- 1Reproductive Medicine Center, The Affiliated Chenggong Hospital of Xiamen University, Xiamen, Fujian, China
- 2Medical College, Xiamen University, Xiamen, Fujian, China
Objective: To investigate whether using Zishen Yutai Pills (ZYP) following embryo transfer would affect the live birth rate in frozen-thawed embryo transfer (FET) cycles.
Methods: A retrospective analysis was performed on 15044 FET cycles in the Reproductive Medicine Center of The Affiliated Chenggong Hospital of Xiamen University from January 2013 to December 2020. Patients who used Zishen Yutai Pills were defined as Zishen Yutai Pills Group (ZYP, n=2735), while patients who did not use them were defined as Non- Zishen Yutai Pills Group (Non-ZYP, n=12309). The propensity score matching method was used to control for potential confounders between the two groups, and logistic regression analysis was also used to assess whether using ZYP would affect the live birth rate.
Results: After propensity score matching, basic characteristics were similar between the two groups. Using ZYP did not increase the pregnancy rate (51.5% vs. 52.7%, P=0.372), and live birth rate (43.0% vs. 44.7%, P=0.354). This was also confirmed by the logistic regression analysis results (OR=0.95, 95%CI=0.85-1.06). In the subgroup analysis of the endometrial preparation protocols, however, it was found that the use of ZYP in patients with natural cycles increased the live birth rate (47.4% vs. 41.5%, P=0.004). A significant interaction between endometrial preparation and ZYP was found (OR=1.38, 95%CI=1.07-1.79) in the multivariate model.
Conclusion: The use of ZYP may not improve the live birth rate of unselected patients in FET cycles. However, a future study is needed on the effect of ZYP in natural cycles for endometrial preparation.
Introduction
Assisted reproductive technologies (ART) provide an important option for infertility treatment, but they are also expensive, complex, and not without significant risk of failure (1). Many adjuvants and supplements were used in the procedure of ART and infertility treatment in an attempt to optimize the pregnancy outcome or improve the live birth rate (2). Herbal therapy represents an important aspect of the use of adjuvants and supplements in infertility treatment. A recent survey suggested that over two-thirds of women seeking infertility used herbal therapy previously and over half of women used herbal medicine and supplement currently (3). In China and East Asia, Chinese herbal medicine (CHM) has been used to treat various diseases including infertility, and is reported to enhance the success of live births in infertile couples treated with ART (4–7), possibly by improving oocyte quality, ovarian function, and endometrial receptivity (8). However, the evidence remained biased and heterogeneous, warranting further investigations to justify the use of CHMs in ART treatment.
Zishen Yutai Pill (ZYP) is one of the few representative CHMs that are supported by RCT evidence (9–11). A recent meta-analysis of RCTs suggested that ZYP may improve clinical symptoms such as human chorionic gonadotropin, progesterone, estradiol, duration of abdominal pain, duration of vaginal bleeding, and fibrinogen in women with threatened miscarriage (12). For patients receiving ART treatment, a rate ratio of 1.14 (95% CI 1.00–1.30) for clinical pregnancy in comparison with placebo treatment was reported when ZYP was administrated from the preceding menstrual cycle of the fresh embryo transfer (13). These data suggested that ZYP may play a role in complementary treatment in ART. However, it is still less known whether the use of ZYP would benefit ART-related clinical scenarios other than fresh embryo transfer, such as patients receiving frozen embryo transfer (FET).
FET is an important scenario of ART treatment. In the past decade, with the improvement of cryopreservation technology and increasing demand to reduce ovarian hyperstimulation syndrome, promote single embryo transfer, optimize endometrial receptivity, and preserve fertility, more and more patients choose FET (14). There is still a lack of evidence supporting the use of ZYP in FET cycles. The present study aims to investigate whether ZYP has an impact on live birth rates in people with FET cycles. In this study, 15044 FET cycles were included, of which 2735 cycles used ZYP. A propensity score matching method was used to account for comparability between the two groups by balancing the biases and confounders, and binary logistic regression was carried out to confirm the findings.
Materials and methods
Institutional review board approval for this study was obtained from The Affiliated Chenggong Hospital of Xiamen University. Informed consent was not necessary because this retrospective research was based on non-identifiable records.
Study subjects
This retrospective study was performed at the reproductive medicine center of The Affiliated Chenggong Hospital of Xiamen University. Patients who underwent FET cycles from 2013 to 2020 were enrolled and retrospectively analyzed. Exclusion criteria:(a) cycles with missing or incomplete data, (b) patients with uterine factors like uterine adhesion, uterine autonomy, and previous cesarean scar diverticulum (PCSD). A total of 15,044 FET cycles were included, of which 2,735 cycles used ZYP. Cycles using ZYP were defined as the ZYP group and cycles without ZYP were defined as the non-ZYP group.
Embryo vitrification and thawing
Ovarian stimulation protocols, embryo culture procedures, and vitrification protocol used for embryo cryopreservation were all as we previously described (15), and embryo thawing was carried out using the corresponding thawing kit. Embryos were placed in the blastocyst culture medium (K-SIBM, Cook) and cultured in an incubator at 37°C with 6% CO2 until transfer. Survival of thawed embryos was assessed under an inverted microscope depending on whether blastocysts showed severely damaged cellular content or not.
Endometrial preparation and embryo transfer
The natural cycle (NC) or modified natural cycle (mNC), the hormone replacement therapy (HRT) cycle, and the HRT cycle in combination with gonadotropin-releasing hormone agonist (GnRHa) downregulation were used for endometrial preparation.
