
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 23 August 2024
Sec. Clinical Diabetes
Volume 15 - 2024 | https://doi.org/10.3389/fendo.2024.1379127
This article is part of the Research TopicHeadache and other symptoms in special populationsView all 20 articles
Introduction: Migraine, a debilitating neurological disorder characterized by recurrent headaches, affects over 1.1 billion individuals globally. Diabetes mellitus (DM), a chronic metabolic condition marked by high blood sugar levels, affects 463 million individuals according to the International Diabetes Federation. Our study aimed to evaluate the association between migraine and DM and to identify several demographic, socioeconomic, and lifestyle factors, as well as medical and psychiatric comorbidities, associated with migraine among individuals with DM.
Methods: This cross-sectional study is based on data from the European Health Interview Surveys conducted in 2009, 2014, and 2019 in Hungary. Pearson’s chi-squared tests and multiple logistic regression models were used to assess associations. Statistical significance was set at p<0.05.
Results: In multiple regression analyses, we found no significant association between DM and migraine after adjusting for socioeconomic status, various health conditions, and lifestyle factors (OR=0.84, 95% CI: 0.66-1.06). However, adults with DM who had comorbid conditions including stroke (OR=2.08, 95% CI: 1.06-4.08), low back pain (OR=3.52, 95% CI: 2.13-5.84), and depression (OR=4.91, 95% CI: 2.84-8.47) were significantly more likely to suffer from migraine.
Discussion: Our study found no significant difference in the prevalence of migraine among adults with and without diabetes mellitus. However, several comorbidities were found to be significantly associated with migraine occurrence in those with DM. Thus, the study’s results highlight the need for proper management of diabetes, especially in terms of comorbidities, to mitigate migraine risk factors and improve patient outcomes.
Migraine is characterized by recurrent attacks of pulsatile, moderate-severe intensity headache, located on one side of the head, and accompanied by photo- and phonophobia, and/or nausea/vomiting (1–3). The global prevalence of migraine is 14-15%, and recent studies have indicated an increasing temporal trend in the last decades (4, 5).
Migraine-related disability burden is substantial. Migraine accounts for 4.9% of total years lived with disability (YLDs). Migraine is the leading cause of disability among young adult women, and most frequently affects individuals during the most productive years. Migraine is associated with impaired quality of life (5–8).
Moreover, there is evidence that migraine is comorbid with a range of conditions including depression, anxiety, and/or pain disorders. Migraine is also associated with increased prevalence of cardiovascular risk factors, such as hypertension, increased cholesterol level, smoking, and obesity (7, 9–13).
However, previous studies have yielded conflicting results regarding the association between migraine and diabetes mellitus (DM). Some studies have reported a lower (14, 15) prevalence of migraine in individuals with DM, while others have suggested similar (16–19) or higher (9, 20) rates compared with those without DM. Despite the limited and controversial results, some studies (19, 21) have suggested that socioeconomic factors and lifestyle habits may play a role in the link between migraine and DM. The possible underlying mechanisms involved in this potential link are not completely understood and require additional investigation (21).
Due to the increasing burden of migraine and DM worldwide, a better understanding of the complex relationship between these conditions, as well as examining potential risk factors could help inform clinical care. Our study aimed to estimate the prevalence of migraine in individuals with DM in a representative sample of the Hungarian adult population and to evaluate the association between migraine and several demographic, socioeconomic, and lifestyle factors, as well as medical and psychiatric comorbidities, among individuals with DM.
A cross-sectional study was carried out using data from the European Health Interview Surveys (EHIS) conducted in Hungary in 2009, 2014, and 2019. The datasets were obtained from the Hungarian Central Statistical Office (22). Individualized weights were applied to address non-response bias and achieve a representative sample to the Hungarian population (23). A standardized questionnaire, used across all participating EU Member States, was utilized to collect comprehensive data on health status. We integrated data from all three survey years including difference participants from each wave to perform the analysis to increase the statistical power of the results.
The final dataset consists of 12,025 participants living in private households. Individuals aged 35 years and older were included in this study.
