
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Endocrinol. , 13 May 2024
Sec. Cancer Endocrinology
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1382066
The syndrome of inappropriate antidiuretic hormone secretion (SIADH) is frequent in lung cancer patients. Here, we report a case with persistent hyponatremia, which suggested malignant SIADH and facilitated an early diagnosis of small cell lung cancer (SCLC). A combined radio-chemotherapy led to a partial remission and resolution of SIADH. An early relapse was indicated by reoccurring severe hyponatremia and increased copeptin levels, which were used as surrogate markers for the antidiuretic hormone (ADH). As palliative immunochemotherapy, together with fluid restriction and solute substitution, were unable to control hyponatremia, treatment with the ADH V2-receptor antagonist tolvaptan was initiated. Over time, the dose of tolvaptan needed to be increased, paralleled by a well-documented exponential increase of copeptin levels. In summary and conclusion, this is a rare case of a secondary failure to tolvaptan with unique documentary evidence of increasing copeptin levels. This observation supports the hypothesis that exceedingly high ADH levels may lead to competitive displacement of tolvaptan from the V2 receptor.
Hyponatremia due to the syndrome of inappropriate ADH secretion (SIADH) is frequent in lung cancer patients (1). SIADH results from a variety of conditions such as pain, stress, infections, drugs, and CNS disorders, or it can be of paraneoplastic etiology. Malignant SIADH is caused by extra-hypothalamic production of the antidiuretic hormone (ADH), also called arginine vasopressin (AVP). Alternative causes of hyponatremia, such as adrenal insufficiency, need to be considered and essentially ruled out before SIADH and, more specifically, malignant SIADH can be diagnosed (2, 3).
The laboratory findings of SIADH include reduced serum sodium concentration (Na < 135 mmol/l) and low effective serum osmolality with an inappropriately high urine osmolality and continued renal sodium excretion, i.e., > 30 mmol/l (2, 4). Particularly in patients on diuretics, the fractional uric acid excretion with a cut-off of ≥ 12% has an excellent diagnostic value for the diagnosis of SIADH (5). Nonetheless, SIADH remains a diagnosis of exclusion, and low solute intake in relativity to water intake is an important differential diagnosis (2).
Copeptin, the c-terminal part of the precursor peptide of ADH, is a reliable surrogate marker for the less stable ADH (6). Copeptin has a limited value to differentiate between SIADH and other causes of hyponatremia, such as hypovolemic and hypervolemic hyponatremia (7), and mean copeptin values do not differ in SIADH of malignancy versus SIADH of other causes. Nevertheless, the highest measured values were found in malignant SIADH (8).
Tolvaptan is an orally effective ADH V2-receptor antagonist, which adds to the armamentarium to treat paraneoplastic SIADH, mainly if fluid restriction, salt tablets, urea, or hypertonic saline are ineffective or poorly tolerated. Considering the high price of this drug, it should be used judiciously. Tolvaptan binds competitively with 1.8 times greater affinity than ADH to the human V2-receptor (9), which results in decreased aquaporin-2 molecules in the apical membrane and thereby decreased permeability for water (9, 10). As a result, free water clearance is normalized and allows correction of hyponatremia.
In the present study, we report a case of a patient with paraneoplastic SIADH due to SCLC with secondary failure to tolvaptan paralleled by a sharp increase in copeptin levels.
A 63-year-old woman was hospitalized in October 2019 with hyponatremia of 122 mmol/l. She was diagnosed with migraine, and the hyponatremia without a thorough workup was interpreted as pain-induced SIADH. Fluid restriction raised the serum sodium concentration to 130 mmol/l, and the patient was discharged with a fixed dose of flunarizine to treat her migraine and supplementary rizatriptan for acute attacks.
