
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 08 April 2024
Sec. Pediatric Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1383812
Background: Recent studies suggest a link between the Klotho protein, sex hormones, and insulin-like growth factor-1 (IGF-1), indicating that α-Klotho levels may rise during puberty, including in central precocious puberty (CPP) cases. This study aimed to explore α-Klotho levels in girls with CPP to assess its potential as a diagnostic and monitoring tool for this condition.
Methods: In total, 139 girls, comprising 82 patients diagnosed with CPP and 57 healthy prepubertal controls, were enrolled in this study. From March 2020 to May 2023, we assessed both α-Klotho levels and clinical parameters. α-Klotho concentrations were measured using an α-Klotho ELISA kit. For the girls with CPP, we additionally analyzed samples taken 6 months after GnRH agonist treatment.
Results: α-Klotho levels were higher in the CPP group compared with the control (CPP group: 2529 ± 999 ng/mL; control group: 1802 ± 675 pg/mL) (P < 0.001), and its level modest decreased after 6 months of GnRH agonist treatment (2147± 789 pg/mL) (P < 0.001). The association between α-Klotho and IGF-1 SDS, follicular stimulating hormone and baseline luteinizing hormone was assessed by partial correlation after adjusting for age, BMI SDS (r= 0.416, p= <0.001; r= 0.261, p= 0.005; r= 0.278, p= 0.002), respectively. Receiver operating characteristic curve analysis identified an α-Klotho cut-off differentiating CPP from controls, with a cut-off of 1914 pg/mL distinguishing girls with CPP from controls with a sensitivity of 69.5% and specificity of 70.2%; the area under the curve was 0.723.
Conclusion: The findings of our study are the first step towards deciphering the role of α-Klotho in puberty induction. With additional data and further research, α-Klotho could potentially be utilized as a significant diagnostic and monitoring tool for CPP.
Central precocious puberty (CPP) is a relatively uncommon but significant pediatric endocrine disorder characterized by premature activation of the hypothalamic-pituitary-gonadal axis, resulting in the early onset of pubertal development, typically before the age of 8 years in girls (1). CPP not only leads to physical and psychological challenges for affected children and their families but also necessitates early diagnosis and appropriate management to mitigate potential long-term health consequences (2). Although tools for CPP diagnosis have been established (3, 4), continuous research is being conducted on new markers to improve diagnosis and monitoring (5–7). Understanding the causes and progression of CPP can aid the development of enhanced diagnostic tools and contribute to the advancement of improved treatment options.
The Klotho protein is encoded by the Klotho gene and exists in multiple forms, including transmembrane and soluble forms. The soluble form of Klotho protein is released into the circulation and exerts endocrine functions (8). Notably, Klotho is predominantly expressed in the hypothalamus-pituitary-ovary axis, which is associated with reproductive endocrine-related diseases (9). In line with this, previous studies have shown that in mice, Klotho is moderately expressed in ovarian and pituitary tissues, with a positive correlation with basal LH levels (10). Furthermore, a 2007 study on female mice reported a potential relationship between estradiol and Klotho (11), and a study conducted in 2022 confirmed a connection between α-Klotho and sex hormones among American male participants (12). Recent research has also shown a strong relationship between the Klotho protein and the GH/insulin-like growth factor-1 (IGF-1) system (13). It can be postulated that the levels of sex hormones, IGF-1, and Klotho are organically interconnected. Based on these studies, we hypothesize that α-Klotho may provide valuable insights into the pathophysiology of CPP, its progression, and the effectiveness of therapeutic interventions. This study aimed to investigate the levels of α-Klotho in girls diagnosed with CPP and evaluate its utility as a diagnostic and monitoring marker.
