- 1Center for Reproductive Medicine, Department of Pediatrics, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, China
- 2Bengbu Medical College, Bengbu, Anhui, China
Aims: Studies on the association between serum lead levels and parathyroid function in adolescents are lacking. Therefore, in this study, we elucidated the possible association between blood lead levels (BLLs) and the parathyroid hormone (PTH) in adolescents aged 12–19 years in the United States.
Methods: In this study, information from the database of the National Health and Nutrition Examination Survey was utilized. The study included 3919 participants from survey cycles between 2003–2004 and 2005–2006. Multivariable linear regression analysis was performed to determine the correlation between BLLs and PTH. Furthermore, smooth curve fitting was utilized to analyze the dose–response relationship between BLLs and PTH.
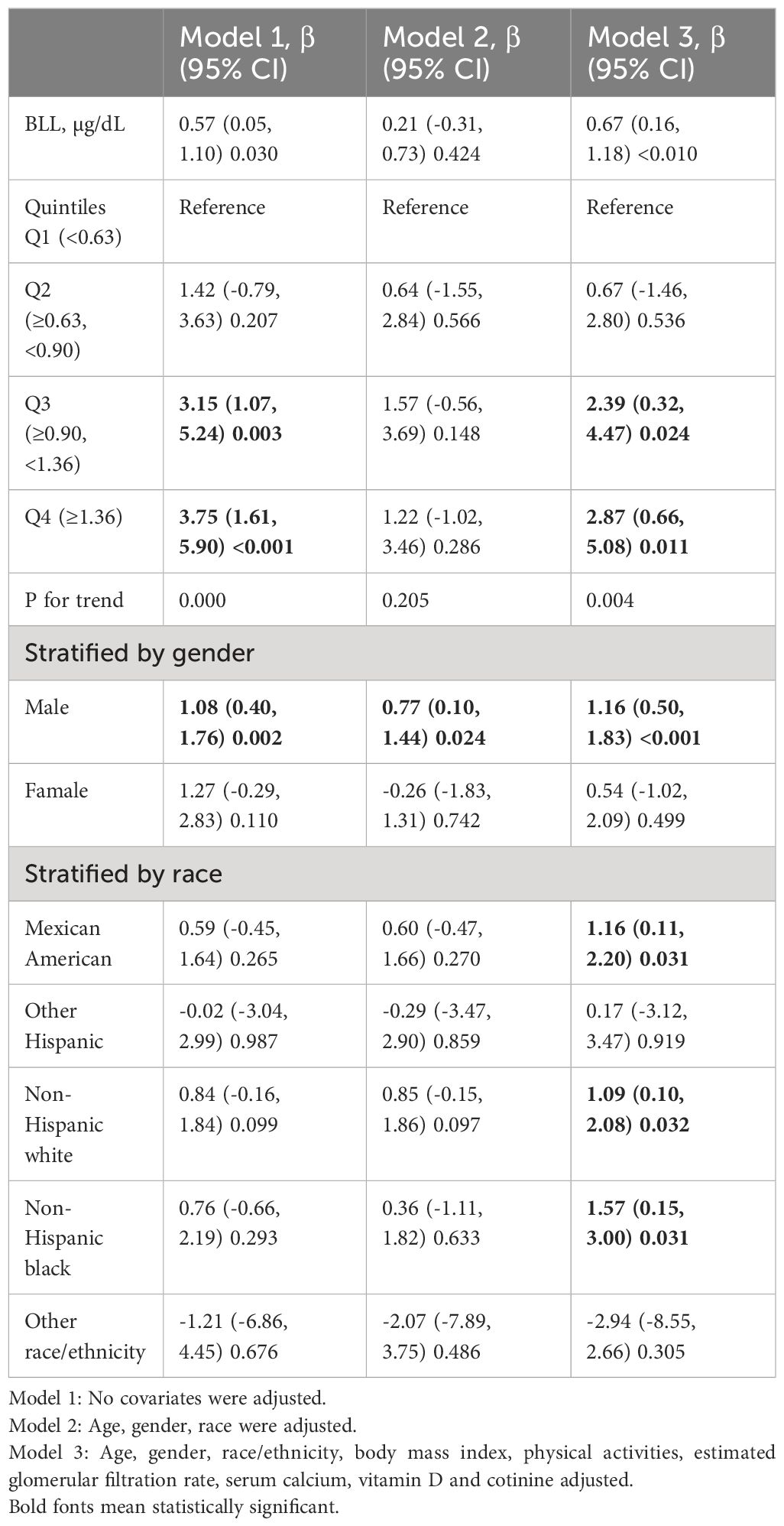
Results: Multivariable linear regression analysis revealed that every 1 μg/dL increase in BLLs was associated with 0.67 pg/mL increase in PTH (β = 0.67, 95% CI: 0.16–1.18, p < 0.01). However, sex-stratified subgroup analysis revealed that this positive association was only observed in males (β = 1.16, 95% CI: 0.50–1.83 p < 0.01). Smooth curve fitting revealed a positive correlation between BLLs and PTH.
