- 1Endocrinology ASL Nord-West, Spedali Riuniti, Livorno, Italy
- 2Department of Surgery, UO Pathology, University of Pisa, Pisa, Italy
- 3Department of Surgery, Endocrine Surgery, University of Pisa, Pisa, Italy
Background: Tyrosine kinase inhibitors (TKIs) and immunotherapy have been proposed for advanced metastatic anaplastic thyroid cancer (ATC). We report a case of BRAF V600E-mutated ATC in which lenvatinib (L) plus pembrolizumab (P) enabled neoadjuvant treatment.
Case presentation: A male patient aged 65 years presented with a rapidly enlarging left latero-cervical mass. Fine needle aspiration was suggestive of ATC. Surgical consultation excluded radical surgery. While awaiting molecular profile analysis and considering the fast evolution of the disease, treatment with L and P was started. L was started at a dose of 14 mg daily, while P was started at the standard regimen (200 mg every 3 weeks). After 1 month, computerized tomography showed a reduction in the mass with almost complete colliquative degeneration, and the carotid artery wall was free from infiltration. Radical surgery was performed. Histology confirmed papillary thyroid cancer (PTC) in the left lobe and ATC with extensive necrosis in the left latero-cervical lymph node metastasis. The margins were free of tumors (R0). A BRAF V600E mutation was present in both PTC and ATC. At the 1-year follow-up, the patient was free of disease.
Conclusion: L and P in combination also appeared to be effective as a neoadjuvant treatment for BRAF V600E-mutated ATC. This combination treatment could be used when there is an opportunity for complete resection of the cancer, and as soon as possible. The intermediate dose of 14 mg of L appeared to be well tolerated and effective.
Introduction
Anaplastic thyroid cancer (ATC) is one of the most aggressive human cancers. It usually presents at an advanced stage, and the median survival is approximately 4 months (1, 2). Complete resection can be achieved in only a minority of patients. Incomplete resection followed by multimodal therapy, i.e., external beam radiotherapy (EBRT) and chemotherapy or EBRT alone, has been shown to ameliorate the prognosis in some but not all studies, above all depending on the disease stage (1–3). Recently, tyrosine kinase inhibitors (TKIs) and immunotherapy have been proposed and used for advanced metastatic ATC (1, 2, 4–9). A number of interesting reports have shown that the combination of TKIs and pembrolizumab (P) can have an impact on survival in advanced and metastatic forms. The use of highly selective TKIs is dependent on the presence of specific mutations in the tumor. Dabrafenib (D) and trametinib (T) especially, block selectively and consequently two enzymes of the MAP kinase cascade, and they have been used in BRAF V600E-mutated ATC. The use of D and T has significantly changed the treatment of ATC (10); in fact, the molecular profile is one of the most important first diagnostic tools in these cancers. On the other hand, the multi-TKI lenvatinib (L) has been used in BRAF wild-type cases. In almost all cases published, TKIs plus immunotherapy have been used in metastatic cancer (stage IVC) after surgical intervention and/or after EBRT and chemotherapy. In a few cases, this association was used for neoadjuvant purposes, but no patients were cured after 1 year. We report a case of ATC in which L+P enabled neoadjuvant treatment followed by a complete resection of the tumor, with the patient free of disease at 1 year. In this case, L was also used, although the cancer was BRAF V600E-mutated.
Case report: diagnosis, treatment, and follow-up
A male patient, aged 65 years, presented with a rapidly enlarging left latero-cervical mass. The mass was hard/wooden, fixed to the neck structure, and extended from the jaw angle to the base of the neck. Ultrasonography showed a hypoechoic mass with a maximum size of approximately 7 cm in the latero-cervical II and III compartments and a hypoechoic nodule with a maximum size of 2 cm in the left thyroid lobe. Fine needle aspiration (FNA) showed a smear suggesting papillary thyroid cancer (PTC) on the thyroid nodule and ATC on the latero-cervical mass (confirmed by a large needle biopsy). CT showed a large mass with a colliquative component (Figure 1), completely infiltrating the jugular vein and, most importantly, infiltrating the carotid artery wall. A double surgery consultation excluded the possibility of radical surgery and raised some doubts regarding the outcome of a surgical intervention.