Two months later, in December 2019, the patient was readmitted with severe hyponatremia of 113 mmol/l. She suffered from headaches, nausea, and vertigo. Her serum osmolality was 236 mOsm/kg with a urine osmolality of 464 mOsm/kg and urine sodium < 20 mmol/l, which supported the clinical diagnosis of hypovolemia. After the attempt to correct hypovolemia with chicken soup and bouillon, the urine sodium concentration increased to 113 mmol/l, but serum sodium remained low at 120 mmol/l. Together with a high fractional excretion of uric acid of 32%, we diagnosed concomitant and persistent SIADH. In addition to fluid restriction, we initiated therapy with urea to facilitate free water clearance. Further, workup in this former smoker included a CT scan of the thorax (not shown) followed by a PET-CT scan (Figure 1), which revealed a pulmonary lesion in the right upper lobe. The biopsy obtained from the tumor resulted in the diagnosis of limited disease SCLC.
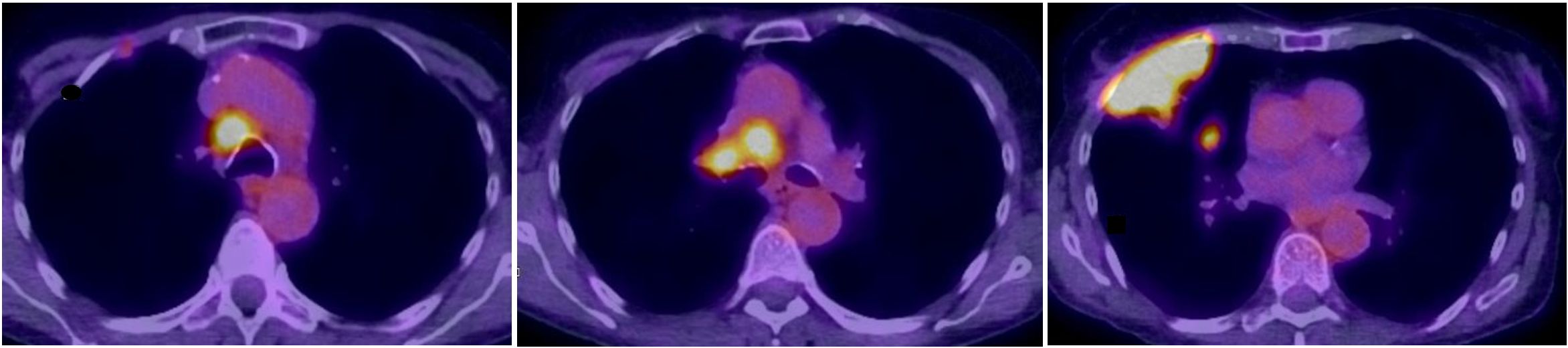
Figure 1 Fusion FDG-PET/CT axial slices with a large lesion in the upper lobe infiltrating the thoracic wall with a small lung metastasis and pathologic high FDG-uptake in mediastinal, hilar lymph nodes.
The patient received a combined radio-chemotherapy with cisplatin and etoposide between January and April 2020. During that period, she demonstrated a good partial remission, and her sodium levels remained between 130 and 141 mmol/l from March to June 2020. After that, the patient showed a drop of her serum sodium concentration to a minimum of 113 mmol/l. A CT scan of the thorax and abdomen revealed new nodules in the lung (not shown), multiple lesions in the liver, and one lesion in the pancreas (Figure 2A). The cerebral MRI did not indicate cerebral metastases. We obtained a liver biopsy, and the pathological examination was consistent with SCLC. The patient received palliative immunochemotherapy with atezolizumab, carboplatin, and etoposide. With fluid restriction, bouillon, and urea, serum sodium concentration increased to 124 mmol/l.