Conclusions: In adolescents in the United States, BLLs are positively correlated with PTH, particularly in males.
Introduction
Lead is a harmful heavy metal that is ubiquitous in the environment; its toxicity remains an important public health concern because populations are still exposed to low lead levels (1–3). As per the Centers for Disease Control and Prevention, there are no safe blood lead levels (BLLs) for children because lead exhibits toxicity even at low levels. Exposure to low levels of lead can exert deleterious effects on multiple organ systems in the human body (4, 5). Furthermore, prolonged lead exposure can result in detrimental health outcomes in adults, encompassing cardiovascular diseases, renal impairment, reproductive disorders, and neurological dysfunction (6–9). Moreover, in expectant women, lead exposure may lead to complications such as preterm birth or low birth weight (10, 11).
Exposure to lead in the environment during childhood and the resultant health issues are considered public health disasters (12). Compared with adults, children are at a higher risk of lead exposure because they possess enhanced absorption capabilities and limited excretion capacities (13). Lead exposure among children is associated with blood pressure, endocrine-disrupting activity, cognitive impairment, developmental delay, decreased intelligence quotient, and behavioral issues (14–16).
The parathyroid hormone (PTH) is a polypeptide that plays a key role in regulating calcium and phosphate levels in the body. In the parathyroid gland, PTH is synthesized and subsequently cleaved to exclusively generate the active state.
Studies have revealed that lead affects blood PTH levels. Kristal-Boneh et al. (16) and Potula et al. (17) confirmed the correlation between lead exposure in the workplace and levels of PTH in the bloodstream, noting a significant rise in PTH among individuals with occupational lead exposure. However, most studies are limited owing to their small sample size; furthermore, the findings are contradictory (18, 19). In addition, there is little information regarding the effects of serum lead on PTH levels in the general population with low-level exposure.
Research has indicated that lead disrupts the metabolism of vitamin D through its impact on the expression of metabolizing enzymes (20), and there is a negative correlation between BLL and free 25(OH)D, and a positive correlation between BLL and Vitamin D binding protein in healthy adolescents (21). These results suggest that lead may affect PTH levels through its involvement in vitamin D metabolism.
Increasing evidence indicates elevated lead levels in the general population. Therefore, further elucidating the possible association between BLLs and calcium homeostasis markers such as PTH is essential. Previous individual primary studies have presented inconclusive evidence of this relationship.
Considering limited studies in children and adolescents, in this current investigation, we investigated the potential correlation among exposure to low BLLs and PTH levels in children and teenagers residing in the United States. For this, we used data from the National Health and Nutrition Examination Survey (NHANES) from 2003 to 2006.
Methods
Study design
NHANES is a nationwide survey that collects information on the nutritional and health statuses of United States individuals who are not residing in institutions. This program started as a sequence of surveys during the early 1960s and has been continuously running since 1999. Around 5,000 people from different regions nationwide are included in this survey each year. This study was approved by the Ethics Review Board of the National Center of Health Statistics (NCHS). All participants provided their consent after being fully informed. Parents/guardians provided written informed consent for children under 18 years of age, while individuals aged 18 or above autonomously provided their own signature on the document.
Study population
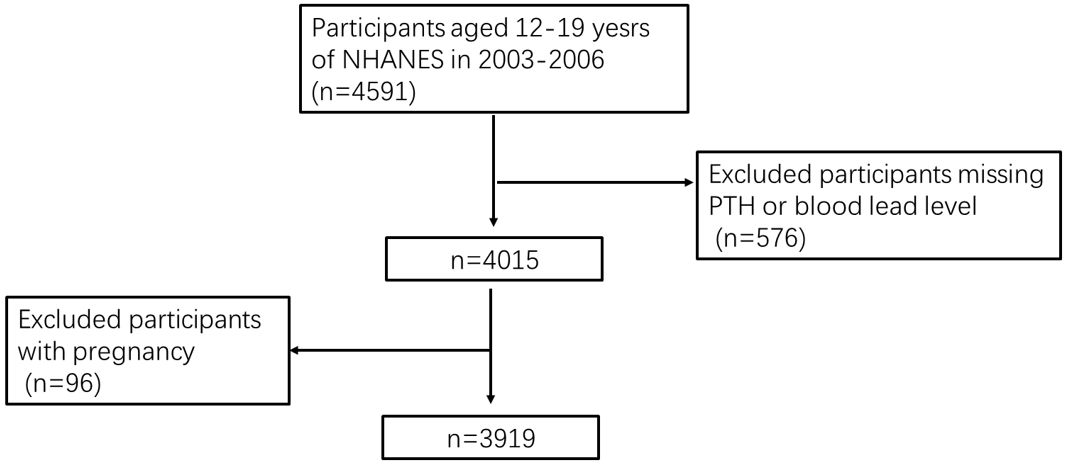
NHANES data from 2003–2004 to 2005–2006 were used because this was the only period when information on PTH measurements was collected. We only focused on individuals aged 12–19 years with information on blood PTH and lead levels. Among the eligible participants who met this criterion, 4,015 participants were included. However, 96 pregnant women were excluded from the final sample size; as a result, a cohort comprising 3,919 participants was selected for further examination.
