- 1Department of Maternal and Child Health and Precision Medicine, University Research Institute, Athens, Greece
- 2Department of Integrative and Complementary Medicine, Universal Health, Sydney, NSW, Australia
- 3Faculty of Health Sciences, Durban University of Technology, Durban, South Africa
This case report presents a novel, non-pharmacological treatment of Type 2 Diabetes in a 46-year-old male, demonstrating improvements in blood chemistry and psychometric markers after 8 treatments using a Mind-Body Intervention (MBI) called Neuro-Emotional Technique (NET). The patient presented with a diagnosis of Type 2 Diabetes (T2D), pain, psychosocial indicators of stress and anxiety, and a score of 4 on the ACE-Q (Adverse Childhood Experiences Questionnaire) that is consistent with a predisposition to chronic disease and autoimmune disorders. Glucose levels for this patient were above normal levels (typically between 10-15mmol/L where optimal range is between 4-10mmol/L) for at least two months prior to the 4-week NET intervention period, despite the standard use of conventional antidiabetic medications (insulin injections). The patient exhibited numerous indictors of chronic stress that were hypothesised to be underlying his medical diagnosis and a series of 8 NET treatments over a period of 4 weeks was recommended. Psychometric tests and glucose measurements were recorded at baseline (prior to treatment), 4 weeks (at the conclusion of treatment) and at 8 weeks (4 weeks following the conclusion of treatment). Results show that glucose levels were reduced, and self-reported measures of depression, anxiety, stress, distress and pain all decreased from high and extreme levels to within normal ranges after 4 weeks, with ongoing improvement at 8 weeks. McEwen described the concept of allostatic load and the disruptive effects that cumulative stress can have on both mental and physical health. It is hypothesized that NET reduces allostatic load thereby fortifying homeostasis and the salutogenic stress response mechanisms involved in recovery from chronic illness, possibly via the Psycho-Immune-Neuroendocrine (PINE) network. Further studies with larger sample sizes are required to establish whether these results could be extrapolated to a wider population, however the results of this case suggest that it may be beneficial to consider co-management of T2D with an MBI such as NET.
Introduction
This case report describes a novel, non-pharmacological Mind-Body Intervention (MBI) called Neuro-Emotional Technique (NET) that was utilised in the treatment of a 46-year-old male with Type 2 Diabetes (T2D). After 4 weeks of NET treatment, glucose levels stabilized and psychometric tests demonstrated improvements in pain, stress and emotional wellbeing.
The diabetes epidemic
T2D is a chronic, progressive metabolic condition that occurs when the body becomes resistant to the normal effects of insulin and gradually loses its ability to produce enough insulin in the pancreas, leading to a range of serious physical symptoms and even life-threatening complications (1–6). Even pre-diabetic increases in fasting glucose have been shown to increase all cause risk of death (7), and a diagnosis of T2D is statistically associated with an approximately two-fold increase in mortality, primarily due to cardiovascular complications (8).
Medical literature currently states that there is no known cure for T2D and remission is extremely rare, reported in only about 0.1% of cases (9, 10). T2D affects about 10% of the population and is a major health problem (11). Globally, the incidence of T2D has increased by 70% in recent decades, closely associated with the rising prevalence of obesity and sedentary behaviour (12–16), even in children (12, 17). The estimated economic cost of diabetes in the US alone in 2022 was $412.9 billion and people diagnosed with diabetes have individual health care costs 2.6 times greater than those without (18). The rise of T2D is projected to continue globally from 537 million sufferers in 2021 to 873 million in 2045 (equivalent to about 1 in every 8 adults) (19–21).
Conventional medical treatment of T2D
The conventional medical management of T2D typically involves a multifaceted approach including life-long education, a balanced diet, frequent exercise, weight management, regular medical check-ups, and continuous blood glucose monitoring, all used as fundamental strategies to help control blood sugar levels (22). When lifestyle modification alone is insufficient, a pharmacological approach involving self-administered insulin injections is typically recommended for life (23). Diabetic pharmacotherapy is aimed at preventing or delaying microvascular and macrovascular complications; however the benefits of anti-diabetic medication may vary depending on patient-specific baseline risk factors (24). While 85.6% of adults diagnosed with diabetes are treated with diabetes medication, only about 50% achieve the target level of glycated haemoglobin (HbA1c), usually due to poor medication adherence (25). The long-term strategy of a conventional medical approach to T2D is a slow intensification of pharmacological therapy “as disease progression advances and β-cell function declines (26)” implying no expectation of improvement, only deterioration.
Mental health symptoms (such as depression) and chronic stress commonly occur in conjunction with T2D and ought to be considered in the management of the condition (27–30). Adverse Childhood Experiences (ACEs) have also been found to strongly correlate with incidence of T2D (31–33), however this association is rarely considered in conventional methods of treatment and prevention (34), representing an area of research that warrants additional investigation.
To our knowledge, no medical literature currently exists to describe the potential effects of NET on T2D. Furthermore, while diabetes research over the last 20 years has focused primarily on the influence of obesity and inflammation (35), comparatively few studies have assessed the influence of non-pharmacological modalities on T2D (for example, Mindfulness-Based Interventions (MBIs) such as yoga, meditation, biofeedback, Mindfulness-Based Stress Reduction (MBSR) etc.), highlighting a need for further contributions to this field. In this case report, therefore NET, a Precision Body-Mind Intervention (PBMI), is presented as a non-pharmacological alternative, or co-management option, for the treatment of T2D.
Patient information
The personal information of the patient has been de-identified, as per the CARE case report guidelines, and written informed consent was given for the publication of personal health information in print and digital format.
The patient described in this case report is a 46-year-old male reporting high levels of stress due to a demanding workload as a builder and as a father to two children.
The patient had never experienced NET treatment, nor had he utilised any other stress relieving MBIs or non-pharmacological treatment modalities to address his diabetes.
Clinical findings
Prior to the commencement of the treatment period, the treating practitioner conducted a thorough review of the patient’s medical, family, and psychosocial history.
The patient reported that on May 11, 2023, his blood glucose level was recorded as 28.5 mmol/L and the patient’s general practitioner (GP) provided a diagnosis of Type 2 Diabetes (T2D). [A result of greater than 11.1mmol/L on a random blood sugar test is suggestive of diabetes (36)]. The patient was prescribed and began to self-administer insulin injections. He was also advised by his GP to follow a diabetic eating plan including high fibre / low GI (Glycaemic Index) foods, low salt, minimal sugar and saturated fats, and drinking plenty of water (37). The patient maintained this for two months prior to the treatment period and no other lifestyle or dietary changes (including food type, frequency, or quantity of food intake) occurred during the 4-week NET intervention, or during the 4-week follow up period.
It was noted that the patient wore a Continuous Glucose Monitor (CGM), providing real-time measurements of interstitial glucose as a biofeedback mechanism to assist with insulin self-administration. Despite the use of insulin injections, the patient reported difficulty maintaining glucose levels within the healthy range of 4-10mmol/L on a day-to-day basis.
A review of the patient’s family history revealed that the patient’s mother and uncle (on his mother’s side) have been previously diagnosed with T2D. The patient had sought no additional treatment for stress or diabetes since his diagnosis.
Primary concerns and symptoms
The patient reported experiencing early symptoms approximately 7-8 months prior to receiving the T2D diagnosis, including anxiety, poor sleep, losing temper easily, dizziness and irrationality. As his symptoms progressed the patient experienced rapid weight loss (from 84kg down to 68kg), insomnia (no more than 4 hours of restless sleep per night), debilitating anxiety, polyuria (needing to urinate 6-8 times every night), constipation, fatigue, physical pain and discomfort, moodiness, and a desire to withdraw from all social contact and activities. The patient described himself as “not a nice person to be around.”
Examination and assessment
The practitioner performed a postural analysis and physical examination of the spine and musculoskeletal system including range of motion tests and palpation, which were unremarkable. There were no red flags or contraindications identified in the examination and assessment.
As part of the routine intake process for many new patients at this practice, a biopsychosocial assessment was conducted that included the following psychometric tests: ACE-Q (Adverse Childhood Experiences Questionnaire) (38), DASS-21 (Depression, Anxiety and Stress Scale) (39), DRAM (Distress and Risk Assessment Method) (40, 41) and SF-MPQ (Short Form McGill Pain Questionnaire) (42). The results of these tests indicated that this patient was experiencing high levels of chronic stress, distress and pain and had a history of childhood trauma.
Diagnosis and recommendations
Based on the patient’s health history, the prior medical diagnosis of T2D and clinical findings revealing indicators of chronic stress, the patient was considered a suitable candidate for NET. The practitioner recommended a schedule of 8 treatments at a frequency of two 15-minute sessions per week for 4 weeks. A review of the patient’s progress planned for the 8th visit with a further follow-up assessment conducted 4 weeks later.