In NC cycles, transvaginal ultrasonography was used to track follicle growth from cycle days 9 to 11. Luteinizing hormone and estradiol levels were checked every three days once the leading follicle’s diameter reached 14 mm. The day when a spontaneous LH surge was noted was considered as the ovulation day (day 0) and luteal phase support was started on the same day. Blastocyst transfer was scheduled for the fifth day after ovulation and cleavage period embryo transfer was scheduled for the third day after ovulation. In cases of modified NC cycles, patients were given 6500 units of HCG on the day of the LH surge and an intramuscular progesterone injection of 40 mg/day was also started on the same day, the second day after the LH surge was considered as ovulation day (day 0) and embryo transfer was scheduled.
In HRT cycles, administration of oral estradiol valerate was carried out using a daily dose of 6 mg between cycle days 1 and 14. As soon as the endometrial thickness reached 7-8 mm (designated as day 0), a progesterone injection (40 mg) was administered.
In GnRHa-HRT cycles, GnRHa was injected on the first day of the menstrual cycle, and estrogen administration was initiated on the first day of the second menstrual cycle.
For all cycles, embryo transfers were carried out on days 3 or 5 under transabdominal ultrasound guidance using a Guardia Access Embryo Transfer catheter (K-JETS-7019-SIVF, Cook, IN, USA). If there was no available blastocyst on day 5, and day 6 transfers were scheduled. Up to week ten of pregnancy, luteal support was maintained. Patients with an elevated hCG level underwent an ultrasound examination 28 days after embryo transfer, and the gestational sac was determined to be a clinical pregnancy.
ZYP medication
ZYP (Guangzhou BaiyunshanZhongyi Pharmaceutical Co., Ltd., Sinopharm Z44020008, Specifications: 5g/bag, 6 bags/box) was used on the day of embryo transfer in FET cycles, 3 times/day, 5g/time, until the 14th day after embryo transfer when the biochemical pregnancy test was carried out. The treatment continued for 3 months if the pregnancy was confirmed. The daily dosage adheres to the manufacturer`s instructions (https://www.gz111.com/product/41.htm) and previous studies (13, 16). Because there were no clear indications for the use of ZYP in FET cycles by the time of the study, the assignment of patients was based on the preference of the clinicians.
Statistical analysis
The primary outcome was the live birth rate (LBR). Secondary outcomes of the study included clinical pregnancy rate (PR) and miscarriage rate (MR).
To minimize the effect of confounders and covariates, the outcomes were evaluated in both a propensity score (PS) matched cohort and an unmatched cohort. The selection of covariates was based on experience and existing knowledge with the guidance of a directed acyclic graph with DAGitty software (Supplementary Figure S1). Potential confounders and selection biases were accounted for by propensity score matching (17).
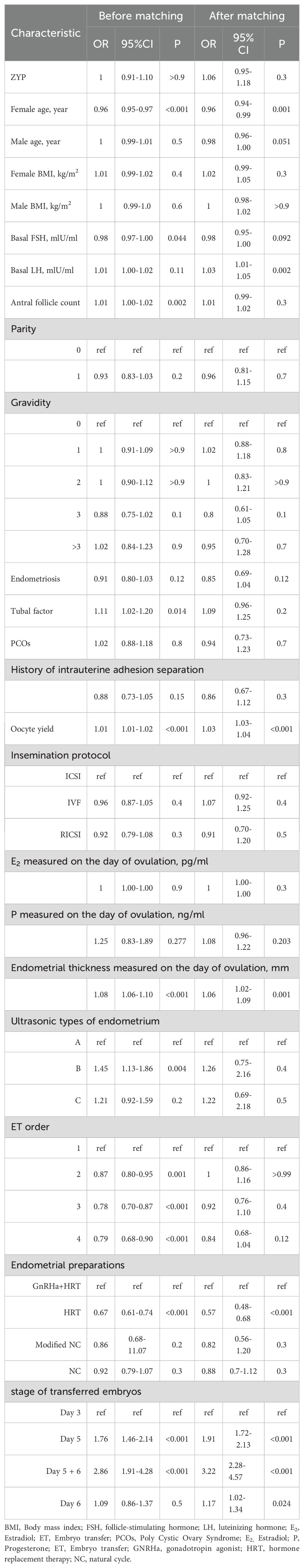
Propensity scores were calculated using logistic regression based on potential variables related to the prognosis (18). The variables included age, Body Mass Index (BMI), basal endocrine parameters (FSH, LH, and AFC), previous maternal history, infertility-related diagnoses (including polycystic ovary syndrome [PCOS], history of intrauterine adhesion separation, endometriosis, and tubal factor), previous embryo transfer attempts, endometrial preparation protocols, oocyte yield, insemination protocols, E2 level, and endometrial thickness measured on the day of ovulation, ultrasonic types of the endometrium, the number and quality of the transferred embryo, the day of transfer. A one-to-one nearest neighbor matching method without substitution is performed to match data between group ZYP and non-ZYP with a caliper width equal to 0.03 (19). To investigate whether differences in medication regimens between ZYP and non-ZYP groups and the day of embryo transfer affected live birth, the two groups were stratified and PS was matched separately for each subgroup. Standard differences (D) were calculated to evaluate the balance of the distribution of the baseline characteristics between the two groups before and after PS matching using a cobalt package. D < 0.1 was used as the threshold to indicate a negligible difference in the mean or prevalence of a covariate between exposure groups. The distribution of propensity scores was also identical after matching (Supplementary Figure S2).
Multivariate logistic regression was used to confirm the outcome of PS matching. A multivariate logistic analysis was carried out following matching in an attempt to adjust for potential residual confoundings. We also used binary logistic regression analysis to assess the association between ZYP and live birth and adjusted for important covariates and potential confounders.
Continuous variables are represented by medians (first quartile, third quartile), while categorical variables are represented as n (%). Continuous variables were analyzed using the Wilcoxon test, and categorical variables were analyzed using the Chi-square test or Fisher’s exact test, P <0.05 was considered to be significant. All analyses were performed in R.
