
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 31 May 2024
Sec. Pediatric Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1385002
This article is part of the Research TopicMetabolic Associated Fatty Liver Disease (MAFLD) in childhood: a new challengeView all 9 articles
 Kyungchul Song1
Kyungchul Song1 Eun Gyung Seol2
Eun Gyung Seol2 Hyejin Yang3
Hyejin Yang3 Soyoung Jeon3
Soyoung Jeon3 Hyun Joo Shin4
Hyun Joo Shin4 Hyun Wook Chae1
Hyun Wook Chae1 Eun-Kyung Kim4
Eun-Kyung Kim4 Yu-Jin Kwon5*
Yu-Jin Kwon5* Ji-Won Lee6,7*
Ji-Won Lee6,7*Introduction: Metabolic dysfunction-associated steatotic liver disease (MASLD) presents a growing health concern in pediatric populations due to its association with obesity and metabolic syndrome. Bioelectrical impedance analysis (BIA) offers a non-invasive and potentially effective alternative for identifying MASLD risk in youth with overweight or obesity. Therefore, this study aimed to assess the utility of BIA for screening for MASLD in the youth.
Method: This retrospective, cross-sectional study included 206 children and adolescents aged <20 years who were overweight and obese. The correlations between anthropometric measurements and BIA parameters and alanine aminotransferase (ALT) levels were assessed using Pearson’s correlation analysis. Logistic regression analysis was performed to examine the associations between these parameters and ALT level elevation and MASLD score. Receiver operating characteristic (ROC) curves were generated to assess the predictive ability of the parameters for MASLD.
Results: Pearson’s correlation analysis revealed that waist-to-hip ratio (WHR), percentage body fat (PBF), and BIA parameters combined with anthropometric measurements were correlated with ALT level. Logistic regression revealed that WHR, skeletal muscle mass/WHR, PBF-WHR, fat-free mass/WHR, and appendicular skeletal muscle mass/WHR were correlated with ALT level elevation after adjusting for age, sex, and puberty. WHR, PBF-WHR, and visceral fat area (VFA)-WHR were positively correlated with the MASLD score in the total population after adjusting for age, sex, and puberty. PBF-WHR and VFA-WHR were correlated with the MASLD score even in youth with a normal ALT level. The cutoff points and area under the ROC curves were 34.6 and 0.69 for PBF-WHR, respectively, and 86.6 and 0.79 for VFA-WHR, respectively.
Discussion: This study highlights the utility of combining BIA parameters and WHR in identifying the risk of MASLD in overweight and obese youth, even in those with a normal ALT level. BIA-based screening offers a less burdensome and more efficient alternative to conventional MASLD screening methods, facilitating early detection and intervention in youth at risk of MASLD.
Nonalcoholic fatty liver disease (NAFLD) is a chronic liver condition characterized by the accumulation of excess fat in the liver, encompassing a spectrum from simple steatosis to nonalcoholic steatohepatitis and hepatic fibrosis (1, 2). NAFLD has been associated with cardiovascular and metabolic risk factors as well as the development of hepatic cirrhosis and hepatic cancer, even in children and adolescents (1, 3, 4). A meta-analysis revealed a global increase in the prevalence of NAFLD from 4.62% in 2000 to 9.02% in 2017 among children and adolescents (5). Notably, in Korea, the prevalence of NAFLD increased from 45.8% to 62.5% during the outbreak of coronavirus disease 2019 among children and adolescents with obesity (6).
Early screening for NAFLD is crucial for prevention of severe complications, given that NAFLD is often asymptomatic and is reversible with appropriate management during the initial stages (1, 4). Therefore, the Endocrine Society recommends alanine transaminase (ALT) screening for early detection of NAFLD in children with overweight and obesity (7). The North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition recommends imaging studies, including ultrasonography, for NAFLD screening in children with abnormal ALT levels (8).
However, the necessity for blood sampling for ALT screening creates a potential burden on children. Furthermore, some children diagnosed with NAFLD may exhibit normal ALT levels, which can be elevated in various liver-related conditions (1). Consequently, initiatives have been undertaken to explore biomarkers to compensate for the limitations of ALT screening or imaging studies (1, 9). Despite these efforts, additional research in pediatric populations is warranted.
While body mass index (BMI) serves as a diagnostic tool for obesity, individuals whose BMI falls within the normal range may still present a metabolically unhealthy profile commonly associated with obesity; conversely, some individuals who are overweight or obese may be metabolically healthy (10). According to a recent study on the Korean population, the prevalence of metabolically healthy adolescents with overweight or obesity has remained steady, while the number of adolescents with overweight or obesity increased (11). Identifying metabolically healthy individuals with overweight is encumbered by the inherent limitations of BMI measurements, as they lack the ability to distinguish between body fat and muscle mass (12). A meta-analysis reported that bioelectrical impedance analysis (BIA) is a practical method for evaluating body fat in children and adolescents (13). However, investigations on the associations of BIA parameters with pediatric fatty liver disease are limited.
Recently, international experts have released a consensus statement regarding the renaming of fatty liver disease to metabolic dysfunction-associated steatotic liver disease (MASLD) (14). This new terminology reflects the association this condition with hepatic steatosis and the inclusion of at least one out of five cardiometabolic risk factors, which are associated with elements of metabolic syndrome. While this new concept enables the assessment of MASLD as a cardiometabolic risk factor by emphasizing the close pathophysiological link between fatty liver and metabolic dysfunction and insulin resistance, investigations on MASLD in pediatrics are limited. Considering adverse trends in cardiovascular risk factors, including metabolic syndrome, screening strategies for MASLD in pediatric patients are needed (2, 15).
