- 1Department of Digestive Endoscopy, The First Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, China
- 2School of Traditional Chinese Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, China
- 3The Third School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 4School of Basic Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
Objective: This study aims to analyze the causal relationship between autoimmune thyroiditis (AIT) and inflammatory bowel disease (IBD) using bidirectional Mendelian randomization (MR).
Methods: Single nucleotide polymorphisms were obtained from FinnGen. Exposure-outcome causality was assessed using inverse variance weighted, MR-Egger, and weighted median. MR-Egger intercept, Cochran’s Q, and leave-one-out sensitivity analysis were used to evaluate horizontal pleiotropy, heterogeneity, and robustness, respectively.
Results: Forward analysis revealed no significant association between AIT and the risk of ulcerative colitis (UC) (odds ratio [OR] 1.008, 95% confidence interval [CI] 0.986 to 1.03, p = 0.460) or Crohn’s disease (CD) (OR 0.972, 95% CI 0.935 to 1.010, p = 0.143). Reverse analysis showed that UC (OR 0.961, 95% CI 0.783 to 1.180, p = 0.707) was not associated with AIT risk, while CD (OR 2.371, 95% CI 1.526 to 3.683, p < 0.001) was linked to an increased risk of AIT. Intercept analysis and Cochran’s Q test indicated no horizontal pleiotropy or heterogeneity. Sensitivity analysis confirmed the robustness of the MR results.
Conclusion: This MR analysis suggests that CD, but not UC, is a risk factor for AIT, whereas AIT is not associated with the risk of IBD. Proactive prevention and treatment of CD can help mitigate the risk of AIT.
1 Introduction
Inflammatory bowel disease (IBD) is an immune-mediated chronic inflammatory disease of the gastrointestinal tract, primarily consisting of Crohn’s disease (CD) and ulcerative colitis (UC) (1, 2). Epidemiological studies show that the prevalence of IBD is more than 0.3% in Western countries and continues to rise annually (3, 4). IBD presents with gastrointestinal symptoms, including abdominal pain, diarrhea, and rectal bleeding, which may be accompanied by systemic symptoms or extraintestinal organ involvement in some patients (5, 6). Chronic and recurrent inflammation in IBD elevates the risk of cardiovascular disease such as myocardial infarction, heart failure, stroke, and mental disorders (7, 8), imposing significant burdens on individuals, families, and society.
Autoimmune thyroiditis (AIT) is an autoimmune disease characterized by abnormal thyroid function resulting from the immune system’s attack on thyroid tissue (9, 10). It is characterized by overproduction of thyroid autoantibodies and lymphocytic infiltration in thyroid tissue (11). Epidemiological research shows that the prevalence of AIT is 3% to 5% and has shown a consistent increase over the years (12). Fatigue, bradycardia, and chills are the main clinical manifestations of AIT and contribute significantly to a decline in patients’ quality of life (13). As research progressed, investigators identified potential links between AIT and IBD (14), including common genetic factors, immune abnormalities, and inflammatory processes (14–16). In past observational studies, several studies have explored the relationship between IBD and AIT (17–20), but the causal relationship is unclear due to limitations in study design. Therefore, more robust methods are warranted to investigate this association thoroughly.
Mendelian randomization (MR) is an innovative method for analyzing the causal effect of exposures on outcomes by leveraging genetic variation (21). Compared to traditional methods, MR is less susceptible to issues like reverse causation and confounding biases (22). Therefore, in this study, we employed MR to assess the causal relationship between AIT and IBD and conducted an analysis integrating clinical data.
2 Materials and methods
2.1 Study design
The MR study is grounded in three fundamental assumptions (23), as illustrated in Figure 1. The association assumption necessitates that single nucleotide polymorphisms (SNPs) are strongly correlated with exposure. The independence assumption requires that SNPs are independent of confounding variables. The exclusivity assumption demands that the SNPs influence the outcome solely through the exposure and not via other pathways.
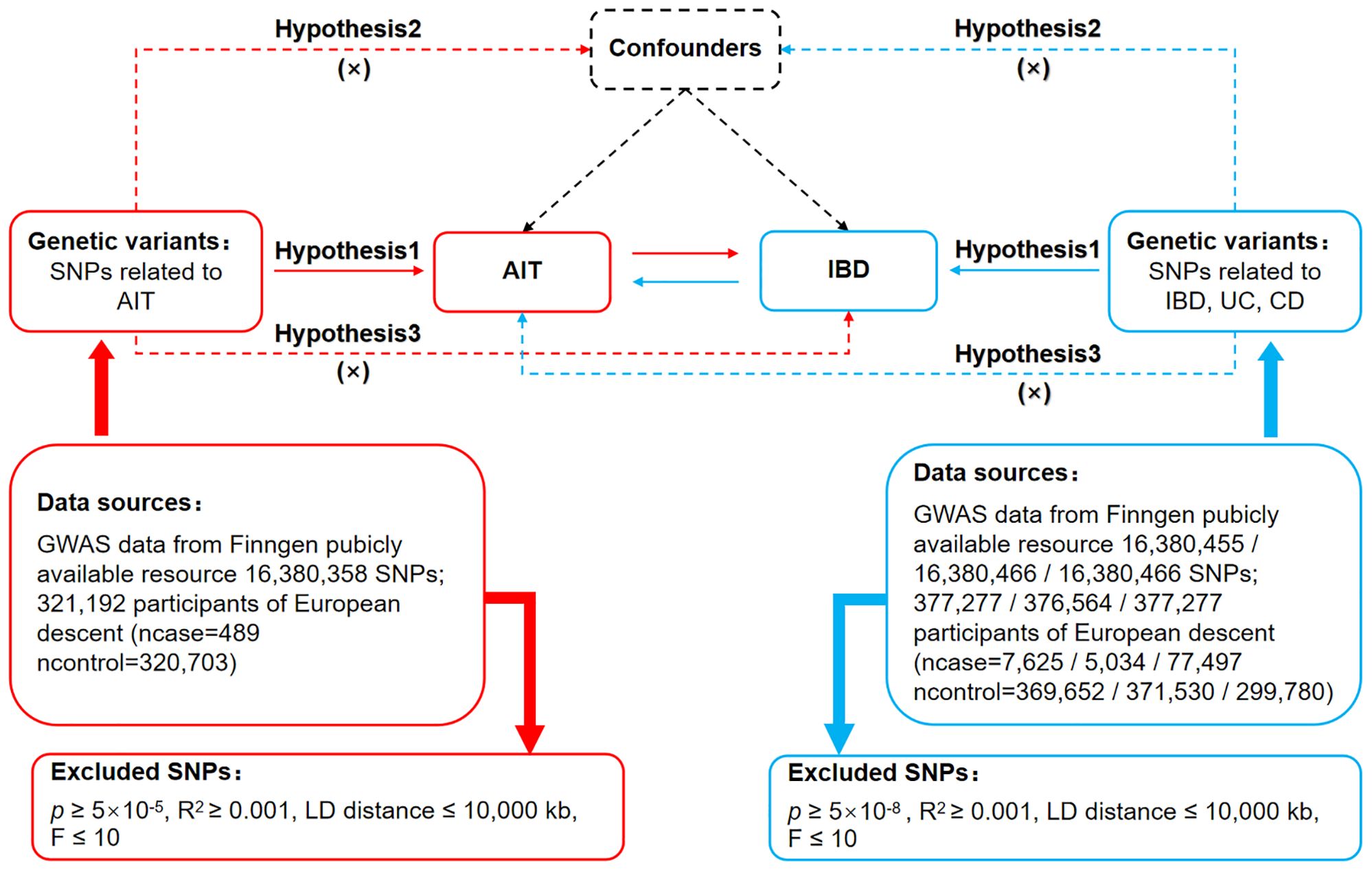
Figure 1. Bidirectional MR design for causal analysis of AIT and IBD. AIT, autoimmune thyroiditis; IBD, inflammatory bowel disease.