The patient fully adhered to the treatment recommendations by attending all 8 treatments and there were no adverse or unanticipated events during the treatment period. There were no barriers to the patient receiving the planned care, nor were there any changes to the therapeutic intervention throughout the treatment period.
Timeline
In the nine months preceding the NET treatment, the patient retrospectively reported experiencing a gradual worsening of various symptoms. Two months prior, the patient was diagnosed with T2D after returning high serum glucose results, commencing insulin injections and lifestyle changes.
On July 14, 2023, a baseline assessment was conducted, with 24-hour continuous glucose monitor (CGM) readings ranging from 10 to 15 mmol/L, exceeding the healthy range despite two months of consistent lifestyle modifications and insulin use. Baseline measurements were recorded, and the patient commenced NET treatment.
After eight NET treatment sessions, conducted twice per week for four weeks, the 24-hour glucose readings on August 13, 2023, were within the normal range (between 4 and 10 mmol/L). Post-treatment psychometric test results were recorded for comparison with the baseline measurements.
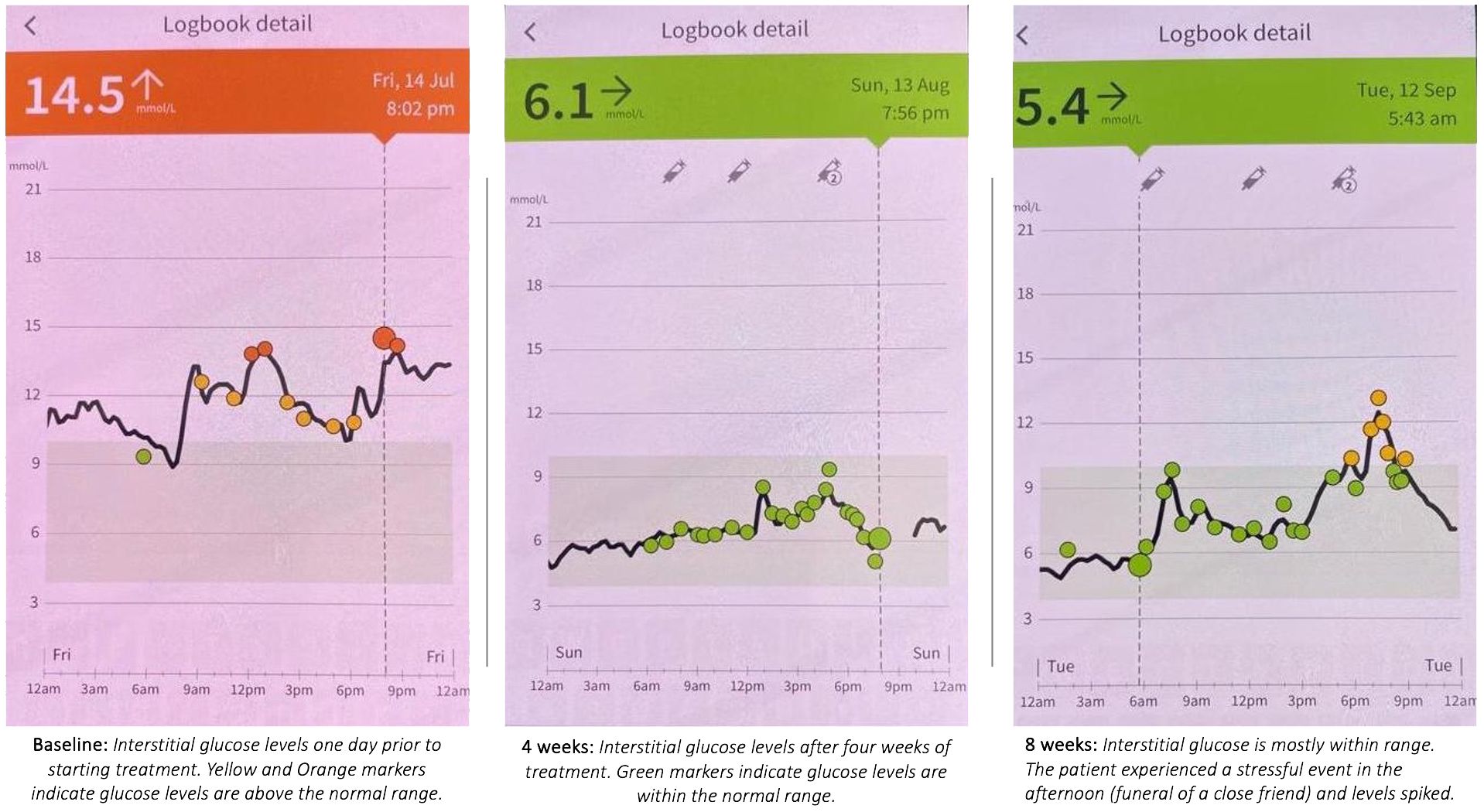
On September 13, 2023, four weeks after the NET treatment concluded, with no further treatment or lifestyle modifications, 24-hour glucose and psychometric tests were assessed again. The patient had attended the funeral of a close friend the day before, and the physiological response to this stressful event may explain the observed afternoon glucose spike. Notably, all psychometric and pain scores stabilized or continued to improve in the four weeks following the NET treatment.
Therapeutic intervention: the NET treatment protocol
As appropriate, NET is utilised in the treating clinic as a stress-reduction modality where stress plays a significant role as potential underlying factor for autoimmune and/or chronic illness (43).
Neuro-Emotional Technique (NET) is a fast and precise 15-step stress-reduction Mind-Body Intervention aimed at improving physical and emotional health (44). The NET methodology is used to find and remove unresolved, dormant stress patterns in the neurophysiology of the patient called NEC’s (Neuro-Emotional Complexes) (44) that drive adverse bodily responses, potentially contributing to chronic illness (45, 46). NECs are unique to the conditioned, experiential, and emotional reality of each patient and can be quickly identified and relieved, reducing the impact and role of stress that may be underlying the patient’s presenting health condition (44).
To achieve this objective, NET combines elements of numerous health fields, such as pulse assessment in traditional Chinese medicine, cognitive behavioural psychology, gentle spinal adjustments and a reliable feedback method known as the muscle test (47, 48).
Reversing (or extinguishing) classically conditioned painful emotional responses to trauma-related stimuli is a primary objective of the treatment. These stimuli have the unique capacity to replicate a physiological stress response even in the absence of the initial stressor or stressors. The goal of clearing or reducing NEC’s using NET protocols is comparable to exposure therapy and other common cognitive behavioural therapies for severe stress (49).
It is hypothesised that NET works by regulating the homeostatic mechanisms of the Hypothalamic-Pituitary-Adrenal (HPA) Axis and the Psycho-Immune-Neuroendocrine (PINE) Network (50–54) thereby relieving allostatic load, as explored further in the Discussion.
Published research demonstrating these effects includes an RCT where NET was shown to lower inflammatory blood markers and relieve pain in a cohort of chronic low back pain sufferers (55), and another revealed reduced activity in brain areas involved with traumatic memories and distress (such as the anterior cingulate gyrus, parahippocampus, insula and brainstem) in a cohort of cancer survivors (56). For a more detailed description of NET we refer the reader to previously published detailed explanations of the technique as applied in standardised clinical research settings (57–62).
Results
The patient experienced relief of all symptoms reported including a reduction of physical pain/discomfort as well as a decrease in mood dysregulation (anxiety, irritation, losing temper, irrationality etc.) He reported normalisation of sleep and nocturia, and his symptoms of anxiety, fatigue, constipation and dizziness ceased. The results of the psychometric tests, self-reported pain assessment and interstitial glucose measurements are as follows:
The adverse childhood experiences questionnaire
The Adverse Childhood Experiences (ACE) Questionnaire is a popular assessment instrument made to gauge the severity of adversity in childhood and its potential to affect a person's physical and mental health in adulthood (38). The patient in this case report returned a score of 4 on the ACE-Q, which lies within what we call “the auto-immune risk zone” because any ACE score greater than 2 represents a 70-80% risk of experiencing an auto-immune condition in adulthood (63), including a strong correlation with a predisposition to T2D (31–34).
The depression, anxiety and stress scale
The DASS-21 (Depression, Anxiety and Stress Scale) Questionnaire is a popular psychometric tool for assessing and quantifying the severity of a person's symptoms of depression, anxiety, and stress (64). It is a reliable self-report questionnaire that enables respondents to provide information about their emotional health (39, 65). Higher scores in each of the three categories indicate symptoms that are more serious in nature (64).
