- 1Department of Neurosurgery, The Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, Jiangsu, China
- 2Department of Neurosurgery, Binhai County People’s Hospital Affiliated to Kangda College of Nanjing Medical University, Yancheng, Jiangsu, China
Purpose: The interaction between the renin-angiotensin system (RAS) and the acute ischemic stroke (AIS) is definite but not fully understood. This study aimed to analyze the risk factors of AIS and explore the role of serum indicators such as angiotensin I (Ang I) in the prognosis of patients undergoing endovascular thrombectomy (EVT).
Patients and methods: Patients with AIS who underwent EVT and healthy controls were retrospectively enrolled in this study, and the patients were divided into a good or a poor prognosis group. We compared Ang I, blood routine indexes, biochemical indexes, electrolyte indexes, and coagulation indexes between patients and controls. We used univariate and multivariate logistic regression analyses to evaluate possible risk factors for AIS and the prognosis of patients undergoing EVT. Independent risk factors for the prognosis of patients undergoing EVT were identified through multifactorial logistic regression analyses to construct diagnostic nomograms, further assessed by receiver operating characteristic curves (ROC).
Results: Consistent with previous studies, advanced age, high blood glucose, high D-dimer, and high prothrombin activity are risk factors for AIS. In addition, Ang I levels are lower in AIS compared to the controls. The level of Ang I was higher in the good prognosis group. Furthermore, we developed a nomogram to evaluate its ability to predict the prognosis of AIS after EVT. The AUC value of the combined ROC model (Ang I and albumin-globulin ratio (AGR)) was 0.859.
Conclusions: In conclusion, advanced age, high blood glucose, high D-dimer, and high prothrombin activity are risk factors for AIS. The combined Ang I and AGR model has a good predictive ability for the prognosis of AIS patients undergoing arterial thrombectomy.
Introduction
Acute ischemic stroke (AIS) is a disorder of blood flow supply to brain tissue caused by various reasons, which is characterized by high morbidity, disability, and mortality (1). According to statistics, the number of deaths due to ischemic stroke (IS) in the world ranks first in the number of deaths from cardiovascular and cerebrovascular diseases. It is one of the third leading causes of death in the world. China is a country with a high incidence of stroke. The incidence of stroke was 170/100,000 and 620/100,000 in males and females, respectively, and the prevalence rate was 620/100,000.
There are many pathogenic factors and complex pathological mechanisms of AIS. At present, it is generally believed that the occurrence of AIS is caused by atherosclerosis. Oxidative stress and vascular endothelial dysfunction are the primary pathogenesis of atherosclerosis. In recent years, with the deepening of research, people have found that the renin-angiotensin system (RAS) activation is closely related to AIS, which can directly or indirectly induce the occurrence and influence the development of AIS.
RAS is an endocrine regulatory system composed of peptide hormones and corresponding enzymes as the main components. It comprises six parts: renin, angiotensinogen, angiotensin, angiotensin-converting enzyme, angiotensin receptor, and aldosterone. Renin is an acid-hydrolytic protease produced by the juxtaglomerular cells of the kidney. Sodium depletion, sympathetic excitation, and decreased renal blood flow stimulate the release of renin, which acts on the hepatic production of angiotensinogen to convert it to angiotensin I (Ang I). Ang I has no biological activity and is further hydrolyzed to angiotensin II (Ang II) by the action of angiotensin-converting enzyme I. Ang II can be further decomposed into angiotensin (1–7) and angiotensin III and angiotensin IV by the step of angiotensin-converting enzyme II and aminopeptidase A, respectively (2).
Over-activation of RAS can produce a series of pathophysiological effects. At present, it is believed that circulating RAS mainly acts through the following two axes: (1) angiotensin-converting enzyme 1-Ang II-AT1R axis: the binding of Ang I-IV to AT1R leads to vasoconstriction, tissue cell fibrosis, and oxidative stress; (2) Angiotensin-converting enzyme 2-Ang (1–7)-Mas receptor axis: The binding of angiotensin domain with AT2R and Ang (1–7) with Mas receptor can resist the pathological effects of AT1R, dilate blood vessels, anti-inflammation, anti-tissue cell fibrosis, reduce cell apoptosis, and have protective effects on heart, brain, kidney, blood vessels and other organs (2). Studies on the pathological mechanism of RAS causing AIS mainly focus on Ang II, and the relationship between other components of RAS and AIS is relatively few.
The preferred treatment for AIS is intravenous thrombolysis within the time window. Still, due to the strict time window, the proportion of patients who can benefit from it is relatively low, and the treatment effect is poor (3). In recent years, with the continuous development of various endovascular therapy (EVT) devices and techniques, EVT has shown a good application prospect in treating AIS. Some domestic and foreign AIS treatment guidelines (4) recommend EVT as the first choice of treatment when intravenous thrombolysis is contraindicated or ineffective. Studies have shown that the overexpression of inflammatory factors and abnormal secretion of neurohormones in the pathogenesis of AIS aggravates the degree of neurological impairment and affects the prognosis of patients (5).
At present, the content of clinical evaluation of the development and prognosis of AIS mainly includes serological indicators and imaging examinations, among which serological indicators have the advantages of being simple, fast, and highly accurate and have become an essential means to guide clinical diagnosis and treatment. The primary objective of this study is to analyze the risk factors related to the occurrence of AIS, and the secondary aim is to explore the role of serum indicators such as neurohormones, blood routine, liver and kidney function, electrolytes, and coagulation function in the prognosis of patients undergoing endovascular thrombectomy (EVT).
Materials and methods
Patients
In this study, the alpha value was 0.05, the beta value was 0.2, and the test power was 0.8. The difference of Ang I between the patients and the controls was expected to be about 1–3 ng/ml. According to the 1:1 ratio between the case group and the control group, about 33 patients should be enrolled. Ultimately, seventy-two patients with AIS who underwent EVT at the Neurosurgery department of the First People’s Hospital of Lianyungang from December 2022 to October 2023 were retrospectively enrolled in this study. At the same time, 60 subjects who underwent regular physical examinations during the same period were included, and a total of 132 cases were included. This study met the criteria outlined in the Declaration of Helsinki. The ethics committee approved it, and the institutional review board of the First People’s Hospital of Lianyungang (ethics numbers: SHSY-IECKY-4.0/18–68/01 and ZDKYSB077). Written informed consent was obtained from this study’s patients and their relatives.
Inclusion and exclusion criteria
Inclusion criteria: (1). Age over 18 years old; (2). AIS diagnosed by imaging (CT and MRI); (3). Undergo EVT.
Exclusion criteria: (1). Cerebral hemorrhage was confirmed by imaging examination; (2). Patients who have received thrombolytic therapy; (3). Concurrent diagnosis of other malignant tumors that may seriously affect survival; (4). Accompanied with severe infectious diseases or liver and kidney dysfunction; (5). Severe bleeding tendency; (6). Patients with previous IS and severe motor dysfunction.
Based on the above criteria, 7 patients accept thrombolytic therapy, 2 patients had severe liver and kidney dysfunction, and 3 patients had IS previously and severe motor dysfunction. We excluded 12 patients, and 60 patients were finally included in this study. According to the above criteria, we divided the 120 subjects into two groups: normal group and disease group. To further analyze the effect of different variables on the prognosis of patients with AIS who underwent EVT, 60 patients in the disease group were divided into good prognosis group (0–2 scores) and poor prognosis group (3–5 scores) according to the modified Rankin scale score at discharge.
Study variables
The clinical data of inpatients in the Department of Neurosurgery of the First People’s Hospital of Lianyungang were collected retrospectively, including age, sex, blood routine indexes, biochemical indexes, electrolyte indexes, and coagulation indexes. Blood samples were collected within 72 hours after surgery for further analysis. All blood samples were obtained from either the left or right femoral vein. Rapidlab 1200 series equipment (Laboratory equipment of the First People’s Hospital of Lianyungang City) was used to analyze blood samples. Ang I ELISA assay: To measure the concentration of Ang I, quantitative factor high-sensitivity ELISA [R&D; Human angiotensin: BY-EH111540 (sensitivity 0.1 ng/mL)] was used to detect the concentration of Ang I in serum. Patients who underwent EVT were followed up by outpatient examination or telephone.
