- 1Department of Cardiology, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Department of Cardiology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
- 3Department of Public Education, Zhangzhou Institute of Technology, Zhangzhou, China
- 4Department of Radiology, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
Background: Stress hyperglycemia ratio (SHR) has shown a predominant correlation with transient adverse events in critically ill patients. However, there remains a gap in comprehensive research regarding the association between SHR and mortality among patients experiencing cardiac arrest and admitted to the intensive care unit (ICU).
Methods: A total of 535 patients with their initial ICU admission suffered cardiac arrest, according to the American Medical Information Mart for Intensive Care (MIMIC)-IV database. Patients were stratified into four categories based on quantiles of SHR. Multivariable Cox regression models were used to evaluate the association SHR and mortality. The association between SHR and mortality was assessed using multivariable Cox regression models. Subgroup analyses were conducted to determine whether SHR influenced ICU, 1-year, and long-term all-cause mortality in subgroups stratified according to diabetes status.
Results: Patients with higher SHR, when compared to the reference quartile 1 group, exhibited a greater risk of ICU mortality (adjusted hazard ratio [aHR] = 3.029; 95% CI: 1.802-5.090), 1-year mortality (aHR = 3.057; 95% CI: 1.885-4.958), and long-term mortality (aHR = 3.183; 95% CI: 2.020-5.015). This association was particularly noteworthy among patients without diabetes, as indicated by subgroup analysis.
Conclusion: Elevated SHR was notably associated with heightened risks of ICU, 1-year, and long-term all-cause mortality among cardiac arrest patients. These findings underscore the importance of considering SHR as a potential prognostic factor in the critical care management of cardiac arrest patients, warranting further investigation and clinical attention.
Introduction
Approximately 420,000 individuals experience out-of-hospital cardiac arrest annually in the United States, with respiratory failure being the predominant cause in most cases (1, 2). Unfortunately, only 25% of these cases result in survival and discharge from the hospital. Even with a successful return of spontaneous circulation following out-of-hospital cardiac arrest, approximately 80% of patients remain comatose (3). Notably, cardiac arrest-associated central nervous system damage stands out as the primary cause of long-term disability and mortality among survivors of the acute resuscitation phase (4, 5). The persistent risk of ischemia/reperfusion injury affecting various organs continues to be a significant concern, impacting patient outcomes (1, 6–8). The timely administration of cardiopulmonary resuscitation (CPR) and defibrillation has been proven crucial for maintaining sustained autonomic circulation and improving survival rates. Consequently, there is an urgent need to identify effective and straightforward indicators that can accurately predict the prognosis of patients who have undergone cardiac arrest.
Stress hyperglycemia, a transient metabolic response characterized by elevated glucose levels in emergency situations, had been reported in studies involving patients in critical conditions (9, 10). This phenomenon had been associated with instability, perforation, and exacerbation of myocardial ischemia in atherosclerotic plaques, which are among the leading causes of cardiac arrest (11, 12). Despite its significance, there is currently no universally accepted definition of stress hyperglycemia (13). Patients with this condition are typically classified as having known diabetes, newly diagnosed diabetes, or hyperglycemia related to the hospital environment (14). In previous research, fasting glucose or initial blood glucose levels upon admission were commonly used as indicators of stress hyperglycemia. However, these measures often failed to consider the baseline glucose levels preceding the onset of cardiovascular-related diseases, particularly in emergency situations such as cardiac arrest (15, 16). Introducing the stress hyperglycemia ratio (SHR), a novel indicator calculated by dividing the measurement of admission blood glucose (ABG) by HbA1c, offered a more comprehensive assessment (17–19). Studies have indicated a strong association between SHR and adverse clinical outcomes in critically ill patients (20, 21). Nonetheless, limited research has explored the relationship between SHR and mortality in patients experiencing cardiac arrest.
Accordingly, the purpose of the present investigation was to examine the association between SHR and ICU, 1-year and long-term all-cause mortality in American Medical Information Mart for Intensive Care IV (MIMIC-IV) cohort cardiac arrest patients admitted to the intensive care unit (ICU).
Methods
Sources of data
This retrospective study utilized data from the MIMIC-IV database (22), established and maintained by the Massachusetts Institute of Technology’s Laboratory for Computational Physiology (23). The MIMIC database is an extensive, one-stop, publicly accessible database comprising data about patients admitted to critical care sections at a major tertiary care facility (23). Since 2008, 76,540 intensive care unit (ICU) patients have been entered into the Beth Israel Deaconess Medical Center in Boston database. Access to the database was granted to one of our group’s authors (Record ID: 5,076,556) upon successful completion of the Collaborative Institutional Training Initiative (CITI) program course. The data extraction process was executed utilizing PostgreSQL version 6.11.1 software.