In this study, we aimed to investigate the utility of BIA in identifying different metabolic profiles among individuals who are overweight and obese, providing valuable insights into more effective and less burdensome screening methods for MASLD in the pediatric population. Therefore, our study focused on the development of MASLD-related parameters using anthropometric measurements and BIA parameters that are specifically tailored for children and adolescents who are overweight and obese. Considering the metabolic diversity within this population, our objectives were to (1) examine the associations of BIA parameters with ALT level and MASLD, (2) compare the associations between MASLD and traditional anthropometric data and BIA parameters, and (3) establish valid cutoff values for BIA-related parameters for the prediction of MASLD.
This retrospective, cross-sectional study investigated participants aged <20 years who visited the Department of Pediatrics or Department of Family Medicine at Yongin Severance Hospital with a chief complaint of overweight or obesity from March 2020 to July 2023. Among them, 208 participants who underwent BIA were enrolled. Among these participants, those with missing ALT data (n=2) were excluded. None of the participants were alcohol drinkers or had hepatitis B virus and hepatitis C virus infection. Finally, 206 participants (107 boys and 99 girls) were enrolled.
Height measurements were acquired with a precision of 0.1 cm, whereas body weight was measured using an electronic scale accurate to 0.01 kg. BMI was computed by dividing weight in kilograms by the square of the height in meters (kg/m2). Height, weight, and BMI were expressed with respect to the standard deviation scores (SDSs) established by the 2017 Korean National Growth Charts (16). Waist circumference (WC) was measured by placing a measuring tape horizontally at the midpoint between the lowest rib and the iliac crest. Hip circumference was measured around the widest point of the hips, i.e., around the buttocks.
The waist-to-height ratio (WHtR) was calculated by dividing WC by height, while the waist-to-hip ratio (WHR) was calculated by dividing WC by hip circumference. Obesity was defined as BMI above the 95th percentile, and overweight was defined as a BMI between the 85th percentile and the 95th percentile according to the 2017 Korean National Growth Charts (16, 17). Participants with normal BMI, BMI below the 85th percentile, were not included in this study. Puberty was identified by any pubertal development corresponding to a Tanner stage of ≥2 (18). Blood samples were collected from the antecubital vein after an 8-h fast and were then processed and immediately refrigerated. The serum levels of aspartate transaminase and ALT were measured using an absorbance assay with a Roche Cobas 8000 c702 (Roche Diagnostics, Mannheim, Germany). The concentrations of hepatitis B surface antigen and anti-HCV antibodies were measured using a Roche Cobas 8000 c702 (Roche Diagnostics, Mannheim, Germany).
Skeletal muscle mass (SMM), fat-free mass (FFM), appendicular skeletal muscle mass (ASM), percentage body fat (PBF), WHtR, WHR, and visceral fat area (VFA) were assessed using the InBody720 body composition analyzer (Biospace, Seoul, South Korea). ALT level elevation was defined as ALT level >26 IU/L for male participants and >22 IU/L for female participants, excluding individuals with hepatitis B or C viral infection and alcohol drinkers (6, 19).
We established the following BIA parameters: 1) SMM divided by BMI SDS (SMM/BMI SDS), SMM divided by WHtR (SMM/WHtR), and SMM divided by WHR (SMM/WHR). FFM and ASM were calculated in the same manner: 2) PBF × BMI SDS (PBF-BMI SDS), PBF × WC (PBF-WC), PBF ×WHtR (PBF-WHtR), and PBF × WHR (PBF-WHR). The VFA was calculated in the same manner.
The diagnosis of steatotic liver disease was based on abdominal ultrasonography performed with a C1–8 MHz convex transducer of the Aplio i800 (Canon Medical Systems, Otawara, Japan) and C1–6 MHz of the LOGIQ E10 (GE Healthcare, Wauwatosa, WI, USA) by a pediatric radiologist with 14 years of experience. Participants were categorized into four groups based on the presence and severity of steatotic liver disease determined by assessing the degree of liver tissue echogenicity, the level of contrast between the liver and right kidney, and the visibility of vascular structures (1, 20). A hepatic fat accumulation grade of 1–3 was considered indicative of steatotic liver disease, while a hepatic fat accumulation grade of 0 indicated normal conditions.
According to international consensus, MASLD is defined as a steatotic liver disease with the presence of at least one of five cardiometabolic risk factors (14). Because all the participants in our study were overweight or obese, we defined steatotic liver disease as MASLD.
All continuous variables are presented as means ± standard deviations, while categorical variables are presented as numbers (percentages). Continuous variables were compared using the independent t test, while categorical variables were compared using the chi-squared test. Pearson’s correlation was employed to determine the associations between the BIA parameters and the WHR and ALT. Logistic regression analysis was performed to determine the associations of WHR alone and WHR combined with BIA parameters with ALT elevation and MASLD. The area under the receiver operating characteristic (ROC) curve (AUC) was computed to assess the diagnostic efficacy of these parameters for identifying MASLD. The data were analyzed using R, version 4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org, accessed on October 31, 2022). Statistical significance was set at p values ≤0.05.
This study adhered to the ethical guidelines outlined in the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of Yonsei University Yongin Severance Hospital (IRB number: 9–2023-0071); the requirement for informed consent was waived.
