- 1Department of Orthopedics, Shengjing Hospital of China Medical University, Shenyang, China
- 2Department of Nursing, Shengjing Hospital of China Medical University, Shenyang, China
- 3Department of Neurosurgery, Shengjing Hospital of China Medical University, Shenyang, China
Background: Chronic kidney disease (CKD) is a common complication among individuals with hypertension. We aimed to identify the prevalence of CKD and the sex and race disparities within the hypertensive population in the United States from 2001–2016.
Methods: A total of 16,148 participants with hypertension were included, representing 561,909,480 individuals from the U.S. population between 2001 and 2016, as documented in the National Health and Nutrition Examination Survey. The prevalence of albuminuria and CKD stage were assessed using survey-weighted general linear regression analysis. Heterogeneity in the CKD stage among the hypertensive population, stratified by sex and race, was identified through survey-weighted logistic regression analysis.
Results: Overall, the prevalence of albuminuria remained stable (p for trend = 0.3196), and changes in the CKD stage were minimal (p for trend > 0.05) from 2001–2016. In the analysis of CKD stage heterogeneity by sex and race, the prevalence of CKD was higher among women than men and higher among individuals of other races combined than non-Hispanic Whites, but the differences were not statistically significant.
Conclusion: The overall CKD stage within the hypertensive population plateaued between 2001 and 2016. Our findings highlight the importance of continuous monitoring and potential refinement of renoprotection strategies in individuals with hypertension to mitigate the persistent burden of CKD and address health disparities among different demographic groups.
1 Introduction
Chronic kidney disease (CKD) is a leading cause of the global burden of noncommunicable diseases, contributing substantially to morbidity and mortality and affecting <10% of the worldwide population (1–3). Unhealthy lifestyle choices and comorbidities, including diabetes, hypertension, and poor dietary habits, accelerate the progression of CKD (4, 5). These associations are particularly pronounced within the hypertensive population owing to the interconnected pathophysiological mechanisms linking hypertension and CKD. Specifically, sustained hypertension can exacerbate kidney function deterioration, and CKD can complicate blood pressure management. The complex pathophysiology of hypertension in CKD involves reduced nephron mass, increased sodium retention, activation of the sympathetic nervous system, and the renin-angiotensin-aldosterone system (RAAS) (6–8). Current guidelines recommend a stringent blood pressure target of <130/80 mmHg for individuals with CKD via lifestyle modification and the use of multiple antihypertensive drugs (8, 9). Analyzing the prevalence of CKD and sex and race disparities is crucial for assessing the adequacy of renoprotection strategies in the hypertensive population. Therefore, we investigated changes in CKD trends and assessed CKD heterogeneity by sex and race within the hypertensive population, utilizing the National Health and Nutrition Examination Survey (NHANES) database spanning 2001–2016.
2 Materials and methods
2.1 Study population
The NHANES is a comprehensive and rigorous database designed to assess the physical and nutritional well-being of noninstitutionalized populations in the United States. The National Center for Health Statistics (NCHS) developed and conducted the NHANES, gathering data on participant demographics, dietary intake, health, and physical examinations through household interviews and mobile examination centers. The NHANES updates its data every two years, making it freely accessible on its website (www.cdc.gov/nchs/nhanes/). Hypertension is defined as an abnormal blood pressure level (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg), a self-reported physician diagnosis of hypertension, or the use of antihypertensive medications (10, 11). The hypertensive population with complete kidney function data (including urine creatinine, urine albumin, and serum creatinine) consisted of 16,546 individuals from 2001–2016 in the NHANES database. We excluded participants with missing body mass index (BMI) (n=364), serum total cholesterol (n=7), and hemoglobin A1c (n=27) information. Ultimately, 16,148 participants with hypertension were enrolled. All data used in this study were taken from the NHANES publicly available database (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx). The NCHS and Research Ethics Review Committee approved the participation of human individuals in the NHANES study. Written informed consent was provided by each participant.
2.2 Measurement of renal function
We utilized the equation developed by the Chronic Kidney Disease Epidemiology Collaboration to calculate the estimated glomerular filtration rate (eGFR) (12). Albuminuria was defined as a urinary albumin-to-creatinine ratio ≥30 mg/g (13). The CKD stage was characterized by an eGFR ≥60 mL/min/1.73 m2 with albuminuria or an eGFR <60 mL/min/1.73 m2. The severity of CKD was categorized into four stages: no CKD (no albuminuria and eGFR ≥60 mL/min/1.73 m2); mild CKD (presence of albuminuria and eGFR ≥60 mL/min/1.73 m2); moderate CKD (30 mL/min/1.73 m2 ≤ eGFR <60 mL/min/1.73 m2); and advanced (eGFR <30 mL/min/1.73 m2) (14, 15).
