- 1Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy
- 2Istituto Auxologico Italiano, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Experimental Laboratory for Auxo-endocrinological Research, Piancavallo-Verbania, Italy
- 3Istituto Auxologico Italiano, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Laboratory of Metabolic Research, Piancavallo-Verbania, Italy
- 4Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 5Istituto Auxologico Italiano, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Experimental Laboratory for Auxo-endocrinological Research, Milan, Italy
Introduction: Prader-Willi syndrome (PWS) is a rare disease, which shows a peculiar clinical phenotype, including obesity, which is different from essential obesity (EOB). Metabolomics might represent a valuable tool to reveal the biochemical mechanisms/pathways underlying clinical differences between PWS and EOB. The aim of the present (case-control, retrospective) study was to determine the metabolomic profile that characterizes PWS compared to EOB.
Methods: A validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) targeted metabolomic approach was used to measure a total of 188 endogenous metabolites in plasma samples of 32 patients with PWS (F/M = 23/9; age: 31.6 ± 9.2 years; body mass index [BMI]: 42.1 ± 7.0 kg/m2), compared to a sex-, age- and BMI-matched group of patients with EOB (F/M = 23/9; age: 31.4 ± 6.9 years; BMI: 43.5 ± 3.5 kg/m2).
Results: Body composition in PWS was different when compared to EOB, with increased fat mass and decreased fat-free mass. Glycemia and HDL cholesterol were higher in patients with PWS than in those with EOB, while insulinemia was lower, as well as heart rate. Resting energy expenditure was lower in the group with PWS than in the one with EOB, a difference that was missed after fat-free mass correction. Carrying out a series of Tobit multivariable linear regressions, adjusted for sex, diastolic blood pressure, and C reactive protein, a total of 28 metabolites was found to be associated with PWS (vs. non-PWS, i.e., EOB), including 9 phosphatidylcholines (PCs) ae, 5 PCs aa, all PCs aa, 7 lysoPCs a, all lysoPCs, 4 acetylcarnitines, and 1 sphingomyelin, all of which were higher in PWS than EOB.
Conclusions: PWS exhibits a specific metabolomic profile when compared to EOB, suggesting a different regulation of some biochemical pathways, fundamentally related to lipid metabolism.
1 Introduction
Prader-Willi syndrome (PWS) is a rare disease, which shows a variable clinical phenotype, including muscle hypotonia and failure to thrive during infancy, short stature, intellectual disability, behavioral abnormalities, hyperphagia, and obesity in childhood and adulthood (1, 2). Apart from growth hormone (GH) deficiency (GHD) (3), other endocrine diseases are frequently observed, including hypothyroidism and hypogonadism (4).
PWS is due to some abnormality in the so-called Prader-Willi critical region (PWCR) at 15q11-q13. In particular, three main genetic patterns have been identified: paternal 15q11-q13 deletion (del15) (65-75% of cases), maternal uniparental disomy 15 (UPD15) (20-30% of cases), and imprinting center defect (1-3%) (5).
Though clinical studies with conflicting results have been published, PWS-associated obesity exhibits clinical characteristics that are somewhat different from those of essential obesity (EOB), including body composition (particularly, adipose tissue), neuroendocrine system (e.g., ghrelin, oxytocin, and adiponectin), response to body weight reduction programs, occurrence of obesity-related comorbidities such as type 2 diabetes mellitus (T2DM), and long-term cardiovascular prognosis (6–12).
Evaluation of metabolome, i.e., the total of metabolites that, in the human organism, serve as substrates or by-products of enzymatic or non-enzymatic reactions, has become, in the last decade, a valuable tool to understand the biochemical pathways that are altered in patients affected by specific diseases. With the spread and technological improvements of -omics platforms, it is now possible to characterize the metabolomic profiling in manifold biofluids, including plasma and urine samples, which can be easily and repeatedly collected in any subject. This methodological approach is extremely attractive due to the potential diagnostic and/or therapeutic implications (13).
Surprisingly, while a huge number of metabolomics-based clinical studies have been carried out in EOB (14), to the best of our knowledge, PWS has never been investigated by this innovative methodology so far.
So, the aim of the present (case-control, retrospective) study was to compare the metabolomic profiles in patients with PWS vs. EOB. We hypothesized that a different metabolome, compared to EOB, could explain some aspects of the biochemistry and, so, the peculiar clinical phenotype in PWS.
2 Materials and methods
2.1 Study design
The present case-control study was retrospective, based on the use of plasma samples already available.
Before enrolment, patient selection was the initial phase of the study to evaluate inclusion/exclusion criteria and to verify the presence of the patient’s consent (see below for details).
2.2 Subjects
Adults of both sexes were selected from the patients’ population admitted to the Division of Auxology (PWS) and the Division of Metabolic Diseases (EOB) of Istituto Auxologico Italiano, Piancavallo-Verbania, Italy, for a 3-week in-hospital multidisciplinary body weight reduction program.
First of all, patients affected by PWS were selected; then, age-, sex- and body mass index (BMI)-matched patients affected by EOB were identified and included in the control group.
PWS patients showed the typical clinical phenotype of the syndrome, this diagnosis being confirmed by cytogenetic analysis (del15 = 20 cases; UPD15 = 12 cases).
The inclusion criteria were: (1) individuals of both sexes, aged ≥18 years; (2) individuals with or without metabolic syndrome (see below for its definition); (3) individuals having a BMI >30 kg/m2 (for both groups, i.e., PWS and EOB). The exclusion criteria were: (1) secondary causes of obesity (e.g., steroid-induced obesity), apart from PWS; (2) individuals with systolic blood pressure (SBP) ≥180 mmHg and diastolic blood pressure (DBP) ≥110 mmHg; (3) cardiovascular, psychiatric, neurological, or other (relevant) medical diseases evident in the previous 6 months; (4) individuals (and/or their parents) who refused to sign the consent form.
The study protocol was approved by the Ethical Committee (EC) of the Istituto Auxologico Italiano, IRCCS, Milan, Italy (EC code: 2023_03_21_08; research project code: 01C316; acronym: METABOPWS).
2.3 Resting energy expenditure
Resting energy expenditure (REE) was determined after an overnight fast using an open-circuit, indirect computerized calorimetry (Vmax 29, Sensor Medics, Yorba Linda, Ca, USA) with a rigid, transparent, ventilated canopy.