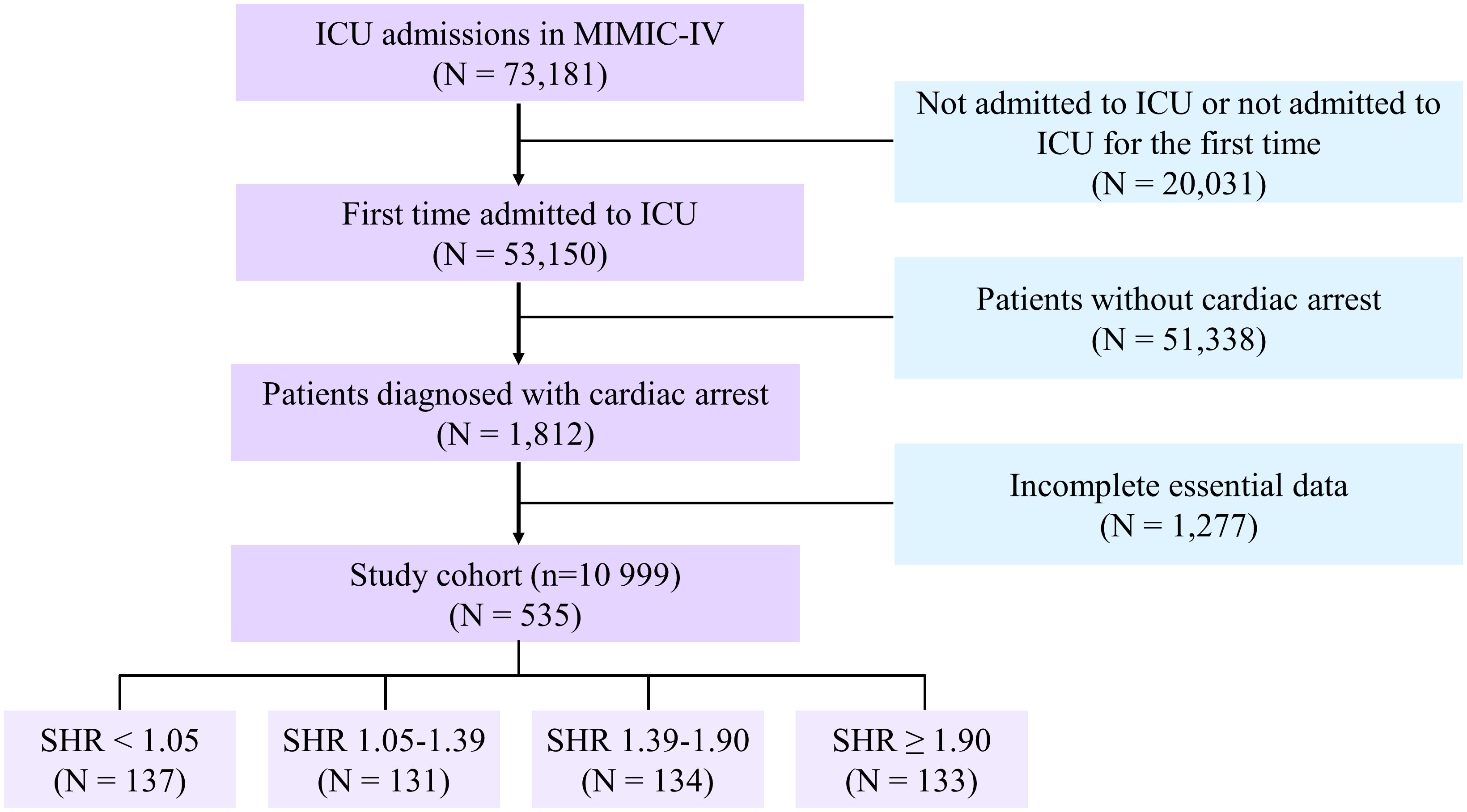
We obtained clinical data from ICU-admitted adults older than 18 years for the first time for the purpose of analysis. The individuals who were found to have cardiac arrest, as classified by the International Classification of Diseases (ICDS) 9th and 10th editions (ICD-9 codes “4275”, “I46”, “I462”, “I468”, “I469”), satisfied the requirements for this study. As screening criteria, the following exclusion criteria were applied: (1) patients younger than 18 years of age; (2) patients whose survival outcome data were unavailable; (3) insufficient or absent critical laboratory findings (glycated hemoglobin [HbA1c] or glucose on admission). All laboratory variables and disease severity scores were extracted from the data generated within the first 24 hours after the patient entered the ICU. Finally, 535 cardiac arrest patients were included in American MIMIC-IV (Figure 1).
Outcomes and definitions
The study’s outcomes were ICU mortality, 1-year mortality and long-term mortality, with the MIMIC-IV cohort having a maximum follow-up of 12.1 years. The SHR index was established using the following formula: SHR = (admission glucose) (mmol/L) divided by (1.59 * HbA1c [%] - 2.59).
Statistical analysis
When analyzing normally distributed values, we represented continuous data using the mean (standard deviation). For non-normally distributed values, we used the median (interquartile range). Categorical data was displayed as quantities and percentages. In order to compare the groups, appropriate statistical tests such as ANOVA, the Kruskal-Wallis test for continuous variables, or the chi-square test for categorical data were utilized. SHR was assessed as a categorical variable: quartiles 1 to 4. To calculate the cumulative hazard of mortality from all causes, the Kaplan–Meier method was applied. In order to ascertain the correlation between SHR level and ICU mortality, 1-year mortality, and long-term mortality, multivariable Cox regression models were employed. These models accounted for age, gender, hypertension, diabetes mellitus, chronic kidney disease, acute myocardial infarction (AMI), cerebrovascular disease, peptic ulcer disease, and chronic obstructive pulmonary disease (COPD). The correlation between SHR and ICU mortality, 1-year mortality, and long-term mortality was also examined using restricted cubic splines (RCS). In presenting the findings, hazard ratios (HR) and confidence intervals (CI) of 95% are employed. In addition, subgroup analyses were performed to ascertain whether SHR influenced mortality in intensive care units, mortality at one year, and mortality over the long term in subgroups stratified according to diabetes status. The statistical analysis was performed utilizing the R software (version 4.2.1). P values with both tails containing less than 0.05 were considered statistically significant.
