- 1Department of Pediatrics, China-Japan Friendship Hospital, Beijing, China
- 2Nursing Department, The Fourth Affiliated Hospital of China Medical University, Shengyang, China
- 3Graduate School of Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
Background: The objective of this study was to explore the association between the ratio of serum creatinine to cystatin C to waist circumference (CCR/WC) and hypertension.
Methods: The study utilized data extracted from the China Health and Retirement Longitudinal Study. In the cross-sectional analysis, logistic regression analyses were employed to examine the association between the CCR/WC ratio and hypertension. By utilizing restricted cubic splines, potential non-linear associations between the CCR/WC ratio and hypertension were explored. In the longitudinal analysis, the association between CCR/WC quartiles (Q1–Q4) and the risk of new-onset hypertension was evaluated by Cox proportional-hazards models.
Results: In total, 7,253 participants were enrolled. The study unveiled an inverse association with hypertension, demonstrating an odds ratio (OR) of 0.29 (95% confidence interval [CI]: 0.23–0.37, P < 0.001). Among males, an OR of 0.38 (95% CI: 0.25–0.58, P < 0.001) was observed, while among females, an OR of 0.41 (95% CI: 0.28–0.60, P < 0.001) was noted. There was an absence of a nonlinear association between the CCR/WC ratio and hypertension. Cox regression analysis unveiled a reduced risk of hypertension in Q3 (Hazard ratios [HR]: 0.69, 95% CI: 0.58–0.82, P < 0.001) and Q4: (HR: 0.70, 95% CI: 0.59–0.83, P < 0.001) in compared to the Q1 of the CCR/WC ratio, and sex-specific analysis yielded consistent results.
Conclusion: This study emphasizes the potential association between an elevated CCR/WC ratio and a reduced risk of hypertension.
Introduction
As rapid socio-economic development unfolds, the aging of the Chinese population is further exacerbated (1). The considerable expansion of the aging population will significantly amplify the societal burden of disease and healthcare requirements (2, 3), with particular emphasis on the pervasive attention given to hypertension among these chronic conditions. Hypertension is a noteworthy global public health concern, with a relatively elevated prevalence of 15.4% in the elderly population (4). Unmanaged hypertension can result in severe health complications, encompassing conditions such as heart disease, stroke, renal disorders, visual impairments, among others (5–7). Early identification and intervention of hypertension are crucial.
The pathogenesis of hypertension remains incompletely elucidated, and the risk of hypertension is linked to a variety of factors, including, age, sex, dietary habits, weight, physical activity, smoking, and drinking (8). Abdominal obesity emerges as a significant risk factor for hypertension (9), and leads to the accumulation of fat around visceral organs, known as visceral fat, which can produce various bioactive substances, influencing metabolism and increasing the risk of hypertension (10, 11). Research findings indicate an association between sarcopenia and hypertension (12). The presence of sarcopenia further contributes to the occurrence of obesity, as sarcopenia leads to reduced physical activity and an increased risk of obesity, a condition referred to as sarcopenic obesity (13). The presence of sarcopenic obesity independently represents a risk factor for hypertension (14). Therefore, in assessing the risk of hypertension, mere estimation of obesity alone falls short; consideration should also be given to sarcopenia.
Creatinine is a waste product generated from muscle metabolism and is primarily excreted from the body through the kidneys (15). Cystatin C is a petite protein synthesized by nucleated cells in the body at a consistently constant rate (16). Previous studies have recognized the creatinine to cystatin C ratio (CCR) not only as an alternative marker for sarcopenia and muscle mass assessment (17–20), but also as being associated with mortality among patients in intensive care (21), as well as with low handgrip strength in older adults (22). Recent research has unveiled a certain correlation between the CCR ratio and hypertension (23). Furthermore, waist circumference (WC) measurement is commonly utilized to evaluate abdominal obesity, where an increased waist circumference may signify a higher accumulation of fat in the abdominal area (24). Compared to the body mass index (BMI), WC serves as a convenient measurement for assessing fat distribution, and exhibits a stronger correlation with visceral adipose tissue (25).
Thus, this study hypothesizes a connection between the CCR to WC ratio (CCR/WC) and hypertension. The principal aim of this study is to examine the association between the CCR/WC and hypertension among elderly Chinese adults, employing both cross-sectional and longitudinal research study design. The results derived from this study will assist physicians in gaining a more comprehensive understanding of whether patients belong to the high-risk category for hypertension, thereby facilitating the implementation of suitable interventions.
Materials and methods
Study population
The dataset employed in this study was sourced from the China Health and Retirement Longitudinal Study (CHARLS). CHARLS is a comprehensive longitudinal research initiative, and comprised of 10,257 households and 17,708 middle-aged and elderly participants from 450 villages or urban communities across 150 counties or districts in 28 provinces. It serves as a valuable resource for understanding health and retirement dynamics in China. The detailed information on the sampling design of CHARLS has been published previously (26). CHARLS initiated with a baseline survey in 2011, followed by biennial face-to-face individual interviews, culminating in four waves of follow-up assessments through 2018.
This study comprises two components. In the cross-sectional part, 4,594 participants were excluded based on specific criteria. These criteria included missing age in wave 1 (n=84), absence of creatinine data (n=211), lack of cystatin C information (n=2,757), missing WC data (n=1,342), abnormal WC (n=124), cancer (n=42), and absence of CCR/WC information (n=34). Ultimately, 7,253 participants were included in the cross-sectional study. Abnormal WC and CCR/WC were defined as values greater than three times the interquartile range. In the second part, a longitudinal cohort analysis, we excluded 2,139 participants based on the following criteria: lost follow-up (n=246), missing hypertension (n=14), and having hypertension at baseline (n=1,879). A total of 5,089 participants were included in the cohort study. The study’s flowchart is depicted in Figure 1.
