- The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Background: Anaplastic thyroid cancer (ATC) is highly invasive, prone to distant metastasis (DM), and has a very poor prognosis. This study aims to construct an accurate survival prediction model for ATC patients with DM, providing reference for comprehensive assessment and treatment planning.
Methods: We extracted data of ATC patients with DM diagnosed between 2004 and 2019 from the SEER database, randomly dividing them into a training set and a validation set in a ratio of 7:3. Univariate and multivariate Cox regression analyses were sequentially performed on the training set to identify independent prognostic factors for overall survival (OS) and construct nomograms for 3-month, 6-month, and 8-month OS for ATC patients with DM based on all identified independent prognostic factors. Receiver operating characteristic (ROC) curve analysis, decision curve analysis (DCA) curve analysis, and calibration curves were separately plotted on the training and validation sets to demonstrate the model’s performance. Furthermore, patients were stratified into high- and low-risk groups based on their risk scores, and the Kaplan-Meier (KM) survival curves were used to illustrate the survival differences between the two groups.
Results: A total of 322 patients were included in this study. Univariate and multivariate Cox regression analyses identified five independent prognostic factors for OS in ATC patients with DM: surgery, tumor size, age, chemotherapy, and radiotherapy. Nomograms for 3-month, 6-month, and 8-month OS were established based on these factors. The training set AUC values (3-month AUC: 0.767, 6-month AUC: 0.789, 8-month AUC: 0.795) and validation set AUC values (3-month AUC: 0.753, 6-month AUC: 0.798, 8-month AUC: 0.806) as well as the calibration curves demonstrated excellent applicability and accuracy of the model. Additionally, the DCA curves indicated substantial clinical net benefit of the model. The KM curves also confirmed the model’s excellent stratification ability for patient OS.
Conclusion: The nomogram developed in this study accurately predicts OS for ATC patients with DM. It can assist clinicians in formulating appropriate treatment strategies for these patients.
1 Introduction
Anaplastic thyroid cancer (ATC) is a rare but highly aggressive malignancy (1). Although it accounts for less than 2% of all types of thyroid cancer, it contributes to more than 50% of the annual mortality rate associated with thyroid cancer (2, 3). The median survival time for ATC is approximately four months (4), and the disease-specific mortality rate ranges from 98% to 99% (5, 6). The most notable clinical feature of ATC is its high invasiveness, which leads to local infiltration and metastasis to regional lymph nodes or distant organs (7). Around 50% of ATC patients are diagnosed with distant metastasis (DM) at the time of diagnosis (8, 9), resulting in a significantly worse prognosis. Current treatment options for ATC patients with DM mainly include surgery, radiotherapy, and chemotherapy (10). The selection of precise treatment strategies for individual patients relies on their comprehensive systemic evaluation and survival prediction. Given the highly malignant nature and rapid disease progression of this condition, it is crucial to develop appropriate assessment tools for ATC patients with DM to facilitate accurate management planning.
Although previous studies have contributed some practical clinical prediction tools (11–13), these tools encompassed all ATC patients. However, patients with ATC and DM often exhibit distinct clinical characteristics and biological behaviors compared to the overall population, representing a significant proportion of all ATC patients. Therefore, there is an urgent need to establish a precise clinical prediction tool specifically tailored to ATC patients with DM.
In this study, we included multicenter patient data from the Surveillance, Epidemiology, and End Results (SEER) database to evaluate independent prognostic factors influencing overall survival (OS) in ATC patients with DM. We constructed a nomogram to predict OS for this patient population and confirmed the excellent performance of the model through a series of evaluation metrics.
2 Materials and methods
2.1 Data source
The patient cohort for this study was derived from ATC patients with DM diagnosed between 2004 and 2019 in the SEER database. The clinical information of all patients was extracted from the SEER Cancer Database (http://www.seer.cancer.gov) using SEER*Stat software (https://seer.cancer.gov/seerstat/, version 8.4.2). As patient data in the SEER database is de-identified, local ethical review was not required for this study.
2.2 Patient selection criteria
The inclusion criteria for the patient cohort in this study were as follows: (1) primary tumor site in the thyroid; (2) histological diagnosis codes according to the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) as 8020/3 and 8021/3; (3) presence of distant metastasis, classified as stage IVC according to the eighth edition of the AJCC. Patients meeting any of the following criteria were excluded from this study: (1) cases provided by autopsy or death reports; (2) survival time of 0; (3) missing clinical information. All radiotherapeutic modalities involved in this study were external beam radiation therapy.
2.3 Statistical analysis
Considering the extremely high disease-specific mortality rate in the study population and the potential side effects of treatments such as radiation and chemotherapy, OS was chosen as the study endpoint. Firstly, all patients were randomly divided into training and validation sets in a 7:3 ratio, and the Chi-square/Wilcoxon test confirmed no statistical differences in baseline clinical characteristics between the two sets. Subsequently, univariate Cox regression analysis and multivariate Cox regression analysis were performed in the training set to determine the independent prognostic factors for OS in ATC patients with DM. A nomogram was constructed based on the selected independent risk factors. To evaluate the accuracy and clinical utility of the nomogram, receiver operating characteristic (ROC) curves, decision curve analysis (DCA) curves, and calibration curves were simultaneously plotted in both the training and validation sets. The ROC curve was used to assess the accuracy and recall of the model; the DCA curve was used to evaluate the clinical net benefit; the calibration curve was used to assess the prediction accuracy and consistency of the model. Risk scores for each patient were calculated based on the model, and patients were stratified into high- and low-risk groups using the median risk score. The Kaplan-Meier (KM) survival curves were employed to demonstrate the model’s ability to stratify patient prognosis. Finally, a web-based dynamic nomogram was published for readers’ use. All statistical analyses and visualizations in this study were performed using R software (version 4.3.1). A two-sided P value <0.05 was considered statistically significant.
3 Results
3.1 Patient characteristics
This study assimilated 322 qualified patients afflicted with ATC, with a median survival duration of three months. The Kaplan-Meier survival plot pertaining to the patient cohort in this investigation, juxtaposed with ATC sufferers devoid of distant metastasis, is demonstrated in Figure 1 (Figure 1). As shown in Table 1, all patients in the study cohort had advanced T stage (T3a and above), with the majority of patients classified as T4b (72.4%). Similarly, the majority of patients had lymph node metastasis, with N1b being the most common stage (56.8%). Most patients received surgical treatment (48.1%), chemotherapy (51.2%), and radiotherapy (63.4%). The mean age for all patients stands at 68.2 (± 12.1). All patients were randomly allocated to the training and validation sets in a 7:3 ratio, and there were no statistically significant differences in clinical variables between the two groups (p>0.05, Table 1).