While awaiting the results of the molecular profile analysis performed on the smear and considering the fast evolution of the disease, treatment with L and P was started. Based on our previous experience (11), L was started at a lower dose, i.e., 14 mg instead of 24 mg, and P was started at the standard regimen (200 mg every 3 weeks). After 1 month (specifically 32 days after starting L and 10 days after the second infusion of P), clear clinical benefits were observed: a slight visual reduction and, most importantly, a change in the consistency of the mass, which now appeared soft and mobile. CT showed a mild reduction, but with almost complete colliquative degeneration and with the carotid artery wall free of infiltration (Figure 2).
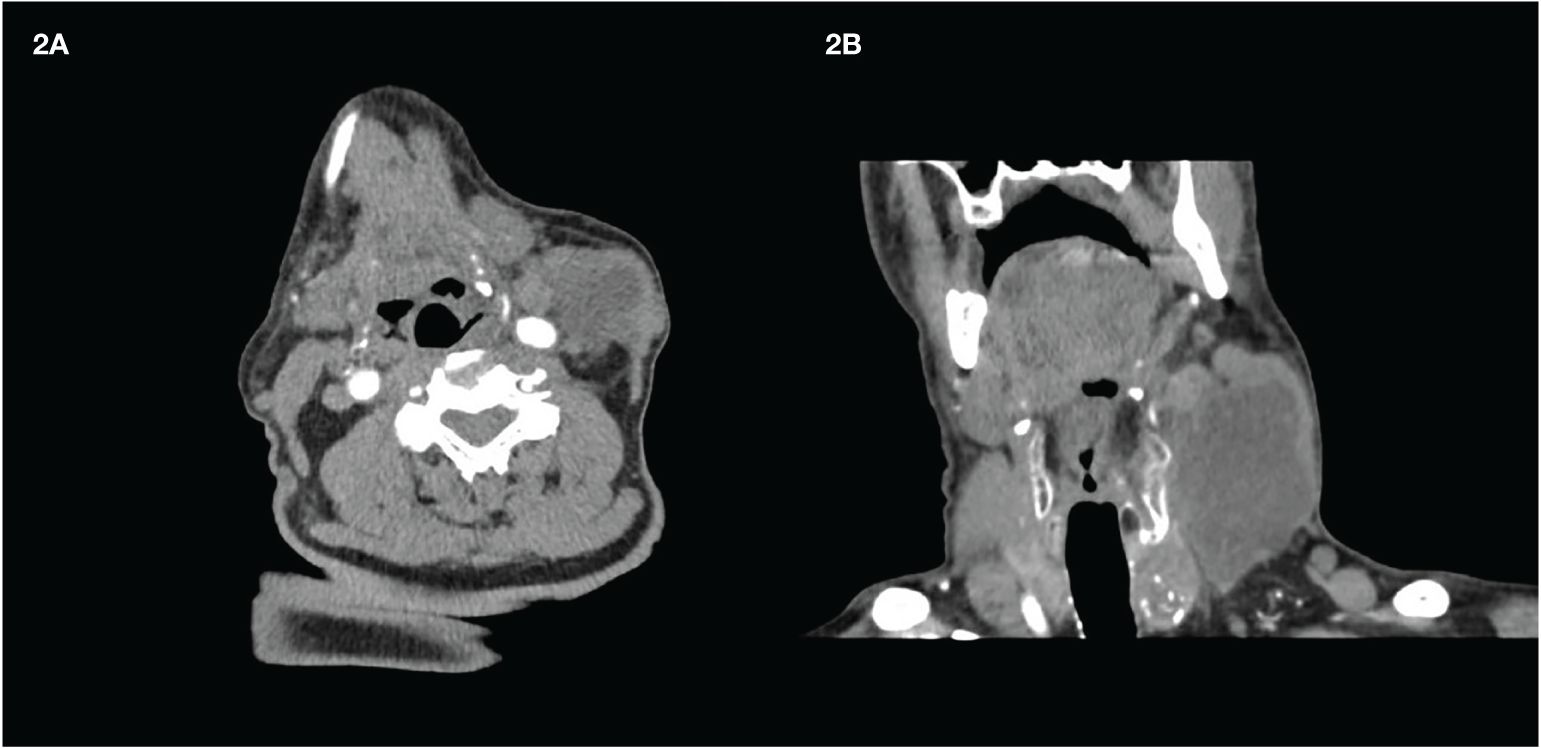
Figure 2 Latero-cervical mass after 1 month of treatment. (A) Transversal scan. (B) Coronal scan. The transverse scan shows the detachment from the carotid artery wall. Almost the whole mass appears dark due to the presence of colliquative necrosis.