This study included 139 girls, comprising 82 girls diagnosed with CPP and 57 healthy prepubertal control girls. Patients with a known brain tumor, those exposed to cranial irradiation, and those presenting central nervous system-related symptoms were excluded from the study. Patients diagnosed with CPP were treated with subcutaneous administration of leuprorelin acetate (1.875 mg (0.5 vial) for <20 kg, 2.81 mg (0.75 vial) for 20-30 kg, 3.75 mg (1 vial) for ≥30 kg) every 4 weeks. Medical records were collected for both groups, including data before CPP diagnosis and 6 months after the initiation of GnRH agonist treatment. This study was approved by the Institutional Review Board of Hallym University Kangdong Sacred Heart Hospital (Institutional Review Board No. 2020-05-010-009).
The criteria for diagnosing CPP in this study required the fulfillment of all three of the following: breast development occurring before the age of 8 years. Bone age (BA) was advanced by 1 year compared to chronological age (CA). A peak luteinizing hormone (LH) concentration of ≥ 5 IU/L during the GnRH stimulation test.
The following laboratory parameters were measured in the study participants: LH, follicular stimulating hormone (FSH), Estradiol, IGF-1, alkaline phosphatase (ALP), calcium, phosphate, α-Klotho. Serum levels of α-Klotho were quantified using an α-Klotho enzyme-linked immunosorbent assay (ELISA) kit from Immuno-Biological Laboratories Co., Japan.
Statistical analyses were performed using SPSS version 21.0. To compare continuous variables between groups (control, before, and 6 months after GnRH agonist treatment), the t-test was used for independent and paired samples. Pearson’s correlation analysis was conducted to explore the relationship between α-Klotho levels and other clinical variables.
Multivariable logistic regression analysis was performed to investigate the association between serum α-Klotho levels and CPP diagnosis. Receiver operating characteristic (ROC) curves were constructed to assess the diagnostic performance of serum α-Klotho levels in distinguishing prepubertal control girls from girls with CPP.
The clinical, hormonal, and metabolic parameters of controls and girls with CPP at diagnosis are presented as mean ± standard deviation values. Girls with CPP exhibited significantly higher levels of LH, estradiol, ALP, and IGF-1 SDS than control girls (Table 1). The α-Klotho levels in control girls were significantly lower (mean: 1802 pg/mL) than those in girls diagnosed with CPP (mean: 2529 pg/mL), showing a statistically significant difference (p = 0.001).
Partial correlation analysis adjusted for age and BMI (body mass index) SDS was performed to investigate the relationship between α-Klotho and other clinical variables (Table 2). Results demonstrated that α-Klotho had a significant positive correlation with basal LH, basal FSH, and estradiol. Notably, IGF-1 SDS displayed a stronger correlation, with a coefficient of 0.416, which was higher than that of the other variables.
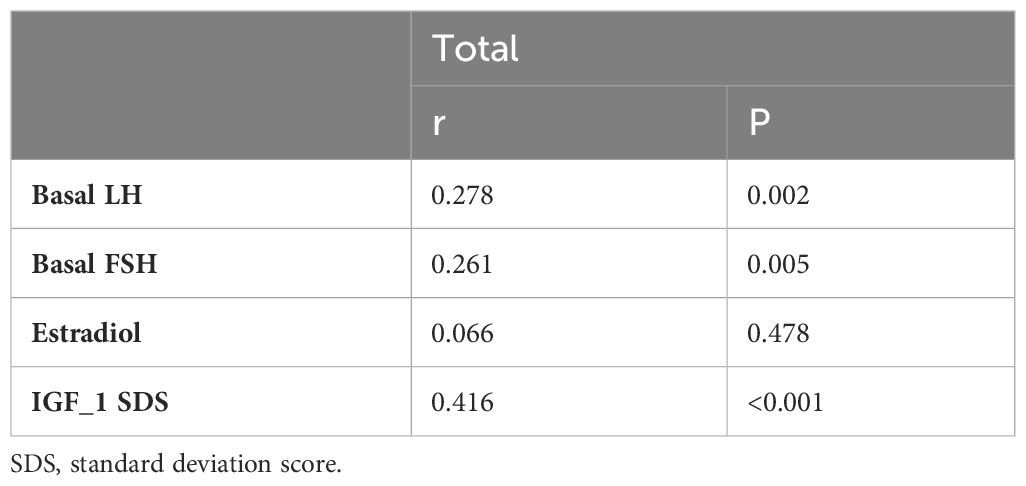
Table 2 α-Klotho relationship with LH, FSH, estradiol, and IGF-I SDS studied by partial correlation with adjustment for age and BMI SDS.