Results
Comparison of baseline data between ZYP group and Non-ZYP group before and after PSM
A total of 15,044 patients were collected, including 12,309 cases in the non-ZYP group and 2,735 cases in the ZYP group. As shown in Table 1, there were significant differences in the data before matching, including the female age, male age, female BMI, parity, PCOS, history of intrauterine adhesion separation, oocyte yield, E2 level measured on the day of ovulation, ultrasonic types of the endometrium, order of ET, endometrial preparation protocols, stage of transferred embryos (P<0.05). After PS matching, there were 2733 cases in the ZYP group and 2733 cases in the non-ZYP group, and there was no significant statistical significance in all indicators (D<0.1). Distributions of the PSs before and after PS matching were shown in Supplementary Material (Supplementary Figure S2).
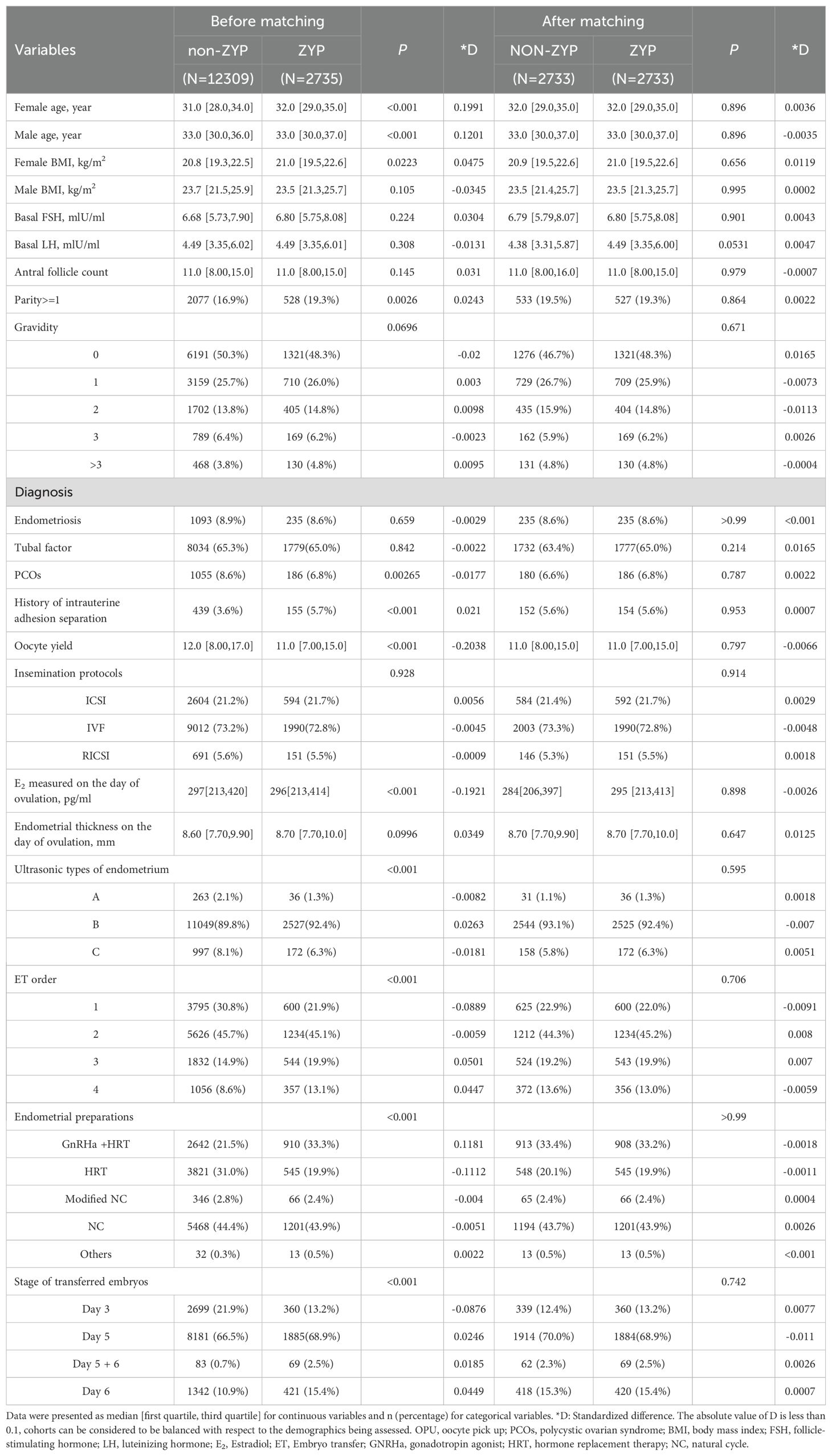
Table 1. Comparison of baseline characteristics between ZYP group and Non-ZYP group before and after PSM.