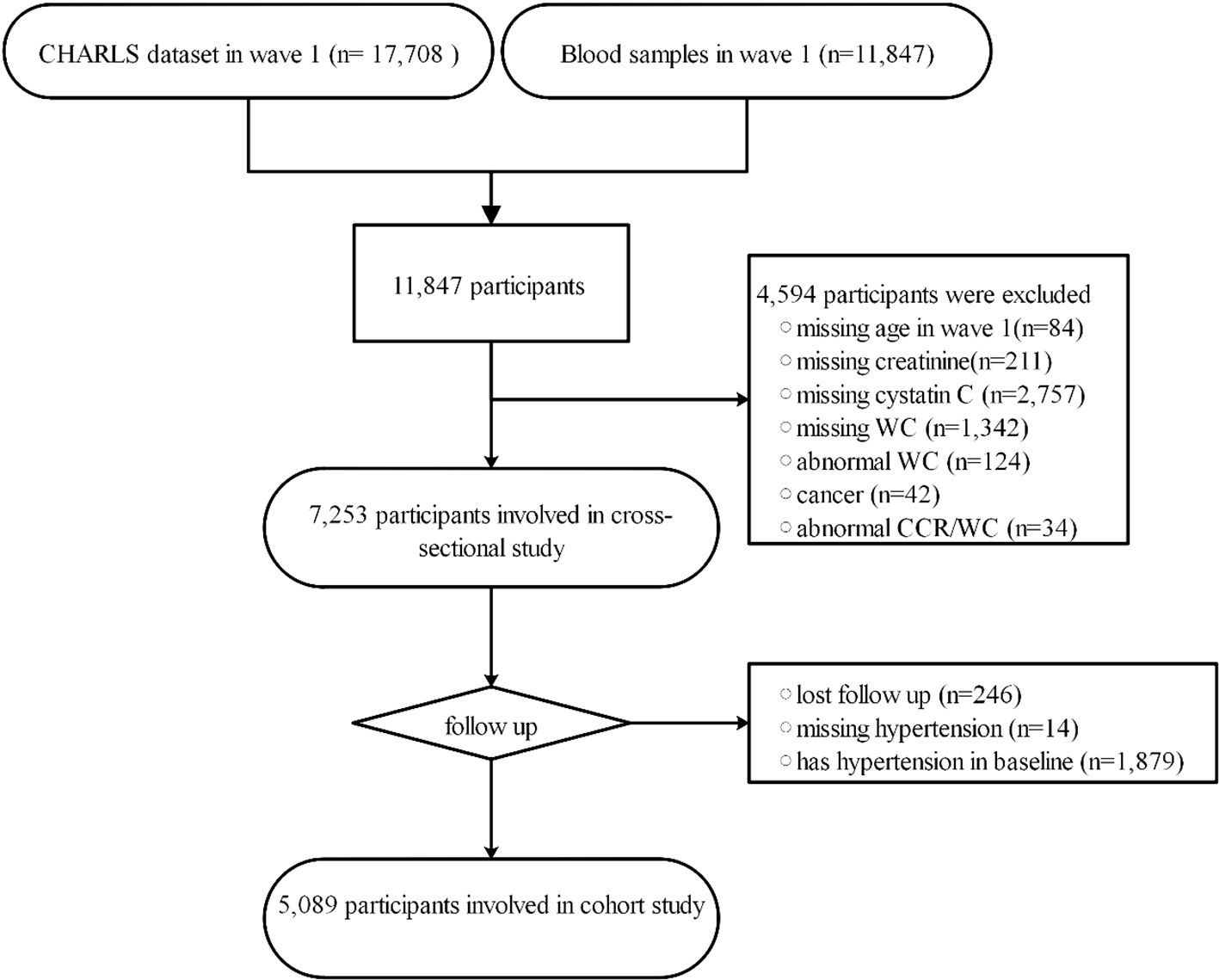
Figure 1 The workflow of study. CCR/WC: Ratio of Serum Creatinine to Cystatin C to Waist Circumference; CHARLS, China Health and Retirement Longitudinal Study; WC, waist circumference.
The study design of CHARLS received approval from the Peking University Ethics Review Committee (IRB-00001052-11014). All participants provided informed consent before the initiation of interviews. This study constitutes a secondary analysis based on existing data, and as such, ethical approval and informed consent were not required.
CCR/WC measurement
Blood samples were collected following an overnight fast. Creatinine measurements were performed utilizing the rate-blanked and compensated Jaffe creatinine assay. Cystatin C measurements were performed using immunoturbidimetric assay with particle enhancement. Comprehensive assay protocols are available in the CHARLS Blood Test Data User Manual (https://charls.charlsdata.com).
The procedure for measuring WC involved placing a measuring tape horizontally at the level of the participant’s waist, aligned with the navel, while the participant stood still, maintained calm breathing, and held their breath at the end of exhalation. The CCR was determined by dividing the creatinine value (mg/dL) by the cystatin C value (mg/L) and multiplying the result by 100, and the CCR/WC was calculated by dividing the CCR by the WC in centimeters (27). The ratio of CCR/WC was obtained at baseline in 2011.
Hypertension assessment
The assessment of hypertension was conducted through self-reported questionnaire interviews, wherein all participants were asked to respond to the question: ‘Have you been diagnosed with hypertension by a doctor?’
Covariates
The study encompassed various covariates, including age (in years), sex, residence, marital status, and educational level (elementary school or below/high school or below/vocational training or below/college or below), smoking status (yes/no), drinking (yes/no), activities of daily living (ADL), and the occurrence of diverse chronic conditions, including diabetes (yes/no), lung disease (yes/no), cardiovascular disease (yes/no), stroke (yes/no), kidney disease (yes/no), and asthma (yes/no). ADL was characterized by self-reported challenges and the requirement for assistance in a minimum of one ADL, encompassing tasks like dressing, bathing, eating, mobility (getting in and out of bed), toileting, and bowel control. Moreover, this study incorporated the following covariates related to glucose metabolism (blood glucose and glycated hemoglobin [HbA1c]), lipid profiles (total cholesterol [TC], low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], and triglycerides [TG]), and uric acid levels (UA).
Statistical analysis
Continuous variables, following a normal distribution, are reported as mean ± standard deviation (SD). Between-group differences in continuous variables were analyzed using either the t-test or Wilcoxon test. Categorical variables are presented as counts (percentages), and group differences for categorical variables were assessed through the chi-square test or Fisher’s exact test.
In the baseline cross-sectional analysis, the link between hypertension and CCR/WC was examined using a logistic regression model, controlling for potential confounding factors, and odds ratios (ORs) were calculated, accompanied by their respective 95% confidence intervals (CIs). The selection of confounding factors was determined based on prior knowledge and meaningful results from univariate analysis (P < 0.05). Restricted cubic spline analysis was employed to explore possible non-linear associations between the CCR/WC with hypertension, aiming to identify potential inflection points and elucidate curvature patterns. Regarding the sensitivity analysis, we excluded individuals with kidney conditions, chronic lung conditions, and asthma at baseline to facilitate a reassessment of the association between hypertension and CCR/WC.
In the cohort study, participants with a pre-existing diagnosis of hypertension were excluded from the analysis. Data on the development of hypertension during follow-up were documented at follow-up visits 2 to visits 4. According to quartiles of CCR/WC, the CCR/WC was divided into four groups. The Kaplan-Meier method was employed to analyze the survival probabilities across different quartiles of the CCR/WC. Additionally, Cox proportional hazards models, controlling for confounding factors, were utilized to assess the association between CCR/WC quartiles with the risk of developing hypertension. Hazard ratios (HRs) with 95% Cis were calculated, offering a quantitative representation of the association between CCR/WC quartiles and hypertension risk.
Statistical analyses were performed using the R software (version 4.1.3). Significance testing was conducted with two-tailed P values, where a threshold of <0.05 denoted statistical significance.