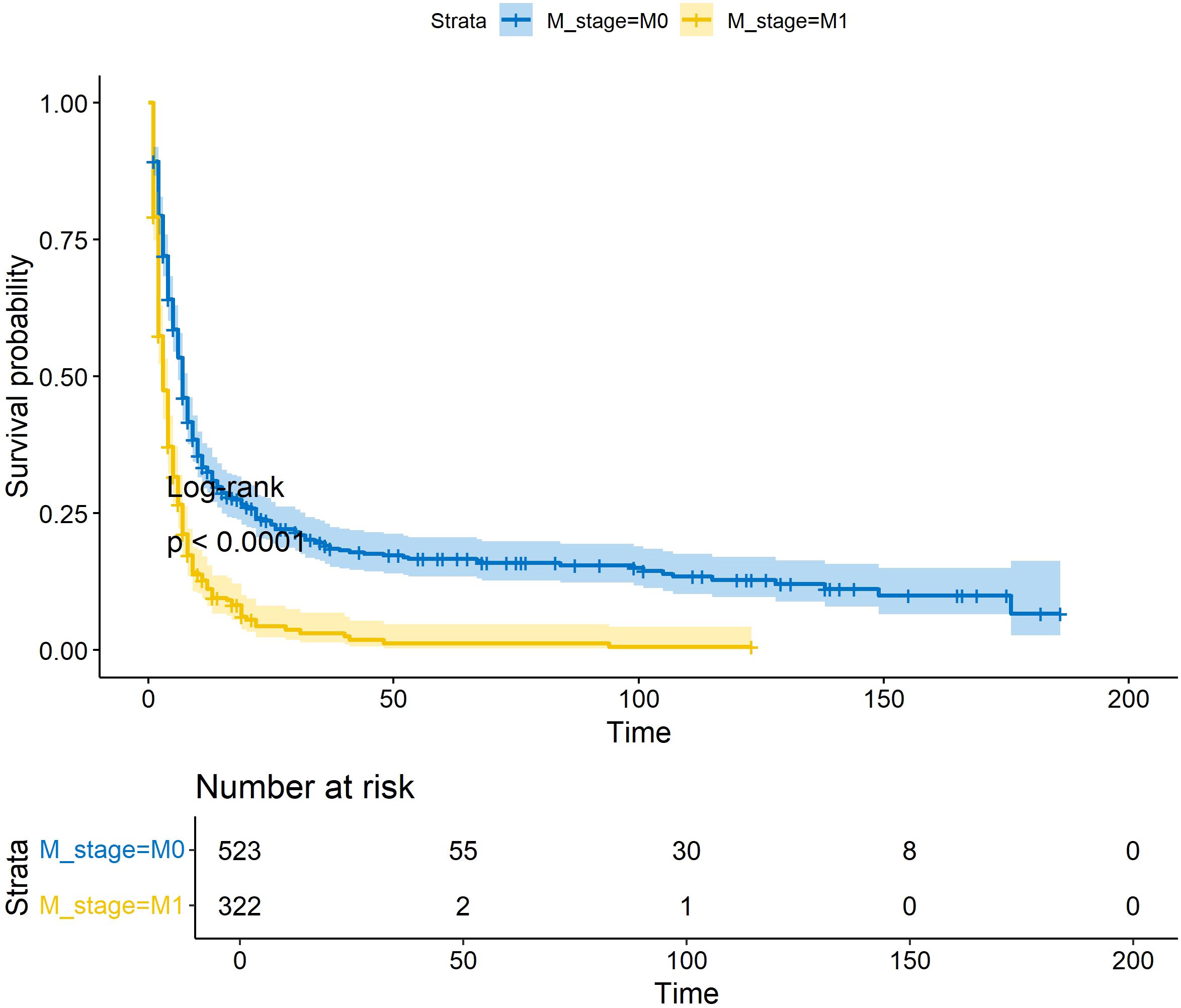
Figure 1 KM survival curves for OS in ATC patients with/without distant metastases. * p<0.05; ** p<0.01; *** p<0.001.
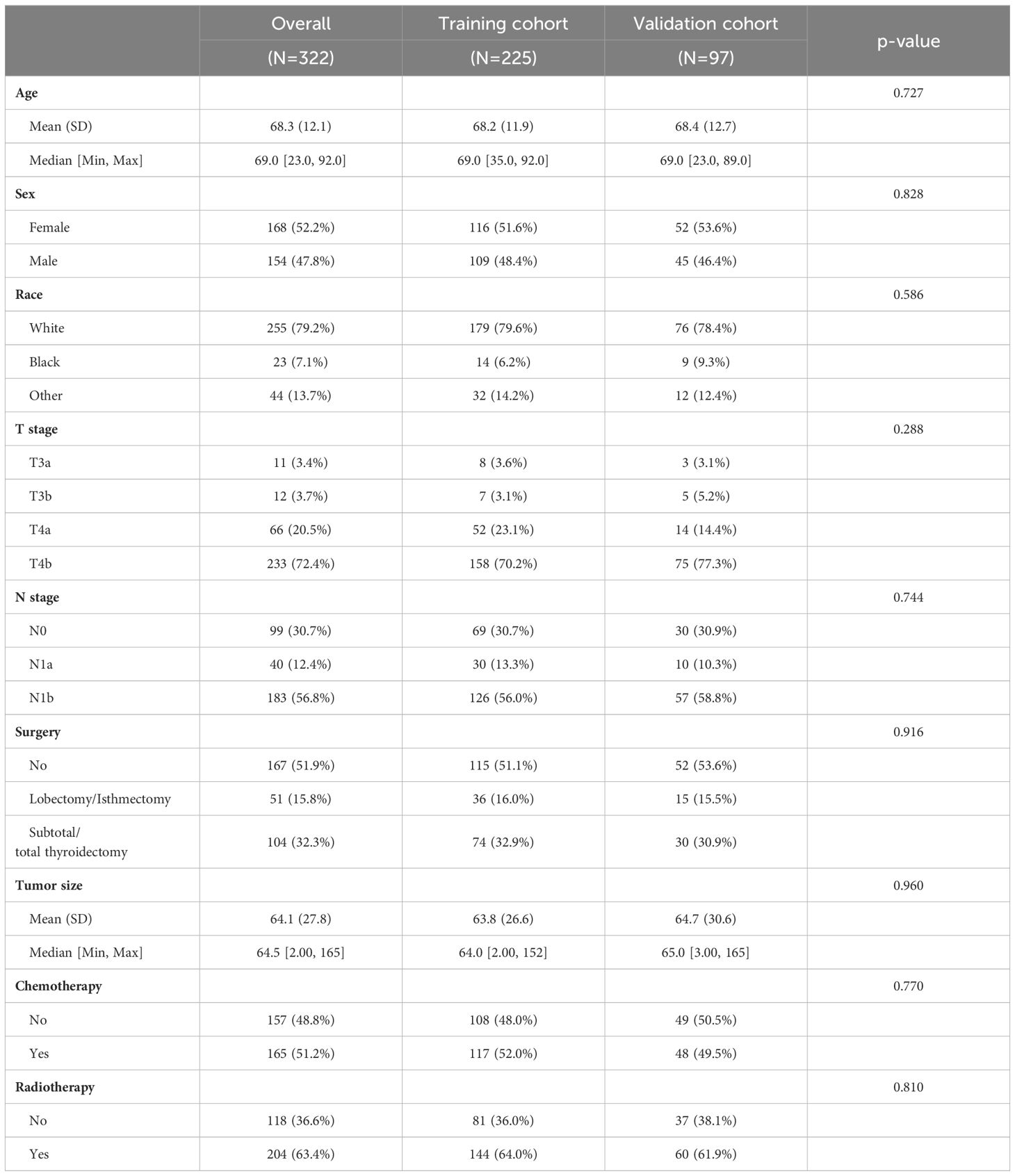
Table 1 Demographic and clinicopathological characteristics of ATC patients with distant metastases.