Table 1 presents the clinical characteristics of the study population with respect to sex and pubertal status. The proportion of puberty and obesity was greater in girls than in boys. In boys, the WC, WHtR, WHR, SMM, SMM/WC, SMM/WHtR, SMM/WHR, FFM, FFM/WC, FFM/WHtR, FFM/WHR, ASM, ASM/WC, ASM/WHtR, ASM/WHR, VFA, VFA-BMI SDS, VFA-WC, VFA-WHtR, VFA-WHR, and ALT level were greater, while PBF was lower than that in girls. The prevalence of obesity was greater in the pubertal group than in the prepubertal group. WHR, SMM, SMM/BMI SDS, SMM/WC, SMM/WHtR, SMM/WHR, FFM, FFM/BMI SDS, FFM/WC, FFM/WHtR, FFM/WHR, ASM, ASM/BMI-SDS, ASM/WC, ASM/WHtR, and ASM/WHR were greater in the puberty group than in the prepubertal group. In contrast, WHR, PBF, PBF-WHtR and PBF-WHR were lower in the pubertal group than in the prepubertal group.
WHR was the only anthropometric factor correlated with ALT in the total population (r=0.25, p=0.001), boys (p=0.030), the prepubertal group (p=0.041), and the pubertal group (p=0.024) (Table 2). Among the BIA parameters, ALT level was significantly correlated with SMM/WC, PBF, PBF-WHtR, PBF-WHR, and FFM/WC in boys and with PBF and the PBF-WHR in girls. No significant correlation was detected between these parameters and ALT level in the prepubertal group. In the pubertal group, ALT level exhibited significant correlations with the PBF, PBF-WC, PBF-WHtR, and PBF-WHR.
Considering the significance of WHR among the anthropometric variables, logistic regression analysis was performed to determine its association with ALT elevation by selecting indicators that integrated WHR into the representative BIA parameters after adjusting for age, sex (if applicable) and puberty (if applicable) (Table 3). In the total population, WHR (per 100) (odds ratio [OR], [95% confidence interval (CI)]=1.09 [1.02–1.17], p=0.017), SMM/WHR, PBF-WHR, FMM/WHR, and ASM/WHR were significantly associated with ALT level elevation after adjusting for age, sex, and puberty. According to the sex-specific analysis, ALT level elevation was significantly associated with PBF-WHR (OR [95% CI]=1.13 [1.04–1.23], p=0.005) and VFA-WHR (OR [95% CI]=1.01 [1.00–1.02], p=0.045) in boys and with SMM/WHR (OR [95% CI]=0.83 [0.73–0.94], p=0.004), FFM/WHR (OR [95% CI]=0.89 [0.83–0.96], p=0.003), and ASM/WHR (OR [95% CI]=0.81 [0.70–0.94], p=0.005) in girls after adjusting for age and puberty. In the pubertal group, significant associations were detected between WHR (per 100) (OR [95% CI]=1.09 [1.01–1.18], p=0.024), SMM/WHR (OR [95% CI]=0.92 [0.85–0.99], p=0.021), PBF-WHR (OR [95% CI]=1.10 [1.04–1.17], p=0.002), FFM/WHR (OR [95% CI]=0.95 [0.91–0.99], p=0.018), ASM/WHR (OR [95% CI]=0.89 [0.81–0.98], p=0.019), and VFA-WHR (OR [95% CI]=1.01 [1.00–1.02], p=0.039), and ALT level elevation after adjusting for age and sex, while no such significant associations were detected in the prepubertal group.
Figure 1 shows the logistic regression analysis results for WHR and WHR combined with BIA parameters for the diagnosis of MASLD by abdominal ultrasonography in the total population (Figure 1A), boys (Figure 1B), girls (Figure 1C), prepubertal group (Figure 1D), and pubertal group (Figure 1E). In the overall population, the WHR (per 100; OR [95% CI]=1.18 [1.04–1.35]), PBF-WHR (OR [95% CI]=1.19 [1.05–1.33]), and VFA-WHR (OR [95% CI]=1.03 [1.01–1.05]) showed significant associations with MASLD after adjusting for age, sex, and puberty. The ORs (95% CIs) were 1.17 (1.01–1.36) for PBF-WHR and 1.03 (1.01–1.06) for VFA-WHR in boys and 1.21 (1.00–1.47) for PBF-WHR and 1.03 (1.00–1.06) for VFA-WHR in girls. In the prepubertal group, the combined WHR and BIA parameters did not exhibit any significant association with MASLD. However, in the pubertal group, MASLD was significantly associated with the PBF-WHR (OR [95% CI]=1.19 [1.05–1.35]) and VFA-WHR (OR [95% CI]=1.03 [1.01–1.05]).