Surgical methods of endovascular thrombectomy
The patient was conventionally given local anesthesia, and if the patient has agitation, general anesthesia or intravenous combined anesthesia will be selected. The patient was asked to take the supine position, the femoral artery puncture was performed, and the 8F artery sheath was inserted. The whole brain digital subtraction angiography (DSA) was performed to determine the infarction site. For patients without large artery occlusion, 100,000 U urokinase was injected into the artery on the opposite side of the lesion or the poor development side. Then 20, 000 U/min urokinase was maintained according to the patient’s condition. The vital signs of the patients were closely monitored. For patients with large artery occlusion, an 8F MPA1 guide catheter was placed at the distal end of the common carotid artery, and a 5F-125 Naven intermediate catheter was placed at the distal end of the internal carotid artery along the guide catheter. Under the guidance of a 0.014 microguide wire, a Rebar-18 microcatheter was used to pass through the occlusion vessel to the distal end of the thrombus. Microcatheter angiography was performed to confirm the patency of the thrombus site and the distal end of the occluded vessel. The Solitaire AB stent was placed through the microcatheter. After 5 minutes, to ensure the full release of the stent, the stent was withdrawn with the microcatheter, and the blood was quickly aspirated with a 50 mL syringe. DSA examination was performed again after thrombectomy to confirm vascular recanalization.
Statistical analysis
Customarily distributed values were calculated as parametric tests and mean ± standard deviation, while non-normally distributed values were calculated as median. Categorical variables were analyzed by chi-square or Fisher’s exact test, and continuous variables were analyzed by unpaired t-test. The chi-square or Kruskal-Wallis test was used to evaluate the correlation between Ang I, blood routine indexes, biochemical indexes, electrolyte indexes, coagulation indexes, and clinicopathological features. The ROC curve and the area under the curve (AUC) were used to compare the ability of the models to predict AIS and outcome status. Univariate and multivariate logistic regression analyses were used to analyze the risk factors of AIS. Multivariate logistic regression was used to analyze the independent risk factors for the prognosis of AIS, and a diagnostic nomogram was constructed. All data were analyzed by SPSS software (version 27.0), GraphPad Prism software (version 8.3.1), and RStudio software (4.3.0). A value of 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
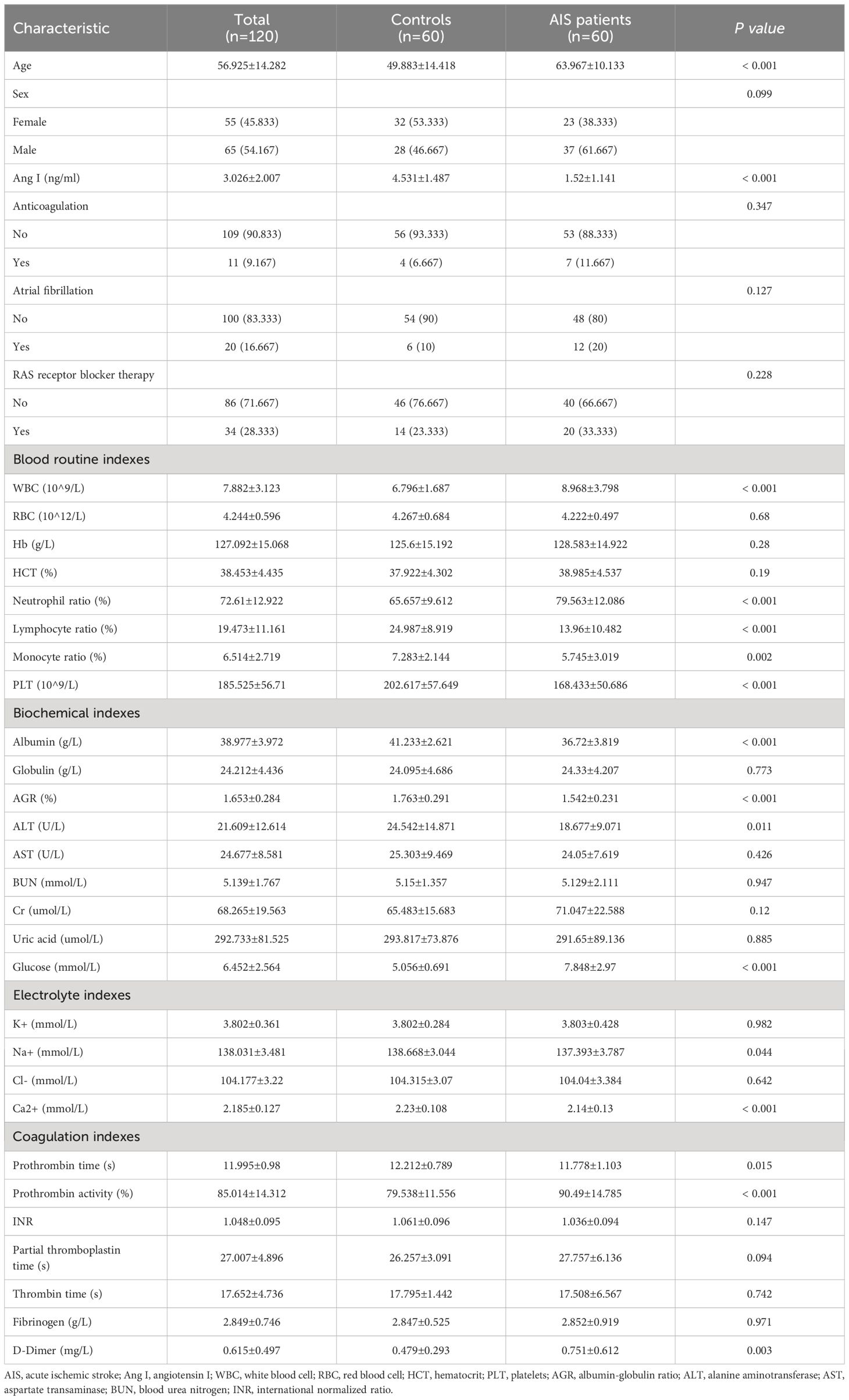
Figure 1; Table 1 show the demographic data and various clinical information data between the controls and disease groups included in the study and the levels of significance of differences between groups. The median age of 120 patients was 56.925 ± 14.282 years old. The median age of 60 controls was 49.883 ± 14.418 years old, and the median age of 60 patients was 63.967 ± 10.133 years old (p<0.001). The level of Ang I in the disease group (1.52 ± 1.141) was significantly lower than that in the control group (4.531 ± 1.487) (p<0.001). WBC, neutrophil ratio, lymphocyte ratio, monocyte ratio, PLT in blood routine indexes; albumin, albumin-globulin ratio (AGR), ALT, glucose in biochemical indexes; K+, Ca2+ in electrolyte; prothrombin time, prothrombin activity and D-dimer all had significant differences between the normal group and the disease group (p<0.05).