The study was conducted with strict adherence to ethical guidelines, following the principles set forth in the Declaration of Helsinki. Ethical approval was secured from the Ethics Committee of the University of Debrecen, under the approval number 5609-2020, ensuring compliance with Regulation 2016/679.
Self-reported information on variables was used in the study. Participants were identified as subjects with diabetes who answered yes to both the following questions: “Have you had this condition (diabetes) in the past 12 months?” and “Has your physician confirmed the diagnosis?”. Respondents were considered as suffering from migraine if they answered yes to the following questions: “Have you had this condition (migraine) in the past 12 months?” and “Has your physician confirmed the diagnosis?”.
In the current study, a range of demographic, socio-economic, and health-related variables were analyzed. We examined socio-demographic factors, including gender (male, female) and age groups (35-64 years, and 65+ years). Educational background was categorized as completion of primary, secondary, and tertiary education. For income status, we analyzed the data across five groups: low income, lower middle-class income, middle-class income, upper middle-class income, and high income.
Self-perceived health status was measured on a scale from 1 to 3, where higher numbers indicated worse health. Self-reported height and weight were used to estimate the body mass index (BMI), which was categorized into three groups: underweight or normal (BMI < 25), overweight (25 ≤ BMI < 30), and obese (BMI ≥ 30).
Among lifestyle factors, smoking habits (smokers and non-smokers) and alcohol consumption (heavy drinkers, regular drinkers, occasional drinkers, and non-drinkers) were investigated.
Data on the presence of chronic conditions affecting the participants during the last 12 months were also collected regarding respiratory diseases (including asthma and bronchitis), acute myocardial infarction (AMI), coronary artery disease (CAD), stroke, high blood pressure, high cholesterol level, mental health disorders (including depression and anxiety), and low back pain.
Descriptive statistics were presented to describe the characteristics of the study populations with and without DM using absolute numbers and weighted proportions. Pearson’s chi-squared tests were used to test differences between groups. Multiple logistic regression models with binary outcomes were performed to measure the association between presence of DM and migraine, and to explore the socioeconomic, health-related and lifestyle factors, as well as medical and psychiatric comorbidities, associated with migraine among individuals with DM. Results of the logistic regression models are presented as odds ratios (OR) and 95% confidence intervals (CI) are presented. Sampling weights were applied using the “svy” command in Stata.
The statistical analyses were performed using STATA IC Version 18.0 (StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC). A p-value less than 0.05 was considered statistically significant.
More than half of individuals with DM were female (54.97%), and most of them belonged to the older age group (51.69%). A significantly better self-reported health status was reported by those with DM compared to those without DM (good rated health DM 36.74% vs non-DM 13.53%). Individuals with DM reported significantly higher BMI (obesity: 46.66% vs 24.38%, p<0.001), higher prevalence of comorbidities, lower education and income levels, and were more likely to be non-smokers (81.03% vs 73%, p<0.001) and non-drinkers (42.76% vs. 32.1%, p<0.001) than individuals without DM (Table 1).
