
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 26 June 2024
Sec. Thyroid Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1385747
This article is part of the Research Topic Advances in precision medicine in the management of thyroid nodules and thyroid cancer View all 38 articles
Background: For patients with anaplastic thyroid cancer (ATC) without mutational driver genes, chemotherapy is suggested to be the first-line treatment option. However, the benefits of chemotherapy in treating ATC are limited. In this analysis, we collected the prospective data reported since 2010 to analyze the emerging chemotherapy-based treatments in ATC comprehensively.
Methods: For this updated analysis, we searched PubMed (MEDLINE), Web of Science, Embase, and Cochrane CENTRAL databases from 1 January 2010 to 7 February 2024 for prospective clinical studies that contained chemotherapy-based treatments. This analysis was done to pool overall survival (OS), progression-free survival (PFS), objective response rates (ORRs), disease control rates (DCRs), and grade 3 or worse treatment-related adverse events (TRAEs).
Results: Six prospective clinical trials with 232 patients were included. Chemotherapy was commonly combined with targeted therapy or radiotherapy. The pooled median OS was 6.0 months (95% CI 4.1–9.7), and the median PFS was 3.2 months (95% CI 1.9–6.0) in patients with ATC who received chemotherapy-based strategies. The integrated ORR and DCR were 21% (95% CI 15%–27%) and 64% (95% CI 55%–72%), respectively. Regarding the grade 3 or worse TRAE, the pooled incidence was 68% (95% CI 47%–86%).
Conclusion: Although the emerging chemotherapy-based treatments showed antitumor activity in patients with ATC, these strategies failed to prolong the survival time substantially. More practical, safe, and novel therapeutic regimens for patients with ATC warrant further investigations.
Anaplastic thyroid cancer (ATC) is recognized for its rare incidence, high aggressiveness, and poor prognosis. For patients without mutational driver genes (e.g., BRAF V600E, NTRK, and RET), the recommended systemic therapeutic option in the National Comprehensive Cancer Network (NCCN) guideline was chemotherapy, including paclitaxel, doxorubicin, carboplatin, and cisplatin (1).
Retrospectively, doxorubicin plus cisplatin chemotherapy has been applied in ATC since 1985. The median overall survival (OS) was approximately 6 months, with an objective response rate (ORR) of up to 26% (2). In terms of taxane-based treatments, clinicians have begun to use these strategies since 2000. In Kenneth B. Ain’s study, the median OS was also nearly 6 months, but the ORR was over 50% (3).
Nevertheless, the efficacy of cytotoxic drugs is limited. The total lifetime of patients with ATC is hard to exceed half a year. In the “Systemic therapy: cytotoxic chemotherapy” section of the American Thyroid Association Guidelines for Management of Patients with ATC (2021), the cited latest record studying cytotoxic drugs in ATC was published in 2010 (4). We are eager to find out whether novel cytotoxic drugs have been explored to treat ATC after 2010.
Therefore, we conducted this updated analysis to collect and synthesize the efficacy and safety data of emerging chemotherapy-based treatments reported in prospective studies from January 2010 to February 2024. Through this analysis, we intend to provide more insights into future studies.
For this analysis, we searched PubMed (MEDLINE), Web of Science, Embase, and Cochrane CENTRAL databases to identify prospective studies from 1 January 2010 to 7 February 2024. The search terms included “chemotherapy or taxane or paclitaxel or docetaxel or taxel or doxorubicin or cisplatin or carboplatin or mitoxantrone or cyclophosphamide or fluorouracil” and “anaplastic thyroid cancer”. The study type was defined as “clinical trial” or “randomized clinical trial”. This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline (5).
The inclusion criteria were as follows: (1) patients were pathologically diagnosed as ATC; (2) patients were treated with cytotoxic chemotherapy-based treatments; (3) data of survival outcomes, response rates, and incidences of treatment-related adverse events (TRAEs) were available; and (4) enrolled studies were prospective clinical trials published in English. Meeting abstracts were excluded.
The following data were extracted from enrolled trials: first authors’ names, year of publication, study design, dose of drugs, median survival time, survival rates, response rates, and TRAEs. Kaplan–Meier survival curves were captured from the original studies, and the Scanlt software was used to digitize these curves.