3.2 Prognostic factors for ATC patients with DM
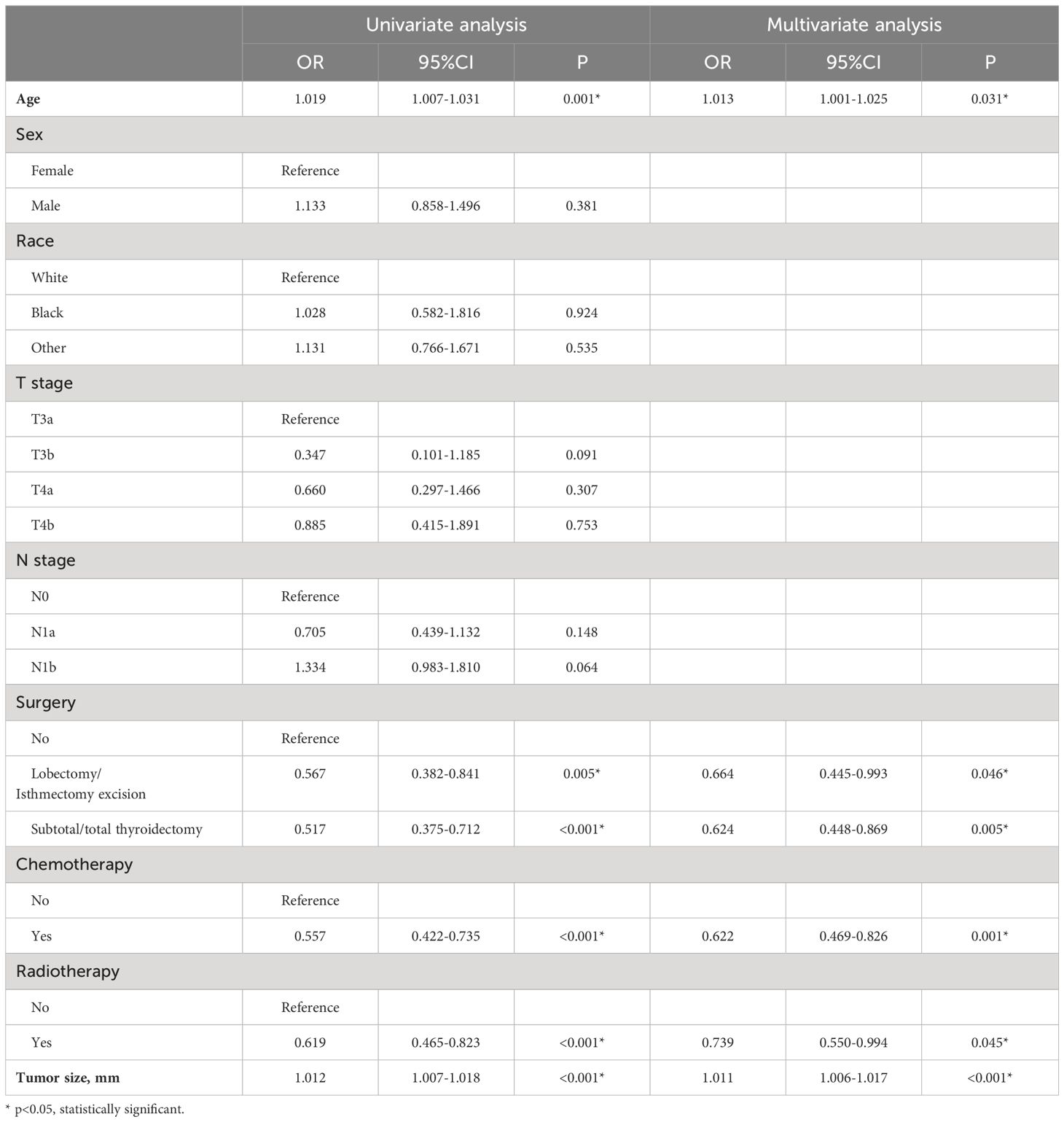
To explore potential clinical prognostic factors associated with OS in ATC patients with DM, we performed univariate Cox regression analysis on nine potential factors, revealing five variables significantly associated with OS: age, tumor size, surgery, chemotherapy, and radiotherapy. Further multivariate Cox regression analysis (Table 2) demonstrated that age, tumor size, surgery, chemotherapy, and radiotherapy were independent prognostic factors for OS in ATC patients with DM. Specifically, chemotherapy (OR=0.622, 95%CI=0.469-0.826), and radiotherapy (OR=0.739, 95%CI=0.550-0.994) were protective factors for patient OS. However, T stage and N stage were not independent prognostic factors for OS (P>0.05).
3.3 Prognostic nomogram development and validation
Based on the Cox regression analysis results of the five independent prognostic factors, we constructed a nomogram for predicting 3-month, 6-month, and 8-month OS in ATC patients with DM (Figure 2A). Each predictive variable was assigned a specific score based on its position on the corresponding scale, and the cumulative sum of all “points” yielded the “total points,” which could be further converted into the probability of death at a specific time point for a particular patient. We also provided a demonstration of survival prediction using randomly selected patient data (Figure 2B). Additionally, we published an online dynamic nomogram (https://jzxwlh.shinyapps.io/DynNomapp/) (Figure 3).