Results
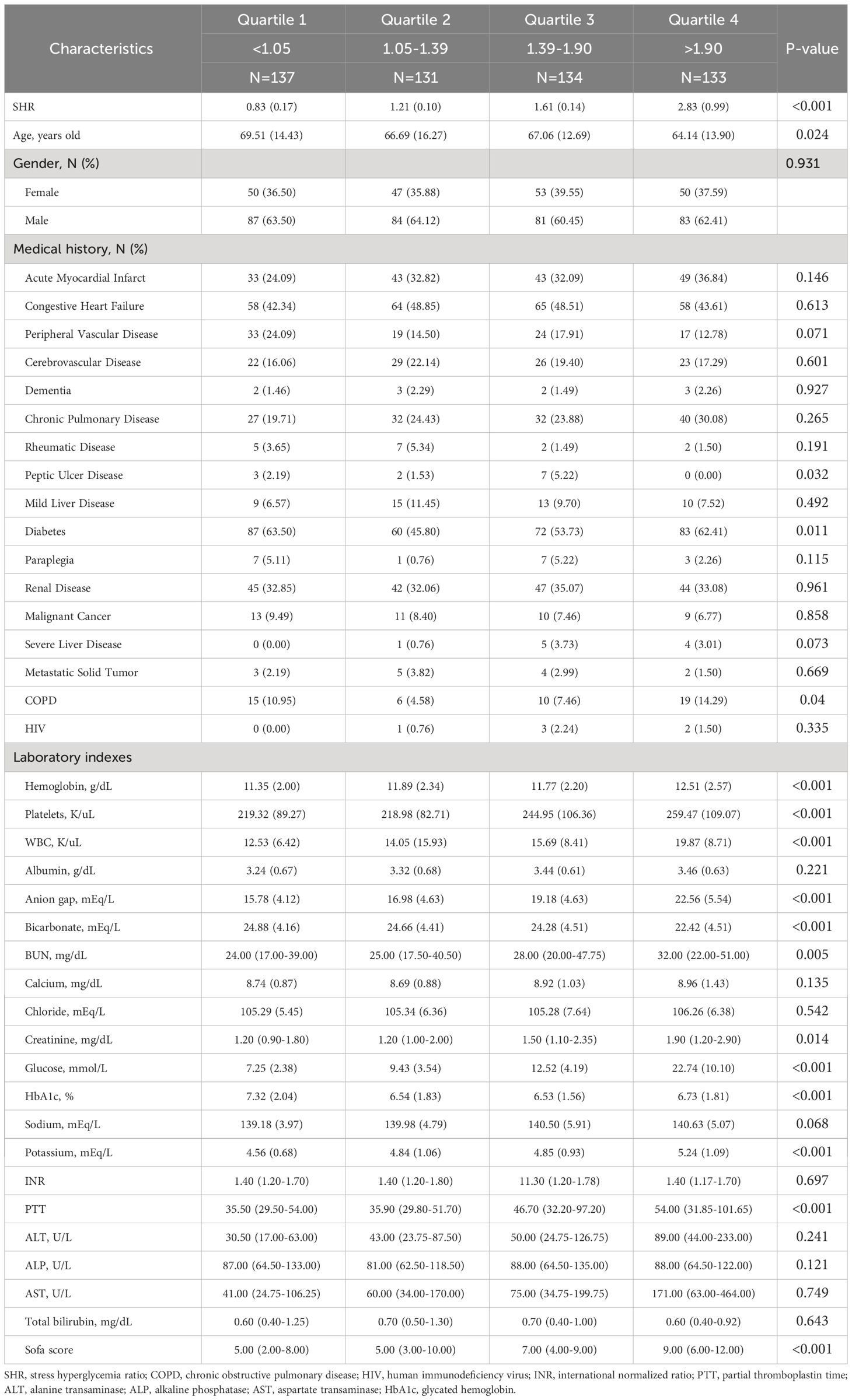
The MIMIC-IV cohort enrolled 535 cardiac arrest patients (mean age [SD]: 66.9 ± 14.4 years; 62.6% male). In the MIMIC-IV cohort, patients were categorized into four groups according to their SHR level: quartile 1 [N =137], SHR<1.05; quartile 2 [N =131], 1.05≤SHR<1.39; quartile 3 [N =134], 1.39≤SHR<1.90; and quartile 4 [N =133], SHR≥1.90).
A total of 65.2% (N =349) of the patients in the MIMIC-IV cohort were diagnosed with hypertension, 56.4% (N =302) with diabetes, 60.9% (N =326) with chronic heart failure (CHF), 27.5% (N= 147) with AMI, and 16.3% (N =87) with CKD. Further elaboration on the foundational data of these groups could be found in Table 1.
All-cause mortality was observed in 169 patients (31.6%) of the American MIMIC-IV cohort; the greatest mortality rate was observed in quartile 4 (N = 53, 39.8%). The higher SHR was associated with higher mortality. The outcomes of Kaplan–Meier analyses of survival were illustrated in Figure 2.