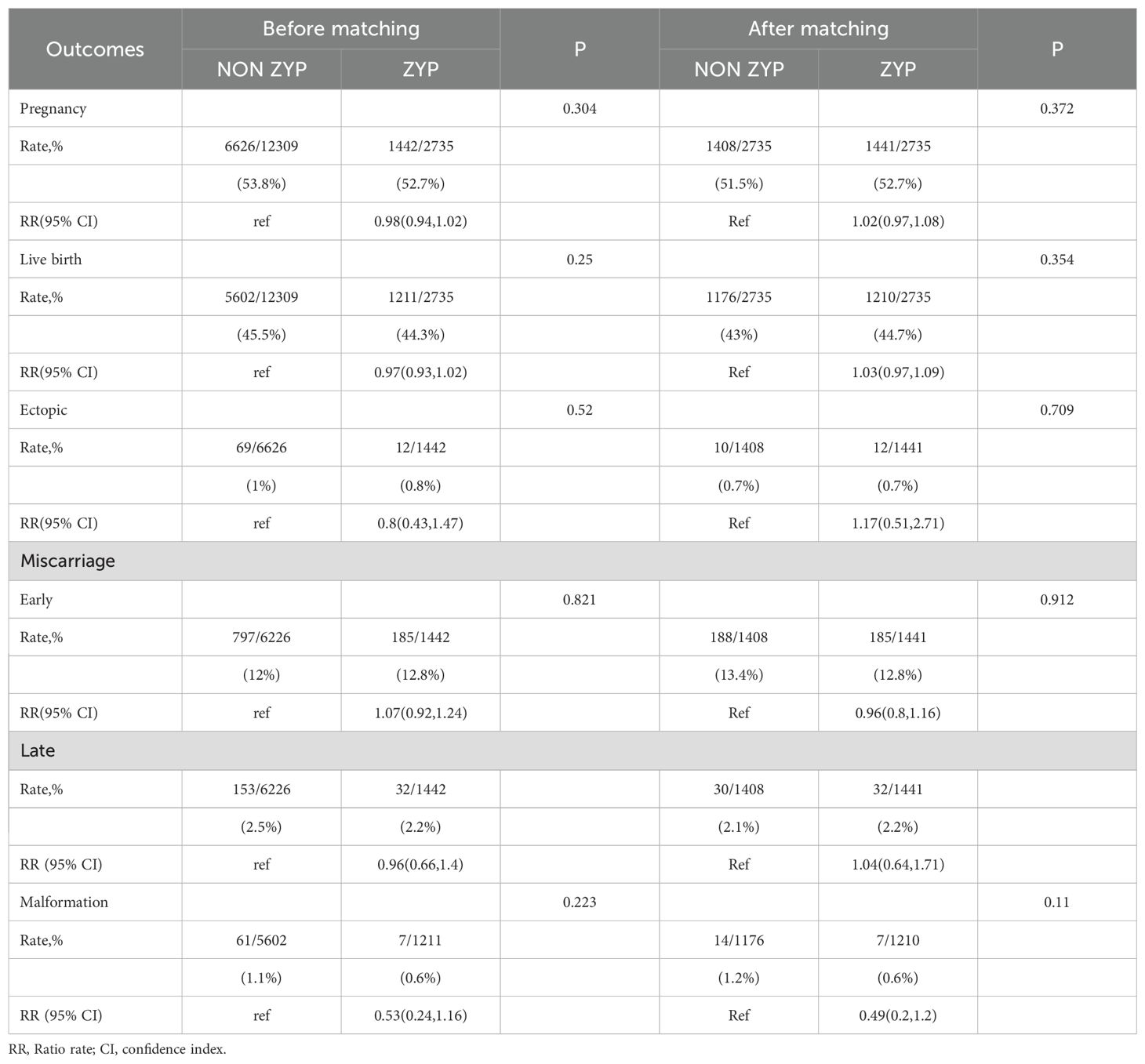
Comparison of pregnancy outcomes between ZYP and non-ZYP groups before and after PSM
Results in Table 2 showed that administration of ZYP does not appear to affect clinical outcomes. Before matching, pregnancy rates [52.7% vs 53.8%, RR=0.98, (95% CI 0.94-1.02)], live birth rates [44.3% vs 45.5%, RR=0.97, (95%CI 0.93-1.02)], ectopic rates [0.8% vs 1.0%, RR=0.8, (95%CI 0.43-1.47)], and malformation rates [0.6% vs 1.1%, RR=0.53, (95% CI, 0.24-1.16)] were slightly lower in ZYP group, and the early miscarriage rate (15.0% vs 14.5%) was slightly higher than that in the non-ZYP group, but there was no significant difference (P>0.05). After matching, the pregnancy rates [52.7%vs51.5%, RR=1.02, (95% CI, 0.97-1.08)], live birth rates [44.7%vs43.0%, RR=1.03, (95% CI, 0.97-1.09)], ectopic pregnancy rates [0.8%vs0.7%, RR=1.17, (95% CI, 0.51-2.71)], and late miscarriage rates [2.2%vs2.1%, RR=1.04, (95% CI, 0.64-1.71)] were higher in the ZYP group, and the early miscarriage rates [12.8%vs13.4%, RR=0.96, (95% CI, 0.8-1.16)], and malformation rates [0.6%vs1.2%, RR=0.49, (95% CI, 0.2-1.2)] were slightly lower than those in the non-ZYP group. None of the differences were statistically significant (P>0.05).
Summary of binary logistic regression analysis results
The binary logistic regression analysis with whether or not live birth was the dependent variable, and the Hosmer-Lemeshow test of the final model showed that the model fit was good. The adjusted OR value of the pre-matching ZYP group for the non-ZYP group was 1.27 (95% CI=0.94~1.70). After matching, the OR value was 0.95 (95% CI=0.85~1.06), the regression coefficient was -0.049 (P=0.391), and the difference was not significant. Results were shown in Table 3.