Surgical resection was then performed 8 days after the discontinuation of L and when complete macroscopic resection was possible. The crucial issue after the surgical intervention was whether or not to continue with the treatment. While also awaiting the results of histology, it was decided to continue with L+P; thus, therapy was restarted after 8 days. However, possibly due to the large extent of the surgical intervention (removal of the sternocleidomastoid muscle and the jugular vein), wound dehiscence was observed after 2 weeks, and all drug treatments had to be stopped.
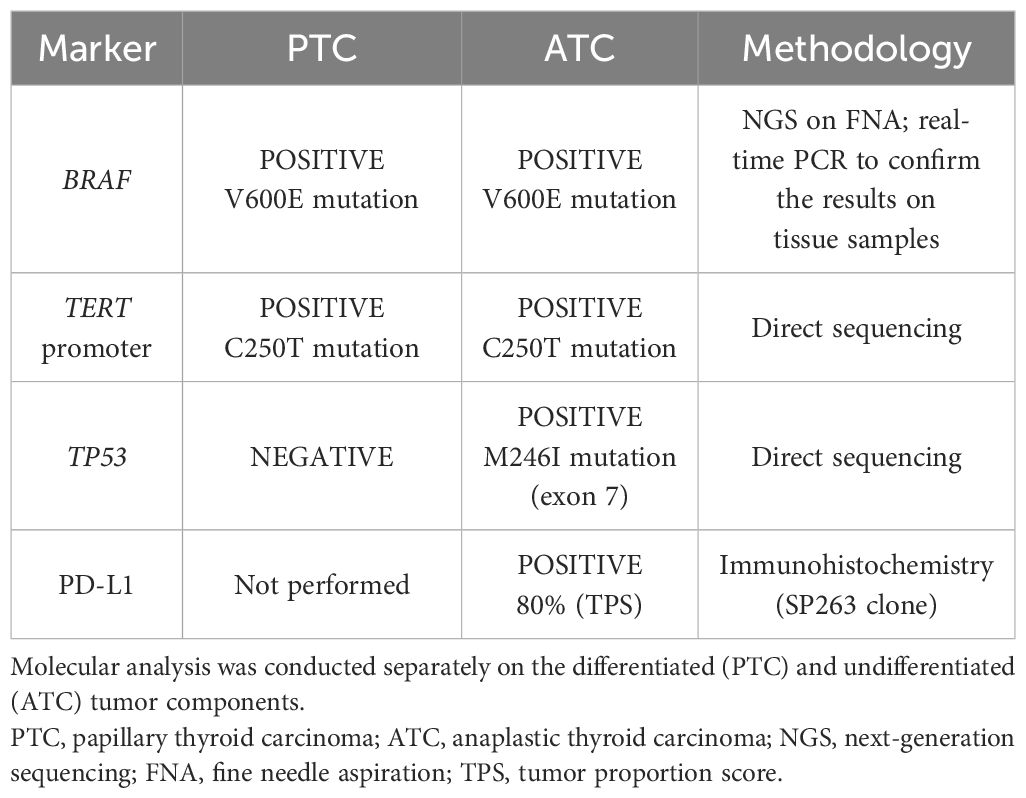
Histology confirmed the coexistence of differentiated and undifferentiated tumor histotypes. In the right lobe, three PTC microfoci (maximum size, 0.2 cm) were detected, along with two lymph nodes with PTC metastases. In the left lobe, a 3-cm classic PTC and a 2.4-cm tall cell subtype PTC were observed. Three perithyroidal lymph nodes were positive for PTC metastases, while the supraclavicular lymph nodes sampled were free from metastases. The residual tumor mass infiltrating the pre-thyroidal muscles was reported as ATC with extensive tumor necrosis. On the other hand, in a large left latero-cervical lymph node metastasis, the coexistence of differentiated and undifferentiated tumor components was observed. The excision margins were free of tumor (R0). Considering the R0 resection, medical treatment was not started again after the recovery of the wound dehiscence. To investigate the relationship between the differentiated and undifferentiated tumors, molecular analysis of the BRAF, TERT, and TP53 genes was performed separately on the two tumor components of the latero-cervical lymph node metastasis. BRAF V600E and TERT promoter mutations were present both in differentiated thyroid cancer and in ATC, while the TP53 mutation was detected only in ATC. Details on the genetic profile of the tumors are reported in Table 1.