Reconstructed OS and progression-free survival (PFS) curves were pooled and analyzed using the metaSurvival package of the R software. The detailed protocol has been described in Christophe Combescure’s study (6).
Pooled ORRs, disease control rates (DCRs), and incidences of TRAEs with 95% CIs were conducted using the meta package of the R software (version 4.2.2). Heterogeneity was quantified using I2 statistic percentages (low: I2 < 50%, p < 0.05). Single-arm analyses were done with the random-effects model.
Funnel plots and Egger’s test (p < 0.01) were used to assess publication bias.
The systematic search returned 109 potentially relevant records. After deduplication and screening, six clinical trials comprising 232 patients were identified and included in the analysis (Figure 1) (7–12).
Table 1 summarizes the characteristics of the trials. Median age ranged from 56 to 71. Cytotoxic drugs included paclitaxel, docetaxel, doxorubicin, and carboplatin. The combination of chemotherapy with targeted therapy (pazopanib, fosbretabulin, and efatutazone) was applied in three trials. Although radiotherapy was pre-designed in three of the six trials (7–9), patients in the other three trials received radiotherapy either (10–12).
Survival outcomes reported in the trials are summarized in Table 2. The median OS ranged from 2.8 months (docetaxel + doxorubicin plus pembrolizumab and radiotherapy) to 7.3 months (paclitaxel plus placebo and radiotherapy), the median PFS ranged from 1.4 months (docetaxel) to 3.3 months (paclitaxel + carboplatin plus fosbretabulin), and the 1-year survival rates ranged from 8.7% (paclitaxel + carboplatin) to 37.1% (paclitaxel plus pazopanib and radiotherapy).
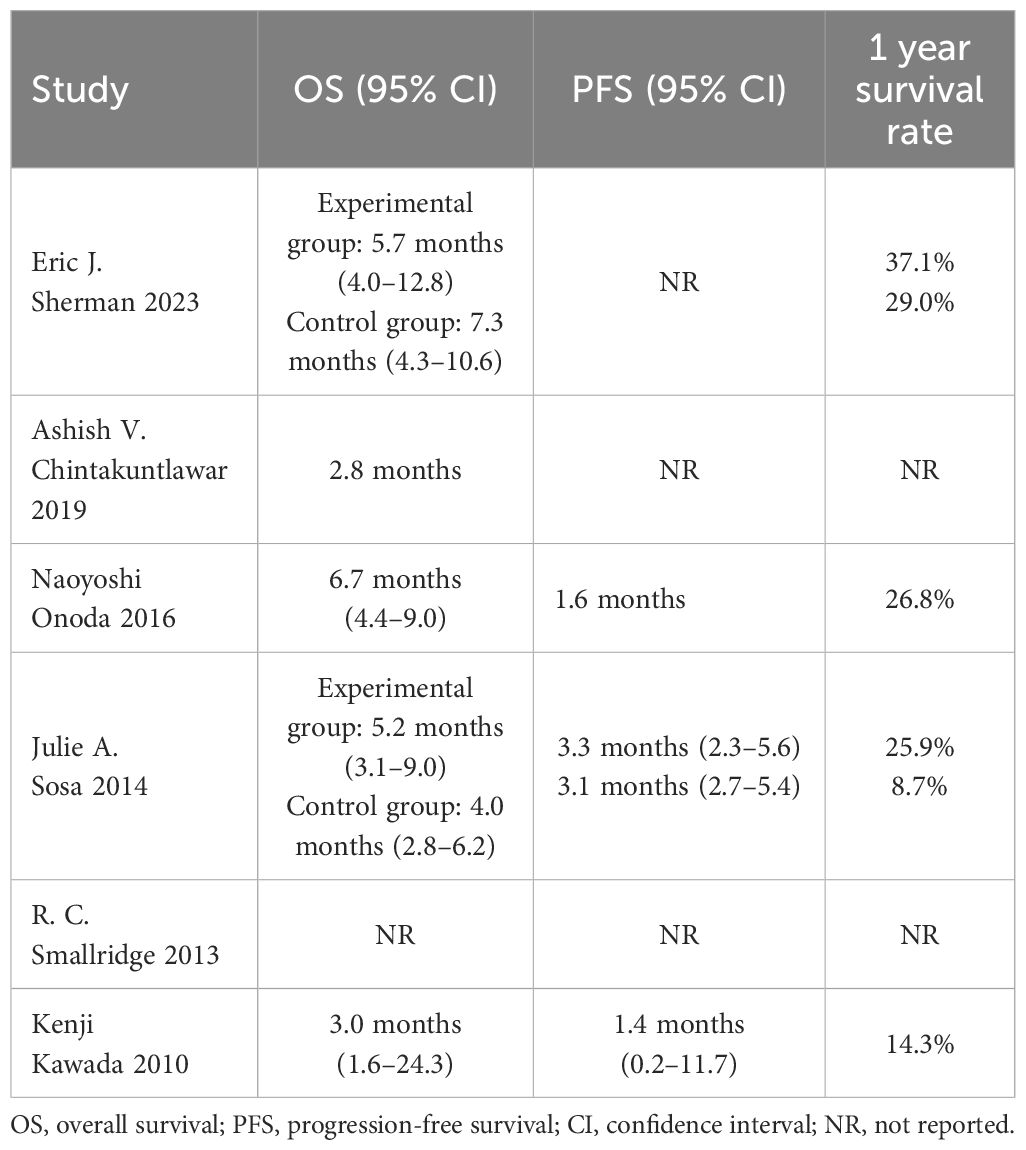
Table 2 Survival outcomes of anaplastic thyroid cancer patients treated with chemotherapy-based treatments.
OS data were derived from the OS curves reported in four of the six trials (7, 9–11). The pooled median OS was 6.0 months (95% CI 4.1–9.7) (Figure 2A). The 6-month and 1-year OS rates were 50% (95% CI 37–68) and 30% (95% CI 18–50), respectively.