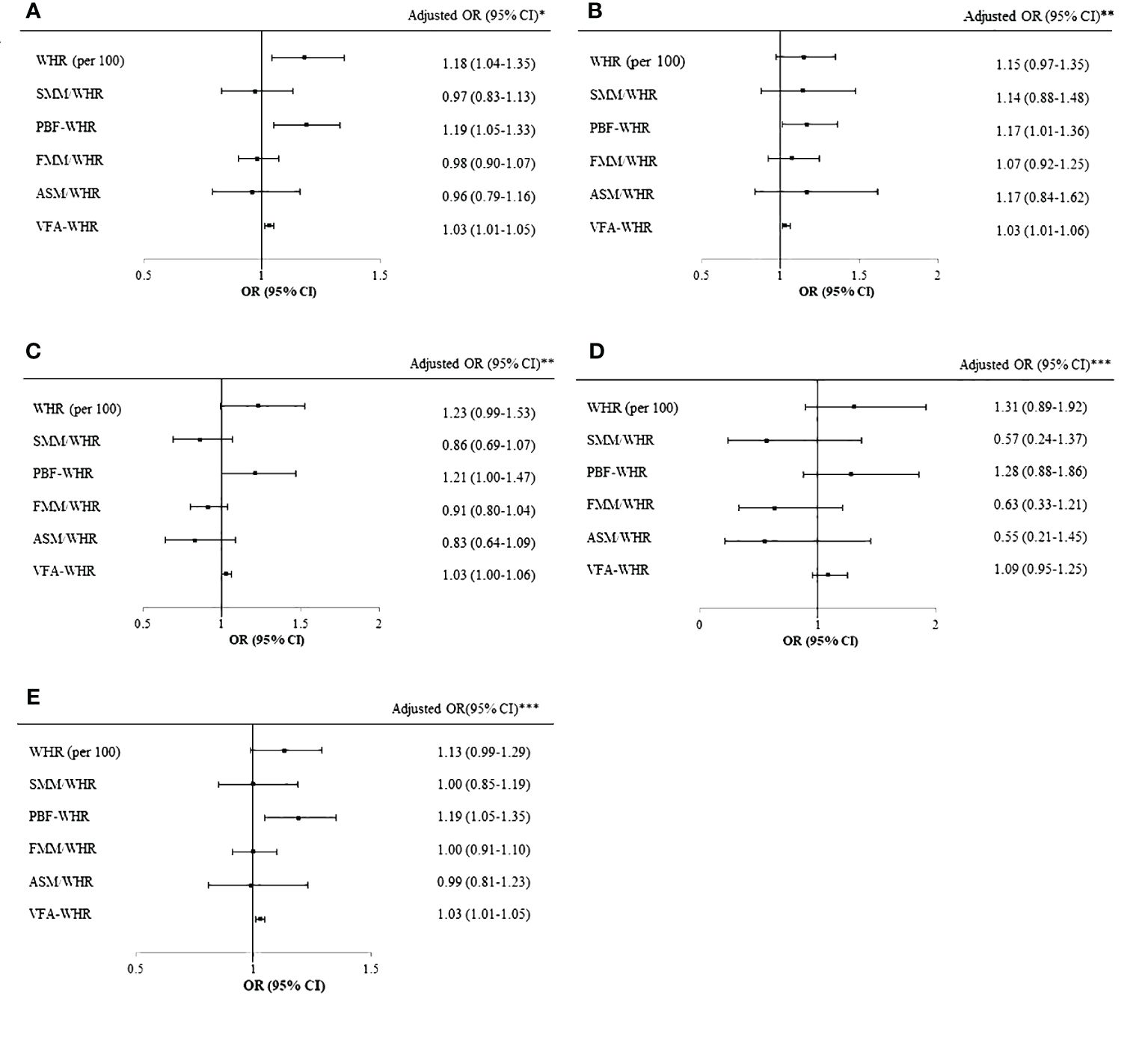
Figure 1 Logistic regression of WHR and BIA parameters for MASLD. (A) Logistic regression of WHR and BIA parameters for MASLD in the total cohort after adjusting for age, sex, and puberty. (B) Logistic regression of WHR and BIA parameters for MASLD in boys after adjusting for age and puberty. (C) Logistic regression of WHR and BIA parameters for MASLD in girls after adjusting for age and puberty. (D) Logistic regression of WHR and BIA parameters for MASLD in the prepubertal group after adjusting for age and sex. (E) Logistic regression of WHR and BIA parameters for MASLD in the pubertal group after adjusting for age and sex. *Adjusted for age, sex, and puberty. **Adjusted for age and puberty. ***Adjusted for age and sex. WHR, waist-to-hip ratio; BIA, bioelectrical impedance analysis; MASLD, metabolic dysfunction associated steatotic liver disease; SMM, skeletal muscle mass; PBF, percentage of body fat; FFM, fat-free mass; ASM, appendicular skeletal mass; VFA, visceral fat area; BMI, body mass index; SDS, standard deviation score.
Logistic regression analysis was performed to investigate the associations of the MASLD with WHR and combined parameters in individuals with normal ALT levels. The analysis revealed that PBF-WHR (OR [95% CI]=1.30 [1.05–1.60]) and VFA-WHR (OR [95% CI]=1.05 [1.01–1.10]) were significantly associated with MASLD (Figure 2).
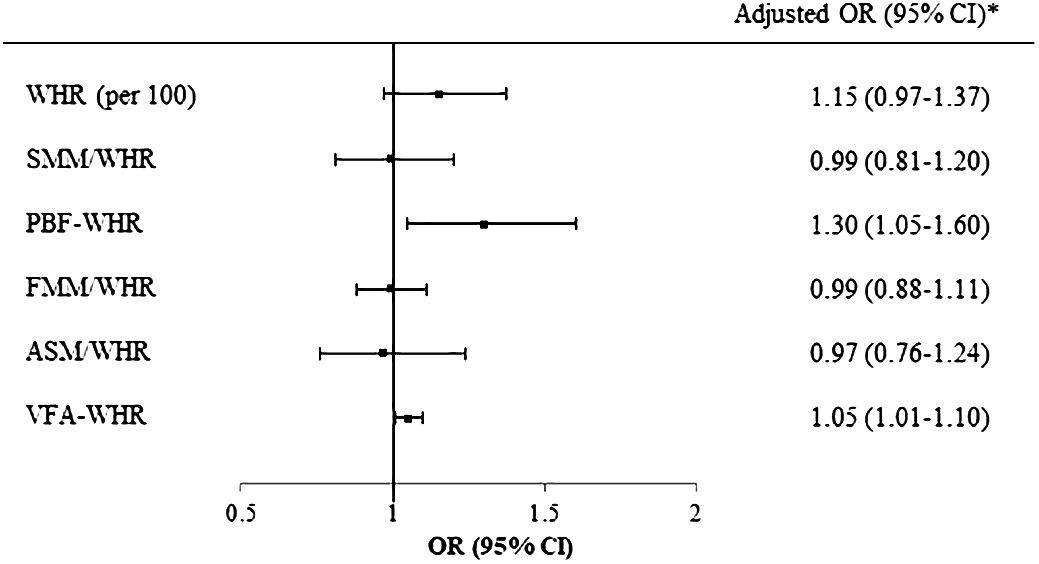
Figure 2 Logistic regression of WHR and BIA parameters for MASLD in participants with normal ALT. *Adjusted for age, sex, and puberty. WHR, waist-to-hip ratio; BIA, bioelectrical impedance analysis; MASLD, metabolic dysfunction-associated steatotic liver disease; ALT, alanine aminotransferase; SMM, skeletal muscle mass; PBF, percentage of body fat; FFM, fat-free mass; ASM, appendicular skeletal mass; VFA, visceral fat area.
