- 1Department of Cardiology, First Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong, China
- 2Clinical Research Center, First Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong, China
- 3Shantou University Medical College, Shantou, Guangdong, China
Background: Thyroid hormones significantly influence cardiovascular pathophysiology, yet their prognostic role in acute aortic dissection (AAD) remains inadequately explored. This study assesses the prognostic value of thyroid hormone levels in AAD, focusing on the mediating roles of renal function and coagulation.
Methods: We included 964 AAD patients in this retrospective cohort study. Utilizing logistic regression, restricted cubic splines, and causal mediation analysis, we investigated the association between thyroid hormones and in-hospital mortality and major adverse cardiovascular events (MACEs).
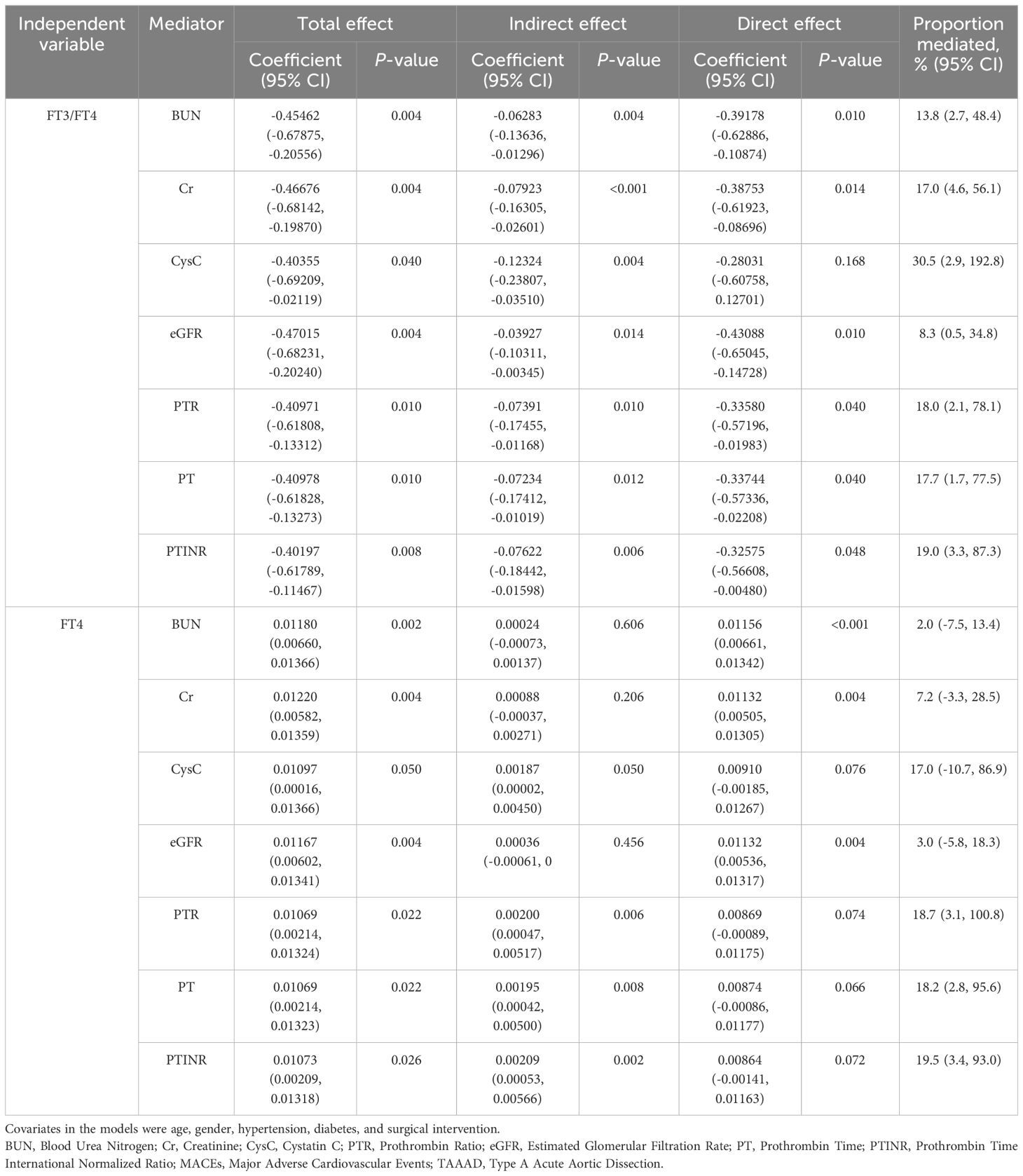
Results: In AAD patients overall, an increase of one standard deviation in FT4 levels was associated with a 31.9% increased risk of MACEs (OR 1.319; 95% CI 1.098–1.584) and a 36.1% increase in in-hospital mortality (OR 1.361; 95% CI 1.095–1.690). Conversely, a higher FT3/FT4 ratio was correlated with a 20.2% reduction in risk of MACEs (OR 0.798; 95% CI 0.637–0.999). This correlation was statistically significant predominantly in Type A AAD, while it did not hold statistical significance in Type B AAD. Key renal and coagulation biomarkers, including blood urea nitrogen, creatinine, cystatin C, prothrombin time ratio, prothrombin time, and prothrombin time international normalized ratio, were identified as significant mediators in the interplay between thyroid hormones and MACEs. The FT3/FT4 ratio exerted its prognostic influence primarily through the mediation of renal functions and coagulation, while FT4 levels predominantly impacted outcomes via a partial mediation effect on coagulation.
Conclusion: FT4 levels and the FT3/FT4 ratio are crucial prognostic biomarkers in AAD patients. Renal function and coagulation mediate the association between the thyroid hormones and MACEs.
1 Introduction
Acute aortic dissection (AAD) is a life-threatening cardiovascular condition with a prognosis influenced by various factors, including the patient’s overall health status, disease severity, and chosen treatment modalities. Despite advancements in understanding these contributory factors, mortality rates in AAD patients remain alarmingly high (1, 2). Therefore, identifying novel prognostic indicators and comprehensively understanding their roles in adverse outcomes are crucial.
Thyroid hormones are recognized as metabolic regulators with intricate interactions within the cardiovascular system. These interactions manifest through various mechanisms, including dyslipidemia, endothelial dysfunction, alterations in blood pressure, and direct effects on myocardial tissue (3, 4). Evidence has shown that thyroid hormones are associated with adverse prognostic outcomes in cardiovascular patients (5–9). Among the thyroid hormones, free triiodothyronine (FT3) and free thyroxine (FT4) are pivotal in influencing both physiological and pathological cardiovascular processes. The FT3/FT4 ratio, a marker for thyroid hormone metabolism and conversion from T4 to T3, has demonstrated prognostic utility in various cardiovascular diseases (10–12). Previous small-scale studies have explored the effects of T3 or T4 on postoperative acute kidney injury (13) and postoperative delirium (14), as well as the relationship between high-thyroid stimulating hormone (TSH) subclinical hypothyroidism and postoperative mortality in AAD patients (15). However, a comprehensive examination of the prognostic significance of the FT3/FT4 ratio in AAD patients remains limited.
Renal function and coagulation are critical determinants of cardiovascular disease prognosis. Impaired renal function is associated with increased mortality and adverse cardiovascular events (16, 17), while coagulation abnormalities can lead to thromboembolic events and worsen cardiovascular outcomes (18–20). Given the known interactions between thyroid function, renal function (21–24), and coagulation (25–27), we hypothesize that thyroid function may influence AAD prognosis through its effects on renal function and coagulation, which serve as mediating factors in this relationship.
The primary objective of this study is to elucidate the relationship between thyroid hormones and adverse outcomes in AAD patients, while also assessing the mediating roles of renal function and coagulation, to offer insight into the potential biological mechanisms of AAD.
2 Method
2.1 Study population
The study cohort comprised 1,305 patients diagnosed with AAD at the First Affiliated Hospital of Shantou University Medical College between 2015 and 2022, and only the initial admission of each patient was considered. Serum FT3 and FT4 levels were evaluated upon admission. Exclusion criteria encompassed missing FT3, FT4, or computed tomography angiography data, prior hyperthyroidism, pregnancy, Marfan syndrome, concurrent malignant tumor, or being underage. Following these criteria, a total of 964 patients were enrolled for this investigation (Figure 1). This retrospective study conforms to the stipulations of the Declaration of Helsinki, and received approval from the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College.