PFS data were reconstructed from the PFS curves published in two of the six trials (10, 11). The pooled median PFS was 3.2 months (95% CI 1.9–6.0) (Figure 2B). The 6-month and 1-year PFS rates were 31% (95% CI 10–64) and 10% (95% CI 1–58), respectively.
Similarly, response rates and grade 3 or worse safety data were pooled-analyzed. A total of 192 patients from five trials were enrolled in the analysis of ORR (7, 9–12). The pooled ORR was 21% (95% CI 15–27) (Figure 3A). DCR data from 142 patients in four trials were integrated (9–12). The pooled DCR was 64% (95% CI 55–72) (Figure 3B).
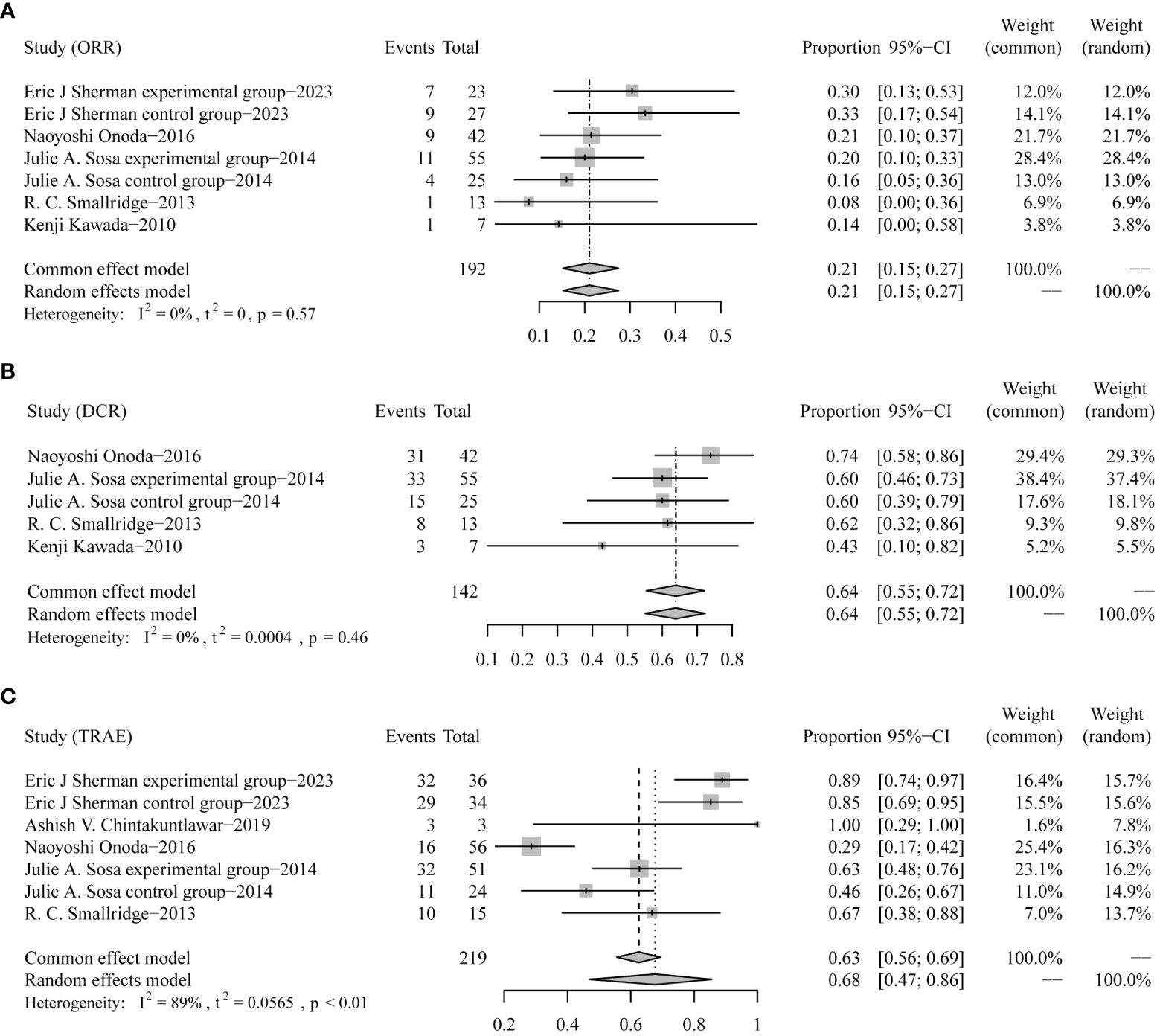
Figure 3 Forest plots for objective response rates [ORRs, (A)], disease control rates [DCRs, (B)], and incidences of grade 3 or worse treatment-related adverse events [TRAEs, (C)].
In terms of TRAE data collected from five trials involving 219 patients (7–11), the pooled rate of grade 3 or worse TRAE was 68% (95% CI 47–86) (Figure 3C).
Funnel plots and Egger’s tests failed to find any publication bias during the analyses of ORR (Figures 4A, B), DCR (Figures 4C, D), and TRAE (Figures 4E, F).
