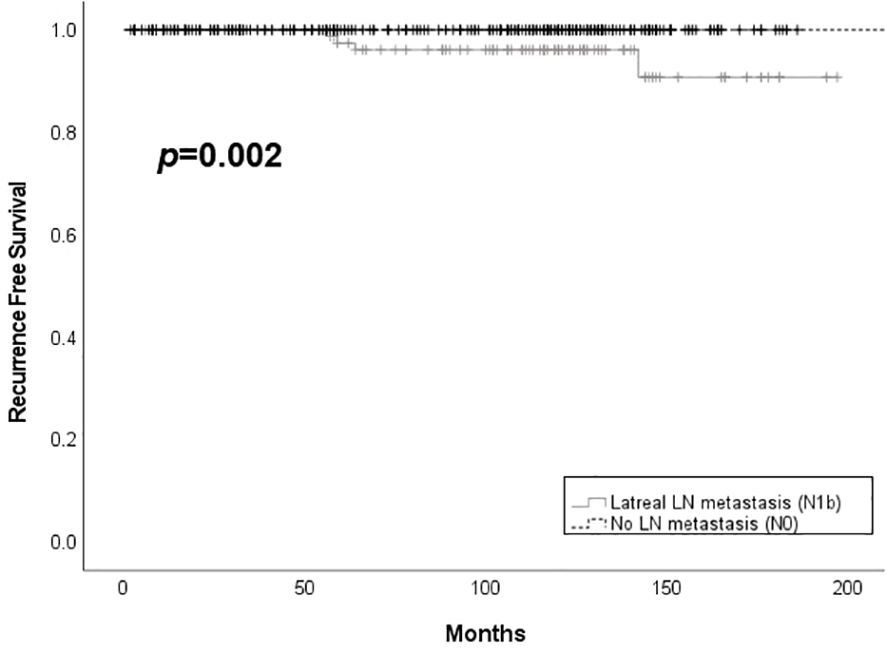
Figure 2. Structural recurrence free survival according to the presence of lateral lymph node metastasis among patients with PTMC. LN, lymph node.
The tumor locations addressed by US are summarized in Table 2. The N1b group showed more frequently upper lobe tumor location compared to the N0 group (38.9% vs. 16.0%, p < 0.001). There was no significant difference of the locations with the presence of invasion to adjacent organs including capsule, muscle, trachea, and RLN between N1b and N0 groups. US features of the main thyroid tumor are summarized in Table 3. Echogenicity, composition, and margin showed no differences between N1b and N0 groups. Macrocalcification was more frequently observed in the N1b group compared to the N0 group, but the difference was not significant (p = 0.053). The N1b group had a higher percentage of non-parallel shape compared to the N0 group (80.0% vs. 66.0%, p = 0.013).
US findings of background thyroid gland is summarized in Supplementary Table 1. There were no differences in echogenicity, sonographic feature, margin, and AP diameter between the two groups. US features for suspicious LN are summarized in Supplementary Table 2. Among patients with PTMC with lateral LN metastasis, the rate of suspicious LNs observed on both sides of the neck was 3.3%. The most frequently observed US feature of suspicious LN was echogenic foci (calcification), followed by cortical hyperechogenicity (55.6% and 24.4%, respectively). Cystic changes were observed in 12.2% of a total of 90 patients, and all of them showed no malignant cells on FNA and all of them showed no malignant cells on FNA. Washout fluid Tg was obtained and evaluated in all suspicious LN with cystic change and the range for washout fluid Tg was 9.70–659.0 ng/ml.
Multivariate regression analysis was performed for both pathological and sonographic variables exhibiting significant differences. ETE, multifocality, upper lobe tumor location, and non-parallel shape were independent risk factors for lateral LN metastasis in patients with PTMC (Table 4).