A multivariable logistic regression analysis was performed to assess the impact of α-Klotho on the diagnosis of CPP (Table 3). The basal LH, estradiol, Klotho variables were used in their log-transformed and standardized forms. In the first step, controlling for age and BMI, α-Klotho exhibited a statistically significant association with CPP diagnosis. In the second step, adding basal LH, estradiol, and α-Klotho as variables, α-Klotho remained significantly associated with CPP diagnosis (odds ratio: 2.24, p < 0.05).
ROC curve analysis was performed to assess the diagnostic performance of serum α-Klotho levels in discriminating between prepubertal control girls and girls with CPP (Figure 1). The optimal cut-off value for α-Klotho was determined to be 2017 pg/mL, yielding a sensitivity of 64% and specificity of 71%. The area under the curve (AUC) was 0.723, indicating the statistically significant diagnostic potential of α-Klotho in identifying CPP (p < 0.001).
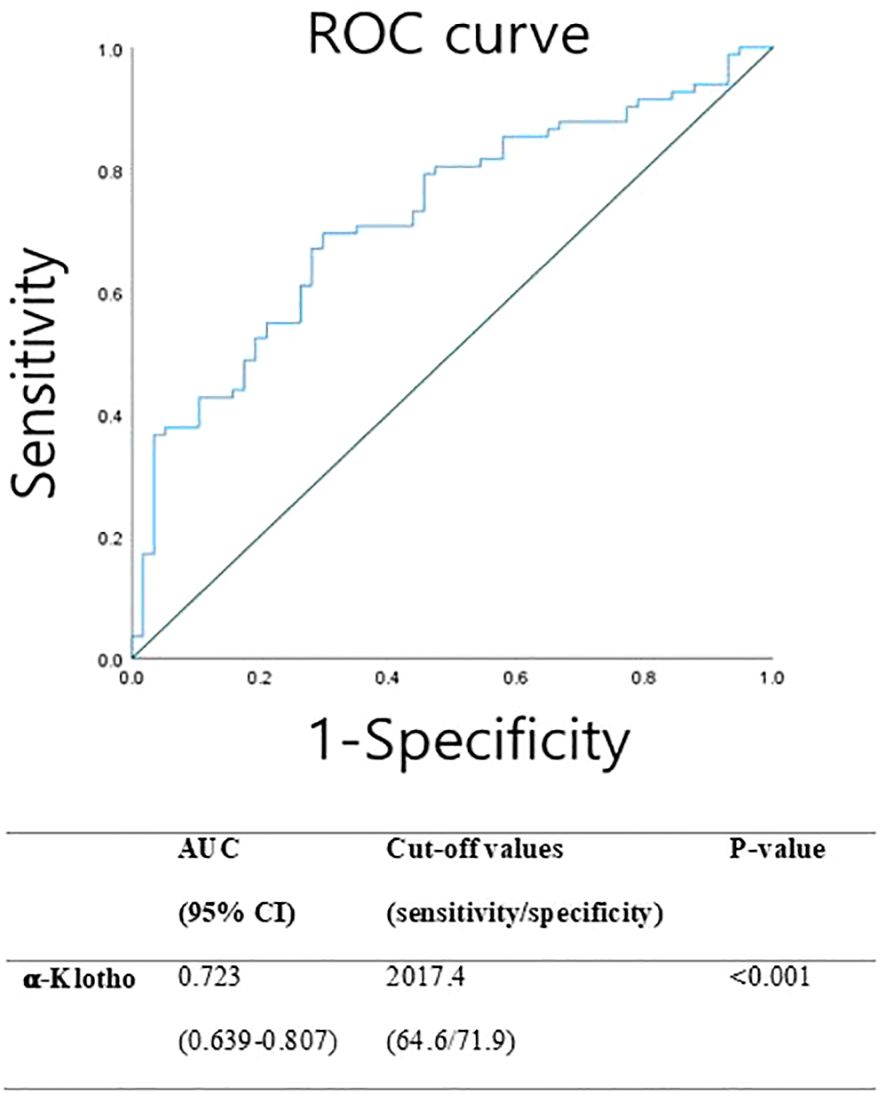
Figure 1 Receiver operating characteristic (ROC) curve representing the sensitivity and specificity of α-klotho for screening CPP.