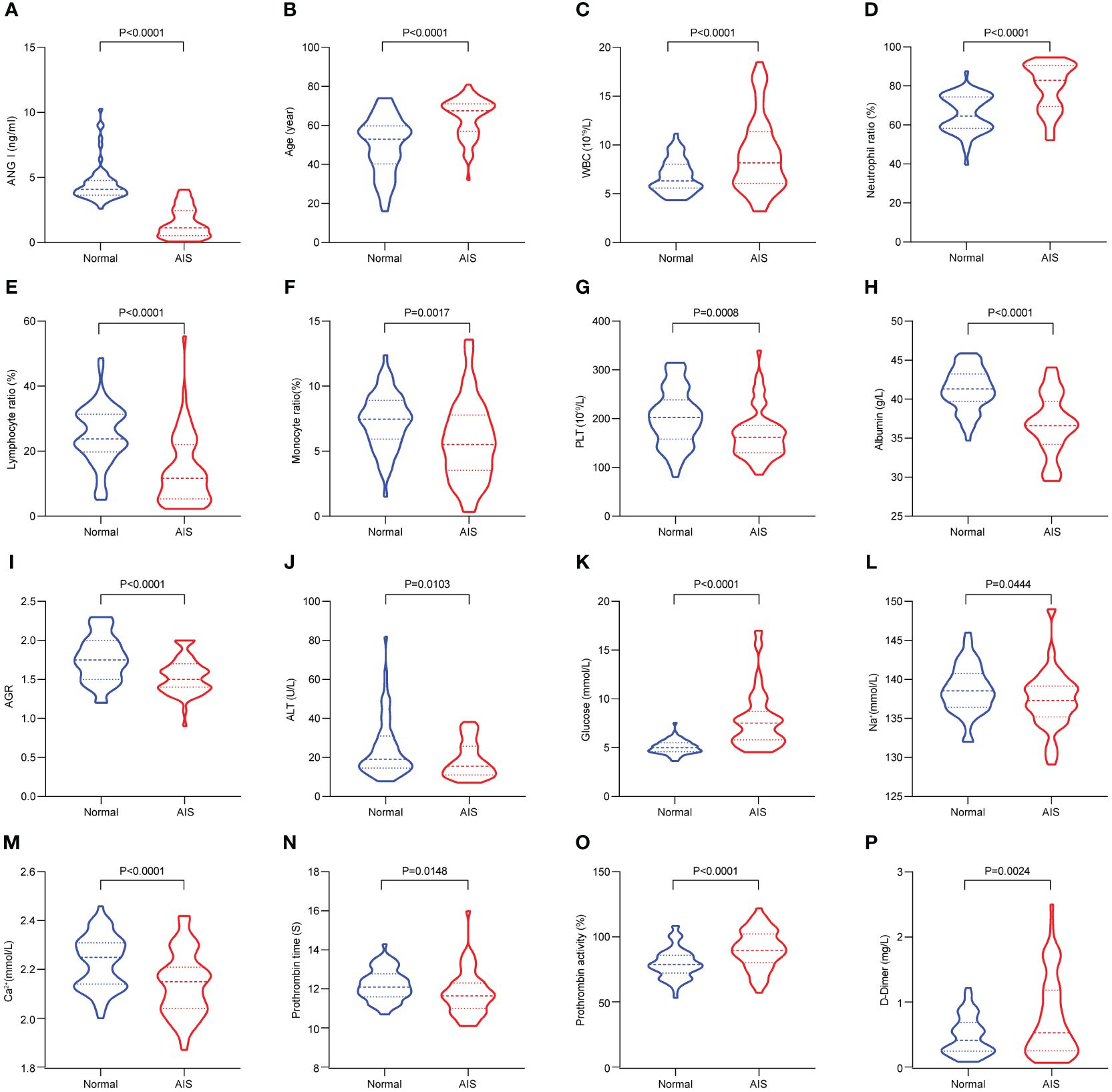
Figure 1 The significant differences in demographic data and clinical information between the controls and AIS patients. (A) Comparison of Ang I. (B) Comparison of age. (C) Comparison of WBC. (D) Comparison of neutrophil ratio. (E) Comparison of lymphocyte ratio. (F) Comparison of monocyte ratio. (G) Comparison of PLT. (H) Comparison of albumin. (I) Comparison of AGR. (J) Comparison of ALT. (K) Comparison of globulin. (L) Comparison of Na+. (M) Comparison of Ca2+. (N) Comparison of prothrombin time. (O) Comparison of prothrombin activity. (P) Comparison of D-Dimer. AIS, acute ischemic stroke; Ang I, angiotensin I; WBC, white blood cell; PLT, platelets; AGR, albumin-globulin ratio; ALT, alanine aminotransferase.
Risk factors for AIS
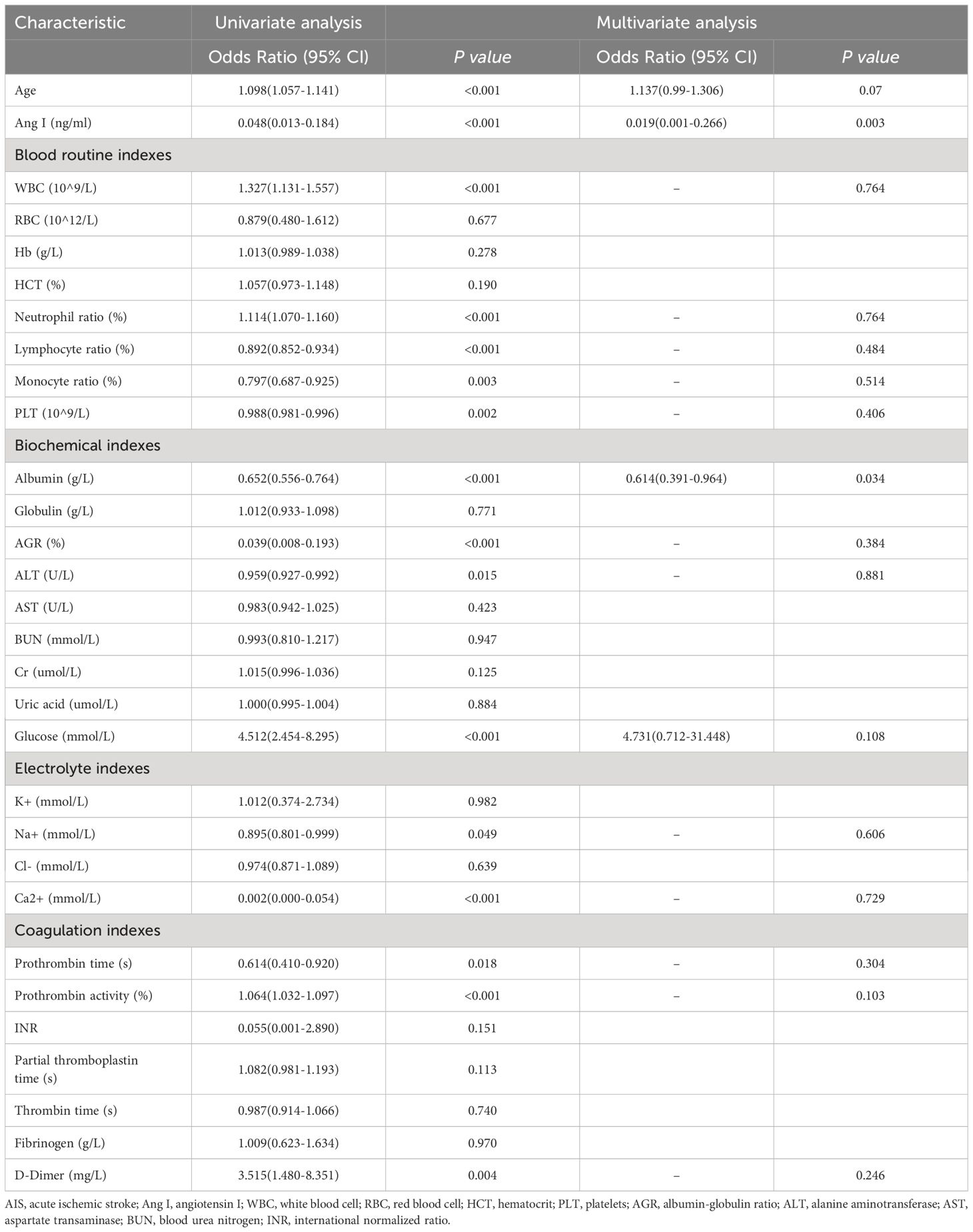
We explored the influencing factors of AIS occurrence by univariate and multivariate Logistic regression analyses (Table 2). Multivariate Logistic regression analysis showed that older age (OR: 1.098, 95%CI: 1.057–1.141, p<0.001), high leukocyte expression (OR: 1.327, 95%CI: 1.131–1.557, p<0.001), high neutrophil ratio (OR: 1.114, 95%CI: 1.070–1.160, p<0.001), hyperglycemia (OR: 4.512, 95%CI: 2.454–8.295, p<0.001), high prothrombin activity (OR: 1.064, 95%CI: 1.032–1.097, p<0.001), high D-dimer (OR: 3.515, 95%CI: 1.480–8.351, p=0.004) might be more likely to have AIS.