Measurement of BLLs and PTH
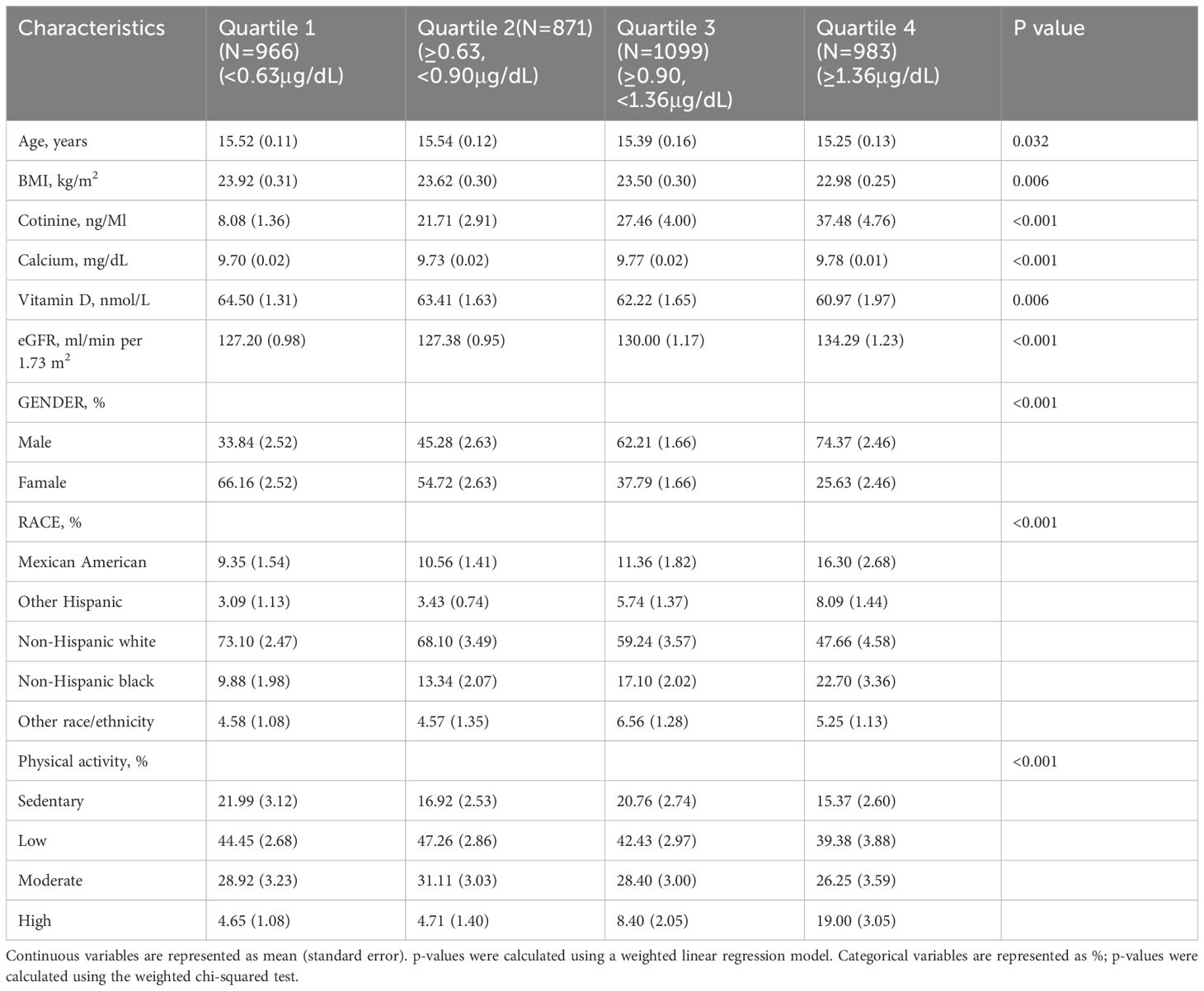
Blood samples were obtained during the physical examination, preserved at a freezing temperature of -20°C, and transported to a central laboratory. Immunological assays were performed to measure serum PTH levels. The protocols outlined on the NHANES website were used (Data Documentation, Codebook, and Frequencies, Parathyroid Hormone). Inductively coupled plasma mass spectrometry was used to measure lead levels in whole blood samples. We treated BLL concentrations as continuous variables and categorized them into quartiles: <0.63μg/dL, 0.63–0.90μg/dL, 0.9–1.36μg/dL, and ≥1.36μg/dL, respectively.