2.2 Data collection and definitions
Data were retrospectively extracted from electronic medical records including demographic variables (e.g., age and gender) and pertinent clinical history variables, including hypertension, diabetes mellitus, atrial fibrillation, coronary artery disease, and smoking status. Laboratory tests included thyroid function tests (FT3, FT4, and TSH); renal function tests, such as blood urea nitrogen (BUN), creatinine (Cr), and cystatin C (CysC); and a lipid profile encompassing low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), and triglycerides (TRIG). Additionally, serum albumin, coagulation markers, and a complete blood count were assessed. We also calculated estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (28, 29).
Due to the critical condition of many patients with AAD, anthropometric measurements, such as height and weight, were often unattainable. Consequently, owing to the elevated proportion of missing data, body mass index (BMI) was not included as a variable. Instead, the controlling nutritional status (CONUT) score, a validated instrument for nutritional risk assessment, was utilized as a covariate. The CONUT score is a validated tool for screening and identifying malnutrition in hospitalized patients, calculated based on serum albumin levels, total cholesterol, and total lymphocyte count (30, 31).
Detailed records were maintained for all therapeutic interventions, including surgical procedures, thoracic endovascular aortic repair (TEVAR), and pharmacological treatments. The pharmacological regimens included β-adrenergic blockers, angiotensin-converting enzyme inhibitors (ACEIs), calcium channel blockers (CCBs), and statins. Based on computed tomography angiography results, patients were categorized as either Type A acute aortic dissection (TAAAD) or Type B acute aortic dissection (TBAAD).
2.3 Endpoints
The primary endpoints of this study were in-hospital mortality and major adverse cardiovascular events (MACEs). MACEs were defined as a composite of acute myocardial infarction, repeat revascularization, heart failure, stroke, vascular reconstruction (primarily coronary artery bypass grafting and percutaneous coronary intervention), and all-cause mortality.
2.4 Statistical analysis
For continuous variables, those with normal or near-normal distributions are represented as mean (standard deviation, SD), while those with skewed distributions are described using the median (interquartile range, IQR). Categorical variables are denoted as numbers (percentages, %). The chi-squared test was utilized for categorical variables, ANOVA for normally distributed continuous variables, and the Kruskal-Wallis test for skewed continuous variables.
Non-linear associations between FT3, FT4, or the FT3/FT4 ratio and in-hospital mortality, as well as MACEs, were assessed using restricted cubic splines (RCS). Furthermore, we standardized (Z-score) the FT3, FT4, and FT3/FT4 ratio, then included them in the univariate and multivariate logistic analyses to investigate the odds ratios (OR) and 95% confidence intervals (CI) for each SD increase. Model 1 adjusted for age, gender, AAD type, hypertension, diabetes, surgical intervention, and TEVAR. Model 2 incorporated additional variables such as smoking, use of CCBs, and β-blockers, and Model 3 further adjusted for TSH, CKMB, WBC, AST, and CONUT score. Subgroup analyses were stratified according to the type of aortic dissection (TAAAD or TBAAD). Additionally, causal mediation analysis was used to probe the mediating effects of BUN, Cr, CysC, eGFR, prothrombin time (PT), prothrombin time ratio (PTR), and prothrombin time international normalized ratio (PTINR) on the FT3/FT4 ratio-MACEs association, as well as their mediating effects on the FT4-MACEs association, employing the ‘mediation’ and ‘bruceR’ packages in R.
All analyses were performed using R (version 4.3.0), and a p-value of <0.05 was considered statistically significant.
3 Results
3.1 Participant characteristics
Patient demographics and clinical characteristics are listed in Table 1. Of the 964 patients with acute aortic dissection (AAD) enrolled, 478 were diagnosed with TAAAD and 486 with TBAAD. The average age of the cohort was 58 years, with a noticeable male preponderance. When examining medical histories, hypertension was prominently present in 76% of the cohort. There were no statistically significant differences between the TAAAD and TBAAD subgroups in relation to hypertension, diabetes mellitus, atrial fibrillation, or coronary artery disease. However, therapeutic interventions showed a significant disparity between the TBAAD and TAAAD subgroups, particularly regarding the incidence of surgical or endovascular stent-graft procedures. Medication use was also markedly elevated in the TBAAD subgroup. Distinct differences between the subgroups emerged in various laboratory indices. A significant difference in smoking prevalence was observed between the TBAAD and TAAAD groups, as well.
3.2 Thyroid hormone levels, MACEs and in-hospital mortality in AAD patients
The relationship between thyroid hormone levels and the incidence of MACEs as well as in-hospital mortality in patients with AAD was assessed. Initially, unadjusted RCS were employed for visual representation of these associations. As illustrated in Figure 2, a progressive escalation in the incidence rates of both MACEs and in-hospital mortality was discernible with incremental elevations in serum FT4 concentrations. In contrast, higher serum FT3 levels showed an inverse association. Notably, the FT3/FT4 ratio displayed an S-shaped non-linear correlation with adverse prognostic outcomes in AAD patients. The curve was characterized by a more gradual change at its extremities, contrasting with the significant decline observed in the mid-range, especially between the 0.25 and 0.45 values.
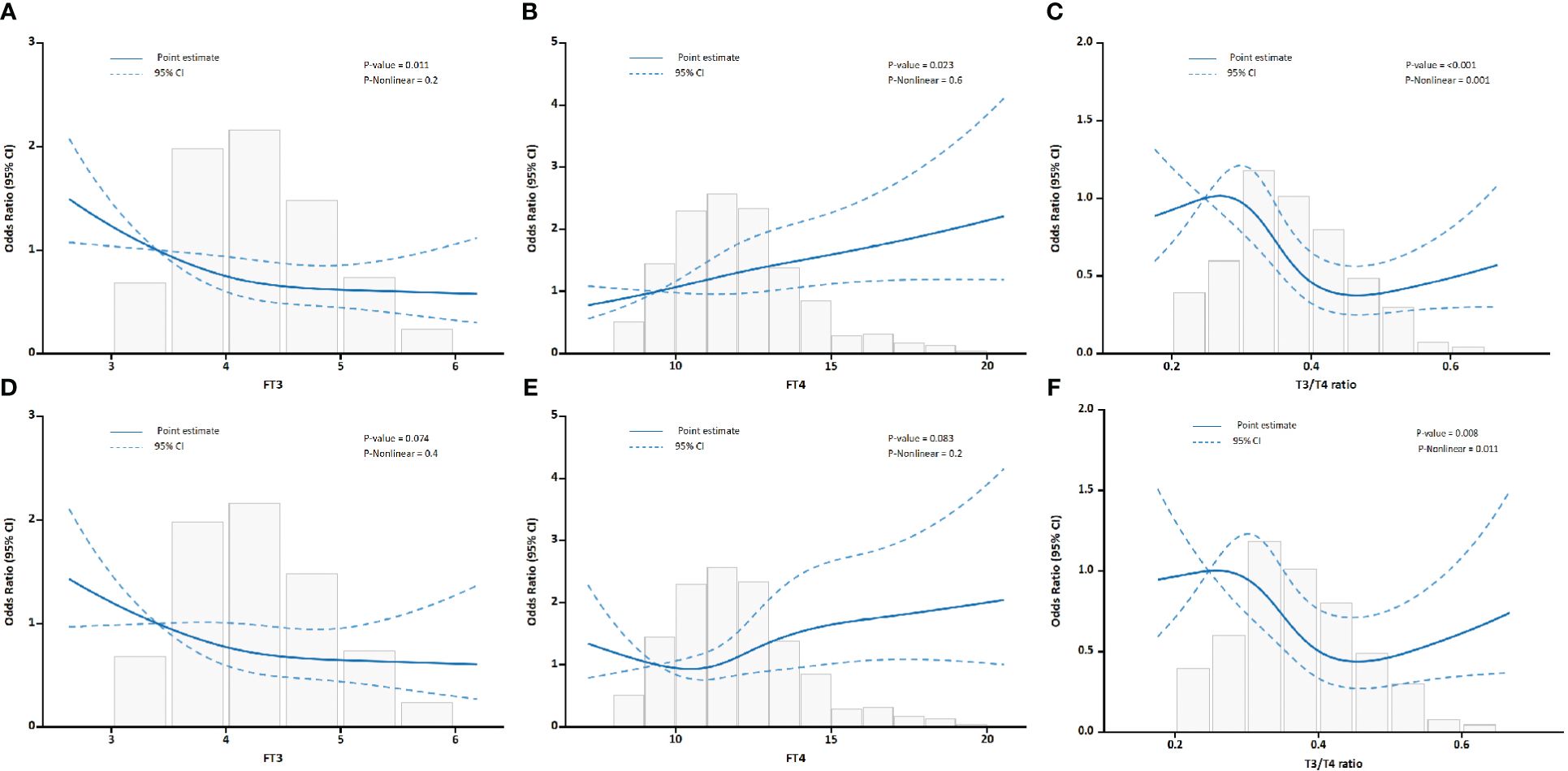
Figure 2 Association between FT3, FT4, and the FT3/FT4 ratio with outcomes in all AAD patients. (A) Association of FT3 with MACEs. (B) Association of FT4 with MACEs. (C) Association of the FT3/FT4 ratio with MACEs. (D) Association of FT3 with in-hospital mortality. (E) Association of FT4 with in-hospital mortality. (F) Association of the FT3/FT4 ratio with in-hospital mortality.