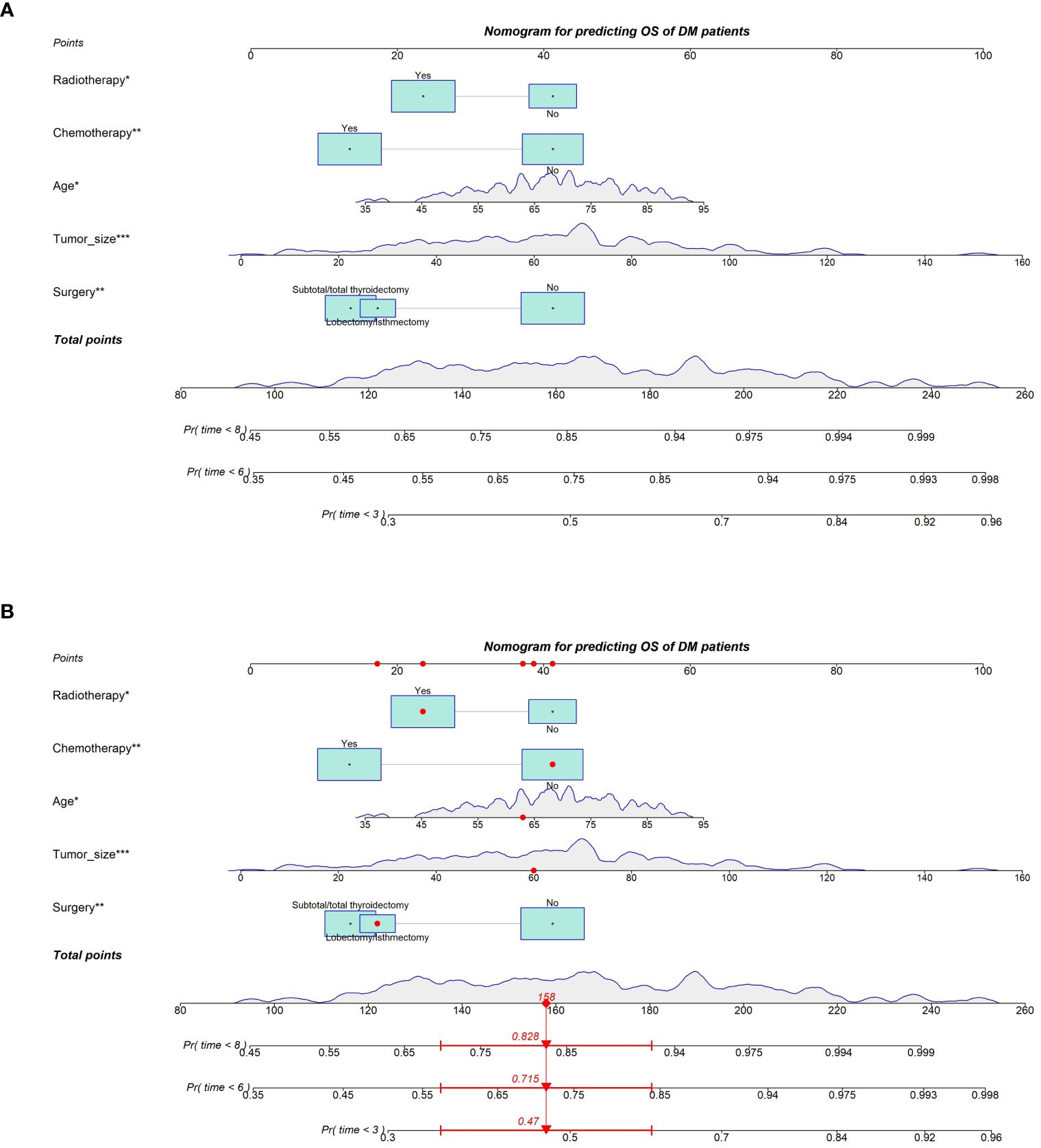
Figure 2 Prognostic nomogram predicting OS at 3, 6 and 8 months in SIC ATC patients with distant metastases (A). Schematic of patient application model for prediction (B).
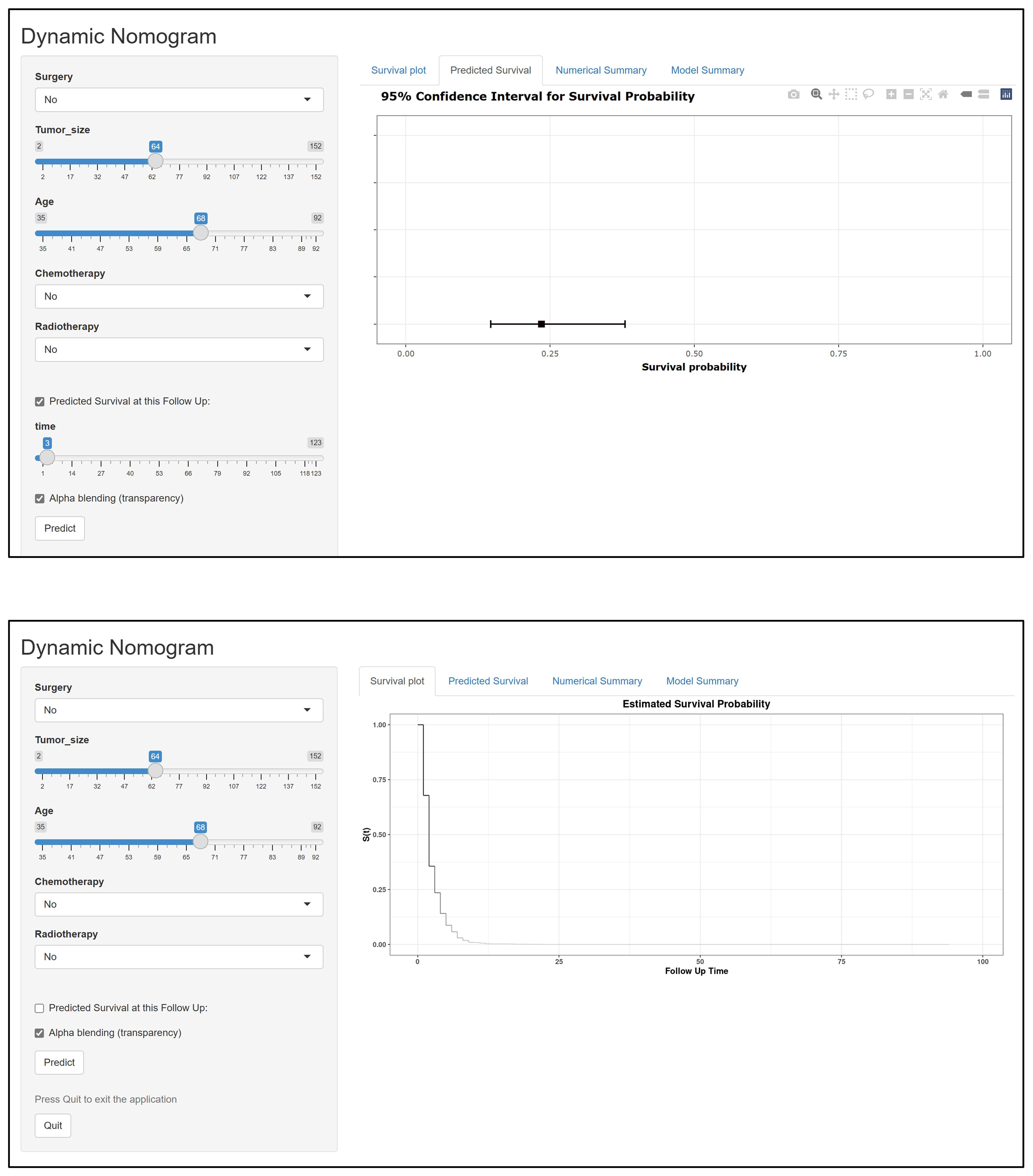
Figure 3 Schematic diagram of the interface for using the web version of the dynamic prediction model.