Among a total of 90 patients with lateral LN metastasis, 29 cases (32.2%) had skip metastasis (Table 5). There were no significant differences between skip metastasis and stepwise metastasis in age, sex, ETE, and the location of LN metastasis. However, a higher frequency of upper lobe tumor location was observed in skip metastasis compared to stepwise metastasis (55.2% vs. 31.1%, p = 0.029). Conversely, bilaterality and multifocality were more frequently found in the stepwise metastasis group (6.9% vs. 36.1%, p = 0.003, 24.1% vs. 54.1%, p = 0.008, respectively) and a greater number of metastatic LNs were observed in the stepwise metastasis group (2.9 ± 2.6 vs. 7.9 ± 4.7, p < 0.001).
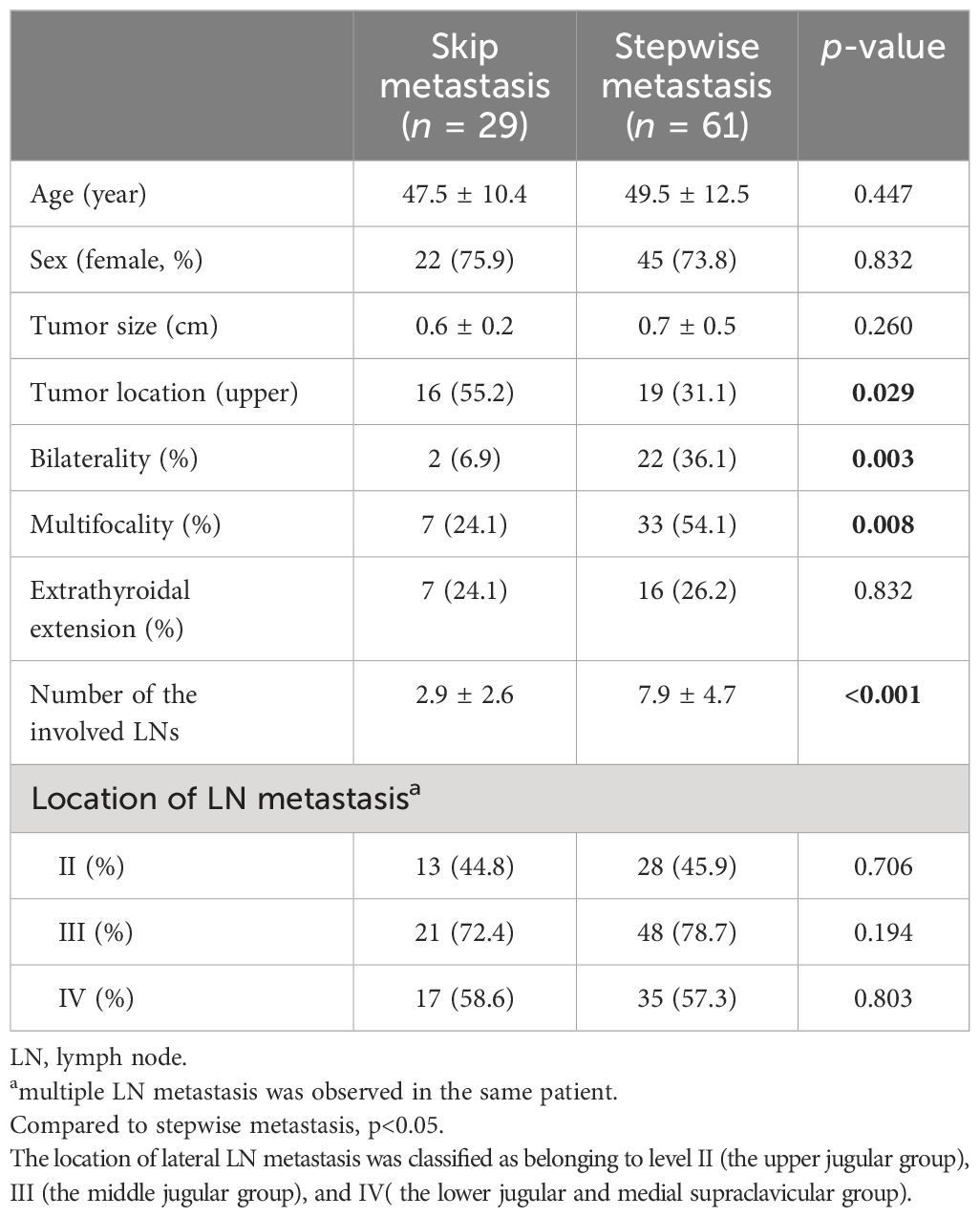
Table 5. Clinicopathological findings between skip metastasis and stepwise metastasis in papillary thyroid microcarcinoma patients with lateral lymph node metastasis.