2.4 Anthropometric measurements
A scale with a stadiometer was used to determine height (with a precision of 0.1 cm) and weight (with a precision of 0.1 kg) (Wunder Sa.Bi., WU150, Trezzo sull’Adda, Italy). Waist circumference (WC) was measured with a flexible tape in a standing position, halfway between the inferior margin of the ribs and the superior border of the crista, while hip circumference (HC) was measured at the largest parts around the buttocks. Body composition was measured by bioimpedance analysis (Human-IM Scan, DS-Medigroup, Milan, Italy) after 20 minutes of supine resting. BMI (weight in kg divided by height in meters squared), fat mass (FM), and fat-free mass (FFM) were determined in all subjects.
2.5 Biological sample collection
Blood samples were collected from patients, following a standardized protocol, at the beginning of the body weight reduction program (T0). The same types of tubes and consumables for each cluster of parameters were used throughout the entire duration of the study to improve consistency.
Blood samples were collected in lithium heparin tubes at around 8:00 AM after an overnight fast. Cells were separated from plasma with centrifugation (20-24°C for 10 minutes at 2500 g) within 2 hours from the blood collection. Plasma was then transferred in pre-cold tubes and put in ice to preserve the metabolome. Each tube was vortexed for at least 10 seconds, divided into aliquots, and stored at -20°C.
Plasma samples were delivered from our Laboratories, located in Piancavallo-Verbania, to Milan while keeping the samples frosted, where they were stored at -20°C until the metabolomic analyses.
2.6 Metabolic, biochemical
Total cholesterol (T-C), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), glucose, insulin, and C-reactive protein (CRP) were measured.
Colorimetric enzymatic assays (Roche Diagnostics, Monza, Italy) were used to determine serum T-C, LDL-C, HDL-C, and TG levels. The sensitivities of the assays were 3.86 mg/dL [1 mg/dL = 0.03 mmol/L], 3.87 mg/dL [1 mg/dL = 0.03 mmol/L], 3.09 mg/dL [1 mg/dL = 0.03 mmol/L] and 8.85 mg/dL [1 mg/dL = 0.01 mmol/L], respectively.
Serum glucose level was measured by the glucose oxidase enzymatic method (Roche Diagnostics, Monza, Italy). The sensitivity of the method was 2 mg/dL [1 mg/dL = 0.06 mmol/L]. Serum insulin concentration was determined by a chemiluminescent immunometric assay, using a commercial kit (Elecsys Insulin, Roche Diagnostics, Monza, Italy). The sensitivity of the method was 0.2 µU/mL [1 µU/mL = 7.18 pmol/L].
The intra- and inter-assay coefficients of variation (CVs) were the following: 1.1% and 1.6% for T-C, 1.2% and 2.5% for LDL-C, 1.8% and 2.2% for HDL-C, 1.1% and 2.0% for TG, 1.0% and 1.3% for glucose, and 1.5% and 4.9% for insulin.
CRP was measured using an immunoturbidimetric assay (CRP RX, Roche Diagnostics GmbH, Mannheim, Germany). The sensitivity of the method was 0.03 mg/dL.
For each patient, the homeostatic model assessment of insulin resistance (HOMA-IR) was also calculated, according to the following formula: (insulin [μU/mL] × glucose [mmol/L])/22.5 (15).
2.7 Evaluation of blood pressure
Blood pressure was measured on the right arm, using a sphygmomanometer with appropriate cuff size, with the subject in a seated position and relaxed condition. The procedure was repeated three times at 10 min intervals in between; the means of the three values for SBP and DBP were recorded.
2.8 Definition of metabolic syndrome
According to the International Diabetes Federation (IDF) criteria for diagnosis of metabolic syndrome in adults (16), our patients were considered positive for the presence of metabolic syndrome if they had abdominal obesity plus two or more of the following factors: (i) increased TG level, (ii) reduced HDL-C levels, (iii) increased BP levels, (iv) increased fasting glucose levels or previously diagnosed T2DM. For cut-offs of each parameter and further information (e.g., use of lipid/glucose-lowering drugs) see ref (16).
2.9 Metabolomics analyses
The metabolomics profile of plasma samples collected from subjects was assessed with a targeted approach, in particular a liquid chromatography-tandem mass spectrometry method (LC–MS/MS, Sciex 550 Qtrap) implementing the Absolute IDQ p180 kit (Biocrates Life Sciences AG, Innsbruck, Austria). With this method, a total of 188 metabolites was quantified, among which 21 amino acids, 21 biogenic amines, the sum of hexose (H1), 40 acylcarnitines, 15 sphingolipids, and 90 glycerophospholipids among which 14 lyso-phosphatidylcholines (LysoPC), 38 diacylphosphatidylcholine (PC aa), and 38 acyl-alkylphosphatidylcholine (PC ae). The analytical details used in our analyses were extensively reported previously (17).
2.10 Data elaboration and statistical analyses
Demographic, biochemical, and clinical parameters were expressed as frequency and percentage for categorical variables and in terms of mean ± standard deviation (SD) or median and first quartile — third quartile [q1;q3] as appropriate for continuous variables. The normal distribution of continuous variables was tested by graphical inspection.
The differences in each parameter between patients with PWS and those with EOB were analyzed by the t-test or Wilcoxon sum-rank test for continuous variables. In the case of a categorical variable, the chi-squared test was applied.
For each metabolite, data <LOD were replaced with the minimum limit of detections (LOD) values divided by 2 (i.e., LOD/2); then, descriptive statistics were performed, including mean ± SD and the following percentiles: 5°, 25°, 50°, 75° and 95°. Only metabolites with at least 20% of observations greater than the LOD were considered for the following statistical analyses. Metabolite concentrations were log-transformed (base e) to ensure normal distribution and then standardized by performing an auto-scale (i.e., each value was subtracted by the mean and divided by the SD), to make the fold-change (FC) of all different metabolites comparable.