We plotted ROC curves in both the training and validation sets to evaluate the sensitivity and specificity of the model. The results showed that the model achieved AUC values of 0.767, 0.789, and 0.795 for 3-month, 6-month, and 8-month OS, respectively, in the training set (Figure 4A), and AUC values of 0.753, 0.798, and 0.806 for 3-month, 6-month, and 8-month OS, respectively, in the validation set (Figures 4A, B), confirming the excellent accuracy of the model. Calibration curves (Figure 5) and DCA curves (Figure 6) were plotted in both the training and validation sets. The calibration curves visually demonstrated the accurate performance of the nomogram in predicting OS at different time points, while the DCA curves confirmed the outstanding performance of the nomogram in real clinical practice. KM survival curves (Figure 7) illustrated the model’s excellent stratification ability for patient OS in both the training and validation sets.
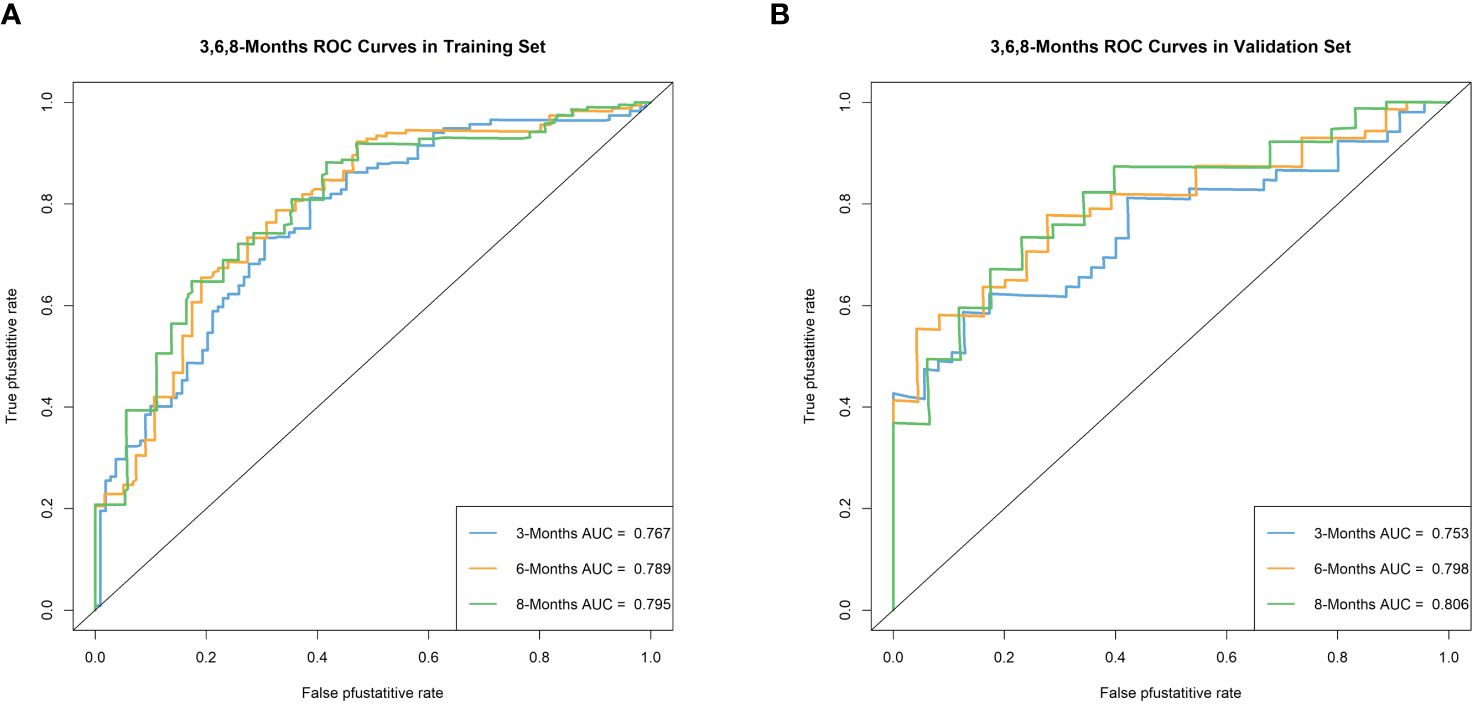
Figure 4 Time-dependent ROC curve analysis of the OS nomogram for the 3-, 6-, and 8-month in the training set (A) and the validation set (B).
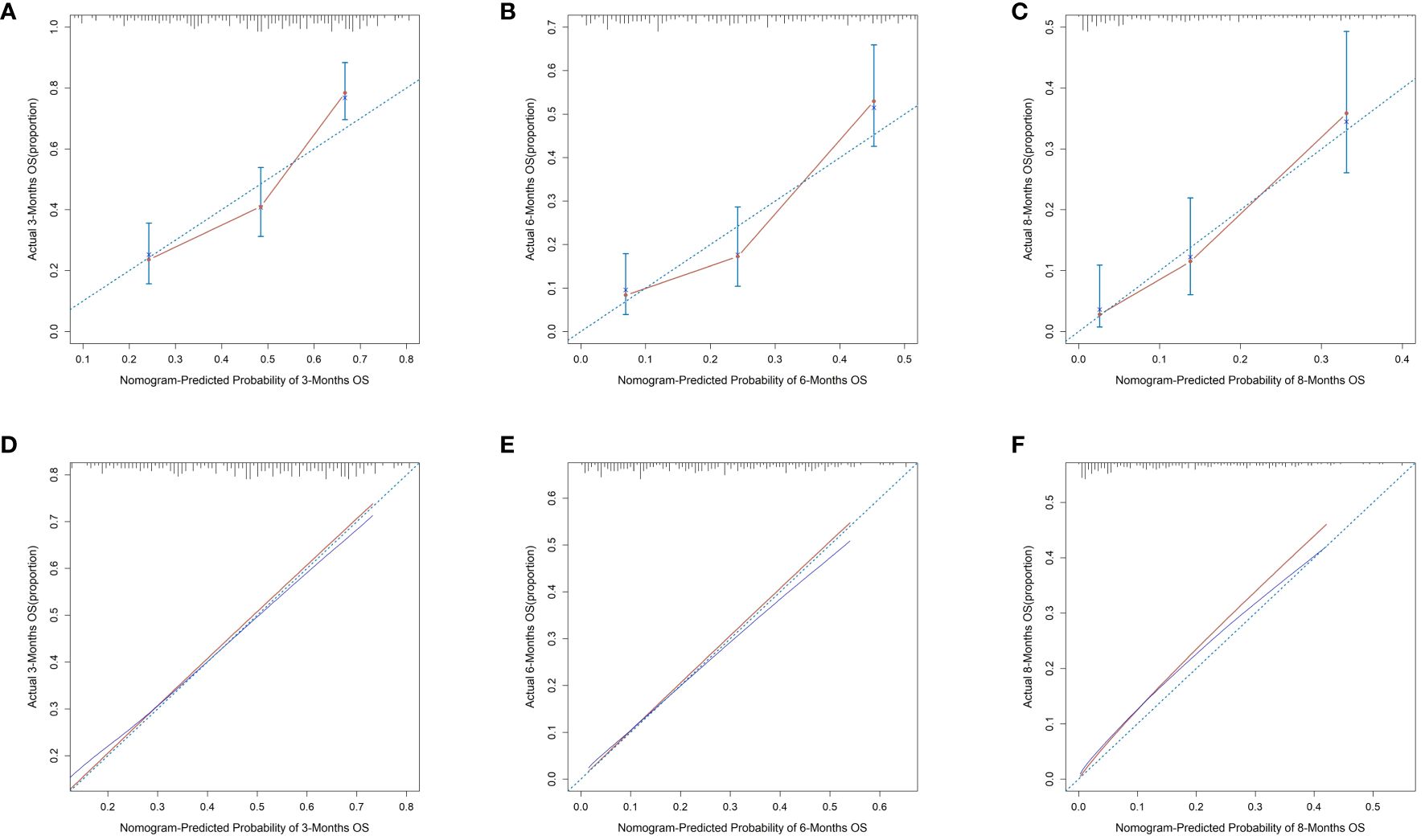
Figure 5 Calibration curves for 3-, 6-, and 8-month OS prediction nomogram in the training set (A–C) and validation set (D–F).