This study focused on identifying risk factors for lateral LN metastasis in patients with PTMC. We evaluated clinicopathologic features and US findings not only in the main thyroid tumor but also in the background thyroid gland. Additionally, this study aimed to evaluate predictors of skip metastasis in patients with PTMC. The results revealed that ETE, tumor multifocality, upper lobe tumor location, and non-parallel tumor shape were independent predictors for lateral LN metastasis in patients with PTMC. Importantly, all these predictors can be assessed non-invasively using US, making it a valuable tool for risk stratification before the decision of AS.
PTC, the most common type of differentiated thyroid cancer, has seen a significant increase in incidence over the last few decades (22), resulting in considerable economic and emotional burden. As a result, AS is increasingly recommended as a suitable management option for low-risk PTC, particularly PTMC, given its favorable prognosis (23). The most important part of AS is to find appropriate criteria for AS, which could reduce the possibility of disease progression during observation. The definition of disease progression under AS is divided into two sections: tumor size growth and the emergence of new LN metastasis (14). The lower frequency of LN metastasis and the lack of significant benefit from prophylactic central LN dissection have supported lobectomy and even AS strategies in patients with PTMC. However, the presence of lateral LN metastasis can alter the treatment approach, with reported rates of up to 39.5% when prophylactic LN dissection is performed (24). This distinction can significantly impact the management strategy choice between AS and immediate surgery (IS) for patients with low-risk PTC. Moreover, LN metastasis has been linked to distant metastasis and recurrence in PTC patients, further emphasizing the importance of detecting and addressing LN involvement in the disease management (3, 25, 26). Our study demonstrated that patients with PTMC without LN metastasis (N0) at diagnosis showed no structural recurrence during an average follow-up period of 94.6 months, which could be safe candidates for AS.
In a Korean study analyzing 5,656 patients with PTMC, male gender was identified as one of the independent predictors for lateral LN metastasis, with the difference of loco-regional recurrence according to nodal stage (27). Similarly, a Japanese AS cohort study revealed that young age (<40 years) was an independent risk factor for newly developed LN metastasis among low-risk patients with PTMC (28). Young age and male gender are well-known predictors in patients with PTC; however, a Korean study demonstrated higher recurrence rates and mortality in male patients compared to female patients with PTC, but male gender was not an independent prognostic factor for recurrence in propensity score-matched patients with PTMC (29). In this study, we performed the PSM for age and sex among patients with PTMC; thus, the age and sex variables for the risk of LN metastasis could not be analyzed. Nevertheless, special attention is warranted to identify lateral LN metastasis in patients with young age and male gender.
In a Korean study analyzing 3,578 patients with PTMC, central LN metastasis, ETE, and multifocality were identified as significant risk factors for lateral LN metastasis (30). Our study also found similar trends, with multifocality and ETE being related to lateral LN metastasis, although central LN metastasis could not be evaluated due to the study’s design comparing N1b with N0. Many studies have shown that multifocality is a risk factor for LN metastasis in PTC and patients with PTMC (7, 31, 32), and there are still controversies regarding its relationship with prognosis. A recent study suggested that minimal ETE is linked to lateral LN metastasis (33), but there is no association between minimal ETE and recurrence among patients with PTMC (34). A future prospective study could explain the relationship between multifocality and clinical outcome in patients with PTMC.