Figure 3 displays the ROC curve along with the corresponding cutoff points for WHR, PBF-WHR, and VFA-WHR in the total population. The AUC (95% CI) and associated cutoff points were 0.65 (0.51–0.78) and 0.88 for WHR, 0.69 (0.56–0.81) and 34.6 for PBF-WHR, and 0.79 (0.69–0.89) and 86.6 for VFA-WHR, respectively.
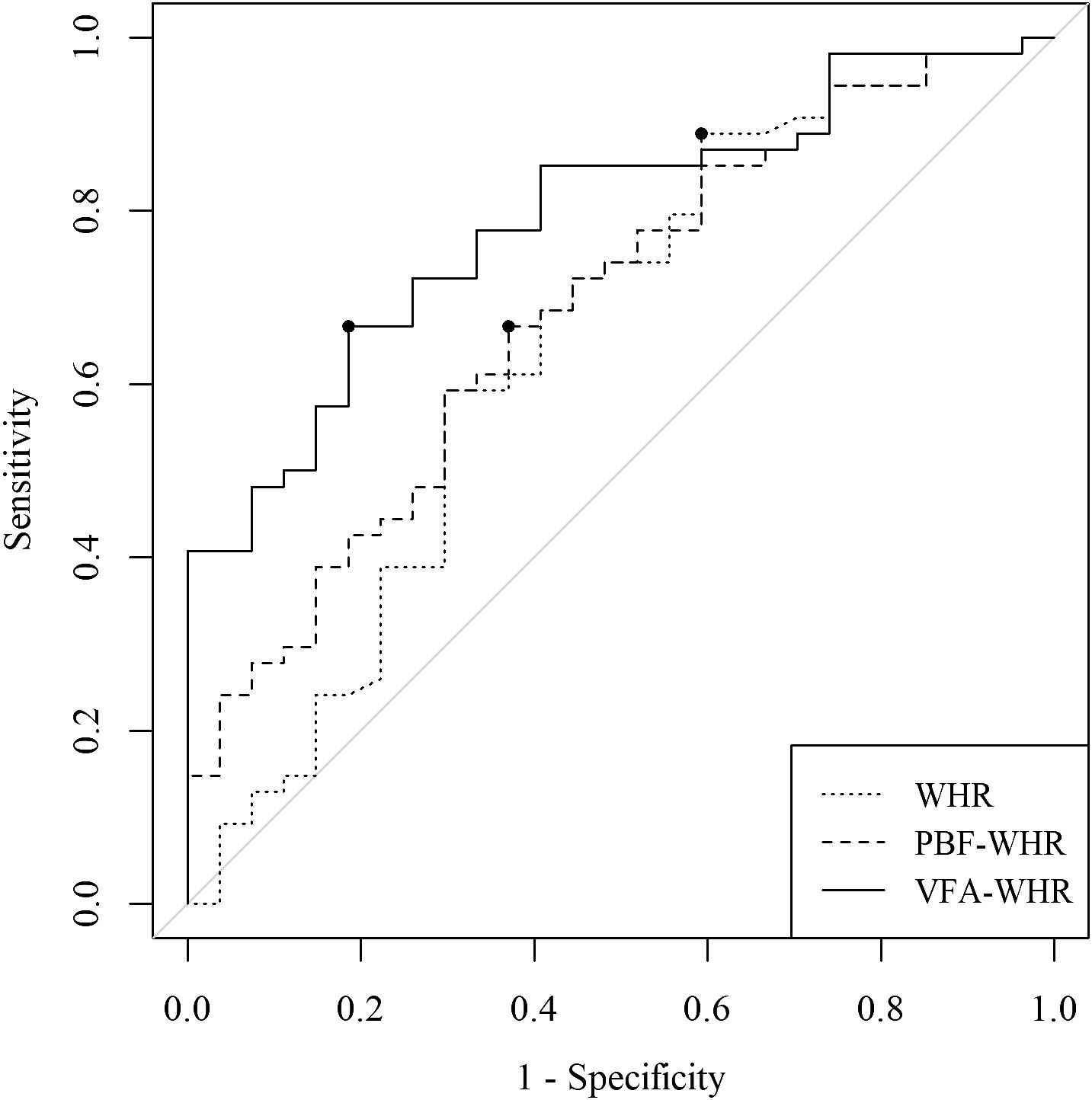
Figure 3 Comparison of the AUC and the corresponding cutoff points for WHR, PBF-WHR, and VFA-WHR. The dots on the curves represent the cutoff points for WHR, PBF-WHR, and VFA-WHR. AUC, area under the receiver operating characteristic curve; WHR, waist-to-hip ratio; SMM, skeletal muscle mass; PBF, percentage of body fat; VFA, visceral fat areaTables.
The present study demonstrated that WHR and the combination of BIA parameters with WHR were correlated with ALT level, ALT elevation, and MASLD among youth with overweight and obesity. These relationships were more prominent in the pubertal group. In addition, BIA parameters combined with WHR were stronger predictors than WHR alone. Among the combined parameters, PBF-WHR and VFA-WHR were correlated with the MASLD, even after adjusting for age, sex, and puberty. Moreover, these relationships were significant in youth with normal ALT levels.