2.2 Data sources
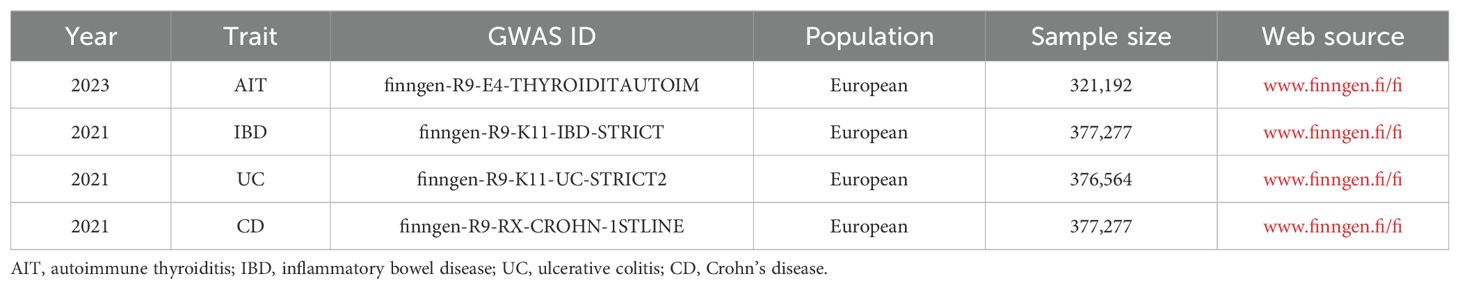
FinnGen (www.finngen.fi/fi) is a publicly available database, which compiles samples from the nationwide network of Finnish bio-banks and digital healthcare data from the national health registry (24). FinnGen provided datasets for AIT, IBD, UC, and CD in this MR study. Among them, the AIT dataset was finngen-R9-E4-THYROIDITAUTOIM, including 321,192 Europeans; the IBD dataset was finngen-R9-K11-IBD-STRICT, encompassing 377,277 Europeans; the UC dataset was finngen-R9-K11-UC-STRICT2, comprising 376,564 Europeans; and the CD dataset was finngen-R9-RX-CROHN-1STLINE, containing 377,277 Europeans, as detailed in Table 1. As these datasets are publicly available, this MR study does not require additional ethical approval.
2.3 Selection of genetic instrument variables
First, a significance level of p < 5 × 10-5 was applied to search for SNPs in the AIT dataset, and p < 5 ×10-8 for SNPs in IBD and its subtypes, adhering to the association assumption. Second, constraints of kb = 10,000 and R2 < 0.001 were imposed to mitigate the interference of linkage disequilibrium. Third, a limit of F ≤ 10 was set to identify SNPs with strong correlation, where F = [R2/(1 – R2)]*[(N – K – 1)/k], with K representing the number of paired samples, N the total number of samples, and R2 the cumulative explained variance. Fourth, PhenoScanner (www.phenoscanner.medschl.cam.ac.uk) and Google Scholar were used to exclude SNPs with confounding factors to fulfill the independence assumption. Fifth, mismatched SNPs were excluded based on the effect allele frequency during the adjustment of allele directions between exposure and outcome. Sixth, MR-Pleiotropy Residual Sum and Outlier method (MR-PRESSO) was employed to exclude outlier SNPs (p < 1) to ensure the accuracy of causal inference.
2.4 Data analysis
The STROBE-MR guidelines served as a guiding method (25). R 4.3.1 software with the “TwoSampleMR (0.5.7)” package installed, was used for all operations of MR analysis. Inverse variance weighted (IVW) was chosen as the primary tool due to its ability to allow unbiased causal analysis without pleiotropy (26). Weighted median, sensitive to outliers, and MR-Egger, capable of analyzing data in the presence of pleiotropy, were set as secondary tools. The intercept of MR-Egger was also utilized to analyze horizontal pleiotropy, necessary to satisfy the exclusivity assumption (p ≥ 0.05). Cochran’s Q and leave-one-out analyses were employed for heterogeneity and sensitivity analysis, respectively. There was no heterogeneity in the results at p ≥ 0.05, and the results were robust when the combined effect sizes were not significantly altered.
3 Results
3.1 Genetic instrument variables
Following association, independence, and exclusivity tests, 53 SNPs for AIT, 31 SNPs for IBD, 25 SNPs for UC, and 28 SNPs for CD were included in this study, as shown in Supplementary Table S1. After removing mismatched and outlier SNPs, the SNPs for each exposure-outcome group are presented in Supplementary Table S2.