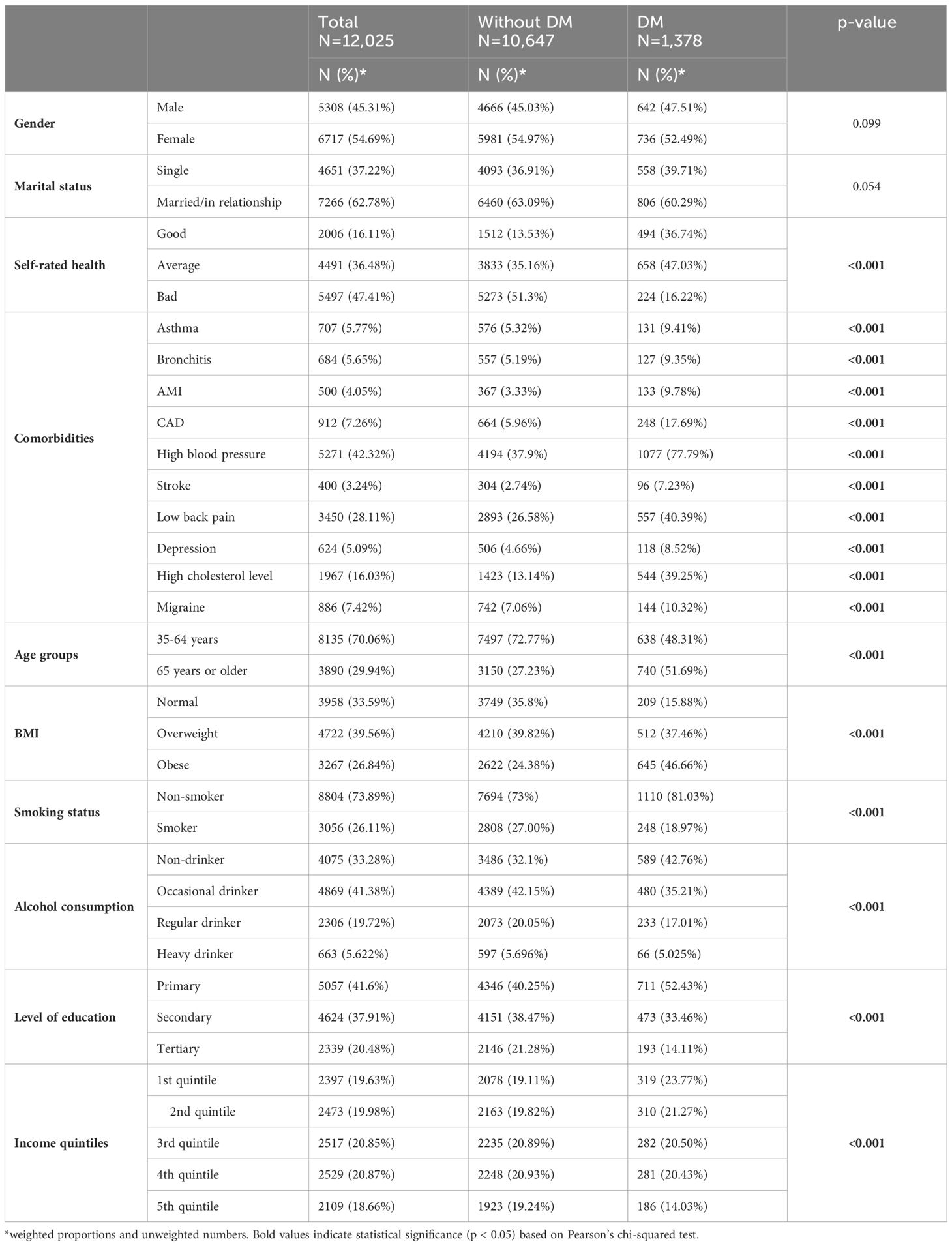
Table 1. The distribution of health-related and sociodemographic factors among participants with and without diabetes mellitus.
Individuals with DM showed a significantly higher overall prevalence of migraine compared to those without DM (10.32% vs. 7.06%, p<0.001). Female participants reported a higher prevalence of migraine than males, particularly among those with DM (14.31% vs. 5.93%). Single individuals with DM reported a higher rate of migraine compared to those in a relationship (13.15% vs. 8.24%, p=0.023). Individuals with DM and depression had an increased prevalence of migraine (43.7%) than those without DM (32.81%) (p<0.001). In those with both DM and comorbidities including bronchitis, AMI, CAD, low back pain, and high cholesterol level, there was a significantly increased prevalence of migraine compared to those without DM. Regarding socioeconomic and lifestyle factors, a significantly higher migraine prevalence was reported among those with primary education (14.93% and 10.65% in DM and non-DM patients, p=0.012) compared with those with higher education, and among smokers (13.51% in DM and 7.6% in non-DM patients, p=0.006) compared with non-smokers (Table 2).