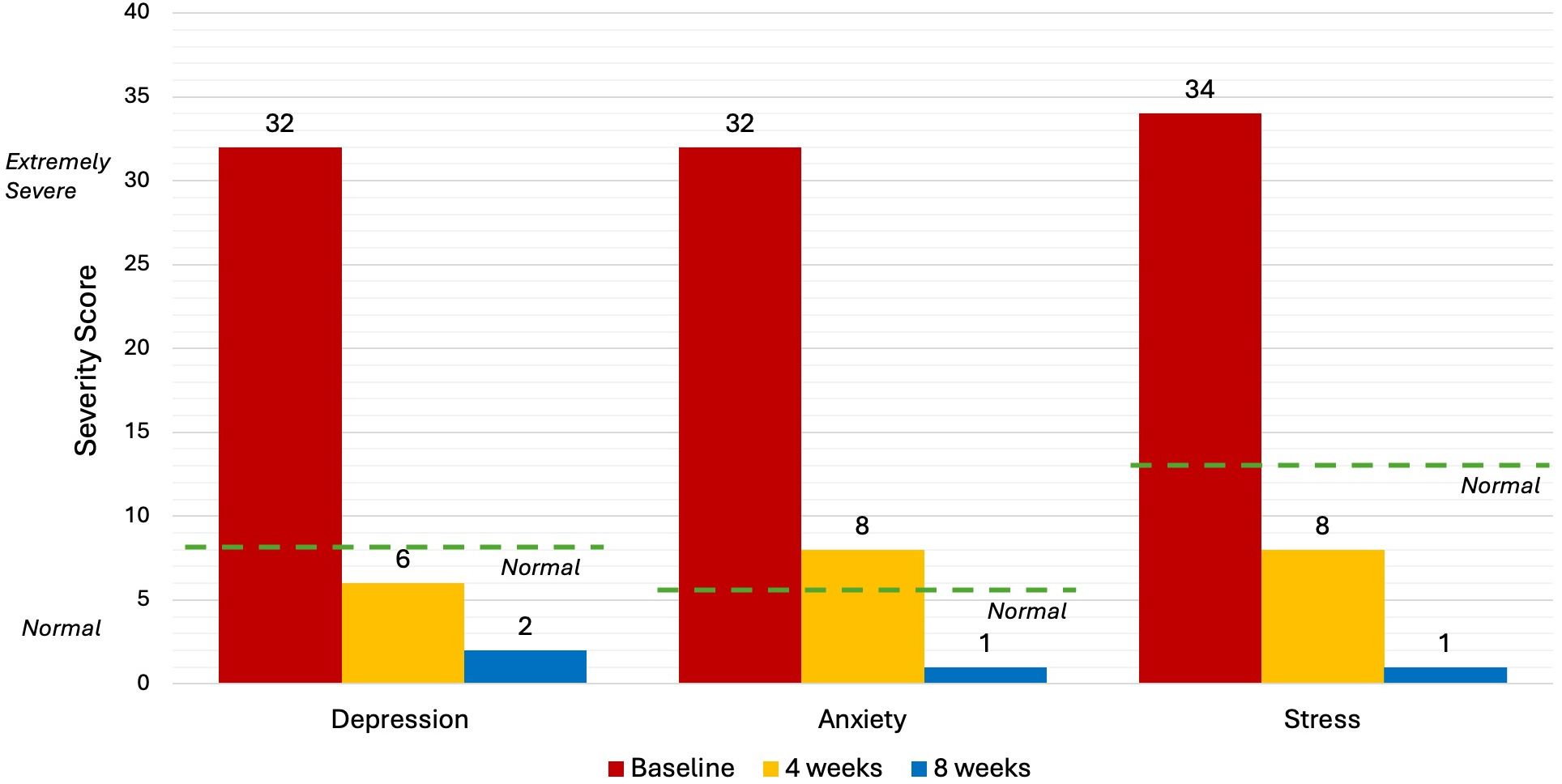
Five levels of severity, from “normal” to “extremely severe”, are utilised in the interpretation of DASS-21 results. “normal” scores are: Depression (<9), Anxiety (<7) and Stress (<14). “Extremely severe” scores are: Depression (28+), Anxiety (20+) and Stress (34+). People with an "extremely severe" score on the DASS-21 are likely dealing with severe psychological symptoms that may seriously impair their ability to operate on a day-to-day basis, as well as their general wellbeing and quality of life (39).
Upon commencement of the treatment, DASS-21 scores for this patient were “extremely severe” in each of the three categories: Depression (32), Anxiety (32) and Stress (34). After 8 NET sessions (4 weeks) the patient completed a second DASS-21 evaluation and returned “normal” results in each category. After 8 weeks, the scores in each category further decreased indicating continued improvement after the treatment period (Figure 1).
The distress and risk assessment method
The DRAM (Distress and Risk Assessment Method) is a practical means of assessing the level of psychological distress in patients with pain (40, 41). It is a validated measuring tool consisting of two questionnaires, the MZDI [Modified Zung Depression Index (66)] and the MSPQ [Modified Somatic Perception Questionnaire (67)]. The MZDI is designed to give a quantitative measure of a person’s level of depression while the MSPQ is a measure of somatic awareness and anxiety, with the combined results of the MZDI and the MSPQ tallied to provide a DRAM score.
Typically, a high DRAM score is considered a poor predictor of treatment success (68). The four DRAM classifications as per Main et al. (40) are: Normal = MZDI < 17; At Risk = MZDI 17-33 and MSPQ < 12; Distressed Depressive = MZDI > 33 and Distressed Somatic = MDZI > 33 + MSPQ > 12 (40).
The baseline DRAM scores for the patient in this case report were an MSPQ (distress) of 26 and a MZDI (depression) of 34, resulting in a classification of “Distressed Somatic/ Depressive”. The MSPQ (distress) score dropped to 6 and then 2 after 4 and 8 weeks respectively. The MZDI dropped from 34 to 16 and then 6 at the same intervals. The patient’s overall DRAM classification changed to “Normal” after 4 weeks of NET treatment, with further improvements noted at 8 weeks (Figure 2).
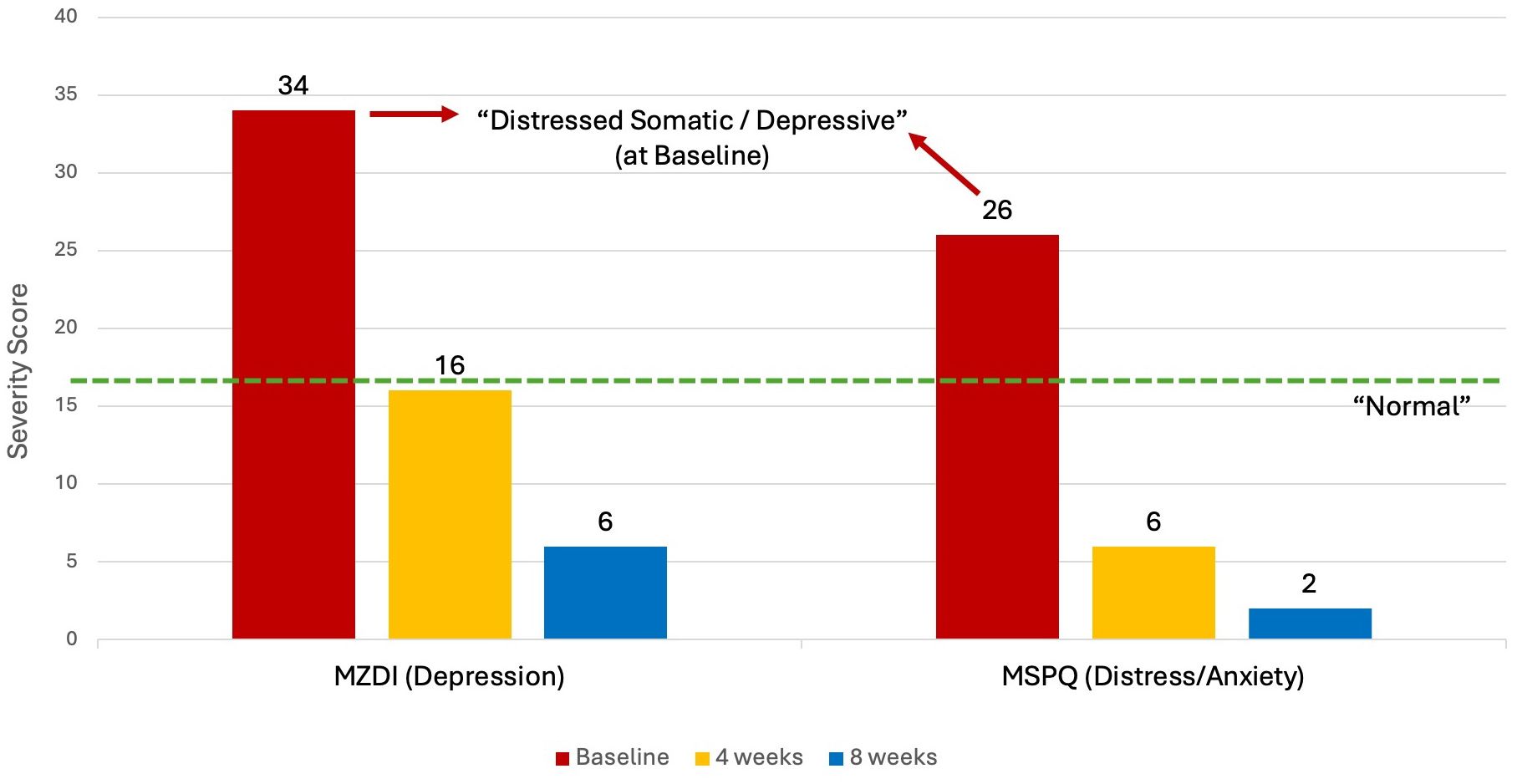
Figure 2 DRAM at Baseline, 4 weeks and 8 weeks. DRAM, Distress and Risk Assessment Method; MZDI, Modified Zung Depression Index; MSPQ, Modified Somatic Perception Questionnaire.