In our study, WHR emerged as the sole anthropometric indicator correlated with ALT level in the total population. WHR accounts for both waist and hip measurements, making it a more comprehensive indicator of body fat distribution. The inclusion of hip circumference provides additional information about fat distribution in the lower body, which is relevant because individuals with a higher WHR tend to store more fat in the abdominal area, particularly around the visceral organs, a characteristic feature of individuals at risk for steatotic liver disease (1, 21). Pimenta et al. reported that WHR is strongly correlated with abdominal body fat and body fat distribution (22). A population-based study reported that WHR is a more accurate predictor of severe liver disease than BMI (23). Among anthropometric measurements, including BMI, WC, and WHtR, Zheng et al. reported that WHR was the most powerful predictor of NAFLD in adults (24).
The associations between BIA parameters and pediatric NAFLD have been investigated in several studies. A Japanese study reported that VFA was correlated with NAFLD in children (25). In another Japanese study, magnetic resonance imaging revealed that BIA parameters are associated with total psoas muscle surface area in children with obesity (26). A Chinese study reported that BIA was superior to anthropometric indices in terms of predicting NAFLD among children (27). Based on this evidence, we explored the associations of MASLD with BIA parameters as well as BIA parameters combined with anthropometric measurements. In our study, PBF-WHR and VFA-WHR were associated with MASLD in all subgroups except the prepubertal group, while WHR, SMM/WHR, FMM/WHR, and ASM/WHR were not significantly correlated with MASLD. Therefore, the inclusion of PBF-WHR and VFA-WHR in our study was of significant value for the prediction of MASLD. Choi et al. reported that PBF and the fat mass index are associated with ALT levels, whereas FFM is not (28). The robust relationships between PBF-WHR and VFA-WHR with MASLD can be explained as follows. First, excessive visceral fat accumulation resulting from the infiltration of macrophages among hypertrophied adipocytes is closely associated with the elevation of inflammatory cytokine levels and diminished production of adipokines such as adiponectin (29). A previous study also reported that PBF is closely associated with chronic inflammation in the youth (30). Chronic inflammation plays a crucial role in the development of steatotic liver disease by inducing insulin resistance and promoting the accumulation of lipids in the liver (29). VFA and PBF are linked to insulin resistance and steatotic liver disease via reduced hepatic clearance of insulin (exacerbating hyperinsulinemia), increased production of triglyceride-rich lipoproteins, and elevated hepatic glucose production (31). Notably, examination of the relationship between ALT level elevation and BIA-related parameters revealed that fat-related indicators were significantly associated in boys, while muscle-related indicators were significantly associated in girls. This suggests a potential sex-based difference in the effect of fat and muscle on the occurrence of hepatic fat deposition.
Although ALT is a valuable biomarker, it is not a reliable or accurate predictor of the presence of MASLD (32). Therefore, steatotic liver disease can be underdiagnosed in youth with normal ALT levels, given that the current guidelines recommend imaging studies such as ultrasonography for NAFLD screening, primarily in children with abnormal ALT levels (8). Moreover, a cohort study reported that 14% of patients with NAFLD had normal ALT levels (33). In our study, PBF-WHR and VFA-WHR were correlated with MASLD even in youth with normal ALT levels, whereas WHR alone was not significantly associated with MASLD. These findings suggest that including BIA parameters in the selection criteria for MASLD screening, even in patients with normal ALT levels, could be a beneficial approach for enhancing diagnostic accuracy.
The effectiveness of BIA parameters was notably greater in the pubertal group than in the prepubertal group; our study also revealed significant differences in most BIA parameters between the prepubertal and pubertal groups. This difference can be attributed to the significant changes in body composition, including muscle and fat, that occur during puberty (34). Puberty is a period marked by rapid growth, hormonal changes, and alterations in body composition (35, 36). These changes, including the development of lean muscle mass and the redistribution of fat, can affect the accuracy and relevance of BIA measurements. During puberty, individuals experience substantial growth in muscle mass, which affects BIA-derived measurements (37). Additionally, the redistribution of fat, especially in the abdominal and visceral regions, is characteristic of puberty and influences the parameters related to body fat in BIA. Thus, BIA parameters have become more informative and relevant for assessing metabolic health and risk factors, including steatotic liver disease, in pubertal individuals owing to these dynamic changes in body composition during puberty (36). A longitudinal study reported that the change in energy expenditure during puberty was associated with body composition change (38). The accuracy of BIA in capturing these changes makes it a valuable tool for understanding the associations between body composition and health outcomes during this critical developmental stage.
This study has some limitations. First, our study had a retrospective design and was limited to the Korean population. Second, MASLD was diagnosed using ultrasonography, although biopsy constitutes the gold standard for the diagnosis of steatotic liver disease. Finally, genetic and environmental factors, including nutrition and physical activity, were not considered. However, we propose novel parameters involving the integration of WHR with BIA parameters and show that these parameters are superior to simple anthropometric measurements for the prediction of MASLD even in young patients with normal ALT levels. To the best of our knowledge, this study is the first to experimentally demonstrate the potential of combined parameters using anthropometric data and BIA parameters, indicating the need for more studies with large samples to validate the usefulness of these parameters.
In conclusion, our study demonstrated that BIA parameters combined with WHR exhibited significant correlations with ALT level, ALT elevation, and MASLD and that these relationships were more pronounced in pubertal individuals. The combination of BIA parameters related to fat composition, such as PBF and VFA with WHR, demonstrated stronger associations with MASLD than did WHR alone. Moreover, these relationships were sustained even in youth with normal ALT levels, suggesting the potential of BIA parameters for MASLD screening in such cases. In summary, our study highlights the value of BIA parameters combined with WHR as a promising approach for MASLD screening in youth with overweight and obesity, providing a less burdensome and potentially more effective alternative to traditional methods.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Institutional Review Board of Yonsei University Yongin Severance Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The requirement for informed consent was waived.