After 6 months of GnRH agonist treatment in girls with CPP, significant reductions were observed in LH, estradiol, ALP, IGF-1 SDS, and α-Klotho levels. Particularly, α-Klotho levels decreased from an average of 2529 pg/mL before treatment to 2147 pg/mL after treatment (p = 0.001) (Table 4, Figure 2).
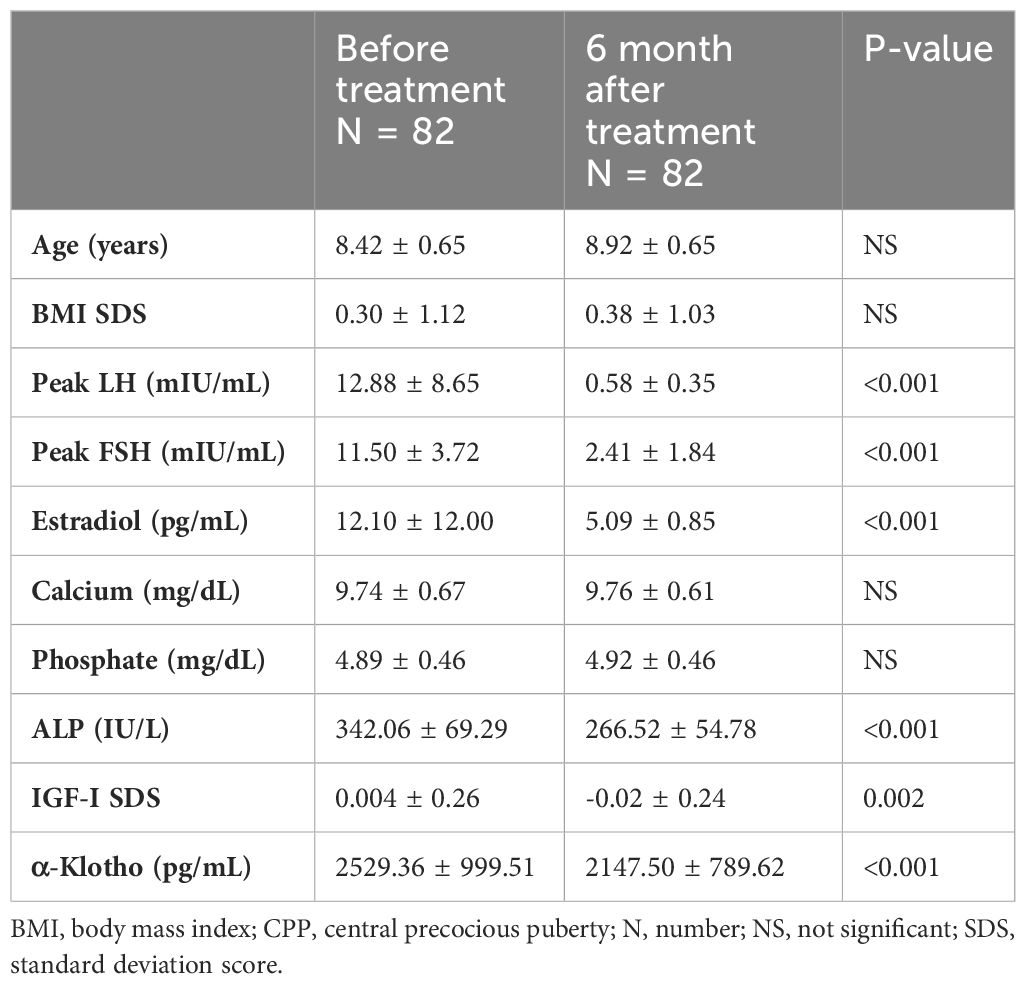
Table 4 Clinical, hormonal, and metabolic parameters of girls with CPP at diagnosis and after 6 months of GnRHa.