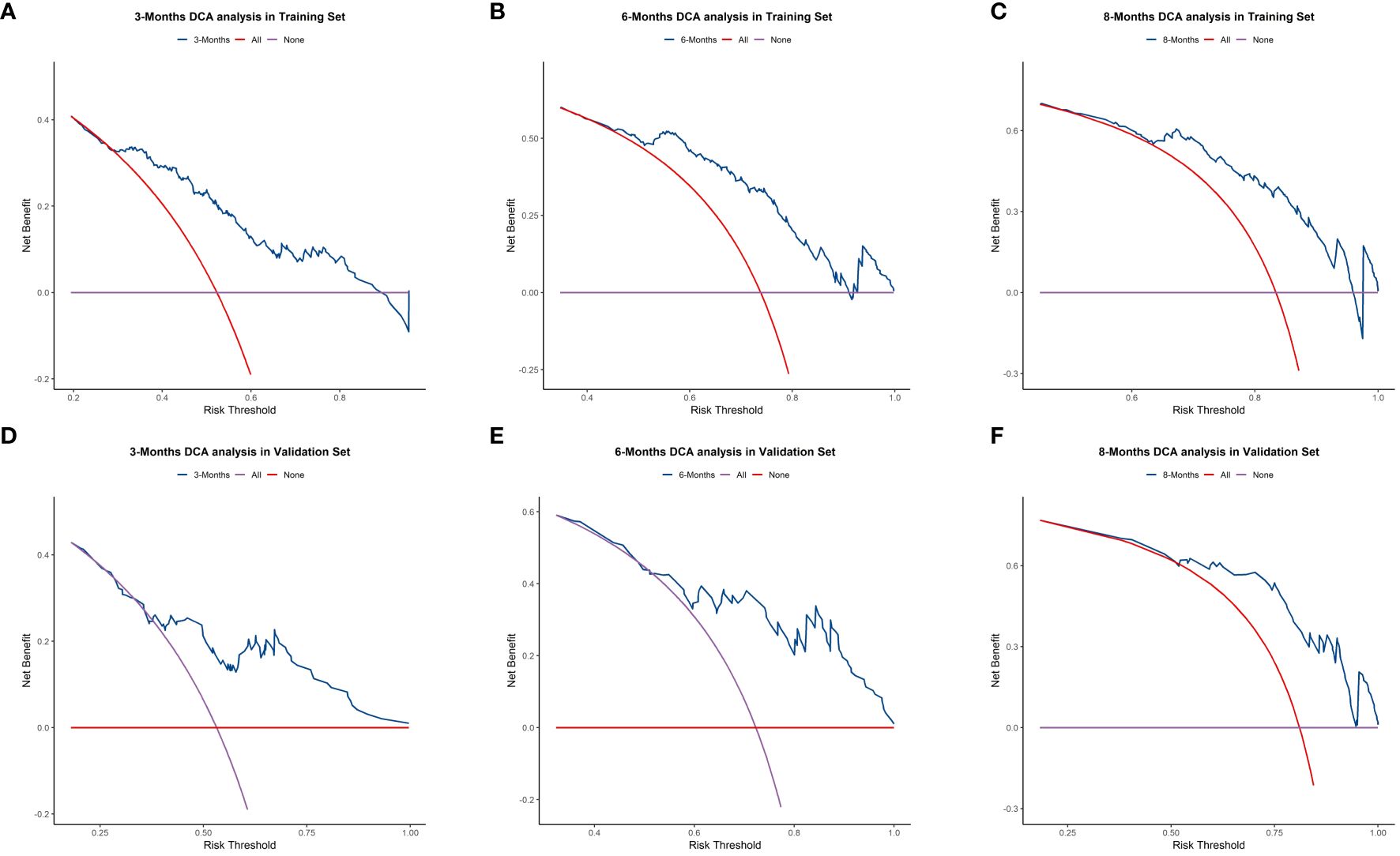
Figure 6 Decision Curve Analysis (DCA) curves for 3-, 6-, and 8-month OS prediction nomogram in the training set (A–C) and validation set (D–F).
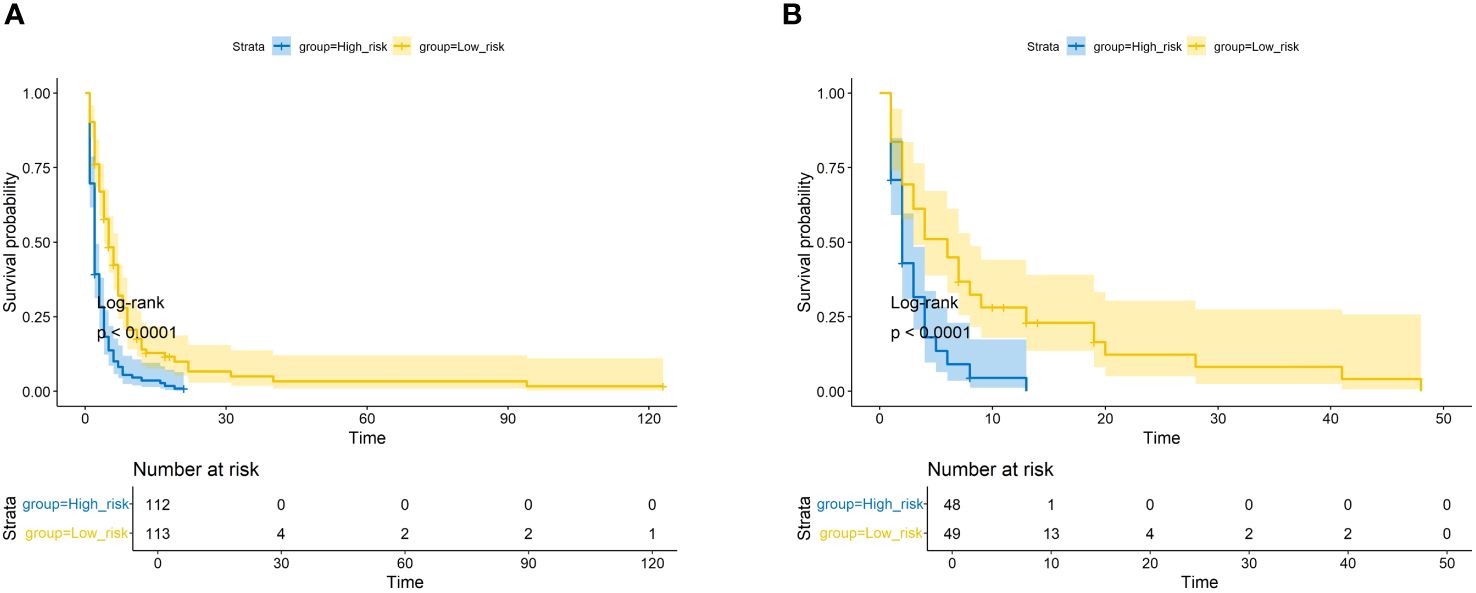
Figure 7 The Kaplan–Meier survival curves of the patients in the training set (A) and in the validation set (B).
