
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 01 May 2024
Sec. Diabetes: Molecular Mechanisms
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1383035
This article is part of the Research TopicNovel Insights into the Pathophysiology of Diabetes-related Complications: Implications for Improved Therapeutic Strategies, Volume IIView all 37 articles
Background: Diabetic retinopathy (DR) is a microvascular complication of diabetes, severely affecting patients’ vision and even leading to blindness. The development of DR is influenced by metabolic disturbance and genetic factors, including gene polymorphisms. The research aimed to uncover the causal relationships between blood metabolites and DR.
Methods: The two-sample mendelian randomization (MR) analysis was employed to estimate the causality of blood metabolites on DR. The genetic variables for exposure were obtained from the genome-wide association study (GWAS) dataset of 486 blood metabolites, while the genetic predictors for outcomes including all-stage DR (All DR), non-proliferative DR (NPDR) and proliferative DR (PDR) were derived from the FinnGen database. The primary analysis employed inverse variance weighted (IVW) method, and supplementary analyses were performed using MR-Egger, weighted median (WM), simple mode and weighted mode methods. Additionally, MR-Egger intercept test, Cochran’s Q test, and leave-one-out analysis were also conducted to guarantee the accuracy and robustness of the results. Subsequently, we replicated the MR analysis using three additional datasets from the FinnGen database and conducted a meta-analysis to determine blood metabolites associated with DR. Finally, reverse MR analysis and metabolic pathway analysis were performed.
Results: The study identified 13 blood metabolites associated with All DR, 9 blood metabolites associated with NPDR and 12 blood metabolites associated with PDR. In summary, a total of 21 blood metabolites were identified as having potential causal relationships with DR. Additionally, we identified 4 metabolic pathways that are related to DR.
Conclusion: The research revealed a number of blood metabolites and metabolic pathways that are causally associated with DR, which holds significant importance for screening and prevention of DR. However, it is noteworthy that these causal relationships should be validated in larger cohorts and experiments.
Diabetic retinopathy (DR) stands as the predominant and severe ocular complication arising from diabetes mellitus. It ranks as the foremost contributor to irreversible yet preventable vision impairment among the working-age adult population (1). With the rapid increase in the incidence of diabetes worldwide, the number of people with DR is expected to rise to about 161 million by 2045 (2). According to the classification of Airlie House, DR is classified into non-proliferative DR (NPDR) and proliferative DR (PDR) (3). NPDR is the early manifestation of DR, characterized primarily by retinal microaneurysms, hemorrhages, hard exudates, and cotton lint spots (3). PDR is the advanced stage of DR, characterized by retinal neovascularization (3). The pathogenesis of DR is complex and includes multiple contributing factors such as oxidative stress, inflammation, angiogenesis, intestinal flora dysregulation, and neurodegeneration (4). Common risk factors for DR include diabetes course (5), elevated blood glucose levels (6), high lipid levels (7), and hypertension (8). Despite the extensive research conducted on DR, the mechanisms and risk factors for DR are still not fully understood.
Presently, an expanding body of research suggests a close correlation between metabolic disturbance and DR (9, 10). Furthermore, metabolomics is a powerful tool that greatly aids in identifying differential metabolites in DR (10, 11). These metabolites are often potential biomarkers and targets for the disease and can be utilized for screening, prediction, and treatment of DR (12, 13). However, exploring causal relationships between metabolites and DR is challenging due to limited sample sizes and confounding factors. Randomized controlled trials (RCTs) are generally recognized as the best evidence for epidemiological studies, but they require substantial resources and time, and ethical concerns may sometimes make them impractical. As an alternative, Mendelian randomization (MR) study explores the causality of exposure on outcome by employing single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) (14). Recently, genome-wide association study (GWAS) has updated metabolic phenotypes that created a genetically determined metabolites (GDMs) atlas (15). There is no MR study investigating the causal relationships between circulating metabolites and DR. Our research aims to identify blood metabolites associated with DR and provide new perspectives on its biological processes.
A two-sample MR analysis was utilized to assess the causality of human circulating metabolites on the risk of DR. Summary data for the exposures (486 blood metabolites) and outcomes (DR) were both sourced from GWAS. Ensuring the effectiveness of MR analysis requires satisfying three assumptions: (1) There should be a close connection between genetic variations and exposure; (2) the genetic variations ought to be independent of confounders related to exposure and outcome; (3) the genetic variations ought to be unrelated to outcome and only affect outcome through exposure. To prevent sample duplication, the hereditary information of metabolites and DR was derived from distinct datasets (Figure 1). The report followed the STROBE-MR statement (16).
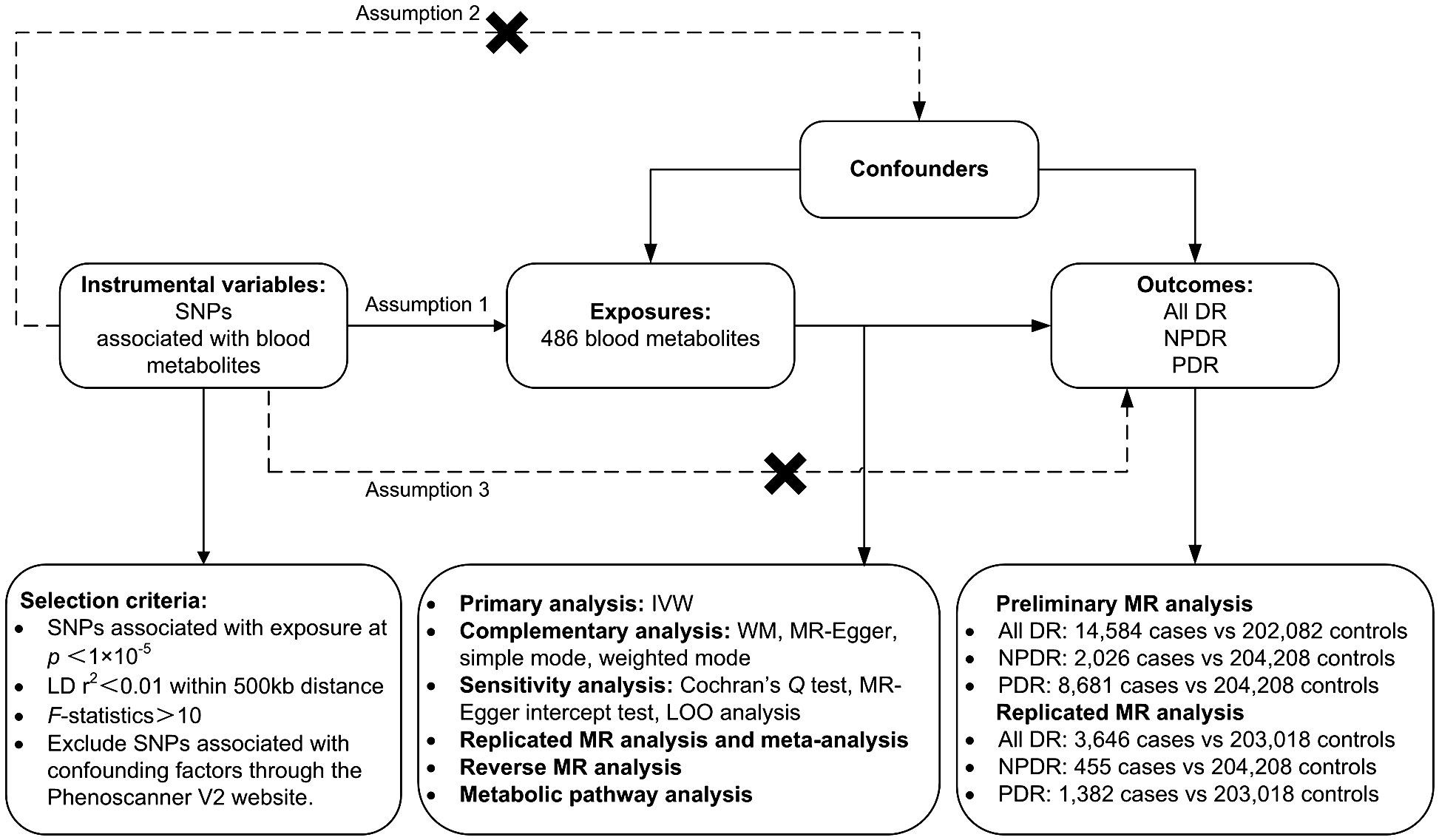
Figure 1 Study design, datasets, assumptions of the Mendelian randomization (MR) study of the associations between 486 blood metabolites and diabetic retinopathy. nSNPs, number of single nucleotide polymorphisms; LD, linkage-disequilibrium.