The short form McGill pain questionnaire
The Short Form McGill Pain Questionnaire (SF-MPQ) is a reliable and valid assessment of a person’s intensity and frequency of physical discomfort (42). It includes four primary components: Present Pain Intensity (PPI) scale, the Visual Analogue Scale (VAS) and 15 pain descriptors (11 Sensory, 4 Affective) that can be scored on an intensity scale where 0 = none, 1 = mild, 2 = moderate and 3 = severe.
The results of the SF-McGill showed a 100% decrease in Present Pain Intensity (PPI) from a score of 4/5 to 2/5 at 4 weeks to 0/5 at 8 weeks (Figure 3A). Affective (emotional) pain dropped by 85% from 7/12 to 1/12 at 4 weeks and this improvement was sustained at 8 weeks (Figure 3B). Sensory (physical) pain was reduced by 80% from 18/33 to 9/33 at 4 weeks and further decreased to 3/33 at 8 weeks (Figure 3C). The Visual Analogue Scale (VAS) fell from 4/5 to 2/5 at 4 weeks and 0.5/5 at 8 weeks (Figure 3D).
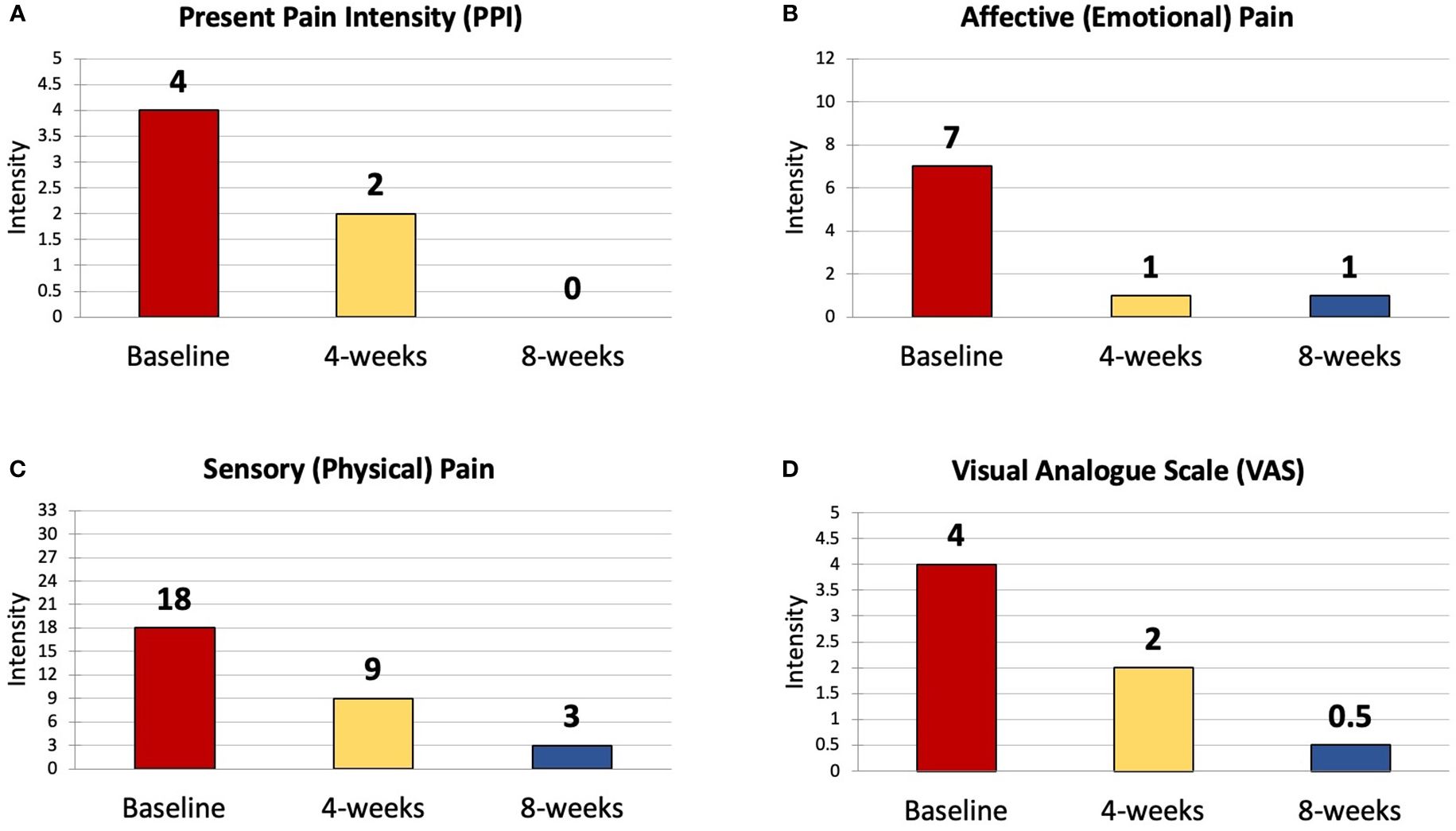
Figure 3 The Four Components of the SF-MPQ - Results at Baseline, 4 weeks and 8 weeks. SF-MPQ, Short Form McGill Pain Questionnaire; PPI, Present Pain Intensity; VAS, Visual Analogue Scale. (A) Present Pain Intensity (PPI); (B) Affective (Emotional) Pain Intensity; (C) Sensory (Physical) Pain Intensity; (D) Visual Analogue Scale (VAS) Pain Rating.
Interstitial glucose
The patient wears a continuous glucose monitoring (CGM) sensor called “FreeStyle Libre 2” which is a medical device registered with the Therapeutic Goods Administration (TGA) in Australia (69). The sensor is placed on the patient’s skin allowing constant detection of glucose levels in the Interstitial Fluid (ISF). ISF glucose levels are comparable to and follow blood glucose levels after a period of time known as “the lag” (70). Interstitial glucose levels are transmitted to the FreeStyle LibreLink app on the patient’s mobile device. Data from the sensor was gathered from the patient’s phone to compare ISF glucose levels at baseline and then after 4 and 8 weeks of treatment (Figure 4).
The optimal zone for glucose levels is between 4.0-7.0mmol/L (fasting) and 5.0-10.0mmol/L (postprandial) (71). Prior to commencing treatment, the patient’s baseline levels were above the healthy range for almost the entire 24-hour period (Figure 4, Baseline). After four weeks of treatment, glucose measurements across a full 24-hour period are entirely within the optimal range 4.0-10mmol/L. At 8 weeks interstitial glucose remains mostly within the optimal range, however there is a spike above optimal levels late in the day following a stressful event - the funeral of a close friend. This rise in glucose levels practically demonstrates a connection between biopsychosocial stress and subsequent adaptation of the PINE network, as explored in the Discussion.
Discussion
Considering the anticipated global rise of T2D, and the mixed success and poor prognosis of the conventional medical approach to treatment, it is appropriate to investigate the potential benefits of adjunct or alternative treatments (such as MBIs) that may better address the underlying physiological dysfunction and chronic stress at the root of the condition.