2.3 Covariates
Covariates for the multivariable model were selected based on clinical experience and prior research. These covariates included age, sex, race, BMI, serum glucose, hemoglobin A1c, and total cholesterol levels. Diabetes was defined as either a physician-diagnosed condition or the use of hypoglycemic medications or insulin (16). The presence of hyperlipidemia was established when participants had previously received a diagnosis from a physician or were using lipid-lowering medications (17, 18). Smoking status was categorized as never smoked (never smoked or smoked <100 cigarettes in their lifetime), ever smoked (smoked >100 cigarettes in their lifetime), or unknown (19, 20).
2.4 Statistical analyses
We adhered to the NHANES analysis guidelines for conducting our statistical analyses, accounting for the complex sample design and employing design variables to determine nationally representative estimates. To enhance statistical accuracy, two-year survey cycles were consolidated into four-year intervals. Continuous variables are reported as survey-weighted means with 95% confidence intervals (95% CIs), whereas categorical variables are presented as survey-weighted percentages with 95% CIs. To assess trends in kidney function changes during the four-year survey periods (2001–2004, 2005–2008, 2009–2012, and 2013–2016), survey-weighted linear regression was used. Survey-weighted logistic regression analysis was performed, and odds ratios (ORs) were calculated to assess cross-sectional heterogeneity in CKD stages by sex and race. We conducted all analyses using R version 3.4.3 (http://www.Rproject.org; The R Foundation) and Empower software (www.empowerstats.com; X&Y Solutions Inc., Boston, MA, USA). Statistical significance was set at p<0.05.
3 Results
3.1 Baseline characteristics of participants with hypertension from 2001–2016
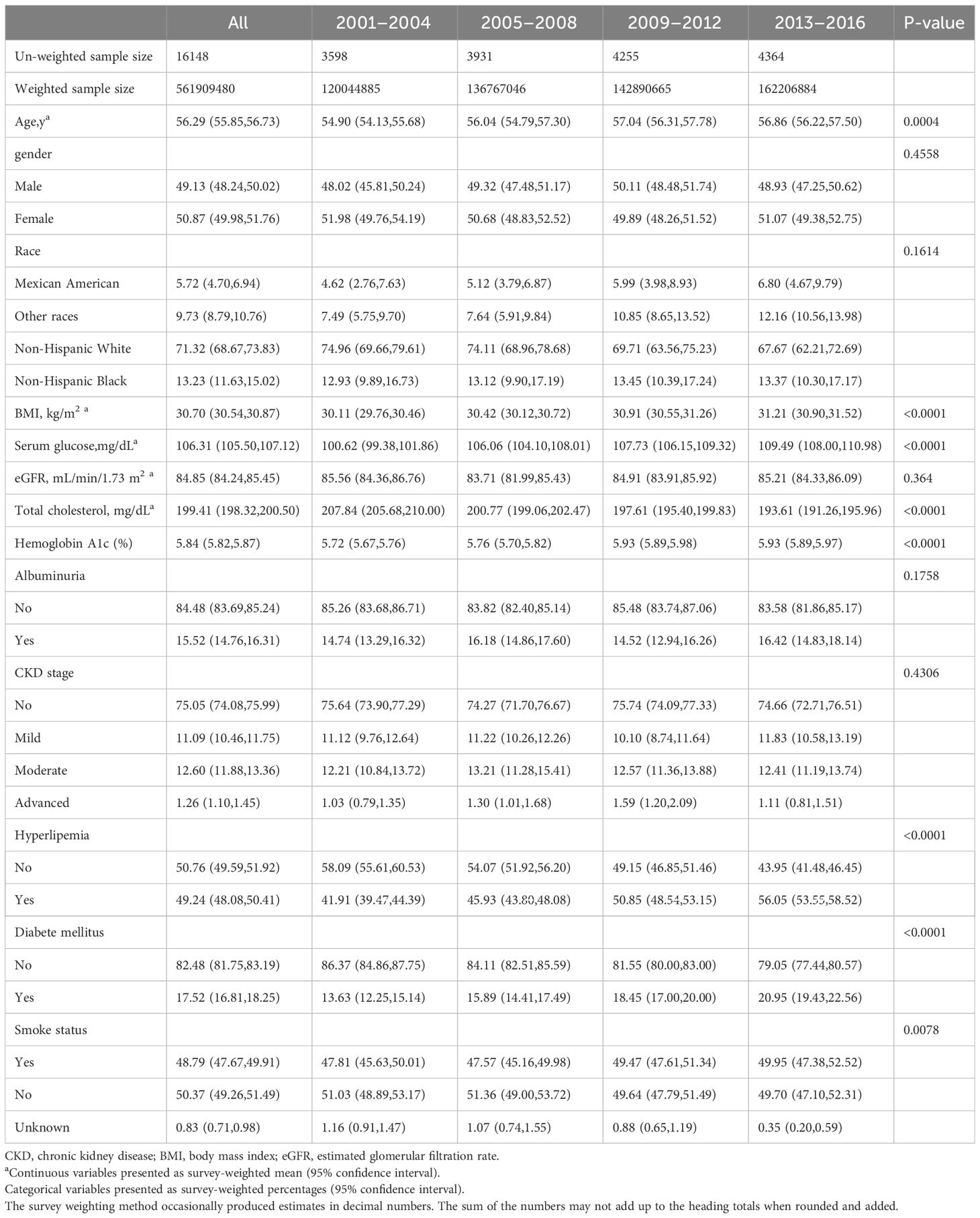