Genetic variations of 486 blood metabolites were obtained through comprehensive genetic scanning and metabolic analyses performed by Shin et al. (15). These publicly available data were derived from the GWAS Catalog (http://metabolomics.helmholtz-muenchen.de/gwas/). The dataset identifies about 2.1 million SNPs from 7,824 adults across two European cohorts (TwinsUK and KORA cohorts). According to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (17), 309 known metabolites are categorized into 8 pathways: amino acids, lipids, cofactors and vitamins, carbohydrates, nucleotides, energy, peptides and xenobiotics. The chemical properties of another 177 unknown metabolites have yet to been determined (Supplementary Table 1).
Based on different stages of DR, outcomes are categorized into all-stage DR (All DR), NPDR, and PDR. The summary dataset of genetic variants related to DR was sourced from FinnGen (https://r5.finngen.fi/). The GWAS IDs for the outcomes are as follows: All DR (finn-b-DM_RETINOPATHY); NPDR (finn-b-DM_BCKGRND_RETINA); PDR (finn-b-DM_RETINA_PROLIF). The characteristics of these summary datasets are presented in Table 1.
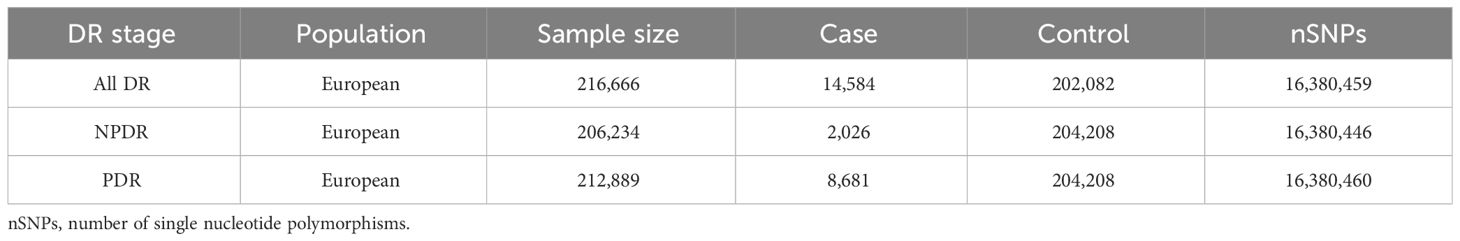
Table 1 Characteristics of the summary datasets for diabetic retinopathy in preliminary MR Analysis.
We established several criteria for screening IVs associated with blood metabolites. Firstly, considering the relatively small number of SNPs associated with metabolites, we lowered the significance threshold to p <1×10–5 to ensure a comprehensive conclusion (18). Secondly, r2 < 0.01 within 500-kilobase (kb) distance was set as linkage-disequilibrium (LD) threshold (19, 20) and the SNP of the moderate frequency palindrome structure was excluded. Thirdly, we employed F-statistic to evaluate the power of IVs. In general, IVs with F-statistic > 10 were used for subsequent MR analysis (19). The above criteria have been applied in previous literatures (18, 20). In addition, we examined these SNPs on the Phenoscanner V2 website (http://www.phenoscanner.medschl.cam.ac.uk/) to evaluate whether they are related to common confounders for DR, such as Type 1 Diabetes (5), Type 2 Diabetes (T2D) (21), Diabetic nephropathy (DN) (22), HbA1c (6), blood pressure (8), fasting glucose (6), fasting insulin (23), Total Cholesterol (7), Body Mass Index (24) and smoking (25). SNPs related to the above confounders (p < 1×10–5) were excluded to satisfy the MR assumptions.
We primarily evaluated the causality of 486 metabolites on DR through random‐effects inverse variance weighted (IVW) method, which yields the most reliable estimation results when all chosen SNPs serve as valid IVs (14). If IVs challenge the MR assumptions, the results could be inaccurate. Consequently, we conducted the subsequent sensitivity analyses to ensure the reliability of our results: (1) Cochran’s Q test was employed to assess heterogeneity among SNPs; (2) MR-Egger intercept was calculated to detect horizontal pleiotropy; (3) Supplementary analyses including MR-Egger, weighted median (WM), weighted mode and simple mode were used to guarantee the stability and dependability of the conclusion; (4) Leave‐one‐out (LOO) method was employed to assess whether the results were greatly influenced by individual SNP. The analyses were carried out utilizing the TwoSampleMR and MRPRESSO packages in the R software (version 4.3.2). The threshold of significance was set as P < 0.05.
To ensure the reliability and stability of the preliminary MR analysis results, we replicated the MR analysis using three additional DR datasets following the above steps. The summary data for DR in the repetitive analysis were also obtained from the FinnGen database, and the GWAS IDs for these datasets are as follows: All DR (finn-b-H7_RETINOPATHYDIAB), NPDR (finn-b-DM_BCKGRND_RETINA_NONPROLIF) and PDR (finn-b-H7_RETINOPATHYDIAB_PROLIF). The characteristics of these summary datasets are presented in Table 2. Through a meta-analysis of two datasets, we conclusively identified blood metabolites causally linked to DR. The Review Manager (version 5.4) was used for the meta-analysis with random-effects IVW model.
To further explore the causality of DR on circulating metabolites, we performed a reverse MR analysis using DR as the exposure and the identified metabolites as the outcomes. p < 5×10–8 and r2 < 0.001 within 10,000-kilobase (kb) distance were set as clumping threshold to extract DR-related IVs.
To clarify the roles of circulating metabolites in the pathogenesis of DR, we conducted Metaboanalyst 6.0 (https://www.Metaboanalyst.ca/) for metabolic pathway analysis. Metabolite names were standardized to HMDB IDs based on the HMDB database (https://hmdb.ca/) for metabolic pathway analysis. P < 0.05 was selected as the significance threshold.
The filtered IVs comprised SNPs ranging from 2 to 481 (Glutamate had the fewest IVs, with 2 SNPs, while 2-methoxyacetaminophen sulfate had the most IVs, with 481 SNPs). These SNPs associated with metabolites exhibited F statistics greater than 10 (Supplementary Tables 2, 3, 4), and they were not related to above confounding factors after examination by the Phenoscanner V2 website.
To provide a better understanding of metabolic changes, 177 unknown metabolites were excluded, while 309 metabolites with known structure and function were included. We estimated the causality of 309 blood metabolites on DR and found 39 significant associations, corresponding to 26 different metabolites, which included eight metabolites in the amino acid pathways, five in the lipid metabolism pathways, six in the xenobiotic pathways, two in the nucleotide pathways, two in the cofactors and vitamins pathways, one in the energy pathways, two in the peptide pathways (Figure 2). The complete results were provided in Supplementary Table 5. The causality of 486 metabolites on All DR, NPDR and PDR were shown in Supplementary Tables 6, 7, 8 respectively.
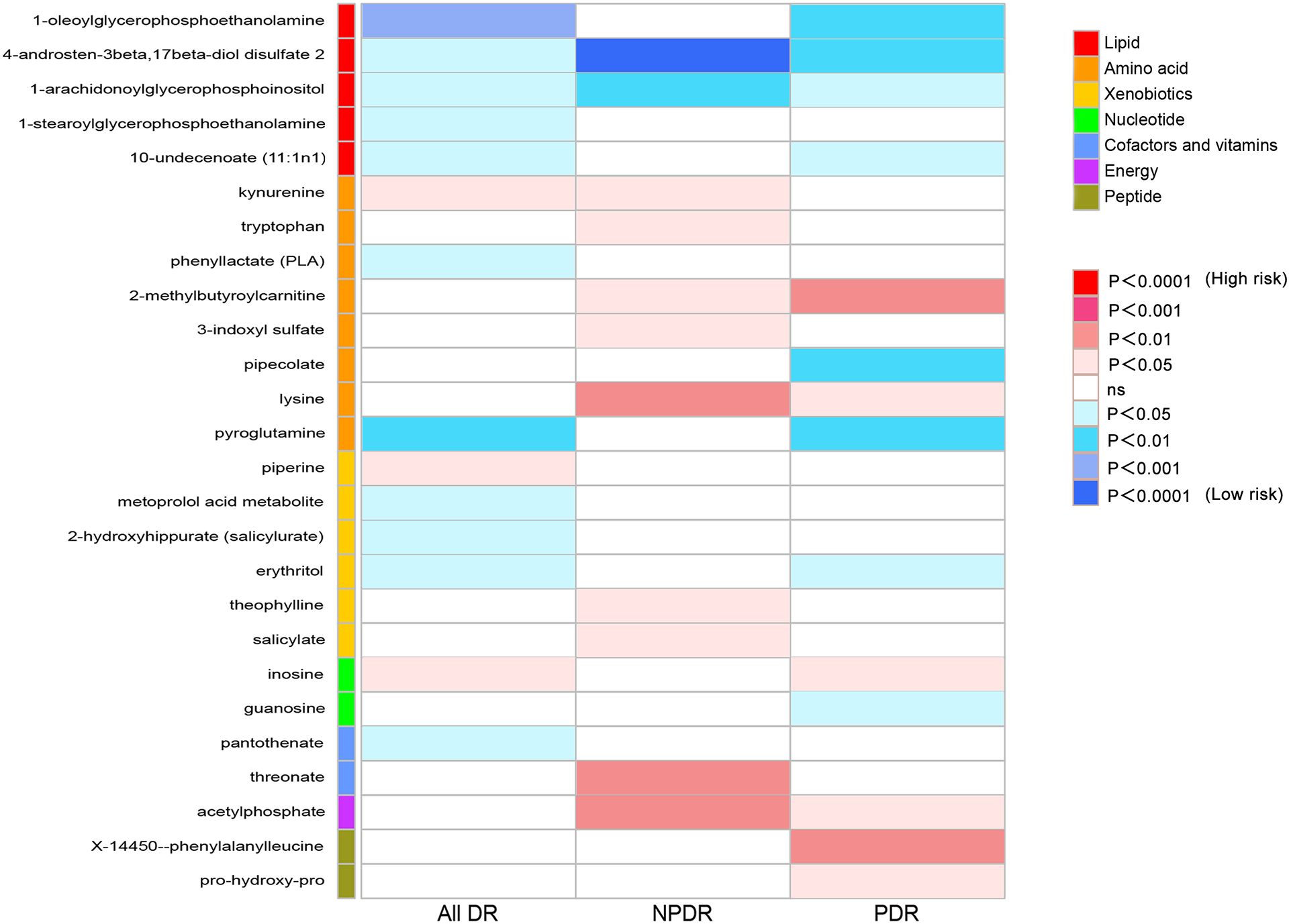
Figure 2 Heat map of causal associations between blood metabolites and diabetic retinopathy (derived from IVW analysis, p < 0.05). IVW, inverse-variance weighted.