Outcomes of MBIs on T2D
MBIs commonly examined in the scientific literature as potential treatments for T2D include mindfulness training programs such as Kabat-Zinn’s MBSR (Mindfulness-Based Stress Reduction), Mindful Awareness Programs (MAP’s), Cognitive Behavioural Therapy (CBT) and Medical Nutrition Therapy (MNT) (72–74) but may also include more dynamic, movement-based practices such as yoga, tai chi and qigong (75). MBIs have been found in systematic reviews to not only reduce psychological stress, but also induce physiological changes including parasympathetic activation, lower cortisol secretion, down-regulate pro-inflammatory genes and pathways, delay the rate of ageing, counter the effects of chronic stress, and reduce blood glucose, which are all risk factors for T2D (76).
However, benefits vary greatly between interventions and there is significant heterogeneity between studies making it difficult to generalise the effects, influences and cost-effectiveness of MBIs as a whole (75, 77). For example, one systematic review found that in older, working-aged adults MBSR may help reduce participants’ perception of depression immediately following the intervention, but there paradoxically appeared to be no effect on perceived levels of anxiety or stress (78). Moreover, a systematic review of Randomised Controlled Trials (RCTs) assessing the efficacy of psychological interventions on T2D showed no conclusive improvements in levels of Diabetes Related Distress (DRD) or Health Related Quality of Life (HRQoL), with only slight improvements in diabetic blood markers after a median treatment duration of 6 months (79). Another literature review of MBSR research studies ironically revealed no conclusive evidence of lasting stress reduction beyond the treatment period and did not detect effects on the diabetic blood marker HbA1c (80). Authors Hackett and Steptoe identified that stress-relieving MBIs improved symptoms of depression and DRD but a positive effect on glycaemic control was less certain across a range of studies (30), however Yang et al. identified several studies showing “MBIs can significantly contribute to blood glucose control” (76). Future studies with larger cohorts over longer time periods may help to clarify some of these inconsistencies.
Allostatic load and T2D
Interestingly, the NET treatment evidenced in this case report resulted in consistent improvements across a range of measurable psychological markers and lowered glucose levels, all within a comparatively short treatment period of just 4 weeks. We hypothesise that this is due to the precise physiological reduction of dormant emotional stressors facilitated specifically by the NET treatment, thereby lowering the allostatic load that may have been a significant contributor to the accumulated physiological dysfunction that eventually led to the manifestation of T2D (30).
McEwen’s allostatic load concept refers to the cumulative burden of chronic stress and challenges from life events, and the resultant difficulty for the body to return to homeostasis (81). Dysregulation of glucose metabolism, neuroendocrine function, diurnal cortisol release and chronic low-grade inflammation (LGI) can result via disruption of the PINE Network, thereby contributing to the development of chronic conditions such as T2D (30). Emerging research suggests that both T1D and T2D may express aspects of autoimmunity [loss of self-tolerance (82)] primarily due to pervasive LGI, both in the pancreas and system-wide throughout the body (83, 84). Alongside metabolic dysregulation, a vicious cycle then results: increasing cytokine (inflammatory cell) production destroying pancreatic β-cells, in turn leading to the release of autoimmune “self” antigens that further impair insulin secretion and promote hyperglycaemia, triggering even more inflammation (85, 86). Understanding what can cause allostatic load to chronically increase beyond physiological tolerance, setting off these metabolic and immune disease processes, may inform an understanding of why a stress relieving MBI like NET can benefit T2D.
Adverse childhood experiences and T2D
Allostatic overload can occur due to micro-stressful events (MSEs) such as chronic work stress, financial pressure, health crises such as depression, or the presence of persistent and unresolved early life stressors (ELS), such as those quantified in the ACE-Q. People who report four or more ACE’s are statistically more likely to report chronic health conditions compared to those who report experiencing zero adverse childhood events (32, 87–91). ELS and Adverse Childhood Experiences (ACEs) can predispose sufferers specifically to T2D (34, 92–94), especially the presence of any of the following: undergoing childhood economic adversity, suffering abuse (verbal, physical or sexual) or having a family member incarcerated (33). The addition of a single ACE equates to an approximately 11% increase in the odds of diabetes (95) and an ACE-Q score of 4 or more is associated with a 2.1 times greater risk of diabetes (31).
Stress, NET and T2D
A review of the physiological response to chronic stressors, such as unresolved ELS or childhood mistreatment (CM), may explain these associations between ACE scores and increased risk of T2D (96). Initially, glucocorticoid hormones, such as cortisol, are released by the adrenal medulla in response to a stressor, increasing insulin secretion (97). Glucocorticoids influence glucose homeostasis by inducing the release of glucose and lipids into the circulation, in turn activating the Sympathetic Nervous System (SNS). The SNS acts then to release adrenaline, increasing heart rate and blood pressure, lowering heart rate variability, mobilising energy and releasing pro-inflammatory cytokines. Repeated or sustained stimulation of the allostatic system can cause an overabundance of glucose and lipids relative to the cellular energy demands, constituting a form of metabolic stress that can promote insulin resistance, weight gain and an amplified immune reactivity due to the presence of heightened inflammation (30, 83, 98). The “Biological Embedding of Childhood Adversity” model has been proposed to explain how childhood stress becomes epigenetically “programmed” into macrophages, consequentially endowing cells with pro-inflammatory tendencies, thereby exaggerating cytokine responses and decreasing sensitivity to inhibitory hormone signals, altering endocrine and autonomic patterns that, in conjunction with resulting genetic and lifestyle influences, ultimately results in chronic inflammation that fosters chronic disease (99).
Inflammation is both a key marker of chronic stress and a contributing factor in the pathophysiology of T2D (100). CRP (C-Reactive Protein), a blood marker for inflammation, is known to independently elevate in adults 20 years after experiences of childhood maltreatment or trauma (101). The assessment of ACEs in this case report is appropriate and informative because increased serum CRP levels not only predict the risk of T2D, small increases in CRP can also predict the likelihood of cardiovascular events – a common comorbidity of T2D (102). NET can be appropriately considered as a treatment option because the technique has been shown in a RCT by two of the authors to reduce CRP levels, as well as other inflammatory blood markers associated with stress [such as tumour necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6 (55)], all of which can be predictive of T2D (100).
The resolution or reduction of dormant stressors for a patient with unresolved childhood trauma – and an associated decrease in stress-induced inflammation and allostatic load - may, at least in part, explain the effectiveness of the NET treatment in this case report. A similar treatment effect has been proposed in a published case report of a hypothyroidism patient with a high ACE score who demonstrated normalisation of thyroid blood markers and psychometric stress following NET treatment (54).
Strengths and weaknesses
A weakness of this case report is that the treatment was performed by a single practitioner on a single patient, therefore the results cannot yet be generalised for other practitioners, or to a wider population of T2D sufferers. It is possible that NET may not be a suitable intervention or co-management strategy for all sufferers of T2D. Another weakness is that additional data from the patient’s CGM was not available for publishing, making it difficult to ascertain further insights into the precise mechanisms and future trends associated with the patient’s response to treatment.
Strengths of this case report include reliable outcome measures with pre- and post-treatment changes that could help to establish protocols for larger RCT based studies. In future studies, more pre- and post-treatment CGM data (for example, “average glucose” and “time in range”), measures of additional blood markers such as Fasting Blood Glucose, Fasting Insulin, HbA1c, inflammatory cytokines such as (TNF-α, IL-1β, IL-6, IL-8 and CRP) and the assistance of an experienced biostatistician could be garnered for deeper insights into the physiological responses to NET treatment.
Should the results of this case report be reproduced in longitudinal, randomised controlled trials with a greater number of patients and practitioners involved, NET may be identified as a reliable co-management intervention to assist sufferers of adverse childhood events and chronic stress-based illnesses such as T2D.
Conclusion
This case report describes the effect of a new, stress-relieving MBI called NET on psychological and physiological markers of T2D, resulting in normalisation of symptoms, stress, and glucose levels over a 4-week treatment period. The changes in objective and subjective measures (including reduction in physical pain, psychological distress and interstitial glucose levels) were sustained, and many indicators showed continued improvement, 4 weeks after the treatment period concluded. The allostatic load created by early life stress (ELS) – represented by a high score on the Adverse Childhood Experiences (ACE) questionnaire that correlates with increased likelihood of auto-immune disorders such as Type 2 Diabetes (T2D) – may have been discharged by the stress-relieving and inflammation-lowering effects of the NET treatment. In similar cases where conventional pharmacological management alone is insufficient for moderating symptoms and blood glucose levels associated with T2D, NET may prove to be a valuable co-management strategy to address underlying physiological stressors that exacerbate or cause the condition.