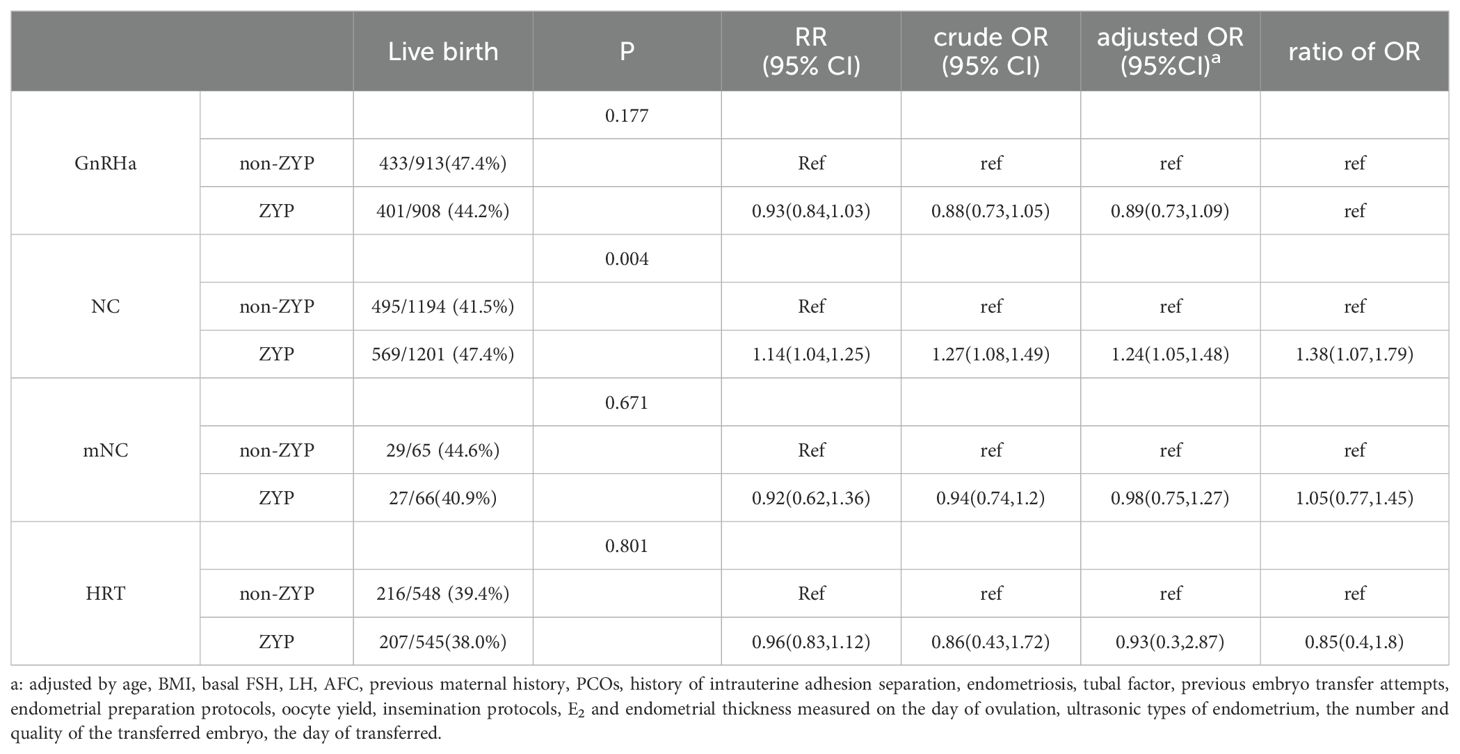
Endometrial preparation protocols of subgroup analysis
When stratified according to endometrial preparation protocols, an increase in live birth rates in natural cycle patients was associated with ZYP use (P<0.05). In NC cycles, the OR that described the association between live birth and ZYP treatment increased was 1.14 times (RR = 1.14, 95% Cl 1.04-1.25) than that in the GnRH-HRT cycles, indicating that the live birth rate in the ZYP group was 1.14 times that of the non-group. The use of ZYP benefited more natural cycle patients, resulting in a 5.9% increase in live birth rates (41.5% vs. 47.4%). The association between other endometrial preparations and live birth rate was not significant. Results were shown in Table 4.
Discussion
Similar to many of its CHM counterparts, ZYP is considered to be a multi-functional adjuvant therapy for reproductive medicine and infertility treatment (20). Its role may include a curative effect for luteal dysfunction, miscarriage, and recurrent spontaneous abortion, which may be relevant to embryo implantation and development following transfer. These effects may theoretically benefit the patients receiving FET. In the present study, we found a neutral effect of administrating ZYP following embryo transfer in FET cycles. The evidence may not support the routine use of ZYP in FET cycles as an adjuvant during early luteal phase support. However, for patients receiving natural cycle endometrial preparation, a positive association between ZYP administration and live birth was found.
Previous studies showed that the positive effects of ZYP treatment on IVF outcomes are exclusively based on fresh embryo transfer cycles, and the duration of ZYP treatment is much longer than the present study. In fresh cycles, patients generally start using ZYP before oocyte retrieval. For instance, in the study of Chen et al. (13), patients receiving the gonadotropin-releasing hormone analogs long protocol, and both the ZYP and placebo were administered orally three times per day in a dose of 5 g from the day of down-regulation. For patients receiving GnRH antagonist protocol, both the ZYP and placebo were administered orally three times per day in a dose of 5 g from day 19-23 in a previous cycle until the day of the pregnancy test (2 weeks after ET). Patients with long protocol, also oral ZYP from the date of down-regulation, 5 g/time, 3 times/d until 35 days after embryo transfer in Bai’s study (21). The early start of ZYP treatment allows the adjuvant to target several reproduction-related processes of the cycle, including follicular development, endometrial growth, and embryo implantation. Clinical observations showed that ZYP may benefit the patient by increasing the oocyte yield and availability of good-quality embryos (22). Therefore, in fresh cycles, the multiple roles of ZYP were difficult to figure out due to the subsequential embryo transfer following oocyte development. In contrast, FET separates the oocyte collection and embryo transfer in different cycles. As the availability of the embryos is determined by previous ovarian stimulation cycles, the role of ZYP in FET cycles is limited to supporting endometrial growth and embryo implantation, allowing an evaluation of the effect of ZYP on these processes. Although our data did not support a generalized benefit of ZYP treatment following embryo transfer, the role of ZYP administration during endometrial preparation could not be excluded, as the treatment may be associated with a higher endometrial thickness (23).