In a multiple regression analysis, adjusting for age and gender, we found a significant association between DM and migraine (OR=1.55, 95% CI: 1.26-1.90) in the total sample, suggesting that individuals with DM had a 55% higher likelihood of experiencing migraines compared to those without DM. However, when we further adjusted for a broader range of factors, including socioeconomic status, various health conditions, and lifestyle factors, this association was no longer significant (OR=0.84, 95% CI: 0.66-1.06). Table 3 shows the results of multiple regression analysis among DM participants. Individuals with DM and with comorbid conditions including stroke (OR=2.08, 95% CI: 1.06-4.08), low back pain (OR=3.52, 95% CI: 2.13-5.84) and depression (OR=4.91, 95% CI: 2.84-8.47) were significantly more likely to suffer from migraine according to the results of a weighted multiple logistic regression model. Furthermore, in the DM population, individuals with secondary education reported significantly lower likelihood of having migraine (OR=0.43, 95% CI: 0.23-0.82) compared to those with primary education. The other variables showed no significant association with migraine prevalence (Table 3).
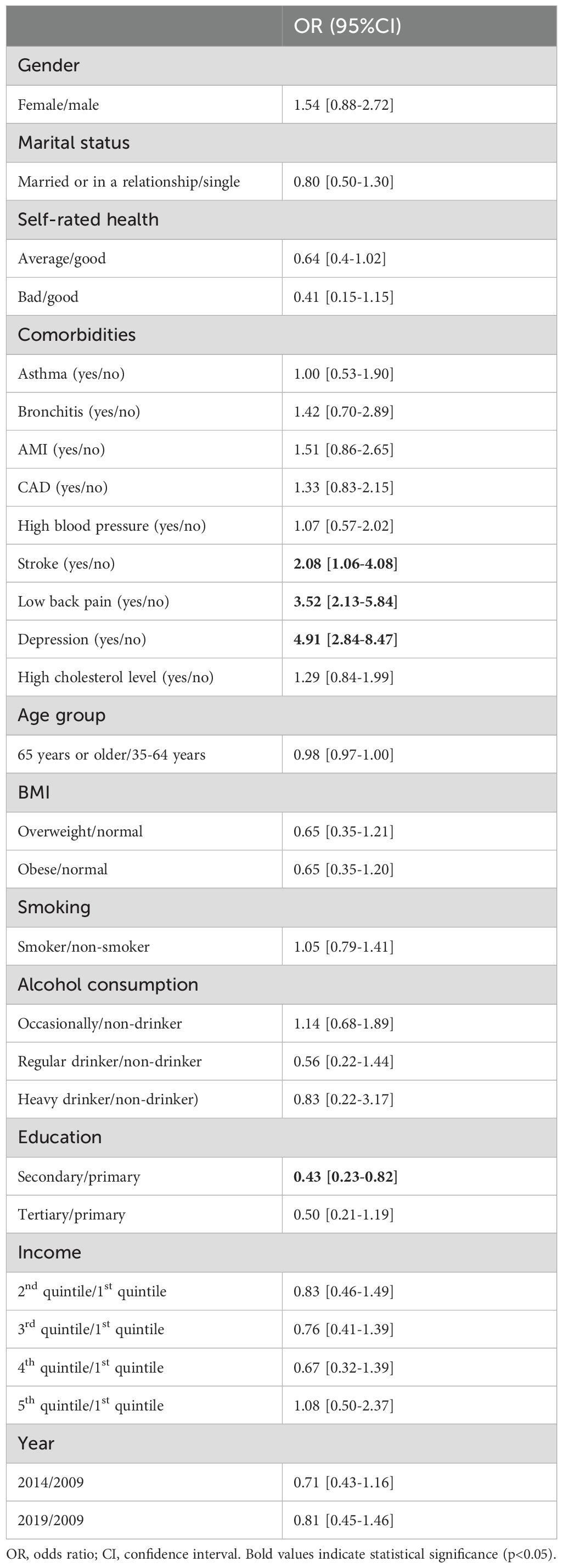
Table 3. Weighted multiple logistic regression models to identify the factors associated with migraine.
Though our study found an association between DM and migraine when adjusting for age and gender, our study found no significant differences in the prevalence of migraine among adults with DM compared to those without DM after adjusting for several socioeconomic, lifestyle, and health-related variables, which is consistent with the literature (19). We also showed that among those with DM, stroke, low back pain, depression and educational attainment were significantly associated with increased migraine prevalence.