We enrolled 16,148 participants with hypertension, representing 561,909,480 individuals in the U.S. population. The mean age was 56.29 years, with 49.13% being men. In terms of race, non-Hispanic Whites, non-Hispanic Blacks, Mexican Americans, and other races constituted 71.32%, 13.23%, 5.72%, and 9.73%, respectively. Participants with hypertension exhibited a trend toward poor glycemic control, with the proportion of participants with diabetes increasing from 13.63% to 20.95% between 2001 and 2016 and serum glucose levels rising from 100.62 mg/dL to 109.49 mg/dL. The mean eGFR among participants with hypertension was 84.85 mL/min/1.73 m2, and the albuminuria ratio was 15.52%. Detailed information on the basic characteristics and renal function of participants with hypertension from 2001–2016 in the NHANES database is provided in Table 1.
3.2 Prevalence of albuminuria and CKD status in participants with hypertension from 2001–2016
Between 2001 and 2016, the trend in albuminuria exhibited fluctuations but showed no significant change, with albuminuria accounting for 14.74% of the hypertensive population from 2001–2004, 16.18% from 2005–2008, 14.52% from 2009–2012, and 16.42% from 2013–2016 (p for trend 0.3196; Figure 1A). The change in prevalence for most CKD stages was minimal, with the prevalence of CKD stages 1–4 remaining relatively stable: no CKD stage (p for trend 0.7315), mild CKD stage (p for trend 0.6466), moderate CKD stage (p for trend 0.9281), and advanced CKD stage (p for trend 0.6352; Figure 1B). Overall, the albuminuria and CKD stage did not improve significantly in the hypertensive population over time.
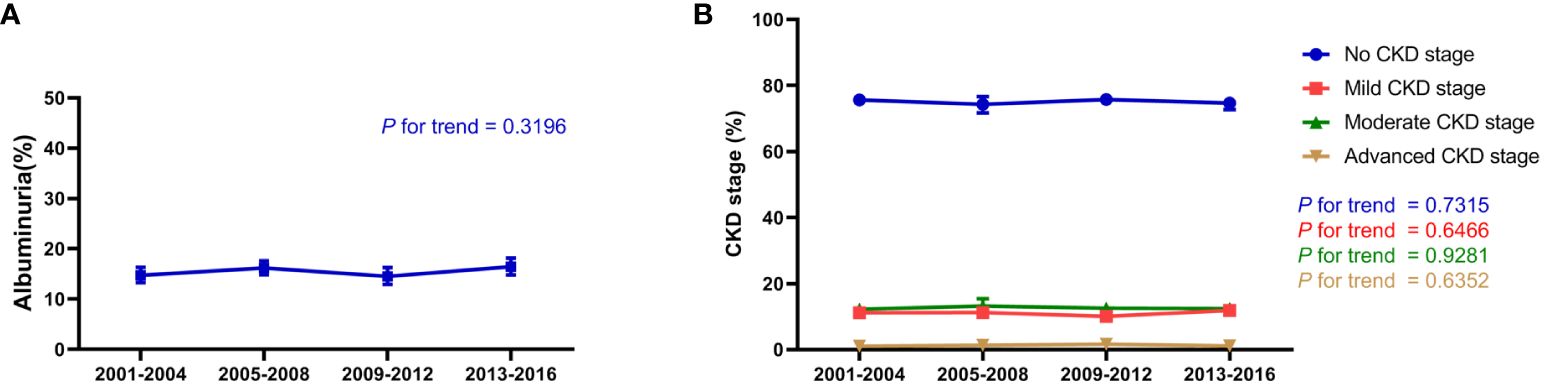
Figure 1 Renal function trend for participants with hypertension in the NHANES (2001–2016). (A) Change in albuminuria ratio in participants with hypertension from 2001–2016; (B) Change in CKD stage, including no CKD, mild CKD, moderate CKD, and advanced CKD in participants with hypertension from 2001–2016. The estimates are presented as survey-weighted means with 95% confidence intervals. The P for trend was analyzed using survey-weighted linear regression. NHANES, National Health and Nutrition Examination Survey; CKD, chronic kidney disease.