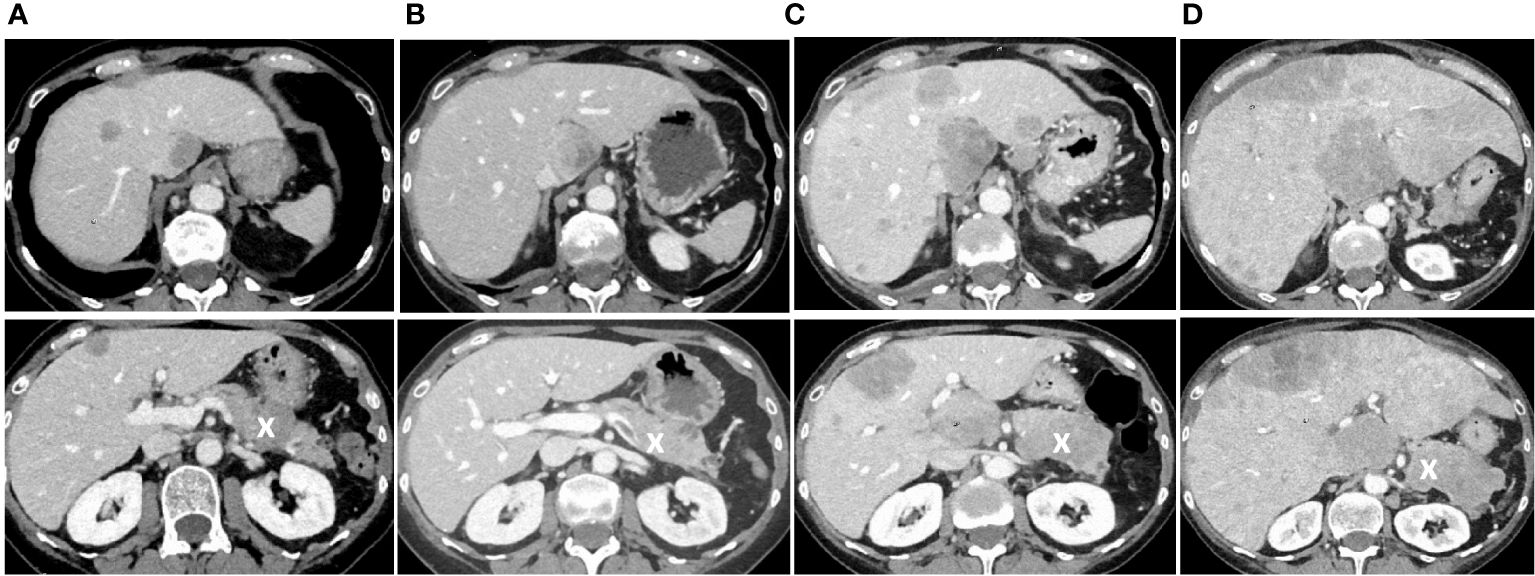
Figure 2 Follow-up CT scans showing tumor progression with hepatic and pancreatic metastases. (A) Follow-up CT scan three months after the end of the initial chemotherapy showing new liver metastases and a new lesion in the pancreas, marked with an X. (B) Follow-up CT scan seven months after the end of initial chemotherapy at the time when increasing doses of tolvaptan were necessary to control hyponatremia. (C) CT scan nine months after the end of chemotherapy at a time when tolvaptan was not effective anymore, and one month later (D) when the patient had beginning signs of liver failure and died shortly after that. In all images, the pancreas is marked with an X.
In September 2020, the patient had a urine osmolality of 813 mOsm/kg and did not respond sufficiently to urea and hypertonic saline solution (NaCl 3%) (Figure 3). Therefore, we initiated tolvaptan, starting with a dose of 15 mg. We observed a rapid increase in urine volume, and urine osmolality dropped to 50 mosmol/l within hours, requiring a volume substitution with electrolyte-free water, e.g. glucose 5%. Serum sodium increased from 124 mmol/l to 134 mmol/l within 24 hours (Figure 3). In the follow-up, increasing doses of tolvaptan - paralleled with a rapid increase of copeptin levels (Figure 4) - were necessary to control hyponatremia. To better interpret and classify the copeptin levels of our patient, we analyzed 932 copeptin concentrations measured in clinical routine of all patients from January 2019 to March 2021 (B·R·A·H·M·S Copeptin proAVP KRYPTOR assay). Of these values, 92% ranged between 0 and 50 pmol/l, 4.7% between 50 and 100 pmol/l, and 1.5% from 100 to 150 pmol/l, respectively. Five out of the 15 values above 150 pmol/l and the highest three values were measured in our patient.