After adjusting for confounding variables, as shown in Table 2, each SD increase in the FT3/FT4 ratio corresponded to a 20.2% decrease in the risk of MACEs (OR, 0.798; 95% CI, 0.637–0.999). Conversely, each SD increase in FT4 level was associated with a 31.9% increased risk for MACEs (OR 1.319; 95% CI, 1.098–1.584). The association between FT3 and MACEs became insignificant after further multivariable adjustments. This implies that the FT3/FT4 ratio may be a more accurate representation of thyroid hormone metabolic variations linked to MACEs in AAD patients than FT3 or FT4 alone. For in-hospital mortality, both FT3 and the FT3/FT4 ratio showed inverse relationships in univariate analyses. However, these associations were not significant in multivariable models. FT4 consistently correlated positively with in-hospital mortality after adjusting for all confounding variables.
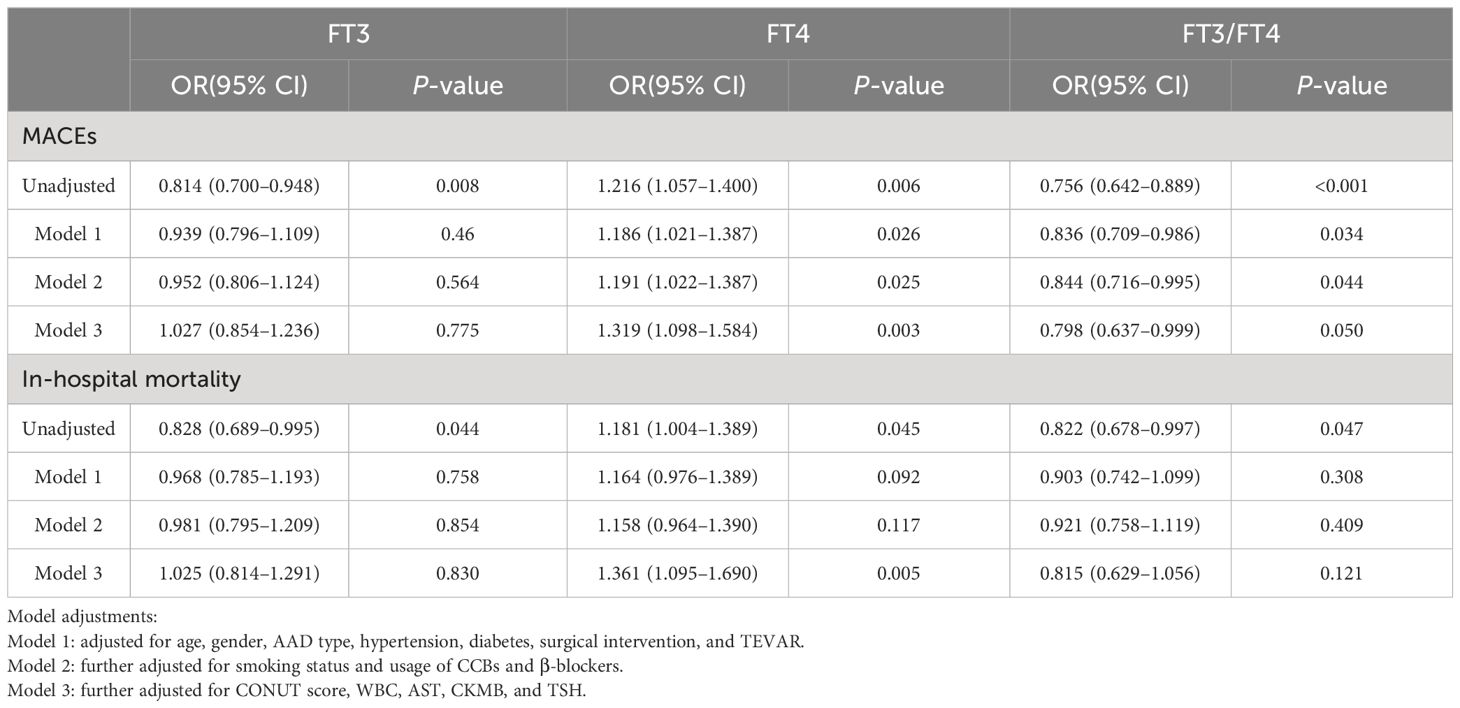
Table 2 Odds ratios for MACEs and in-hospital mortality associated with FT3, FT4, and FT3/FT4 levels (per SD increase) in AAD patients.
3.3 Interplay between thyroid hormone levels and subtypes of AAD: a stratified analysis based on Stanford classification
Patients were categorized into Stanford Type A and Type B subgroups, with separate analyses conducted for each. The pertinent statistical outcomes are presented in Tables 3 and 4.
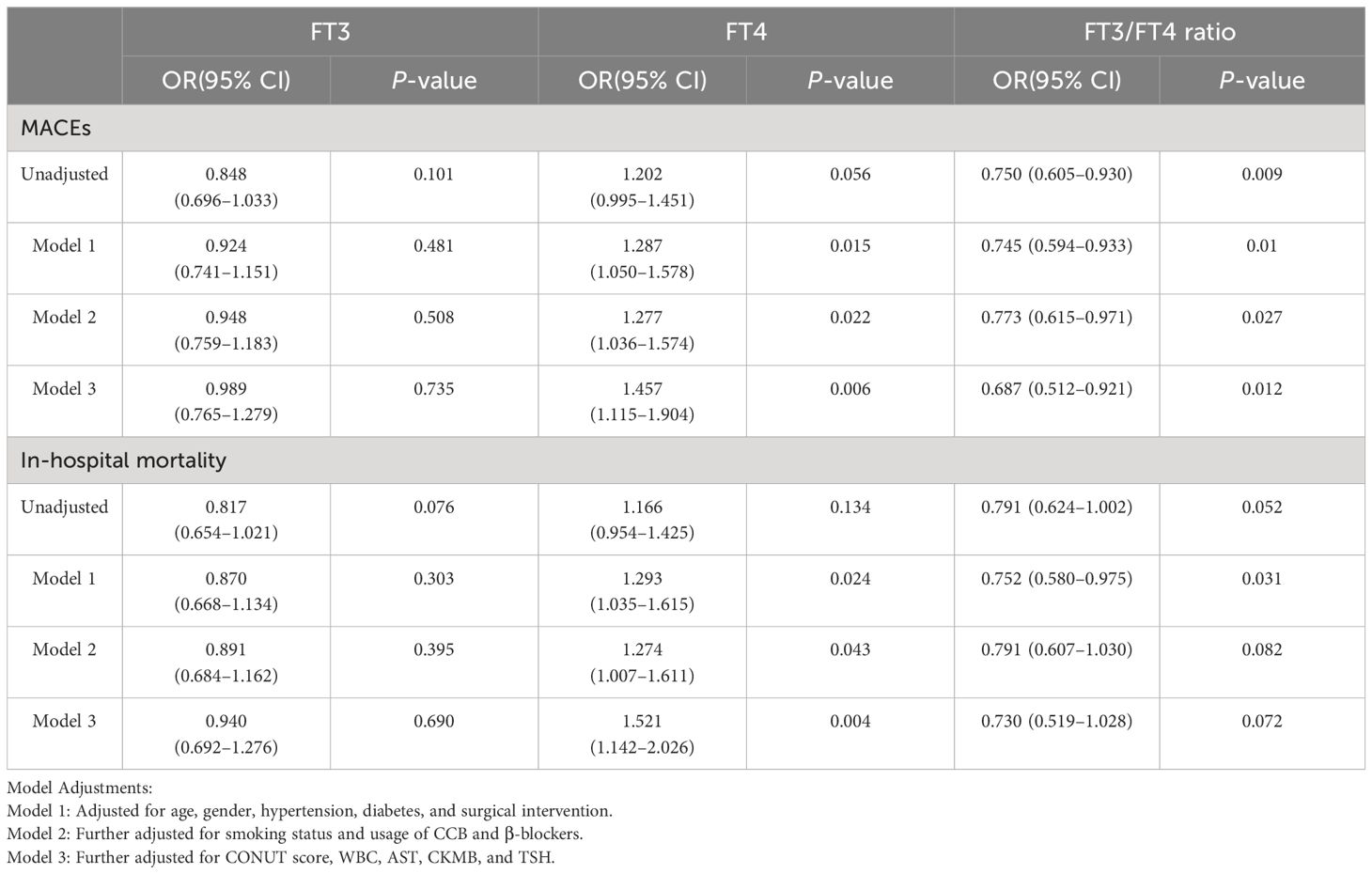
Table 3 Odds ratios for MACEs and in-hospital mortality associated with FT3, FT4, and FT3/FT4 levels (per SD increase) in TAAAD patients.