In our study, we observed a higher prevalence of migraine among females with DM compared to those without DM. Migraine occurs more commonly in the female population, with a prevalence three times higher in females compared to males. Additionally, after menopause, the prevalence of migraine in females decreases (24). The observed gender and temporal disparity of migraine is related in part to the complex role of estrogen in migraine pathophysiology. Estrogen influences serotonin reuptake and regulation, and fluctuations of estrogen in the menstrual cycle can affect migraine frequency (25, 26). Therefore, stabilization of estrogen levels post-menopause is associated with decreased migraine frequency (26, 27, 29, 30).
Our study did not identify an increased risk of diabetes and migraine when adjusting for demographics and lifestyle factors. However, possible links between migraine and DM exist. Several studies have investigated the role of glucose dysregulation in the pathophysiology of migraine and headache disorders in DM. A recent study found that plasma glucose levels were higher in individuals with migraine (with and without aura) during migraine attacks (31). In addition, previous research has suggested that shared genetics and biological mechanisms may contribute to migraine and glucose-related traits (32, 33). Insulin plays a role in the pathophysiological mechanism in migraine, as it is associated with glucose metabolism and directly affects the secretion of gonadotropins by the hypothalamus. In addition, chronic sleep deprivation in those with migraine can cause hyperinsulinism due to higher postprandial glucose levels with abnormal insulin sensitivity (34). As a consequence, good sleep hygiene, as well as dietary patterns that include small, frequent meals high in protein and vegetables and low in carbohydrates may contribute to migraine prevention by minimizing blood glucose fluctuations (35).
The overlap of comorbid conditions with migraine in individuals with DM, including depression and chronic pain, is well-recognized in the scientific literature. Our study’s finding that migraine occurrence is more common in individuals with depression is consistent with a growing body of research underscoring the bidirectional relationship between migraine and depression (36). According to Holt et al. (2014), up to one third of individuals with DM experience depressive symptoms, leading to decreased quality of life (37), particularly affecting those of younger age, female gender, and lower educational levels.
Our study showed that in individuals with DM, lower back pain significantly increased the risk of comorbid migraine, which is consistent with the literature (38–40). This may be related in part to frequent use of acute analgesics for headache and other pain conditions, which can lead to medication overuse headache as a complicating factor for those with an underlying headache disorder (38, 39). Furthermore, experiencing one chronic pain condition has been associated with an increased risk of an additional chronic pain condition, raising the possibility of shared underlying mechanisms. Central sensitization to pain, in which the nervous system develops altered responsiveness to and processing of pain stimuli, leads to increased pain perception (40). For individuals with DM alone, Aldossari et al. (2020) highlighted a correlation with chronic pain, which may be related in part to comorbid diabetic neuropathy and inflammation (41).
The present study found that in those with DM and history of stroke, there is an increased risk of migraine compared to those without DM. The literature suggests that migraine with aura is associated with an increased risk of stroke (42) due to complex, multifactorial etiologies, related in part to alterations in cerebral blood flow, inflammation, platelet hyperaggregability, and endothelial activation (43). However, only 20% of individuals with migraine have migraine with aura (44). The increased prevalence of migraine in those suffering from both stroke and DM may be explained by the presence of metabolic risk factors including hypertension and obesity, which are known to be associated with migraine (45). Another explanation may be that individuals with DM and stroke may have reduced physical activity levels. Regular physical activity has a prophylactic effect on the frequency of migraine (46).
The strength of this study lies in its use of a representative database that covers a large and diverse population. Additionally, the availability of data on sociodemographic characteristics, lifestyle, and health-related variables allowed us to account for potential confounding factors in our analyses. Probability sampling and weighting contributed to a reduced non-response bias and external validity of the study.
However, the study has several limitations. First, the questionnaire was self-reported, so there may have been variable biases and inaccuracies in the data used. Second, the diagnoses of migraine and diabetes were based on self-reporting, relying on recall. Thus, bias from underreporting or overreporting may have occurred. Third, the cross-sectional study design does not allow causal inferences. In addition, regarding the diagnosis of stroke, the distinction between ischemic and hemorrhagic stroke was not made. Diabetes severity was also not noted, and type 1 and type 2 DM were not differentiated.