Variables
The covariates analyzed were age, body mass index (BMI), serum calcium levels, cotinine levels, vitamin D levels, and estimated glomerular filtration rate (eGFR). Furthermore, sex, race/ethnicity, physical activities, and other relevant covariate acquisition processes available in the NHANES dataset (http://cdc.gov/nchs/nhanes) were included as categorical variables.
Race/ethnicity was self-reported by the participants. The participants were divided as follows: Mexican American, Other Hispanic, non-Hispanic white, non-Hispanic black, or others. BMI was calculated by dividing the weight in kilograms by the height in meters squared. The Chronic Kidney Disease Epidemiology Collaboration equation based on serum creatinine levels was used to determine eGFR (22). The accelerometer recorded the intensity and frequency of movement. Per-minute activity counts were calculated. The average weekly duration of moderate-to-vigorous physical activity (MVPA) was measured. MVPA was defined as counts per minute ≥ 2020 (23).
Statistical analysis
R (http://www.R-project.org) and EmpowerStats (http://www.empowerstats.com) were used to perform statistical analyses. The significance level was p < 0.05. Sample weights were used according to the analytical guidelines provided by NCHS for estimating all values to ensure data representativeness for the civilian noninstitutionalized US population. Continuous variables are represented as mean (standard error) using a weighted linear regression model. Categorical variables are represented as % using the weighted chi-squared test. Three multivariable linear regression models were constructed: model 1, without any adjusted covariates; model 2, adjusted for age, sex, and race; and model 3, adjusted for all covariates presented in Table 1. In addition, subgroup analyses were performed. A weighted generalized additive model and smooth curve fitting were employed to account for potential linear relationships.
Results
Participant selection
Figure 1 illustrates the flow chart of participant selection. After excluding participants with missing information on blood PTH or lead levels, 576 adolescents aged 12–19 years were excluded. Furthermore, 96 pregnant women were excluded. Finally, 3,919 eligible adolescents were included in this study.
Baseline characteristics
Table 1 summarizes the demographic profiles of the participants in different BLL quartiles. Individuals with the highest BLLs were younger and predominantly male; they exhibited lower BMI and vitamin D levels. In contrast, they exhibited higher eGFR, cotinine levels, and calcium levels than those with the lowest BLLs.
Association between BLLs and PTH
Table 2 summarizes the correlation between blood PTH and BLLs. According to the non-adjusted model, BLLs and PTH levels show a positive association. Furthermore, the multivariable-adjusted model revealed a positive correlation between BLLs and PTH. Each 1 μg/dL increase in BLLs corresponded to a 0.67 pg/mL rise in PTH (β = 0.67, 95% CI: 0.16–1.18, p < 0.01). In addition, in both models 1 and 3, when considering blood lead as a categorical variable (quartiles), the participants in the highest BLL quartile exhibited higher PTH levels compared with those in the lowest quartile (Q3: β = 3.15, 95% CI: 1.07–5.24, p=0.003; Q4: β = 3.75, 95% CI: 1.61–5.90, p < 0.01 and Q3: β = 2.39, 95% CI: 0.32–4.47, p = 0.024; Q4: β = 2.87, 95% CI: 0.66–5.08, p = 0.011, respectively). Next, sex-stratified subgroup analysis was performed. A significant association was observed between BLLs and PTH levels in males, as indicated by consistent findings in all multivariable linear regression models. However, this association was not statistically significant in females (Table 2). Furthermore, race-stratified subgroup analysis revealed that this positive association persisted among Mexican American individuals (β = 1.16, 95% CI: 0.11–2.20, p = 0.031), non-Hispanic white individuals (β = 1.09, 95% CI: 0.10–2.08, p = 0.032), and non-Hispanic black groups (β = 1.57, 95% CI: 0.15–3.00, p = 0.031).
Moreover, a weighted generalized additive model was employed to consider the linear association and verify the results, while employing techniques for fitting smooth curves. (Figures 2, 3). We further conducted analysis by excluding 8 subjects with serum lead levels ≥10μg/dL, and found that the results remained the same (Supplementary Figures 1, 2).