We used Tobit regression models to evaluate the differences in the metabolite concentrations between PWS and EOB. This approach was chosen because the Tobit regression model is designed to estimate linear relationships with left- or right-censoring dependent variables (18, 19). In our case, metabolite concentrations lower than the LOD were considered as the left-censored values. A Tobit regression model was applied for each metabolite: the standardized natural logarithm of the metabolite concentrations was the dependent variable, while the independent variable of interest was the condition of PWS (or non-condition of PWS, i.e., EOB). In the multivariable models, gender, DBP, and CRP were chosen as adjustment covariates, considering the results of previous univariate Tobit linear regressions, built to evaluate the relationships of each metabolite concentration with demographic, biochemical, and clinical characteristics in the entire population. From each multivariable Tobit model, we estimated the geometric means for the PWS group and the control groups (EOB) with the relative confidence interval (95% CI). The ratio of the geometric means was used to obtain the Fold-Change (FC). Due to the high number of comparisons, we applied a multiple comparison correction method based on the Benjamini–Hochberg False Discovery Rate (FDR) to calculate the FDR-adjusted p-value (FDR p-value).
A volcano plot of log2 (FC) vs -log10(FDR p-value) was used to display the results. Only for the 28 analytes with significant FDR p-values we reported the box-plots to visualize the original distribution of metabolite concentrations (without standardization) in PWS and EOB groups.
Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
3 Results
Patients with PWS (F/M = 23/9; age: 31.6 ± 9.2 years; BMI: 42.1 ± 7.0 kg/m2) and with EOB (F/M = 23/9; age: 31.4 ± 6.9 years; BMI: 43.5 ± 3.5 kg/m2) were recruited, being the two groups comparable in terms of sex, age, BMI, and metabolic syndrome prevalence. Table 1 reports descriptive statistics of demographic, biochemical, and clinical data referred to patients with PWS or EOB, including the corresponding inter-group comparisons.
When considering body composition, patients with PWS exhibited lower FFM than those with EOB (p<0.0001), as well as a lower FM (p=0.0060), being similar the corresponding values expressed in percentage. REE was lower in patients with PWS than in those with EOB (p<0.0001), a difference that was missed after correcting the REE value by the subject’s FFM (i.e., energy efficiency).
Though SBP and DBP were similar in both groups (PWS and EOB), without any difference in the percentages of subjects treated with antihypertensives, HR was lower in patients with PWS than in those with EOB (p<0.0001).
When considering glucometabolic homeostasis, glycemia and insulinemia were, respectively, higher and lower in patients with PWS than in those with EOB (glucose: p=0.0052; insulin: =0.0019), though the percentage of the subjects treated with antidiabetic drugs was higher in the former than the latter ones (p=0.0389). Nevertheless, there were no differences in HOMA-IR and HbA1c when comparing the two groups (i.e., PWS vs. EOB).
While there were no differences in T-C, LDL-C, and TG, HDL-C levels were higher in patients with PWS than in those with EOB (p=0,0186), being the two groups similarly treated with antilipidemic drugs.
Supplementary Table S1, included in the Supplementary Material, reports descriptive statistics of all metabolites measured in the plasma for the two groups.
Carrying out Tobit univariate linear regression models, several metabolites belonging to different chemical classes were identified to be associated with PWS, including glycerophospholipids (7 lysoPCs a, 12 PCs aa, 13 PCs ae, all lysoPCs and all PCs), sphingolipids (1 sphingomyelin, SM) and 3 acylcarnitines, all of which were higher in patients with PWS than in those with EOB (at least p<0.05) (Supplementary Table S2 in the Supplementary Material).
Carrying out Tobit multivariable linear regression models, adjusted for sex, DBP, and CRP, several metabolites belonging to different chemical classes were identified to be associated with PWS, including 7 lysoPCs a, 9 PCs ae, 5 PCs aa, all lysoPCs, all PCs aa, 4 acylcarnitines, and 1 SM, all of which were higher in PWS than EOB (at least p<0.05) (Table 2 for statistically significant metabolites and Supplementary Table S3 for statistically non-significant metabolites).
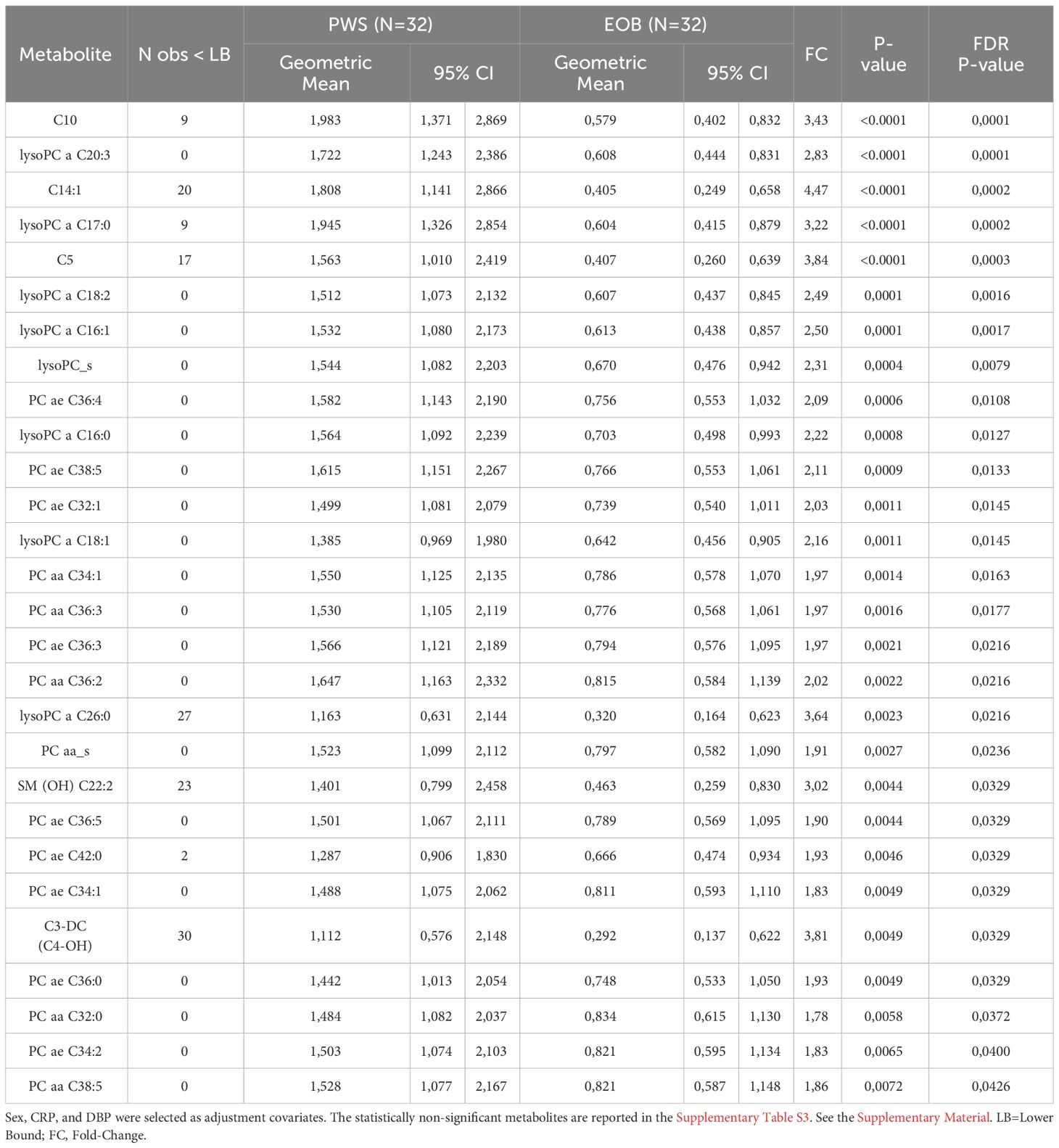
Table 2 Multivariable Tobit regression models used to evaluate the association of PWS with metabolites expressed in at least 20% of the population.