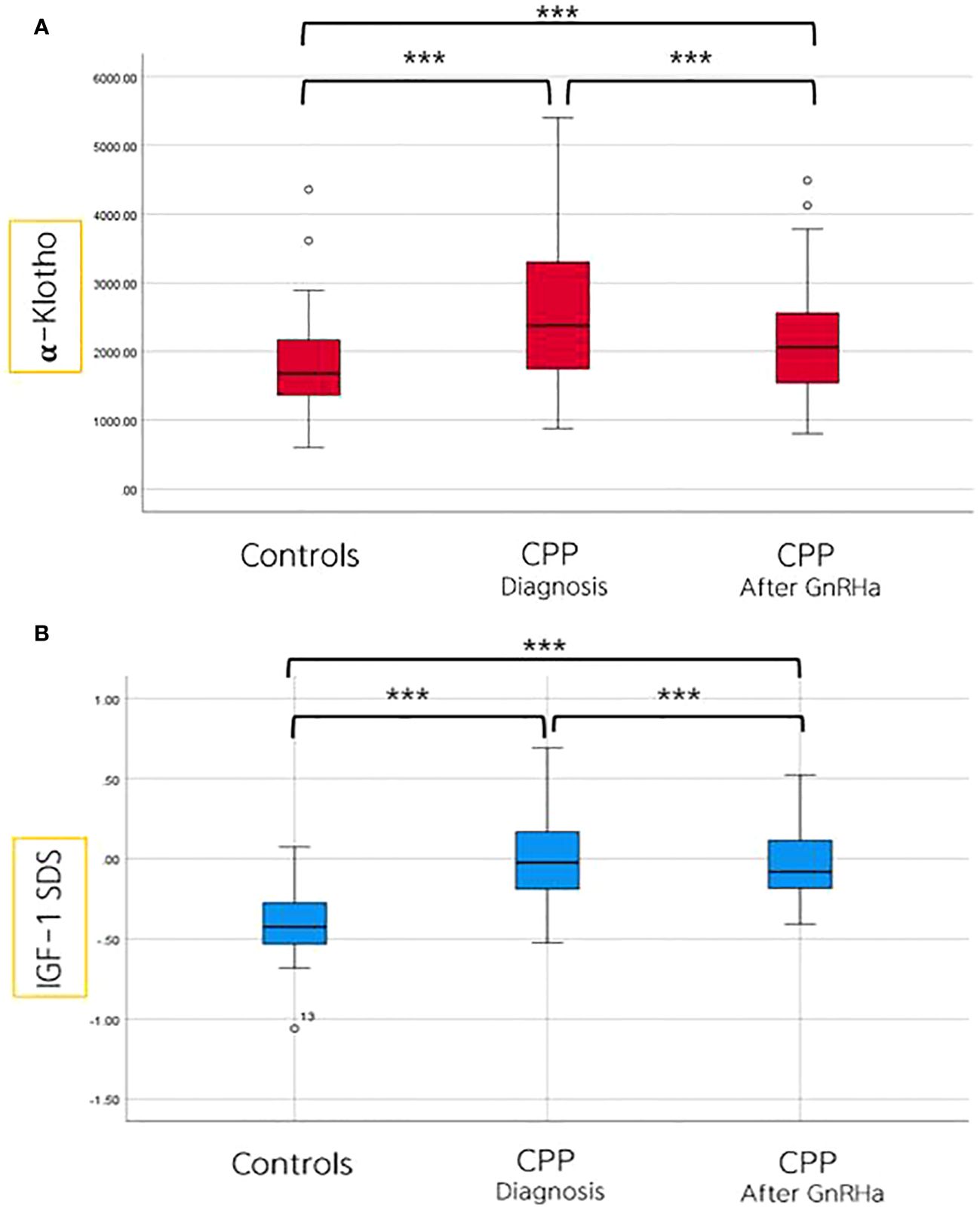
Figure 2 Comparison of α-Klotho (A) and IGF-I SDS (B) levels among controls, and in girls with CPP, both before and after 6 months of GnRHa treatment. ***p value ≤ 0.001.