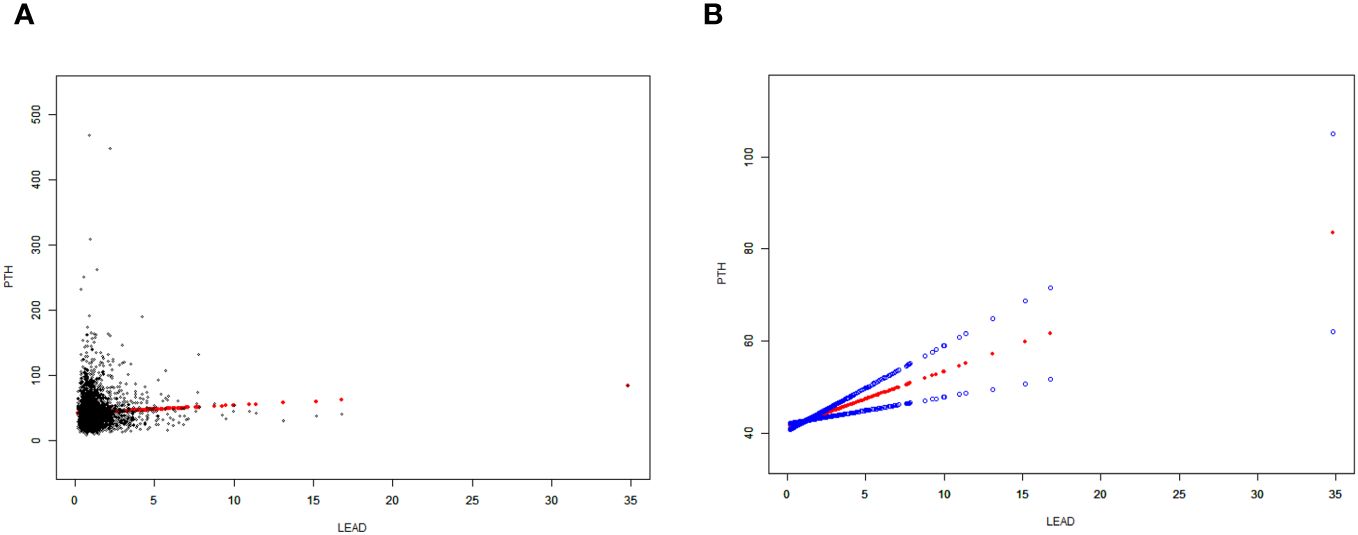
Figure 2 Correlation between BLLs and PTH levels. (A) Each data point is represented using a black dot. (B) The smoothed curve fit between the variables is represented by a solid red line, whereas the 95% confidence interval derived from the fit is indicated by the blue bands. Age, gender, race/ethnicity, body mass index (BMI), physical activities, estimated glomerular filtration rate (eGFR), serum calcium, vitamin D and cotinine were adjusted.
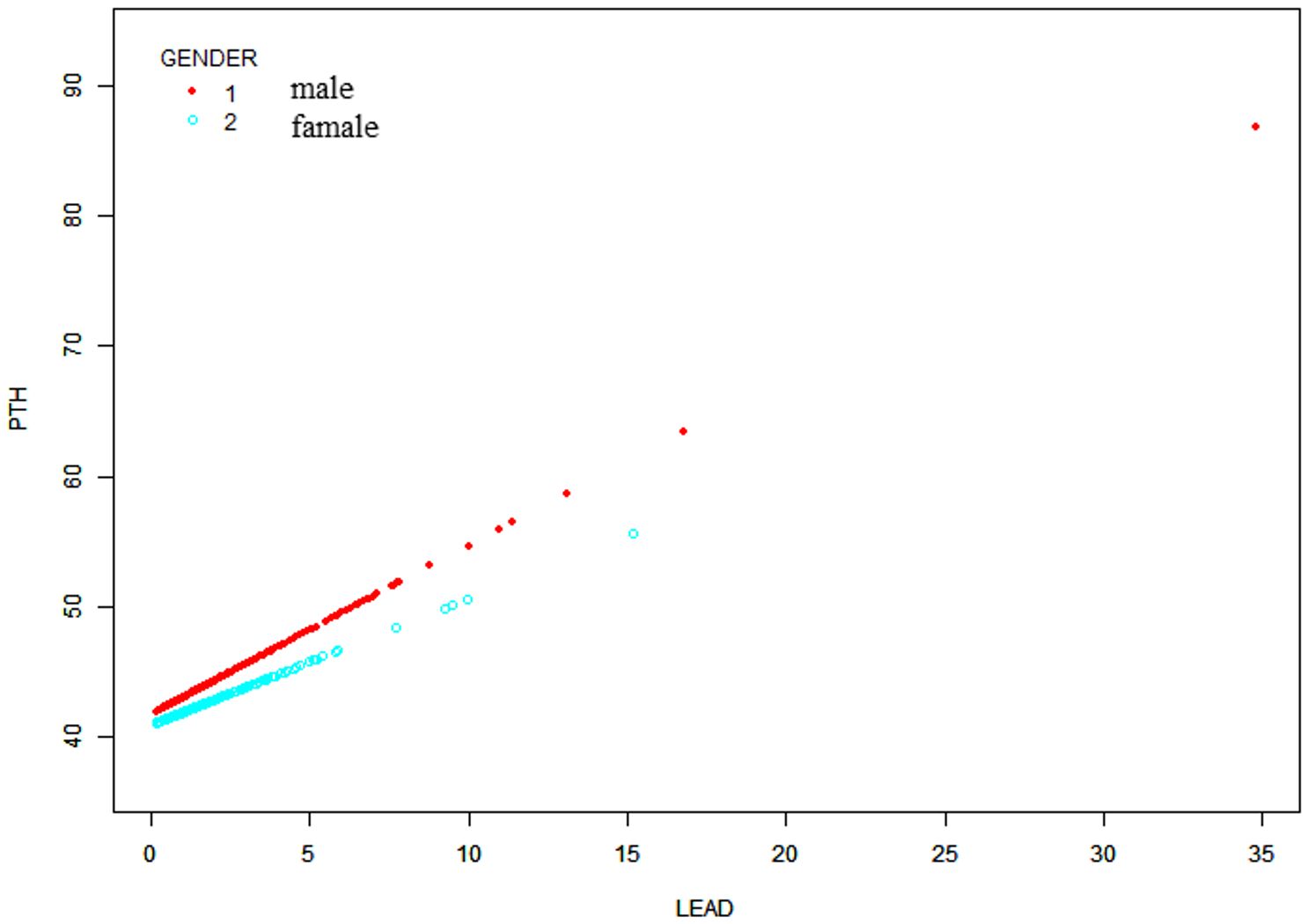
Figure 3 Sex-stratified association between BLLs and PTH levels. Age, race/ethnicity, BMI, physical activity, eGFR, serum calcium, vitamin D and cotinine were adjusted.