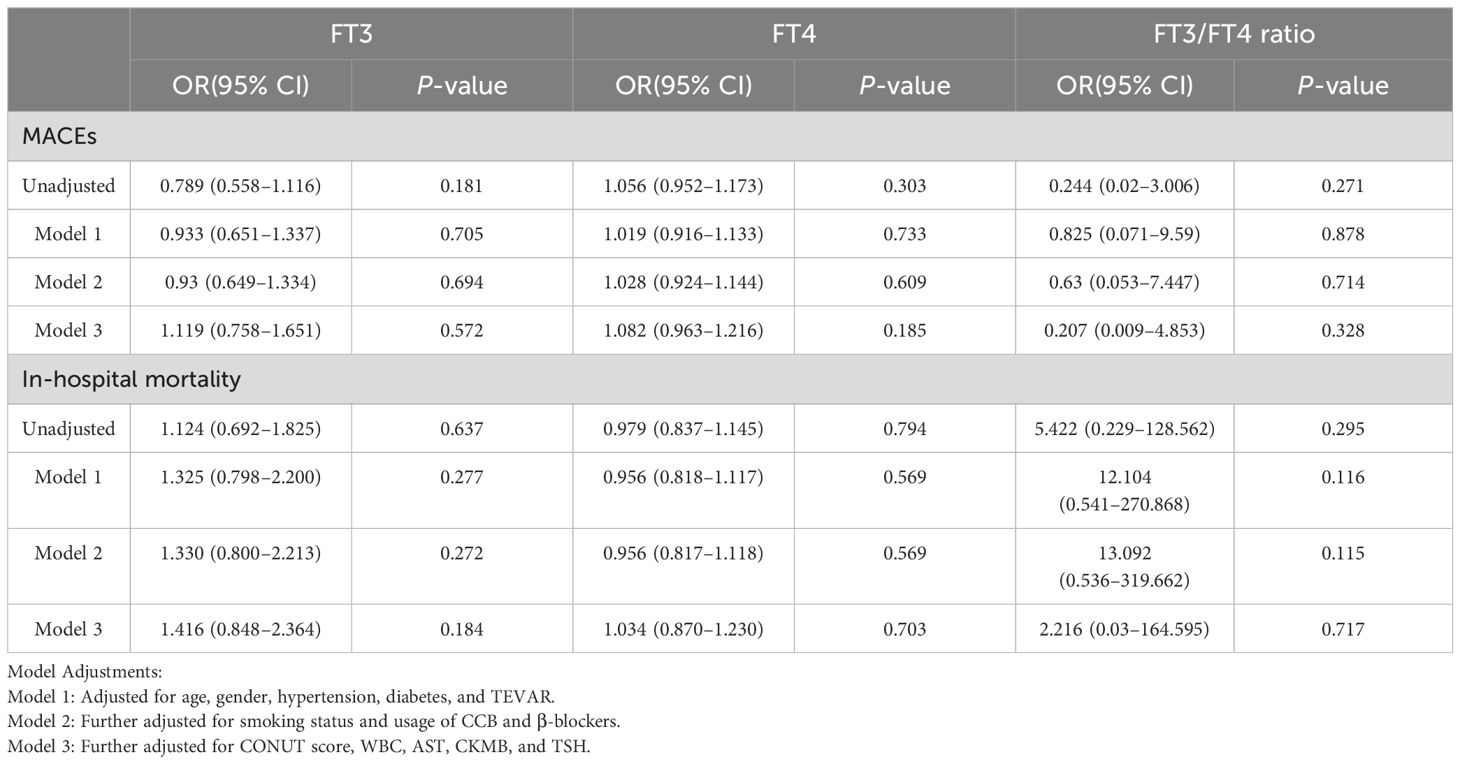
Table 4 Odds ratios for MACEs and in-hospital mortality associated with FT3, FT4, and FT3/FT4 levels (per SD increase) in TBAAD patients.
In the analysis of TAAAD patients, our data demonstrated a consistent association, mirroring the observations made in the overall AAD cohort. Specifically, with each increase of one SD in the FT3/FT4 ratio, we identified a 31.3% reduction in risk of MACEs (OR: 0.687; 95% CI: 0.512–0.921). Conversely, an increase in FT4 level was significantly correlated with an elevated risk of adverse outcomes. Each SD increment in FT4 was associated with a 45.7% increase in risk of MACEs (OR: 1.457; 95% CI: 1.115–1.904) and a 52.1% increase in in-hospital mortality risk (OR: 1.521; 95% CI: 1.142–2.026). However, these observed relationships were conspicuously absent in the TBAAD cohort.
This detailed observation underscores the complex relationship between thyroid hormone levels and acute aortic dissection, and suggests that the nature of this relationship may vary among different AAD subtypes.
3.4 Linear relationship between FT4, FT3/FT4 ratio and MACEs in TAAAD patients
In the subgroup analysis, we observed a significant association between FT4 levels and the FT3/FT4 ratio (per 1 SD increase) with the occurrence of MACEs in TAAAD patients. Then, we employed a RCS model to evaluate the potential non-linear relationships. The RCS model for FT4 (Figure 3A, nonlinearity P-value = 0.2) and the RCS model for the FT3/FT4 ratio (Figure 3B, nonlinearity P-value = 0.2) both indicated no significant nonlinear relationships.