Results
Participant characteristics
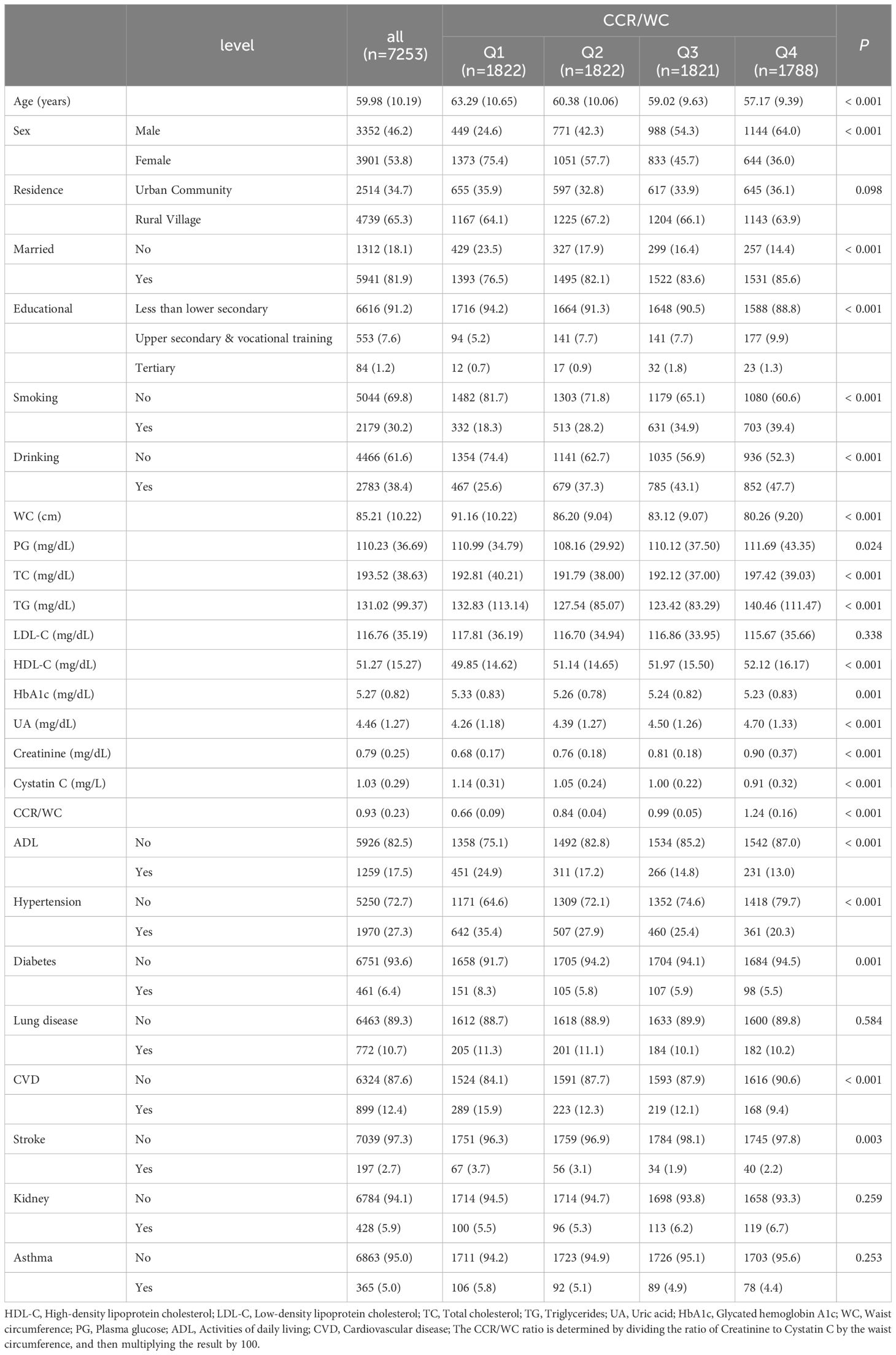
In the present study, 7,253 individuals participated in the cross-sectional analysis, as outlined in Table 1. The mean age was 59.98 years with a standard deviation of 10.19 years. Overall, 46.2% were male, and 53.8% were female. The proportion of participants residing in urban communities was 34.7%, while those in rural villages accounted for 65.3%. Hypertensive individuals comprised 27.3% of the total population. The proportion of participants with education levels beyond high school and vocational training was lower in the lowest quartile (Q1) (5.2%) and higher in the highest quartile (Q4) (9.9%). Most participants had education levels below elementary school. The smoking prevalence was lower in the lowest quartile (18.3%) and higher in the highest quartile (39.4%). Drinking was less common in the lowest quartile (25.6%) and more prevalent in the highest quartile (47.7%). The proportion of hypertensive individuals was lower in the CCR/WC lowest quartile (20.3%) and higher in the Q4 quartile (79.7%).
Association of CCR/WC with hypertension at baseline
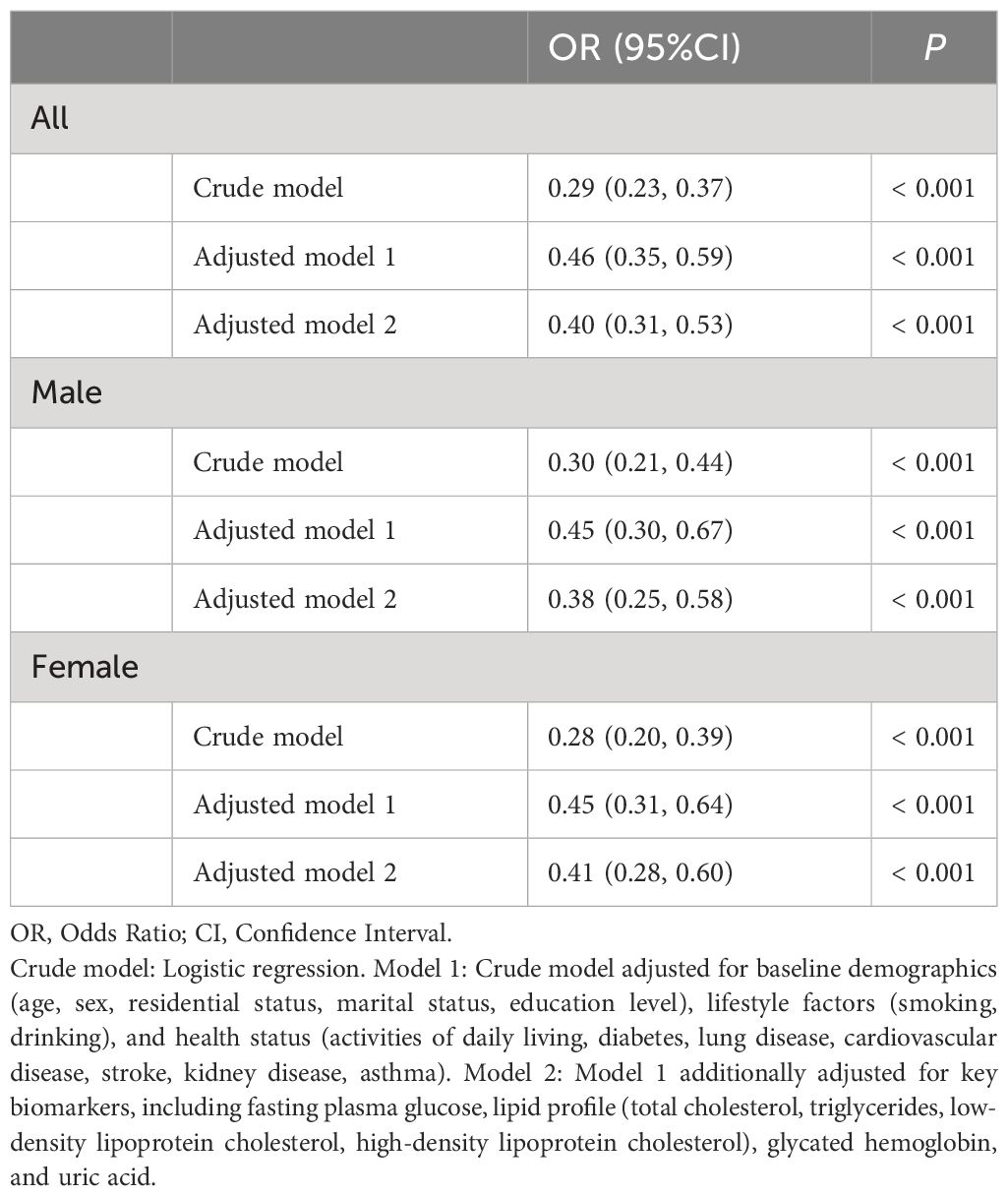
Table 2 illustrates the association between CCR/WC and baseline hypertension. In the crude model, CCR/WC showed a significant inverse association with hypertension (OR: 0.29, 95% CI: 0.23–0.37, P < 0.001). After adjusting for various covariates in model 1, the association remained significant (OR: 0.46, 95% CI: 0.35–0.59, P < 0.001). Further adjustment in model 2 yielded an OR of 0.40 (95% CI: 0.31–0.53, P < 0.001). Among males, the crude model demonstrated a