Firstly, we identified 14 blood metabolites associated with All DR, as shown in Figure 3A. They are as follows: 1-oleoylglycerophosphoethanolamine [OR = 0.41, 95%CI = (0.26, 0.66), p = 0.0002]; kynurenine [OR = 1.77, 95%CI = (1.09, 2.85), p = 0.0200]; erythritol [OR = 0.69, 95%CI = (0.50, 0.94), p = 0.0206]; 1-stearoylglycerophosphoethanolamine [OR = 0.64, 95%CI = (0.43, 0.94), p = 0.0222]; 10-undecenoate (11:1n1) [OR = 0.80, 95%CI = (0.66, 0.97), p = 0.0235]; inosine [OR = 1.10, 95%CI = (1.01, 1.21), p = 0.0287]; piperine [OR = 1.30, 95%CI = (1.03, 1.64), p = 0.0293]; phenyllactate (PLA) [OR = 0.65, 95%CI = (0.43, 0.97), p = 0.0353]; 1-arachidonoylglycerophosphoinositol [OR = 0.70, 95%CI = (0.50, 0.99), p = 0.0408]; 2-hydroxyhippurate (salicylurate) [OR = 0.95, 95%CI = (0.90, 1.00), p = 0.0462]; pantothenate [OR = 0.72, 95%CI = (0.52, 1.00), p = 0.0484]; pyroglutamine [OR = 0.71, 95%CI = (0.57, 0.89), p = 0.0030]; metoprolol acid metabolite [OR = 0.98, 95%CI = (0.96, 1.00), p = 0.0140]; 4-androsten-3beta,17beta-diol disulfate 2 [OR = 0.65, 95%CI = (0.44, 0.97), p = 0.0364].
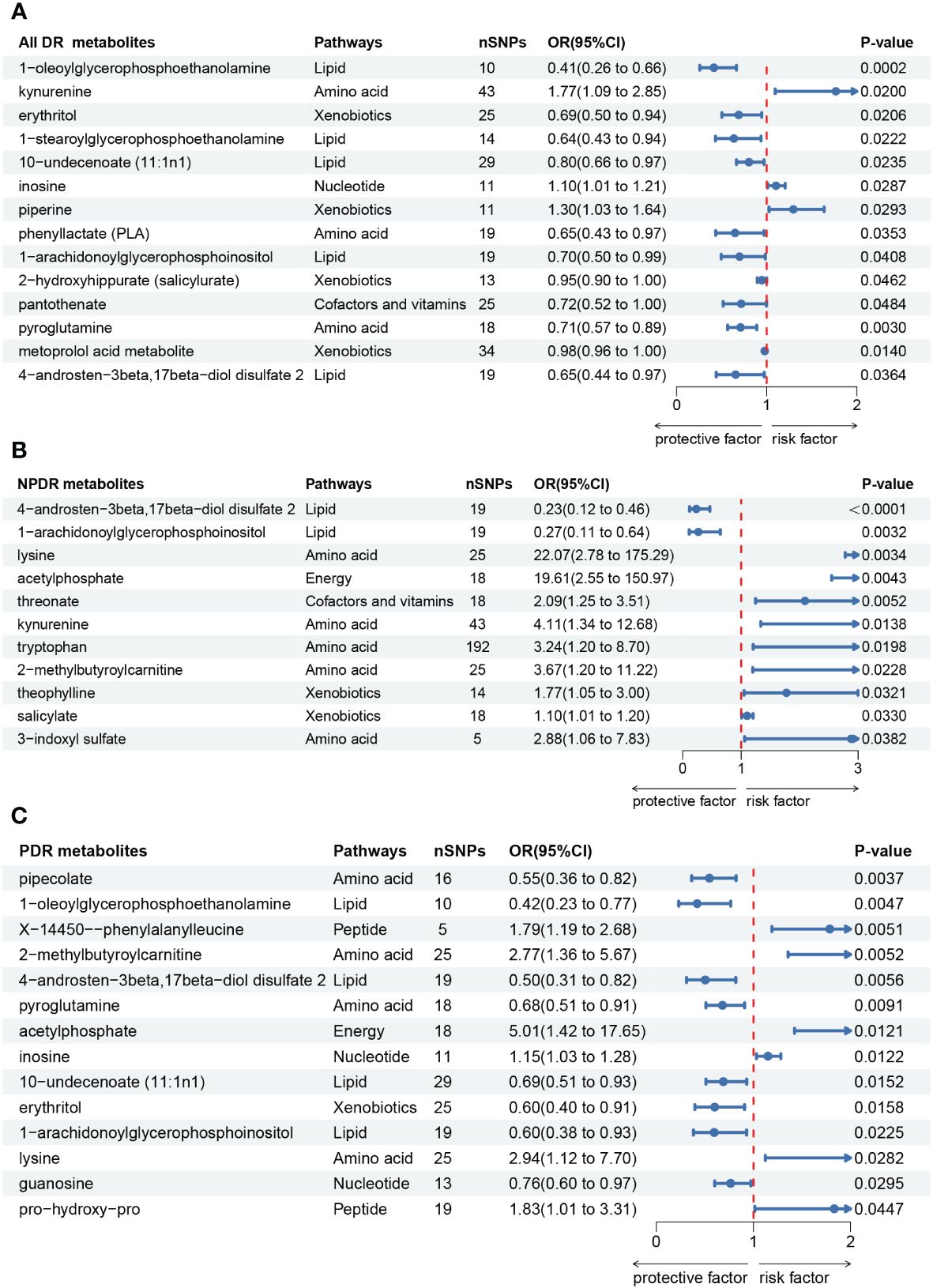
Figure 3 Forest plot of Mendelian randomization (MR) associations of blood metabolites on the risk of diabetic retinopathy (derived from IVW analysis, p < 0.05). (A) all-stage diabetic retinopathy (All DR); (B) non-proliferative diabetic retinopathy (NPDR); (C) proliferative diabetic retinopathy (PDR). IVW, inverse-variance weighted; 95%CI, 95% confidence interval; OR, odds ratio; nSNPs, number of single nucleotide polymorphisms.
Secondly, we identified 11 blood metabolites associated with NPDR, as shown in Figure 3B. They are as follows: 4-androsten-3beta,17beta-diol disulfate 2 [OR = 0.23, 95%CI = (0.12, 0.46), p < 0.0001]; 1-arachidonoylglycerophosphoinositol [OR = 0.27, 95%CI = (0.11, 0.64), p = 0.0032]; lysine [OR = 22.07, 95%CI = (2.78, 175.29), p = 0.0034]; acetylphosphate [OR = 19.61, 95%CI = (2.55, 150.97), p = 0.0043]; threonate [OR = 2.09, 95%CI = (1.25, 3.51), p = 0.0052]; kynurenine [OR = 4.11, 95%CI = (1.34, 12.68), p = 0.0138]; tryptophan [OR = 3.24, 95%CI = (1.20, 8.70), p = 0.0198]; 2-methylbutyroylcarnitine [OR = 3.67, 95%CI = (1.20, 11.22), p = 0.0228]; theophylline [OR = 1.77, 95%CI = (1.05, 3.00), p = 0.0321]; salicylate [OR = 1.10, 95%CI = (1.01, 1.20), p = 0.0330]; 3-indoxyl sulfate [OR = 2.88, 95%CI = (1.06, 7.83), p = 0.0382].