Figure 1 shows the Volcano plot of metabolomics data, while Figure S1, included in the Supplementary Material, represents the box-plots referring to the distribution of the 28 metabolites associated with PWS (as described above).
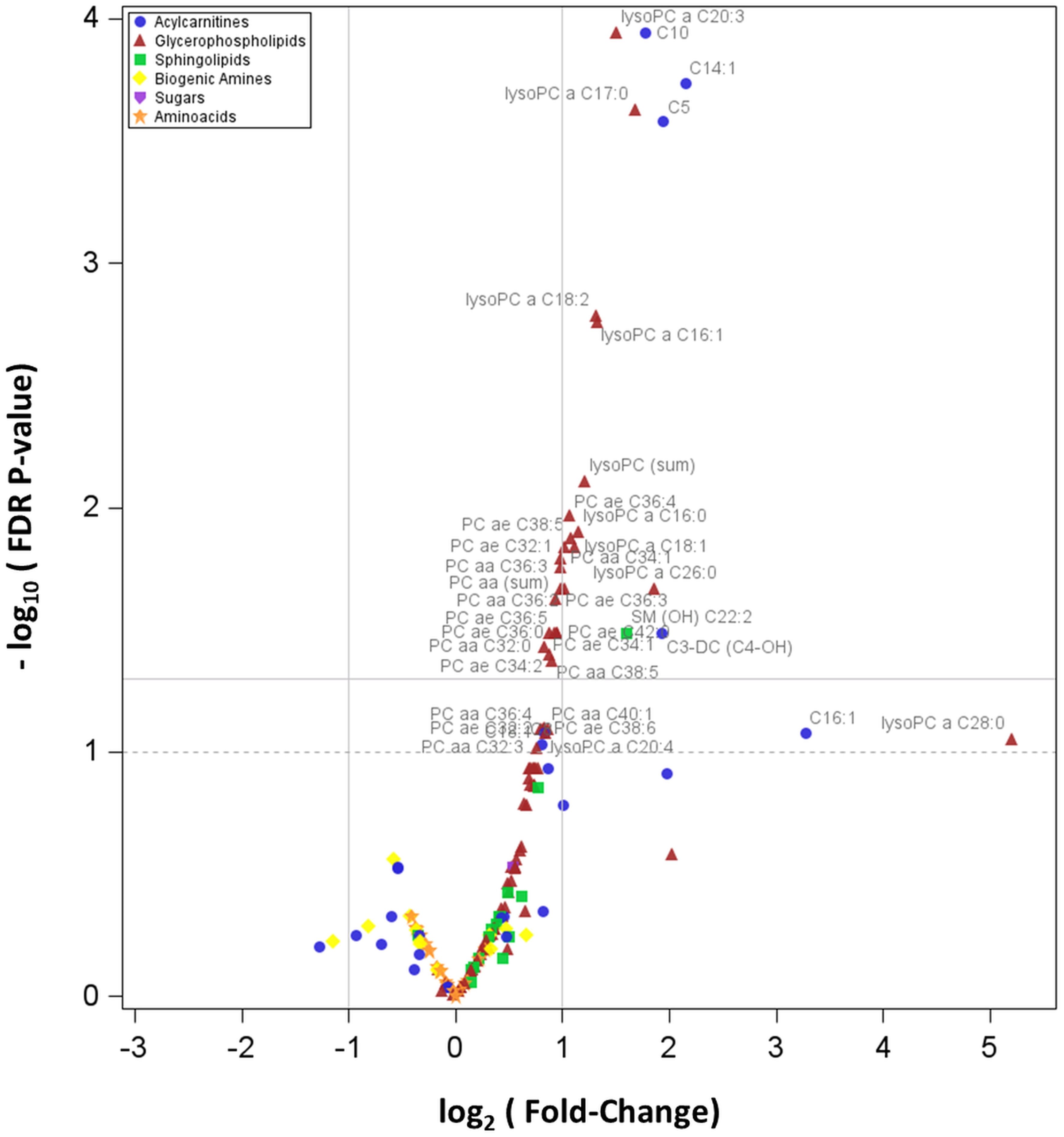
Figure 1 Volcano plot showing how plasma concentrations of each metabolite are differentially expressed between PWS and EOB groups. In particular, the graph reports the values of the 166 detectable metabolites. The horizontal line states the –log10 of the FDR 0.05, being the horizontal dot line the –log10 of the FDR p-value 0.10; the two vertical lines indicate the log2 of the Fold-Change (FC) values 0.5 and 2.0.
4 Discussion
In the present study, the plasma metabolomic profile of adult patients with PWS has been determined in comparison with that of a control group, consisting of sex-, age- and BMI-matched subjects with EOB, being similar the prevalence of metabolic syndrome in both groups. When considering the results of a series of multivariable linear regressions, adjusted for some covariates such as sex, DBP, and CRP, plasma levels of many lysoPCs and PCs, together with those of some acylcarnitines and one SM, were shown to be associated with PWS (vs. non-PWS, i.e., EOB), with a FC ranging from 1.83 to 4.47, indicating higher levels of all metabolites in PWS than EOB group.