Patient perspective
“After receiving this treatment for my Type 2 Diabetes, my life has changed in many positive ways. Mentally, I feel I can cope much better with everyday life and its challenges. Mentally and emotionally, I feel "reset", a lot more positive and less foggy than I did before treatment. I would describe my experience of the treatment I received as pivotal, because it helped me change my mind-set about my condition and more positive as a result. I feel acceptance and accepting that I now have to balance my insulin injections & food intake. This has given me relief, as well as provided me with the confidence that I am able to manage and regulate my condition. I realise that I now prioritise health and lifestyle as much as I prioritise work. All around, I am happier with myself, and more balanced.”
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written consent was received from the patient for the publishing of the case report. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PB: Conceptualization, Data curation, Methodology, Project administration, Writing – review & editing. RD: Writing – original draft, Writing – review & editing. HP: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACE, Adverse Childhood Experiences; ACE-Q, Adverse Childhood Experiences Questionnaire; BPS, Biopsychosocial; CBT, Cognitive Behavioural Therapy; CGM, Continuous Glucose Monitoring; CM, Childhood Mistreatment; CRP, C-Reactive Protein; DASS, Depression, Anxiety and Stress Scale; DRAM, Distress and Risk Assessment Method; DRD, Diabetes Related Distress; ELS, Early Life Stress; GI, Glycaemic Index; HbA1c, Glycated haemoglobin; HPA Axis, Hypothalamic-Pituitary-Adrenal Axis; HRQoL, Health-Related Quality of Life; IL-1, Interleukin-1; IL-6, Interleukin-6; ISF, Interstitial Fluid; MAPs, Mindful Awareness Programs; MBI, Mindfulness-Based Intervention; MBSR, Mindfulness-Based Stress Reduction; mmol/L, millimoles per litre; MNT, Medical Nutrition Therapy; MSE, Micro-Stressful Events; MSPQ, Modified Somatic Perception Questionnaire; MZDI, Modified Zung Depression Index; NEC, Neuro-Emotional Complex; NET, Neuro-Emotional Technique; PBMI, Precision Body-Mind Intervention; PINE Network, Psycho-Immune-Neuroendocrine Network; RCT, Randomised Controlled Trial; SF-MPQ, Short Form McGill Pain Questionnaire; SNS, Sympathetic Nervous System; T2D, Type 2 Diabetes; TCM, Traditional Chinese Medicine; TGA, Therapeutic Goods Administration; TNF-, Tumour Necrosis Factor-α.
References
1. Australian Institute of Health and Welfare. Diabetes: Australian facts (2023). Available online at: https://www.aihw.gov.au/reports/diabetes/diabetes/contents/what-is-diabetes (Accessed 14 December 2023).
2. Mayo Clinic. Type 2 diabetes. Available online at: https://www.mayoclinic.org/diseases-conditions/type-2-diabetes/symptoms-causes/syc-20351193 (Accessed 14 December 2023).
3. Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KM, et al. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabetes Endocrinol. (2014) 2:867–74. doi: 10.1016/S2213-8587(14)70161-5
4. Rao Kondapally Seshasai S, Kaptoge S, Thompson and e. al. A. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. (2011) 364:829–41. doi: 10.1056/NEJMoa1008862
5. Tomic D, Morton JI, Chen L, Salim A, Gregg EW, Pavkov ME, et al. Lifetime risk, life expectancy, and years of life lost to type 2 diabetes in 23 high-income jurisdictions: a multinational, population-based study. Lancet Diabetes Endocrinol. (2022) 10:795–803. doi: 10.1016/S2213-8587(22)00252-2
6. Brutsaert EF. Complications of diabetes mellitus, in: MSD manuals (2022). Available online at: https://www.msdmanuals.com/home/hormonal-and-metabolic-disorders/diabetes-mellitus-dm-and-disorders-of-blood-sugar-metabolism/complications-of-diabetes-mellitus (Accessed 05 Oct 2023).
7. Yi S, Park S, Lee Y, Park H, Balkau and J. Yi B. Association between fasting glucose and all-cause mortality according to sex and age: a prospective cohort study. Sci Rep. (2017) 7:8194. doi: 10.1038/s41598-017-08498-6
8. Nwaneri C, Cooper H, Bowen-Jones D. Mortality in type 2 diabetes mellitus: magnitude of the evidence from a systematic review and meta-analysis. Br J Diabetes Vasc Dis. (2013) 13:192–207. doi: 10.1177/1474651413495703
9. Corporate Author: Diabetes Victoria. Diabetes victoria. In: Type 2 diabetes. Victoria, Australia: Diabetes Victoria. Available at: https://www.diabetesvic.org.au/Type-2.
10. BMJ. Losing weight can reverse type 2 diabetes, but is rarely achieved or recorded. In: BMJ newsroom. London, United Kingdom: BMJ Publishing Group Ltd. (2024). Available at: https://www.bmj.com/company/newsroom/losing-weight-can-reverse-type-2-diabetes-but-is-rarely-achieved-or-recorded/.
11. Taylor S, Yazdi Z, Beitelshees A. Pharmacological treatment of hyperglycemia in type 2 diabetes. J Clin Invest. (2021) 131:e142243. doi: 10.1172/JCI142243
12. Geiss LS, Wang J, Cheng and e. al. YJ. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. (2014) 312:1218–26. doi: 10.1001/jama.2014.11494
13. Danaei G, Finucane MM, Lu Y. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2•7 million partipants. Lancet. (2011) 378:31–40. doi: 10.1016/S0140-6736(11)60679-X
14. Twito O, Frankel M, Nabriski D. Impact of glucose level on morbidity and mortality in elderly with diabetes and pre-diabetes. World J Diabetes. (2015) 6:345–51. doi: 10.4239/wjd.v6.i2.345
15. World Health Organisation. The top 10 causes of death (020). Available online at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (Accessed 18 Sept 2023).
16. Zhou B, Lu Y, Hajifathalian KEA. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4•4 million participants. Lancet. (2016) 387:1513–30. doi: 10.1016/S0140-6736(16)00618-8
17. Kaptoge S, Sun L, Walker M, Spackman S, Pennells L, Seshasai SRK, et al. Life expectancy associated with different ages at diagnosis of type 2 diabetes in high-income countries: 23 million person-years of observation, Lancet Diabetes Endocrinol. (2023) 11:731–742. doi: 10.1016/S2213-8587(23)00223-1
18. Parker ED, Lin J, Mahoney T, Ume N, Yang G, Gabbay RA, et al. Economic costs of diabetes in the U.S. @ in 2022. Diabetes Care. (2024) 47:26–43. doi: 10.2337/dci23-0085
19. International Diabetes Federation. Diabetes facts & Figures. Available online at: https://idf.org/about-diabetes/diabetes-facts-figures/ (Accessed 18 Sept 2023).
20. Seuring T, Archangelidi O, Suhrcke M. The economic costs of type 2 diabetes: a global systematic review. Pharmacoeconomics. (2015) 33:811–31. doi: 10.1007/s40273-015-0268-9
21. Guariguata L, Whiting DR, Hambleton and J. Beagley I. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. (2014) 103:137–49. doi: 10.1016/j.diabres.2013.11.002
22. Wang X, Kang J, Lui Q, Tong T, Quan H. Fighting diabetes mellitus: pharmacological and non-pharmacological approaches. Curr Pharm Des. (2020) 2639:4992–5001. doi: 10.2174/1381612826666200728144200
23. Emilie White P. Treatments for diabetes (Non-pharmacologic) (2023). Available online at: https://www.endocrinologyadvisor.com/ddi/treatments-for-diabetes-nonpharmacologic/ (Accessed 15 December 2023).
24. Shi Q, Nong K, Vandvik P, Guyatt G, Schnell O, Rydén L, et al. Benefits and harms of drug treatment for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. (2023) 381:e074068. doi: 10.1136/bmj-2022-074068
25. Edelman S, Polonsky W. Type 2 diabetes in the real world: the elusive nature of glycemic control(2017). Available online at: https://diabetesjournals.org/care/article/40/11/1425/37008/Type-2-Diabetes-in-the-Real-World-The-Elusive (Accessed 13 Jun 2024).
26. Stolar M. Defining and achieving treatment success in patients with type 2 diabetes mellitus. Mayo Clin Proc. (2010) 85:S50–9. doi: 10.4065/mcp.2010.0471
27. Royal Australian College of General Practitioners (RACGP). Management of type 2 diabetes: A handbook for general practice (2020). Available online at: https://www.racgp.org.au/getattachment/41fee8dc-7f97-4f87-9d90-b7af337af778/Management-of-type-2-diabetes-A-handbook-for-general-practice.aspx (Accessed 20 Sept 2023).