Thirdly, we identified 14 blood metabolites associated with PDR, as shown in Figure 3C. They are as follows: pipecolate [OR = 0.55, 95%CI = (0.36, 0.82), p = 0.0037]; 1-oleoylglycerophosphoethanolamine [OR = 0.42, 95%CI = (0.23, 0.77), p = 0.0047]; X-14450–phenylalanylleucine [OR = 1.79, 95%CI = (1.19, 2.68), p = 0.0051]; 2-methylbutyroylcarnitine [OR = 2.77, 95%CI = (1.36, 5.67), p = 0.0052]; 4-androsten-3beta,17beta-diol disulfate 2 [OR = 0.50, 95%CI = (0.31, 0.82), p = 0.0056]; pyroglutamine [OR = 0.68, 95%CI = (0.51, 0.91), p = 0.0091]; acetylphosphate [OR = 5.01, 95%CI = (1.42, 17.65), p = 0.0121]; inosine [OR = 1.15, 95%CI = (1.03, 1.28), p = 0.0122]; 10-undecenoate (11:1n1) [OR = 0.69, 95%CI = (0.51, 0.93), p = 0.0152]; erythritol [OR = 0.60, 95%CI = (0.40, 0.91), p = 0.0158]; 1-arachidonoylglycerophosphoinositol [OR = 0.60, 95%CI = (0.38, 0.93), p = 0.0225]; lysine [OR = 2.94, 95%CI = (1.12, 7.70), p = 0.0282]; guanosine [OR = 0.76, 95%CI = (0.60, 0.97), p = 0.0295]; pro-hydroxy-pro [OR = 1.83, 95%CI = (1.01, 3.31), p = 0.0447].
In sensitivity analysis, the results of complementary analyses including MR-Egger, weighted median (WM), simple mode and weighted mode are shown in Supplementary Table 5. We did not observe horizontal pleiotropy in 39 significant associations by MR-Egger intercepts (Supplementary Table 5). However, we found significant heterogeneity for some metabolites by Cochrane’s Q-test (Supplementary Table 5). They are as follows: All DR: kynurenine (IVW: Q = 72.71, p = 0.0023; MR-Egger: Q = 71.76, p = 0.0021) and 4-androsten-3beta,17beta-diol disulfate 2 (IVW: Q = 39.20, p = 0.0027; MR-Egger: Q = 36.59, p = 0.0038); NPDR: kynurenine (IVW: Q = 61.55, p = 0.0261; MR-Egger: Q = 60.21, p = 0.0268); PDR: 2-methylbutyroylcarnitine (IVW: Q = 40.90 p = 0.0171; MR-Egger: Q = 40.61, p = 0.0131); 4-androsten-3beta,17beta-diol disulfate 2 (IVW: Q = 35.71, p = 0.0077; MR-Egger: Q = 30.03, p = 0.0261); 10-undecenoate (11:1n1) (IVW: Q = 45.60, p = 0.0192; MR-Egger: Q = 41.72, p = 0.0351). To reduce the impact of heterogeneity on the results, we used the random-effects IVW method to calculate causal effects of metabolites mentioned above on DR. Finally, the LOO analysis showed that the overall effect of the metabolites was not strongly influenced by any single SNP (Figure 4). In addition, the scatter plots and funnel plots of MR analyses for the identified blood metabolites are shown in Figures 5, 6.

Figure 4 Leave-one-out analysis of Mendelian randomization (MR) analyses between blood metabolites and diabetic retinopathy. (A) all-stage diabetic retinopathy (All DR); (B) non-proliferative diabetic retinopathy (NPDR); (C) proliferative diabetic retinopathy (PDR).
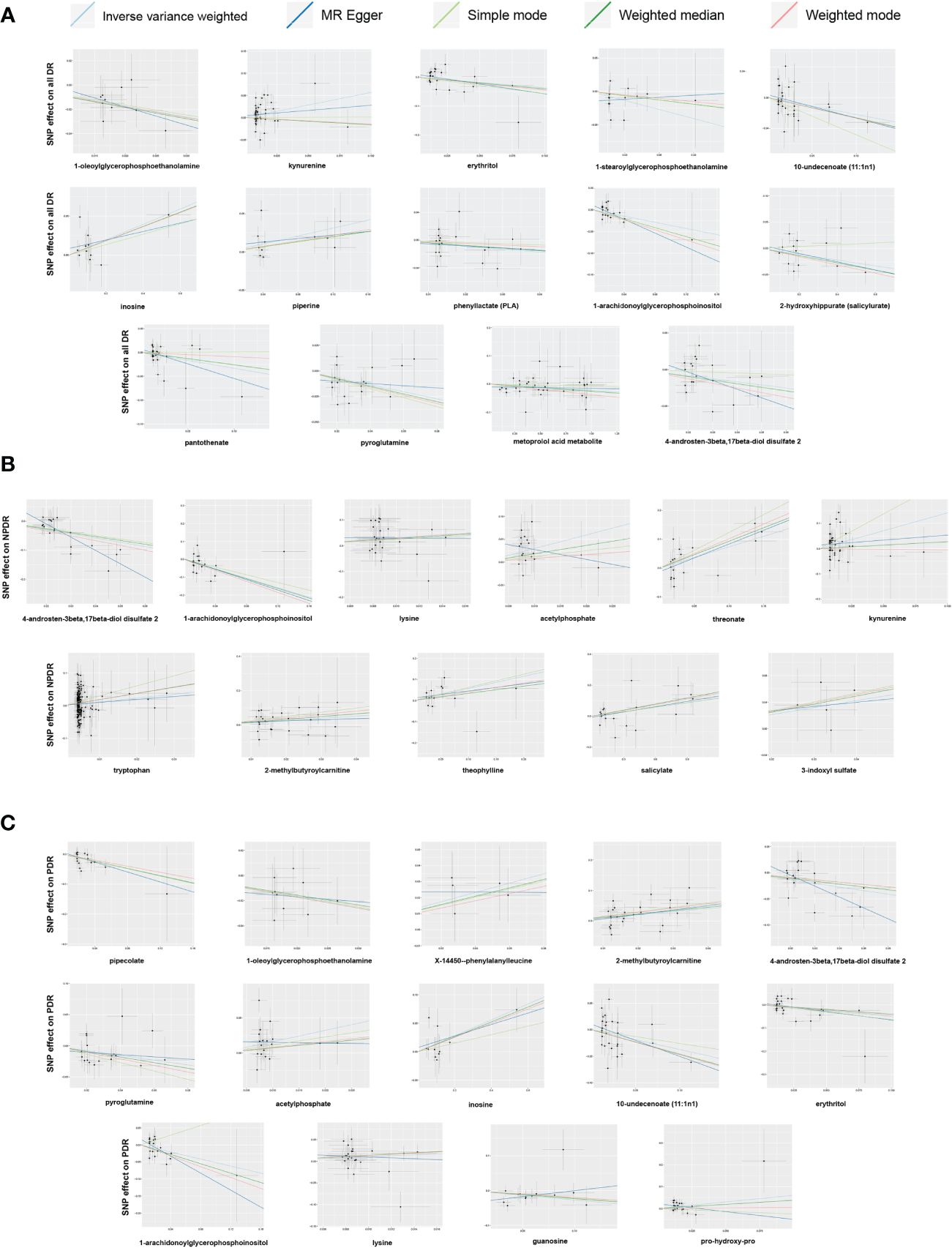
Figure 5 Scatter plots of Mendelian randomization (MR) analyses between blood metabolites and diabetic retinopathy. (A) all-stage diabetic retinopathy (All DR); (B) non-proliferative diabetic retinopathy (NPDR); (C) proliferative diabetic retinopathy (PDR).