significant inverse association (OR: 0.30, 95% CI: 0.21–0.44, P < 0.001). Adjusting for covariates in model 1 resulted in an OR of 0.45 (95% CI: 0.30–0.67, P < 0.001). Model 2, with further adjustments, showed an OR of 0.38 (95% CI: 0.25–0.58, P < 0.001). Among females, the crude model yielded an OR of 0.28 (95% CI: 0.20–0.39, P < 0.001). In model 1, adjusting for covariates, the OR was 0.45 (95% CI: 0.31–0.64, P < 0.001). Model 2, with additional adjustments, showed an OR of 0.41 (95% CI: 0.28–0.60, P < 0.001). Sensitivity analysis revealed consistent findings with the exclusion of participants having baseline kidney disease, chronic lung disease, or asthma in Supplementary Table 1.
The findings of RCS indicated that there was no non-linear association between the hypertension and CCR/WC. As the CCR/WC increased, a decrease in the OR was observed, indicating a protective effect of the CCR/WC ratio against hypertension, particularly when the CCR/WC ratio exceeded 0.663, as illustrated in Figure 2.
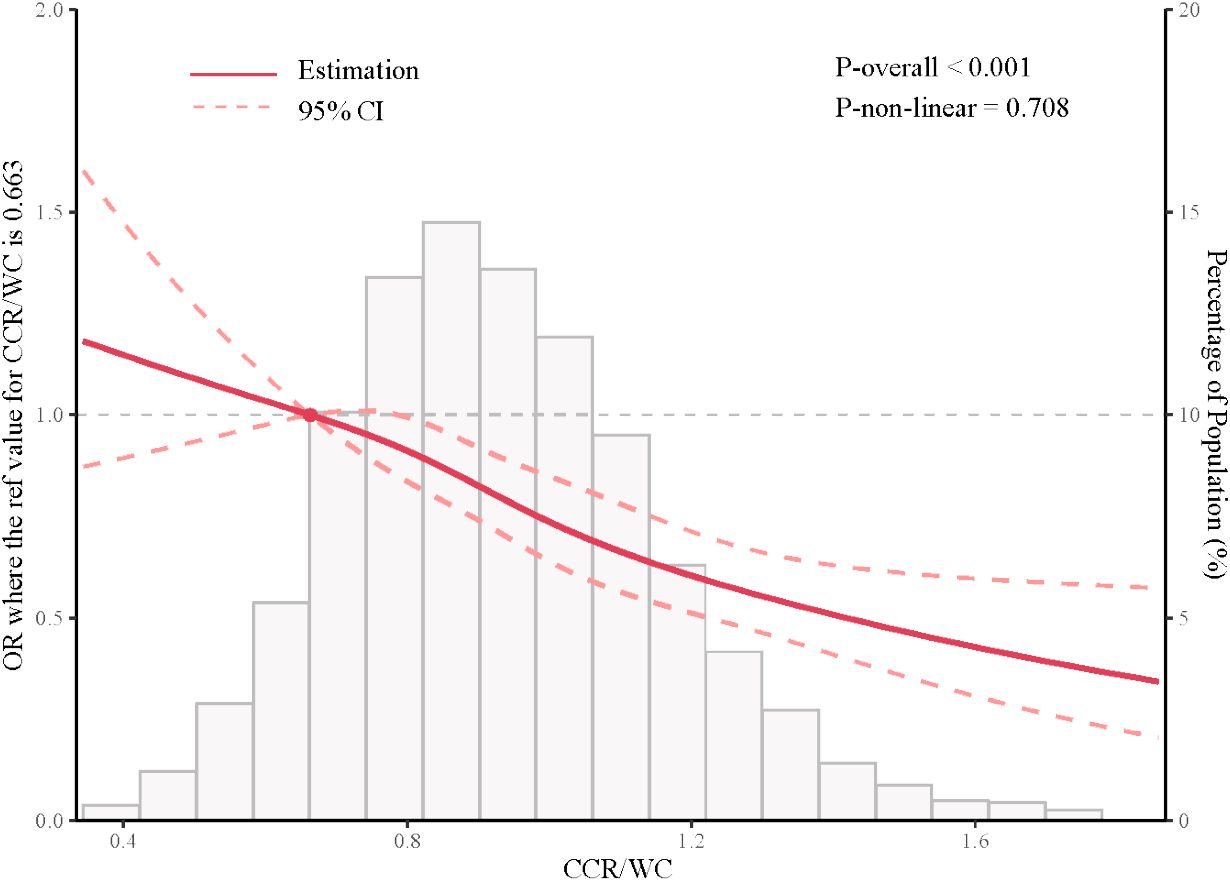
Figure 2 Restricted cubic spline analysis depicting the association between CCR/WC ratio and hypertension. CCR/WC: serum creatinine to cystatin C to waist circumference ratio.
Risk of new onset hypertension of CCR/WC quartiles
A longitudinal analysis was conducted with 5,089 participants free from hypertension at baseline, exploring the association between the CCR/WC and the onset of new hypertension. The CCR/WC was stratified into four groups according to quartiles, and survival analysis demonstrated notable variations in survival rates among the distinct CCR/WC groups (Figure 3, P < 0.001).