28. Gonzalez J, Peyrot M, McCarl L, Collins E, Serpa L, Mimiaga and S. Safren M. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. (2008) 31:2398–403. doi: 10.2337/dc08-1341
29. Shiloah E, Rapoport M. Psychological stress and new onset diabetes. Pediatr Endocrinol Rev. (2006) 3:272–5.
30. Hackett R, Steptoe A. Type 2 diabetes mellitus and psychological stress - a modifiable risk factor. Nat Rev Endocrinol. (2017) 139:547–60. doi: 10.1038/nrendo.2017.64
31. Hughes K, Ford K, Bellis M. Adverse Childhood Experiences (ACEs) and Diabetes | A brief review (2020). Available online at: https://phwwhocc.co.uk/wp-content/uploads/2021/06/PHWBangor-ACEs-Diabetes-Factsheet-5-1.pdf (Accessed 6 February 2024).
32. Huang H, Yan P, Shan Z. Adverse childhood experiences and risk of type 2 diabetes: a systematic review and meta-analysis. Metabolism. (2015) 6411:1408–18. doi: 10.1016/j.metabol.2015.08.019
33. Zhu S, Shan S, Liu W, Li S, Hou L, Huang X, et al. Adverse childhood experiences and risk of diabetes: A systematic review and meta-analysis. J Glob Health. (2022) 12:4082. doi: 10.7189/jogh.12.04082
34. Huffhines L, Noser A, Patton S. The link between adverse childhood experiences and diabetes. Curr Diabetes Rep. (2016) 166:54. doi: 10.1007/s11892-016-0740-8
35. Zyoud S, Shakhshir M, Koni A, Abushanab A, Shahwan M, Jairoun A, et al. Mapping the global research landscape on insulin resistance: Visualization and bibliometric analysis. World J Diabetes. (2022) 13:786–98. doi: 10.4239/wjd.v13.i9.786
36. Kudva Y, Mayo Clinic. Diabetes (2023). Available online at: https://www.mayoclinic.org/diseases-conditions/diabetes/diagnosis-treatment/drc-20371451.
37. Healthy Eating Channel. Diabetes and healthy eating. Available online at: https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/diabetes-and-healthy-eating (Accessed 15 Sept 2023).
38. Felitti VJ, Anda RF, Nordenberg D. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. (1998) 14:245–58. doi: 10.1016/S0749-3797(98)00017-8
39. Lovibond PF, Lovibond SH. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. (1995) 333:335–43. doi: 10.1016/0005-7967(94)00075-U
40. Main CJ, Wood PL, Hollis S, Spanswick and G. Waddell CC. The distress and risk assessment method. Spine. (1992) 17:42–52. doi: 10.1097/00007632-199201000-00007
41. Hobby J, Lutchman L, Powell and D. Sharp J. The distress and risk assessment method (DRAM). J Bone Joint Surg. (2001) 83:19–21. doi: 10.1302/0301-620X.83B1.0830019
42. Melzack R. The short-form mcGill pain questionnaire. Pain. (1987) 30:191–7. doi: 10.1016/0304-3959(87)91074-8
43. Sharif K, Watad A, Coplan L, Lichtbroun B, Krosser A, Lichtbroun M, et al. The role of stress in the mosaic of autoimmunity: An overlooked association. Autoimmun Rev. (2018) 1710:967–83. doi: 10.1016/j.autrev.2018
45. Monti D, Tobia A, Stoner M, Wintering N, Matthews M, Conklin CJ, et al. Changes in cerebellar functional connectivity and autonomic regulation in cancer patients treated with the Neuro Emotional Technique for traumatic stress symptoms. J Cancer Surviv. (2018) 12:145–53. doi: 10.1007/s11764-017-0653-9
46. Selye H. A syndrome produced by diverse nocuous agents. Nature. (1936) 138:32. doi: 10.1038/138032a0
47. Pollard H, Lakay B, Tucker F, Watson and P. Bablis B. Interexaminer reliability of the deltoid and psoas muscle test. J Manipulative Physiol Ther. (2005) 28:52–6. doi: 10.1016/j.jmpt.2004.12.008
48. Monti D, Sinnott J, Marchese M, Kunkel EJ, Greeson JM. Muscle test comparisons of congruent and incongruent self-referential statements. Percept Mot Skills. (1999) 88:1019–28. doi: 10.2466/pms.1999.88.3.1019
49. Foa EB, Jaycox LH. “Cognitive-behavioral theory and treatment of posttraumatic stress disorder”. In: Spiegel D, editor. Efficacy and cost-effectiveness of psychotherapy. Arlington, VA, United States: American Psychiatric Publishing, Inc. (1999).
50. Nold V, Allers K. Consequences of chronic stress on the PINE network, in: (2020). Available online at: https://www.intechopen.com/chapters/76550 (Accessed 11 Oct 2023).
51. Stapelberg N, Neumann D, Shum D, McConnell and I. Hamilton-Craig H. From physiome to pathome: A systems biology model of major depressive disorder and the psycho-immune-neuroendocrine network. Curr Psychiatry Rev. (2015) 11:32–62. doi: 10.2174/1573400510666140619211733
52. Stapelberg N, Neumann D, Shum D, Headrick JP. Health, pre-disease and critical transition to disease in the psycho-immune-neuroendocrine network: Are there distinct states in the progression from health to major depressive disorder? Physiol Behav. (2019) 198:108–19. doi: 10.1016/j.physbeh.2018.10.014
53. Stapelberg N, Pratt R, Neumann D, Shum D, Brandis S, Muthukkumarasamy V, et al. From feedback loop transitions to biomarkers in the psycho-immune-neuroendocrine network: detecting the critical transition from health to major depression. Neurosci Behav Rev. (2018) 90:1–15. doi: 10.1016/j.neubiorev.2018.03.005
54. Bablis P, Day R, Bablis and H. Pollard S. Treatment of hypothyroidism and stress using neuro-emotional technique (NET): A case study. Cureus. (2024) 164:e58231. doi: 10.7759/cureus.58231
55. Bablis P, Pollard H, Rosner AL. Stress reduction via neuro-emotional technique to achieve the simultaneous resolution of chronic low back pain with multiple inflammatory and biobehavioural indicators: A randomized, double-blinded, placebo controlled trial. J Integr Med. (2022) 202:135–44. doi: 10.1016/j.joim.2021.12.001
56. Monti D, Tobia A, Stoner M, Wintering N, Matthews M, He X, et al. Neuro emotional technique effects on brain physiology in cancer patients with traumatic stress symptoms: preliminary findings. J Cancer Surviv. (2017) 11:438–46. doi: 10.1007/s11764-017-0601-8
57. Bablis P, Pollard H, Monti D. Resolution of anovulation infertility using neuro emotional technique: a report of 3 cases. J Chiropr Med. (2006) 5:13–21. doi: 10.1016/S0899-3467(07)60128-1
58. Bablis P, Pollard H, Bonello R. Neuro Emotional Technique for the treatment of trigger point sensitivity in chronic neck pain sufferers: a controlled clinical trial. Chiropr Osteopat. (2008) 164:4. doi: 10.1186/1746-1340-16-4
59. Karpouzis F, Pollard H, Bonello R. A randomised controlled trial of the Neuro Emotional Technique (NET) for childhood Attention Deficit Hyperactivity Disorder (ADHD): a protocol. Trials. (2009) 10:6. doi: 10.1186/1745-6215-10-6
60. Bablis P, Pollard H. Anxiety and depression profile of 188 consecutive new patients presenting to a Neuro-Emotional Technique practitioner. J Altern Complement Med. (2009) 152:121–7. doi: 10.1089/acm.2007.0805
61. Bablis P, Pollard H. A mind–body treatment for hypothyroid dysfunction: A report of two cases. Complementary Therapies Clin Pract. (2009) 15:67–71. doi: 10.1016/j.ctcp.2009.01.004
62. Bablis P, Pollard H. Hypothyroidism: A new model for conservative management in two cases. Chiropractic J Aust. (2004) 34:11–8.
63. Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. (2009) 71:243–50. doi: 10.1097/PSY.0b013e3181907888
64. Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. (2005) 44:227–39. doi: 10.1348/014466505X29657
65. Antony M, Bieling P, Cox B, Enns and R. Swinson M. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol Assess. (1998) 10:176–81. doi: 10.1037/1040-3590.10.2.176
66. Zung W. A self-rating depression scale. Arch Gen Psychiatry. (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
67. Main CJ. The modified somatic perception questionnaire (MSPQ). P Psychosom Res. (1983) 27:503–4. doi: 10.1016/0022-3999(83)90040-5
68. Serrano-García A, Fernández-González M, Betegón-Nicolás J, Villar-Pérez J, Lozano-Muñoz A, Hernández-Encinas J, et al. Evaluation of dram score as a predictor of poor postoperative outcome in spine surgery. J Clin Med. (2020) 9:3825. doi: 10.3390/jcm9123825
69. Abbott Diabetes Care. Abbott’s freeStyle libre 2 registered for use by children and adults living with diabetes in Australia, in: Abbott diabetes care (2021). Available online at: https://www.freestylelibre.com.au/freestyle-libre-2-registration (Accessed 10 Oct 2023).