3.2 Bidirectional MR analysis
3.2.1 Impact of AIT on IBD
The MR analysis indicated no association between AIT and the risk of IBD: IVW (OR, 0.993; 95% CI, 0.975 to 1.011; p = 0.442), MR-Egger (OR, 0.988; 95% CI, 0.941 to 1.037; p = 0.626), and weighted median (OR, 0.988; 95% CI, 0.963 to 1.014; p = 0.355), as the forest plot depicted in Figure 2 and the scatter plot depicted in Figure 3A. Moreover, no horizontal pleiotropy was observed in the result (p = 0.833), as detailed in Supplementary Table S3.
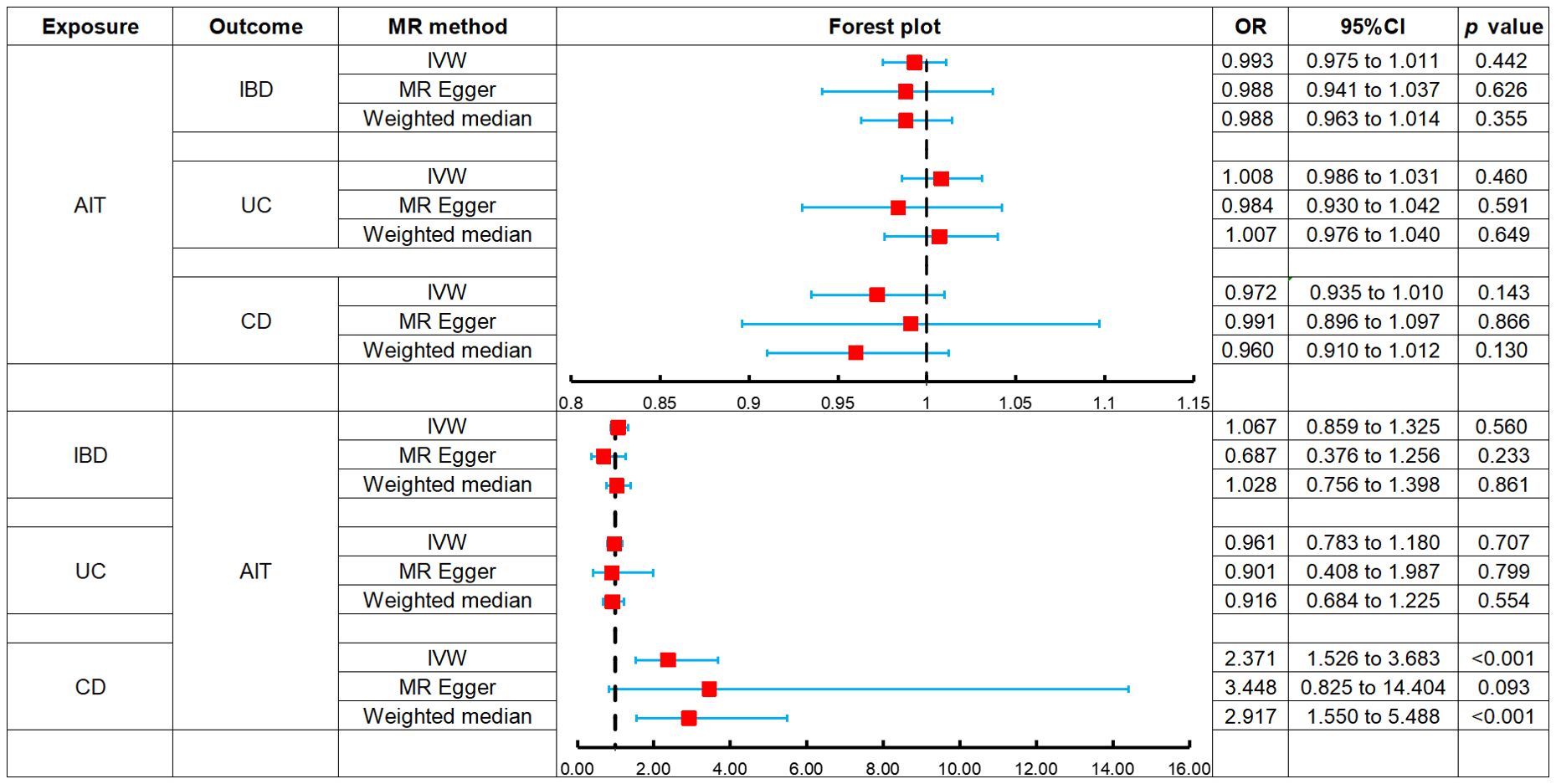
Figure 2. Forest plots of MR analysis on the causal relationship between AIT and IBD. AIT, autoimmune thyroiditis; IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease.