The higher Ang I (OR: 0.048, 95%CI: 0.013–0.184, p<0.001), the higher lymphocyte ratio (OR: 0.892, 95%CI: 0.852–0.934, p<0.001), higher monocyte ratio (OR: 0.797, 95%CI: 0.687–0.925, p=0.003), higher PLT (OR: 0.988, 95%CI: 0.981–0.996, p=0.002), and higher albumin (OR: 0.797, 95%CI: 0.687–0.925, p=0.002). 0.652, 95%CI 0.556–0.764, p<0.001), AGR (OR: 0.039, 95%CI: 0.008–0.193, p=0.049), ALT (OR: 0.959, 95%CI: 0.927–0.992, p=0.015), and Na+ (OR: 0.959, 95%CI: 0.927–0.992, p=0.015). 0.895, 95%CI = 0.801–0.999, p=0.049), and the higher Ca2+ (OR: 0.002, 95%CI = 0.000–0.054, p<0.001) and longer prothrombin time (OR<: 0.614, 95%CI: 0.410–0.920, p=0.018) might reduce the risk of AIS.
Demographic and clinical characteristics of patients with different prognosis
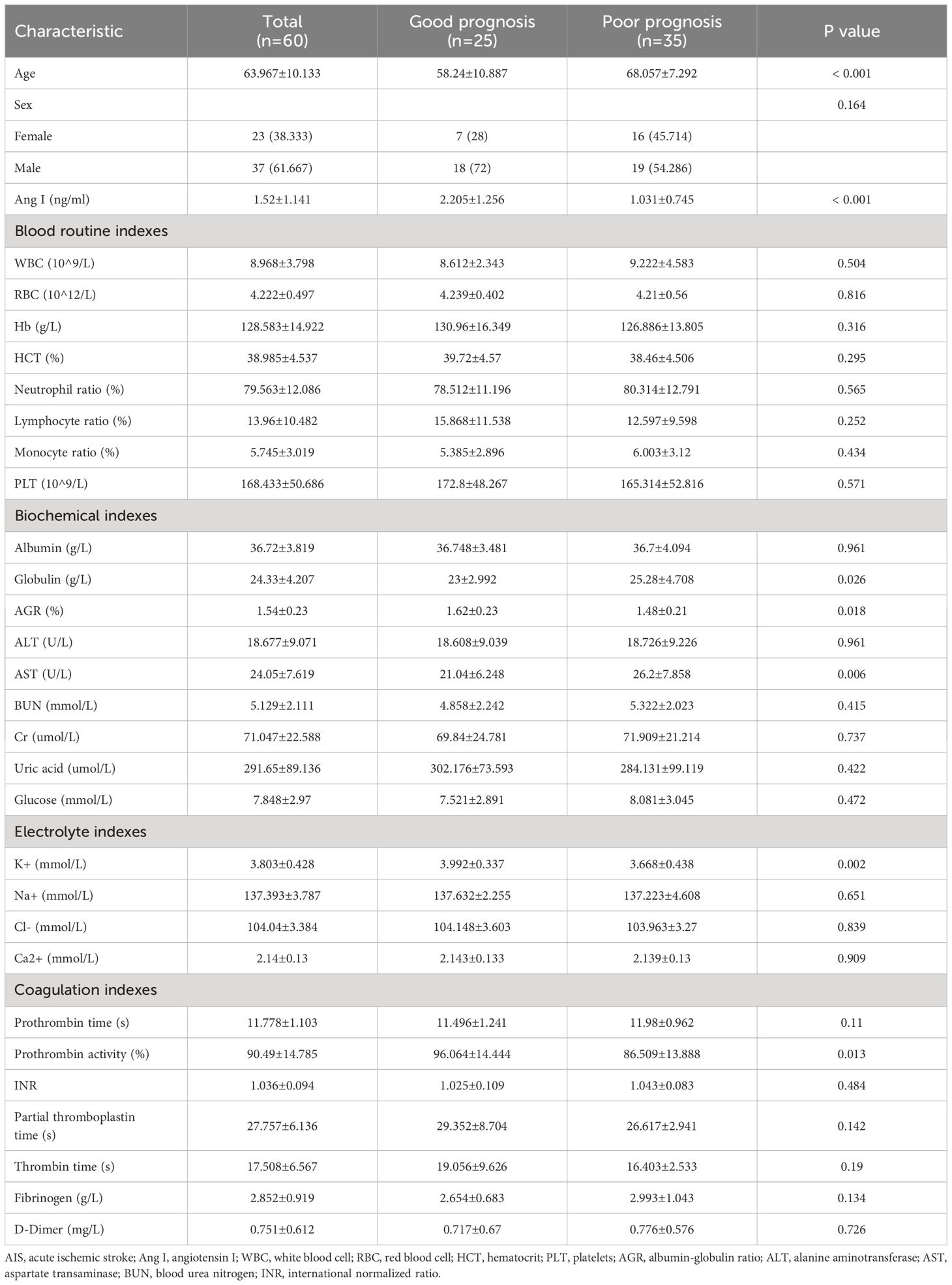
Table 3; Figure 2 show the demographic data and various clinical information data between the good and poor prognosis groups of AIS patients and the levels of significance of differences between groups. The median age of 60 patients was 63.967 ± 10.133 years old. The median age of 25 patients in the good prognosis group was 58.24 ± 10.887 years old, and the median age of 35 patients in the poor prognosis group was 68.057 ± 7.292 years old (p<0.001). The level of Ang I was significantly different between the good prognosis group (2.205 ± 1.256) and the poor prognosis group (1.031 ± 0.745) (p<0.001). Significant differences existed in the expression of globulin, AGR, ALT, K+, and prothrombin activity between the good and poor prognoses groups (p<0.05).
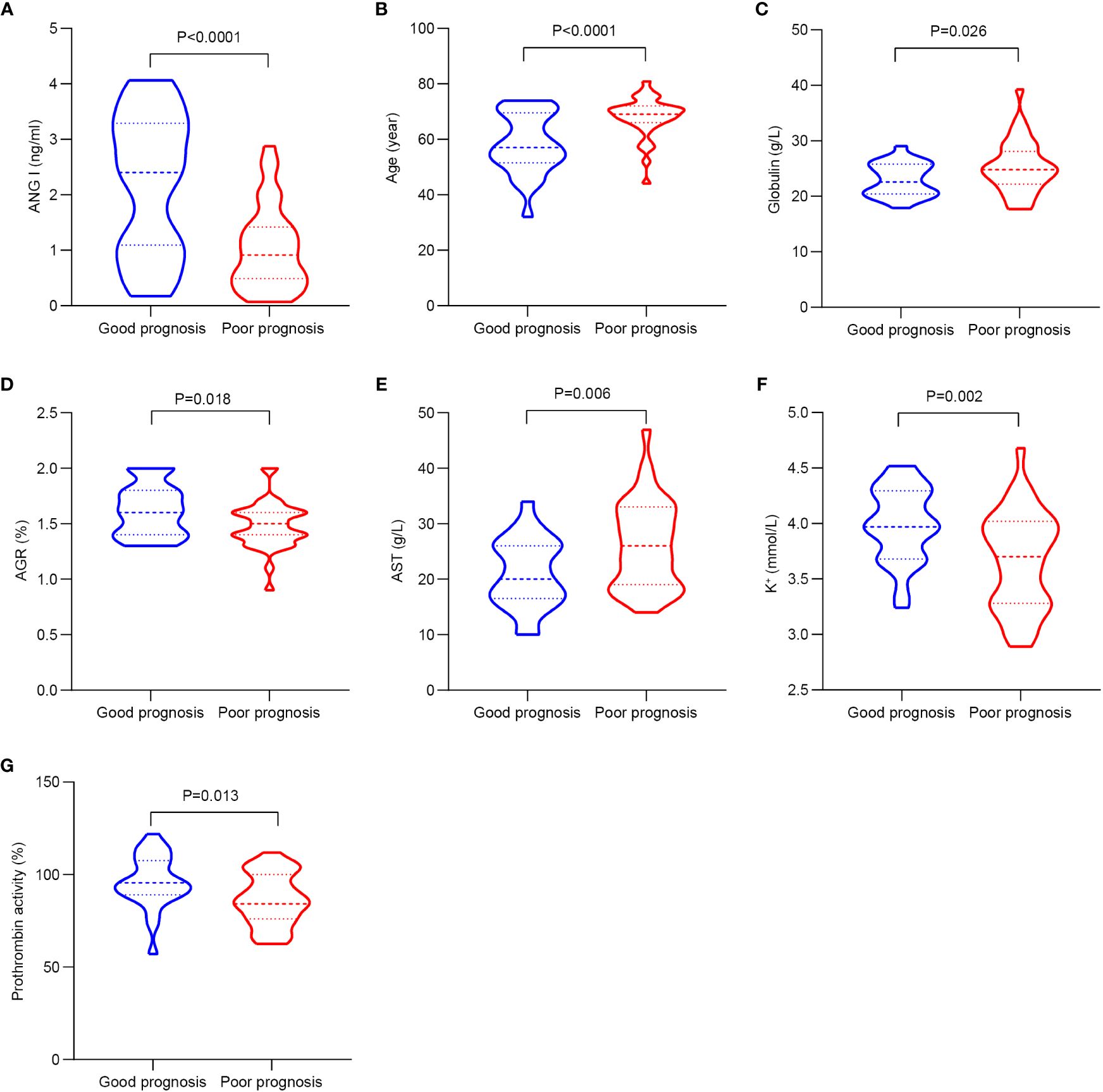
Figure 2 The significant differences in demographic data and clinical information between the good and poor prognosis groups of AIS patients. (A) Comparison of Ang I. (B) Comparison of age. (C) Comparison of globulin. (D) Comparison of AGR. (E) Comparison of AST. (F) Comparison of K+. (G) Comparison of prothrombin activity. AIS, acute ischemic stroke; Ang I, angiotensin I; AGR, albumin-globulin ratio; AST, aspartate transaminase.