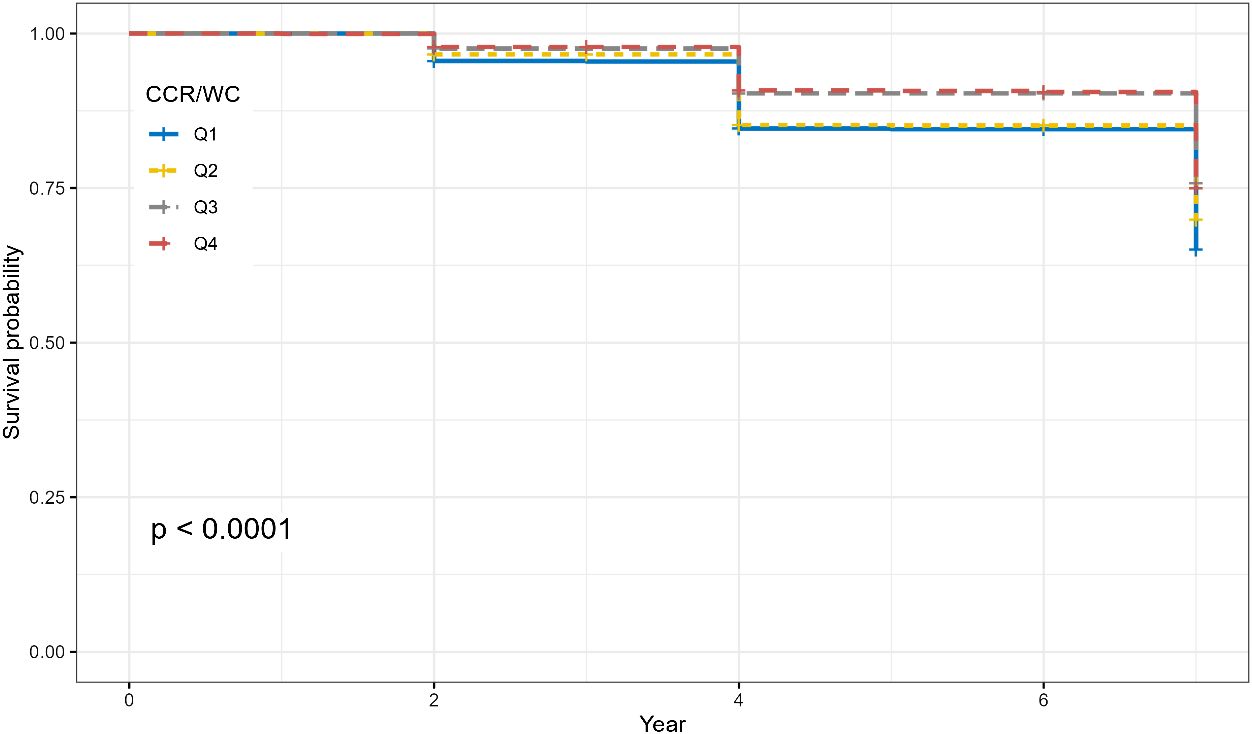
Figure 3 Survival curves illustrating the impact of CCR/WC quartiles on the risk of hypertension. The P value was calculated by the log-rank test.
Furthermore, the results from the Cox regression model demonstrated that in the overall population, individuals in the second quartile (Q2) had a hazard ratio (HR) of 0.84 (95% CI: 0.73–0.98, P = 0.026). Participants in the third quartile (Q3) demonstrated a significantly lower risk with an HR of 0.64 (95% CI: 0.55, 0.75; P < 0.001). The fourth quartile (Q4) also displayed a reduced risk with an HR of 0.66 (95% CI: 0.56–0.77, P < 0.001). After adjusting for multiple covariates, including age, sex, residence, marital status, education, lifestyle factors, and comorbidities, the inverse association persisted. Participants in Q3 still exhibited a reduced risk of new-onset hypertension with an HR of 0.68 (95% CI: 0.57–0.80, P < 0.001). Further adjustments for metabolic and biochemical factors strengthened the observed associations. In the overall population, Q3 and Q4 maintained a reduced risk of new-onset hypertension, with HRs of 0.69 (95% CI: 0.58–0.82, P < 0.001) and 0.70 (95% CI: 0.59–0.83, P < 0.001), respectively. Sex-specific analysis yielded consistent results, indicating that the inverse association between CCR/WC and new-onset hypertension remained robust across different models (Table 3).
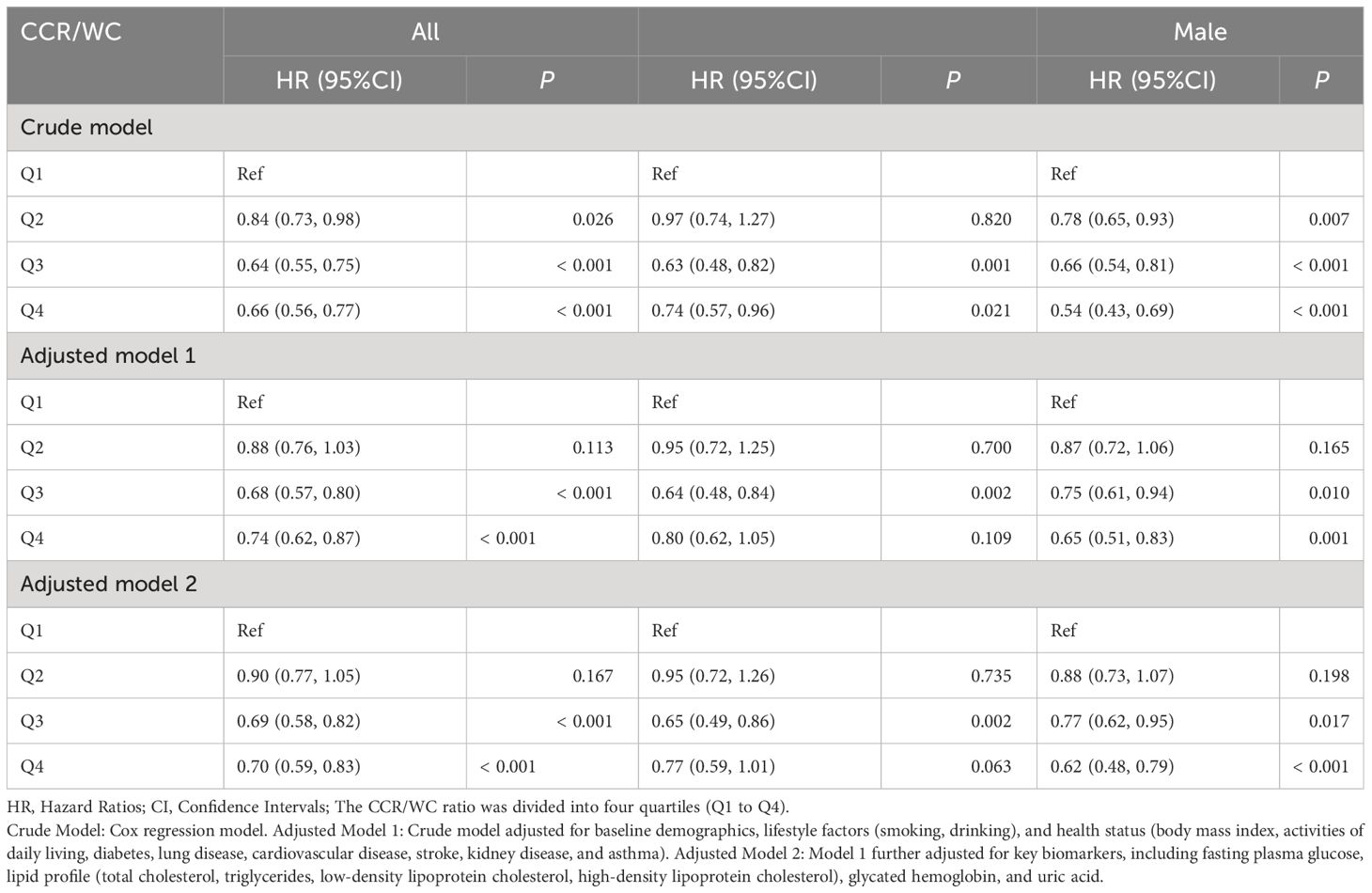
Table 3 Association between baseline CCR/WC and new-onset hypertension in longitudinal cohort analysis.