3.3 Heterogeneity of CKD stage in participants with hypertension by sex and race
Further analysis of CKD stage heterogeneity in participants with hypertension by sex and race from 2001–2016 was conducted. When adjusted for age, race, BMI, serum glucose, hemoglobin A1c, total cholesterol, hyperlipidemia, diabetes mellitus, and smoking, we observed that women had a higher prevalence of CKD than men, but the differences were not statistically significant. The adjusted ORs and 95% CIs for women were 1.18 (0.96, 1.46) in the 2001–2004 survey, 1.25 (0.96, 1.63) in the 2005–2008 survey, 1.11 (0.90, 1.37) in the 2009–2012 survey, and 1.22 (0.93, 1.51) in the 2013–2016 survey (Figure 2). Similarly, when adjusted for age, sex, BMI, serum glucose, hemoglobin A1c, total cholesterol, hyperlipidemia, diabetes mellitus, and smoking, participants of other races combined with hypertension had a higher CKD ratio relative to non-Hispanic White participants, and the differences were also not statistically significant. The adjusted ORs and 95% CIs for other races were 1.10 (0.83, 1.46) in the 2001–2004 survey, 1.14 (0.86, 1.52) in the 2005–2008 survey, 1.05 (0.82, 1.35) in the 2009–2012 survey, and 0.95 (0.75, 1.18) in the 2013–2016 survey (Figure 3). The disparity in CKD stage between non-Hispanic Whites and other races diminished over the years.