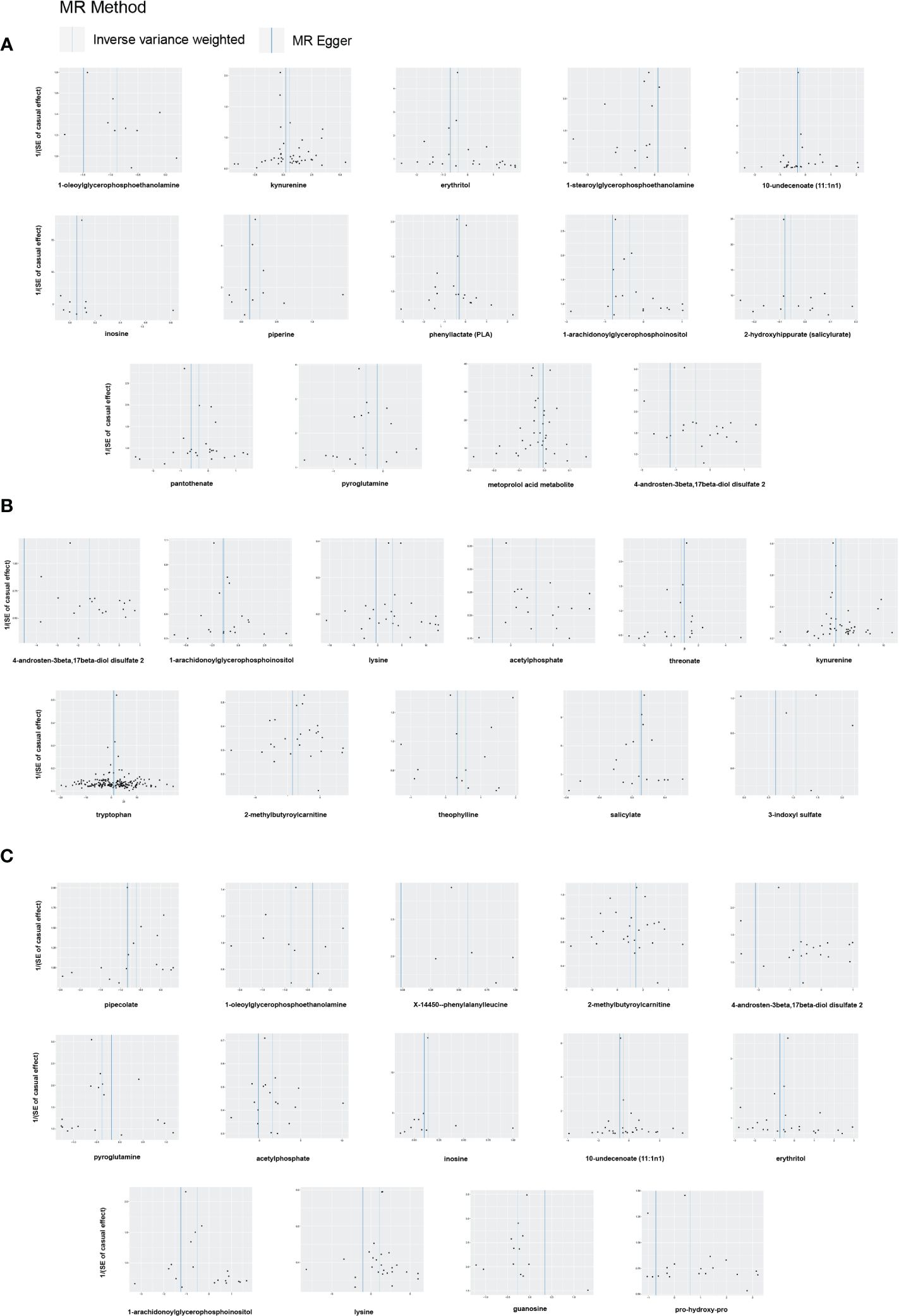
Figure 6 Funnel plots of Mendelian randomization (MR) analyses between blood metabolites and diabetic retinopathy. (A) all-stage diabetic retinopathy (All DR); (B) non-proliferative diabetic retinopathy (NPDR); (C) proliferative diabetic retinopathy (PDR).
To improve the credibility of the results, we performed a replicated MR analysis using three additional GWAS datasets for DR. As anticipated, we discovered the candidate metabolites with trends analogous to those in the preliminary MR analysis, and without horizontal pleiotropy (Supplementary Table 9). In addition, the replicated MR results for 486 metabolites are presented in Supplementary Tables 10, 11, 12 respectively. Through a meta-analysis of the results from two MR analyses, we ultimately identified 34 significant correlations involving 21 blood metabolites, among which 13 were associated with All DR, 9 with NPDR, and 12 with PDR (Figure 7). Pipecolate, 2-hydroxyhippurate (salicylurate), salicylate, threonate and pro-hydroxy-pro were excluded due to non-significant results in the meta-analysis.

Figure 7 Meta-analysis of the causal associations between blood metabolites and diabetic retinopathy. (A) all-stage diabetic retinopathy (All DR); (B) non-proliferative diabetic retinopathy (NPDR); (C) proliferative diabetic retinopathy (PDR). 95% CI, 95% confidence interval; OR, odds ratio.
Among the 21 ultimately identified metabolites, we found a reverse causal relationship between pantothenate and All DR [OR = 1.011, 95%CI = (1.000, 1.023), p = 0.0466], while 1-arachidonoylglycerophosphoinositol exhibited reverse causal effects with both NPDR [OR = 0.992, 95%CI = (0.987, 0.998), p = 0.0109] and PDR [OR = 0.988, 95%CI = (0.979, 0.998), p = 0.0143]. The results of reverse MR analysis and sensitivity analysis are shown in Supplementary Table 13. In addition, the scatter plots, funnel plots and LOO analysis of reverse MR analyses for the identified blood metabolites are shown in Figure 8.
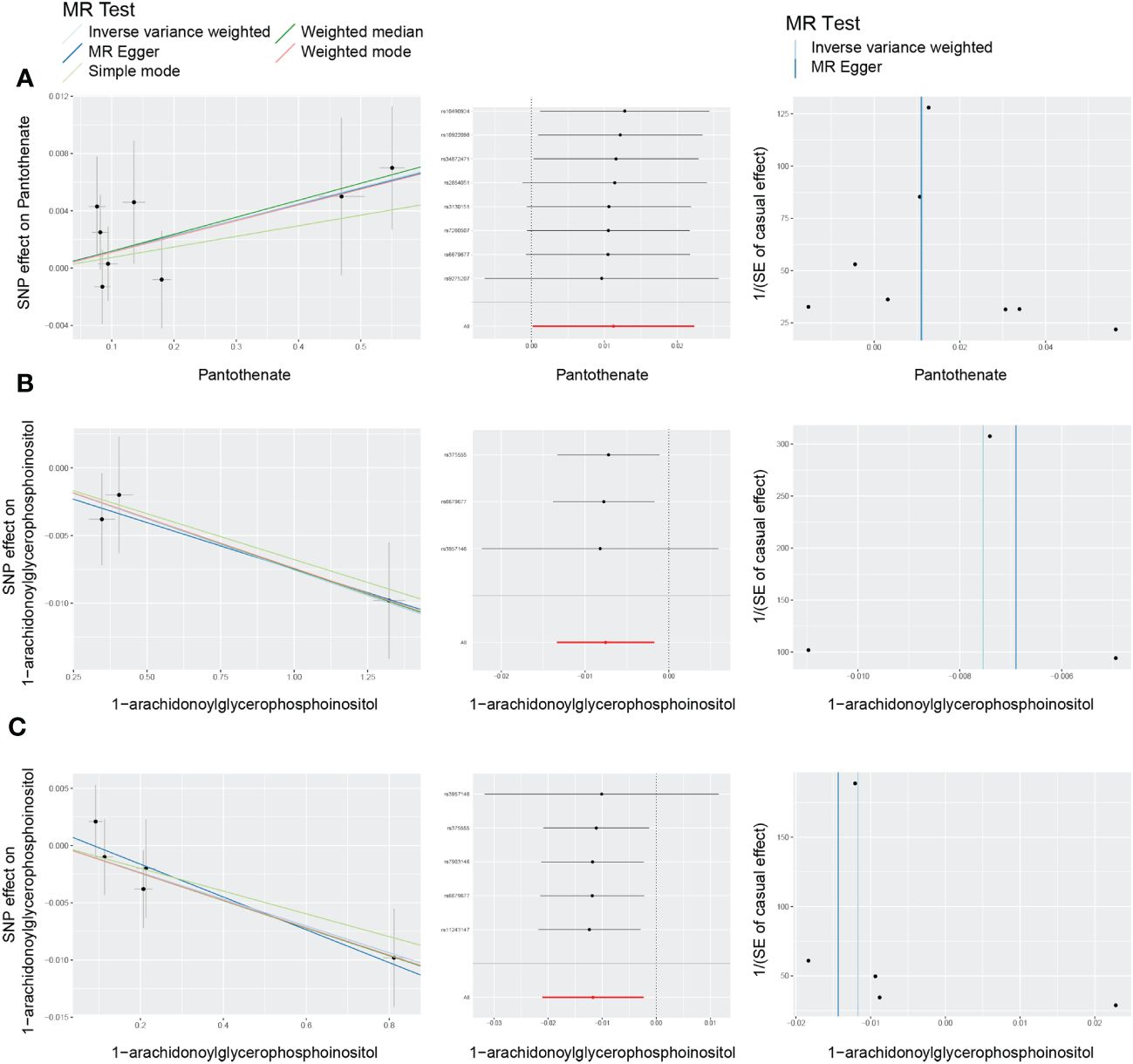
Figure 8 Scatter plots, funnel plots and leave-one-out (LOO) analysis of reverse Mendelian randomization (MR) analyses between blood metabolites and diabetic retinopathy. (A) all-stage diabetic retinopathy (All DR); (B) non-proliferative diabetic retinopathy (NPDR); (C) proliferative diabetic retinopathy (PDR).