Our findings demonstrated that girls with CPP exhibited significantly higher α-Klotho levels than prepubertal control girls in the same age group. Furthermore, we observed a modest decrease in α-Klotho levels in girls with CPP after 6 months of GnRH agonist treatment.
A previous study reported that children before and during puberty had median α-Klotho level of 2399 pg/mL (range, 762–5866 pg/mL), whereas the prepubertal group had a mean level of 1875 pg/mL (range, 372–5694 pg/mL) (14). In our study, the mean α-Klotho levels aligned with those findings, showing that girls with CPP had levels of 2529 ± 999 pg/mL and prepubertal girls had levels of 1802 ± 675 pg/mL. Our study specifically compared girls of the same age, enabling us to demonstrate a significant increase in α-klotho levels coinciding with the onset of puberty, thereby excluding the influence of aging on α-Klotho. To the best of our knowledge, this is the first investigation of α-Klotho levels in Korean girls, and we believe that continued data collection can establish these findings as valuable references for future medical research.
Our findings revealed that α-Klotho had a significant positive correlation with basal LH, FSH, and IGF-SDS, particularly after adjusting for age and BMI. This adjustment was performed because α-Klotho levels may be influenced by age and BMI (15–18). Previous studies have also supported the involvement of the insulin-like growth factor system in the initiation and progression of puberty, with documented positive correlations between α-Klotho and IGF-1 levels, indicating a closely regulated interplay between these factors (19–21). Specifically, it has been suggested that IGF-1 may stimulate Klotho secretion (22, 23). In addition, our multivariable logistic regression analysis for CPP revealed that α-Klotho is a significant factor influencing the outcome, similar to basal LH and estradiol. Furthermore, a previous study demonstrated the correlation between basal LH, FGF23, Klotho, and IGF-1 with rapidly progressive CPP, suggesting their potential importance in the rate of sexual development in girls (24). Building upon this body of literature and our own results, we hypothesized that an increase in LH, FSH, and IGF-1 levels during the onset of puberty may lead to higher α-Klotho levels. However, future large-scale studies with larger sample sizes are required to enhance the confidence in these associations.
Moreover, our study confirmed a modest decrease in α-Klotho levels 6 months after GnRH agonist treatment. The decline in levels, following the inhibitory treatment, suggests that elevated α-Klotho levels may be associated with hormonal changes in girls with CPP. Tracking α-Klotho levels may offer valuable insights for guiding potential future treatment directions and could serve as a promising monitoring marker. Through ongoing research efforts, we anticipate contributing to a better understanding of the pathological mechanisms of CPP and the development of more effective treatment strategies.
Nevertheless, this study had some limitations that warrant consideration. We were unable to assess α-Klotho levels in boys, and further investigation is required to determine whether similar patterns are observed in males. Additionally, the study did not evaluate α-Klotho levels after discontinuation of GnRH agonist treatment, which could provide insights into the long-term effects of treatment.