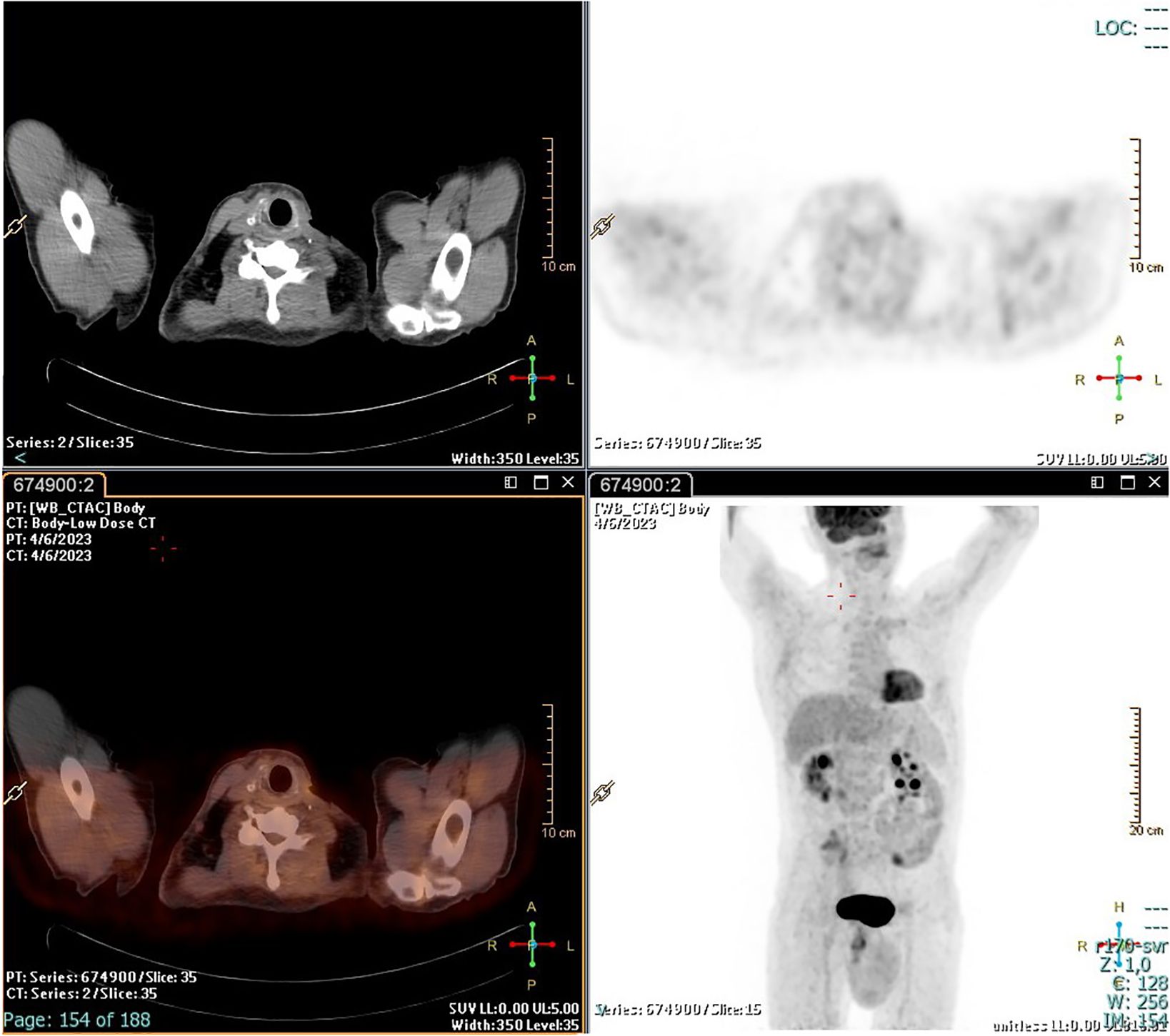
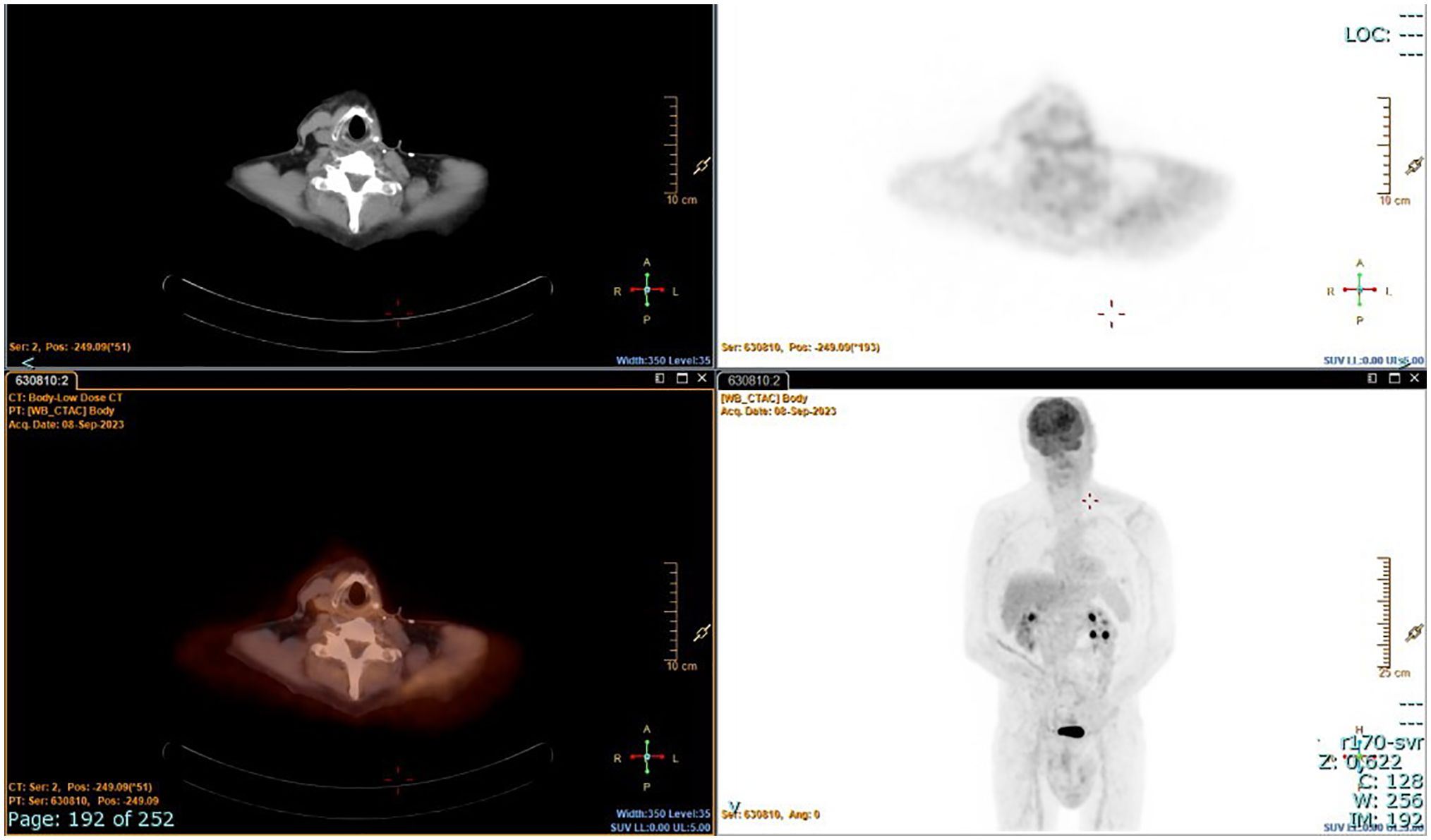
The patient underwent radioiodine treatment with an activity of 130 mCi (patients prepared with L-T4 withdrawal), and a whole-body scan showed only minimal post-surgical remnants. Subsequent 18FDG/PET-CT performed after 4 months showed no residual disease (Figure 3). At the 1-year follow-up, the patient was free of disease: 18FDG/PET-CT was negative (Figure 4), and basal and stimulated thyroglobulin (Tg) was undetectable.