To the best of our knowledge, no one has so far applied a metabolomic methodology to (biochemically) investigate the peculiar clinical phenotype in PWS compared to EOB. For the first time, in the limitations of our small patients’ sample, we have fundamentally documented an alteration in lipid metabolism, particularly the biochemical pathways of acylcarnitines and lysoPCs/PCs.
Understanding the molecular mechanism(s) underlying metabolomics-derived biochemical results is not easy, but, in the present discussion, we would like to tentatively provide some explanations.
As well-known, obesity is characterized by a lipid excess, with increased plasma non-esterified fatty acids (NEFAs), with ensuing lipid accumulation into different peripheral tissues, such as the liver and skeletal muscle (20, 21). This deleterious process occurs not only as a result of increased NEFA availability, but also reduced FA oxidation (FAO) (22). Indeed, the rate of FAO, the so-called mitochondrial β-oxidation, has been reported to be reduced in the obese state, particularly during fasting (23). The proposed mechanism underlying this reduction is malonyl-CoA inhibition of carnitine-palmitoyl-transferase-1 (CPT1)-mediated entry of NEFA into mitochondria through acylcarnitine translocase (24). This molecular explanation is apparently congruent with our finding of increased C3-DC-acyl carnitine (i.e., malonylcarnitine), which would suggest an intracellular availability of malonyl-CoA.
Anyway, as demonstrated by animal and human studies (25), in obesity there is an “intrinsic” decrease in mitochondrial FAO capacity rather than an acylcarnitines generation (26).
In the present study, a general increase in plasma acylcarnitines levels was found in both EOB and PWS groups (when compared to the corresponding ranges in the normal-weight population) (17, 27), suggesting an impairment in mitochondrial FAO capacity rather than CPT1 function. Furthermore, malonyl-CoA, an inhibitor of CPT1, has not the same biochemical role as malonylcarnitine (C3-DC-acylcarnitine), generated by CPT1.
The acylcarnitine profile, as determined through our metabolomic approach, includes substrates of both fatty acid oxidation and amino acid β-oxidation (28). In particular, long-chain acylcarnitines, containing up to 20 carbons, accumulate in response to incomplete/inefficient FAO. In contrast, amino acid catabolism produces short-chain acylcarnitine species such as C3-acylcarnitine, C4-acylcarnitine, and C5-acylcarnitine. Importantly, the accumulation of medium- and short-chain acylcarnitines can also derive from the intermediate and ultimate steps (or rounds) of an incomplete/inefficient FAO (29). In the present study, differently from patients with EOB, increased levels of medium- (i.e., C10 and C14:1) and short-chain (i.e., C3-DC, C4-OH, and C5) acylcarnitines were found in the group of patients with PWS, suggesting a more incomplete/inefficient mitochondrial FAO, even in the intermediate/ultimate steps of the oxidative shortening of the acyl chain.
To date, we are unable to explain the molecular reasons for this different impairment of FAO in EOB (initial steps of FAO) vs. PWS (intermediate/ultimate steps of FAO). In this regard, our patients with PWS had less FFM (kg) in comparison to patients with EOB (30, 31). If FAO is predominantly carried out by skeletal muscle (32–34), the increased plasma short-chain acylcarnitines levels, found in our subjects with PWS, might depend on an incomplete/inefficient FAO related to a reduced amount of skeletal muscle (22).
Reportedly, plasma levels of branched-chain amino acid (BCAA) and short-chain acylcarnitines are increased in the obese state, mainly due to a dysfunctional BCAA catabolic pathway (27, 35). In particular, C5-acyl-CoA (i.e., isovaleryl-CoA) derives from leucine and isoleucine catabolism, from which C5-acylcarnitine (i.e., isovalerylcarnitine) is generated. In patients with PWS, a more evident sarcopenia has been documented due to GHD, hypogonadism, and muscle hypotonia (36), suggesting a skeletal muscle catabolic or anti-anabolic state that might represent another explanation for the higher short-chain acylcarnitines levels in patients with PWS than in those with EOB.
Lecithin cholesterol acyl transferase (LCAT) is a plasma enzyme that esterifies cholesterol. This enzyme, primarily produced in the liver, is associated with specific lipoproteins, being the majority bound to HDL-C and, to a lesser extent, to LDL-C (37). LCAT has two different catalytic activities that account for its ability to esterify cholesterol: the first is a phospholipase A2 activity, which cleaves FAs from the sn-2 position of PCs; the second is a transesterification activity, which transfers the cleaved FA to the hydroxyl group on the A-ring of cholesterol, with the production of a molecule of lysoPC. Apolipoprotein A-I (apoA-I), which is predominantly expressed in HDL-C, is an activating factor of LCAT by modifying the presentation of its lipid substrates. As cholesteryl esters are more hydrophobic than free cholesterol, cholesteryl esters formed by LCAT transfer from the surface of lipoproteins to the hydrophobic core. This process transforms the small pre-β-HDL (the so-called nascent HDL) into mature HDL (i.e., larger, spherical-shaped α-migrating particles) (38).
Based on the metabolomic results obtained in the present study, the higher levels of lysoPCs and PCs are supposed to be related to an increase in (HDL-associated) LCAT activity in PWS than EOB. This putative explanation is congruent with the higher HDL-C levels in patients with PWS than in those with EOB.
After its esterification by LCAT, cholesteryl esters on HDL can be transferred to apoB-containing lipoproteins (such as LDL) by cholesteryl ester transfer protein (CETP) (38). In the present study, though the statistical significance was not reached, LDL-C and T-C levels were higher in patients with PWS than in those with EOB. This finding might be an indirect consequence of LCAT hyperactivity, cholesteryl esters enriched HDL and enhanced CETP-mediated transfer of cholesteryl esters from HDL to LDL, with the ensuing increase in T-C levels.
More difficult is to identify the causes of this (supposed) PWS-related LCAT hyperactivity (vs. EOB). These might be found among several factors recognized as specific for PWS compared to EOB, including abundance, distribution, and type of FM, the elevation of adiponectin and ghrelin and reduction of oxytocin and irisin levels, GHD, and impairment in the autonomic nervous system, all of which have been demonstrated to exert some effects on lipid metabolism (such as lysoPcs/PCs, NEFA, HDL-C, etc) (6–12). Based on the available information about the PWS patients recruited in the present study, apart from GHD and GH therapy, we cannot determine which factor(s) is of utmost importance, and what is the underlying mechanism(s) of PWS-specific metabolomic differences (vs. EOB) as above described.