Our study sheds light on the intriguing role of α-Klotho in the context of CPP. Elevated α-Klotho levels in girls with CPP and their subsequent decrease following GnRH agonist treatment highlight its potential significance as a diagnostic and monitoring marker. The positive correlation between IGF-I and LH implies a complex interplay between hormones during the pubertal process, which warrants further investigation. While these findings are promising, additional research is required to fully elucidate the mechanisms underlying α-Klotho’s involvement in early puberty and its clinical utility as a diagnostic tool.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Institutional Review Board of Kangdong Sacred Heart Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
J-HP: Investigation, Methodology, Writing – original draft. E-SN: Conceptualization, Methodology, Writing – original draft. IH: Conceptualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We are grateful to all participants who volunteered their time and effort to participate in this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ALP, alkaline phosphatase; AUC, area under the curve; BA, bone age; CA, chronological age; CPP, central precocious puberty; FSH, follicular stimulating hormone; IGF-I, insulin-like growth factor-1;
LH, luteinizing hormone; ROC, receiver operating characteristic.
1. Styne DM, Grumbach MM. Puberty: Ontogeny, Neuroendocrinology, Physiology, and Disorders. In: Williams Textbook of Endocrinology. New York, USA: Elsevier (2011). p. 1054–201.
2. Soliman A, Alaaraj N, De Sanctis V, Alyafei F, Ahmed S, Hamed N. Long-term health consequences of central precocious/early puberty (Cpp) and treatment with gn-rh analogue: A short update. Acta BioMed. (2023) 94:e2023222. doi: 10.23750/abm.v94i6.15316
3. Kandemir N, Demirbilek H, Özön ZA, Gönç N, Alikaşifoğlu A. Gnrh stimulation test in precocious puberty: single sample is adequate for diagnosis and dose adjustment. J Clin Res Pediatr Endocrinol. (2011) 3:12–7. doi: 10.4274/jcrpe.v3i1.03
4. Kim SJ, Kim JH, Hong YH, Chung IH, Lee EB, Kang E, et al. 2022 Clinical practice guidelines for central precocious puberty of Korean children and adolescents. Ann Pediatr Endocrinol Metab. (2023) 28:168–77. doi: 10.6065/apem.2346168.084
5. Lee SY, Kim JM, Kim YM, Lim HH. Single random measurement of urinary gonadotropin concentration for screening and monitoring girls with central precocious puberty. Ann Pediatr Endocrinol Metab. (2021) 26:178–84. doi: 10.6065/apem.2040208.104
6. Rhie YJ, Lee KH, Eun SH, Choi BM, Chae HW, Kwon AR, et al. Serum kisspeptin levels in Korean girls with central precocious puberty. J Korean Med Sci. (2011) 26:927–31. doi: 10.3346/jkms.2011.26.7.927
7. Kutlu E, Özgen İT, Bulut H, Koçyiğit A, Otçu H, Cesur Y. Serum irisin levels in central precocious puberty and its variants. J Clin Endocrinol Metab. (2021) 106:e247–54. doi: 10.1210/clinem/dgaa720
8. Dalton GD, Xie J, An S-W, Huang C-L. New insights into the mechanism of action of soluble klotho. Front Endocrinol (Lausanne). (2017) 8:323. doi: 10.3389/fendo.2017.00323
9. Xie T, Ye W, Liu J, Zhou L, Song Y. The emerging key role of Klotho in the hypothalamus–pituitary–ovarian axis. Reprod Sci. (2021) 28:322–31. doi: 10.1007/s43032-020-00277-5
10. Olauson H, Mencke R, Hillebrands JL, Larsson TE. Tissue expression and source of circulating αKlotho. Bone. (2017) 100:19–35. doi: 10.1016/j.bone.2017.03.043