Discussion
This study, based on a population of elderly Chinese individuals, utilized both cross-sectional and longitudinal cohort designs to explore the association between hypertension and CCR/WC. The study results suggest that the CCR/WC serves as a protective factor against hypertension, which was further corroborated through longitudinal analysis. Sex-specific analysis revealed no significant differences in this protective effect between males and females. Additionally, restricted cubic spline analysis indicated the absence of a non-linear association between hypertension and the CCR/WC.
To our knowledge, this study represents the initial exploration into the association between the risk of hypertension and the CCR/WC. CCR is acknowledged as an indicator of muscle wasting, serving as an alternative measure for assessing muscle mass (28, 29). Prior investigations have proposed cystatin C as a predictive biomarker in pulmonary hypertension (30). However, these investigations did not incorporate the independent influences of muscle mass and adipose tissue measurements when evaluating the risk of hypertension. Prior studies have also suggested a link between abdominal obesity and an elevated risk of hypertension (31). Moreover, obesity may contribute to lipid abnormalities, potentially heightening the risk of conditions related to hypertension (32). Hence, we utilized the CCR/WC to discern between lean body mass and abdominal obesity in this investigation. Our study reveals that a higher CCR/WC ratio is associated with a decreased risk of hypertension development. A cross-sectional study may introduce potential reverse causality. Therefore, we followed up with individuals who did not have hypertension at baseline. The results from longitudinal analysis substantiated a causal link between the CCR/WC and the development of hypertension. The CCR/WC ratio shows potential as a predictive marker for conditions influenced by both muscle mass and obesity. Its validation in various contexts is a prospect for future exploration.
Several potential mechanisms explain the association between CCR/WC, and hypertension. Firstly, Creatinine and cystatin C independently serve as markers of renal health (33). CCR, leveraging both markers, yields a more stable and reliable glomerular filtration rate (GFR) metric, enhancing the accuracy of renal function assessment (34). An elevated level of CCR may suggest an increase in the GFR (35–37), which plays a pivotal role in maintaining fluid balance and regulating blood pressure (38). Enhanced GFR facilitates the effective excretion of sodium and waste, thereby aiding in the prevention of volume overload—a key contributor to the development of hypertension (39). Consequently, the heightened GFR, as indicated by an increased CCR, ostensibly lowers the risk of hypertension through effective fluid balance management and blood pressure regulation. This aspect potentially elucidates a portion of the mechanism underlying the association between the CCR/WC ratio and hypertension. Additionally, muscle tissue is a critical component of the body’s metabolism (40), and CCR/WC indirectly reflects muscle mass. A relatively higher CCR/WC ratio may indicate more muscle mass, which is essential for glucose metabolism and insulin sensitivity (41). Thus, individuals with an elevated CCR/WC may be better equipped to maintain normal metabolism, reducing the risk of hypertension. Finally, abdominal obesity constitutes a risk factor for hypertension (42), and considering it in conjunction with CCR/WC may offer a more thorough comprehension of the pathophysiology underlying hypertension. A higher CCR/WC ratio may reflect lower abdominal fat accumulation, helping to reduce visceral fat accumulation. Visceral fat represents a substantial source of bioactive substances and might contribute to the development of hypertension (43). However, additional research is needed to fully uncover the precise pathways and underlying factors contributing to these associations.
While our study contributes valuable insights, it is important to recognize its limitations. Firstly, although we controlled for numerous covariates, there are still some variables related to hypertension that were not taken into consideration, such as dietary habits (44), albuminuria (45), physical activity (46), and information on antihypertensive treatments such as ACE inhibitors, ARBs, and aldosterone antagonists. In future studies, it will be essential to incorporate these variables into hypertension-related analyses. Secondly, our study is observational, relying on self-reported questionnaires for data collection, including hypertension, which may introduce sampling errors and information bias. Future research should aim for more accurate data collection methods by employing randomized controlled trials, exploring biological mechanisms, and conducting intervention trials to more precisely establish causality. Thirdly, our study includes a higher proportion of residents from rural areas. These individuals may face limitations in accessing healthcare resources and possibly have lower health awareness. To address this, we adjusted for place of residence in our analysis and employed professionally trained personnel to conduct one-on-one interviews. This approach was designed to minimize information bias stemming from definitions of hypertension and awareness of comorbid conditions. Lastly, we utilized four wave data of CHARLS. Subsequent study should involve greater sample sizes, longer term, and multicenter studies to determine the longitudinal changes and development of hypertension associated with CCR/WC ratio. Despite these limitations, our study provides valuable insights into the association between CCR/WC ratio and hypertension in elderly individuals and lays the foundation for further research in this field. Despite these constraints, our study offers significant findings regarding the association between the hypertension and CCR/WC in Chinese older adults, establishing the foundation for further research in this field.
The findings of our study carry noteworthy implications for both clinical practice and public health policy. Firstly, our findings offer clinicians a simple yet potentially predictive tool. The CCR/WC ratio can be utilized for screening and assessing hypertension risk, particularly in the elderly population, who may be at a higher risk for hypertension (47). Secondly, based on the CCR/WC ratio, clinicians can personalize treatment plans more effectively. For hypertensive individuals with higher CCR/WC ratios, a greater emphasis on managing muscle mass and abdominal obesity, possibly through tailored dietary and exercise programs, may be warranted to control hypertension (48). Lastly, the findings of this study bear importance in shaping public health policies. Governments and health authorities may consider incorporating the CCR/WC ratio as part of hypertension risk assessment, including it in routine health check-ups and community health promotion activities. By enhancing public awareness and understanding of the CCR/WC ratio, it is possible to facilitate the early detection and management of hypertension on a broader scale.
Conclusions
In conclusion, our study illuminates the association between hypertension and CCR/WC in elderly Chinese adults, and an elevated CCR/WC is linked to a diminished risk of hypertension. Our study implies that supervising and enhancing the CCR/WC could be a valuable strategy in the control and management of hypertension.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://charls.charlsdata.com/index/zh-cn.html.