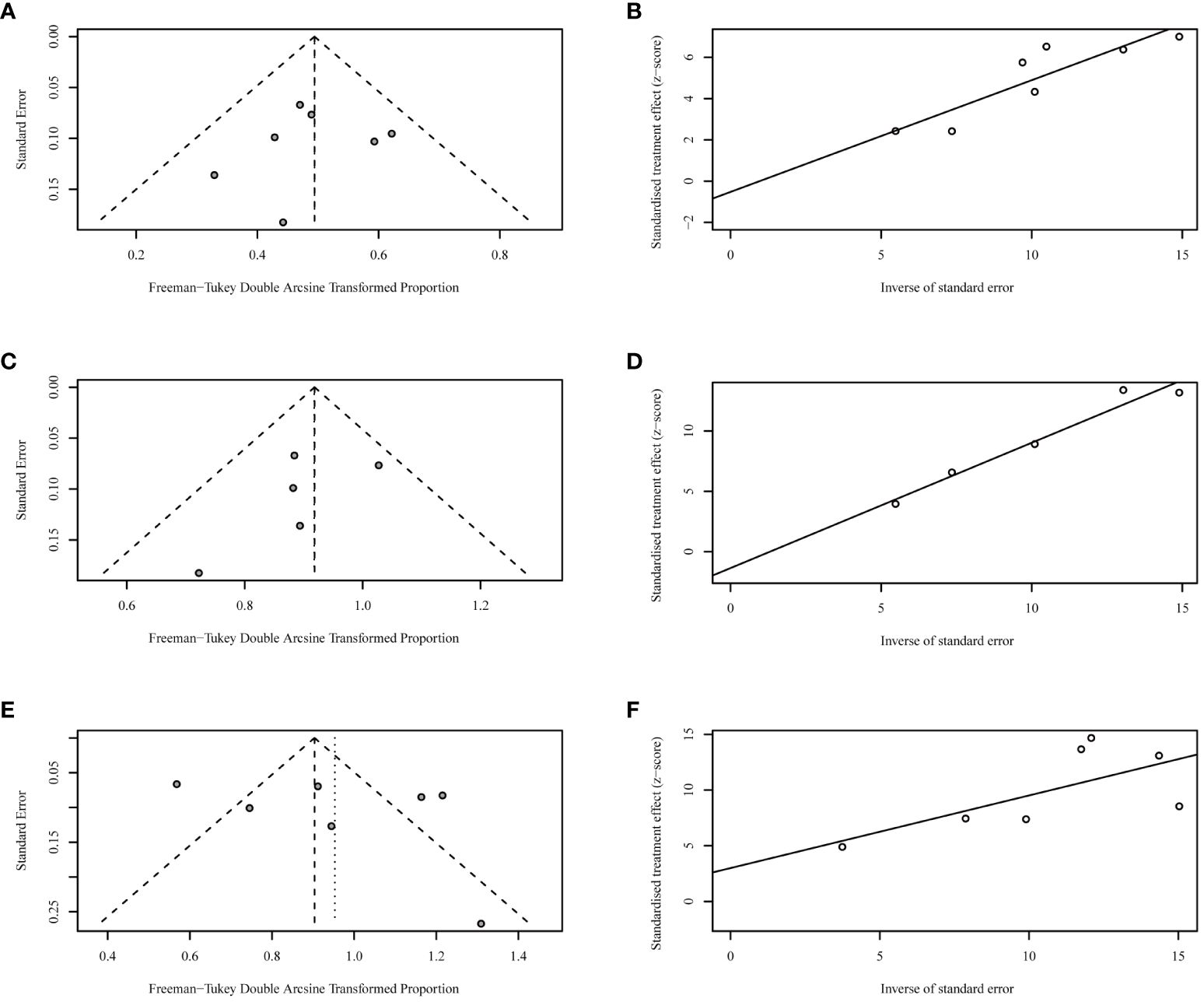
Figure 4 Funnel plots and Egger’s tests for objective response rates [ORRs, (A, B)], disease control rates [DCRs, (C, D)], and incidences of grade 3 or worse treatment-related adverse events [TRAEs, (E, F)].
In this updated analysis of emerging chemotherapy-based treatment for patients with ATC, the total lifetime remains within 6 months even if targeted therapy, immunotherapy, or radiotherapy were added, and DCR was over 60%. To overcome the issues, basic researchers and physicians still need to do numerous explorations.
Among the eligible trials, cytotoxic drugs included only paclitaxel, docetaxel, doxorubicin, and carboplatin. Such selections indicate that clinicians or the drug industry may be more confident in these drugs. According to the search strategy described in the Methods section, mitoxantrone, cyclophosphamide, and fluorouracil have also been administered in patients with ATC (13–15). However, substantial improvements in survival outcomes were not identified when patients with ATC were treated with these drugs. Since novel-designed cytotoxic drugs have been approved to treat both solid and hematological tumors, like antibody–drug conjugates brentuximab vedotin (16) and sacituzumab govitecan (17), whether antibody–drug conjugates can help to enhance the responses and prolong survival outcome in ATC deserves further investigations.