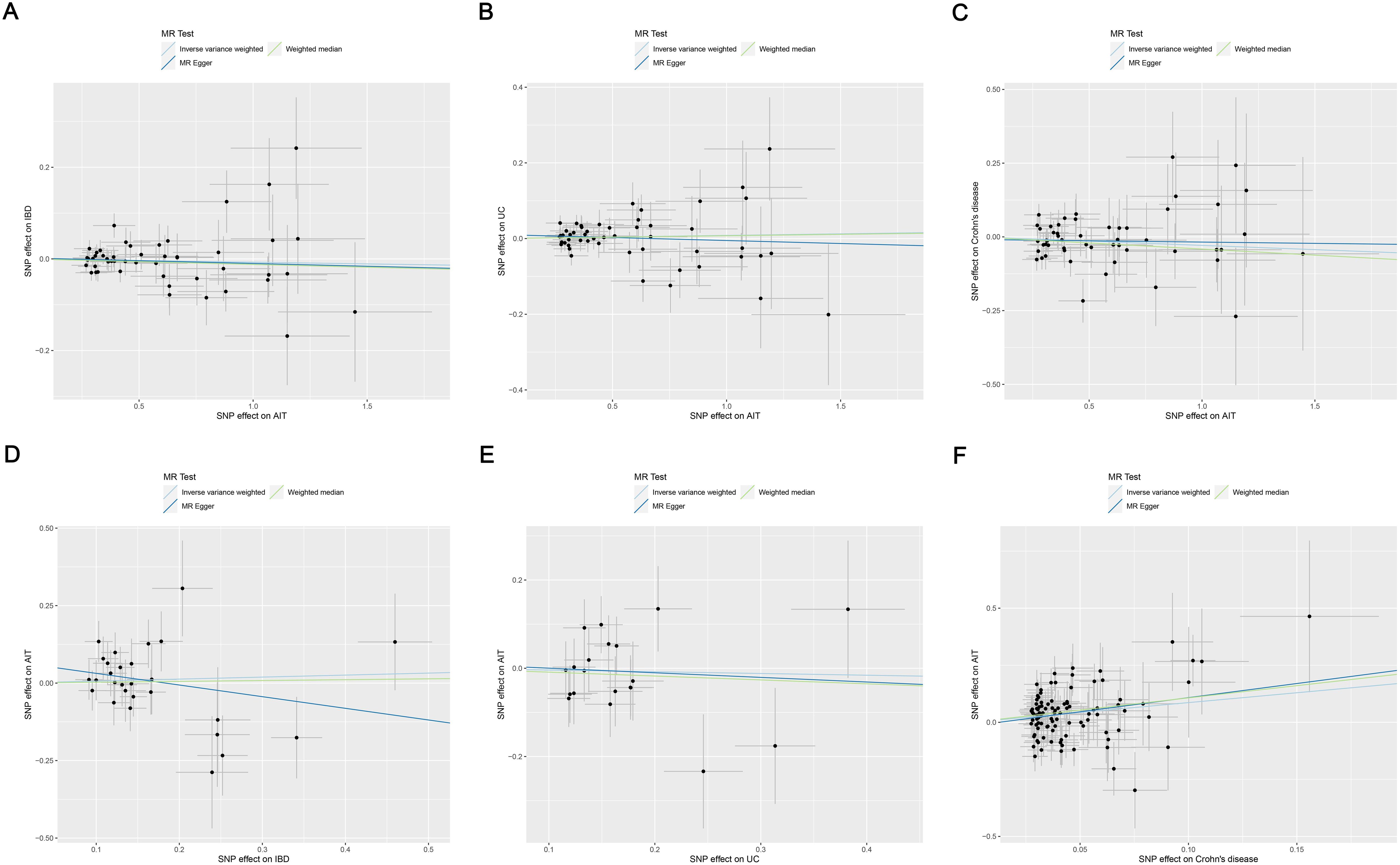
Figure 3. Scatter plots of MR analysis on the causal relationship between AIT and IBD. (A) AIT on IBD; (B) AIT on UC; (C) AIT on CD; (D) IBD on AIT; (E) UC on AIT; (F) CD on AIT. CD on AIT. AIT, autoimmune thyroiditis; IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease.
3.2.2 Impact of AIT on UC
The MR analysis revealed no association between AIT and the risk of UC: IVW (OR, 1.008; 95% CI, 0.986 to 1.031; p = 0.460), MR-Egger (OR, 0.984; 95% CI, 0.930 to 1.042; p = 0.591), and weighted median (OR, 1.007; 95% CI, 0.976 to 1.040; p = 0.649), as the forest plot depicted in Figure 2 and the scatter plot depicted in Figure 3B. Moreover, no horizontal pleiotropy was observed in the result (p = 0.375), as detailed in Supplementary Table S3.
3.2.3 Impact of AIT on CD
The MR analysis demonstrated no association between AIT and the risk of CD: IVW (OR, 0.972; 95% CI, 0.935 to 1.010; p = 0.143), MR-Egger (OR, 0.991; 95% CI, 0.896 to 1.097; p = 0.866), and weighted median (OR, 0.960; 95% CI, 0.910 to 1.012; p = 0.130), as the forest plot depicted in Figure 2 and the scatter plot depicted in Figure 3. Moreover, no horizontal pleiotropy was observed in the results (p = 0.676), as detailed in Supplementary Table S3.
3.2.4 Impact of IBD on AIT
The MR analysis revealed no association between IBD and the risk of AIT: IVW (OR, 1.067; 95% CI, 0.859 to 1.325; p = 0.560), MR-Egger (OR, 0.687; 95% CI, 0.376 to 1.256; p = 0.233), and weighted median (OR, 1.028; 95% CI, 0.756 to 1.398; p = 0.861), as the forest plot depicted in Figure 2 and the scatter plot depicted in Figure 3. Moreover, no horizontal pleiotropy was observed in the results (p = 0.139), as detailed in Supplementary Table S3.
3.2.5 Impact of UC on AIT
The MR analysis demonstrated no association between UC and the risk of AIT: IVW (OR, 0.961; 95% CI, 0.783 to 1.180; p = 0.707), MR-Egger (OR, 0.901; 95% CI, 0.408 to 1.987; p = 0.799), and weighted median (OR, 0.916; 95% CI, 0.684 to 1.225; p = 0.554), as the forest plot depicted in Figure 2 and the scatter plot depicted in Figure 3. Moreover, no horizontal pleiotropy was observed in the results (p = 0.869), as detailed in Supplementary Table S3.
3.2.6 Impact of CD on AIT
Both IVW (OR, 2.371; 95% CI, 1.526 to 3.683; p < 0.001) and weighted median (OR, 2.917; 95% CI, 1.550 to 5.488; p < 0.001) indicated that CD was associated with an increased risk of AIT, while MR-Egger (OR, 3.448; 95% CI, 0.825 to 14.404; p = 0.093) did not observe such a causal relationship, as the forest plot depicted in Figure 2 and the scatter plot depicted in Figure 3F. Moreover, no horizontal pleiotropy was observed in the results (p = 0.560), as detailed in Supplementary Table S3.
3.3 Heterogeneity and sensitivity analysis
Cochran’s Q test suggested no heterogeneity in the MR analysis results (p ≥ 0.05), as the funnel plots depicted in Figure 4 and the results of Cochran’s Q depicted in Supplementary Table S4. Sensitivity analysis confirmed the robustness of the results, as shown in Figure 5.