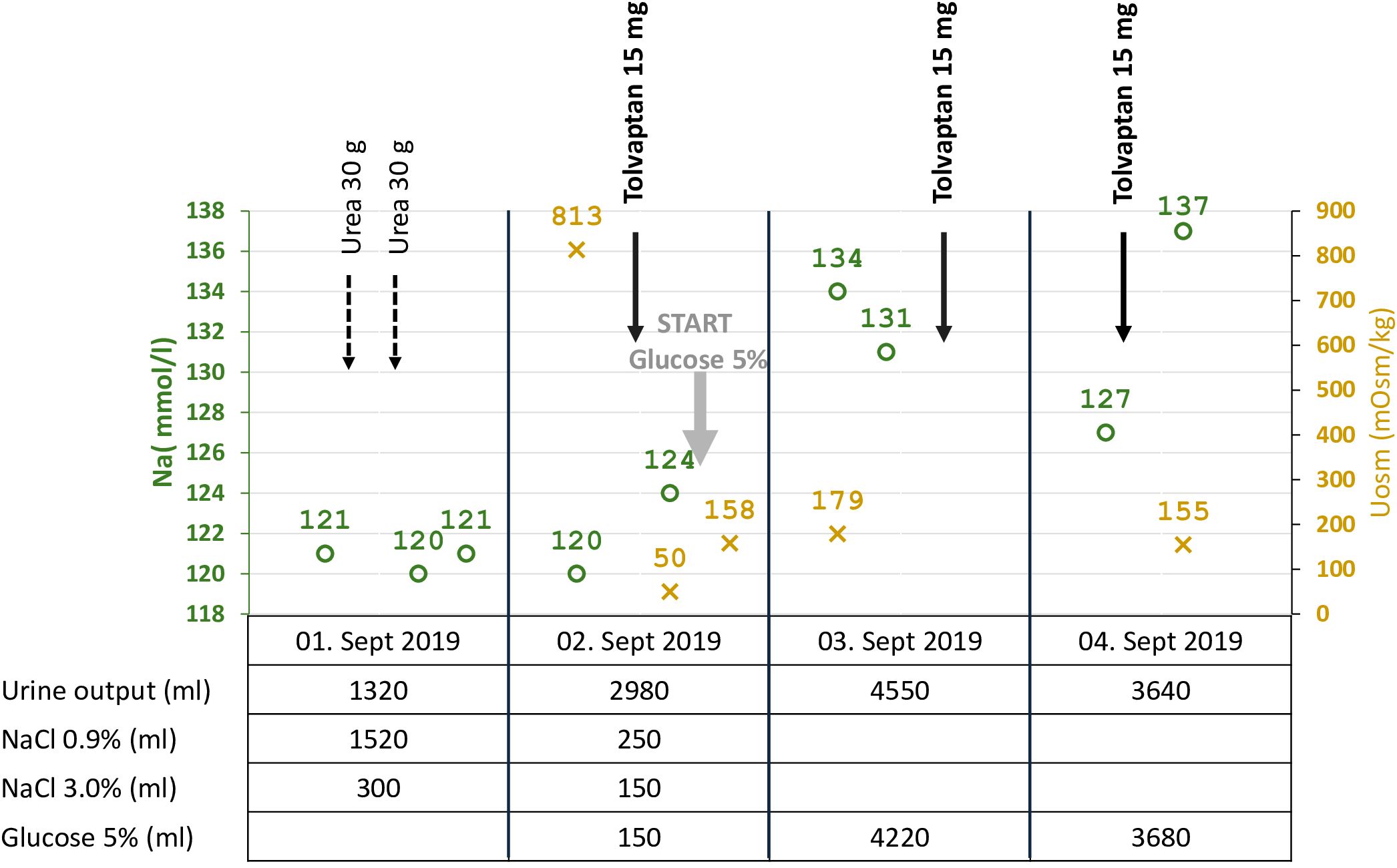
Figure 3 Start of tolvaptan: Serum sodium, urine osmolality, urine output, and volume/solute management. Summary of critical data before and after the start of tolvaptan. Within a few hours after the first dose of tolvaptan, the urine volume increased, paralleled by a drop in urine osmolarity from 813 to 50 mOsm/kg, and substitution of electrolyte-free water (glucose 5%) was started at a serum sodium concentration of 124 mmol/l to prevent too fast correction of hyponatremia.