11. Öz OK, Hajibeigi A, Howard K, Cummins CL, Van Abel M, Bindels RJ, et al. Aromatase deficiency causes altered expression of molecules critical for calcium reabsorption in the kidneys of female mice. J Bone Miner Res. (2007) 22:1893–902. doi: 10.1359/jbmr.070808
12. Zhang Z, Qiu S, Huang X, Jin K, Zhou X, Lin T, et al. Association between testosterone and serum soluble α-klotho in us males: A cross-sectional study. BMC Geriatr. (2022) 22:570. doi: 10.1186/s12877-022-03265-3
13. Sato T, Komaba H, Nagatani T, Watanabe T, Kishida Y, Fukagawa M. The pituitary is a candidate organ that modulates circulating klotho levels. J Endocr Soc. (2019) 3:52–61. doi: 10.1210/js.2018-00223
14. Gkentzi D, Efthymiadou A, Kritikou D, Chrysis D. Fibroblast growth factor 23 and Klotho serum levels in healthy children. Bone. (2014) 66:8–14. doi: 10.1016/j.bone.2014.05.012
15. Manya H, Akasaka-Manya K, Endo T. Klotho protein deficiency and aging. Geriatr Gerontol Int. (2010) 10:S80–S7. doi: 10.1111/j.1447-0594.2010.00596.x
16. Carreras-Badosa G, Puerto-Carranza E, Mas-Parés B, Gómez-Vilarrubla A, Gómez-Herrera B, Díaz-Roldán F, et al. Higher levels of serum α-klotho are longitudinally associated with less central obesity in girls experiencing weight gain. Front Endocrinol (Lausanne). (2023) 14:1218949. doi: 10.3389/fendo.2023.1218949
17. Prud’homme GJ, Kurt M, Wang Q. Pathobiology of the klotho antiaging protein and therapeutic considerations. Front Aging. (2022) 3:931331. doi: 10.3389/fragi.2022.931331
18. Buchanan S, Combet E, Stenvinkel P, Shiels PG. Klotho, aging, and the failing kidney. Front Endocrinol (Lausanne). (2020) 11:560. doi: 10.3389/fendo.2020.00560
19. Fortes MR, Li Y, Collis E, Zhang Y, Hawken RJ. The Igf1 pathway genes and their association with age of puberty in cattle. Anim Genet. (2013) 44:91–5. doi: 10.1111/j.1365-2052.2012.02367.x
20. Daftary SS, Gore AC. The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing hormones neurones during postnatal development. J Neuroendocrinol. (2004) 16:160–9. doi: 10.1111/j.0953-8194.2004.01149.x
21. Rosales Nieto CA, Ferguson MB, Macleay CA, Briegel JR, Martin GB, Thompson AN. Selection for superior growth advances the onset of puberty and increases reproductive performance in ewe lambs. Animal. (2013) 7:990–7. doi: 10.1017/s1751731113000074
22. Caicedo D, Díaz O, Devesa P, Devesa J. Growth hormone (GH) and cardiovascular system. Int J Mol Sci. (2018) 19:290. doi: 10.3390/ijms19010290
23. Rubinek T, Shahmoon S, Shabtay-Orbach A, Ben Ami M, Levy-Shraga Y, Mazor-Aronovitch K, et al. Klotho response to treatment with growth hormone and the role of Igf-I as a mediator. Metabolism. (2016) 65:1597–604. doi: 10.1016/j.metabol.2016.08.004
Keywords: α-Klotho, IGF-1, central precocious puberty, GnRHa, puberty
Citation: Park J-H, Noh E-S and Hwang IT (2024) α-Klotho levels in girls with central precocious puberty: potential as a diagnostic and monitoring marker. Front. Endocrinol. 15:1383812. doi: 10.3389/fendo.2024.1383812
Received: 08 February 2024; Accepted: 25 March 2024;
Published: 08 April 2024.
Edited by:
Semra Çaglar Çetinkaya, University of Health Sciences, TürkiyeReviewed by:
Anastasios Serbis, University of Ioannina, GreeceCopyright © 2024 Park, Noh and Hwang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eu-Seon Noh, YmJhbmFuZXQxQG5hdmVyLmNvbQ==; Il Tae Hwang, aXRod2FuZzgzQGtkaC5vci5rcg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.