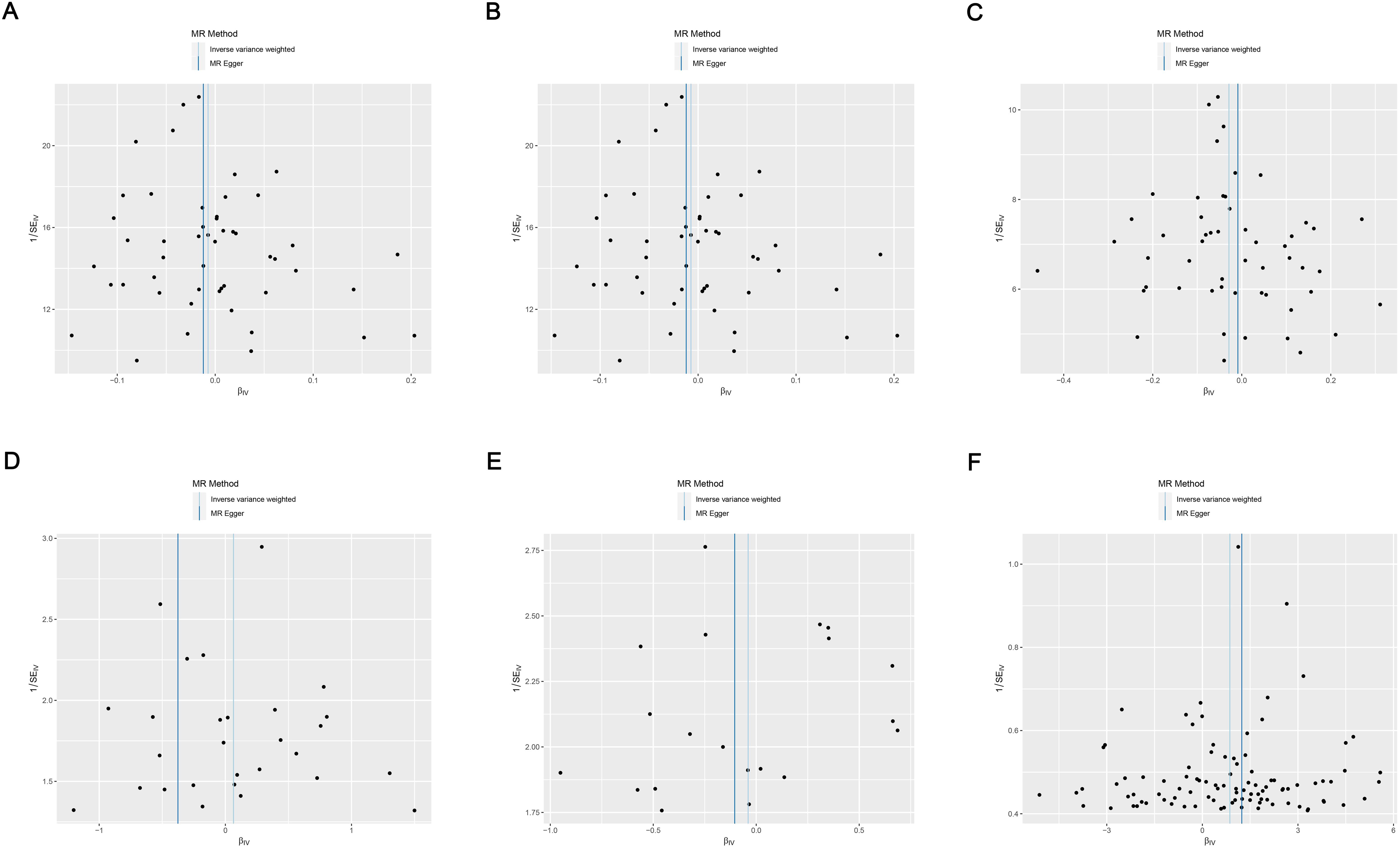
Figure 4. Funnel plots of heterogeneity analysis on the causal relationship between AIT and IBD. (A) AIT on IBD; (B) AIT on UC; (C) AIT on CD; (D) IBD on AIT; (E) UC on AIT; (F) CD on AIT. AIT, autoimmune thyroiditis; IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease.
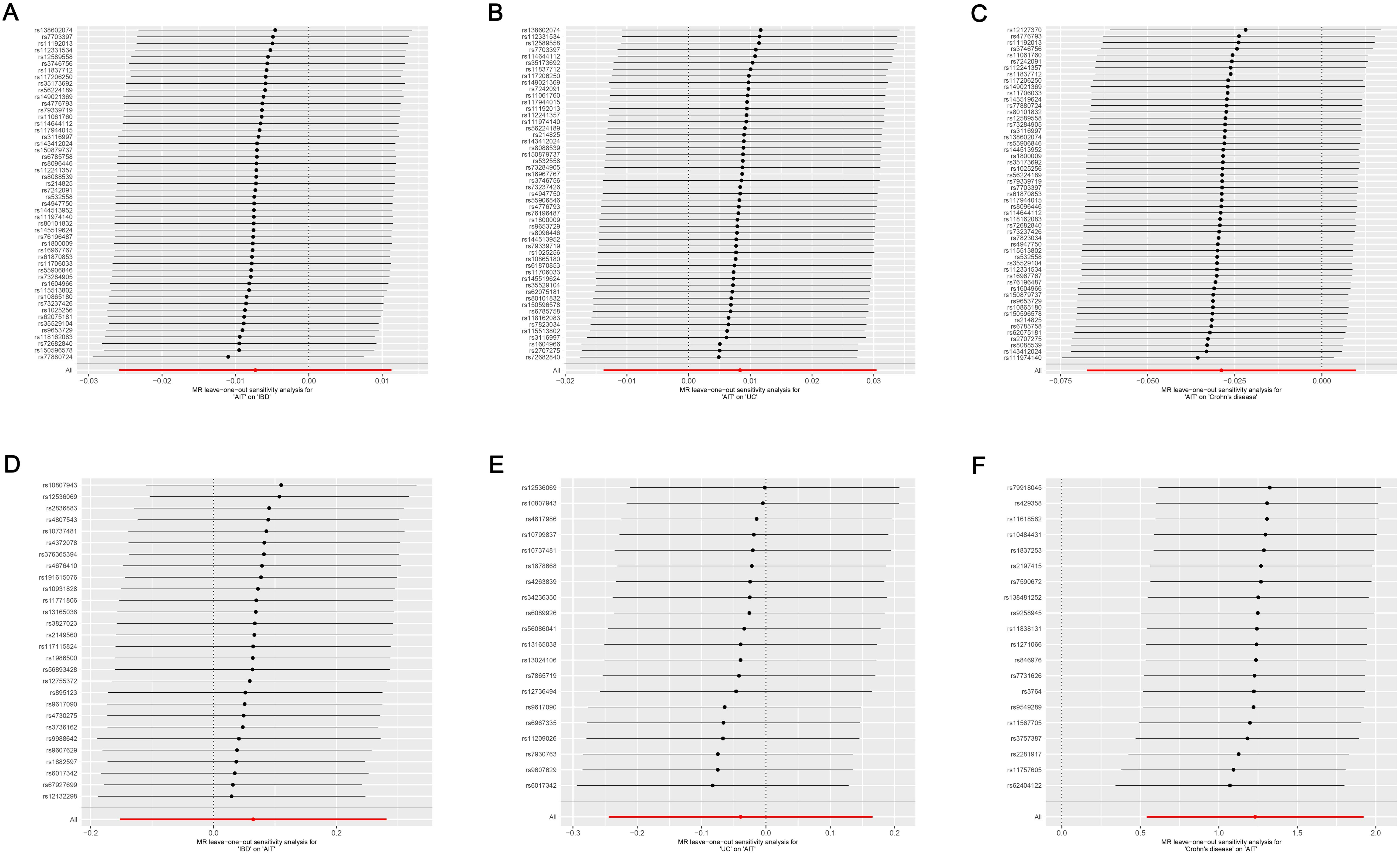
Figure 5. Leave-one-out sensitivity analysis on the causal relationship between AIT and IBD. (A) AIT on IBD; (B) AIT on UC; (C) AIT on CD; (D) IBD on AIT; (E) UC on AIT; (F) CD on AIT. AIT, autoimmune thyroiditis; IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease.