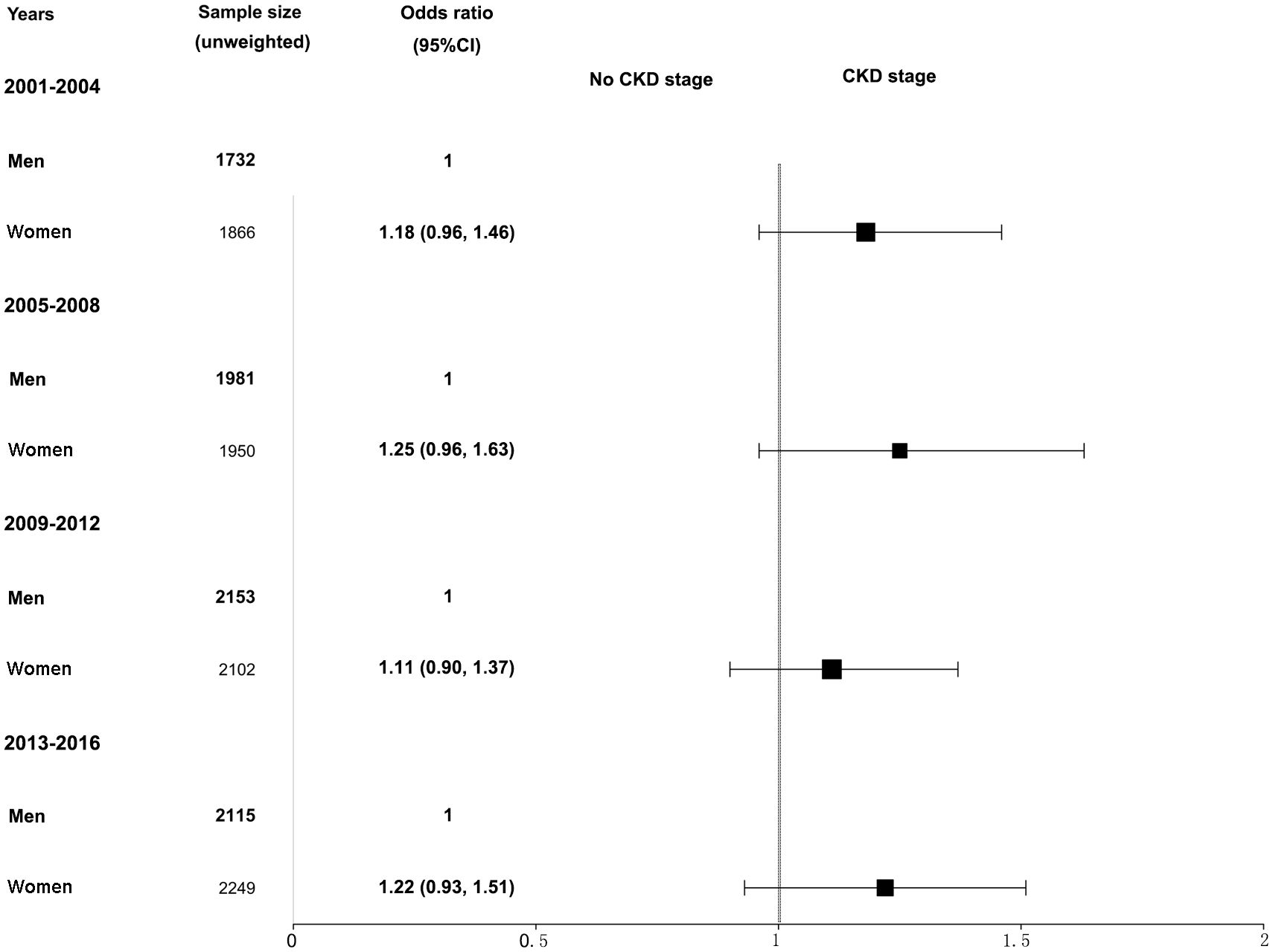
Figure 2 Heterogeneity in the CKD stage in participants with hypertension by sex in the NHANES (2001–2016). The CKD stage was classified as no CKD or existing CKD. An odds ratio >1 indicates a higher risk of CKD. The reference group was male. The analysis results were adjusted for all covariates (age, race, BMI, glucose, total cholesterol, hemoglobin A1c, hyperlipidemia, diabetes mellitus, and smoking status) except for the corresponding subgroup variable, sex. NHANES, National Health and Nutrition Examination Survey; CKD, chronic kidney disease; BMI, body mass index.
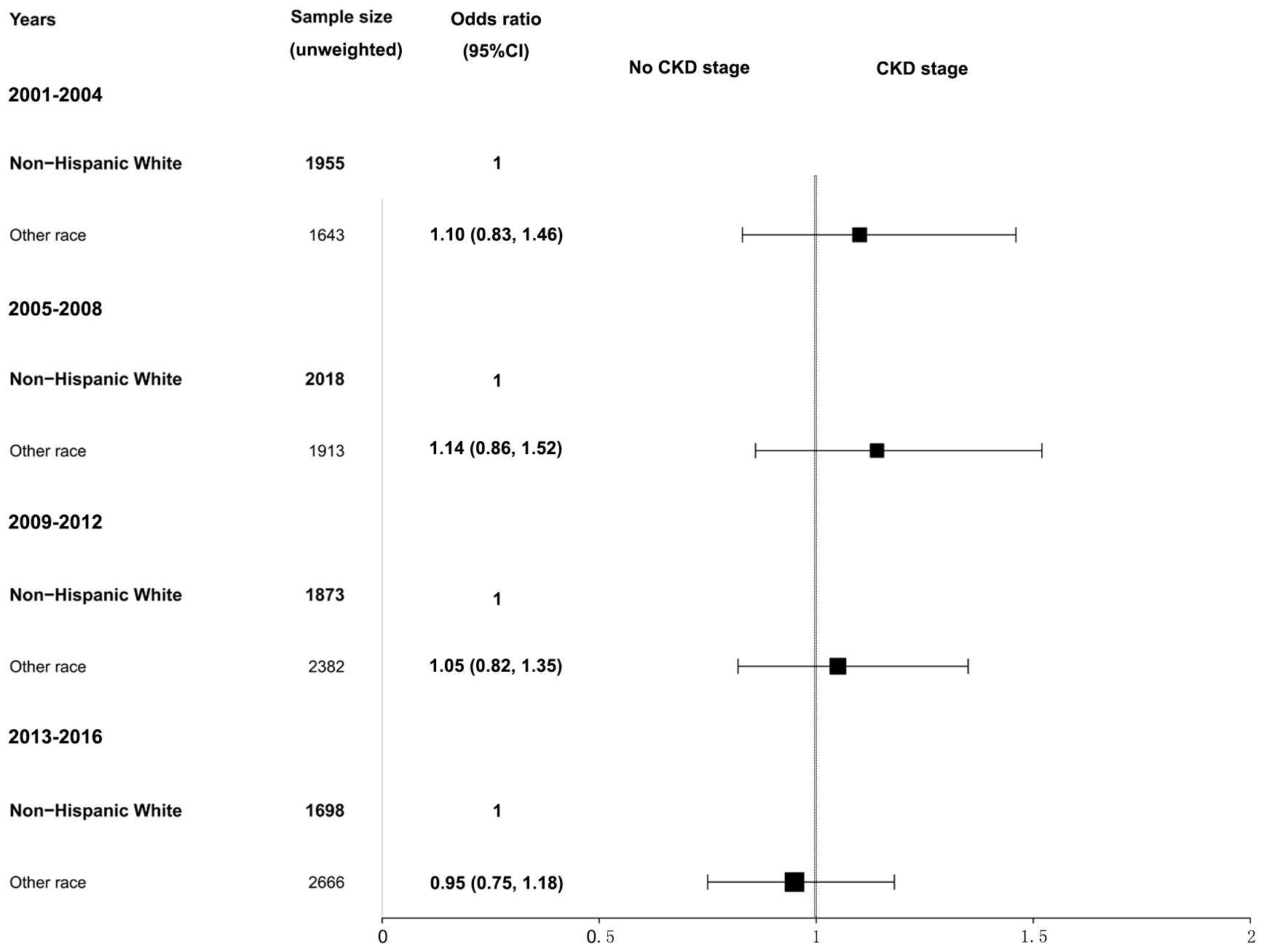
Figure 3 Heterogeneity in the CKD stage in participants with hypertension by race in the NHANES (2001–2016). The CKD stage was classified as no CKD or existing CKD. An odds ratio >1 indicates a higher risk of CKD. The reference group was non-Hispanic White. The analysis results were adjusted for all covariates (age, sex, BMI, glucose, total cholesterol, hemoglobin A1c, hyperlipidemia, diabetes mellitus, and smoking status) except for the corresponding subgroup variable, race. NHANES, National Health and Nutrition Examination Survey; CKD, chronic kidney disease; BMI, body mass index.