Identification of prognostic factors for AIS
To further explore the clinical diagnostic predictive value of serum Ang I, blood routine indexes, biochemical indexes, electrolyte indexes, and coagulation indexes for the prognosis of AIS, the good prognosis group was used as negative samples, and the poor prognosis group was used as positive samples. The ROC curve diagnostic analysis model was established. Ang I, K+, and prothrombin activity all had high diagnostic and predictive value. The AUC of Ang I was 0.763, 95%CI was 0.633–0.894, p<0.001; The AUC of K+ in electrolyte was 0.715, 95%CI was 0.585–0.846, p=0.005; The AUC of prothrombin activity in coagulation function was 0.705, 95%CI was 0.568–0.841, p=0.007 (Table 4; Figure 3).
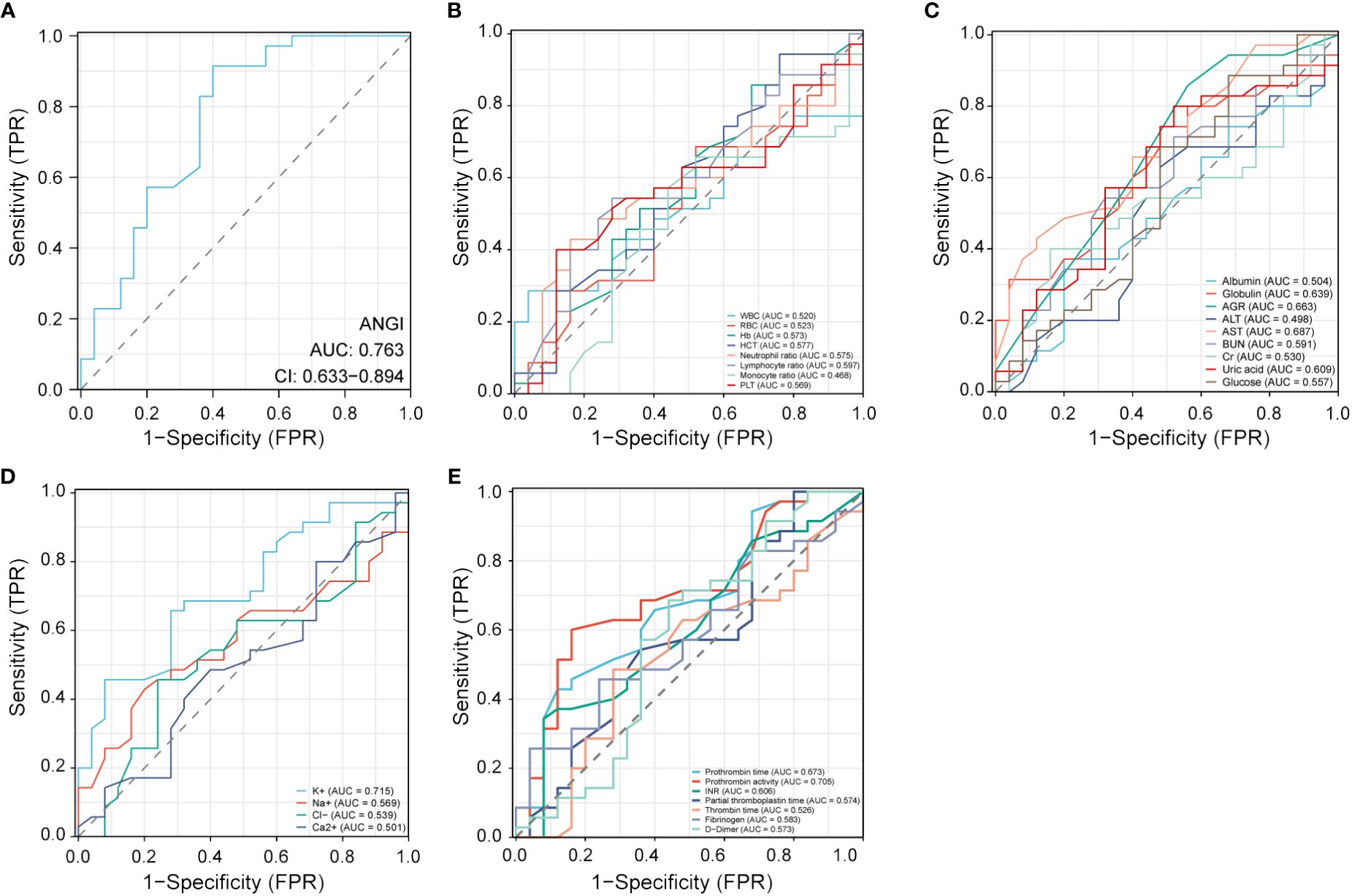
Figure 3 Receiver operating characteristic (ROC) curve analysis of different factors for the prognosis of AIS. (A) Ang I. (B) Blood routine indexes. (C) Biochemical indexes. (D) Electrolyte indexes. (E) Coagulation indexes. AIS, acute ischemic stroke; Ang I, angiotensin I; WBC, white blood cell; RBC, red blood cell; HCT, hematocrit; PLT, platelets; AGR, albumin-globulin ratio; ALT, alanine aminotransferase; AST, aspartate transaminase; BUN, blood urea nitrogen; INR, international normalized ratio.