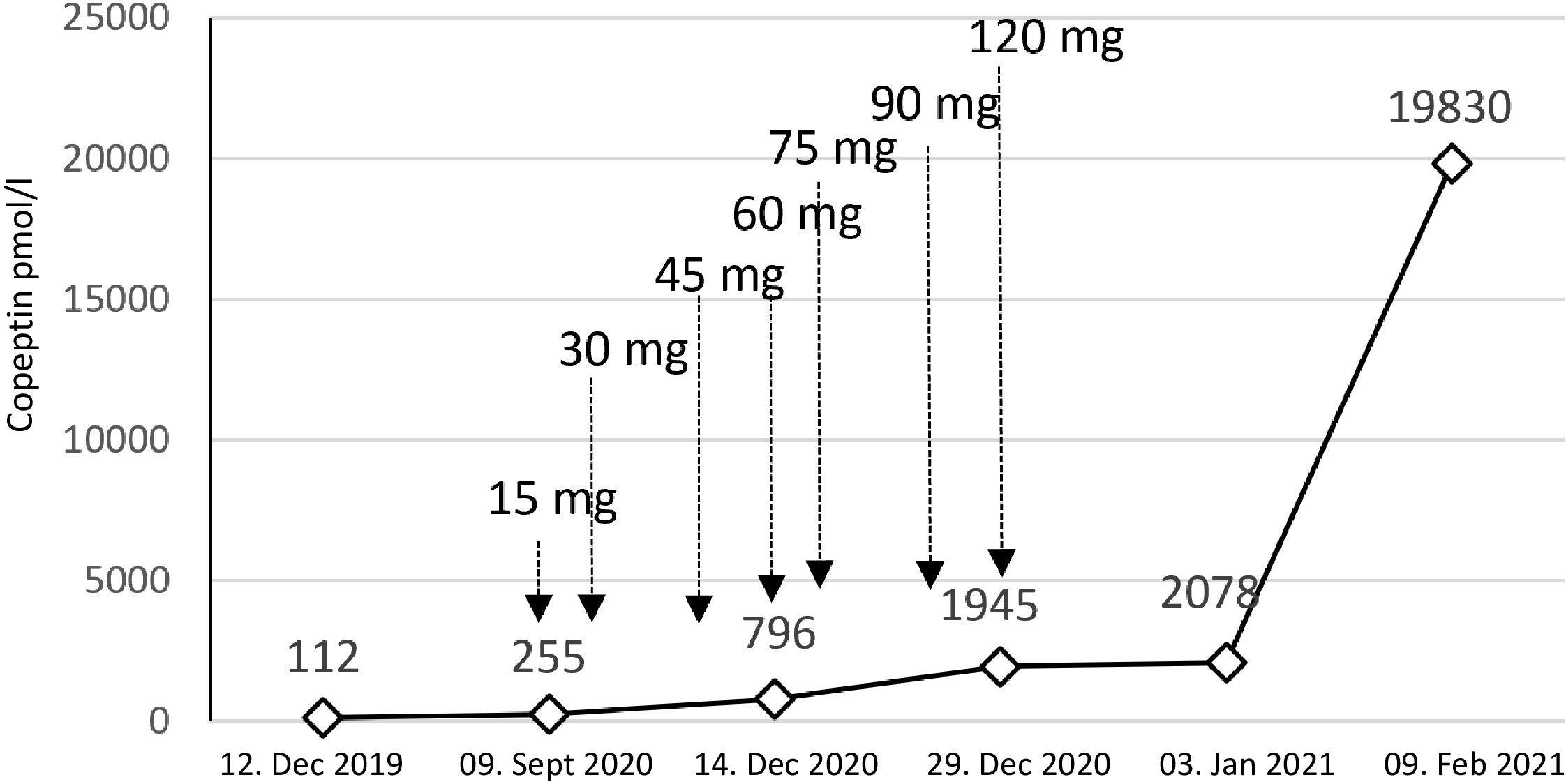
Figure 4 Copeptin values and tolvaptan doses. The figure shows the dramatic increase in copeptin over time. Treatment with tolvaptan was started with a dose of 15 mg per day. We needed to rapidly increase the dose to a maximum of 120 mg per day.