4 Discussion
In this study, we revealed a static prevalence of albuminuria and a plateaued prevalence of CKD among participants with hypertension between 2001 and 2016. Furthermore, we observed that women exhibited poorer renoprotection relative to men. We also observed that the prevalence of CKD was higher in other races combined compared with that in the non-Hispanic White population; however, the differences were not statistically significant.
Hypertension is a cause and a factor in the progression of CKD (21, 22). A decline in eGFR is associated with the activation of the RAAS, which exacerbates salt and water retention and heightens salt sensitivity (23). We observed that the CKD stage in the hypertensive population remained relatively stable from 2001–2016. According to the 2017 American College of Cardiology guidelines, all individuals with CKD and hypertension should be treated with a blood pressure target of 130/80 mmHg, regardless of albuminuria (24). Health guidelines from institutions such as the National Institute for Health and Care Research and the UK Renal Association recommend a target of <140/90 mmHg in cases with albuminuria <1g/d and a target of <130/80 mmHg in cases with increased urinary protein leakage. From a therapeutic perspective, angiotensin-converting enzyme inhibitors may be used as first-line agents in patients with hypertension and non-proteinuric CKD (25). Albuminuria is a crucial consideration in hypertension treatment for individuals with CKD, and RAAS blockade appears to offer a blood pressure-independent reduction in albuminuria (26, 27). Implementing this intensive hypertension target in patients with hypertension and CKD would require accurate blood pressure measurement, an understanding of patient preferences and concurrent medical conditions, as well as accurate monitoring of the adverse effects of therapy (28).
The influence of sex on the CKD stage in the hypertensive population remains uncertain. Genetic diversity in the RAAS and α-adrenergic receptors may partly explain the CKD heterogeneity between men and women with hypertension (29, 30). Sex hormones also play a role in vascular reactivity (31). Evidence from previous studies has indicated that young men have a higher prevalence of hypertension around the fifth decade of life than women of similar ages until menopause, causing the incidence of hypertension in women to surpass that in men (30, 31). According to an analysis of the 1999–2000 NHANES, White men have a more favorable situation than women regarding hypertension control owing to increasing treatment rates (32). Variations in access to healthcare, health education, and insurance may contribute to racial heterogeneity, resulting in differences in hypertension control. The development of health education programs aimed at preventing and treating hypertension and the expansion of universal health insurance coverage for non-White races may help reduce disparities in CKD stages based on race and sex.
This study had certain limitations. First, variations in urine albumin and blood creatinine testing methods from 2001–2016 might have introduced bias into the results of this study. Second, owing to the limited sample size of non-White races, we categorized races into non-Hispanic Whites and other races when analyzing racial heterogeneity. The lack of detail in racial categorization may miss the opportunity to illustrate the true burden of CKD in hypertensive populations of non-White races. Third, the inclusion of analytic covariates lacked treatment-related variables, such as antihypertensive medication use and type. Fourth, the eGFR value was based on single measurements, potentially excluding some cases of kidney disease. Finally, to obtain more reliable estimates, we combined survey periods into four-year intervals, and it remains uncertain whether the current findings correspond to the 2017–2020 results. Further validation is warranted when the NHANES data is updated.