The study did not find an increased risk of diabetes and migraine when adjusting for demographics and lifestyle factors. However, there were increased risks of comorbidities of low back pain, stroke, and depression in individuals with DM and migraine compared to those without DM. This highlights the complexities of these conditions, and raises the importance of addressing vascular risk factors and chronic pain in those with DM and migraine. Further longitudinal and perhaps genetic research is needed to unravel the underlying mechanisms between DM and migraine.
The datasets of the European Health Interview Survey for this study are available upon request from the Hungarian Central Statistical Office (https://www.ksh.hu). Requests to access these datasets should be directed to Karolyne Tokaji, S2Fyb2x5bmUuVG9rYWppQGtzaC5odQ==.
The studies involving humans were approved by Ethics Committee of the University of Debrecen, under the approval number 5609-2020. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
EV: Writing – original draft. AG: Writing – original draft. EF: Writing – review & editing. CN: Writing – review & editing. NK: Conceptualization, Writing – review & editing. AN: Conceptualization, Formal analysis, Methodology, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Global Burden of Disease (GBD). (2024). accessed January 25, 2024. Available online at: https://www.healthdata.org/research-analysis/gbd.
2. National Institute of Neurological Disorders and Stroke. Migraine. (2024). Available at: https://www.ninds.nih.gov/health-information/disorders/migraine.
3. Amiri P, Kazeminasab S, Nejadghaderi SA, Mohammadinasab R, Pourfathi H, Araj-Khodaei M, et al. Migraine: A review on its history, global epidemiology, risk factors, and comorbidities. Front Neurol. (2022) 12:800605. doi: 10.3389/fneur.2021.800605
4. Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. (2020) 21:137. doi: 10.1186/s10194-020-01208-0
5. Steiner TJ, Stovner LJ. Global epidemiology of migraine and its implications for public health and health policy. Nat Rev Neurol. (2023) 19:109–17. doi: 10.1038/s41582-022-00763-1
6. Linde M, Stovner LJ, Zwart JA, Hagen K. Time trends in the prevalence of headache disorders. The Nord-Trondelag Health Studies (HUNT 2 and HUNT 3). Cephalalgia. (2011) 31:585–96. doi: 10.1177/0333102410391488
7. Fernández-de-las-Peñas C, Palacios-Ceña D, Salom-Moreno J, López-de-Andres A, Hernández-Barrera V, Jiménez-Trujillo I, et al. Has the prevalence of migraine changed over the last decade (2003–2012)? A spanish population-based survey. PloS One. (2014) 9:e110530. doi: 10.1371/journal.pone.0110530
8. Lantéri-Minet M, Duru G, Mudge M, Cottrell S. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia. (2011) 31:837–50. doi: 10.1177/0333102411398400
9. Bigal ME, Kurth T, Santanello N, Buse D, Golden W, Robbins M, et al. Migraine and cardiovascular disease: a population-based study. Neurology. (2010) 74:628–35. doi: 10.1212/WNL.0b013e3181d0cc8b
10. Scher AI, Terwindt GM, Picavet HSJ, Verschuren WMM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology. (2005) 64:614–20. doi: 10.1212/01.WNL.0000151857.43225.49
11. Kurth T, Ridker PM, Buring JE. Migraine and biomarkers of cardiovascular disease in women. Cephalalgia. (2008) 28:49–56. doi: 10.1111/j.1468-2982.2007.01467.x
12. Ford ES, Li C, Pearson WS, Zhao G, Strine TW, Mokdad AH. Body mass index and headaches: findings from a national sample of US adults. Cephalalgia. (2008) 28:1270–6. doi: 10.1111/j.1468-2982.2008.01671.x
13. Bigal ME, Kurth T, Hu H, Santanello N, Lipton RB. Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology. (2009) 72:1864–71. doi: 10.1212/WNL.0b013e3181a71220
14. Aamodt AH, Stovner LJ, Midthjell K, Hagen K, Zwart JA. Headache prevalence related to diabetes mellitus. The Head-HUNT study. Eur J Neurol. (2007) 14:738–44. doi: 10.1111/j.1468-1331.2007.01765.x
15. Cook NR, Benseñor IM, Lotufo PA, Lee IM, Skerrett PJ, Chown MJ, et al. Migraine and coronary heart disease in women and men. Headache. (2002) 42:715–27. doi: 10.1046/j.1526-4610.2002.02173.x
16. Burch RC, Rist PM, Winter AC, Buring JE, Pradhan AD, Loder EW, et al. Migraine and risk of incident diabetes in women: a prospective study. Cephalalgia. (2012) 32:991–7. doi: 10.1177/0333102412453954
17. Haghighi FS, Rahmanian M, Namiranian N, Arzaghi SM, Dehghan F, Chavoshzade F, et al. Migraine and type 2 diabetes; is there any association? J Diabetes Metab Disord. (2016), 15:37. doi: 10.1186/s40200-016-0241-y
18. Davey G, Sedgwick P, Maier W, Visick G, Strachan DP, Anderson HR. Association between migraine and asthma: matched case-control study. Br J Gen Pract. (2002) 52:723–7.