During the further follow-up, the patient’s symptomatic hyponatremia with migraine attacks led to frequent hospitalizations. We registered a peak copeptin value of 19’830 pmol/l (Figure 4), and repeated CT scans showed further progression of the metastatic SCLC (Figure 2B-D). The patient died due to disseminated liver metastases and liver failure in February 2021.
In the case reported here, persistent hyponatremia suggested malignant SIADH and facilitated the diagnosis of SCLC. A combined radio-chemotherapy led to a partial remission and resolution of SIADH during a few months. An early relapse was indicated by reoccurring severe hyponatremia, after which we started therapy with tolvaptan at a time when palliative immunochemotherapy, together with fluid restriction and solute substitution, was unable to control hyponatremia. The initial response to 15 mg tolvaptan was strong, and the substitution of free water was necessary to prevent rapid correction of hyponatremia with the risk of osmotic demyelination (Figure 3). This suggests, that a starting dose of 7.5 mg tolvaptan may be preferred over 15 mg in the setting of chronic SIADH as the lower dose may be sufficient and the risk of a too rapid correction is diminished. Over time, the dose of tolvaptan needed to be increased, which can be explained by a well-documented exponential increase in copeptin levels (Figure 4). Although a strong correlation between ADH levels and tumor burden has not been observed consistently (11), repetitive CT scans in our patient (Figure 2) showed a link between increasing copeptin levels and tumor progression. Mechanistically, very high ADH levels may result in a competitive replacement of tolvaptan at the V2 receptor, as suggested in previous cases of two SCLC patients (12).
Following its approval in 2009, tolvaptan has been successfully applied in patients with SIADH due to SCLC. The first case reports were published in 2011 (13, 14). Amongst published cases, we identified four patients with SCLC and SIADH, where the initial effect of tolvaptan vanished (12, 15, 16). In two cases published in 2018 (3, 12), the patients showed an initial response to fluid restriction. Later, they were started on tolvaptan 7.5 mg daily with a good response. However, with cancer progression, tolvaptan with doses of up to 60 mg daily was not effective anymore. In the second patient, the copeptin value was 6088 pmol/l, i.e., extremely high as in the patient reported here. In 2019, a third case was reported (16) of a 48-year-old male with recurrent metastatic SCLC and hyponatremia of 107 mmol/l. Serum Na raised to 125 mmol/l with fluid restriction and NaCl 3%. He was then started on tolvaptan 15 mg daily, which was increased to 30 mg, then 60 mg, and finally to twice daily 60 mg without a response, and urine osmolality remained high at > 900 mOsm/kg. Tolvaptan treatment was discontinued, and serum sodium rose to 140 mmol/l with increasing doses of urea (up to 60 g per day), oral NaCl, and KCl combined with furosemide. A fourth case reported in 2022 (15) described a 61-year-old male with a locally advanced SCLC. He had symptomatic hyponatremia of 111 mmol/l. Symptoms subsided after receiving hypertonic saline, and sodium raised to 120 mmol/l. After that, he received tolvaptan 30 mg daily. During the next three months, he was readmitted multiple times with acute on chronic hyponatremia despite an increase of tolvaptan to 45 mg. Treating physicians then diagnosed disease progression with new liver metastases.
In summary, this case illustrates the importance of identifying underlying malignancy in a patient with persistent SIADH, as it can be the first manifestation of a malignant disease. When anticancer therapy and supportive measures fail to treat hyponatremia, a V2 receptor antagonist such as tolvaptan may enable the correction of hyponatremia, but this requires careful observation of the treatment response. This case demonstrates the rare observation of a secondary failure to tolvaptan associated with tumor progression and exponentially increasing copeptin levels, which supports the hypothesis of competitive displacement of tolvaptan from the V2 receptor. In conclusion, resistance to tolvaptan in malignant SIADH and a rise in copeptin levels, if measured, may be used as markers of tumor progression.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SM: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation, Formal analysis, Data curation, Conceptualization. SJ: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation. SP: Writing – review & editing, Visualization. HS: Writing – review & editing, Validation. AJ: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Burst V, Grundmann F, Kubacki T, Greenberg D, Rudolf A, Salahudeen J, et al. Euvolemic hyponatremia in cancer patients. Report of the Hyponatremia Registry: an observational multicenter international study. Support Care Cancer. (2017) 25:2275–83. doi: 10.1007/s00520-017-3638-3
2. Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol Dial Transplant. (2014) 29 Suppl 2:i1–i39. doi: 10.1093/ndt/gfu040
3. Garrahy A, Cuesta M, Thompson CJ. Physiopathology, Diagnosis, and Treatment of Inappropriate ADH Secretion and Cerebral Salt Wasting Syndrome. Casanueva FF, Ghigo E, editors. Springer Cham: Hypotalamic-Pituitary Diseases: Springer (2018) p. 405–31.