The supposed effect on oocyte development might play a key role in the pharmacological mechanisms of ZYP during the IVF procedure. A previous study have found the expression of bone morphogenetic protein 15 (BMP15) and growth differentiation factor 9 (GDP9) in the follicular fluid (FF), these two factors are paracrine regulators for oocyte development and are very important for its development (24). Both BMP15 and GDF9 stimulate follicle growth and granule cell proliferation (25). Furthermore, the expression of these two factors is mediated by the phosphatidylinositol 3 kinase/protein kinase B (PI3K/AKT) pathway (26). A metabolomics study found that the content of PI3K/AKT-related metabolites could be upregulated in patients taking ZYP (11). Therefore, ZYP upregulates the expression of BMP15 and GDF9 in FF by changing the PI3K/AKT content, thereby improving oocyte quality (27). However, molecular targets such as PI3K/AKT may also be involved in endometrial growth and embryo implantation (28). A similar mechanism may also affect the endometrium and improve the endometrial receptivity (29). Although the majority of the previous studies focused on the potential effects of ZYP on ovaries and endometrium, the complexity of herbal materials made a difficult to study the synergistic effects of traditional Chinese medicine (TCM) prescriptions. Like other types of traditional medicine, TCM is expected to affect the biological system rather than the specific molecular targets (30). A recent metabonomics study suggested that blood concentrations of metabolites such as tauroursodeoxycholic acid, L-asparagine, L-glutamic acid, kynurenic acid, 11-deoxycorticosterone, melatonin glucuronide, and hydroxytyrosol, indicating changes in lipid and amino acid metabolism (11). It may suggest a wider mechanism of ZYP actions. To better understand the mechanism and target of therapy, the investigators and manufacturers should provide a more detailed picture of how the therapy affects each targeted organ and process.
A stratified analysis of endometrial preparation regimens showed that the live birth rate was significantly higher in the ZYP group than in the non-ZYP group (41.5% vs. 47.4%) for NC patients. Endometrial preparation is an integral part of FET, and the most commonly used regimens are NC and HRT (31). The HRT regimens use exogenous estradiol and progesterone to support endometrial growth and differentiation, lacking a normal follicular dynamic and corpus luteum which are present in the NC cycles. The corpus luteum is associated with endometrial development, and luteal insufficiency can lead to endometrial dysplasia and may impair the role of ZYP in improving implantation support in normal pregnancy (32). The ovary is an active endocrine and paracrine organ that controls the pregnancy beyond generating the female gametes, and the ovary may be the target of ZYP (20). Analyses of the blood components in ZYP-treated animals may suggest that the mechanism of effects may include neuroactive ligand-receptor interaction and steroid hormone biosynthesis, and these effects might be weakened when the luteal function is insufficient (33).
On the one hand, a study revealed that the HRT cycle has a substantial impact on placental development compared with the modified natural cycles (mNC), resulting in a higher incidence of preeclampsia, postpartum hemorrhage, and Cesarean section (34). Another study showed that exogenous estrogen and progesterone administration in artificial cycles may interfere with the readiness of the endometrium (35). It has been shown that ZYP can improve the outcomes of a fresh cycle by upregulating the expression of the homologous frame gene A10 (HOXA10) and improving the uterine receptivity impaired by ovarian stimulation (9). To benefit the patients with HRT, the timing of ZYP administration may be earlier than the day following ET, possibly covering the entire cycle period as what has been done in an ovarian stimulation cycle.
The main advantage of this study is that we have substantially large sample sizes of data compared to previous studies. There are also some limitations because it is a retrospective study, confounded by unknown or unmeasured factors. The results are also possibly biased. Since there was a lack of clear indications, the patient assignment may be a significant source of bias. For instance, the patients who chose to receive ZYP may have a poorer prognosis or be less confident, seeking adjuvants for solutions to uncertainty. Nevertheless, we use the PSM method to correct potential conjuring factors as much as possible to minimize possible bias. On the other hand, however, the differences in endometrial preparation may hint at a potential selection bias. The observed interaction between endometrial preparation and ZYP treatment could also be explained by unmeasured confounding in different populations.
The belief in traditional medicine is common in patients seeking fertility treatment. According to a large cross-sectional survey, traditional Chinese medicine is the top fertility treatment option for infertile couples (29.4%) (36). The belief in the effects of TCM may also be a potential motive driving patients to receive ZYP in FET cycles. The beliefs of patients may also lead to potential bias and confounding in several aspects. The patients who intend to receive ZYP may be biased toward those with longer disease courses, higher desire, or better informed concerning traditional medicine. According to a previous review, patients who have stronger beliefs in the effectiveness of TCM beliefs may be less likely to adhere to self-management and medication (11). It could suggest unknown or unmeasured confounding to the outcomes. For instance, these patients may also seek other TCM adjuvants outside the ART program. The patients` belief in efficacy may be also associated with a placebo effect (37). Since the study was retrospective, the placebo effect is also a reason for caution.
In conclusion, our study suggests that ZYP may not be a routine adjuvant in FET cycles. However, the positive association between live birth rate and ZYP treatment in NC cycles warrants further investigation into the subgroups of patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional review board of The Affiliated Chenggong Hospital of Xiamen University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XY: Conceptualization, Data curation, Formal analysis, Investigation, Resources, Software, Writing – original draft, Writing – review & editing. JLC: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. LJ: Writing – review & editing, Data curation, Formal analysis, Investigation. XJ: Funding acquisition, Writing – review & editing, Data curation, Investigation, Software. ZL: Writing – review & editing, Data curation, Investigation. JHC: Writing – review & editing, Investigation. KC: Writing – review & editing, Data curation. CY: Writing – review & editing, Data curation. JG: Writing – review & editing, Formal analysis. CM: Writing – review & editing, Software. JR: Funding acquisition, Writing – review & editing, Formal analysis, Investigation, Methodology. LL: Writing – review & editing, Data curation, Formal analysis, Investigation, Software, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant number 22176159), the Xiamen Medical Advantage subspecialty construction project (grant number 2018296), and the Xiamen Natural Science Foundation (grant number 20231302).