KS: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft. ES: Data curation, Resources, Writing – review & editing. HY: Formal analysis, Methodology, Writing – review & editing. SJ: Methodology, Writing – review & editing. HS: Methodology, Writing – review & editing. HC: Methodology, Writing – review & editing. E-KK: Methodology, Writing – review & editing. Y-JK: Conceptualization, Formal analysis, Investigation, Methodology, Writing – review & editing. J-WL: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Korea Evaluation Institute of Industrial Technology (KEIT) grant funded by the Korean government (MOTIE) (20018384) (to J-WL).
The authors would like to thank InBody Corporation for providing the BIA equipment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Song K, Kim HS, Chae HW. Nonalcoholic fatty liver disease and insulin resistance in children. Clin Exp Pediatr. (2023) 66(12):512–9. doi: 10.3345/cep.2022.01312
2. Song K, Park G, Lee HS, Lee M, Lee HI, Ahn J, et al. Trends in prediabetes and non-alcoholic fatty liver disease associated with abdominal obesity among Korean children and adolescents: based on the Korea national health and nutrition examination survey between 2009 and 2018. Biomedicines. (2022) 10(3):584. doi: 10.3390/biomedicines10030584
3. Song K, Park G, Lee HS, Lee M, Lee HI, Choi HS, et al. Comparison of the triglyceride glucose index and modified triglyceride glucose indices to predict nonalcoholic fatty liver disease in youths. J Pediatr. (2022) 242:79–85.e1. doi: 10.1016/j.jpeds.2021.11.042
4. Crespo M, Lappe S, Feldstein AE, Alkhouri N. Similarities and differences between pediatric and adult nonalcoholic fatty liver disease. Metabolism. (2016) 65:1161–71. doi: 10.1016/j.metabol.2016.01.008
5. Li J, Ha A, Rui F, Zou B, Yang H, Xue Q, et al. Meta-analysis: global prevalence, trend and forecasting of non-alcoholic fatty liver disease in children and adolescents, 2000–2021. Aliment Pharmacol Ther. (2022) 56:396–406. doi: 10.1111/apt.17096
6. Song K, Yang J, Lee HS, Kim SJ, Lee M, Suh J, et al. Changes in the Prevalences of Obesity, Abdominal Obesity, and Non-Alcoholic Fatty Liver Disease among Korean Children during the COVID-19 Outbreak. Yonsei Med J. (2023) 64:269–77. doi: 10.3349/ymj.2022.0540
7. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2017) 102:709–57. doi: 10.1210/jc.2016–2573
8. Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the expert committee on NAFLD (ECON) and the north american society of pediatric gastroenterology, hepatology and nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. (2017) 64:319–34. doi: 10.1097/mpg.0000000000001482
9. Song K, Lee HW, Choi HS, Park G, Lee HS, Kim SJ, et al. Comparison of the modified tyG indices and other parameters to predict non-alcoholic fatty liver disease in youth. Biol (Basel). (2022) 11(5):685. doi: 10.3390/biology11050685
10. Shea JL, King MT, Yi Y, Gulliver W, Sun G. Body fat percentage is associated with cardiometabolic dysregulation in BMI-defined normal weight subjects. Nutr Metab Cardiovasc Dis. (2012) 22:741–7. doi: 10.1016/j.numecd.2010.11.009
11. Kim HY, Kim JH. Temporal trends in the prevalence of metabolically healthy overweight and obesity in Korean youth: data from the Korea National Health and Nutrition Examination Survey 2011–2019. Ann Pediatr Endocrinol Metab. (2022) 27:134–41. doi: 10.6065/apem.2142192.096
12. Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. (2019) 129:3978–89. doi: 10.1172/jci129186
13. Chula de Castro JA, Lima TR, Silva DAS. Body composition estimation in children and adolescents by bioelectrical impedance analysis: A systematic review. J Bodyw Mov Ther. (2018) 22:134–46. doi: 10.1016/j.jbmt.2017.04.010
14. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. (2023) 79:1542–56. doi: 10.1016/j.jhep.2023.06.003
15. Park SI, Suh J, Lee HS, Song K, Choi Y, Oh JS, et al. Ten-year trends of metabolic syndrome prevalence and nutrient intake among Korean children and adolescents: A population-based study. Yonsei Med J. (2021) 62:344–51. doi: 10.3349/ymj.2021.62.4.344
16. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. (2018) 61:135–49. doi: 10.3345/kjp.2018.61.5.135
17. Choe J, Kim J, Moon JS. Cutoff values of body mass index for severe obesity in Korean children and adolescents: the 99th percentile versus 120% of the 95th percentile. Ann Pediatr Endocrinol Metab. (2023) 28:131–7. doi: 10.6065/apem.2244058.029
18. Yang EH, Jo HY, Park SJ, Yoo HW, Choi SH, Kim HY, et al. Effect of gonadotropin-releasing hormone agonist treatment on near final height in girls with central precocious puberty and early puberty. Ann Pediatr Endocrinol Metab. (2023) 28:49–53. doi: 10.6065/apem.2142250.125
19. Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. (2010) 138:1357–64. doi: 10.1053/j.gastro.2009.12.052
20. Song K, Son NH, Chang DR, Chae HW, Shin HJ. Feasibility of ultrasound attenuation imaging for assessing pediatric hepatic steatosis. Biol (Basel). (2022) 11(7):1087. doi: 10.3390/biology11071087