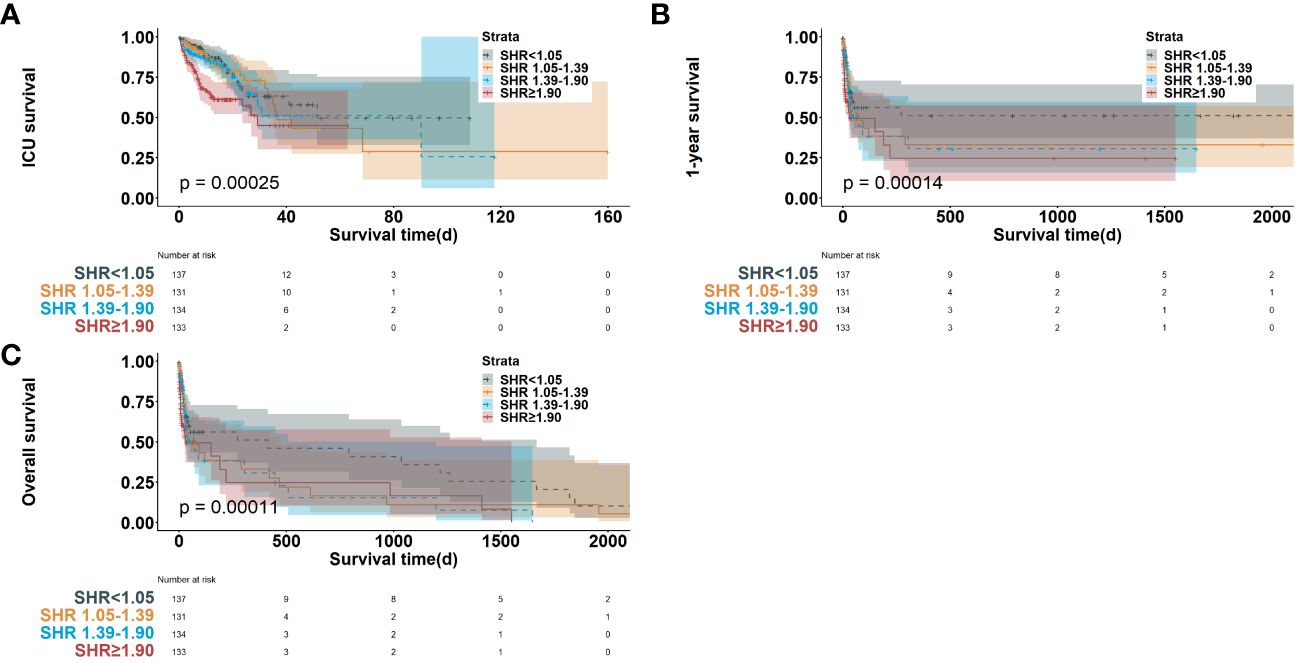
Figure 2 Kaplan-Meier survival curve of survival probability in cardiac arrest patients in ICU mortality (A), 1-year mortality (B) and long-term mortality (C). SHR, stress hyperglycemia ratio; ICU, intensive care unit.
The results of the RCS analyses revealed an L-shaped association between the SHR and all-cause mortality during the follow-up period (P value for nonlinearity >0.05 for all, Supplementary Figure 1). In the MIMIC-IV cohort, the SHR corresponding to the lowest risk on RCS was 1.39 of ICU mortality, 1-year mortality, and long-term mortality (Supplementary Figure 1).
Subgroup analyses were conducted to assess the relationship between SHR and all-cause mortality based on diabetes status (Table 2; Figure 3). SHR in quartile 4 was significantly associated with an increased risk of ICU mortality in the MIMIC-IV cohort (HR = 3.239, 95% CI: 1.671-6.278, P < 0.001; HR = 2.678, 95% CI: 1.140-6.290, P = 0.024) compared to SHR in quartile 1 among patients with or without diabetes. Comparable results were found that SHR in quartile 4 was strongly related with higher 1-year mortality (patients with diabetes: HR [95% CI] = 3.138 [1.709-5.764], P < 0.001; patients without diabetes: HR [95% CI] = 2.620 [1.146-5.992], P = 0.022) and long-term mortality (patients with diabetes: HR [95% CI] = 3.204 [1.801-5.700], P < 0.001; patients without diabetes: HR [95% CI] = 2.829 [1.233-6.494], P = 0.014). The P value for interaction was 0.410, 0.379, and 0.471 for ICU mortality, 1-year mortality, and long-term mortality, respectively.