Ethics statement
The studies involving humans were approved by Peking University Ethics Review Committee (IRB-00001052-11014). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YY: Methodology, Conceptualization, Writing – original draft, Writing – review & editing. QS: Conceptualization, Writing – original draft, Writing – review & editing. SM: Data curation, Writing – original draft, Writing – review & editing. XDL: Data curation, Writing – original draft, Writing – review & editing. XML: Data curation, Writing – original draft, Writing – review & editing. QZ: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by MOE Key Laboratory of Population Health Across Life Cycle (No: JK20225), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No: 2021-I2M-1-049), and Chinese Academy of Medical Sciences Clinical and Translational Medicine Research Project (No: 2021-I2M-C&T-B-089).
Acknowledgments
We express our gratitude to the CHARLS research team for providing us with the data utilized in this study. Specifically, the analysis was conducted using information and data obtained from the Harmonized CHARLS dataset and Codebook, Version D as of June 2021, which were developed by the Gateway to Global Aging Data. Notably, the development of the Harmonized CHARLS was funded by the National Institute on Aging (R01 AG030153, RC2AG036619, R03 AG043052).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1375232/full#supplementary-material
References
1. Wang Z, Wei L, Zhang X, Qi G. Impact of demographic age structure on energy consumption structure: Evidence from population aging in mainland China. Energy. (2023) 273:127226. doi: 10.1016/j.energy.2023.127226
2. Zhang R, Lu Y, Shi L, Zhang S, Chang F. Prevalence and patterns of multimorbidity among the elderly in China: a cross-sectional study using national survey data. BMJ Open. (2019) 9:e024268. doi: 10.1136/bmjopen-2018-024268
3. Yip W, Fu H, Chen AT, Zhai T, Jian W, Xu R, et al. 10 years of health-care reform in China: progress and gaps in universal health coverage. Lancet. (2019) 394:1192–204. doi: 10.1016/S0140-6736(19)32136-1
4. Fan Z-Y, Yang Y, Zhang C-H, Yin R-Y, Tang L, Zhang F. Prevalence and patterns of comorbidity among middle-aged and elderly people in China: A cross-sectional study based on CHARLS data. Int J Gen Med. (2021) 14:1449–55. doi: 10.2147/IJGM.S309783
5. Cheung CY, Biousse V, Keane PA, Schiffrin EL, Wong TY. Hypertensive eye disease. Nat Rev Dis Primers. (2022) 8:14. doi: 10.1038/s41572-022-00342-0
6. Balwan WK, Kour S. A systematic review of hypertension and stress-the silent killers. Sch Acad J Biosci. (2021) 6:150–4. doi: 10.36347/sajb.2021.v09i06.002
7. Zoccali C, Mark PB, Sarafidis P, Agarwal R, Adamczak M, Bueno de Oliveira R, et al. Diagnosis of cardiovascular disease in patients with chronic kidney disease. Nat Rev Nephrol. (2023) 19(11):733–46. doi: 10.1038/s41581-023-00747-4
8. Humbert X, Licaj I, Fedrizzi S, Alexandre J, Menotti A, Manrique A, et al. Relationship between lifestyle factors and hypertension: A cross-sectional analysis from the Gubbio study. Acta Cardiologica. (2023) 78:565–73. doi: 10.1080/00015385.2022.2088170
9. Zhao Y, Qin P, Sun H, Liu Y, Liu D, Zhou Q, et al. Metabolically healthy general and abdominal obesity are associated with increased risk of hypertension. Br J Nutr. (2020) 123:583–91. doi: 10.1017/S0007114519003143
10. Feng Y, Yang X, Li Y, Wu Y, Han M, Qie R, et al. Metabolic Score for Visceral Fat: A reliable indicator of visceral obesity for predicting risk for hypertension. Nutrition. (2022) 93:111443. doi: 10.1016/j.nut.2021.111443
11. Fan Y, He D, Liu S, Qiao Y, Gao H, Xin L. Association between visceral adipose index and risk of hypertension in a middle-aged and elderly Chinese population. Nutrition Metab Cardiovasc Dis. (2021) 31:2358–65. doi: 10.1016/j.numecd.2021.04.024
12. Bai T, Fang F, Li F, Ren Y, Hu J, Cao J. Sarcopenia is associated with hypertension in older adults: a systematic review and meta-analysis. BMC geriatrics. (2020) 20:1–9. doi: 10.1186/s12877-020-01672-y
13. Zamboni M, Rubele S, Rossi AP. Sarcopenia and obesity. Curr Opin Clin Nutr Metab Care. (2019) 22:13–9. doi: 10.1097/MCO.0000000000000519
14. Park SH, Park JH, Song PS, Kim DK, Kim KH, Seol SH, et al. Sarcopenic obesity as an independent risk factor of hypertension. J Am Soc Hypertension. (2013) 7:420–5. doi: 10.1016/j.jash.2013.06.002
15. Kashani K, Rosner MH, Ostermann M. Creatinine: From physiology to clinical application. Eur J Internal Med. (2020) 72:9–14. doi: 10.1016/j.ejim.2019.10.025
16. Jha P, Jha AK, Dayal VM, Jha SK, Kumar A. Baseline serum cystatin C as a marker of acute kidney injury in patients with acute-on-chronic liver failure. Indian J Gastroenterol. (2021) 40:563–71. doi: 10.1007/s12664-021-01201-8
17. Zheng C, Wang E, Li J-S, Xie K, Luo C, Ge Q-Y, et al. Serum creatinine/cystatin C ratio as a screening tool for sarcopenia and prognostic indicator for patients with esophageal cancer. BMC geriatrics. (2022) 22:1–11. doi: 10.1186/s12877-022-02925-8
18. Hirai K, Tanaka A, Homma T, Goto Y, Akimoto K, Uno T, et al. Serum creatinine/cystatin C ratio as a surrogate marker for sarcopenia in patients with chronic obstructive pulmonary disease. Clin Nutr. (2021) 40:1274–80. doi: 10.1016/j.clnu.2020.08.010
19. Tang T, Xie L, Hu S, Tan L, Lei X, Luo X, et al. Serum creatinine and cystatin C-based diagnostic indices for sarcopenia in advanced non-small cell lung cancer. J Cachexia Sarcopenia Muscle. (2022) 13:1800–10. doi: 10.1002/jcsm.12977
20. Rizk JG, Streja E, Wenziger C, Shlipak MG, Norris KC, Crowley ST, et al. Serum creatinine-to-cystatin-C ratio as a potential muscle mass surrogate and racial differences in mortality. J Renal Nutr. (2023) 33:69–77. doi: 10.1053/j.jrn.2021.11.005
21. Jung C-Y, Joo YS, Kim HW, Han SH, Yoo T-H, Kang S-W, et al. Creatinine–cystatin C ratio and mortality in patients receiving Intensive Care and Continuous kidney Replacement Therapy: a retrospective cohort study. Am J Kidney Dis. (2021) 77:509–16. doi: 10.1053/j.ajkd.2020.08.014
22. Tan L, Li R, Hu X, Zhu Y, Bao T, Zuo Y, et al. Serum creatinine/cystatin C ratio as a case-finding tool for low handgrip strength in Chinese middle-aged and older adults. Sci Rep. (2020) 10:14028. doi: 10.1038/s41598-020-71028-4
23. Liao L, Shi S, Ding B, Zhang R, Tu J, Zhao Y, et al. The relationship between serum creatinine/cystatin C ratio and mortality in hypertensive patients. Nutr Metab Cardiovasc Dis. (2024) 34(2):369–76.