19. López-de-Andrés A, Luis del Barrio J, Hernández-Barrera V, de Miguel-Díez J, Jimenez-Trujillo I, Martinez-Huedo MA, et al. Migraine in adults with diabetes; is there an association? Results of a population-based study. Diabetes Metab Syndr Obes. (2018) 11:367–74. doi: 10.2147/DMSO
20. Split W, Szydlowska M. Headaches in non insulin-dependent diabetes mellitus. Funct Neurol. (1997) 12:327–32.
21. Rivera-Mancilla E, Al-Hassany L, Villalón CM, MaassenVanDenBrink A. Metabolic aspects of migraine: association with obesity and diabetes mellitus. Front Neurol. (2021) 12:686398. doi: 10.3389/fneur.2021.686398
22. Eurostat. European health interview survey - methodology. accessed January 25, 2024. Available online at: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=European_health_interview_survey_-_methodology.
23. Hintzpeter B, Finger JD, Allen J, Kuhnert R, Seeling S, Thelen J, et al. European Health Interview Survey (EHIS) 2 - Background and study methodology. J Health Monit. (2019) 4:66–79. doi: 10.25646/6228
24. Lebedeva ER. Chapter Nine - Sex and age differences in migraine treatment and management strategies. In: Moro E, Arabia G, Tartaglia MC, Ferretti MT, editors. International Review of Neurobiology. Amsterdam, Netherlands: Academic Press (2022). p. 309–47. Available at: https://www.sciencedirect.com/science/article/pii/S0074774222000745. Sex and Gender Differences in Neurological Disease; vol. 164.
25. Bolay H, Ozge A, Saginc P, Orekici G, Uludüz D, Yalın O, et al. Gender influences headache characteristics with increasing age in migraine patients. Cephalalgia. (2015) 35:792–800. doi: 10.1177/0333102414559735
26. Sacco S, Ricci S, Degan D, Carolei A. Migraine in women: the role of hormones and their impact on vascular diseases. J Headache Pain. (2012) 13:177–89. doi: 10.1007/s10194-012-0424-y
27. Kneavel M. Relationship between gender, stress, and quality of social support. Psychol Rep. (2021) 124:1481–501. doi: 10.1177/0033294120939844
29. Graves BS, Hall ME, Dias-Karch C, Haischer MH, Apter C. Gender differences in perceived stress and coping among college students. PloS One. (2021) 16:e0255634. doi: 10.1371/journal.pone.0255634
30. Stubberud A, Buse DC, Kristoffersen ES, Linde M, Tronvik E. Is there a causal relationship between stress and migraine? Current evidence and implications for management. J Headache Pain. (2021) 22:155. doi: 10.1186/s10194-021-01369-6
31. Zhang DG, Amin FM, Guo S, Vestergaard MB, Hougaard A, Ashina M. Plasma glucose levels increase during spontaneous attacks of migraine with and without aura. Headache: J Head Face Pain. (2020) 60:655–64. doi: 10.1111/head.13760
32. Islam MR, Nyholt DR. Glucose-related traits and risk of migraine—A potential mechanism and treatment consideration. Genes (Basel). (2022) 13:730. doi: 10.3390/genes13050730
33. Islam MR, Nyholt DR. Cross-trait analyses identify shared genetics between migraine, headache, and glycemic traits, and a causal relationship with fasting proinsulin. Hum Genet. (2023) 142:1149–72. doi: 10.1007/s00439-023-02532-6