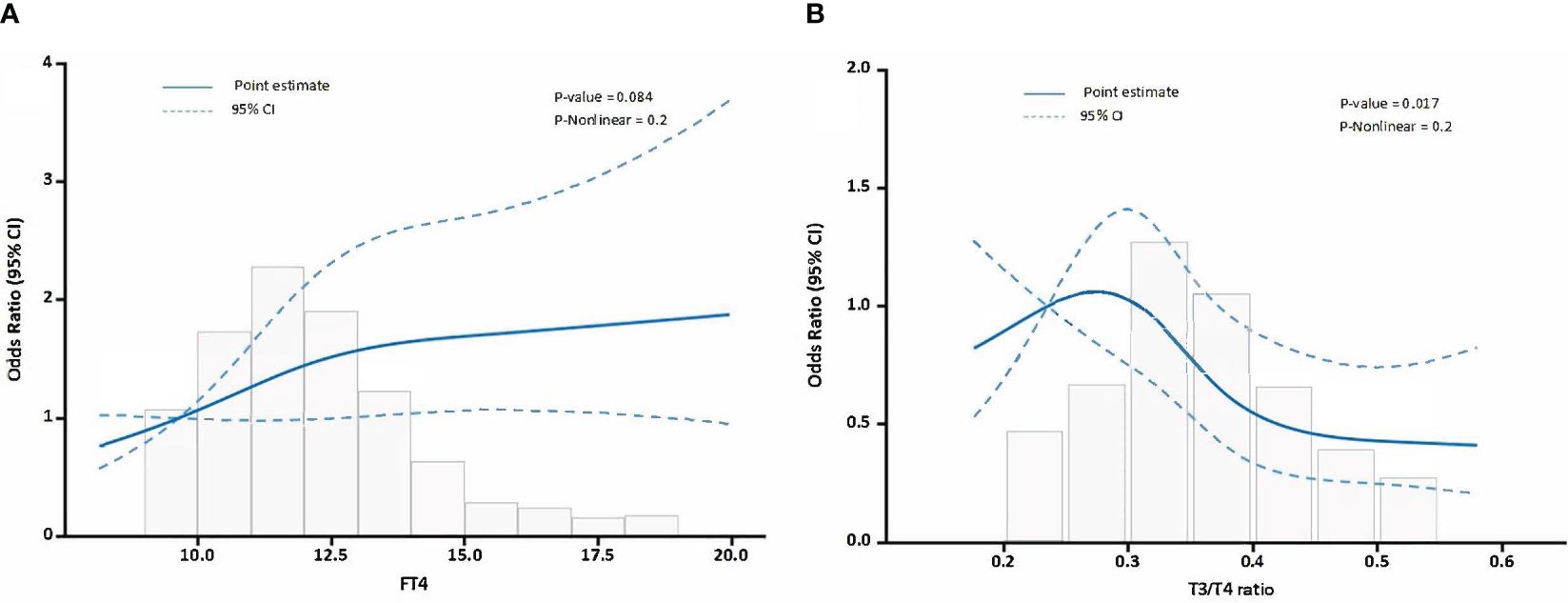
Figure 3 The relationship between FT4, FT3/FT4 ratio and MACEs in TAAAD patients. (A) illustrates the relationship between FT4 levels and MACEs, (B) delineates the relationship between the FT3/FT4 ratio and MACEs. Odds ratios are indicated by solid lines and 95% CIs by shaded areas.
3.5 Mediating roles of renal function and coagulation in MACEs
Logistic regression analyses have revealed a robust impact of FT4 and FT3/FT4 ratio on MACEs in TAAAD patients. Given these findings, we examined the mediating roles of biomarkers, including BUN, Cr, CysC, eGFR, PT, PTR, and PTINR, in the adverse effects of FT4 and the FT3/FT4 ratio on MACEs in TAAAD patients. Table 5 demonstrates that BUN, Cr, CysC, eGFR, PTR, PT, and PTINR serve as partial mediators in the relationship between the FT3/FT4 ratio and the incidence of MACEs. Among these, CysC stands out with the most pronounced mediating proportion, constituting 30.5%. Conversely, FT4 predominantly mediates its effect by influencing coagulation function indicators.
3.6 Multivariable mediation effects on the association between FT3/FT4 ratios and MACEs
The above results show that the FT3/FT4 ratio, through mediation by renal function markers and coagulation profiles, impacts the incidence of MACEs in TAAAD patients. To enhance the precision of these findings, we advanced to a multivariate parallel mediation model, incorporating CysC and PTINR—mediators with significant effect sizes in the initial analysis—to formulate a multiple mediator model without assuming inter-mediator interactions. Figure 4 presents the mediation model parameters in TAAAD patients with the FT3/FT4 ratio as the predictor, CysC and PTINR as the mediators, and MACEs as the outcome, adjusting for sex, age, hypertension, diabetes, and surgery. The model corroborates the findings from the simple mediation analysis. The results substantiate the mediating roles of CysC and PTINR in the association between the FT3/FT4 ratio and MACEs, with a notably stronger mediating effect by CysC (mediating proportion 29.5%).
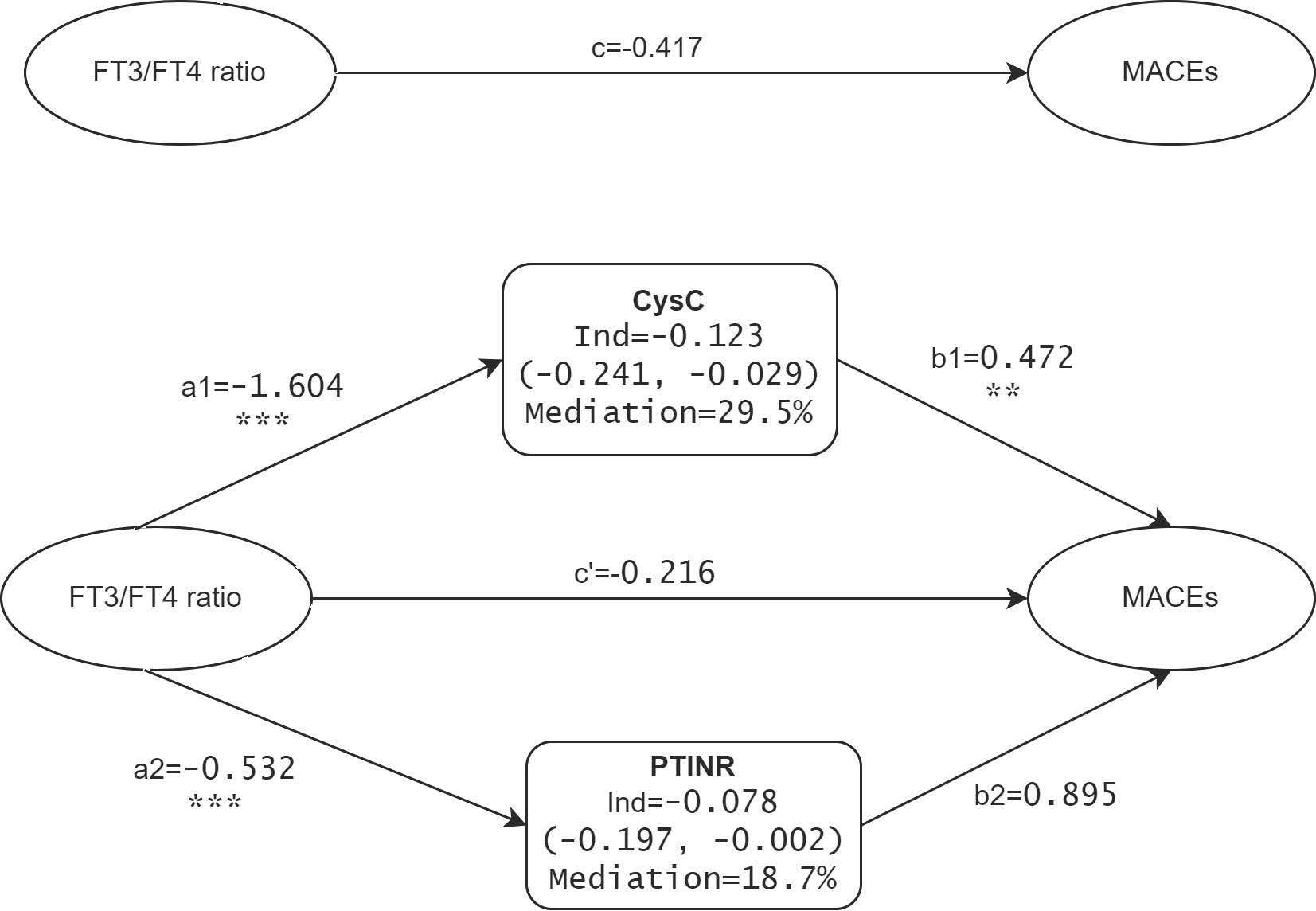
Figure 4 Mediation effects of CysC and PTINR on the FT3/FT4 ratio-MACEs association in TAAAD patients. a, b, c, and c’ are standardized regression coefficients; c=total effect; c’=direct effect; Ind=indirect effect. ** p <.01, *** p <.001.