Discussion
In the present study, we utilized data from a representative sample of US adolescents aged 12–19 years to elucidate the potential association between BLLs and serum PTH levels. We observed that BLL is positively associated with PTH. Their relationships remained significant after adjustment for confounding factors. However, the potential role may differ by gender. We observed linear relationships between them, our multivariable linear regression analysis showed similar trends. Furthermore, race-stratified subgroup analysis revealed that this positive association persisted among most racial groups, including Mexican American, non-Hispanic white, and non-Hispanic black groups.
Research on the impact of lead on the endocrine system mainly focuses on individuals who are exposed to lead in their occupation and animal models used in experiments (24–27). In an 18-month (January 2017–July 2018), cross-sectional, case–control study, PTH levels and BLLs were significantly increase in 90 lead-exposed participants compared with controls (19). Similarly, in a cross-sectional survey including 146 people, Kristal-Boneh et al. reported that participants with occupational lead exposure exhibited substantial compensatory increases in PTH levels (16). Notably, the association demonstrated statistical significance among males while not achieving significance among females. In addition, a meta-analysis reported alterations in PTH levels following exposure to lead in the workplace (18). The pooled results revealed that the lead-exposed group had lower PTH levels than the control group. However, these results did not reach statistical significance, with unacceptable heterogeneity levels.
A NHANES study revealed that lead is positively associated with PTH in the general population aged ≥18 years (28). However, another study involving 105 children concluded that children with sufficient nutritional status and low-to-moderate lead exposure do not exhibit significant changes in vitamin D metabolism, as well as calcium and phosphorus balance, along with bone mineral content (29). In the present study, we revealed a stable positive association between BLLs and PTH in males aged 12–19 years.
The potential impact of lead exposure on parathyroid function may vary depending on gender. Baecklund et al. (30) revealed that BLLs were lower in women than in men (median 24 vs. 30 µg Pb/L). These sex disparities can be attributed to differences in calcium metabolism and calmodulin, renal sensitivity, iron storage status, and genetic factors between males and females (31, 32). Nevertheless, additional studies are warranted to examine the possible mechanisms or factors.
In the body, PTH is a central regulator of calcium homeostasis and plays a vital role in bone metabolism. It governs serum calcium levels by affecting the bones, kidneys, and intestine; however, its levels are regulated by the feedback mechanism of calcium levels (33). PTH stimulates calcium release from the bones into the bloodstream. Furthermore, it affects calcium reabsorption to enhance renal retention. Elevated BLLs may disrupt the hydroxylation of 25OH-vitamin D in the kidneys via the 1-α hydroxylase enzyme, resulting in the decreased production of active vitamin D [1,25 (OH)2D] (34). This subsequently results in decreased calcium levels.
Therefore, lead has the potential to act as a disruptor of the endocrine system and contribute to hormonal imbalances (15, 18, 24, 28). In this study, elevated BLLs could have decreased vitamin D levels, which, in turn, decreased serum calcium levels and increased PTH levels among lead-exposed individuals. However, our research revealed a positive correlation between lead levels and serum calcium levels, contradicting the previously mentioned mechanism by which lead affects PTH via serum calcium.
Our study has many strengths. First, a positive relationship was observed between BLL and PTH in adolescents; this has not been reported in prior research. Second, we examined a more extensive and representative multiracial population sample obtained from the NHANES, with rigorous measures and adjustment for important covariates.
Nevertheless, it is crucial to acknowledge the constraints of this study. Firstly, given its cross-sectional design, we cannot draw any causal inferences between blood lead levels (BLL) and parathyroid hormone (PTH). Secondly, the NHANES dataset does not include information regarding lead concentrations in bones, a commonly utilized measure for assessing cumulative lead exposure. In children, lead is primarily accumulated in trabecular bone, and its turnover rate is high (35).