A total of 5 significant associations corresponding to 4 metabolic pathways were identified through metabolic pathway analysis (Figure 9). “Pantothenate and CoA biosynthesis” (p = 0.038) may be involved in the biological process of All DR. “Tryptophan metabolism” (p = 0.004) and “Biotin metabolism” (p = 0.025) may be involved in the biological process of NPDR. “Purine metabolism” (p = 0.011) and “Biotin metabolism” (p = 0.025) may be involved in the biological process of PDR (Supplementary Table 14).
Among the 486 blood metabolites, we ultimately identified 13 metabolites associated with All DR, 9 with NPDR, and 12 with PDR, totaling 21 metabolites associated with DR. These findings have significant implications for future research in identifying novel biomarkers and targets for DR, and may inspire new preventive and therapeutic strategies.
Diabetes is widely recognized as a significant global public health issue, leading to microvascular complications such as DN and DR (21, 26). Approximately one-third of diabetes patients develop DR (21). Recent studies revealed that the pathological processes of DR were associated with long-term metabolic disturbances (9, 27, 28). With the advancement of metabolomics, metabolites and metabolic pathways associated with DR are continuously being discovered (10, 27, 29). These crucial metabolites are often considered potential biomarkers for disease prediction. In human studies, various biofluids such as circulating blood (serum and plasma), aqueous humor, vitreous body are used for the detection of metabolites. Due to its ease of acquisition and low invasiveness, circulating blood is the most commonly used sample. It offers a comprehensive profile of metabolic characteristics that can help identify potential biomarkers of DR.
We found causal relationships between seven metabolites in the amino acid metabolic pathway and DR. Amino acids participate in energy metabolism and regulate various metabolic pathways through gluconeogenesis (30). Previous studies have highlighted the significant role of amino acids in DR (30–32). Our findings specifically focused on the roles of tryptophan (TRP) and kynurenines (KYN) in DR. TRP serves as a primary origin for a range of bioactive molecules, including KYN, 5-hydroxytryptamine, melatonin, niacin and indoles (33). The TRP–KYN pathway constitutes the principal route for TRP conversion in central and peripheral tissues (33). The rate-limiting enzymes responsible for the initial step of the TRP-KYN pathway is indoleamine 2,3-dioxygenases (IDO), which is associated with immune response and inflammation (34). Previous research suggested that patients with DR exhibited higher level of retinal IDO compared to non-diabetic patients, and the loss of IDO has been shown to inhibit capillary degeneration in diabetic mice (35). Praveen et al. (36) observed a significant elevation in KYN levels and IDO mRNA levels in the serum of DR patients compared to healthy individuals, with a more pronounced increase in PDR patients. Meanwhile, multiple metabolomics analyses of plasma/serum from DR patients have also identified TRP and KYN as potential biomarkers of DR (10, 37). Indoxyl sulfate, a uremic toxin produced by bacterial decomposition of intestinal amino acids, especially TRP, has been reported to be involved in the metabolic disorder of DN (38). Our research suggested that 3-indoxyl sulfate may serve as a potential risk factor for DR, although further confirmation is required through experiments. Pyroglutamine, a cyclic derivative of glutamine, has been reported to be associated with kidney function and T2D (39). Our study identified pyroglutamine as a potential protective factor for DR.
Lysine primarily undergoes metabolic processes via the saccharopine pathway, with a smaller fraction proceeding through the pipecolic acid pathway (40). Our research identified lysine as a potential risk factor for DR, while pipecolate (pipecolic acid) is considered a potential protective factor against DR in the preliminary MR analysis. A metabolomic analysis observed increased levels of lysine in the vitreous bodies of DR patients compared to the normal population (41). Vidhya et al. (42) also reported a significant elevation of lysine in the vitreous bodies of PDR patients. In vivo experiment, lysine was found to promote the differentiation of retinal pericytes into adipocytes, exerting a protective effect on PDR (42), which contradicts with our research finding. As for pipecolic acid, Luo et al. (12) found that the serum levels of pipecolic acid were lower compared to those in healthy population, and it exhibited a negative correlation with blood glucose and glycated hemoglobin. Wang et al. (43) also observed reduced pipecolic acid in plasma and vitreous body of PDR patients through metabolomic analysis. In vivo experiment, pipecolic acid was found to alleviate ferroptosis in DR by inhibiting the GPX4-YAP signaling pathway, thereby preventing the progression of DR (12). Although the meta-analysis result for pipecolate was not significant, it may have a certain potential effect in the development of DR.
The disruption of lipid metabolism is generally recognized to is connected with the onset and development of DR (44). The study revealed that the lipid metabolites causally associated with DR are predominantly lyso-phospholipids, including 1-stearylglycerolphosphatethanolamine and 1-arachidonicglycerolphosphateinositol, which are both protective factors for DR. Meanwhile, the reverse MR analysis found that both NPDR and PDR can to some extent impact 1-arachidonicglycerolphosphateinositol. The role of sex hormones in diabetic retinopathy is currently uncertain (45). Gangwar et al. (46) found that diabetic patients with hypogonadism exhibited an longer course of diabetes, higher levels of HbA1c and an increased risk of DR. Our study revealed that 4-androsten-3beta,17beta-diol disulfate, an androgenic steroid, can exert a protective effect against any stage of DR.
Additionally, our research has demonstrated the causal relationships between xenobiotics and DR at the genetic level. Erythritol is recommended as a diabetic-safe sweetener due to its metabolic inertness and antioxidant properties and can displayed an endothelium-protective effect in diabetic rats (47). Metabonomic studies have also identified erythritol as a biomarker of diabetes and impaired fasting glucose (11, 48, 49). Piperine is a natural alkaloid from black pepper, and it has been found to protect the retina of diabetic mice by enhancing PEDF expression and suppressing HIF-1/VEGFA pathway (50).
Limited research has been conducted on the role of cofactors and vitamins in DR. Threonate is a metabolic product of vitamin C. A metabolomic analysis showed that the plasma levels of threonate were lower in NPDR patients compared to healthy people (51). The preliminary MR analysis considered threonine as a protective factor, but its meta-analysis result was not significant. Pantothenate, also known as Vitamin B5 or anti-stress vitamin, serves as the precursor of coenzyme A. Wang et al. (52) conducted a comparison of the serum from 15 PDR patients and 15 NPDR patients by untargeted metabolomics and found significant differences in pantothenate levels. Our study further established the bidirectional genetic association between pantothenate and DR.
Our study also identified several potential correlations between DR and nucleotide compounds, such as inosine and guanine. In the purine metabolic cycle, adenosine is initially phosphorylated to adenine, then rapidly deaminated to inosine, and ultimately undergoes a series of reactions to produce nitric oxide (NO) (53). For diabetic patients, NO plays a crucial role in microvascular dysfunction, directly causing lipid and protein peroxidation, tissue damage, ultimately leading to vascular leakage (54). Xia et al. (55) observed the plasma levels of inosine in DR patients were higher than those in the healthy population and diabetic patients without retinopathy. A metabolomics analysis of retina in diabetic mice indicated that, compared to other purine metabolites, adenosine, guanine, and inosine served as excellent biomarkers for predicting DR with higher sensitivity, specificity, and accuracy (56).
Acetylphosphate, as a marker of mitochondrial activity, is considered a metabolic intermediate in the generation of citric acid cycle precursors and often mediates nonenzymatic acetylation (57). Previous studies revealed that acetylation of proteins such as mitofusin 2 (58), histones (59), and P65 (60) contributes to the development of DR. Our study suggested that acetylphosphate may be a risk factor for DR and the acetylation it mediates holds promise as a potential focus for further investigation.
The study identified several metabolic pathways related to DR, some of which have been demonstrated in previous studies (43, 55, 61). As mentioned above, pantothenate and CoA biosynthesis, tryptophan metabolism and purine metabolism may be involved in the biological mechanism for DR (10, 52, 56, 62). In our study, lysine has been identified as a metabolite associated with DR. Given that lysine is involved in the activation of biotin (63), biotin metabolism may play a role in diabetic retinopathy.