4 Discussion
As an autoimmune disease, AIT is frequently associated with other organ-specific or systemic autoimmune diseases (27, 28). Recently, the correlation between AIT and IBD has received increasing attention from researchers (16, 18). Relevant studies indicate that patients with AIT exhibit a significantly elevated risk for autoimmune diseases such as autoimmune gastritis, rheumatoid arthritis, and celiac disease but not an increased risk of IBD (19). However, some studies suggest that thyroid dysfunction often affects the gastrointestinal system, potentially exacerbating IBD conditions (20). The association between AIT and IBD remains contentious. This MR analysis indicated that CD, but not UC, was associated with an increased risk of AIT, while AIT was not associated with either UC or CD risk. These results were free of horizontal pleiotropy and heterogeneity, and were confirmed to be robust by sensitivity analysis.
Our results demonstrate that AIT is not associated with the risk of IBD and its subtypes. Although reports on to the effect of AIT on the risk of IBD are scarce, the available clinical study supports our findings. The cohort study by Fallahi et al. (19) assessed the prevalence of other autoimmune diseases in 3,069 white AIT patients between 1993 and 2008. And they also found that the prevalence of UC and CD in AIT patients was 0.55% and 0.39%, respectively, which was not significantly different from that of the general population controls (19). This evidence points to the fact that AIT is not a risk factor for IBD. However, due to the limited clinical evidence available, further studies are necessary to validate this result.
Additionally, previous clinical studies have mainly concluded that IBD and UC are not associated with the risk of AIT, which supports our findings. A cross-sectional study in California showed that although IBD increased the risk of autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, and psoriasis, it did not elevate the prevalence of AIT compared to controls (0.25% vs. 0.21%) (29). An Israeli retrospective study analyzed the prevalence of autoimmune diseases in 12,625 patients with IBD and found that IBD was associated with all autoimmune diseases except AIT (30). Shizuma et al. (31) reported no significant difference in the prevalence of thyroid dysfunction between patients with IBD and the general population, which indirectly supports the idea that IBD is not associated with the risk of AIT. Moreover, Bernstein et al. (32) further noted that the risk of AIT was comparable to that of controls in both UC patients (PR, 1.58; 95% CI, 0.79 to 3.20) and CD patients (PR, 0.96; 95% CI, 0.47 to 2.00) after analyzing 8,072 IBD cases in the University of Manitoba IBD Database. Casella et al. (33) also found a relatively low incidence of hypothyroidism in patients with UC (1.9%), suggesting that UC is not associated with AIT risk. In summary, these pieces of evidence point to the fact that IBD is not a risk factor for AIT, which is consistent with the results of this MR analysis.
Interestingly, our study revealed an association between CD and an increased risk of AIT, supported by several clinical research. Shah et al. (16) first reported three cases of AIT associated with CD in 1998, suggesting that CD may be involved in the pathogenesis of AIT. Messina et al. (34) noted that patients with CD were more likely to have increased thyroid volume and uneven parenchymal structure compared to normal individuals, and these abnormal changes did not completely normalize even after treatment. A cross-sectional study by Bardella et al. (18) reported that the incidence of AIT in patients with UC was comparable to that of controls (OR, 2.2; 95% CI, 0.3 to 7.8), whereas the incidence of AIT in patients with CD was significantly higher than that of controls (OR, 4.4; 95% CI, 1.2 to 11.0). Moreover, the logistic regression analysis by Kappelman et al. (35) found that the risk of hypothyroidism was significantly higher in patients with CD than in the general population (OR 2.9, 95% CI 1.4 to 6.1), while the risk of hypothyroidism in UC patients was similar to that in the general population. These pieces of evidence point to CD, rather than UC, as a potential risk factor for AIT, consistent with our analysis results. Meanwhile, it also suggests that the insignificant effect of IBD on AIT is mediated by UC.
The differential impact of UC and CD on the risk of AIT may stem from differences in their pathogenesis (36). The pathology of UC is characterized by continuous and diffuse inflammation of the colonic mucosa (37). It is marked by an atypical T helper cell (Th2)-like response, secretion of cytokines such as IL-13, and increased expression of Th17 (36, 38). However, the pathological manifestations of CD include transmural inflammation and epithelial granulomas (39). It is characterized by a helper T-cell (Th1) response, elevated levels of IFN-γ and TNF-α, and tissue infiltration by Th17 cells (40). AIT shares an immune response with CD, and their pathogenesis involves CD4+ T lymphocytes of the Th1 phenotype, contrasting with the Th2 phenotype of UC (18, 40, 41). IFN-γ secreted by Th1 makes the intestinal barrier more susceptible to damage by disrupting tight junction proteins (42), subsequently affecting other organs in the body, and this may be a potential mechanism by which CD increases the risk of AIT.