4 Discussion
The primary findings of our study indicate that FT4 levels and FT3/FT4 ratios are associated with MACEs and in-hospital mortality in AAD patients. Elevated FT4 levels increase the risk of MACEs and all-cause mortality, whereas a higher FT3/FT4 ratio is associated with reduced risk of MACEs. This association was particularly pronounced in TAAAD patients. Mediating analysis suggested that both renal function and coagulation acted as intermediaries in the relationship between the FT3/FT4 ratio and MACEs, with renal function playing a more significant role. Conversely, FT4 predominantly influenced MACEs indirectly through its impact on coagulation. These findings offer a new perspective on the role of thyroid function in determining the prognosis of aortic dissection patients.
Thyroid hormones exert direct effects on the cardiovascular system. They manifest direct genomic actions on cardiomyocytes, modulating the expression of target genes, and also exhibit non-genomic effects on ion channels within cardiomyocyte membranes (4). Even subtle variations in thyroid hormone concentrations can perturb cardiovascular physiology, leading to manifestations such as endothelial dysfunction, alterations in blood pressure, myocardial dysfunction, and lipid anomalies (3). Extensive literature underscores the nexus between thyroid hormones and progression of cardiovascular ailments, such as the independent predictive value of hypothyroidism or low T3 syndrome for cardiovascular adverse events and/or death in AMI patients (5, 32), the relationship between thyroid function and risk of atrial fibrillation and stroke (33), and the relationship between thyroid function and incidence and mortality of atherosclerotic cardiovascular disease (34).
Our study finds that high levels of FT4 and low FT3/FT4 ratios are associated with a high incidence of MACE in TAAAD patients. This association is likely due to reduced peripheral conversion of FT4 into FT3, suggesting the possible presence of low T3 syndrome in these patients. Low T3 syndrome reflects a state of non-thyroidal illness, where reduced levels of T3 are an adaptive response to acute illness but may also contribute to adverse outcomes (35, 36). It would be interesting to assess the role of low T3 syndrome in AAD patients, explore the underlying mechanisms, and consider its potential as a therapeutic target. Future studies should focus on these aspects to better understand and manage the condition.
We find that thyroid hormones can induce alterations in both renal function and coagulation. This is reflected by altered levels of CysC, BUN, Cr, and various coagulation parameters, such as PT and PTINR, ultimately impacting the prognosis of AAD patients. Such findings align with existing research. For instance, studies have reported the influence of FT3 levels on postoperative acute renal failure in aortic dissection patients (13), suggesting that thyroid function may play a role in AAD patient mortality through renal impairment.
The mechanisms behind this effect are multifaceted. Thyroid hormones are known to impact renal function through various mechanisms, including affecting renal blood flow, the RAS system in the kidneys, renal tubular ion transport, and glomerular filtration rate (37). Studies have found that higher FT4 levels are associated with risk of chronic kidney disease (CKD) and a rapid decline in eGFR (38). Conversely, higher FT3 levels are associated with a decrease in all-cause mortality and renal function indicators (39). A low FT3/FT4 ratio is associated with an increased mortality rate and worsening prognosis in CKD patients (40), and this ratio also associates with the risk of diabetic nephropathy (41). These renal abnormalities can severely impact AAD patients, increasing risks of in-hospital mortality, stroke, and renal failure (42, 43).
Furthermore, both in vitro and clinical studies confirm that thyroid hormones, particularly free hormones such as FT4, significantly influence coagulation functions. Elevated levels of FT4 are associated with an augmented risk of thrombosis, indicative of a heightened likelihood of thromboembolic diseases (44–46), and thyroid hormones regulate coagulation processes through various mechanisms. For instance, 3,5,3’-triiodothyronine (T3) interacts with nuclear thyroid hormone receptors, affecting the synthesis and secretion of coagulation factors, vascular endothelial functions, platelet activity, and the fibrinolytic system (47). Beyond genomic actions, recent findings have highlighted that nongenomic mechanisms, initiated at the L-thyroxine (T4) receptor on platelet integrin αvβ3, also exhibit prothrombotic properties. These T4-induced mechanisms entail platelet activation and the production of cytokines and chemokines, such as CX3CL1, with procoagulant activities. Abnormal coagulation further elevates the risk of adverse outcomes in AAD patients (20, 48).
While it is theoretically plausible that thyroid hormones could affect AAD patient prognosis, literature on this topic is sparse. The phenomenon of ‘over-adjustment’ in statistical models (49), where mediating variables might dilute or obscure the effect of the independent variable, is one possible factor among others that could contribute to the limited reports in the literature. Thus, when analyzing thyroid hormone impact on AAD prognosis, it is crucial to avoid including these mediating variables as corrective covariates.
Although our study provides insightful findings, it has some limitations. Firstly, due to its observational nature, we cannot assert causality between thyroid hormone levels and clinical outcomes in AAD patients. Longitudinal studies or randomized controlled trials would be more definitive in this regard. Secondly, our analysis was based on single-time measurements of thyroid hormone levels, renal function, and coagulation. Future studies might benefit from multiple measurements over time to account for potential fluctuations in these parameters. Thirdly, although we adjusted for a wide range of confounding factors, there might be other unmeasured variables that could have influenced our results. For instance, vascular calcification and atherosclerosis have been reported as independent risk factors for MACEs in aneurysm patients (50–54); the presence of a bicuspid aortic valve (55), a family history of aortic dissection or other aortic pathologies (56), and patient compliance data (57) could also influence prognosis. However, our study did not collect data on these factors. Future research should consider incorporating these factors to better understand their impact on the prognosis of AAD patients. Lastly, the findings from our single-center study may not be generalizable to other settings. Multicenter studies would help to validate our results and enhance their applicability to a broader population.
Nevertheless, our findings hold significant theoretical and practical value. They propose potential pathways through which thyroid hormones may influence AAD prognosis via renal and coagulation functions. This insight not only enhances our understanding of aortic dissection prognosis, but also suggests new biological mechanisms, aiding in risk stratification and personalized treatment for AAD patients. However, it is imperative to note that while our findings indicate a significant association, causative relationships should be interpreted with caution given the inherent limitations of observational research.
5 Conclusion
FT4 and the FT3/FT4 ratio are independent predictors of MACEs in AAD patients. The findings are especially relevant for TAAAD patients. Renal and coagulation functions serve as mediators in these associations. Our results underscore the importance of considering the FT3/FT4 ratio alongside FT4 in the prognostic evaluation of AAD patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College (B-2022-207). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XS: Formal analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. SW: Data curation, Funding acquisition, Investigation, Methodology, Writing – original draft. JY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – original draft. HY: Formal analysis, Investigation, Validation, Writing – original draft. SZ: Investigation, Methodology, Writing – original draft. HW: Investigation, Methodology, Writing – original draft. SY: Conceptualization, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. WL: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the following funding: Guangdong Province Science and Technology Special Fund (2021–88-45), Guangdong Province Science and Technology Special Fund (2022–124-6), 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (2020LKSFG19B).
Acknowledgments
We would like to thank Dr. Stanley Li Lin, Department of Cell Biology and Genetics, Shantou University Medical College, for his helpful comments and English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AAD, Acute Aortic Dissection; ACEIs, Angiotensin-Converting Enzyme Inhibitors; ALB, Albumin; AST, Aspartate Aminotransferase; BUN, Blood Urea Nitrogen; CCBs, Calcium Channel Blockers; CI, Confidence Interval; CK, Creatine Kinase; CKMB, Creatine Kinase-MB; CONUT, Controlling Nutritional Status; Cr, Creatinine; CysC, Cystatin C; eGFR, Estimated Glomerular Filtration Rate; FBG, Fasting Blood Glucose; Fib, Fibrinogen; FT3, Free Triiodothyronine; FT4, Free Thyroxine; HDL, High-Density Lipoprotein; HDL-C, High-Density Lipoprotein Cholesterol; IQR, Interquartile Range; LDL, Low-Density Lipoprotein; LDL-C, Low-Density Lipoprotein Cholesterol; LY, Lymphocyte Count; MACEs, Major Adverse Cardiovascular Events; OR, Odds Ratio; PT, Prothrombin Time; PTINR, Prothrombin Time International Normalized Ratio; PTR, Prothrombin Time Ratio; RCS, Restricted Cubic Splines; SCI, Scientific Citation Index; SD, Standard Deviation; TAAAD, Type A Acute Aortic Dissection; TBAAD, Type B Acute Aortic Dissection; TC, Total Cholesterol; TEVAR, Thoracic Endovascular Aortic Repair; TRIG, Triglycerides; TSH, Thyroid Stimulating Hormone; UA, Uric Acid; WBC, White Blood Cell Count.