Factors associated with the prognosis of AIS
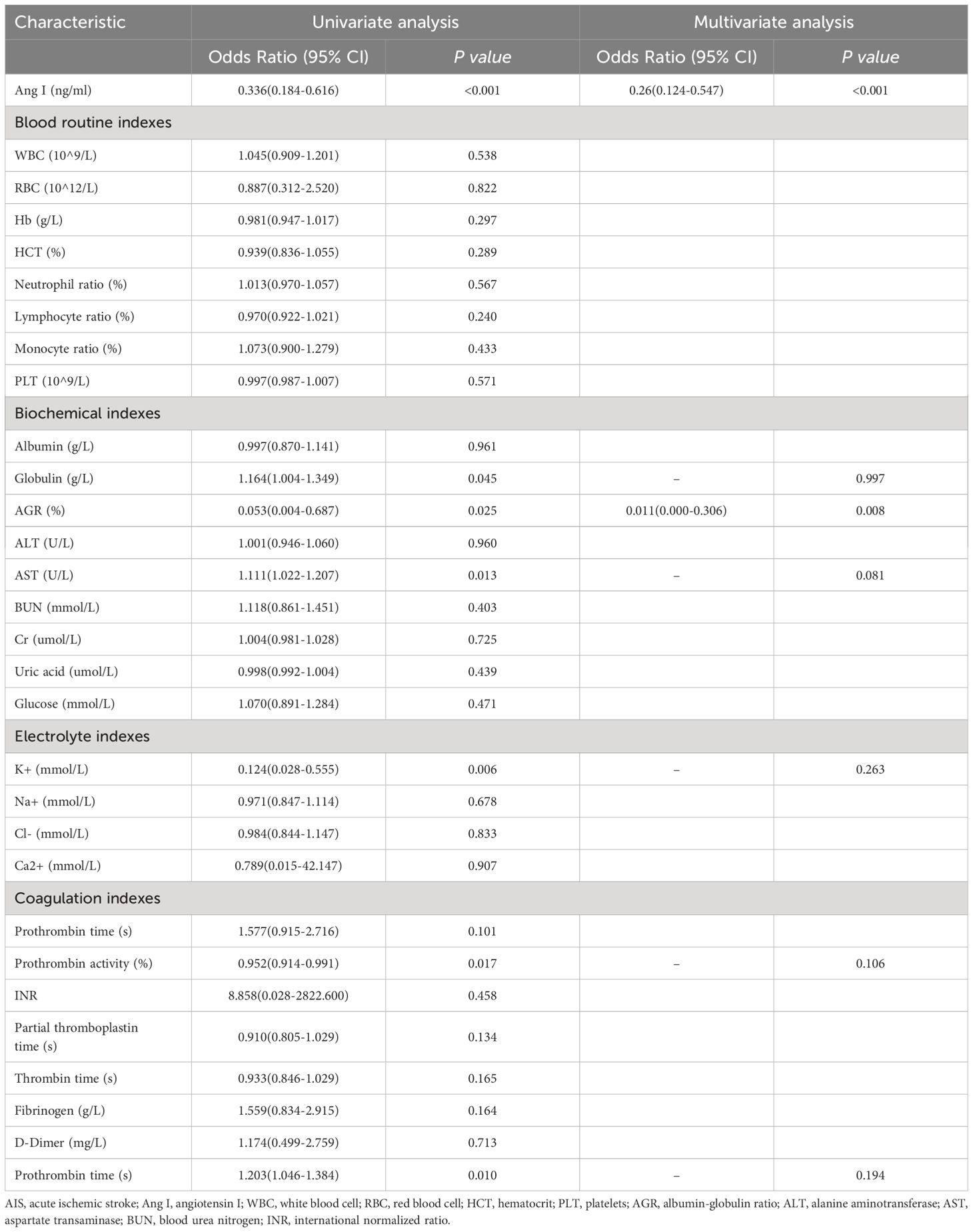
We explored the influencing factors of AIS outcome by univariate and multivariate logistic regression (Table 5). The results showed that the higher Ang I (OR: 0.336, 95%CI: 0.184–0.616, p<0.001), the higher lymphocyte ratio (OR: 0.892, 95%CI: 0.852–0.934, p<0.001), higher AGR (OR = 0.053, 95%CI: 0.004–0.687, p=0.025), higher K+ (OR = 0.124, 95%CI: 0.028–0.555, p=0.006), and higher prothrombin activity (OR = 0.124, 95%CI: 0.028–0.555, p=0.006). 0.952, 95%CI: 0.914–0.991, p=0.017) might have a better prognosis.
High expression of globulin (OR: 1.164, 95%CI: 1.004–1.349, p=0.045), increased expression of ALT (OR: 1.111, 95%CI: 1.022–1.207, p=0.013), and high admission NIHSS score (OR: 1.203, 95%CI: 1.046–1.384, p=0.010) might have a poor prognosis of AIS. Multivariate Logistic regression analysis of variables with significant differences in univariate logistic regression showed that Ang I (OR: 0.260, 95%CI: 0.124–0.547, p<0.001) and high AGR (OR: 0.011, 95%CI: 0.000–0.306, p=0.008) were still statistically significant.
Nomogram construction and validation
According to the results of multivariate logistic regression, Ang I and AGR were selected to construct a diagnostic nomogram for the prognosis of AIS, and the ROC curve was used to verify the diagnostic nomogram to test its predictive efficacy for the prognosis of patients. The AUC value of the combined model was 0.859 (95%CI: 0.765–0.954). According to the above results, it was suggested that the model had a solid predictive ability, as shown in Figure 4.
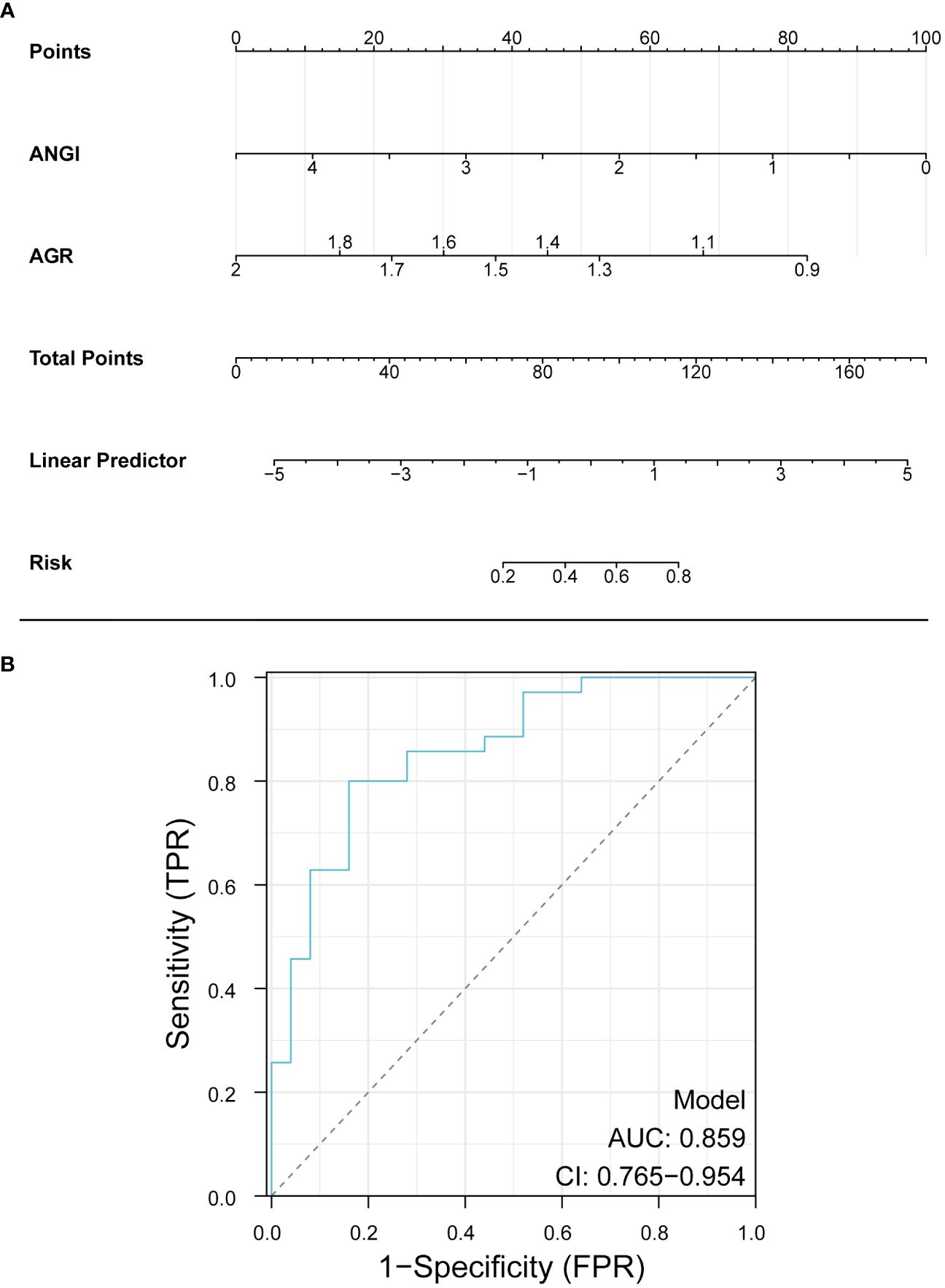
Figure 4 Nomogram for predicting the prognosis of AIS. Ang I, angiotensin I; AGR, albumin-globulin ratio.