At the time of the decision for AS in low-risk patients with PTMC, many histopathological risk factors are not readily available. However, tumor size, ETE, and multifocality can be evaluated by US with some limitations. For instance, capsular invasion on US can be defined as the percentage of the tumor perimeter in contact with the thyroid capsule, and PTC patients with >50% capsular invasion on US show a higher frequency of lateral LN metastasis (35). Loss of the echogenic capsule has been identified as a reliable US value for evaluating capsular invasion in PTC patients, with 75% sensitivity and 65% specificity, but a false discovery rate of 57.1% (36). US may have some limitations in accurately assessing multifocality due to the possibility of occult tumors with small size, and three patients were excluded because tumor was not observed in preoperative US of our study. Despite these challenges, our study showed the association of ETE and multifocality with lateral LN metastasis; thus, careful US evaluation of ETE and tumor number can provide valuable clues for the risk of lateral LN metastasis.
US findings play a critical role in predicting disease progression, especially lateral LN metastasis, in patients with PTMC considering AS because US should be performed in all patients before the decision of AS. Despite its importance, only a few studies have focused on US features for PTMC. A Chinese study highlighted that US features, such as central LN metastasis in the presence of concurrent HT, upper lobe tumor location, lack of a well-defined margin, and the presence of calcifications, were significantly associated with lateral LN metastasis in patients with PTMC (37). Another study identified upper lobe tumor location, microcalcification, and subcapsular lesions (defined as nodules abutting the thyroid capsule without intervening thyroid tissue) as factors associated with lateral LN metastasis (38). In the present study, we found that upper lobe tumor location and a non-parallel shape of the main thyroid tumor were independent predictors for lateral LN metastasis in patients with PTMC. The association between upper lobe tumor location and lateral LN metastasis has been consistently demonstrated in most studies focusing on patients with PTMC (39). Consequently, meticulous US follow-up is essential with a specific focus on detecting lateral LN metastasis in patients with PTMC with tumors located in the upper lobe. The upper lobe tumor location and PTMC were risk factors of skip metastasis (40, 41). This study evaluated risk factors of skip metastasis in patients with PTMC, and upper lobe tumor location was identified as a risk factor. Furthermore, skip metastasis could suddenly occur without other progressive signs including bilaterality, multifocality, and the expansion of metastatic LNs.
Apart from the main characteristics of the thyroid tumor, the background of the thyroid itself could also influence LN metastasis. The association between DTD and PTC has primarily been evaluated in the context of HT. Previous reports have indicated a higher prevalence of PTC in patients with HT compared to those without HT, and some studies have suggested a potential protective effect of HT in terms of recurrence and disease-related mortality of PTC (42). However, controversies still exist regarding this association. In our study, we found that coexisting HT, as determined by pathological results and US findings, showed no association with lateral LN metastasis in patients with PTMC. Similarly, other studies have shown no significant association between HT and LN metastasis or recurrence rate in patients with PTMC when HT was defined based on thyroid autoantibodies or pathology (43). Furthermore, a meta-analysis reported that there was no relationship between HT and LN metastasis in patients with PTMC and that HT had a negative association with LN metastasis in PTC cases larger than 1 cm (44).
This study has several limitations that need to be acknowledged. Firstly, the retrospective design of the study may introduce selection bias and limit the generalizability of the findings. Secondly, the evaluation of vascularity using Doppler US was not conducted due to the lack of data, which could have provided valuable information on the vascularity of the thyroid tumor and gland. Thirdly, age- and sex-matched data were used as controls (N0 group), which means that the effects of age and sex as potential risk factors were not specifically evaluated in this study. Despite these limitations, this study is valuable as it assessed and analyzed US findings of both the main tumor and the thyroid gland, allowing for the identification of potential candidates for AS without relying solely on pathological findings.