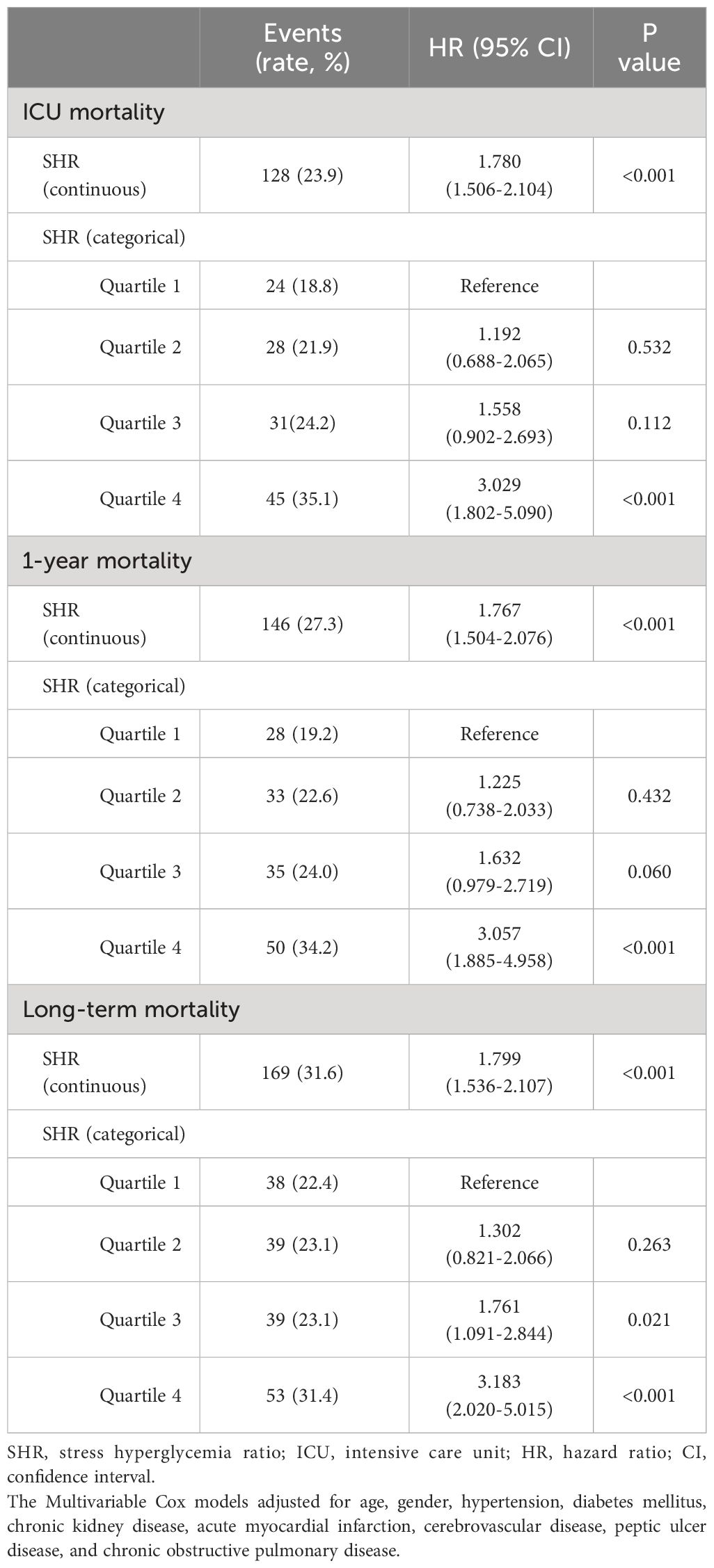
Table 2 Multivariable Cox regression analysis for ICU mortality, 1-year and long-term all-cause mortality.
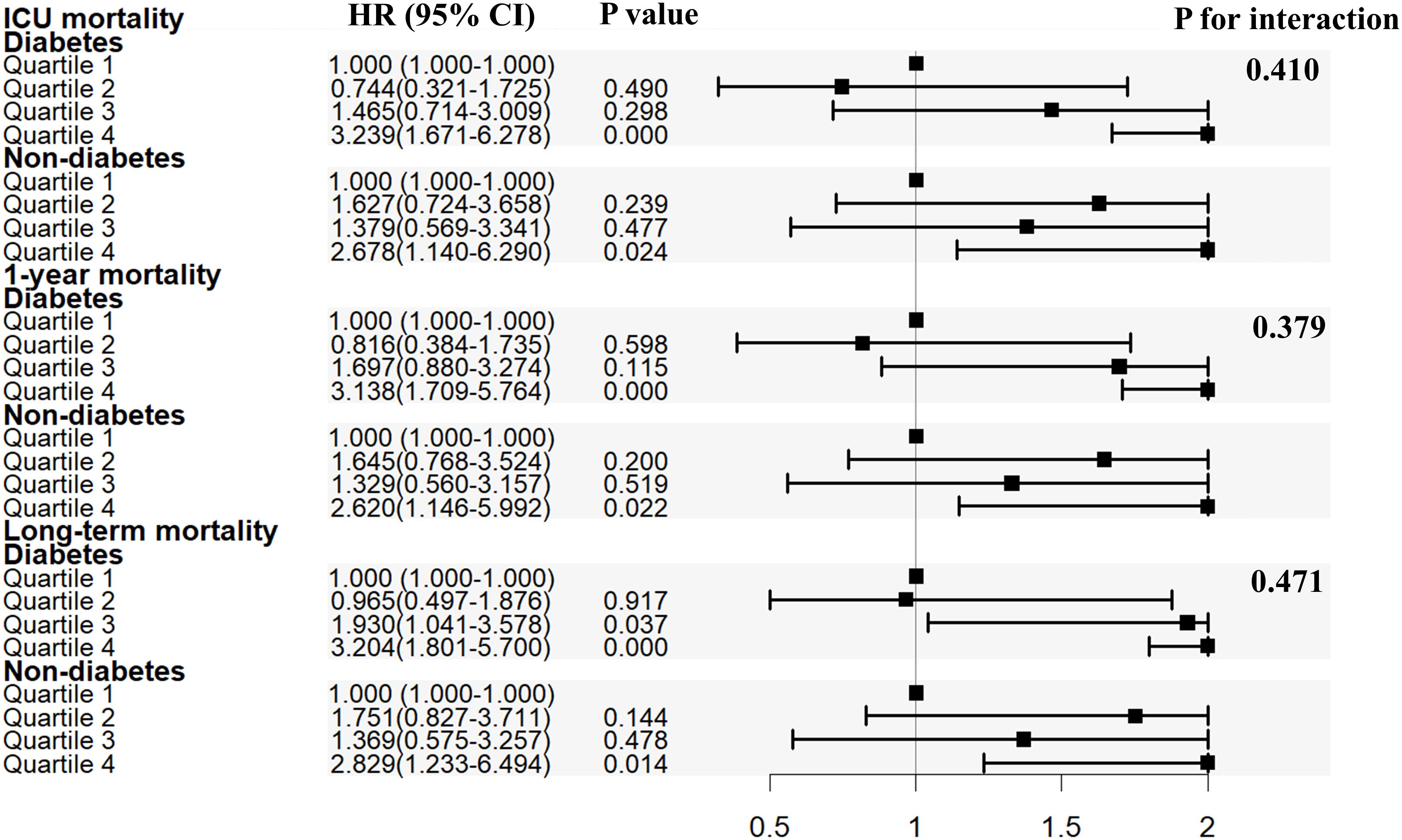
Figure 3 Subgroup analysis for the association of SHR with in ICU mortality, 1-year mortality and overall death. SHR, stress hyperglycemia ratio; ICU, intensive care unit; HR, hazard ratio; CI, confidence interval.