Discussion
TKIs have revolutionized the treatment of advanced iodine-refractory thyroid cancer (1, 2, 12). Many studies have shown that these drugs can also play a role in the treatment of ATC (1, 2). Studies on treatment with multi-target TKIs, such as L, have shown conflicting results regarding the real possibility of significantly ameliorating survival. The first study was encouraging, and L was authorized in Japan for the treatment of ATC (13). However, a subsequent multicenter trial failed to show benefit, and the trial was thus stopped (NCT02657369). In another retrospective study of 23 patients, off-label use of L as a second-line treatment appeared to be more effective in mixed ATC than in pure ATC (14). This discrepancy in the data may have been dependent on the dose of the drug used (11). On the other hand, the results of treatment with D and T appear more interesting in special cases. These drugs act in a sequential manner by blocking the RAF–MEK–ERK enzymes in the MAP kinase cascade, and the clinical results of their use on BRAF V600E-mutated ATC have been encouraging (15–17). Treatment with D+T is now the preferred option for these patients; in fact, their use is widely discussed in the ESMO and ATA guidelines (1, 2). For ATC, there is also a strong rationale for the use of immunotherapy, especially P (18–20). The mutation burden in ATC causes an elevated immune infiltration, which the tumor can react to with the elevated expression of immune checkpoint ligands, including programmed death-ligand 1 (PD-L1). For this reason, P has been used in the treatment of ATC in combination with TKIs. L+P has especially been used in BRAF wild-type cancers, and a further rationale for the use of this combination is that VEGF-A can induce immunosuppression in the tumor microenvironment by inhibiting dendritic cell maturation and by reducing T-cell infiltration (21). On the other hand, D and T+P have been used successfully in BRAF V600E-mutated cases.
In one of the largest series (7), six patients with ATC and two patients with poorly differentiated thyroid cancer (all at stage IVC and with BRAF wild-type) were treated with the L+P combination after surgical intervention, followed by EBRT and systemic chemotherapy. The results were exceptionally good: the best overall response in ATC was a complete response in four out of six patients. There are also a few other reports in which P+L appeared to be effective as salvage treatment in BRAF wild-type cases (5, 6). In another report of BRAF wild-type ATC, treatment with P+L was used as an adjuvant, enabling the resection of the lesion (an apparent R0), but the patient relapsed 4 months later (8). Finally, another study showed some efficacy of treatment with P+L as the first-line therapy (after EBRT) in five patients with ATC. In a later study, the median progression-free survival (PFS) was 4.7 months (range, 0.8–5.9 months), while the median overall survival (OS) was 6.3 months. One patient was still alive at the time of data collection (12.7 months) (22).
Our case shows that treatment with L at a dose of 14 mg and P (which was used in the standard regimen) can also play a neoadjuvant role in BRAF V600E-mutated ATC.
This case gives rise to various speculations. First, this tumor represents a clear example of the anaplastic transformation of differentiated thyroid cancer. As demonstrated in previous studies, anaplastic transformation occurs through complex and heterogeneous mechanisms (23, 24). In this case, the molecular profiles of PTC and ATC clearly suggest that the undifferentiated tumor is derived from its differentiated counterpart. The presence of BRAF and TERT promoter mutations in both tumor components suggests that they may have a common origin and that, over time, the tumor cells clonally evolved by also acquiring the TP53 mutation, ultimately resulting in dedifferentiation.
Second, because the P+L combination can also work in BRAF V600E-mutated cancers, starting with L+P is an option if time is required for molecular profiling, especially considering that the growth of ATC is extremely rapid and that the possible clinical benefit can also be quite rapid. However, as next-generation sequencing (NGS) can be time-consuming, real-time PCR can help to determine such a mutation in a shorter time. Immunohistochemistry could also be used for the diagnosis of the BRAF V600E mutation if other methods are not readily available.
The third issue is the dose of L used. Our previous report (11) showed a very good response with 14 mg in two cases, particularly in one case that was stage IVC and had a partial response with a PFS of 16 months. We speculated that in the complex mechanism of regulating the various MAP kinase cascades, a specific, most effective dose may exist, beyond which a hook effect occurs and the efficacy decreases. In vitro data appear to show that the effects of L on ATC cells are dose-dependent. However, L can act on FGR1–FGR4, PDGFR-b, RET, and c-kit, although its main effect is considered to be on VEGFR-1, VEGFR-2, and VEGFR-3 and, hence, on tumor vascularization. Other experimental models are thus needed, and studies on organoids may provide an answer to the question of the dose effect of L in in vivo ATC. An alternative and simpler hypothesis is that this dose represents a good middle road for these fragile patients. However, 14 mg of L does appear to be effective, with only a few adverse effects.
To the best of our knowledge, this case represents the first in which neoadjuvant treatment with L+P in BRAF V600E-mutated ATC has enabled complete resection of the mass and complete cure of the patient for at least 1 year.
A clinical consideration is that this result was also possible, as we only needed to obtain a small reduction in the mass, i.e., to split the tumor from the deep planes of the neck and especially from the carotid artery. This point should be kept in mind with regard to possible neoadjuvant treatments in these patients, most importantly considering that the optimal aim of surgical intervention is to perform R0 resection.