5 Conclusion
Our findings revealed no significant improvement in the CKD stage among the hypertensive population from 2001–2016. The analysis of CKD stage heterogeneity by sex and race indicated a higher prevalence of CKD in women relative to men and other racial groups compared with non-Hispanic White individuals, but the differences were not statistically significant. Further research and greater efforts are needed to refine renoprotection strategies in individuals with hypertension to mitigate the persistent burden of CKD and address health disparities among different demographic groups.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Author contributions
JS: Data curation, Writing – review & editing, Software. BW: Data curation, Writing – original draft, Software. LJ: Data curation, Writing – original draft, Software. TC: Conceptualization, Data curation, Writing - original draft, Software. LH: Conceptualization, Data curation, Writing – review & editing, Software. WD: Conceptualization, Data curation, Writing – review & editing, Software.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful to the NHANES database for providing access to the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bian J, Liebert A, Bicknell B, Chen XM, Huang C, Pollock CA. Faecal microbiota transplantation and chronic kidney disease. Nutrients. (2022) 14:2528. doi: 10.3390/nu14122528
2. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. Jama. (2007) 298:2038–47. doi: 10.1001/jama.298.17.2038
3. Hurt L, Wright M, Demmler J, VanDerVoort J, Morris S, Brook F, et al. Mild-to-moderate renal pelvis dilatation identified during pregnancy and hospital admissions in childhood: an electronic birth cohort study in wales, uk. PloS Med. (2019) 16:e1002859. doi: 10.1371/journal.pmed.1002859
4. Yan MT, Chao CT, Lin SH. Chronic kidney disease: strategies to retard progression. Int J Mol Sci. (2021) 22:10084. doi: 10.3390/ijms221810084
5. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: A review. Jama. (2019) 322:1294–304. doi: 10.1001/jama.2019.14745
6. Georgianos PI, Agarwal R. Resistant hypertension in chronic kidney disease (Ckd): prevalence, treatment particularities, and research agenda. Curr Hypertens Rep. (2020) 22:84. doi: 10.1007/s11906-020-01081-x
7. Thomas G, Rahman M. Resistant hypertension in ckd. Clin J Am Soc Nephrol. (2021) 16:467–9. doi: 10.2215/cjn.14610920
8. Kalaitzidis RG, Elisaf MS. Treatment of hypertension in chronic kidney disease. Curr Hypertens Rep. (2018) 20:64. doi: 10.1007/s11906–018-0864–0
9. Ku E, Lee BJ, Wei J, Weir MR. Hypertension in ckd: core curriculum 2019. Am J Kidney Dis. (2019) 74:120–31. doi: 10.1053/j.ajkd.2018.12.044
10. Baker JV, Henry WK, Patel P, Bush TJ, Conley LJ, Mack WJ, et al. Progression of carotid intima-media thickness in a contemporary human immunodeficiency virus cohort. Clin Infect Dis. (2011) 53:826–35. doi: 10.1093/cid/cir497
11. Kozlitina J, Zhou H, Brown PN, Rohm RJ, Pan Y, Ayanoglu G, et al. Plasma levels of risk-variant apol1 do not associate with renal disease in a population-based cohort. J Am Soc Nephrol. (2016) 27:3204–19. doi: 10.1681/asn.2015101121
12. Ramalho SHR, Santos M, Claggett B, Matsushita K, Kitzman DW, Loehr L, et al. Association of undifferentiated dyspnea in late life with cardiovascular and noncardiovascular dysfunction: A cross-sectional analysis from the aric study. JAMA Netw Open. (2019) 2:e195321. doi: 10.1001/jamanetworkopen.2019.5321
13. Sauder KA, Stafford JM, Ehrlich S, Lawrence JM, Liese AD, Marcovina S, et al. Disparities in hemoglobin a(1c) testing during the transition to adulthood and association with diabetes outcomes in youth-onset type 1 and type 2 diabetes: the search for diabetes in youth study. Diabetes Care. (2021) 44:2320–8. doi: 10.2337/dc20–2983
14. Chen W, Eisenberg R, Mowrey WB, Wylie-Rosett J, Abramowitz MK, Bushinsky DA, et al. Association between dietary zinc intake and abdominal aortic calcification in us adults. Nephrol Dial Transplant. (2020) 35:1171–8. doi: 10.1093/ndt/gfz134
15. de Jong Y, Fu EL, van Diepen M, Trevisan M, Szummer K, Dekker FW, et al. Validation of risk scores for ischaemic stroke in atrial fibrillation across the spectrum of kidney function. Eur Heart J. (2021) 42:1476–85. doi: 10.1093/eurheartj/ehab059