In conclusion, factors including upper lobe tumor location and non-parallel shape, along with ETE and multifocality, were identified as independent risk factors for lateral LN metastasis in patients with PTMC, and skip metastasis is also more commonly observed in tumors of the upper lobe. Therefore, meticulous US examinations to predict LN metastasis that include an assessment of tumor location, number, orientation shape, and the presence of ETE beyond mere detection of LN metastasis are necessary in the decision-making process for AS in especially when primary tumor was found in upper lobe.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The Institutional Review Board of Chonnam National University Hwasun Hospital (No. CNUHH-2020-271) approved this study. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
JY: Data curation, Formal analysis, Investigation, Writing – original draft. JP: Data curation, Methodology, Writing – original draft. AH: Investigation, Software, Writing – review & editing. HK: Supervision, Validation, Writing – review & editing. H-CK: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by a grant (HCRI 20014) from the Chonnam National University Hwasun Hospital Institute for Biomedical Science.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1392247/full#supplementary-material
1. Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. (2010) 34:28–35. doi: 10.1007/s00268-009-0303-0
2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
3. Jeon MJ, Kim WG, Choi YM, Kwon H, Lee YM, Sung TY, et al. Features predictive of distant metastasis in papillary thyroid microcarcinomas. Thyroid. (2016) 26:161–8. doi: 10.1089/thy.2015.0375
4. Celik H, Akgul O, Yıldız BD, Saylam B, Tez M. Predictors of central lymph node metastasis in papillary thyroid cancer. Ann Ital Chir. (2017) 88:193–7.
5. Chen S, Niu C, Peng Q, Tang K. Sonographic characteristics of papillary thyroid carcinoma with coexistent hashimoto's thyroiditis in the preoperative prediction of central lymph node metastasis. Front Endocrinol (Lausanne). (2021) 12:556851. doi: 10.3389/fendo.2021.556851
6. Ciobanu Apostol D, Giuşcă SE, Căruntu ID, Lozneanu L, Andriescu EC, Moscalu M. Relationships between clinicopathological prognostic factors in papillary thyroid microcarcinoma: a refined analysis based on 428 cases. Int J Clin Exp Pathol. (2017) 10:8944–56.
7. Feng JW, Qu Z, Qin AC, Pan H, Ye J, Jiang Y. Significance of multifocality in papillary thyroid carcinoma. Eur J Surg Oncol. (2020) 46:1820–8. doi: 10.1016/j.ejso.2020.06.015
8. Ito Y, Kudo T, Kobayashi K, Miya A, Ichihara K, Miyauchi A. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: analysis of 5,768 patients with average 10-year follow-up. World J Surg. (2012) 36:1274–8. doi: 10.1007/s00268-012-1423-5
9. Kim WB. A closer look at papillary thyroid carcinoma. Endocrinol Metab (Seoul). (2015) 30:1–6. doi: 10.3803/EnM.2015.30.1.1
10. Lim YC, Koo BS. Predictive factors of skip metastases to lateral neck compartment leaping central neck compartment in papillary thyroid carcinoma. Oral Oncol. (2012) 48:262–5. doi: 10.1016/j.oraloncology.2011.10.006
11. Feng JW, Qin AC, Ye J, Pan H, Jiang Y, Qu Z. Predictive factors for lateral lymph node metastasis and skip metastasis in papillary thyroid carcinoma. Endocr Pathol. (2020) 31:67–76. doi: 10.1007/s12022-019-09599-w
12. Yang Z, Heng Y, Zhao Q, Cao Z, Tao L, Qiu W, et al. A specific predicting model for screening skip metastasis from patients with negative central lymph nodes metastasis in papillary thyroid cancer. Front Endocrinol (Lausanne). (2021) 12:743900. doi: 10.3389/fendo.2021.743900