24. Bojanic D, Ljubojevic M, Krivokapic D, Gontarev S. Waist circumference, waist-to-hip ratio, and waist-to-height ratio reference percentiles for abdominal obesity among Macedonian adolescents. Nutricion hospitalaria. (2020) 37:786–93. doi: 10.20960/nh.03006
25. Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. (2020) 16:177–89. doi: 10.1038/s41574-019-0310-7
26. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol. (2014) 43:61–8. doi: 10.1093/ije/dys203
27. Chen Y, Wen W, Mai Z, Wang M, Chen H, Sun J. The serum creatinine to cystatin C to waist circumference ratios predicts risk for type 2 diabetes: A Chinese cohort study. J Diabetes. (2023) 15(10):808–16. doi: 10.1111/1753-0407.13435
28. Zheng W-H, Zhu Y-B, Yao Y, Huang H-B. Serum creatinine/cystatin C ratio as a muscle mass evaluating tool and prognostic indicator for hospitalized patients: A meta-analysis. Front Med. (2023) 9:1058464. doi: 10.3389/fmed.2022.1058464
29. Yamada Y, Umegaki H, Sugimoto T, Nagae M, Komiya H, Watanabe K, et al. Relationship of creatinine cystatin C ratio with muscle mass and grip strength in memory clinic outpatients. Exp Gerontol. (2022) 168:111935. doi: 10.1016/j.exger.2022.111935
30. Duan A, Huang Z, Zhao Z, Zhao Q, Jin Q, Yan L, et al. The potential of cystatin C as a predictive biomarker in pulmonary hypertension. BMC Pulmonary Med. (2023) 23:1–11. doi: 10.1186/s12890-023-02595-1
31. Dhawan D, Sharma S. Abdominal obesity, adipokines and non-communicable diseases. J Steroid Biochem Mol Biol. (2020) 203:105737. doi: 10.1016/j.jsbmb.2020.105737
32. Zhu X-P, Han G-C, Chen Q, Zhang Z-Y, Wang L-S, Zhang B. Fatty liver is a sensitive early warning for hypertension and its complication in the Chinese population. Clin Exp Hypertension. (2022) 44:306–12. doi: 10.1080/10641963.2022.2029469
33. Chen S, Li J, Liu Z, Chen D, Zhou L, Hu D, et al. Comparing the value of cystatin C and serum creatinine for evaluating the renal function and predicting the prognosis of COVID-19 patients. Front Pharmacol. (2021) 12:587816. doi: 10.3389/fphar.2021.587816
34. Tio MC, Shafi T, Zhu X, Kalantar-Zadeh K, Chan A, Nguyen L. Traditions and innovations in assessment of glomerular filtration rate using creatinine to cystatin C. Curr Opin Nephrol hypertension. (2023) 32:89–97. doi: 10.1097/MNH.0000000000000854
35. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. (2012) 367:20–9. doi: 10.1056/NEJMoa1114248
36. Tsai C-W, Grams ME, Inker LA, Coresh J, Selvin E. Cystatin C–and creatinine-based estimated glomerular filtration rate, vascular disease, and mortality in persons with diabetes in the US. Diabetes Care. (2014) 37:1002–8. doi: 10.2337/dc13-1910
37. Pasala S, Carmody JB. How to use… serum creatinine, cystatin C and GFR. Arch Dial Transplant. Practice. (2017) 102:37–43. doi: 10.1136/archdischild-2016-311062
38. Agarwal R, Light RP. GFR, proteinuria and circadian blood pressure. Nephrol Dialysis Transplantation. (2009) 24:2400–6. doi: 10.1093/ndt/gfp074
39. Shin J, Lee CH. The roles of sodium and volume overload on hypertension in chronic kidney disease. Kidney Res Clin Practice. (2021) 40:542. doi: 10.23876/j.krcp.21.800
40. Chadt A, Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflügers Archiv-European J Physiol. (2020) 472:1273–98. doi: 10.1007/s00424-020-02417-x
41. Jaiswal N, Gavin MG, Quinn Iii WJ, Luongo TS, Gelfer RG, Baur JA, et al. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol Metab. (2019) 28:1–13. doi: 10.1016/j.molmet.2019.08.001
42. Shen C, Zhou Z, Lai S, Tao X, Zhao D, Dong W, et al. Urban-rural-specific trend in prevalence of general and central obesity, and association with hypertension in Chinese adults, aged 18–65 years. BMC Public Health. (2019) 19:1–8. doi: 10.1186/s12889-019-7018-4
43. Chrysant SG. Pathophysiology and treatment of obesity-related hypertension. J Clin Hypertension. (2019) 21:555–9. doi: 10.1111/jch.13518
44. Di Raimondo D, Buscemi S, Musiari G, Rizzo G, Pirera E, Corleo D, et al. Ketogenic diet, physical activity, and hypertension—a narrative review. Nutrients. (2021) 13:2567. doi: 10.3390/nu13082567
45. Lin Y-P. Albuminuria in hypertension. Hypertension Res. (2013) 36:762–4. doi: 10.1038/hr.2013.76
46. Pescatello LS, Buchner DM, Jakicic JM, Powell KE, Kraus WE, Bloodgood B, et al. Physical activity to prevent and treat hypertension: a systematic review. Med Sci Sports Exercise. (2019) 51:1314–23. doi: 10.1249/MSS.0000000000001943
47. Hu L, Huang X, Zhou W, You C, Li J, Li P, et al. Effect of hypertension status on the association between sleep duration and stroke among middle-aged and elderly population. J Clin Hypertension. (2020) 22:65–73. doi: 10.1111/jch.13756
Keywords: serum creatinine, cystatin C, waist circumference, hypertension, abdominal obesity
Citation: Yang Y, Sun Q, Ma S, Li X, Lang X and Zhang Q (2024) Association of serum creatinine to cystatin C to waist circumference ratios and hypertension: evidence from China health and retirement longitudinal study. Front. Endocrinol. 15:1375232. doi: 10.3389/fendo.2024.1375232
Received: 23 January 2024; Accepted: 16 April 2024;
Published: 01 May 2024.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Takafumi Osaka, Kyoto Prefectural University, JapanMorales-Alvarez Mc, University of Alabama at Birmingham, United States
Copyright © 2024 Yang, Sun, Ma, Li, Lang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Zhang, emhhbmdxaWtleWFuQDE2My5jb20=
†These authors have contributed equally to this work
 Yang Yang1†
Yang Yang1† Qi Sun
Qi Sun Qi Zhang
Qi Zhang