Discussion
This was the first investigation into the correlation between the SHR, short and long-term all-cause mortality in individuals who have experienced cardiac arrest. Our findings revealed that (1) elevated levels of SHR in cardiac arrest patients admitted to the ICU were associated with increased ICU-mortality, 1-year and long-term all-cause mortality; (2) Increased SHR was significantly correlated with a greater likelihood of all-cause mortality among non-diabetic cardiac arrest patients. This research established SHR as a straightforward yet effective biomarker for assessing risk among ICU-admitted patients who have experienced cardiac arrest.
It was suggested that the hyperactivated oxidative stress response, insulin resistance, inflammation, cytokine production, and hormonal abnormalities may be responsible for the correlation between stress hyperglycemia and unfavorable outcomes (24), despite the fact that the underlying mechanisms are too complicated to fully comprehend. In vitro (25) and in patients (26) with or without known diabetes, repetitive acute glucose fluctuations would cause increased endothelial apoptosis, endothelial dysfunction, and oxidative stress responses in comparison to less varied excursions.
It was previously believed that transient hyperglycemia during severe illness in adult patients without known diabetes would be beneficial or even innocuous (27). Initial enthusiasm for strict glycemic control in combined medical and ICU had been tempered by subsequent reports (28–30), primarily due to the unacceptable risk of hypoglycemia. In contrast, the findings of a substantial randomized controlled trial (31) demonstrated unequivocal reductions in mortality associated with intensive insulin therapy for ICU patients, regardless of prior diabetes diagnosis. These discoveries have prompted calls for targeted initiatives to identify patients who are particularly susceptible to damage caused by hyperglycemia and are likely to derive benefits from interventions.
The use of admission glucose as a marker for stress hyperglycemia has been questioned, as a high value does not necessarily indicate an acute increase in glucose in response to severe illnesses, particularly in patients with diabetes and inadequate glycemic control (32). By introducing HbA1c, the SHR, an index of relative glycemia, was developed in an effort to obtain new insights into the relationship between hyperglycemia and patient outcomes. Previous studies had demonstrated that the strong relationship between SHR and poor clinical outcomes in acute coronary syndrome (33–36), acute ischemic stroke (13, 37), heart failure and type 2 DM (38). Our study also demonstrated the strong association between SHR and mortality in cardiac arrest patients.
Higher SHR was associated with a greater risk of all-cause mortality among critically ill patients who have cardiac arrest, and this association is similar to those without diabetes. Previous study also found that hyperglycemia was common in both diabetics and non-diabetics in cardiac arrest patients (39). Diabetes patients had relatively insensitive survival odds to blood glucose, with severe hyperglycemia being the factor associated with decreased survival. Survival probabilities were sensitive to hypoglycemia in non-diabetic individuals (39). A retrospective investigation of critically ill patients failed to establish a significant correlation between average blood glucose concentration and mortality in diabetic patients (40).
ICU-admitted patients frequently exhibit hemodynamic instability, necessitating prompt implementation of optimal care and management. In an effort to enhance patient outcomes, our findings highlight the critical nature of meticulous glycemic control in cardiac arrest patients admitted to intensive care units. Moreover, distinct glycemic management strategies should be implemented for patients with abnormally elevated SHR upon admission, particularly those who do not have diabetes. The mechanisms underlying the relationship between stress hyperglycemia and outcomes in critical cardiac arrest patients require additional research.
Limitation
We fully recognized a number of limitations in our research. Due to the retrospective nature of this investigation, information regarding potential confounding variables, such as the duration of diabetes, was absent. This real-world study aimed to establish a substantial correlation between increased SHR and all-cause mortality in critically ill cardiac arrest patients, as well as to elaborate on the findings regarding cohorts from the United States.
Conclusion
The SHR index demonstrates a L-shaped correlation with both ICU, one-year and long-term all-cause mortality in the American MIMIC-IV cohort, according to our research. SHR can serve as a significant prognostic risk indicator for cardiac arrest patients. To investigate the effect of glycemic control according to the SHR on improving outcomes for patients with cardiac arrest, additional prospective studies are required.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was performed according to the guidelines of the Helsinki Declaration. The use of the MIMIC-IV database was approved by the review committee of Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. The data is publicly available (in the MIMIC-IV database), therefore, the ethical approval statement and the requirement for informed consent were waived for this study.