As known, SMs represent the major sphingolipid species in mammalian cells, concentrated in the outer leaflet of the plasma membrane (39). SM plays an important role in maintaining the function and integrity of lipid rafts, which are microdomains in the plasma membrane that are implicated in signal transduction (40, 41). SM is synthesized from ceramide (Cer) and PC by sphingomyelin synthases (SMSs), for which two genes have been identified (i.e., SMS1 and SMS2). Mammalian cells produce many species of SM (42, 43). Liver SMS2 is one of the determinants of plasma SM levels (44). Finally, high plasma SM levels have been associated with coronary artery disease, atherosclerosis, and obesity (45–49).
Among several sphingoid metabolites that were measured in the plasma through our metabolomic approach, only one SM, precisely SM C22:2, was found to be associated with PWS (compared to EOB). We do not know why SM C22;2 levels were higher in patients with PWS than in those with EOB. The most plausible explanation is the general abundance of PCs in PWS compared to EOB, which, as described above, represent substrates for SMS2.
Another hypothesis can be argued: as sphingolipids are known to elicit cellular inflammatory and specifically immune responses via many different molecular mechanisms (46, 50), the distinctive alteration of SM metabolism might be the consequence (or the cause?) of that anomalous activation of the innate immune system that, in PWS, has been demonstrated to be independent from central adiposity and insulin resistance (51).
In recent years, several studies have documented that PWS patients have lower insulinemia and insulin resistance than (BMI-matched) EOB controls, suggesting protected glucose metabolism (11). According to this view, the prevalence of impaired glucose intolerance, T2DM, or metabolic syndrome in PWS tends to be lower when compared to that in EOB controls (6, 10, 52).
In the present study, PWS patients, though more treated with antidiabetic drugs, exhibited lower insulinemia in the context of hyperglycemia, when compared to the group with EOB. No other differences in T2DM biochemical markers, such as HOMA-IR and HbA1c, were found between the PWS and EOB groups. Our results seem to conflict with the commonly accepted view of a favorable glucometabolic homeostasis in PWS (vs. EOB) (11), but, due to the experimental design adopted for the present study, we were forced to strictly match the most relevant demographic, biochemical, and clinical characteristics between PWS and EOB groups, including the diagnosis of metabolic syndrome. This might have introduced an intrinsic (non-avoidable) bias. Furthermore, if abundance, distribution, and type of FM differ in PWS compared to EOB, BMI-matching might have minimized, as demonstrated by the present study and other ones, the typical insulin sensitivity in PWS. Being BMI (as well as FM/FFM) a “gross” parameter, only the use of sophisticated methods capable of evaluating the specific body fat patterning in PWS might solve this issue.
In a recent study by Hou et al. (53), a serum lipidomics analysis was simultaneously explored in PWS, EOB, and normal-weight Chinese children. Results indicated that the total PCs and lysoPCs were deceased in PWS children compared with both EOB and normal-weight groups. In contrast, several SM, Cer, acylcarnitine, and TG species were increased. We do not know the reason(s) for the discrepancy between these results and those reported in our study. Age (children vs. adults), BMI (with higher values in our study), ethnicity (Asian vs. Caucasian), and selection of different gut microbial populations for diet and other environmental factors might be invoked.
Before closing, apart from the limited sample size and the cross-sectional design, some other limitations of our study should be mentioned. First of all, we have determined only the metabolomic profiling in fasting conditions with no specific external interventions. In particular, metabolomic responses to exercise or weight loss might be different in PWS compared to EOB; thus, future studies are mandatory to investigate these research topics. Secondly, we have not determined microbial profiling in feces from any recruited subject. So, we cannot rule out that the changes in metabolite concentrations that were observed in the present study may be related to a different gut microbiota, capable of modifying the host’s metabolism or metabolite absorption from the gut in PWS compared to EOB.
5 Conclusions
By using a metabolomic approach, patients with PWS, compared to those with EOB, exhibit higher plasma levels of specific metabolites belonging to some chemical classes, including manyfold lysoPCs/PCs, few (particularly, short-chain) acylcarnitines, and one SM, suggesting a different regulation of some biochemical pathways, fundamentally related to lipid metabolism. Deciphering the molecular mechanisms underlying the specific metabolomic profile might allow us to understand the peculiar clinical phenotype in patients with PWS (vs. those with EOB). Further studies are mandatory to translate these molecular data into more effective treatments and better clinical management of PWS.
Data availability statement
The datasets used and/or analyzed in the present study are available in the Supplementary Material. Raw data related to this study will be uploaded on Zenodo.org and available upon reasonable request to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Istituto Auxologico Italiano (Milan, Italy). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AR: Conceptualization, Writing – original draft, Writing – review & editing. EP: Data curation, Formal analysis, Methodology, Writing – review & editing. CF: Formal analysis, Writing – review & editing. DC: Data curation, Writing – review & editing. AB: Data curation, Writing – review & editing. GG: Investigation, Writing – review & editing. SM: Data curation, Writing – review & editing. SC: Writing – review & editing. SF: Funding acquisition, Methodology, Writing – review & editing. AS: Conceptualization, Funding acquisition, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Italian Ministry of Health - Ricerca Corrente. The two metabolomics kits used were purchased in the frame of a grant by Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1386265/full#supplementary-material
Abbreviations
AA, amino acids; Arg, arginine; BA, biogenic amines; BCAA, branched-chain amino acids; BMI, body mass index; C0, L-carnitine; EC, Ethical Committee; EOB, essential obesity; CETP, cholesteryl ester transfer protein; Cit, citrulline; CPT1, carnitine-palmitoyl-transferase-1; CRP, C-reactive protein; del15, paternal 15q11-q13 deletion; FA, fat acid; FAO, FA oxidation; FFM, fat free mass; FM, fat mass; Gln, glutamine; GLS, glutaminase; Glu, Glutamic acid; HbA1c, glycated hemoglobin; HDL, high density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; HR, heart rate; LB, lower bound; LCAT, lecithin cholesterol acyl transferase; LC-MS/MS, liquid chromatography tandem mass spectrometry; LDL, low density lipoprotein; Leu, leucine; LOD, limit of detection; lysoPC, lysophos-phatidylcholine; NEFA, not esterified fat acids; obs, observation; PC aa, diacylphosphatidylcholine; PC ae, acylalkylphosphatidylcholine; pctl, percentile; Phe, phenylalanine; q, quartile; REE, resting energy expenditure; SM, sphingolipid; SMS, sphingomyelin synthase; T2DM, type 2 diabetes mellitus; Trp, tryptophan; Tyr, tyrosine; UDP15, maternal uniparental disomy 15; WHR, waist to hip ratio.