The causal correlations between circulating metabolites and DR were assessed for the first time in this MR study. However, there are several restrictions that should be taken into consideration. Firstly, due to the limitations of MR analysis, complete elimination of residual pleiotropy is not achievable, potentially leading to bias. Secondly, the presence of ethnic bias should be noted as the subjects included in both exposure and outcome were of European descent. Hence, caution is warranted when generalizing the findings to other ethnicities. Thirdly, to explore additional blood metabolites associated with DR, we did not correct for P-value by false discovery rate correction, which can result in false positives in multiple tests. Fourthly, the direct pathological site of DR is the retina, and the blood-retinal barrier exhibits strict selectivity in the filtration of metabolites (64), indicating the need for further research to analyze changes in metabolites in vitreous and aqueous humor. Finally, it is worth noting that some metabolites in the metabolic profile of this study have unclear structures and functions, which limits our ability to interpret the results of the MR study.
In conclusion, our study identified 21 circulating metabolites and 4 metabolic pathways associated with DR. These metabolites have the potential to serve as blood biomarkers for DR screening and prevention, as well as potential candidates for further investigation into underlying mechanisms and drug target selection. Both clinical and basic research are required to confirm the roles of these metabolites in DR.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
CY: Writing – original draft, Writing – review & editing. YM: Writing – original draft. MY: Writing – original draft. QJ: Writing – review & editing. JX: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research received financial support from the National Natural Science Foundation of China (grant number no. 82070983).
The authors sincerely thank the GWAS database and the investigators involved for sharing the summary statistics used in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1383035/full#supplementary-material
1. Tan TE, Wong TY. Diabetic retinopathy: looking forward to 2030. Front Endocrinol (Lausanne). (2022) 13:1077669. doi: 10.3389/fendo.2022.1077669
2. Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. (2021) 128:1580–91. doi: 10.1016/j.ophtha.2021.04.027
3. Yang Z, Tan TE, Shao Y, Wong TY, Li X. Classification of diabetic retinopathy: past, present and future. Front Endocrinol (Lausanne). (2022) 13:1079217. doi: 10.3389/fendo.2022.1079217
4. Wei L, Sun X, Fan C, Li R, Zhou S, Yu H. The pathophysiological mechanisms underlying diabetic retinopathy. Front Cell Dev Biol. (2022) 10:963615. doi: 10.3389/fcell.2022.963615
5. Shah VN, Kanapka LG, Akturk HK, Polsky S, Forlenza GP, Kollman C, et al. Time in range is associated with incident diabetic retinopathy in adults with type 1 diabetes: A longitudinal study. Diabetes Technol Ther. (2023) 26:246–51. doi: 10.1089/dia.2023.0486
6. Washirasaksiri C, Srivanichakorn W, Borrisut N, Sitasuwan T, Tinmanee R, Kositamongkol C, et al. Fasting plasma glucose and hba1c levels predict the risk of type 2 diabetes and diabetic retinopathy in a Thai high-risk population with prediabetes. Front Pharmacol. (2022) 13:950225. doi: 10.3389/fphar.2022.950225
7. Li Z, Yuan Y, Qi Q, Wang Q, Feng L. Relationship between dyslipidemia and diabetic retinopathy in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Syst Rev. (2023) 12:148. doi: 10.1186/s13643-023-02321-2
8. Bulum T, Tomić M, Vrabec R, Brkljačić N, Ljubić S. Systolic and diastolic blood pressure are independent risk factors for diabetic retinopathy in patients with type 2 diabetes. Biomedicines. (2023) 11:2242. doi: 10.3390/biomedicines11082242
9. Fernandes Silva L, Hokkanen J, Vangipurapu J, Oravilahti A, Laakso M. Metabolites as risk factors for diabetic retinopathy in patients with type 2 diabetes: A 12-year follow-up study. J Clin Endocrinol Metab. (2023) 109:100–6. doi: 10.1210/clinem/dgad452
10. Ancel P, Martin JC, Doukbi E, Houssays M, Gascon P, Righini M, et al. Untargeted multiomics approach coupling lipidomics and metabolomics profiling reveals new insights in diabetic retinopathy. Int J Mol Sci. (2023) 24:12053. doi: 10.3390/ijms241512053
11. Chen L, Cheng CY, Choi H, Ikram MK, Sabanayagam C, Tan GS, et al. Plasma metabonomic profiling of diabetic retinopathy. Diabetes. (2016) 65:1099–108. doi: 10.2337/db15-0661
12. Luo L, Cai Y, Jiang Y, Gong Y, Cai C, Lai D, et al. Pipecolic acid mitigates ferroptosis in diabetic retinopathy by regulating gpx4-yap signaling. BioMed Pharmacother. (2023) 169:115895. doi: 10.1016/j.biopha.2023.115895
13. Zong GW, Wang WY, Zheng J, Zhang W, Luo WM, Fang ZZ, et al. A metabolism-based interpretable machine learning prediction model for diabetic retinopathy risk: A cross-sectional study in Chinese patients with type 2 diabetes. J Diabetes Res. (2023) 2023:3990035. doi: 10.1155/2023/3990035
14. Pierce BL, Burgess S. Efficient design for mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. (2013) 178:1177–84. doi: 10.1093/aje/kwt084
15. Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. (2014) 46:543–50. doi: 10.1038/ng.2982
16. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the strobe-MR statement. Jama. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
17. Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. (2012) 40:D109–14. doi: 10.1093/nar/gkr988
18. Yun Z, Guo Z, Li X, Shen Y, Nan M, Dong Q, et al. Genetically predicted 486 blood metabolites in relation to risk of colorectal cancer: A mendelian randomization study. Cancer Med. (2023) 12:13784–99. doi: 10.1002/cam4.6022
19. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
20. Yang J, Yan B, Zhao B, Fan Y, He X, Yang L, et al. Assessing the causal effects of human serum metabolites on 5 major psychiatric disorders. Schizophr Bull. (2020) 46:804–13. doi: 10.1093/schbul/sbz138
21. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. (2012) 35:556–64. doi: 10.2337/dc11-1909
22. Suzuki Y, Kiyosawa M. Relationship between diabetic nephropathy and development of diabetic macular edema in addition to diabetic retinopathy. Biomedicines. (2023) 11:1502. doi: 10.3390/biomedicines11051502
23. Kuo JZ, Guo X, Klein R, Klein BE, Weinreb RN, Genter P, et al. Association of fasting insulin and C peptide with diabetic retinopathy in Latinos with type 2 diabetes. BMJ Open Diabetes Res Care. (2014) 2:e000027. doi: 10.1136/bmjdrc-2014-000027
24. Huang Y, Zhang X, Li B, Zhu X, Li C, Zhou C, et al. Association of bmi and waist circumference with diabetic microvascular complications: A prospective cohort study from the UK Biobank and mendelian randomization analysis. Diabetes Res Clin Pract. (2023) 205:110975. doi: 10.1016/j.diabres.2023.110975
25. Li L, Peng X, Jiang N, Yan M, Zheng Z, Zhang D, et al. The influence of smoking on retinal ganglion cell-inner plexiform layer complex in male diabetes. Cutan Ocul Toxicol. (2023) 42:253–7. doi: 10.1080/15569527.2023.2245034
26. Liu XZ, Duan M, Huang HD, Zhang Y, Xiang TY, Niu WC, et al. Predicting diabetic kidney disease for type 2 diabetes mellitus by machine learning in the real world: A multicenter retrospective study. Front Endocrinol (Lausanne). (2023) 14:1184190. doi: 10.3389/fendo.2023.1184190
27. Fang J, Wang H, Niu T, Shi X, Xing X, Qu Y, et al. Integration of vitreous lipidomics and metabolomics for comprehensive understanding of the pathogenesis of proliferative diabetic retinopathy. J Proteome Res. (2023) 22:2293–306. doi: 10.1021/acs.jproteome.3c00007
28. Qian X, Klatt S, Bennewitz K, Wohlfart DP, Lou B, Meng Y, et al. Impaired detoxification of trans, trans-2,4-decadienal, an oxidation product from omega-6 fatty acids, alters insulin signaling, gluconeogenesis and promotes microvascular disease. Adv Sci (Weinh). (2023) 11:e2302325. doi: 10.1002/advs.202302325