Acknowledgments
The authors thank all the staff in our center, especially the embryologists in our lab, for their support in generating this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1379590/full#supplementary-material
References
1. Adamson GD, Dyer S, Chambers G, Ishihara O, De Mouzon J, Kupka M, et al. O-151 ICMART preliminary world report 2018. Hum Reprod. (2022) 37:i89. doi: 10.1093/humrep/deac105.057
2. Pourmoghadam Z, Abdolmohammadi-Vahid S, Pashazadeh F, Leili AM, Ansari F, Yousefi M. Evaluating interventions and adjuncts to optimize pregnancy outcomes in subfertile women: an overview review (vol 28, dmac001, 2022). Hum Reprod Update. (2022) 28:583-600. doi: 10.1093/humupd/dmac001
3. Friedman J, Sheeder J, Lazorwitz A, Polotsky AJ. Herbal supplement use among reproductive-aged women in an academic infertility practice. F&S Rep. (2023) 4:104–11. doi: 10.1016/j.xfre.2022.12.001
4. Xie ZY, Peng ZH, Yao B, Chen L, Xia YB. The effects of acupuncture on pregnancy outcomes of in vitro fertilization: a systematic review and meta-analysis. BMC Complementary Altern Med. (2019) 19:131. doi: 10.1186/s12906-019-2523-7
5. Dan J, Lily L, Bai-Yun Z. Treatment of chinese herbal medicine for female infertility. Int Rev Neurobiol. (2017) 135:233–47. doi: 10.1016/bs.irn
6. Smith JF, Eisenberg ML, Millstein SG, Nachtigall RD, Shindel AW, Wing H, et al. The use of complementary and alternative fertility treatment in couples seeking fertility care: data from a prospective cohort in the United States. Fertility Sterility. (2010) 93:2169–74. doi: 10.1016/j.fertnstert.2010.02.054
7. Ju-Feng X, Inagaki Y, Jian-Feng Z, Ling W, Pei-Pei S. Chinese medicine as complementary therapy for female infertility. Chin J Integr Med. (2017) 23:245–52. doi: 10.1007/s11655-016-2510-5
8. Xie L, Li J, Li Y, Wang B, Xie C, Xia Q, et al. Chinese herbal medicine for assisted reproduction technology: A protocol for a systematic review and meta-analysis. Medicine. (2020) 99:e22009. doi: 10.1097/MD.0000000000022009
9. Gao Q, Han L, Li X, Cai X. Traditional chinese medicine, the zishen yutai pill, ameliorates precocious endometrial maturation induced by controlled ovarian hyperstimulation and improves uterine receptivity via upregulation of HOXA10. Evid Based Complement Alternat Med. (2015) 2015:317586. doi: 10.1155/2015/317586
10. Zhu WJ, Li XM, Li XM. Effect of Zishen Yutai pill on embryo implantation rate in patients undergoing fertilization embryo transfer in vitro. Chin J Integr Med. (2003) 9:15. doi: 10.1007/BF02836345
11. Li L, Ning N, Wei J-A, Huang Q-L, Lu Y, Pang X-F, et al. Metabonomics study on the infertility treated with zishen yutai pills combined with in vitro fertilization-embryo transfer. Front Pharmacol. (2021) 12. doi: 10.3389/fphar.2021.686133
12. Xu L, Tu Q, Wang F, Yan D, Li B, Sun P. Zishen yutai pill as an adjuvant therapy in threatened Miscarriage:A meta-analysis of 23 randomized controlled trials. Heliyon. (2023) 9:e16213. doi: 10.1016/j.heliyon.2023.e16213
13. Chen X, Hao C, Deng W, Bai H, Li Y, Wang Z, et al. Effects of the zishen yutai pill compared with placebo on live births among women in a fresh embryo transfer cycle-A randomized controlled trial. Obstetrics Gynecology: J Am Coll Obstetricians Gynecologists. (2022) 139:192-201. doi: 10.1097/AOG.0000000000004658
14. Singh B, Reschke L, Segars J, Baker VL. Frozen-thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertility sterility. (2020) 113:252–7. doi: 10.1016/j.fertnstert.2019.12.007
15. Liu L, Jiang X, Liu Z, Chen J, Yang C, Chen K, et al. Oocyte degeneration in a cohort adversely affects clinical outcomes in conventional IVF cycles: a propensity score matching study. Front Endocrinol. (2023) 14. doi: 10.3389/fendo.2023.1164371
16. Chen X, Li Y, Zhou J, Wei X, Ning N, Huang Q, et al. Effects of the Zishen Yutai Pill compared with placebo on pregnancy outcomes among women in a fresh embryo transfer cycle: a Post Hoc subgroup analysis of a randomized controlled trial. Front Endocrinol (Lausanne). (2023) 14:1196636. doi: 10.3389/fendo.2023.1196636
17. Agoritsas T, Merglen A, Shah ND, O’donnell M, Guyatt GH. Adjusted analyses in studies addressing therapy and harm: users’ Guides to the medical literature. JAMA. (2017) 317:748–59. doi: 10.1001/jama.2016.20029
18. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. (2011) 46:399–424. doi: 10.1080/00273171.2011.568786
19. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. (2011) 10:150–61. doi: 10.1002/pst.433
20. Maharajan K, Xia Q, Duan X, Tu P, Zhang Y, Liu K. Therapeutic importance of Zishen Yutai Pill on the female reproductive health: A review. J Ethnopharmacol. (2021) 281:114523. doi: 10.1016/j.jep.2021.114523