13. Xiang D, Xie L, Xu Y, Li Z, Hong Y, Wang P. Papillary thyroid microcarcinomas located at the middle part of the middle third of the thyroid gland correlates with the presence of neck metastasis. Surgery. (2015) 157:526–33. doi: 10.1016/j.surg.2014.10.020
14. Sugitani I, Ito Y, Takeuchi D, Nakayama H, Masaki C, Shindo H, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan association of endocrine surgery task force on management for papillary thyroid microcarcinoma. Thyroid. (2021) 31:183–92. doi: 10.1089/thy.2020.0330
15. Ito Y, Miyauchi A, Oda H, Kobayashi K, Kihara M, Miya A. Revisiting low-risk thyroid papillary microcarcinomas resected without observation: was immediate surgery necessary? World J Surg. (2016) 40:523–8. doi: 10.1007/s00268-015-3184-4
16. Abe I, Lam AK. Assessment of papillary thyroid carcinoma with ultrasound examination. Methods Mol Biol. (2022) 2534:17–28. doi: 10.1007/978-1-0716-2505-7_2
17. Grant EG, Tessler FN, Hoang JK, Langer JE, Beland MD, Berland LL, et al. Thyroid ultrasound reporting lexicon: white paper of the ACR thyroid imaging, reporting and data system (TIRADS) committee. J Am Coll Radiol. (2015) 12:1272–9. doi: 10.1016/j.jacr.2015.07.011
18. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol. (2016) 17:370–95. doi: 10.3348/kjr.2016.17.3.370
19. Kim DW, Eun CK, In HS, Kim MH, Jung SJ, Bae SK. Sonographic differentiation of asymptomatic diffuse thyroid disease from normal thyroid: a prospective study. AJNR Am J Neuroradiol. (2010) 31:1956–60. doi: 10.3174/ajnr.A2164
20. Kim DW. A COMPARATIVE STUDY OF REAL-TIME AND STATIC ULTRASONOGRAPHY DIAGNOSES FOR THE INCIDENTAL DETECTION OF DIFFUSE THYROID DISEASE. Endocr Pract. (2015) 21:910–6. doi: 10.4158/EP15646.OR
21. Prativadi R, Dahiya N, Kamaya A, Bhatt S. Chapter 5 ultrasound characteristics of benign vs Malignant cervical lymph nodes. Semin Ultrasound CT MR. (2017) 38:506–15. doi: 10.1053/j.sult.2017.05.005
22. Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. (2021) 9:225–34. doi: 10.1016/S2213-8587(21)00027-9
23. Ito Y, Miyauchi A. Active surveillance as first-line management of papillary microcarcinoma. Annu Rev Med. (2019) 70:369–79. doi: 10.1146/annurev-med-051517-125510
24. Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. (2003) 237:399–407. doi: 10.1097/01.SLA.0000055273.58908.19
25. Bernet V. Approach to the patient with incidental papillary microcarcinoma. J Clin Endocrinol Metab. (2010) 95:3586–92. doi: 10.1210/jc.2010-0698
26. Maksimovic S, Jakovljevic B, Gojkovic Z. Lymph node metastases papillary thyroid carcinoma and their importance in recurrence of disease. Med Arch. (2018) 72:108–11. doi: 10.5455/medarh.
27. Kim SK, Park I, Woo JW, Lee JH, Choe JH, Kim JH, et al. Predictive factors for lymph node metastasis in papillary thyroid microcarcinoma. Ann Surg Oncol. (2016) 23:2866–73. doi: 10.1245/s10434-016-5225-0
28. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. (2014) 24:27–34. doi: 10.1089/thy.2013.0367
29. Lee YH, Lee YM, Sung TY, Yoon JH, Song DE, Kim TY, et al. Is male gender a prognostic factor for papillary thyroid microcarcinoma? Ann Surg Oncol. (2017) 24:1958–64. doi: 10.1245/s10434-017-5788-4
30. Kim K, Zheng X, Kim JK, Lee CR, Kang SW, Lee J, et al. The contributing factors for lateral neck lymph node metastasis in papillary thyroid microcarcinoma (PTMC). Endocrine. (2020) 69:149–56. doi: 10.1007/s12020-020-02251-2
31. Zhang C, Cheng L, Zhu W, Zhuang J, Zhao T, Li X, et al. Construction of a diagnostic model for lymph node metastasis of the papillary thyroid carcinoma using preoperative ultrasound features and imaging omics. J Healthc Eng. (2022) 2022:1872412. doi: 10.1155/2022/1872412
32. Liu Z, Wang L, Yi P, Wang CY, Huang T. Risk factors for central lymph node metastasis of patients with papillary thyroid microcarcinoma: a meta-analysis. Int J Clin Exp Pathol. (2014) 7:932–7.