Conclusions
BLLs are positively correlated with serum PTH levels in adolescents aged 12–19 years, particularly in males.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: http://cdc.gov/nchs/nhanes.
Ethics statement
The studies involving humans were approved by the Ethics Review Board of the National Center of Health Statistics (NCHS). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
BH: Writing – original draft. XW: Validation, Writing – review & editing. HL: Writing – review & editing. QZ: Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Zhejiang Province Health Science and Technology Project (grant no. 2021KY479, 2023KY476) and the General scientific research project of Zhejiang Education Department (grant no. Y202146132).
Acknowledgments
We would like to thank all the staff members of the NHANES study for their valuable contributions. The authors also thank Yi-er College for their contribution to the statistical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1383058/full#supplementary-material
References
1. Schock MR, Hyland RN, Welch MM. Occurrence of contaminant accumulation in lead pipe scales from domestic drinking-water distribution systems. Environ Sci Technology. (2008) 42:4285–91. doi: 10.1021/es702488v
3. Tong S, von Schirnding YE, Prapamontol T. Environmental lead exposure: a public health problem of global dimensions. Bull World Health Organization. (2000) 78:1068–77.
4. Jacob B, Ritz B, Heinrich J, Hoelscher B, Wichmann HE. The effect of low-level blood lead on hematologic parameters in children. Environ Res. (2000) 82:150–9. doi: 10.1006/enrs.1999.4011
5. Téllez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-García A, Schnaas-Arrieta L, et al. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics. (2006) 118:e323–e30.
6. Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease–a systematic review. Environ Health Perspectives. (2007) 115:472–82. doi: 10.1289/ehp.9785
7. Yu C-C, Lin J-L, Lin-Tan D-T. Environmental exposure to lead and progression of chronic renal diseases: a four-year prospective longitudinal study. J Am Soc Nephrol. (2004) 15:1016–22. doi: 10.1097/01.ASN.0000118529.01681.4F
8. Gollenberg AL, Hediger ML, Lee PA, Himes JH, Louis GMB. Association between lead and cadmium and reproductive hormones in peripubertal U. S girls Environ Health Perspect. (2010) 118:1782–7. doi: 10.1289/ehp.1001943
9. Reuben A. Childhood lead exposure and adult neurodegenerative disease. J Alzheimer's Disease: JAD. (2018) 64:17–42. doi: 10.3233/JAD-180267
10. Habibian A, Abyadeh M, Abyareh M, Rahimi Kakavandi N, Habibian A, Khakpash M, et al. Association of maternal lead exposure with the risk of preterm: a meta-analysis. J Matern Fetal Neona. (2022) 35:7222–30. doi: 10.1080/14767058.2021.1946780
11. Zhu M, Fitzgerald EF, Gelberg KH, Lin S, Druschel CM. Maternal low-level lead exposure and fetal growth. Environ Health Perspectives. (2010) 118:1471–5. doi: 10.1289/ehp.0901561
12. Maitre L, Julvez J, López-Vicente M, Warembourg C, Tamayo-Uria I, Philippat C, et al. Early-life environmental exposure determinants of child behavior in Europe: A longitudinal, population-based study. Environ Int. (2021) 153:106523. doi: 10.1016/j.envint.2021.106523
13. Shen X, Rosen JF, Guo D, Wu S. Childhood lead poisoning in China. Sci Total Environment. (1996) 181:101–9. doi: 10.1016/0048-9697(95)04956-8
14. Yao B, Lu X, Xu L, Wang Y, Qu H, Zhou H. Relationship between low-level lead, cadmium and mercury exposures and blood pressure in children and adolescents aged 8-17 years: An exposure-response analysis of NHANES 2007-2016. Sci Total Environment. (2020) 726:138446. doi: 10.1016/j.scitotenv.2020.138446
15. Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev. (2009) 12:206–23. doi: 10.1080/10937400902902062
16. Kristal-Boneh E, Froom P, Yerushalmi N, Harari G, Ribak J. Calcitropic hormones and occupational lead exposure. Am J Epidemiol. (1998) 147:458–63. doi: 10.1093/oxfordjournals.aje.a009471
17. Potula V, Henderson A, Kaye W. Calcitropic hormones, bone turnover, and lead exposure among female smelter workers. Arch Environ Occup Health. (2005) 60:195–204. doi: 10.3200/AEOH.60.4.195-204
18. Upadhyay K, Viramgami A, Bagepally BS, Balachandar R. Association between blood lead levels and markers of calcium homeostasis: a systematic review and meta-analysis. Sci Rep. (2022) 12:1850. doi: 10.1038/s41598-022-05976-4