References
1. Harris KM, Nienaber CA, Peterson MD, Woznicki EM, Braverman AC, Trimarchi S, et al. Early mortality in type A acute aortic dissection: insights from the international registry of acute aortic dissection. JAMA Cardiol. (2022) 7:1009–15. doi: 10.1001/jamacardio.2022.2718
2. Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, et al. Insights from the international registry of acute aortic dissection: A 20-year experience of collaborative clinical research. Circulation. (2018) 137:1846–60. doi: 10.1161/CIRCULATIONAHA.117.031264
3. Razvi S, Jabbar A, Pingitore A, Danzi S, Biondi B, Klein I, et al. Thyroid hormones and cardiovascular function and diseases. J Am Coll Cardiol. (2018) 71:1781–96. doi: 10.1016/j.jacc.2018.02.045
4. Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, Razvi S. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol. (2017) 14:39–55. doi: 10.1038/nrcardio.2016.174
5. Wang W, Wang S, Zhang K, Chen J, Zhang X, Shao C, et al. Hypothyroidism is associated with clinical outcomes in patients with acute myocardial infarction: subgroup analysis of China PEACE study. Endocrine. (2021) 74:128–37. doi: 10.1007/s12020-021-02742-w
6. Larsson SC, Allara E, Mason AM, Michaelsson K, Burgess S. Thyroid function and dysfunction in relation to 16 cardiovascular diseases. Circ Genom Precis Med. (2019) 12:e002468. doi: 10.1161/CIRCGEN.118.002468
7. Kannan L, Shaw PA, Morley MP, Brandimarto J, Fang JC, Sweitzer NK, et al. Thyroid dysfunction in heart failure and cardiovascular outcomes. Circ Heart Fail. (2018) 11:e005266. doi: 10.1161/CIRCHEARTFAILURE.118.005266
8. Holmager P, Schmidt U, Mark P, Andersen U, Dominguez H, Raymond I, et al. Long-term L-Triiodothyronine (T3) treatment in stable systolic heart failure patients: a randomised, double-blind, cross-over, placebo-controlled intervention study. Clin Endocrinol (Oxf). (2015) 83:931–7. doi: 10.1111/cen.12648
9. Cappola AR, Desai AS, Medici M, Cooper LS, Egan D, Sopko G, et al. Thyroid and cardiovascular disease: research agenda for enhancing knowledge, prevention, and treatment. Circulation. (2019) 139:2892–909. doi: 10.1161/CIRCULATIONAHA.118.036859
10. Yuan D, Zhang C, Jia S, Liu Y, Jiang L, Xu L, et al. Predictive value of free triiodothyronine (FT3) to free thyroxine (FT4) ratio in long-term outcomes of euthyroid patients with three-vessel coronary artery disease. Nutr Metab Cardiovasc Dis. (2021) 31:579–86. doi: 10.1016/j.numecd.2020.10.011
11. Gao S, Ma W, Huang S, Lin X, Yu M. Predictive value of free triiodothyronine to free thyroxine ratio in euthyroid patients with myocardial infarction with nonobstructive coronary arteries. Front Endocrinol (Lausanne). (2021) 12:708216. doi: 10.3389/fendo.2021.708216
12. Wang C, Han S, Li Y, Tong F, Li Z, Sun Z. Value of FT3/FT4 ratio in prognosis of patients with heart failure: A propensity-matched study. Front Cardiovasc Med. (2022) 9:859608. doi: 10.3389/fcvm.2022.859608
13. Liu J, Xue Y, Jiang W, Zhang H, Zhao Y. Thyroid hormone is related to postoperative AKI in acute type A aortic dissection. Front Endocrinol (Lausanne). (2020) 11:588149. doi: 10.3389/fendo.2020.588149
14. Zheng GZ, Chen XF, Chen LW, Luo ZR. Thyroid hormone, cortisol, interleukin-2, and procalcitonin regulate postoperative delirium in acute type A aortic dissection patients. BMC Cardiovasc Disord. (2022) 22:503. doi: 10.1186/s12872-022-02962-6
15. Wang SP, Xue Y, Li HY, Jiang WJ, Zhang HJ. High-TSH subclinical hypothyroidism is associated with postoperative mortality in acute type A aortic dissection. Front Endocrinol (Lausanne). (2022) 13:844787. doi: 10.3389/fendo.2022.844787
16. West M, Kirby A, Stewart RA, Blankenberg S, Sullivan D, White HD, et al. Circulating cystatin C is an independent risk marker for cardiovascular outcomes, development of renal impairment, and long-term mortality in patients with stable coronary heart disease: the LIPID study. J Am Heart Assoc. (2022) 11:e020745. doi: 10.1161/JAHA.121.020745
17. Levin A, Lan JH. Cystatin C and cardiovascular disease: causality, association, and clinical implications of knowing the difference. J Am Coll Cardiol. (2016) 68:946–8. doi: 10.1016/j.jacc.2016.06.037
18. Liu Y, Han L, Li J, Gong M, Zhang H, Guan X. Consumption coagulopathy in acute aortic dissection: principles of management. J Cardiothorac Surg. (2017) 12:50. doi: 10.1186/s13019-017-0613-5
19. Li M, Xu S, Yan Y, Wang H, Zheng J, Li Y, et al. Association of biomarkers related to preoperative inflammatory and coagulation with postoperative in-hospital deaths in patients with type A acute aortic dissection. Sci Rep. (2021) 11:18775. doi: 10.1038/s41598-021-98298-w
20. Jiang S, Li Y, Li C, Shu Z, Wu S, Lu X, et al. The association of coagulation indicators with in-hospital acute kidney injury and Malignant events of patients with acute aortic dissection: a retrospective cohort study. J Thorac Dis. (2023) 15:658–67. doi: 10.21037/jtd
21. Iglesias P, Bajo MA, Selgas R, Diez JJ. Thyroid dysfunction and kidney disease: An update. Rev Endocr Metab Disord. (2017) 18:131–44. doi: 10.1007/s11154-016-9395-7
22. Basu G, Mohapatra A. Interactions between thyroid disorders and kidney disease. Indian J Endocrinol Metab. (2012) 16:204–13. doi: 10.4103/2230-8210.93737
23. Wang F, Pan W, Wang H, Zhou Y, Wang S, Pan S. The impacts of thyroid function on the diagnostic accuracy of cystatin C to detect acute kidney injury in ICU patients: a prospective, observational study. Crit Care. (2014) 18:R9. doi: 10.1186/cc13186
24. Ellervik C, Mora S, Ridker PM, Chasman DI. Hypothyroidism and kidney function: A mendelian randomization study. Thyroid. (2020) 30:365–79. doi: 10.1089/thy.2019.0167
25. Squizzato A, Romualdi E, Buller HR, Gerdes VE. Clinical review: Thyroid dysfunction and effects on coagulation and fibrinolysis: a systematic review. J Clin Endocrinol Metab. (2007) 92:2415–20. doi: 10.1210/jc.2007-0199
26. Engelmann B, Bischof J, Dirk AL, Friedrich N, Hammer E, Thiele T, et al. Effect of experimental thyrotoxicosis onto blood coagulation: A proteomics study. Eur Thyroid J. (2015) 4:119–24. doi: 10.1159/000381769
27. Elbers LPB, Fliers E, Cannegieter SC. The influence of thyroid function on the coagulation system and its clinical consequences. J Thromb Haemost. (2018) 16:634–45. doi: 10.1111/jth.13970
28. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
29. Hui E, Yeung CY, Lee PC, Woo YC, Fong CH, Chow WS, et al. Elevated circulating pigment epithelium-derived factor predicts the progression of diabetic nephropathy in patients with type 2 diabetes. J Clin Endocrinol Metab. (2014) 99:E2169–2177. doi: 10.1210/jc.2014-2235
30. Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. (2005) 20:38–45.