In conclusion, L and P in combination appear to be effective as a neoadjuvant treatment for ATC, as well as in BRAF V600E-mutated ATC. Their use could be considered when a complete resection of the cancer is possible, which should likely be done as soon as possible while awaiting the results of molecular analysis. Changing into another TKI could also be a second option if there is a specific mutation and if there is no benefit. A middle dose of 14 mg of L can be well tolerated and appears effective.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DB: Writing – original draft, Writing – review & editing. RF: Writing – original draft. MP: Writing – original draft. PL: Writing – original draft. CG: Writing – original draft. LT: Writing – original draft. EM: Investigation, Writing – original draft. GM: Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2019) 30:1856–83. doi: 10.1093/annonc/mdz400
2. Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr, et al. 2021 American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. (2021) 31:337–86. doi: 10.1089/thy.2020.0944
3. Yang SR, Tsai MH, Hung CJ, Peng SL, Chiu NT, Huang YH, et al. Anaplastic thyroid cancer successfully treated with radiation and immunotherapy: a case report. AACE Clin Case Rep. (2021). doi: 10.1016/j.aace.2021.03.003
4. Sukrithan V, Jain P, Shah MH, Konda B. Kinase inhibitors in thyroid cancers. Endocr Oncol. (2023) 13:e220062. doi: 10.1530/EO-22-0062
5. Luongo C, Porcelli T, Sessa F, De Stefano MA, Scavuzzo F, Damiano V, et al. Combination of lenvatinib and pembrolizumab as savage treatment for paucicellular variant of anaplastic thyroid cancer: a case report. Curr Oncol. (2021) 28:5401–7. doi: 10.3390/curroncol28060450
6. Boudin L, Morvan J-B, Thariat J, Métivier D, Marcy P-Y, Delarbre D. Rationale efficacy and safety evidence of lenvatinib and pembrolizumab association in anaplastic thyroid carcinoma. Curr Oncol. (2022) 29:7718–31. doi: 10.3390/curroncol29100610
7. Dierks C, Seufert J, Aumann K, Ruf J, Klein C, Kiefer S, et al. Combination of lenvatinib and pembrolizumab is an affective treatment option of anaplastic and poorly differentiated thyroid carcinoma. Thyroid. (2021) 31:1076–85. doi: 10.1089/thy.2020.0322
8. Maurer E, Eilsberger F, Wachter S, Riera Knorrenschild J, Pehl A, Holzer K, et al. Mutation-based, short-term “neoadjuvant” treatment allows resectability in stage IVB and C anaplastic thyroid cancer. Eur Arch Otorhinolaryngol. (2023) 280:1509–18. doi: 10.1007/s00405-023-07827-y
9. Locati LD, Colombo E, Dedecjus M, de la Fouchardière C, Sents W, Bongiovanni M, et al. Current picture of anaplastic thyroid cancer patients’ care and meetable needs: a survey of 94 institutions from the EORTC Endocrine and Head and Neck Cancer Groups. Eur J Cancer. (2023) 180:146–54. doi: 10.1016/j.ejca.2022.12.002
10. Busaidy NL, Wang JR, Ferrarotto R, Lu C, Williams MD, Brandon Gunn G, et al. Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000-2019. JAMA Oncol. (2020) 6:1397–404. doi: 10.1001/jamaoncol.2020.3362
11. Barbaro D, Lapi P, Viacava P, Torregrossa L. Low-intermediate dose of lenvatinib in anaplastic thyroid cancer is highly effective and safe. BMJ Case Rep. (2020) 13:e236934. doi: 10.1136/bcr-2020-236934
12. Hamidi S, Hofmann MC, Iyer PC, Cabanillas ME, Hu MI, Busaidy NL, et al. Review article: new treatments for advanced differentiated thyroid cancers and potential mechanisms of drug resistance. Front Endocrinol (Lausanne). (2023) 14:1176731. doi: 10.3389/fendo.2023.1176731
13. Tahara M, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, et al. Lenvatinib for anaplastic thyroid cancer. Front Oncol. (2017) 7:25. doi: 10.3389/fonc.2017.00025
14. Sparano C, Godbert Y, Attard M, Do Cao C, Zerdoud S, Roudaut N, et al. Limited efficacy of lenvatinib in heavily pretreated anaplastic thyroid cancer: a French overview. Endocr Relat Cancer. (2021) 28:15–26. doi: 10.1530/ERC-20-0106
15. Subbiah V, Kreitman RJ, Wainberg ZA, Cho Y, Schellens JHM, Soria JC, et al. Dabrafenib plus trametinib in patients with BRAF V600-mutant anaplastic thyroid cancer: update analysis from the phase II ROAR basket study. Lancet Oncol. (2022) 23:53–64.