21. Bai H, Wang T, Liu X. Study on effects of zishen yutai pill on pregnancy outcome of women undergoing in vitro fertilization-embryo transfer. World Chin Med. (2018) 13(3):658–61. doi: 10.3969/j.issn.1673-7202
22. Li XF, Wang ZQ, Xu HY, Liu H, Sheng Y, Xu J, et al. Effects of Zishen Yutai Pills on in vitro Fertilization-Embryo Transfer Outcomes in Patients with Diminished Ovarian Reserve: A Prospective, Open-Labeled, Randomized and Controlled Study. Chin J Integr Med. (2023) 29(4):291–8. doi: 10.1007/s11655-023-3546-y
23. Li M, Ning N, Liu Y, Li X, Mei Q, Zhou J, et al. The potential of Zishen Yutai pills to facilitate endometrial recovery and restore fertility after induced abortion in rats. Pharm Biol. (2021) 59(1):1505–16. doi: 10.1080/13880209.2021.1993272
24. Heath DA, Pitman JL, Mcnatty KP. Molecular forms of ruminant BMP15 and GDF9 and putative interactions with receptors. Reproduction. (2017) 154:521–34. doi: 10.1530/REP-17-0188
25. Turathum B, Gao EM, Chian RC. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells. (2021) 10:2292. doi: 10.3390/cells10092292
26. Sambit R, Divya G, Christina S, Anindita B, Kushnir VA, Norbert G, et al. Oocyte-derived factors (GDF9 and BMP15) and FSH regulate AMH expression via modulation of H3K27AC in granulosa cells. Endocrinology. (2018) 9:3433–45. doi: 10.1210/en.2018-00609
27. Li XF, Wang ZQ, Xu HY, Liu H, Sheng Y, Xu J, et al. Effects of Zishen Yutai Pills onin vitroFertilization-Embryo Transfer Outcomes in Patients with Diminished Ovarian Reserve: A Prospective, Open-Labeled, Randomized and Controlled Study. Chin J Integr Med. (2023) 29:291–8. doi: 10.1007/s11655-023-3546-y
28. Huang H, Xia L, Xia Y, Yan Y, Jiang Z, Zhao P, et al. Tiaojing cuyun recipe enhances pregnancy outcome via the VEGF/PI3K/AKT/eNOS signaling pathway in EID mice. Dis Markers. (2022) 2022:9461444. doi: 10.1155/2022/9461444
29. You F, Du X, Zhang T, Wang Y, Lv Y, Zeng L. TJZYF improves endometrial receptivity through regulating VEGF and PI3K/AKT signaling pathway. BioMed Res Int. (2022) 2022:9212561. doi: 10.1155/2022/9212561
30. Matos LC, MaChado JP, Monteiro FJ, Greten HJ. Understanding traditional chinese medicine therapeutics: an overview of the basics and clinical applications. Healthcare (Basel). (2021) 9:257. doi: 10.3390/healthcare9030257
31. Glujovsky D, Pesce R, Sueldo C, Quinteiro Retamar AM, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev. (2020) 10:CD006359. doi: 10.1002/14651858.CD006359.pub3
32. Lessey BA, Young SL. What exactly is endometrial receptivity? Fertility Sterility. (2019) 111:611–7. doi: 10.1016/j.fertnstert.2019.02.009
33. Dang L, Zhang C, Su B, Ning N, Huang Q, Zhou S, et al. Mechanisms of action of Zishen Yutai pills in treating premature ovarian failure determined by integrating UHPLC-Q-TOF-MS and network pharmacology analysis. BMC Complementary Med Therapies. (2022) 22:281. doi: 10.1186/s12906-022-03763-2
34. Wang F, Wang Q, Song Y, Ding J, Li H, Meng Q. Programmed frozen embryo transfer cycles are associated with a higher risk of abnormal placental development: a retrospective cohort study of singleton live births. Front Endocrinol. (2023) 14. doi: 10.3389/fendo.2023.1202044
35. Guan Y, Fan H, Styer AK, Xiao Z, Li Z, Zhang J, et al. A modified natural cycle results in higher live birth rate in vitrified-thawed embryo transfer for women with regular menstruation. Syst Biol Reprod Med. (2016) 62:335–42. doi: 10.1080/19396368.2016.1199064
36. Zheng D, Zhou Z, Li R, Wu H, Xu S, Kang Y, et al. Consultation and treatment behaviour of infertile couples in China: a population-based study. Reprod BioMed Online. (2019) 38:917–25. doi: 10.1016/j.rbmo.2018.11.034
Keywords: IVF-FET, Zishen Yutai pill, live birth rate, endometrial preparation, corpus luteum
Citation: Yang X, Cai J, Jiang L, Jiang X, Liu Z, Chen J, Chen K, Yang C, Geng J, Ma C, Ren J and Liu L (2024) Neutral effect of Zishen Yutai Pill on frozen-thawed embryo transfer: a propensity score matching study. Front. Endocrinol. 15:1379590. doi: 10.3389/fendo.2024.1379590
Received: 31 January 2024; Accepted: 13 August 2024;
Published: 29 August 2024.
Edited by:
Bufang Xu, Shanghai Jiao Tong University, ChinaReviewed by:
Dazhi Fan, Foshan Women and Children Hospital, ChinaChuanlong Zhang, Capital Medical University, China
Copyright © 2024 Yang, Cai, Jiang, Jiang, Liu, Chen, Chen, Yang, Geng, Ma, Ren and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianzhi Ren, cmp6MTc0QDEyNi5jb20=; Lanlan Liu, bGFubGFuX2xpdUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Xiaolian Yang1†
Xiaolian Yang1† Jiali Cai
Jiali Cai Kaijie Chen
Kaijie Chen Jianzhi Ren
Jianzhi Ren Lanlan Liu
Lanlan Liu