31. Chen SC, Yang YL, Wu CH, Huang SS, Chan WL, Lin SJ, et al. Association between preoperative nutritional status and clinical outcomes of patients with coronary artery disease undergoing percutaneous coronary intervention. Nutrients. (2020) 12:1295. doi: 10.3390/nu12051295
32. Jabbar A, Ingoe L, Thomas H, Carey P, Junejo S, Addison C, et al. Prevalence, predictors and outcomes of thyroid dysfunction in patients with acute myocardial infarction: the ThyrAMI-1 study. J Endocrinol Invest. (2021) 44:1209–18. doi: 10.1007/s40618-020-01408-0
33. Papaleontiou M, Levine DA, Reyes-Gastelum D, Hawley ST, Banerjee M, Haymart MR. Thyroid hormone therapy and incident stroke. J Clin Endocrinol Metab. (2021) 106:e3890–900. doi: 10.1210/clinem/dgab444
34. Bano A, Chaker L, Mattace-Raso FUS, van der Lugt A, Ikram MA, Franco OH, et al. Thyroid function and the risk of atherosclerotic cardiovascular morbidity and mortality: the rotterdam study. Circ Res. (2017) 121:1392–400. doi: 10.1161/CIRCRESAHA.117.311603
35. Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, et al. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation. (2003) 107:708–13. doi: 10.1161/01.CIR.0000048124.64204.3F
36. He CJ, Zhu CY, Fan HY, Qian YZ, Zhai CL, Hu HL. Low T3 syndrome predicts more adverse events in patients with hypertrophic cardiomyopathy. Clin Cardiol. (2023) 46:1569–77. doi: 10.1002/clc.24156
37. Rhee CM, Brent GA, Kovesdy CP, Soldin OP, Nguyen D, Budoff MJ, et al. Thyroid functional disease: an under-recognized cardiovascular risk factor in kidney disease patients. Nephrol Dial Transplant. (2015) 30:724–37. doi: 10.1093/ndt/gfu024
38. Huang X, Ding L, Peng K, Lin L, Wang T, Zhao Z, et al. Thyroid hormones associate with risk of incident chronic kidney disease and rapid decline in renal function: a prospective investigation. J Transl Med. (2016) 14:336. doi: 10.1186/s12967-016-1081-8
39. Schultheiss UT, Steinbrenner I, Nauck M, Schneider MP, Kotsis F, Baid-Agrawal S, et al. Thyroid function, renal events and mortality in chronic kidney disease patients: the German Chronic Kidney Disease study. Clin Kidney J. (2021) 14:959–68. doi: 10.1093/ckj/sfaa052
40. Zhang L, Wu Y, Nie Y, Lv W, Li Y, Zhu B, et al. The serum free triiodothyronine to free thyroxine ratio as a potential prognostic biomarker of chronic kidney disease in patients with glomerular crescents: A retrospective study. Front Endocrinol (Lausanne). (2022) 13:977355. doi: 10.3389/fendo.2022.977355
41. Zhao X, Sun J, Xin S, Zhang X. Predictive effects of FT3/FT4 on diabetic kidney disease: an exploratory study on hospitalized euthyroid patients with T2DM in China. Biomedicines. (2023) 11:2211. doi: 10.3390/biomedicines11082211
42. Chen X, Bai M, Sun S, Chen X. Outcomes and risk management in type B aortic dissection patients with acute kidney injury: a concise review. Ren Fail. (2021) 43:585–96. doi: 10.1080/0886022X.2021.1905664
43. Brooke V, Goswami S, Mohanty A, Kasi PM. Aortic dissection and renal failure in a patient with severe hypothyroidism. Case Rep Med. (2012) 2012:842562. doi: 10.1155/2012/842562
44. van Zaane B, Squizzato A, Huijgen R, van Zanten AP, Fliers E, Cannegieter SC, et al. Increasing levels of free thyroxine as a risk factor for a first venous thrombosis: a case-control study. Blood. (2010) 115:4344–9. doi: 10.1182/blood-2009-11-253724
45. Maung AC, Cheong MA, Chua YY, Gardner DS. When a storm showers the blood clots: a case of thyroid storm with systemic thromboembolism. Endocrinol Diabetes Metab Case Rep. (2021) 2021:20-0118. doi: 10.1530/EDM-20-0118
46. Debeij J, Dekkers OM, Asvold BO, Christiansen SC, Naess IA, Hammerstrom J, et al. Increased levels of free thyroxine and risk of venous thrombosis in a large population-based prospective study. J Thromb Haemost. (2012) 10:1539–46. doi: 10.1111/j.1538-7836.2012.04818.x
47. Davis PJ, Mousa SA, Schechter GP. New interfaces of thyroid hormone actions with blood coagulation and thrombosis. Clin Appl Thromb Hemost. (2018) 24:1014–9. doi: 10.1177/1076029618774150
48. Paparella D, Rotunno C, Guida P, Malvindi PG, Scrascia G, De Palo M, et al. Hemostasis alterations in patients with acute aortic dissection. Ann Thorac Surg. (2011) 91:1364–9. doi: 10.1016/j.athoracsur.2011.01.058
49. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. (2009) 20:488–95. doi: 10.1097/EDE.0b013e3181a819a1
50. Li Z, Zhao Z, Cai Z, Sun Y, Li L, Yao F, et al. Runx2 (Runt-related transcription factor 2)-mediated microcalcification is a novel pathological characteristic and potential mediator of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. (2020) 40:1352–69. doi: 10.1161/ATVBAHA.119.314113
51. Ouyang L, Su X, Li W, Tang L, Zhang M, Zhu Y, et al. ALKBH1-demethylated DNA N6-methyladenine modification triggers vascular calcification via osteogenic reprogramming in chronic kidney disease. J Clin Invest. (2021) 131:e146985. doi: 10.1172/JCI146985
52. Ouyang L, Yu C, Xie Z, Su X, Xu Z, Song P, et al. Indoleamine 2,3-dioxygenase 1 deletion-mediated kynurenine insufficiency in vascular smooth muscle cells exacerbates arterial calcification. Circulation. (2022) 145:1784–98. doi: 10.1161/CIRCULATIONAHA.121.057868
53. Ouyang L, Zhang K, Chen J, Wang J, Huang H. Roles of platelet-derived growth factor in vascular calcification. J Cell Physiol. (2018) 233:2804–14. doi: 10.1002/jcp.25985
54. Petsophonsakul P, Furmanik M, Forsythe R, Dweck M, Schurink GW, Natour E, et al. Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation. Arterioscler Thromb Vasc Biol. (2019) 39:1351–68. doi: 10.1161/ATVBAHA.119.312787
55. Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. (2014) 370:1920–9. doi: 10.1056/NEJMra1207059
56. Chen SW, Kuo CF, Huang YT, Lin WT, Chien-Chia Wu V, Chou AH, et al. Association of family history with incidence and outcomes of aortic dissection. J Am Coll Cardiol. (2020) 76:1181–92. doi: 10.1016/j.jacc.2020.07.028
Keywords: acute aortic dissection (AAD), thyroid hormones, coagulation, major adverse cardiovascular events (MACEs), causal mediation analysis
Citation: Shen X, Wu S, Yan J, Yan H, Zhou S, Weng H, Yang S and Li W (2024) Prognostic implications of thyroid hormones in acute aortic dissection: mediating roles of renal function and coagulation. Front. Endocrinol. 15:1387845. doi: 10.3389/fendo.2024.1387845
Received: 18 February 2024; Accepted: 17 July 2024;
Published: 02 August 2024.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Liu Ouyang, Georgia State University, United StatesSawan Jalnapurkar, Gadsden Regional Medical Center, United States
Copyright © 2024 Shen, Wu, Yan, Yan, Zhou, Weng, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengli Yang, c2x5YW5nQGNhZXNoYy5jb20uY24=; Weiping Li, d3BsaTgxOEAxMjYuY29t
 Xuejun Shen
Xuejun Shen Shiwan Wu
Shiwan Wu Jingyi Yan
Jingyi Yan Hongle Yan
Hongle Yan Shuyi Zhou1,2,3
Shuyi Zhou1,2,3 Weiping Li
Weiping Li