33. Song RY, Kim HS, Kang KH. Minimal extrathyroidal extension is associated with lymph node metastasis in single papillary thyroid microcarcinoma: a retrospective analysis of 814 patients. World J Surg Oncol. (2022) 20:170. doi: 10.1186/s12957-022-02629-8
34. Moon HJ, Kim EK, Chung WY, Yoon JH, Kwak JY. Minimal extrathyroidal extension in patients with papillary thyroid microcarcinoma: is it a real prognostic factor? Ann Surg Oncol. (2011) 18:1916–23. doi: 10.1245/s10434-011-1556-z
35. Ye L, Hu L, Liu W, Luo Y, Li Z, Ding Z, et al. Capsular extension at ultrasound is associated with lateral lymph node metastasis in patients with papillary thyroid carcinoma: a retrospective study. BMC Cancer. (2021) 21:1250. doi: 10.1186/s12885-021-08875-5
36. Kamaya A, Tahvildari AM, Patel BN, Willmann JK, Jeffrey RB, Desser TS. Sonographic detection of extracapsular extension in papillary thyroid cancer. J Ultrasound Med. (2015) 34:2225–30. doi: 10.7863/ultra.15.02006
37. Zeng RC, Li Q, Lin KL, Zhang W, Gao EL, Huang GL, et al. Predicting the factors of lateral lymph node metastasis in papillary microcarcinoma of the thyroid in eastern China. Clin Transl Oncol. (2012) 14:842–7. doi: 10.1007/s12094-012-0875-2
38. Jeon MJ, Chung MS, Kwon H, Kim M, Park S, Baek JH, et al. Features of papillary thyroid microcarcinoma associated with lateral cervical lymph node metastasis. Clin Endocrinol (Oxf). (2017) 86:845–51. doi: 10.1111/cen.13322
39. Back K, Kim JS, Kim JH, Choe JH. Superior located papillary thyroid microcarcinoma is a risk factor for lateral lymph node metastasis. Ann Surg Oncol. (2019) 26:3992–4001. doi: 10.1245/s10434-019-07587-2
40. Zhao L, Wu F, Zhou T, Lu K, Jiang K, Zhang Y, et al. Risk factors of skip lateral cervical lymph node metastasis in papillary thyroid carcinoma: a systematic review and meta-analysis. Endocrine. (2022) 75:351–9. doi: 10.1007/s12020-021-02967-9
41. Qiu Y, Fei Y, Liu J, Liu C, He X, Zhu N, et al. Prevalence, risk factors and location of skip metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis. Cancer Manag Res. (2019) 11:8721–30. doi: 10.2147/CMAR
42. Xu S, Huang H, Qian J, Liu Y, Huang Y, Wang X, et al. Prevalence of hashimoto thyroiditis in adults with papillary thyroid cancer and its association with cancer recurrence and outcomes. JAMA Netw Open. (2021) 4:e2118526. doi: 10.1001/jamanetworkopen.2021.18526
43. Qu N, Zhang L, Lin DZ, Ji QH, Zhu YX, Wang Y. The impact of coexistent Hashimoto's thyroiditis on lymph node metastasis and prognosis in papillary thyroid microcarcinoma. Tumour Biol. (2016) 37:7685–92. doi: 10.1007/s13277-015-4534-4
Keywords: papillary thyroid microcarcinoma (PTMC), lymph node (LN), extra-thyroidal extension (ETE), upper lobe, non-parallel shape, multifocality
Citation: Yoon JH, Park JY, Hong AR, Kim HK and Kang H-C (2024) Predictors of lateral lymph node metastasis and skip metastasis in patients with papillary thyroid microcarcinoma. Front. Endocrinol. 15:1392247. doi: 10.3389/fendo.2024.1392247
Received: 27 February 2024; Accepted: 03 June 2024;
Published: 02 July 2024.
Edited by:
Lorenzo Scappaticcio, University Hospital “Luigi Vanvitelli”, ItalyReviewed by:
Carla Gambale, University of Pisa, ItalyCopyright © 2024 Yoon, Park, Hong, Kim and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ho-Cheol Kang, ZHJrYW5nQGNob25uYW0uYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.