16. Subbiah V, Kreitman RJ, Wainberg ZA, Cho Y, Schellens JHM, Soria JC, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. (2018) 36:7–13. doi: 10.1200/JCO.2017.73.6785
17. Bueno F, Smulever A, Califano I, Guerra J, Del Grecco A, Carrera JM, et al. Dafrafenib plus trametinib treatment in patients with anaplastic thyroid carcinoma: an argentinian experience. Endocrine. (2023) 80:134–41. doi: 10.1007/s12020-022-03295-2
18. Adam P, Kircher S, Sbiera I, Koehler VF, Berg E, Knosel T, et al. FGF-receptir and PD-L1 in anaplastic and poorly differentiated thyroid cancer: evaluation of the preclinical rationale. Front Endocrinol (Lausanne). (2021) 12:712107. doi: 10.3389/fendo.2021.712107
19. Hatashima A, Archambeau B, Armbruster H, Xu M, Shah M, Konda B, et al. An evaluation of clinical efficacy of immune checkpont inhibitors for patients with anaplastic thyroid carcinoma. Thyroid. (2022) 32:926–36. doi: 10.1089/thy.2022.0073
20. Shih SR, Chen KH, Lin KY, Yang PC, Chen KY, Wang CW, et al. Immunotherapy in anaplastic thyroid cancer: case series. J Formos Med Assoc. (2022) 121:1167–73. doi: 10.1016/j.jfma.2022.01.003
21. Kang Y, Li H, Liu Y, Li Z. Regulation of VEGF-A expression and VEGF-A-targeted therapy in Malignant tumors. J Cancer Res Clin Oncol. (2024) 150:221. doi: 10.1007/s00432-024-05714-5
22. Soll D, Bischoff P, Frisch A, Jensen M, Karadeniz Z, Mogl MT, et al. BMC First effectiveness data of lenvatinib and pembrolizumab as first-line therapy in advanced anaplastic thyroid cancer: a retrospective cohort study. Endocr Disord. (2024) 24:25. doi: 10.1186/s12902-024-01555-y
23. Luo H, Xia X, Kim GD, Liu Y, Xue Z, Zhang L, et al. Characterizing dedifferentiation of thyroid cancer by integrated analysis. Sci Adv. (2021) 7:eabf3657. doi: 10.1126/sciadv.abf3657
24. Odate T, Oishi N, Kawai M, Tahara I, Mochizuki K, Akaishi J, et al. Progression of papillary thyroid carcinoma to anaplastic carcinoma in metastatic lymph nodes: solid/insular growth and hobnail cell change in lymph nodes are predictors of subsequent anaplastic transformation. Endocr Pathol. (2021) 32:347–56. doi: 10.1007/s12022-021-09674-1
Keywords: anaplastic thyroid cancer, lenvatinib, pembrolizumab, BRAF mutation V600, neoadjuvant
Citation: Barbaro D, Forleo R, Profilo MA, Lapi P, Giani C, Torregrossa L, Macerola E and Materazzi G (2024) Neoadjuvant treatment with lenvatinib and pembrolizumab in a BRAF V600E-mutated anaplastic thyroid cancer: a case report. Front. Endocrinol. 15:1389294. doi: 10.3389/fendo.2024.1389294
Received: 21 February 2024; Accepted: 14 June 2024;
Published: 09 July 2024.
Edited by:
Paolo Miccoli, Saint Camillus International University of Health and Medical Sciences, ItalyReviewed by:
Cristina Luongo, University of Naples Federico II, ItalyJeffrey A. Knauf, Cleveland Clinic, United States
Copyright © 2024 Barbaro, Forleo, Profilo, Lapi, Giani, Torregrossa, Macerola and Materazzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniele Barbaro, ZGFuaWVsZWJhcmJhcm8xOTcwQGxpYmVyby5pdA==
 Daniele Barbaro
Daniele Barbaro Raffaella Forleo1
Raffaella Forleo1 Liborio Torregrossa
Liborio Torregrossa