4. Sorensen JB, Andersen MK, Hansen HH. Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in Malignant disease. J Intern Med. (1995) 238:97–110. doi: 10.1111/j.1365-2796.1995.tb00907.x
5. Fenske W, Stork S, Koschker AC, Blechschmidt A, Lorenz D, Wortmann S, et al. Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab. (2008) 93:2991–7. doi: 10.1210/jc.2008-0330
6. Christ-Crain M, Fenske W. Copeptin in the diagnosis of vasopressin-dependent disorders of fluid homeostasis. Nat Rev Endocrinol. (2016) 12:168–76. doi: 10.1038/nrendo.2015.224
7. Nigro N, Winzeler B, Suter-Widmer I, Schuetz P, Arici B, Bally M, et al. Evaluation of copeptin and commonly used laboratory parameters for the differential diagnosis of profound hyponatraemia in hospitalized patients: ‘The Co-MED Study’. Clin Endocrinol (Oxf). (2017) 86:456–62. doi: 10.1111/cen.2017.86.issue-3
8. Winzeler B, Steinmetz M, Refardt J, Cesana-Nigro N, Popovic M, Fenske W, et al. Copeptin is not useful as a marker of Malignant disease in the syndrome of inappropriate antidiuresis. Endocr Connect. (2020) 9:20–7. doi: 10.1530/EC-19-0431
10. Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. (2006) 355:2099–112. doi: 10.1056/NEJMoa065181
11. Workeneh BT, Jhaveri KD, Rondon-Berrios H. Hyponatremia in the cancer patient. Kidney Int. (2020) 98:870–82. doi: 10.1016/j.kint.2020.05.015
12. Garrahy A, Hannon AM, Zia-Ul-Hussnain HM, Williams DJ, Williams CJ. Secondary resistance to tolvaptan in two patients with SIAD due to small cell lung cancer. Eur J Clin Pharmacol. (2018) 74:245–6. doi: 10.1007/s00228-017-2363-7
13. Gross P, Benzing T, Hensen J, Mönig H. [Practical approach to hyponatremia]. Dtsch Med Wochenschr. (2011) 136:1728–32. doi: 10.1055/s-0031-1286066
14. Wakil A, Ng JM, Atkin SL. Investigating hyponatraemia. BMJ. (2011) 342:d1118. doi: 10.1136/bmj.d1118
15. Mandal S, Kannan L. SIADH escape or tolvaptan resistance in progressive small cell lung cancer. J Am Soc Nephrol. (2022) 33:736.
Keywords: hyponatremia, SIADH, antidiuretic hormone, ADH, copeptin, SCLC, lung cancer, tolvaptan
Citation: Menzi S, Jaramillo SD, Pfister S, Schefer H and Jehle AW (2024) Case report: Secondary failure to tolvaptan in a patient with SCLC and paraneoplastic SIADH. Front. Endocrinol. 15:1382066. doi: 10.3389/fendo.2024.1382066
Received: 04 February 2024; Accepted: 22 April 2024;
Published: 13 May 2024.
Edited by:
Andreas Serra, ETH Zürich, SwitzerlandReviewed by:
Stefan Russmann, University of Nicosia, CyprusCopyright © 2024 Menzi, Jaramillo, Pfister, Schefer and Jehle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Werner Jehle, YW5kcmVhcy5qZWhsZUBoaXJzbGFuZGVuLmNo; YW5kcmVhcy5qZWhsZUB1bmliYXMuY2g=
†Present address: Silvia Daniela Jaramillo, Laboratory Medicine, Cantonal Hospital Lucerne, Lucerne, Switzerland
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.