21. Savgan-Gurol E, Bredella M, Russell M, Mendes N, Klibanski A, Misra M. Waist to hip ratio and trunk to extremity fat (DXA) are better surrogates for IMCL and for visceral fat respectively than for subcutaneous fat in adolescent girls. Nutr Metab (Lond). (2010) 7:86. doi: 10.1186/1743–7075-7–86
22. Pimenta NM, Santa-Clara H, Melo X, Cortez-Pinto H, Silva-Nunes J, Sardinha LB. Waist-to-hip ratio is related to body fat content and distribution regardless of the waist circumference measurement protocol in nonalcoholic fatty liver disease patients. Int J Sport Nutr Exerc Metab. (2016) 26:307–14. doi: 10.1123/ijsnem.2014–0256
23. Andreasson A, Carlsson AC, Önnerhag K, Hagström H. Waist/hip ratio better predicts development of severe liver disease within 20 years than body mass index: A population-based cohort study. Clin Gastroenterol Hepatol. (2017) 15:1294–301.e2. doi: 10.1016/j.cgh.2017.02.040
24. Zheng RD, Chen ZR, Chen JN, Lu YH, Chen J. Role of body mass index, waist-to-height and waist-to-hip ratio in prediction of nonalcoholic fatty liver disease. Gastroenterol Res Pract. (2012) 2012:362147. doi: 10.1155/2012/362147
25. Abe Y, Tonouchi R, Hara M, Okada T, Jego EH, Taniguchi T, et al. Visceral fat area measured by abdominal bioelectrical impedance analysis in school-aged Japanese children. J Clin Med. (2022) 11(14):4148. doi: 10.3390/jcm11144148
26. Orkin S, Yodoshi T, Romantic E, Hitchcock K, Arce-Clachar AC, Bramlage K, et al. Body composition measured by bioelectrical impedance analysis is a viable alternative to magnetic resonance imaging in children with nonalcoholic fatty liver disease. JPEN J Parenter Enteral Nutr. (2022) 46:378–84. doi: 10.1002/jpen.2113
27. Lee LW, Yen JB, Lu HK, Liao YS. Prediction of nonalcoholic fatty liver disease by anthropometric indices and bioelectrical impedance analysis in children. Child Obes. (2021) 17:551–8. doi: 10.1089/chi.2021.0054
28. Choi M, Lee S, Bae SH, Chung S. Application of body composition zones in boys with nonalcoholic fatty liver disease. Ann Pediatr Endocrinol Metab. (2019) 24:243–7. doi: 10.6065/apem.2019.24.4.243
29. Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. (2019) 7:715–25. doi: 10.1016/s2213–8587(19)30084–1
30. Pettersson-Pablo P, Nilsson TK, Breimer LH, Hurtig-Wennlöf A. Body fat percentage is more strongly associated with biomarkers of low-grade inflammation than traditional cardiometabolic risk factors in healthy young adults - the Lifestyle, Biomarkers, and Atherosclerosis study. Scand J Clin Lab Invest. (2019) 79:182–7. doi: 10.1080/00365513.2019.1576219
31. Liu C, Li N, Sheng D, Shao Y, Qiu L, Shen C, et al. Increased visceral fat area to skeletal muscle mass ratio is positively associated with the risk of metabolic dysfunction-associated steatotic liver disease in a Chinese population. Lipids Health Dis. (2024) 23:104. doi: 10.1186/s12944–024-02100–5
32. Noureddin M, Loomba R. Nonalcoholic fatty liver disease: Indications for liver biopsy and noninvasive biomarkers. Clin Liver Dis (Hoboken). (2012) 1:104–7. doi: 10.1002/cld.65
33. Manco M, Alisi A, Nobili V. Risk of severe liver disease in NAFLD with normal ALT levels: a pediatric report. Hepatology. (2008) 48:2087–8. doi: 10.1002/hep.22631
34. Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. (2009) 16:10–5. doi: 10.1097/MED.0b013e328320d54c
35. Chotipakornkul N, Onsoi W, Numsriskulrat N, Aroonparkmongkol S, Supornsilchai V, Srilanchakon K. The utilization of basal luteinizing hormone in combination with the basal luteinizing hormone and follicle-stimulating hormone ratio as a diagnostic tool for central precocious puberty in girls. Ann Pediatr Endocrinol Metab. (2023) 28:138–43. doi: 10.6065/apem.2346072.036
36. Rusek W, Baran J, Leszczak J, Adamczyk M, Baran R, Weres A, et al. Changes in children's body composition and posture during puberty growth. Children (Basel). (2021) 8(4):288. doi: 10.3390/children8040288
37. Siervogel RM, Demerath EW, Schubert C, Remsberg KE, Chumlea WC, Sun S, et al. Puberty and body composition. Horm Res. (2003) 60:36–45. doi: 10.1159/000071224
Keywords: child, adolescent, obesity, metabolic dysfunction associated steatotic liver disease, bioelectric impedance
Citation: Song K, Seol EG, Yang H, Jeon S, Shin HJ, Chae HW, Kim E-K, Kwon Y-J and Lee J-W (2024) Bioelectrical impedance parameters add incremental value to waist-to-hip ratio for prediction of metabolic dysfunction associated steatotic liver disease in youth with overweight and obesity. Front. Endocrinol. 15:1385002. doi: 10.3389/fendo.2024.1385002
Received: 11 February 2024; Accepted: 14 May 2024;
Published: 31 May 2024.
Edited by:
Anna Di Sessa, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Xiaolin Wang, Shanghai Jiao Tong University, ChinaCopyright © 2024 Song, Seol, Yang, Jeon, Shin, Chae, Kim, Kwon and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Jin Kwon, ZGlnZGEzQHl1aHMuYWM=; Ji-Won Lee, aW5kaTU2NDVAeXVocy5hYw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.