To further explore the genetic mechanisms through which CD influences AIT, we investigated 20 SNPs related to CD-AIT. Among them, only one SNP has been reported in the literature. The rs4657041 was the lead intronic cis-pQTL for FCGR2A and FCGR2B, which was shared with UC, systemic lupus erythematosus and various cell surface markers of different immune cell populations (43). However, no studies have reported an association between rs4657041 and either CD or AIT. More research is needed in the future to explore the roles and significance of these SNPs.
While this MR analysis enhances the genetic evidence, there are inevitably some limitations. First, as the data were derived from Europeans, our findings may not extrapolate to elucidate the effects of AIT and IBD in other ancestry diversity. Second, this study identified CD as a risk factor for AIT, but the mechanism remains unclear. Third, there is an increased potential for selectivity bias, as there may be under-recognized confounding variables. Acknowledging these limitations, we encourage future researchers to enhance the GWAS database with racially diverse MR research to promote health equity. Moreover, higher-quality future studies are needed to elucidate the causal effects of AIT and IBD, and to explore the molecular biological mechanisms through which CD elevates the risk of AIT.
5 Conclusion
This MR analysis suggests that CD, but not UC, is a risk factor for AIT, whereas AIT is not associated with IBD risk. Active prevention and treatment of CD can help reduce the risk of AIT. More studies are needed to explore the intrinsic link and mechanism of action between AIT and IBD in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
SB: Data curation, Methodology, Validation, Software, Writing – original draft, Writing – review & editing. YY: Conceptualization, Supervision, Writing – original draft. XY: Data curation, Methodology, Writing – original draft. GH: Formal analysis, Writing – original draft. JW: Supervision, Writing – original draft. KT: Methodology, Writing – original draft. YY: Writing – review & editing. JD: Writing – review & editing. CC: Formal analysis, Writing – review & editing. CT: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Hunan University of Chinese Medicine Disciplinary Construction' Revealing the List and Appointing Leaders' Project (22JBZ002).
Acknowledgments
We want to acknowledge the participants and investigators of the FinnGen study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1387482/full#supplementary-material
Abbreviations
AIT, Autoimmune thyroiditis; CD, Crohn’s disease; CI, Confidence interval; IBD, Inflammatory bowel disease; IVW, Inverse variance weighted; MR, Mendelian randomization; OR, Odds ratio; SNP, Single nucleotide polymorphism; UC, Ulcerative colitis.
References
1. Agrawal M, Colombel J-F. Treat-to-target in inflammatory bowel diseases, what is the target and how do we treat? Gastrointest Endosc Clin N Am. (2019) 29:421–36. doi: 10.1016/j.giec.2019.02.004
2. Zilbauer M, Heuschkel R. Disease prognostic biomarkers in inflammatory bowel diseases-A reality check. J Crohns Colitis. (2022) 16:162–5. doi: 10.1093/ecco-jcc/jjab118
3. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. (2017) 390:2769–78. doi: 10.1016/S0140-6736(17)32448-0
4. Agrawal M, Jess T. Implications of the changing epidemiology of inflammatory bowel disease in a changing world. United Eur Gastroenterol J. (2022) 10:1113–20. doi: 10.1002/ueg2.12317
5. El-Matary W, Bernstein CN. Cancer risk in pediatric-onset inflammatory bowel disease. Front Pediatr. (2020) 8:400. doi: 10.3389/fped.2020.00400
6. Cucinotta U, Romano C, Dipasquale V. Pancreatic associated manifestations in pediatric inflammatory bowel diseases. Genes (Basel). (2021) 12:1372. doi: 10.3390/genes12091372
7. Yang X, Yang L, Zhang T, Zhang H, Chen H, Zuo X. Causal atlas between inflammatory bowel disease and mental disorders: a bi-directional 2-sample Mendelian randomization study. Front Immunol. (2023) 14:1267834. doi: 10.3389/fimmu.2023.1267834
8. Sinh P, Cross R. Cardiovascular risk assessment and impact of medications on cardiovascular disease in inflammatory bowel disease. Inflammation Bowel Dis. (2021) 27:1107–15. doi: 10.1093/ibd/izaa258
9. Rahimova RR. Autoimmune thyroiditis (review of literature). Klin Lab Diagn. (2022) 67:286–91. doi: 10.51620/0869-2084-2022-67-5-286-291
10. Hossein-Khannazer N, Kazem Arki M, Keramatinia L, Rezaei-Tavirani M. Low-level laser therapy in the treatment of autoimmune thyroiditis. J Lasers Med Sci. (2022) 13:e34. doi: 10.34172/jlms.2022.34
11. Kyritsi EM, Kanaka-Gantenbein C. Autoimmune thyroid disease in specific genetic syndromes in childhood and adolescence. Front Endocrinol (Lausanne). (2020) 11:543. doi: 10.3389/fendo.2020.00543
12. McLeod DSA, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. (2012) 42:252–65. doi: 10.1007/s12020-012-9703-2
13. Koehler VF, Bojunga J. Autoimmune thyroid disease. Dtsch Med Wochenschr. (2021) 146:1329–36. doi: 10.1055/a-1258-5674
14. Inokuchi T, Moriwaki Y, Takahashi S, Tsutsumi Z, Ka T, Yamamoto T. Autoimmune thyroid disease (Graves’ disease and hashimoto’s thyroiditis) in two patients with Crohn’s disease: case reports and literature review. Intern Med. (2005) 44:303–6. doi: 10.2169/internalmedicine.44.303
15. Wang L, Cao Z-M, Zhang L-L, Dai X-C, Liu Z-J, Zeng Y-X, et al. Helicobacter pylori and autoimmune diseases: involving multiple systems. Front Immunol. (2022) 13:833424. doi: 10.3389/fimmu.2022.833424