4 Discussion
Despite the low incidence of ATC, its highly aggressive nature and poor prognosis have attracted increasing attention from scholars (14). Current mainstream treatment modalities for ATC patients include surgery, chemotherapy, radiotherapy, and other comprehensive treatments. However, the prognosis for ATC patients remains unfavorable (15). Therefore, it is crucial to actively study and explore prognostic factors in ATC patients to assess their risk. In this study, we focused on ATC patients with DM and utilized multicenter data from the SEER database to thoroughly investigate the independent prognostic factors in ATC patients with DM. We also developed a nomogram to assist in determining optimal treatment strategies for these patients. To the best of our knowledge, this study is the first multicenter retrospective study to construct a prognostic model specifically for ATC patients with DM. We further confirmed the excellent performance of the model through a series of evaluation metrics.
Thyroid cancer is the only cancer that considers age as an important prognostic factor for thyroid cancer-specific survival. Similarly, in our study, we observed a similar phenomenon where patients over 75 years of age had significantly worse prognosis compared to other age groups. Our constructed nomogram vividly illustrates this point, where older patients receive higher risk scores. Additionally, we found that larger tumor diameter was directly associated with poorer prognosis. Previous studies have highlighted the importance of tumor diameter differences in determining invasiveness (13), but specific research on the relationship between tumor diameter, distant metastasis, and prognosis in ATC is still lacking.
In our study cohort, we were surprised to discover that unlike most tumors, the extent of primary tumor infiltration and regional lymph node metastasis were no longer independent prognostic factors for ATC patients with DM. Instead, surgery, chemotherapy, and radiotherapy were identified as independent prognostic factors. Similar findings have been reported in previous studies (13). We speculate that this is due to the highly invasive nature of ATC, resulting in the majority of ATC patients with DM being diagnosed at an advanced stage where the status of the primary tumor no longer significantly influences patient prognosis.
Furthermore, we noted that various treatment modalities were independent prognostic factors for OS in ATC patients with DM. This emphasizes the importance of actively receiving treatment after diagnosis to prolong patient survival. Previous research has shown that although surgery is indeed a protective factor for patient OS, it is not sufficient to improve long-term prognosis significantly, leading to ongoing debates regarding the extent of the benefits of surgical treatment (16, 17). The guidelines of the American Thyroid Association (18) explicitly state that the surgical goal for patients with ATC at stages IVA/IVB is to perform a resection of all macroscopically visible tumor (R0 or R1) rather than debulking (R2 resection). Additionally, they mention that a subset of patients at stage IVC may consider surgery to control local disease and alleviate or prevent future complications (for instance, impending airway invasion/obstruction, esophageal invasion/obstruction, laryngeal invasion/obstruction), albeit without elaborating in detail on specific surgical recommendations. In this study, we observed superior overall survival (OS) in patients with stage IVC ATC who underwent total/subtotal thyroidectomy compared to those who had only lobectomy/isthmectomy or didn’t undergo surgery. Thus, based on our analysis, we posit that debulking surgery targeting the primary tumor can enhance patients’ OS to some extent. Concurrently, guidelines from the American Thyroid Association also indicate that radical surgeries (including laryngectomy, tracheotomy, esophagectomy and/or major vascular or mediastinal resection) are generally not recommended due to the poor prognosis of ATC. Such procedures can be selectively contemplated only after comprehensive discussions within a multidisciplinary team, considering factors including patient mutation targets.
In our study, radiotherapy was found to be the most commonly used treatment modality for ATC patients with DM. According to previous literature, a radiation dose of at least 50 Gy is required to significantly improve patient prognosis (19, 20), and Pezzi et al (21) found that patients receiving radiation doses of 60-75 Gy had better survival rate improvements compared to those receiving lower doses. The NCCN guidelines recommend an adjuvant radiation dose of 60-66 Gy (14). Due to the low incidence and high invasiveness of ATC, patient enrollment in clinical trials evaluating various chemotherapy agents is extremely limited, leading to incomplete evaluation of chemotherapy efficacy in ATC (22). Regarding comprehensive treatments, many previous studies have consistently suggested that surgical treatment combined with adjuvant chemoradiotherapy can effectively improve OS in ATC patients (23, 24), aligning with our study results. However, some studies have found that while concurrent chemoradiotherapy and/or chemotherapy can improve survival rates in stage IVA/B ATC patients, the benefit is limited in stage IVC patients (25). Nevertheless, our study found that stage IVC patients who actively pursued various treatment options had significantly better prognoses than those who did not receive any treatment. Therefore, despite generally poorer prognoses for stage IVC ATC patients, we still advocate for their active treatment. Additionally, there is a pressing need for new treatment strategies to improve the prognosis of late-stage ATC patients.
In recent years, targeted therapy and immunotherapy for ATC have garnered increasing attention. Currently, BRAF mutation is identified as the most common somatic mutation in ATC. The drugs Dabrafenib and Trametinib, targeting BRAF and MEK1/2 respectively, work by inhibiting tumor cell proliferation through disruption of the RAF-MEK-ERK signaling pathway. Clinical studies suggest that the confirmed overall response rate to combined Dabrafenib and Trametinib therapy stands at 69%, with independently reviewed results corroborating this finding. This validates the significant clinical efficacy of combined Dabrafenib and Trametinib therapy in patients with BRAF V600E-mutant ATC (26, 27). Immunotherapy for ATC is still in the experimental stage; however, immune checkpoint inhibitors targeting cytotoxic T-lymphocyte-associated antigen 4 and PD-1/PD-L1 have shown promising clinical efficacy (28, 29). Unfortunately, ATC patient data on targeted/immunotherapies are not recorded in the SEER database, limiting our ability to conduct detailed analyses or incorporate these treatment variables into predictive models.