29. Wang Z, Tang J, Jin E, Ren C, Li S, Zhang L, et al. Metabolomic comparison followed by cross-validation of enzyme-linked immunosorbent assay to reveal potential biomarkers of diabetic retinopathy in Chinese with type 2 diabetes. Front Endocrinol (Lausanne). (2022) 13:986303. doi: 10.3389/fendo.2022.986303
30. Xia M, Zhang F. Amino acids metabolism in retinopathy: from clinical and basic research perspective. Metabolites. (2022) 12:1244. doi: 10.3390/metabo12121244
31. Tanase DM, Gosav EM, Botoc T, Floria M, Tarniceriu CC, Maranduca MA, et al. Depiction of branched-chain amino acids (BCAAS) in diabetes with a focus on diabetic microvascular complications. J Clin Med. (2023) 12:6053. doi: 10.3390/jcm12186053
32. Zhang X, Xia M, Wu Y, Zhang F. Branched-chain amino acids metabolism and their roles in retinopathy: from relevance to mechanism. Nutrients. (2023) 15:2161. doi: 10.3390/nu15092161
33. Xue C, Li G, Zheng Q, Gu X, Shi Q, Su Y, et al. Tryptophan metabolism in health and disease. Cell Metab. (2023) 35:1304–26. doi: 10.1016/j.cmet.2023.06.004
34. Tanaka M, Tóth F, Polyák H, Szabó Á, Mándi Y, Vécsei L. Immune influencers in action: metabolites and enzymes of the tryptophan-kynurenine metabolic pathway. Biomedicines. (2021) 9:734. doi: 10.3390/biomedicines9070734
35. Nahomi RB, Sampathkumar S, Myers AM, Elghazi L, Smith DG, Tang J, et al. The absence of indoleamine 2,3-dioxygenase inhibits retinal capillary degeneration in diabetic mice. Invest Ophthalmol Vis Sci. (2018) 59:2042–53. doi: 10.1167/iovs.17-22702
36. Munipally PK, Agraharm SG, Valavala VK, Gundae S, Turlapati NR. Evaluation of indoleamine 2,3-dioxygenase expression and kynurenine pathway metabolites levels in serum samples of diabetic retinopathy patients. Arch Physiol Biochem. (2011) 117:254–8. doi: 10.3109/13813455.2011.623705
37. Yun JH, Kim JM, Jeon HJ, Oh T, Choi HJ, Kim BJ. Metabolomics profiles associated with diabetic retinopathy in type 2 diabetes patients. PloS One. (2020) 15:e0241365. doi: 10.1371/journal.pone.0241365
38. Huang YC, Tsai TC, Chang CH, Chang KT, Ko PH, Lai LC. Indoxyl sulfate elevated lnc-slc15a1-1 upregulating cxcl10/cxcl8 expression in high-glucose endothelial cells by sponging microRNAs. Toxins (Basel). (2021) 13:873. doi: 10.3390/toxins13120873
39. Yousri NA, Suhre K, Yassin E, Al-Shakaki A, Robay A, Elshafei M, et al. Metabolic and metabo-clinical signatures of type 2 diabetes, obesity, retinopathy, and dyslipidemia. Diabetes. (2022) 71:184–205. doi: 10.2337/db21-0490
40. Chang FM. Update current understanding of neurometabolic disorders related to lysine metabolism. Epilepsy Behav. (2023) 146:109363. doi: 10.1016/j.yebeh.2023.109363
41. Wang H, Fang J, Chen F, Sun Q, Xu X, Lin SH, et al. Metabolomic profile of diabetic retinopathy: A GC-TOFMS-based approach using vitreous and aqueous humor. Acta Diabetol. (2020) 57:41–51. doi: 10.1007/s00592-019-01363-0
42. Vidhya S, Ramya R, Coral K, Sulochana KN, Bharathidevi SR. Free amino acids hydroxyproline, lysine, and glycine promote differentiation of retinal pericytes to adipocytes: A protective role against proliferative diabetic retinopathy. Exp Eye Res. (2018) 173:179–87. doi: 10.1016/j.exer.2018.05.004
43. Wang H, Li S, Wang C, Wang Y, Fang J, Liu K. Plasma and vitreous metabolomics profiling of proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. (2022) 63:17. doi: 10.1167/iovs.63.2.17
44. Busik JV. Lipid metabolism dysregulation in diabetic retinopathy. J Lipid Res. (2021) 62:100017. doi: 10.1194/jlr.TR120000981
45. Nuzzi R, Scalabrin S, Becco A, Panzica G. Gonadal hormones and retinal disorders: A review. Front Endocrinol (Lausanne). (2018) 9:66. doi: 10.3389/fendo.2018.00066
46. Gangwar SK, Verma SK, Modi S. Frequency and correlates of hypogonadism in adult males with type 2 diabetes mellitus. Indian J Endocrinol Metab. (2021) 25:320–5. doi: 10.4103/ijem.ijem_239_21
47. den Hartog GJ, Boots AW, Adam-Perrot A, Brouns F, Verkooijen IW, Weseler AR, et al. Erythritol is a sweet antioxidant. Nutrition. (2010) 26:449–58. doi: 10.1016/j.nut.2009.05.004
48. Suhre K, Meisinger C, Döring A, Altmaier E, Belcredi P, Gieger C, et al. Metabolic footprint of diabetes: A multiplatform metabolomics study in an epidemiological setting. PloS One. (2010) 5:e13953. doi: 10.1371/journal.pone.0013953
49. Menni C, Fauman E, Erte I, Perry JR, Kastenmüller G, Shin SY, et al. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes. (2013) 62:4270–6. doi: 10.2337/db13-0570
50. Zhang P, Zhou YD, Tan Y, Gao L. Protective effects of piperine on the retina of mice with streptozotocin-induced diabetes by suppressing HIF-1/VEGFA pathway and promoting PEDF expression. Int J Ophthalmol. (2021) 14:656–65. doi: 10.18240/ijo.2021.05.04
51. Wang X, Yang S, Yang G, Lin J, Zhao P, Ding J, et al. Novel risk score model for non-proliferative diabetic retinopathy based on untargeted metabolomics of venous blood. Front Endocrinol (Lausanne). (2023) 14:1180415. doi: 10.3389/fendo.2023.1180415
52. Wang Z, Tang J, Jin E, Zhong Y, Zhang L, Han X, et al. Serum untargeted metabolomics reveal potential biomarkers of progression of diabetic retinopathy in asians. Front Mol Biosci. (2022) 9:871291. doi: 10.3389/fmolb.2022.871291
53. Li JM, Fenton RA, Cutler BS, Dobson JG Jr. Adenosine enhances nitric oxide production by vascular endothelial cells. Am J Physiol. (1995) 269:C519–23. doi: 10.1152/ajpcell.1995.269.2.C519
54. Toda N, Nakanishi-Toda M. Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res. (2007) 26:205–38. doi: 10.1016/j.preteyeres.2007.01.004
55. Xia J, Wang Z, Zhang F. Association between related purine metabolites and diabetic retinopathy in type 2 diabetic patients. Int J Endocrinol. (2014) 2014:651050. doi: 10.1155/2014/651050
56. Han X, Zhang L, Kong L, Tong M, Shi Z, Li XM, et al. Comprehensive metabolic profiling of diabetic retinopathy. Exp Eye Res. (2023) 233:109538. doi: 10.1016/j.exer.2023.109538
57. Wagner GR, Payne RM. Widespread and enzyme-independent Nϵ-acetylation and Nϵ-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J Biol Chem. (2013) 288:29036–45. doi: 10.1074/jbc.M113.486753
58. Alka K, Kumar J, Kowluru RA. Impaired mitochondrial dynamics and removal of the damaged mitochondria in diabetic retinopathy. Front Endocrinol (Lausanne). (2023) 14:1160155. doi: 10.3389/fendo.2023.1160155
59. Wu J, Hu J, Zhang F, Jin Q, Sun X. High glucose promotes il-17a-induced gene expression through histone acetylation in retinal pigment epithelium cells. Int Immunopharmacol. (2022) 110:108893. doi: 10.1016/j.intimp.2022.108893
60. Kowluru RA, Santos JM, Zhong Q. Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Invest Ophthalmol Vis Sci. (2014) 55:5653–60. doi: 10.1167/iovs.14-14383
61. Kozieł K, Urbanska EM. Kynurenine pathway in diabetes mellitus-novel pharmacological target? Cells. (2023) 12:460. doi: 10.3390/cells12030460
62. Hou XW, Wang Y, Pan CW. Metabolomics in diabetic retinopathy: A systematic review. Invest Ophthalmol Vis Sci. (2021) 62:4. doi: 10.1167/iovs.62.10.4
Keywords: diabetic retinopathy, blood metabolites, Mendelian randomization, metabolic pathway analysis, meta-analysis
Citation: Yang C, Ma Y, Yao M, Jiang Q and Xue J (2024) Causal relationships between blood metabolites and diabetic retinopathy: a two-sample Mendelian randomization study. Front. Endocrinol. 15:1383035. doi: 10.3389/fendo.2024.1383035
Received: 06 February 2024; Accepted: 05 April 2024;
Published: 01 May 2024.
Edited by:
Chunjie Jiang, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Xiaozhu Liu, Capital Medical University, ChinaCopyright © 2024 Yang, Ma, Yao, Jiang and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinsong Xue, MjUwNjg0MTFAIHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.