16. Shah SA, Peppercorn MA, Pallotta JA. Autoimmune (Hashimoto’s) thyroiditis associated with Crohn’s disease. J Clin Gastroenterol. (1998) 26:117–20. doi: 10.1097/00004836-199803000-00006
17. Bonapace ES, Srinivasan R. Simultaneous occurrence of inflammatory bowel disease and thyroid disease. Am J Gastroenterol. (2001) 96:1925–6. doi: 10.1111/j.1572-0241.2001.03896.x
18. Bardella MT, Elli L, De Matteis S, Floriani I, Torri V, Piodi L. Autoimmune disorders in patients affected by celiac sprue and inflammatory bowel disease. Ann Med. (2009) 41:139–43. doi: 10.1080/07853890802378817
19. Fallahi P, Ferrari SM, Ruffilli I, Elia G, Biricotti M, Vita R, et al. The association of other autoimmune diseases in patients with autoimmune thyroiditis: Review of the literature and report of a large series of patients. Autoimmun Rev. (2016) 15:1125–8. doi: 10.1016/j.autrev.2016.09.009
20. Kyriacou A, McLaughlin J, Syed AA. Thyroid disorders and gastrointestinal and liver dysfunction: A state of the art review. Eur J Intern Med. (2015) 26:563–71. doi: 10.1016/j.ejim.2015.07.017
21. Thomassen JQ, Tolstrup JS, Benn M, Frikke-Schmidt R. Type-2 diabetes and risk of dementia: observational and Mendelian randomisation studies in 1 million individuals. Epidemiol Psychiatr Sci. (2020) 29:e118. doi: 10.1017/S2045796020000347
22. Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
23. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. (2018) 362:k601. doi: 10.1136/bmj.k601
24. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
25. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
26. 1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. (2015) 526:68–74. doi: 10.1038/nature15393
27. Lazúrová I, Benhatchi K, Rovenský J, Kozáková D, Wagnerová H, Tajtáková M, et al. Autoimmune thyroid disease and autoimmune rheumatic disorders: a two-sided analysis. Ann N Y Acad Sci. (2009) 1173:211–6. doi: 10.1111/j.1749-6632.2009.04809.x
28. Punzi L, Betterle C. Chronic autoimmune thyroiditis and rheumatic manifestations. Joint Bone Spine. (2004) 71:275–83. doi: 10.1016/j.jbspin.2003.06.005
29. Weng X, Liu L, Barcellos LF, Allison JE, Herrinton LJ. Clustering of inflammatory bowel disease with immune mediated diseases among members of a northern california-managed care organization. Am J Gastroenterol. (2007) 102:1429–35. doi: 10.1111/j.1572-0241.2007.01215.x
30. Bar Yehuda S, Axlerod R, Toker O, Zigman N, Goren I, Mourad V, et al. The association of inflammatory bowel diseases with autoimmune disorders: A report from the epi-IIRN. J Crohns Colitis. (2019) 13:324–9. doi: 10.1093/ecco-jcc/jjy166
31. Shizuma T. Concomitant thyroid disorders and inflammatory bowel disease: A literature review. BioMed Res Int. (2016) 2016:5187061. doi: 10.1155/2016/5187061
32. Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. (2005) 129:827–36. doi: 10.1053/j.gastro.2005.06.021
33. Casella G, De Marco E, Antonelli E, Daperno M, Baldini V, Signorini S, et al. The prevalence of hyper- and hypothyroidism in patients with ulcerative colitis. J Crohns Colitis. (2008) 2:327–30. doi: 10.1016/j.crohns.2008.09.001
34. Messina G, Viceconti N, Trinti B. The clinical and echographic assessment of thyroid function and structure in patients with a chronic inflammatory intestinal disease. Recenti Prog Med. (1999) 90:13–6.
35. Kappelman MD, Galanko JA, Porter CQ, Sandler RS. Association of paediatric inflammatory bowel disease with other immune-mediated diseases. Arch Dis Child. (2011) 96:1042–6. doi: 10.1136/archdischild-2011-300633
36. Reiff C, Kelly D. Inflammatory bowel disease, gut bacteria and probiotic therapy. Int J Med Microbiol. (2010) 300:25–33. doi: 10.1016/j.ijmm.2009.08.004
37. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel J-F. Ulcerative colitis. Lancet. (2017) 389:1756–70. doi: 10.1016/S0140-6736(16)32126-2
38. Du L, Ha C. Epidemiology and pathogenesis of ulcerative colitis. Gastroenterol Clin North Am. (2020) 49:643–54. doi: 10.1016/j.gtc.2020.07.005
39. Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc. (2017) 92:1088–103. doi: 10.1016/j.mayocp.2017.04.010
40. Zhang Y-Z, Li Y-Y. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. (2014) 20:91–9. doi: 10.3748/wjg.v20.i1.91
41. Podolsky DK. Inflammatory bowel disease. N Engl J Med. (2002) 347:417–29. doi: 10.1056/NEJMra020831
42. Ciccocioppo R, Finamore A, Ara C, Di Sabatino A, Mengheri E, Corazza GR. Altered expression, localization, and phosphorylation of epithelial junctional proteins in celiac disease. Am J Clin Pathol. (2006) 125:502–11. doi: 10.1309/DTYR-A91G-8R0K-TM8M
Keywords: autoimmune thyroiditis, inflammatory bowel disease, ulcerative colitis, Crohn’s disease, Mendelian randomization, FinnGen
Citation: Bai S, Yu Y, Yang X, Hu G, Wu J, Tong K, Yin Y, Deng J, Chen C and Tan C (2024) Unequal causality between autoimmune thyroiditis and inflammatory bowel disease: a Mendelian randomization study. Front. Endocrinol. 15:1387482. doi: 10.3389/fendo.2024.1387482
Received: 17 February 2024; Accepted: 30 September 2024;
Published: 24 October 2024.
Edited by:
Giuseppe Murdaca, University of Genoa, ItalyReviewed by:
Cecilia Contreras-Cubas, National Institute of Genomic Medicine (INMEGEN), MexicoCheng-Rong Yu, National Eye Institute (NIH), United States
Copyright © 2024 Bai, Yu, Yang, Hu, Wu, Tong, Yin, Deng, Chen and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanchuan Tan, dGNjQGhudWNtLmVkdS5jbg==; Cong Chen, NzgxOTcyODYzQHFxLmNvbQ==
†These authors have contributed equally to this work
 Siyang Bai
Siyang Bai Yunfeng Yu
Yunfeng Yu Xinyu Yang
Xinyu Yang Gang Hu
Gang Hu Jingyi Wu
Jingyi Wu Keke Tong
Keke Tong Yuman Yin2
Yuman Yin2 Cong Chen
Cong Chen Chuanchuan Tan
Chuanchuan Tan