Author contributions
L-YL: Conceptualization, Data curation, Formal analysis, Writing – original draft. W-HX: Software, Supervision, Validation, Writing – review & editing. J-JL: Conceptualization, Data curation, Writing – review & editing. R-JZ: Conceptualization, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1383993/full#supplementary-material
Supplementary Figure 1 | Restricted cubic spline regression analysis of SHR with in ICU mortality (A), 1-year mortality (B) and overall death (C). SHR, stress hyperglycemia ratio; ICU, intensive care unit.
References
1. Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In-hospital cardiac arrest: A review. Jama. (2019) 321:1200–10. doi: 10.1001/jama.2019.1696
2. Chen N, Callaway CW, Guyette FX, Rittenberger JC, Doshi AA, Dezfulian C, et al. Arrest etiology among patients resuscitated from cardiac arrest. Resuscitation. (2018) 130:33–40. doi: 10.1016/j.resuscitation.2018.06.024
3. Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. Jama. (2004) 291:870–9. doi: 10.1001/jama.291.7.870
4. Lindner TW, Langørgen J, Sunde K, Larsen AI, Kvaløy JT, Heltne JK, et al. Factors predicting the use of therapeutic hypothermia and survival in unconscious out-of-hospital cardiac arrest patients admitted to the ICU. Crit Care. (2013) 17:R147. doi: 10.1186/cc12826
5. Vaahersalo J, Hiltunen P, Tiainen M, Oksanen T, Kaukonen KM, Kurola J, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in Finnish intensive care units: the FINNRESUSCI study. Intensive Care Med. (2013) 39:826–37. doi: 10.1007/s00134-013-2868-1
6. Fugate JE, Brinjikji W, Mandrekar JN, Cloft HJ, White RD, Wijdicks EF, et al. Post-cardiac arrest mortality is declining: a study of the US National Inpatient Sample 2001 to 2009. Circulation. (2012) 126:546–50. doi: 10.1161/CIRCULATIONAHA.111.088807
7. Tsai MS, Huang CH, Wang CH, Cheng HJ, Wu SN, Chang WT, et al. Post-cardiac arrest hydrocortisone use ameliorates cardiac mitochondrial injury in a male rat model of ventricular fibrillation cardiac arrest. J Am Heart Assoc. (2021) 10:e019837. doi: 10.1161/JAHA.120.019837
9. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. (2009) 373:1798–807. doi: 10.1016/S0140-6736(09)60553-5
10. Bellis A, Mauro C, Barbato E, Ceriello A, Cittadini A, Morisco C. Stress-induced hyperglycaemia in non-diabetic patients with acute coronary syndrome: From molecular mechanisms to new therapeutic perspectives. Int J Mol Sci. (2021) 22:775. doi: 10.3390/ijms22020775
11. D'Onofrio N, Sardu C, Paolisso P, Minicucci F, Gragnano F, Ferraraccio F, et al. MicroRNA-33 and SIRT1 influence the coronary thrombus burden in hyperglycemic STEMI patients. J Cell Physiol. (2020) 235:1438–52. doi: 10.1002/jcp.29064
12. Sardu C, Barbieri M, Balestrieri ML, Siniscalchi M, Paolisso P, Calabrò P, et al. Thrombus aspiration in hyperglycemic ST-elevation myocardial infarction (STEMI) patients: clinical outcomes at 1-year follow-up. Cardiovasc Diabetol. (2018) 17:152. doi: 10.1186/s12933-018-0795-8
13. Yuan C, Chen S, Ruan Y, Liu Y, Cheng H, Zeng Y, et al. The stress hyperglycemia ratio is associated with hemorrhagic transformation in patients with acute ischemic stroke. Clin Interv Aging. (2021) 16:431–42. doi: 10.2147/CIA.S280808
14. Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. (2004) 27:553–91. doi: 10.2337/diacare.27.2.553
15. Cui K, Fu R, Yang J, Xu H, Yin D, Song W, et al. The impact of fasting stress hyperglycemia ratio, fasting plasma glucose and hemoglobin A1c on in-hospital mortality in patients with and without diabetes: findings from the China acute myocardial infarction registry. Cardiovasc Diabetol. (2023) 22:165. doi: 10.1186/s12933-023-01868-7
16. Stalikas N, Papazoglou AS, Karagiannidis E, Panteris E, Moysidis D, Daios S, et al. Association of stress induced hyperglycemia with angiographic findings and clinical outcomes in patients with ST-elevation myocardial infarction. Cardiovasc Diabetol. (2022) 21:140. doi: 10.1186/s12933-022-01578-6
17. Schmitz T, Freuer D, Harmel E, Heier M, Peters A, Linseisen J, et al. Prognostic value of stress hyperglycemia ratio on short- and long-term mortality after acute myocardial infarction. Acta Diabetol. (2022) 59:1019–29. doi: 10.1007/s00592-022-01893-0
18. Sia CH, Chan MH, Zheng H, Ko J, Ho AF, Chong J, et al. Optimal glucose, HbA1c, glucose-HbA1c ratio and stress-hyperglycaemia ratio cut-off values for predicting 1-year mortality in diabetic and non-diabetic acute myocardial infarction patients. Cardiovasc Diabetol. (2021) 20:211. doi: 10.1186/s12933-021-01395-3