In the trials enrolled in this analysis, we found that nearly all participants have received radiotherapy. Indirectly, radiotherapy is critically essential for patients with ATC to achieve local disease control since a rapidly growing neck mass can cause asphyxiation. In other solid tumors, radiotherapy has been certified to benefit patients with a tolerable safety profile. In patients with renal cell carcinoma who decline surgery, stereotactic body radiotherapy can be suggested as a safe and effective standard treatment option (18). In advanced lung cancer, the addition of radiotherapy significantly improved response rates compared with immunotherapy alone (19). Therefore, we believe that reasonable palliative radiotherapy may bring unexpected benefits for patients with advanced ATC.
Although immunotherapy has significantly impacted previous therapeutic strategies for patients with cancer, the efficacy of immune checkpoint inhibitors on ATC was limited. In the subset of patients with PD-L1 < 1%, the median OS was 1.6 months (95% CI 1.0–19.6), while for the subset of patients with PD-L1 > 1%, the data were not reached (32364844). Clinicians have started investigating the combination of immunotherapy and radiotherapy (20, 21). In head and neck cancer, a synergistic effect has been found as both irradiated sites and metastatic lesions achieved responses (21). Accordingly, digging for effective combination therapies for patients with ATC is also crucial.
Regarding the grade 3 or worse TRAEs, the incidence (68%) deserves our attention. Increased alanine aminotransferase, dermatitis radiation, dysphagia, and neutropenia were the most common grade 3 or worse TRAEs recorded in the enrolled trials.
In this updated analysis, the emerging chemotherapy-based treatments did not substantially prolong the survival time of patients with ATC. Novel cytotoxic or targeted drugs or creative combination therapies are warranted in future studies.
B-CW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. G-HL: Data curation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. B-HK: Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. R-BC: Formal Analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 82302956 to B-CW).
We truly thank Wang’s group for providing the statistical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1385747/full#supplementary-material
1. Haddad RI, Bischoff L, Ball D, Bernet V, Blomain E, Busaidy NL, et al. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:925–51. doi: 10.6004/jnccn.2022.0040
2. Shimaoka K, Schoenfeld DA, DeWys WD, Creech RH, DeConti R. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer. (1985) 56:2155–60. doi: 10.1002/1097-0142(19851101)56:9<2155::aid-cncr2820560903>3.0.co;2-e
3. Ain KB, Egorin MJ, DeSimone PA. Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Collaborative Anaplastic Thyroid Cancer Health Intervention Trials (CATCHIT) Group. Thyroid. (2000) 10:587–94. doi: 10.1089/thy.2000.10.587
4. Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr., et al. American thyroid association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. (2021) 31:337–86. doi: 10.1089/thy.2020.0944
5. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. doi: 10.1136/bmj.g7647
6. Combescure C, Foucher Y, Jackson D. Meta-analysis of single-arm survival studies: a distribution-free approach for estimating summary survival curves with random effects. Stat Med. (2014) 33:2521–37. doi: 10.1002/sim.6111
7. Sherman EJ, Harris J, Bible KC, Xia P, Ghossein RA, Chung CH, et al. Radiotherapy and paclitaxel plus pazopanib or placebo in anaplastic thyroid cancer (NRG/RTOG 0912): a randomised, double-blind, placebo-controlled, multicentre, phase 2 trial. Lancet Oncol. (2023) 24:175–86. doi: 10.1016/S1470-2045(22)00763-X