34. Hosseinpour M, Maleki F, Khoramdad M, Sullman MJM, Nejadghaderi SA, Kolahi AA, et al. A systematic literature review of observational studies of the bilateral association between diabetes and migraine. Diabetes Metab Syndr. (2021) 15:673–8. doi: 10.1016/j.dsx.2021.03.018
35. Hufnagl KN, Peroutka SJ. Glucose regulation in headache: implications for dietary management. Expert Rev Neurotherapeutics. (2002) 2:311–7. doi: 10.1586/14737175.2.3.311
36. Breslau N, Lipton RB, Stewart WF, Schultz LR, Welch KMA. Comorbidity of migraine and depression: investigating potential etiology and prognosis. Neurology. (2003) 60:1308–12. doi: 10.1212/01.WNL.0000058907.41080.54
37. Holt RIG, de Groot M, Golden SH. Diabetes and depression. Curr Diabetes Rep. (2014) 14:491. doi: 10.1007/s11892-014-0491-3
38. Duckro PN, Schultz KT, Chibnall JT. Migraine as a sequela to chronic low back pain. Headache. (1994) 34:279–81. doi: 10.1111/j.1526-4610.1994.hed3405279.x
39. Mesas AE, González AD, Mesas CE, de Andrade SM, Magro IS, del Llano J. The association of chronic neck pain, low back pain, and migraine with absenteeism due to health problems in Spanish workers. Spine (Phila Pa 1976). (2014) 39:1243–53. doi: 10.1097/BRS.0000000000000387
40. Vivekanantham A, Edwin C, Pincus T, Matharu M, Parsons H, Underwood M. The association between headache and low back pain: a systematic review. J Headache Pain. (2019) 20:82. doi: 10.1186/s10194-019-1031-y
41. Aldossari KK, Shubair MM, Al-Zahrani J, Alduraywish AA, AlAhmary K, Bahkali S, et al. Association between chronic pain and diabetes/prediabetes: A population-based cross-sectional survey in Saudi Arabia. Pain Res Manage. (2020) 2020:8239474. doi: 10.1155/2020/8239474
42. Zhang Y, Parikh A, Qian S. Migraine and stroke. Stroke Vasc Neurol. (2017) 2:160–7. doi: 10.1136/svn-2017-000077
43. Tietjen GE, Maly EF. Migraine and ischemic stroke in women. A narrative review. Headache. (2020) 60:843–63. doi: 10.1111/head.13796
44. Lucas C. Migraine with aura. Rev Neurologique. (2021) 177:779–84. doi: 10.1016/j.neurol.2021.07.010
45. Cheon DY, Han K, Yang YS, Kim Y, Lee SH, Kim C, et al. Associations between migraine and major cardiovascular events in type 2 diabetes mellitus. Cardiovasc Diabetol. (2022) 21:275. doi: 10.1186/s12933-022-01705-3
Keywords: headache disorders, disability, migraine, diabetes mellitus, European Health Interview Survey
Citation: Varga E, Ghanem AS, Faludi E, Nguyen CM, Kovács N and Nagy AC (2024) Medical comorbidities and other factors associated with migraine among individuals with diabetes mellitus in Hungary: a cross-sectional study using European Health Interview Surveys 2009–2019. Front. Endocrinol. 15:1379127. doi: 10.3389/fendo.2024.1379127
Received: 13 March 2024; Accepted: 07 August 2024;
Published: 23 August 2024.
Edited by:
Catherine Stika, Northwestern University, United StatesReviewed by:
Ami Cuneo, University of Washington, United StatesCopyright © 2024 Varga, Ghanem, Faludi, Nguyen, Kovács and Nagy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Attila Csaba Nagy, bmFneS5hdHRpbGFAZXRrLnVuaWRlYi5odQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.