Discussion
In this retrospective study, univariate and multivariate logistic regression were used to analyze the risk factors for AIS. Consistent with previous studies, advanced age, high blood glucose, high D-dimer, and high prothrombin activity are risk factors for acute ischemic AIS. We also found that the increase of white blood cells and the increase of neutrophil ratio in blood routine examination also have specific suggestive significance. In addition, we found that the higher Ang I was, the lower the risk of AIS was. The level of Ang I was higher in the good prognosis group. Significant differences existed in the expression of globulin, AGR, ALT, K+, and prothrombin activity between the good and the poor prognosis group. As previously mentioned, our study found that Ang I and AGR had a non-negligible impact on the prognosis of patients with AIS, so we developed a nomogram to evaluate its ability to predict the prognosis of AIS.
Risk factors for AIS include age, smoking, obesity, atrial fibrillation, hypertension, diabetes, etc. (6), of which hypertension is the most critical risk factor. Excluding other risk factors for AIS, a 10mmHg increase in systolic blood pressure was associated with a 49% increased risk of AIS, and a 5mmHg rise in diastolic blood pressure was associated with a 46% increased risk of AIS (7). Experimental results showed that the vascular shear stress was increased in hypertensive patients compared with normal people, which caused vascular endothelial damage (8). At the same time, endothelial injury also affects NO activity and increases the synthesis and release of endothelin and Ang II, resulting in vasomotor dysfunction (8). Wallace et al. found that after endothelial injury, endothelial cells will release a large number of active substances, including adenosine triphosphate, 5-hydroxytryptamine, etc., which can promote the synthesis and release of endothelin, increasing blood pressure, and then damage endothelial cells again, forming a vicious circle (9). The primary pathogenesis of AIS is as follows: The damage of vascular endothelial cells leads to atherosclerosis, which leads to occlusion of cerebral arteries and cerebral ischemia. Neurons in the ischemic center release the injury-related molecular pattern to activate the immune response (10–12).
Hyperactivation of the local renin-angiotensin-aldosterone system (RAAS) in hypertensive patients is also an important reason for vascular endothelial injury (13). RAAS is an essential regulatory system to maintain the body’s balance of water and electrolyte. Its dysfunction will lead to vasoconstriction and aggravate the degree of ischemic injury in cerebrovascular diseases. And affect the rate of vascular recanalization after treatment (14, 15). The mechanism of RAAS activation is as follows: firstly, angiotensinogen is converted into Ang I under the stimulation of renin, and then Ang I is converted into Ang II by ACE. Ang II increases aldosterone secretion through the sympathetic nervous system, resulting in water and sodium retention and increasing blood pressure (16).
Some studies have shown that the serum Ang II level of patients after intravenous thrombolysis or EVT treatment is significantly lower than that before treatment, and the serum Ang II level of patients in the EVT group is lower than that in intravenous thrombolysis group, suggesting that EVT can significantly reduce the neurohormone level of AIS patients (17–22). How does the level of Ang I change? Our study focused on the changes of Ang I.
Our study found that Ang I levels were lower in AIS patients than in controls and higher in the group with a good prognosis. One study had similar results to ours. The level of Ang I decreased while the level of Ang II remained unchanged, accompanied by an increase in ACE activity. This phenomenon can be explained by the decrease in renin caused by the negative feedback after the rise in blood pressure. Increased ACE activity can convert all available Ang I to Ang II (16). Another reason for maintaining a steady Ang II concentration may be the up-regulation of local AT1 and AT2 receptors in and around the infarct area. Animal experiments have also confirmed that RAAS promotes brain oxidative stress response, further promoting RAAS and sympathetic nervous system activation to mobilize peripheral organ responses (23).
Nomograms are highly effective in areas such as diagnosis and prediction. Compared with traditional scoring systems for IS (24, 25), the nomogram model can estimate the probability of adverse outcomes after EVT, which often provides a better-personalized assessment to help management decisions. In addition, the graph has higher accuracy and better-discriminating ability and is more convenient to use. Several studies have predicted poor outcomes in patients with IS. Zhang et al. established a nomogram model to predict the 3-month risk of death in IS patients with anterior circulation arterial occlusion who successfully received endovascular thrombolysis, composed of age, pretreatment collateral status, baseline blood glucose level, symptomatic intracranial hemorrhage, and baseline National Institutes of Health Stroke Scale score (26). Du et al. determined that age, baseline National Institutes of Health Stroke Scale score, collateral circulation, rapid blood glucose levels, and recirculation were independent predictors of malignant cerebral edema after EVT (27). A study from Japan also focused on whether imaging technology, low relative diffusion-weighted imaging (DWI) signal intensity, can predict good clinical outcomes after EVT in patients with acute IS. Forty-nine patients were included in the analysis, and the results showed that the relative DWI signal intensity of the group with a good prognosis was significantly lower than that of the group with a poor prognosis. Low relative DWI signal intensity was associated with a good prognosis after EVT (28). In this study, Ang I and AGR, two readily available variables, have good discriminative ability, and the AUC value of the combined ROC model is 0.859. Therefore, the combined Ang I and AGR model can predict the prognosis of AIS patients, thus achieving a more accurate therapeutic effect and better serving the patient population.
The small sample size limits our study, and the sample size will be expanded. This is a retrospective single-center study, and the results must be further prospectively verified in multiple centers. Our study has established the role of Ang I in the prognosis of patients undergoing EVT. Still, there was no study of the other components, including angiotensinogen and Ang II, so further studies are needed to explore the mechanisms and other parts of the RAAS system.
Conclusion
The study’s findings suggested advanced age, high blood glucose, high D-dimer, and high prothrombin activity are risk factors in patients with AIS. The higher the ANG I and AGR, the better the prognosis of AIS surgery. Furthermore, the combined ANG I and AGR model has a good predictive ability for the prognosis of patients undergoing arterial thrombectomy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The institutional review board of the First People’s Hospital of Lianyungang. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SY: Conceptualization, Data curation, Formal Analysis, Writing – original draft. KL: Methodology, Software, Visualization, Writing – review & editing. ZH: Validation, Writing – review & editing. YX: Validation, Writing – review & editing. JL: Validation, Writing – review & editing. YS: Funding acquisition, Investigation, Resources, Supervision, Writing – review & editing. AL: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by [Multi-factor analysis of cognitive dysfunction in elderly patients with ischemic stroke after thrombectomy] grant number [L202301].
Acknowledgments
The authors thank the participants in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
RAS, renin-angiotensin system; AIS, acute ischemic stroke; Ang I, angiotensin I; EVT, endovascular thrombectomy; ROC, receiver operating characteristic curves; AGR, albumin-globulin ratio; IS, ischemic stroke; Ang I, angiotensin I; DSA, digital subtraction angiography; AUC, area under the curve; RAAS, renin-angiotensin-aldosterone system.