19. Zhou Q, Yang J, Wang W, Shao C, Hua X, Tang YD. The impact of the stress hyperglycemia ratio on mortality and rehospitalization rate in patients with acute decompensated heart failure and diabetes. Cardiovasc Diabetol. (2023) 22:189. doi: 10.1186/s12933-023-01908-2
20. Mi D, Li Z, Gu H, Jiang Y, Zhao X, Wang Y, et al. Stress hyperglycemia is associated with in-hospital mortality in patients with diabetes and acute ischemic stroke. CNS Neurosci Ther. (2022) 28:372–81. doi: 10.1111/cns.13764
21. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. (2001) 32:2426–32. doi: 10.1161/hs1001.096194
23. Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. (2016) 3:160035. doi: 10.1038/sdata.2016.35
24. Kosiborod M. Hyperglycemia in acute coronary syndromes: From mechanisms to prognostic implications. Endocrinol Metab Clin North Am. (2018) 47:185–202. doi: 10.1016/j.ecl.2017.11.002
25. Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. (2003) 52:2795–804. doi: 10.2337/diabetes.52.11.2795
26. Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. (2008) 57:1349–54. doi: 10.2337/db08-0063
27. Turina M, Christ-Crain M, Polk HC Jr. Diabetes and hyperglycemia: strict glycemic control. Crit Care Med. (2006) 34:S291–300. doi: 10.1097/01.CCM.0000231887.84751.04
28. Tamler R, LeRoith D, Roth J. Intensive insulin therapy in the medical ICU. N Engl J Med. (2006) 354:2069–71. doi: 10.1056/NEJMc060566
29. Malhotra A. Intensive insulin in intensive care. N Engl J Med. (2006) 354:516–8. doi: 10.1056/NEJMe058304
30. Mechanick JI, Scurlock C. Glycemic control and nutritional strategies in the cardiothoracic surgical intensive care unit–2010: state of the art. Semin Thorac Cardiovasc Surg. (2010) 22:230–5. doi: 10.1053/j.semtcvs.2010.10.006
31. van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. (2001) 345:1359–67. doi: 10.1056/NEJMoa011300
32. Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care. (2013) 17:305. doi: 10.1186/cc12514
33. Yang J, Zheng Y, Li C, Gao J, Meng X, Zhang K, et al. The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with acute coronary syndrome: Insight from a large cohort study in asia. Diabetes Care. (2022) 45:947–56. doi: 10.2337/dc21-1526
34. Xu W, Song Q, Wang X, Zhao Z, Meng X, Xia C, et al. Association of stress hyperglycemia ratio and in-hospital mortality in patients with coronary artery disease: insights from a large cohort study. Cardiovasc Diabetol. (2022) 21:217. doi: 10.1186/s12933-022-01645-y
35. Karakasis P, Stalikas N, Patoulias D, Pamporis K, Karagiannidis E, Sagris M, et al. Prognostic value of stress hyperglycemia ratio in patients with acute myocardial infarction: A systematic review with Bayesian and frequentist meta-analysis. Trends Cardiovasc Med. (2023). doi: 10.1016/j.tcm.2023.11.006
36. Alkatiri AH, Qalby N, Mappangara I, Zainal ATF, Cramer MJ, Doevendans PA, et al. Stress hyperglycemia and poor outcomes in patients with ST-elevation myocardial infarction: a systematic review and meta-analysis. Front Cardiovasc Med. (2024) 11:1303685. doi: 10.3389/fcvm.2024.1303685
37. Dai Z, Cao H, Wang F, Li L, Guo H, Zhang X, et al. Impacts of stress hyperglycemia ratio on early neurological deterioration and functional outcome after endovascular treatment in patients with acute ischemic stroke. Front Endocrinol (Lausanne). (2023) 14:1094353. doi: 10.3389/fendo.2023.1094353
38. Zhou Y, Liu L, Huang H, Li N, He J, Yao H, et al. 'Stress hyperglycemia ratio and in-hospital prognosis in non-surgical patients with heart failure and type 2 diabetes. Cardiovasc Diabetol. (2022) 21:290. doi: 10.1186/s12933-022-01728-w
39. Beiser DG, Carr GE, Edelson DP, Peberdy MA, Hoek TL. Derangements in blood glucose following initial resuscitation from in-hospital cardiac arrest: a report from the national registry of cardiopulmonary resuscitation. Resuscitation. (2009) 80:624–30. doi: 10.1016/j.resuscitation.2009.02.011
Keywords: cardiac arrest, stress hyperglycemia ratio, diabetes, mortality, prognosis
Citation: Lian L-Y, Xue W-H, Lu J-J and Zheng R-J (2024) Impact of stress hyperglycemia ratio on mortality in patients with cardiac arrest: insight from American MIMIC-IV database. Front. Endocrinol. 15:1383993. doi: 10.3389/fendo.2024.1383993
Received: 08 February 2024; Accepted: 06 May 2024;
Published: 21 May 2024.
Edited by:
Joaquim Barreto, State University of Campinas, BrazilReviewed by:
Pedro Iglesias, Puerta de Hierro University Hospital Majadahonda, SpainNikolaos Stalikas, University General Hospital of Thessaloniki AHEPA, Greece
Copyright © 2024 Lian, Xue, Lu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ru-Jie Zheng, cmplZXpoQDEyNi5jb20=
 Li-You Lian
Li-You Lian Wei-Hao Xue2
Wei-Hao Xue2 Ru-Jie Zheng
Ru-Jie Zheng