References
1. Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest. (2015) 38:1249–63. doi: 10.1007/s40618-015-0312-9
2. Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. (2012) 14:10–26. doi: 10.1038/gim.0b013e31822bead0
3. Marzullo P, Marcassa C, Campini R, Eleuteri E, Minocci A, Priano L, et al. The impact of growth hormone/insulin-like growth factor-I axis and nocturnal breathing disorders on cardiovascular features of adult patients with Prader-Willi syndrome. J Clin Endocrinol Metab. (2005) 90:5639–46. doi: 10.1210/jc.2005-0829
4. Kim SJ, Cho SY, Jin DK. Prader-Willi syndrome: an update on obesity and endocrine problems. Ann Pediatr Endocrinol Metab. (2021) 26:227–36. doi: 10.6065/apem.2142164.082
5. Butler MG, Hartin SN, Hossain WA, Manzardo AM, Kimonis V, Dykens E, et al. Molecular genetic classification in Prader-Willi syndrome: a multisite cohort study. J Med Genet. (2019) 56:149–53. doi: 10.1136/jmedgenet-2018-105301
6. Grugni G, Crino A, Bedogni G, Cappa M, Sartorio A, Corrias A, et al. Metabolic syndrome in adult patients with Pradere-Willi syndrome. Nutr Metab Cardiovasc Dis. (2013) 23:1134–40. doi: 10.1016/j.numecd.2012.11.006
7. Pacoricona Alfaro DL, Lemoine P, Ehlinger V, Molinas C, Diene G, Valette M, et al. Causes of death in Prader-Willi syndrome: lessons from 11 years’ experience of a national reference center. Orphanet J Rare Dis. (2019) 14:238. doi: 10.1186/s13023-019-1214-2
8. Goldstone AP, Thomas EL, Brynes AE, Bell JD, Frost G, Saeed N, et al. Visceral adipose tissue and metabolic complications of obesity are reduced in Prader-Willi syndrome female adults: evidence for novel influences on body fat distribution. J Clin Endocrinol Metab. (2001) 86:4330–8. doi: 10.1210/jcem.86.9.7814
9. Talebizadeh Z, Butler MG. Insulin resistance and obesity-related factors in Prader-Willi syndrome: comparison with obese subjects. Clin Genet. (2005) 67:230–9. doi: 10.1111/j.1399-0004.2004.00392.x
10. Fintini D, Grugni G, Bocchini S, Brufani C, Di Candia S, Corrias A, et al. Disorders of glucose metabolism in Prader-Willi syndrome: Results of a multicenter Italian cohort study. Nutr Metab Cardiovasc Dis. (2016) 26:842–7. doi: 10.1016/j.numecd.2016.05.010
11. Qian Y, Xia F, Zuo Y, Zhong M, Yang L, Jiang Y, et al. Do patients with Prader-Willi syndrome have favorable glucose metabolism? Orphanet J Rare Dis. (2022) 17:187. doi: 10.1186/s13023-022-02344-3
12. Vlaardingerbroek H, van den Akker ELT, Hokken-Koelega ACS. Appetite- and weight-inducing and -inhibiting neuroendocrine factors in Prader-Willi syndrome, Bardet-Biedl syndrome and craniopharyngioma versus anorexia nervosa. Endocr Connect. (2021) 10:R175–88. doi: 10.1530/EC-21-0111
13. Aderemi AV, Ayeleso AO, Oyedapo OO, Mukwevho E. Metabolomics: A scoping review of its role as a tool for disease biomarker discovery in selected non-communicable diseases. Metabolites. (2021) 11:418. doi: 10.3390/metabo11070418
14. Rangel-Huerta OD, Pastor-Villaescusa B, Gil A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics. (2019) 15:93. doi: 10.1007/s11306-019-1553-y
15. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. (2004) 27:1487–95. doi: 10.2337/diacare.27.6.1487
16. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetes Med. (2006) 23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x
17. Frigerio G, Favero C, Savino D, Mercadante R, Albetti B, Dioni L, et al. Plasma metabolomic profiling in 1391 subjects with overweight and obesity from the SPHERE study. Metabolites. (2021) 11:194. doi: 10.3390/metabo11040194
18. Tobin J. Estimation of relationship for limited dependent variables. Econometrica. (1958) 26:24–36. doi: 10.2307/1907382
19. McDonald JF, Robert AM. The uses of Tobit analysis. Rev Econ Stat. (1980) 62:318–21. doi: 10.2307/1924766
20. Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β-cell dysfunction. Eur J Clin Invest. (2002) 32:14–23. doi: 10.1046/j.1365-2362.32.s3.3.x
21. Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. (1997) 46:1579–85. doi: 10.2337/diabetes.46.10.1579
22. Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. (2000) 279:E1039–44. doi: 10.1152/ajpendo.2000.279.5.E1039
23. Rogge M. The role of impaired mitochondrial lipid oxidation in obesity. Biol Res Nurs. (2009) 10:356–73. doi: 10.1177/1099800408329408
24. McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to Mol anal Eur J Biochem. (1997) 244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x
25. Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obes (Silver Spring). (2010) 18:1695–700. doi: 10.1038/oby.2009.510
26. Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. (2002) 51:2944–50. doi: 10.2337/diabetes.51.10.2944
27. Rigamonti AE, Frigerio G, Caroli D, De Col A, Cella SG, Sartorio A, et al. A metabolomics-based investigation of the effects of a short-term body weight reduction program in a cohort of adolescents with obesity: A prospective interventional clinical study. Nutrients. (2023) 15:529. doi: 10.3390/nu15030529
28. Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem. (2003) 49:1797–817. doi: 10.1373/clinchem.2003.022178
29. Houten SM, Violante S, Ventura FV, Wanders RJ. The biochemistry and physiology of mitochondrial fatty acid β-oxidation and its genetic disorders. Annu Rev Physiol. (2016) 78:23–44. doi: 10.1146/annurev-physiol-021115-105045