19. Batra J, Thakur A, Meena SK, Singh L, Kumar J, Juyal D. Blood lead levels among the occupationally exposed workers and its effect on calcium and vitamin D metabolism: A case-control study. J Family Med Primary Care. (2020) 9:2388–93. doi: 10.4103/jfmpc.jfmpc_271_20
20. Rahman A, Al-Awadi AA, Khan KM. Lead affects vitamin D metabolism in rats. Nutrients. (2018) 10. doi: 10.3390/nu10030264
21. Rahman A, Al-Sabah R, Jallad R, Rao MS. Association of blood lead level with vitamin D binding protein, total and free 25-hydroxyvitamin D levels in middle-school children. Br J Nutr. (2022) 127:982–92. doi: 10.1017/S0007114521001823
22. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
23. Fishman EI, Steeves JA, Zipunnikov V, Koster A, Berrigan D, Harris TA, et al. Association between objectively measured physical activity and mortality in NHANES. Med Sci Sports Exerc. (2016) 48:1303–11. doi: 10.1249/MSS.0000000000000885
24. Doumouchtsis KK, Doumouchtsis SK, Doumouchtsis EK, Perrea DN. The effect of lead intoxication on endocrine functions. J Endocrinological Invest. (2009) 32:175–83. doi: 10.1007/BF03345710
25. Akbal A, Tutkun E, Yılmaz H. Lead exposure is a risk for worsening bone mineral density in middle-aged male workers. Aging Male. (2014) 17:189–93. doi: 10.3109/13685538.2013.836482
26. Baloch S, Kazi TG, Baig JA, Afridi HI, Arain MB. Occupational exposure of lead and cadmium on adolescent and adult workers of battery recycling and welding workshops: Adverse impact on health. Sci Total Environment. (2020) 720:137549. doi: 10.1016/j.scitotenv.2020.137549
27. Zhang Y, Zhou L, Li S, Liu J, Sun S, Ji X, et al. Impacts of lead exposure and chelation therapy on bone metabolism during different developmental stages of rats. Ecotoxicology Environ Safety. (2019) 183:109441. doi: 10.1016/j.ecoenv.2019.109441
28. Sang Z, Zhang H, Ma W, Dong Y, Shi B. Parathyroid hormones in relation to serum cadmium and lead: the NHANES 2003-2006. Environ Sci pollut Res Int. (2023) 30:18491–8. doi: 10.1007/s11356-022-23530-7
29. Koo WW, Succop PA, Bornschein RL, Krug-Wispe SK, Steinchen JJ, Tsang RC, et al. Serum vitamin D metabolites and bone mineralization in young children with chronic low to moderate lead exposure. Pediatrics. (1991) 87:680–7. doi: 10.1542/peds.87.5.680
30. Baecklund M, Pedersen NL, Björkman L, Vahter M. Variation in blood concentrations of cadmium and lead in the elderly. Environ Res. (1999) 80:222–30. doi: 10.1006/enrs.1998.3895
31. Toro-Román V, Robles-Gil MC, Muñoz D, Bartolomé I, Grijota FJ, Maynar-Mariño M. Sex differences in cadmium and lead concentrations in different biological matrices in athletes. Relationship with iron status. Environ Toxicol Pharmacol. (2023) 99:104107. doi: 10.1016/j.etap.2023.104107
32. Jackson LW, Howards PP, Wactawski-Wende J, Schisterman EF. The association between cadmium, lead and mercury blood levels and reproductive hormones among healthy, premenopausal women. Hum Reprod. (2011) 26:2887–95. doi: 10.1093/humrep/der250
33. Goltzman D. Physiology of parathyroid hormone. Endocrinol Metab Clin North Am. (2018) 47:743–58. doi: 10.1016/j.ecl.2018.07.003
34. Schwalfenberg GK, Genuis SJ. Vitamin D, essential minerals, and toxic elements: exploring interactions between nutrients and toxicants in clinical medicine. ScientificWorldJournal. (2015) 2015:318595. doi: 10.1155/2015/318595
Keywords: blood lead levels, parathyroid hormone, adolescents, national health and nutrition examination survey, vitamin D
Citation: He B, Wang X, Luo H and Zhou Q (2024) Association between blood lead levels and parathyroid hormone among United States adolescents aged 12–19: a cross-sectional study. Front. Endocrinol. 15:1383058. doi: 10.3389/fendo.2024.1383058
Received: 06 February 2024; Accepted: 13 June 2024;
Published: 09 July 2024.
Edited by:
Pier Francesco Alesina, Helios Klinikum Wuppertal, GermanyReviewed by:
Rizaldy Taslim Pinzon, Duta Wacana Christian University, IndonesiaGiuseppe Murdaca, University of Genoa, Italy
Abdur Rahman, Kuwait University, Kuwait
Copyright © 2024 He, Wang, Luo and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Zhou, emhvdXFpbjU4MDNAMTI2LmNvbQ==
 Baomei He
Baomei He Xiaowei Wang2
Xiaowei Wang2 Huanjun Luo
Huanjun Luo