References
1. Gravbrot N, McDougall R, Aguilar-Salinas P, Avila MJ, Burket AR, Dumont TM. National trends in endovascular thrombectomy and decompressive craniectomy for acute ischemic stroke: A study using national inpatient sample data from 2006 to 2016. J Clin Neurosci. (2022) 101:234–8. doi: 10.1016/j.jocn.2022.04.027
2. Holappa M, Vapaatalo H, Vaajanen A. Many faces of renin-angiotensin system - focus on eye. Open Ophthalmol J. (2017) 11:122–42. doi: 10.2174/1874364101711010122
3. Marko M, Posekany A, Szabo S, Scharer S, Kiechl S, Knoflach M, et al. Trends of R-tpa (Recombinant tissue-type plasminogen activator) treatment and treatment-influencing factors in acute ischemic stroke. Stroke. (2020) 51:1240–7. doi: 10.1161/strokeaha.119.027921
4. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2018) 49:e46–e110. doi: 10.1161/str.0000000000000158
5. Jafari M, Katlowitz K, de la Garza C, Sellers A, Moore S, Hall H, et al. Impact of systemic inflammatory response syndrome on acute ischemic stroke patients treated with mechanical thrombectomy. J Neurol Sci. (2021) 430:119988. doi: 10.1016/j.jns.2021.119988
6. Alloubani A, Saleh A, Abdelhafiz I. Hypertension and diabetes mellitus as a predictive risk factors for stroke. Diabetes Metab Syndr. (2018) 12:577–84. doi: 10.1016/j.dsx.2018.03.009
7. Kannel WB, Wolf PA, Verter J, McNamara PM. Epidemiologic assessment of the role of blood pressure in stroke: the framingham study. JAMA. (1996) 276:1269–78.
8. Li WJ, Liu Y, Wang JJ, Zhang YL, Lai S, Xia YL, et al. "Angiotensin ii memory" Contributes to the development of hypertension and vascular injury via activation of nadph oxidase. Life Sci. (2016) 149:18–24. doi: 10.1016/j.lfs.2016.02.037
9. Wallace SM, Yasmin, McEniery CM, Mäki-Petäjä KM, Booth AD, Cockcroft JR, et al. Isolated systolic hypertension is characterized by increased aortic stiffness and endothelial dysfunction. Hypertension. (2007) 50:228–33. doi: 10.1161/hypertensionaha.107.089391
10. Hurford R, Sekhar A, Hughes TAT, Muir KW. Diagnosis and management of acute ischaemic stroke. Pract Neurol. (2020) 20:304–16. doi: 10.1136/practneurol-2020–002557
11. Zhao Y, Zhang X, Chen X, Wei Y. Neuronal injuries in cerebral infarction and ischemic stroke: from mechanisms to treatment (Review). Int J Mol Med. (2022) 49. doi: 10.3892/ijmm.2021.5070
12. Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A. Neuroinflammatory mechanisms in ischemic stroke: focus on cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci. (2020) 21(18):6454. doi: 10.3390/ijms21186454
13. Montezano AC, Nguyen Dinh Cat A, Rios FJ, Touyz RM. Angiotensin ii and vascular injury. Curr Hypertens Rep. (2014) 16:431. doi: 10.1007/s11906–014-0431–2
14. Ames MK, Atkins CE, Pitt B. The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med. (2019) 33:363–82. doi: 10.1111/jvim.15454
15. McFall A, Nicklin SA, Work LM. The counter regulatory axis of the renin angiotensin system in the brain and ischaemic stroke: insight from preclinical stroke studies and therapeutic potential. Cell Signal. (2020) 76:109809. doi: 10.1016/j.cellsig.2020.109809
16. Back C, Thiesen KL, Skovgaard K, Edvinsson L, Jensen LT, Larsen VA, et al. Raas and stress markers in acute ischemic stroke: preliminary findings. Acta Neurol Scand. (2015) 131:132–9. doi: 10.1111/ane.12298
17. Ahmetović H, Jerković A, Vidaković MR, Košta V. The comparison of mechanical thrombectomy and symptomatic therapy on early outcome of acute ischemic stroke in patients older than 80 years: A retrospective cohort study. Clin Neurol Neurosurg. (2022) 221:107378. doi: 10.1016/j.clineuro.2022.107378
18. Collette SL, Bokkers RPH, Mazuri A, Lycklama À Nijeholt GJ, van Oostenbrugge RJ, LeCouffe NE, et al. Intra-arterial thrombolytics during endovascular thrombectomy for acute ischaemic stroke in the mr clean registry. Stroke Vasc Neurol. (2023) 8:17–25. doi: 10.1136/svn-2022–001677
19. Pan J, Wu H, Wu T, Geng Y, Yuan R. Association between post-procedure cerebral blood flow velocity and severity of brain edema in acute ischemic stroke with early endovascular therapy. Front Neurol. (2022) 13:906377. doi: 10.3389/fneur.2022.906377
20. Weller JM, Dorn F, Meissner JN, Stösser S, Beckonert NM, Nordsiek J, et al. Endovascular thrombectomy in young patients with stroke. Int J Stroke. (2023) 18:453–61. doi: 10.1177/17474930221119602
21. Tokunaga K, Tokunaga S, Hara K, Yasaka M, Okada Y, Kitazono T, et al. Fluid-attenuated inversion recovery vascular hyperintensity-diffusion-weighted imaging mismatch and functional outcome after endovascular reperfusion therapy for acute ischemic stroke. Interv Neuroradiol. (2022) 30(2):189–94. doi: 10.1177/15910199221113900
22. Zhong W, Chen Z, Yan S, Zhou Y, Zhang R, Luo Z, et al. Multi-mode imaging scale for endovascular therapy in patients with acute ischemic stroke (Meta). Brain Sci. (2022) 12(7):821. doi: 10.3390/brainsci12070821
23. Takahashi H, Yoshika M, Komiyama Y, Nishimura M. The central mechanism underlying hypertension: A review of the roles of sodium ions, epithelial sodium channels, the renin-angiotensin-aldosterone system, oxidative stress and endogenous digitalis in the brain. Hypertens Res. (2011) 34:1147–60. doi: 10.1038/hr.2011.105
24. Ong CJ, Gluckstein J, Laurido-Soto O, Yan Y, Dhar R, Lee JM. Enhanced detection of edema in Malignant anterior circulation stroke (Edema) score: A risk prediction tool. Stroke. (2017) 48:1969–72. doi: 10.1161/strokeaha.117.016733
25. Battey TW, Karki M, Singhal AB, Wu O, Sadaghiani S, Campbell BC, et al. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke. (2014) 45:3643–8. doi: 10.1161/strokeaha.114.006884
26. Zhang X, Yuan K, Wang H, Gong P, Jiang T, Xie Y, et al. Nomogram to predict mortality of endovascular thrombectomy for ischemic stroke despite successful recanalization. J Am Heart Assoc. (2020) 9:e014899. doi: 10.1161/jaha.119.014899
27. Du M, Huang X, Li S, Xu L, Yan B, Zhang Y, et al. A nomogram model to predict Malignant cerebral edema in ischemic stroke patients treated with endovascular thrombectomy: an observational study. Neuropsychiatr Dis Treat. (2020) 16:2913–20. doi: 10.2147/ndt.S279303
28. Kishi F, Nakagawa I, Park H, Kotsugi M, Myouchin K, Motoyama Y, et al. Low relative diffusion weighted image signal intensity can predict good prognosis after endovascular thrombectomy in patients with acute ischemic stroke. J neurointerv Surg. (2022) 14:618–22. doi: 10.1136/neurintsurg-2021–017583
Keywords: acute ischemic stroke, risk factors, endovascular thrombectomy, angiotensin I, nomograms
Citation: Yang S, Li K, Huang Z, Xu Y, Liang J, Sun Y and Li A (2024) Risk factors of acute ischemic stroke and the role of angiotensin I in predicting prognosis of patients undergoing endovascular thrombectomy. Front. Endocrinol. 15:1388871. doi: 10.3389/fendo.2024.1388871
Received: 20 February 2024; Accepted: 29 May 2024;
Published: 11 June 2024.
Edited by:
Alexandre Benani, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Omer Iqbal, Loyola University Chicago, United StatesVladimir Tesar, Charles University, Czechia
Copyright © 2024 Yang, Li, Huang, Xu, Liang, Sun and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aimin Li, bGlhaW1pbl82NTI5QDEyNi5jb20=; Yong Sun, c3VueW9uZ0Buam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Shengkai Yang
Shengkai Yang Kemian Li1,2†
Kemian Li1,2† Jingshan Liang
Jingshan Liang Aimin Li
Aimin Li