30. Bedogni G, Grugni G, Tringali G, Tamini S, Marzullo P, Sartorio A. Assessment of fat-free mass from bioelectrical impedance analysis in men and women with Prader-Willi syndrome: cross-sectional study. Int J Food Sci Nutr. (2019) 70:645–9. doi: 10.1080/09637486.2018.1554623
31. Theodoro MF, Talebizadeh Z, Butler MG. Body composition and fatness patterns in Prader-Willi syndrome: comparison with simple obesity. Obes (Silver Spring). (2006) 14:1685–90. doi: 10.1038/oby.2006.193
32. Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, et al. Peroxisome proliferator-activated receptor-γ co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. (2005) 280:33588–98. doi: 10.1074/jbc.M507621200
33. Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. (2008) 7:45–56. doi: 10.1016/j.cmet.2007.10.013
34. An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. (2004) 10:268–74. doi: 10.1038/nm995
35. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. (2009) 9:311–26. doi: 10.1016/j.cmet.2009.02.002
36. Lafortuna CL, Minocci A, Capodaglio P, Gondoni LA, Sartorio A, Vismara L, et al. Skeletal muscle characteristics and motor performance after 2-year growth hormone treatment in adults with prader-willi syndrome. J Clin Endocrinol Metab. (2014) 99:1816–24. doi: 10.1210/jc.2013-3607
37. Rousset X, Vaisman B, Amar M, Sethi AA, Remaley AT. Lecithin: cholesterol acyltransferase-from biochemistry to role in cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. (2009) 16:163–71. doi: 10.1097/MED.0b013e328329233b
38. Rousset X, Shamburek R, Vaisman B, Amar M, Remaley AT. Lecithin cholesterol acyltransferase: an anti- or pro-atherogenic factor? Curr Atheroscler Rep. (2011) 13:249–56. doi: 10.1007/s11883-011-0171-6
39. Zachowski A. Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem J. (1993) 294:1–14. doi: 10.1042/bj2940001
40. Simons K, Ikonen E. Functional rafts in cell membranes. Nature. (1997) 387:569–72. doi: 10.1038/42408
41. Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. (2002) 110:597–603. doi: 10.1172/JCI0216390
42. Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, et al. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J Lipid Res. (2010) 51:3074–87. doi: 10.1194/jlr.D008532
43. Qu F, Wu CS, Hou JF, Jin Y, Zhang JL. Sphingolipids as new biomarkers for assessment of delayed-type hypersensitivity and response to triptolide. PloS One. (2012) 7:e52454. doi: 10.1371/journal.pone.0052454
44. Liu J, Zhang H, Li Z, Hailemariam TK, Chakraborty M, Jiang K, et al. Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arterioscler Thromb Vasc Biol. (2009) 29:850–6. doi: 10.1161/ATVBAHA.109.185223
45. Hanamatsu H, Ohnishi S, Sakai S, Yuyama K, Mitsutake S, Takeda H, et al. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr Diabetes. (2014) 4:e141. doi: 10.1038/nutd.2014.38
46. Liu J, Huan C, Chakraborty M, Zhang H, Lu D, Kuo MS, et al. Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circ Res. (2009) 105:295–303. doi: 10.1161/CIRCRESAHA.109.194613
47. Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, et al. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. (2000) 20:2614–8. doi: 10.1161/01.ATV.20.12.2614
48. Rigamonti AE, Dei Cas M, Caroli D, Bondesan A, Cella SG, Paroni R, et al. Ceramide risk score in the evaluation of metabolic syndrome: an additional or substitutive biochemical marker in the clinical practice? Int J Mol Sci. (2023) 24:12452. doi: 10.3390/ijms241512452
49. Rigamonti AE, Dei Cas M, Caroli D, De Col A, Cella SG, Paroni R, et al. Identification of a specific plasma sphingolipid profile in a group of normal-weight and obese subjects: A novel approach for a “Biochemical” Diagnosis of metabolic syndrome? Int J Mol Sci. (2023) 24:7451. doi: 10.3390/ijms24087451
50. Nixon GF. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol. (2009) 158:982–93. doi: 10.1111/j.1476-5381.2009.00281.x
51. Viardot A, Sze L, Purtell L, Sainsbury A, Loughnan G, Smith E, et al. Prader-Willi syndrome is associated with activation of the innate immune system independently of central adiposity and insulin resistance. J Clin Endocrinol Metab. (2010) 95:3392–9. doi: 10.1210/jc.2009-2492
52. Krochik AG, Ozuna B, Torrado M, Chertkoff L, Mazza C. Characterization of alterations in carbohydrate metabolism in children with Prader-Willi syndrome. J Pediatr Endocrinol Metab. (2006) 19:911–8. doi: 10.1515/JPEM.2006.19.7.911
Keywords: Prader-Willi syndrome, essential obesity, metabolomics, biochemical pathways, lipid metabolism
Citation: Rigamonti AE, Polledri E, Favero C, Caroli D, Bondesan A, Grugni G, Mai S, Cella SG, Fustinoni S and Sartorio A (2024) Metabolomic profiling of Prader-Willi syndrome compared with essential obesity. Front. Endocrinol. 15:1386265. doi: 10.3389/fendo.2024.1386265
Received: 14 February 2024; Accepted: 29 April 2024;
Published: 15 May 2024.
Edited by:
Andrea P. Rossi, Ca’ Foncello Hospital, ItalyReviewed by:
Vittorio Locatelli, University of Milano-Bicocca, ItalyAntonio Torsello, University of Milano-Bicocca, Italy
Copyright © 2024 Rigamonti, Polledri, Favero, Caroli, Bondesan, Grugni, Mai, Cella, Fustinoni and Sartorio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonello E. Rigamonti, YW50b25lbGxvLnJpZ2Ftb250aUB1bmltaS5pdA==
 Antonello E. Rigamonti
Antonello E. Rigamonti Elisa Polledri
Elisa Polledri Chiara Favero
Chiara Favero Diana Caroli2
Diana Caroli2 Graziano Grugni
Graziano Grugni Stefania Mai
Stefania Mai Silvano G. Cella
Silvano G. Cella Silvia Fustinoni
Silvia Fustinoni Alessandro Sartorio
Alessandro Sartorio