8. Chintakuntlawar AV, Yin J, Foote RL, Kasperbauer JL, Rivera M, Asmus E, et al. A phase 2 study of pembrolizumab combined with chemoradiotherapy as initial treatment for anaplastic thyroid cancer. Thyroid. (2019) 29:1615–22. doi: 10.1089/thy.2019.0086
9. Onoda N, Sugino K, Higashiyama T, Kammori M, Toda K, Ito K, et al. The safety and efficacy of weekly paclitaxel administration for anaplastic thyroid cancer patients: a nationwide prospective study. Thyroid. (2016) 26:1293–9. doi: 10.1089/thy.2016.0072
10. Sosa JA, Elisei R, Jarzab B, Balkissoon J, Lu SP, Bal C, et al. Randomized safety and efficacy study of fosbretabulin with paclitaxel/carboplatin against anaplastic thyroid carcinoma. Thyroid. (2014) 24:232–40. doi: 10.1089/thy.2013.0078
11. Smallridge RC, Copland JA, Brose MS, Wadsworth JT, Houvras Y, Menefee ME, et al. Efatutazone, an oral PPAR-γ agonist, in combination with paclitaxel in anaplastic thyroid cancer: results of a multicenter phase 1 trial. J Clin Endocrinol Metab. (2013) 98:2392–400. doi: 10.1210/jc.2013-1106
12. Kawada K, Kitagawa K, Kamei S, Nada M, Mitsuma A, Sawaki M, et al. The feasibility study of docetaxel in patients with anaplastic thyroid cancer. Japanese J Clin Oncol. (2010) 40:596–9. doi: 10.1093/jjco/hyq025
13. Satake S, Sugawara I, Watanabe M, Takami H. Lack of a point mutation of human DNA topoisomerase II in multidrug-resistant anaplastic thyroid carcinoma cell lines. Cancer Lett. (1997) 116:33–9. doi: 10.1016/s0304-3835(97)04742-3
14. Tallroth E, Wallin G, Lundell G, Lowhagen T, Einhorn J. Multimodality treatment in anaplastic giant cell thyroid carcinoma. Cancer. (1987) 60:1428–31. doi: 10.1002/1097-0142(19871001)60:7<1428::aid-cncr2820600703>3.0.co;2-p
15. Tanaka K, Sugitani I, Fujimoto Y. A novel chemo-radiotherapy with low-dose daily cisplatin, 5-fluorouracil and doxorubicin for anaplastic thyroid carcinoma: a preliminary report. Jpn J Clin Oncol. (2011) 41:1074–8. doi: 10.1093/jjco/hyr095
16. Ong SY, Zain JM. Aggressive T-cell lymphomas: 2024: Updates on diagnosis, risk stratification, and management. Am J Hematol. (2024) 99(3):439–56. doi: 10.1002/ajh.27165
17. Cheng SX, Chen QC, Lin GH, Han YH, Wang BC, Dai Y, et al. An integrated analysis of Sacituzumab govitecan in relapsed or refractory metastatic triple-negative breast cancer. Med (Baltimore). (2023) 102:e34486. doi: 10.1097/MD.0000000000034486
18. Siva S, Louie AV, Kotecha R, Barber MN, Ali M, Zhang Z, et al. Stereotactic body radiotherapy for primary renal cell carcinoma: a systematic review and practice guideline from the International Society of Stereotactic Radiosurgery (ISRS). Lancet Oncol. (2024) 25:e18–28. doi: 10.1016/S1470-2045(23)00513-2
19. Wang BC, Kuang BH, Lin GH. Immunotherapy alone or in combination with stereotactic body radiotherapy in advanced lung cancer: A pooled analysis of randomized clinical trials. J Oncol. (2022) 2022:7506300. doi: 10.1155/2022/7506300
20. Perez B, Aljumaily R, Marron TU, Shafique MR, Burris H, Iams WT, et al. Phase I study of peposertib and avelumab with or without palliative radiotherapy in patients with advanced solid tumors. ESMO Open. (2024) 9:102217. doi: 10.1016/j.esmoop.2023.102217
Keywords: chemotherapy, anaplastic thyroid cancer, survival outcome, response rate, adverse event
Citation: Wang B-C, Lin G-H, Kuang B-H and Cao R-B (2024) Emerging chemotherapy-based treatments in anaplastic thyroid cancer: an updated analysis of prospective studies. Front. Endocrinol. 15:1385747. doi: 10.3389/fendo.2024.1385747
Received: 13 February 2024; Accepted: 03 June 2024;
Published: 26 June 2024.
Edited by:
Cristina Alina Silaghi, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaReviewed by:
Barbara Maria Jarzab, Maria Skłodowska-Curie National Research Institute of Oncology, PolandCopyright © 2024 Wang, Lin, Kuang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bi-Cheng Wang, YmNzbm93ZWxsQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.