16. Charidimou A, Boulouis G, Haley K, Auriel E, van Etten ES, Fotiadis P, et al. White matter hyperintensity patterns in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. (2016) 86:505–11. doi: 10.1212/wnl.0000000000002362
17. Chandra D, Gupta A, Kinney GL, Fuhrman CR, Leader JK, Diaz AA, et al. The association between lung hyperinflation and coronary artery disease in smokers. Chest. (2021) 160:858–71. doi: 10.1016/j.chest.2021.04.066
18. Brand JS, van der Schouw YT, Onland-Moret NC, Sharp SJ, Ong KK, Khaw KT, et al. Age at menopause, reproductive life span, and type 2 diabetes risk: results from the epic-interact study. Diabetes Care. (2013) 36:1012–9. doi: 10.2337/dc12–1020
19. Cabréra L, Auguste A, Michineau L, Joachim C, Deloumeaux J, Luce D. Lung cancer in the french west indies: role of sugarcane work and other occupational exposures. Int J Environ Res Public Health. (2022) 19:13444. doi: 10.3390/ijerph192013444
20. Zou QH, Liu H, Huang CW, Kang LP, Qiu B, Mai JL, et al. Efficacy and safety of gemcitabine and capecitabine combination for patients with previously treated advanced primary pulmonary lymphoepithelioma-like carcinoma: A retrospective single-arm cohort study. Transl Lung Cancer Res. (2023) 12:96–108. doi: 10.21037/tlcr-22–256
21. Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. (2008) 74:867–72. doi: 10.1038/ki.2008.350
22. Pugh D, Gallacher PJ, Dhaun N. Management of hypertension in chronic kidney disease. Drugs. (2019) 79:365–79. doi: 10.1007/s40265–019-1064–1
23. Liu J, Jia W, Yu C. Safety and efficacy of spironolactone in dialysis-dependent patients: meta-analysis of randomized controlled trials. Front Med (Lausanne). (2022) 9:828189. doi: 10.3389/fmed.2022.828189
24. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 Aha/Acc/Hfsa guideline for the management of heart failure: A report of the american college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145:e895–e1032. doi: 10.1161/cir.0000000000001063
25. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 Esc/Esh guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the european society of cardiology and the european society of hypertension: the task force for the management of arterial hypertension of the european society of cardiology and the european society of hypertension. J Hypertens. (2018) 36:1953–2041. doi: 10.1097/hjh.0000000000001940
26. Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: A patient-level meta-analysis. Ann Intern Med. (2003) 139:244–52. doi: 10.7326/0003–4819-139–4-200308190–00006
27. Manley HJ. Role of angiotensin-converting-enzyme inhibition in patients with renal disease. Am J Health Syst Pharm. (2000) 57 Suppl 1:S12–8. doi: 10.1093/ajhp/57.suppl_1.S12
28. Chang AR, Lóser M, Malhotra R, Appel LJ. Blood pressure goals in patients with ckd: A review of evidence and guidelines. Clin J Am Soc Nephrol. (2019) 14:161–9. doi: 10.2215/cjn.07440618
29. Wang JG, Staessen JA. Genetic polymorphisms in the renin-angiotensin system: relevance for susceptibility to cardiovascular disease. Eur J Pharmacol. (2000) 410:289–302. doi: 10.1016/s0014–2999(00)00822–0
30. Duru OK, Li S, Jurkovitz C, Bakris G, Brown W, Chen SC, et al. Race and sex differences in hypertension control in ckd: results from the kidney early evaluation program (Keep). Am J Kidney Dis. (2008) 51:192–8. doi: 10.1053/j.ajkd.2007.09.023
31. Khalil RA. Sex hormones as potential modulators of vascular function in hypertension. Hypertension. (2005) 46:249–54. doi: 10.1161/01.HYP.0000172945.06681.a4
Keywords: prevalence, albuminuria, hypertension, chronic kidney disease, NHANES
Citation: Shen J, Wang B, Jing L, Chen T, Han L and Dong W (2024) Gender and race disparities in the prevalence of chronic kidney disease among individuals with hypertension in the United States, 2001–2016. Front. Endocrinol. 15:1378631. doi: 10.3389/fendo.2024.1378631
Received: 30 January 2024; Accepted: 30 April 2024;
Published: 15 May 2024.
Edited by:
Hongbing Liu, Tulane University, United StatesReviewed by:
Cong Zhao, Tulane University School of Public Health and Tropical Medicine, United StatesShengxu Li, Children’s Minnesota Hospitals and Clinics, United States
Copyright © 2024 Shen, Wang, Jing, Chen, Han and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Han, MjE0MTk1MjkyQHFxLmNvbQ==; Weiwei Dong, ZG9uZ3d3QHNqLWhvc3BpdGFsLm9yZw==
†These authors have contributed equally to this work
 Jing Shen1,2
Jing Shen1,2 Li Jing
Li Jing Weiwei Dong
Weiwei Dong