70. Basu A, Dube S, Veettil S. Time lag of glucose from intravascular to interstitial compartment in type 1 diabetes. J Diabetes Sci Technol. (2015) 9:63–8. doi: 10.1177/1932296814554797
71. Royal Australian College of General Practioners. Management of type 2 diabetes: A handbook for general practice - Clinical Summary (2020). Available online at: https://www.racgp.org.au/getattachment/7a2da393-b76f-405a-a2fe-0c9c0c0d5ac8/Clinical-summary.pdf.aspx (Accessed 10 Oct 2023).
72. Pinhas-Hamiel O, Hamiel D. Cognitive behavioral therapy and mindfulness-based cognitive therapy in children and adolescents with type 2 diabetes. Curr Diabetes Rep. (2020) 20:55. doi: 10.1007/s11892-020-01345-5
73. Ngan H, Chong Y, Chien W. Effects of mindfulness- and acceptance-based interventions on diabetes distress and glycaemic level in people with type 2 diabetes: Systematic review and meta-analysis. Diabetes Med. (2021) 38:e14525. doi: 10.1111/dme.14525
74. Raveendran A, Chacko E, Pappachan J. Non-pharmacological treatment options in the management of diabetes mellitus. Eur Endocrinol. (2018) 14:31–9. doi: 10.17925/EE.2018.14.2.31
75. Buric I, Farias M, Jong J, Mee and I. Brazil C. What Is the molecular signature of Mind-Body Interventions? A systematic review of gene expression changes induced by meditation and related practices. Front Immunol. (2017) 8:670. doi: 10.3389/fimmu.2017.00670
76. Yang H, Koh E, Sung and H. Kang M. Changes induced by Mind-Body Intervention including epigenetic marks and its effects on diabetes. Int J Mol Sci. (2021) 22:1317. doi: 10.3390/ijms22031317
77. Zhang D, Lee E, Mak E, Ho C, Wong S. Mindfulness-based interventions: an overall review. Br Med Bull. (2021) 1381:41–57. doi: 10.1093/bmb/ldab005
78. Li S, Bressington D. The effects of mindfulness-based stress reduction on depression, anxiety, and stress in older adults: A systematic review and meta-analysis. Int J Ment Health Nurs. (2019) 28:635–56. doi: 10.1111/inm.12568
79. Chew B, Vos R, Metzendorf M, Scholten and G. Rutten R. Psychological interventions for diabetes-related distress in adults with type 2 diabetes mellitus. Cochrane Database Systematic Rev. (2023) 9:CD011469. doi: 10.1002/14651858.CD011469.pub2
80. Fisher V, Li W, Malabu U. The effectiveness of mindfulness-based stress reduction (MBSR) on the mental health, HbA1C, and mindfulness of diabetes patients: A systematic review and meta-analysis of randomised controlled trials. Appl Psychol Health Well Being. (2023) 15:1733–49. doi: 10.1111/aphw.12441
81. McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. (1993) 153:2093–101. doi: 10.1001/archinte.1993.00410180039004
82. Kamradt T, Mitchison N. Tolerance and autoimmunity. N Engl J Med. (2001) 344:655–64. doi: 10.1056/NEJM200103013440907
84. Wentworth J, Fourlanos S, Harrison L. Reappraising the stereotypes of diabetes in the modern diabetogenic environment. Nat Rev Endocrinol. (2009) 5:483–9. doi: 10.1038/nrendo.2009.149
85. Franceschi C, Garagnani P, Parini P, Giuliani and A. Santoro C. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. (2018) 14:576–90. doi: 10.1038/s41574-018-0059-4
86. Donath M, Shoelson S. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. (2011) 11:98–107. doi: 10.1038/nri2925
87. Centre For Youth Wellness. ACE-Q user guide for health professionals. Available online at: https://centerforyouthwellness.org/wp-content/uploads/2018/06/CYW-ACE-Q-USer-Guide-copy.pdf (Accessed 9 September 2023).
88. Holman DM, Ports KA, Buchanan ND. The association between adverse childhood experiences and risk of cancer in adulthood: a systematic review of the literature. Pediatrics. (2016) 138:S81–91. doi: 10.1542/peds.2015-4268L
89. Austin A, Herrick H, Proescholdbell and J. Simmons S. Disability and exposure to high levels of adverse childhood experiences effect on health and risk behavior. N C Med J. (2016) 77:30–6. doi: 10.18043/ncm.77.1.30
90. Chang X, Jiang X, Mkandarwire and M. Shen T. Associations between adverse childhood experiences and health outcomes in adults aged 18–59 years. PloS One. (2019) 14:e0211850. doi: 10.1371/journal.pone.0211850
91. Mariani N, Borsini A, Cecil C, Felix and e. al. JF. Identifying causative mechanisms linking early-life stress to psycho-cardio-metabolic multi-morbidity: The EarlyCause project. PloS One. (2021) 16:e0245475. doi: 10.1371/journal.pone.0245475
92. Oraibi O, Ghalibi A, Shami M, Khawaji M, Madkhali K, Yaseen A, et al. Adverse childhood experience as a risk factor for developing type 2 diabetes among the jazan population: A A cross-sectional study. Children. (2023) 10:499. doi: 10.3390/children10030499
93. Zhu S, Shan S, Liu W, Li S, Hou L, Huang X, et al. Adverse childhood experiences and risk of diabetes: A systematic review and meta-analysis. J Glob Health. (2022) 12:4082. doi: 10.7189/jogh.12.04082
94. Ostrem F. Adverse childhood experiences in diabetes care. Lancet: Diabetes Endocrinol. (2022) 10:695–6. doi: 10.1016/S2213-8587(22)00256-X
95. Deschênes SS, Graham E, Kivimäki and N. Schmitz M. Adverse childhood experiences and the risk of diabetes: examining the roles of depressive symptoms and cardiometabolic dysregulations in the whitehall II cohort study. Diabetes Care. (2018) 41:2120–6. doi: 10.2337/dc18-0932v
96. Berens A, Jensen S, Nelson C3. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. (2017) 15:135. doi: 10.1186/s12916-017-0895-4
97. Dallman M, Strack A, Akana S, Bradbury M, Hanson E, Scribner and M. Smith K. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. (1993) 14:303–47. doi: 10.1006/frne.1993.1010
98. de Candia P, Prattichizzo F, Garavelli S, De Rosa V, Galgani M, Di Rella F, et al. Type 2 diabetes: how much of an autoimmune disease? Front Endocrinol (Lausanne). (2019) 10:451. doi: 10.3389/fendo.2019.00451
99. Miller G, Chen E, Parker K. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. (2011) 137:959–97. doi: 10.1037/a0024768
100. Liu C, Feng X, Li Q, Wang Y, Li and M. Hua Q. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine. (2016) 86:100–9. doi: 10.1016/j.cyto.2016.06.028
101. Danese A, Pariante CM, Caspi A, Taylor and R. Poulton A. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Nat Acad Sci. (2007) 104:1319–24. doi: 10.1073/pnas.0610362104
Keywords: type 2 diabetes (T2D), neuro-emotional technique (NET), psycho-immune-neuroendocrine (PINE) network, adverse childhood experiences (ACEs), allostatic load, stress
Citation: Bablis P, Day RR and Pollard H (2024) Treatment of type 2 diabetes and stress using neuro-emotional technique: case report. Front. Endocrinol. 15:1382757. doi: 10.3389/fendo.2024.1382757
Received: 06 February 2024; Accepted: 24 June 2024;
Published: 10 July 2024.
Edited by:
Habib Yaribeygi, Semnan University of Medical Sciences, IranReviewed by:
Giacomo Rossettini, University of Verona, ItalyEric Chun-Pu Chu, EC Healthcare, Hong Kong SAR, China
Copyright © 2024 Bablis, Day and Pollard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryan R. Day, cmRheUB1bml2ZXJzYWxoZWFsdGguY29tLmF1
†ORCID: Peter Bablis, orcid.org/0000-0002-1574-7153
Ryan R. Day, orcid.org/0009-0008-4070-3460
Henry Pollard, orcid.org/0000-0003-0269-5697
 Peter Bablis
Peter Bablis Ryan R. Day
Ryan R. Day Henry Pollard
Henry Pollard