Of course, we must acknowledge certain limitations of our study. Firstly, as a retrospective analysis, we excluded patients with missing clinical information, which may introduce some selection bias. Secondly, although we included multicenter patient data spanning over a decade, the limited incidence of ATC with DM resulted in an inadequate number of patients in our study. Furthermore, as the SEER database does not include information regarding chemotherapy regimens, radiation dosage, and targeted/immunotherapy treatments, our predictive model did not take these factors into consideration. Lastly, we lack external validation sets to further validate the model. We hope that future researchers can include a larger number of patient data to further enhance our current research findings.
5 Conclusion
In this study, we identified the independent prognostic factors for OS in ATC patients with DM through univariate and multivariate Cox regression analysis. We developed a nomogram based on these factors and also released an online version of the dynamic nomogram. Furthermore, we demonstrated the excellent performance of the model through a series of evaluation metrics.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/.
Author contributions
LW: Conceptualization, Software, Writing – original draft. YR: Methodology, Visualization, Writing – original draft. PL: Software, Visualization, Writing – original draft. YL: Data curation, Funding acquisition, Investigation, Software, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work was supported by the National Nature Science Foundation of China (No.82204879, No.82360572) and the General Project of the Science and Technology Department of Jiangxi Province (No.2024BAB206053).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1375176/full#supplementary-material
References
1. Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, et al. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. (2017) 13:644–60. doi: 10.1038/nrendo.2017.76
2. Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. (2012) 22:1104–39. doi: 10.1089/thy.2012.0302
3. Maniakas A, Dadu R, Busaidy NL, Wang JR, Ferrarotto R, Lu C, et al. Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000-2019. JAMA Oncol. (2020) 6:1397–404. doi: 10.1001/jamaoncol.2020.3362
4. Lin B, Ma H, Ma M, Zhang Z, Sun Z, Hsieh IY, et al. The incidence and survival analysis for anaplastic thyroid cancer: a SEER database analysis. Am J Transl Res. (2019) 11:5888–96.
5. Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. (2006) 13:453–64. doi: 10.1245/ASO.2006.05.042
6. Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. (2009) 16:17–44. doi: 10.1677/ERC-08-0154
7. O’Neill JP, Shaha AR. Anaplastic thyroid cancer. Oral Oncol. (2013) 49:702–6. doi: 10.1016/j.oraloncology.2013.03.440
8. Rao SN, Zafereo M, Dadu R, Busaidy NL, Hess K, Cote GJ, et al. Patterns of treatment failure in anaplastic thyroid carcinoma. Thyroid. (2017) 27:672–81. doi: 10.1089/thy.2016.0395
9. Ranganath R, Shah MA, Shah AR. Anaplastic thyroid cancer. Curr Opin Endocrinol Diabetes Obes. (2015) 22:387–91. doi: 10.1097/MED.0000000000000189
10. Giuffrida D, Gharib H. Anaplastic thyroid carcinoma: current diagnosis and treatment. Ann Oncol. (2000) 11:1083–9. doi: 10.1023/A:1008322002520
11. Gui W, Zhu W, Lu W, Shang C, Zheng F, Lin X, et al. Development and validation of a prognostic nomogram to predict overall survival and cancer-specific survival for patients with anaplastic thyroid carcinoma. PeerJ. (2020) 8:e9173. doi: 10.7717/peerj.9173
12. Cui H, Wang R, Zhao X, Wang S, Shi X, Sang J. Development and validation of a nomogram for predicting the early death of anaplastic thyroid cancer: a SEER population-based study. J Cancer Res Clin Oncol. (2023) 149:16001–13. doi: 10.1007/s00432-023-05302-z
13. Bi J, Zhang H. Nomogram predicts risk and prognostic factors for lung metastasis of anaplastic thyroid carcinoma: a retrospective study in the Surveillance Epidemiology and End Results (SEER) database. Transl Cancer Res. (2023) 12:3547–64. doi: 10.21037/tcr
14. Haddad RI, Bischoff L, Ball D, Bernet V, Blomain E, Busaidy NL, et al. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:925–51. doi: 10.6004/jnccn.2022.0040
15. Saini S, Tulla K, Maker AV, Burman KD, Prabhakar BS. Therapeutic advances in anaplastic thyroid cancer: a current perspective. Mol Cancer. (2018) 17:154. doi: 10.1186/s12943-018-0903-0
16. Ito K, Hanamura T, Murayama K, Okada T, Watanabe T, Harada M, et al. Multimodality therapeutic outcomes in anaplastic thyroid carcinoma: improved survival in subgroups of patients with localized primary tumors. Head Neck. (2012) 34:230–7. doi: 10.1002/hed.21721
17. Foote RL, Molina JR, Kasperbauer JL, Lloyd RV, McIver B, Morris JC, et al. Enhanced survival in locoregionally confined anaplastic thyroid carcinoma: a single-institution experience using aggressive multimodal therapy. Thyroid. (2011) 21:25–30. doi: 10.1089/thy.2010.0220
18. Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr., et al. 2021 American thyroid association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. (2021) 31:337–86. doi: 10.1089/thy.2020.0944
19. Wendler J, Kroiss M, Gast K, Kreissl MC, Allelein S, Lichtenauer U, et al. Clinical presentation, treatment and outcome of anaplastic thyroid carcinoma: results of a multicenter study in Germany. Eur J Endocrinol. (2016) 175:521–9. doi: 10.1530/EJE-16-0574
20. Tiedje V, Stuschke M, Weber F, Dralle H, Moss L, Führer D. Anaplastic thyroid carcinoma: review of treatment protocols. Endocr Relat Cancer. (2018) 25:R153–r61. doi: 10.1530/ERC-17-0435
21. Pezzi TA, Mohamed ASR, Sheu T, Blanchard P, Sandulache VC, Lai SY, et al. Radiation therapy dose is associated with improved survival for unresected anaplastic thyroid carcinoma: Outcomes from the National Cancer Data Base. Cancer. (2017) 123:1653–61. doi: 10.1002/cncr.30493
22. Jannin A, Escande A, Al Ghuzlan A, Blanchard P, Hartl D, Chevalier B, et al. Anaplastic thyroid carcinoma: an update. Cancers (Basel). (2022) 14:1061–86. doi: 10.3390/cancers14041061
23. Yin Y, Wang L, Huang C. Surgery combined with adjuvant radiation and chemotherapy prolonged overall survival in stage IVC anaplastic thyroid cancer: a SEER-based analysis. Endocrine. (2024). doi: 10.1007/s12020-023-03662-7
24. Chen J, Tward JD, Shrieve DC, Hitchcock YJ. Surgery and radiotherapy improves survival in patients with anaplastic thyroid carcinoma: analysis of the surveillance, epidemiology, and end results 1983-2002. Am J Clin Oncol. (2008) 31:460–4. doi: 10.1097/COC.0b013e31816a61f3
25. Wächter S, Vorländer C, Schabram J, Mintziras I, Fülber I, Manoharan J, et al. Anaplastic thyroid carcinoma: changing trends of treatment strategies and associated overall survival. Eur Arch Otorhinolaryngol. (2020) 277:1507–14. doi: 10.1007/s00405-020-05853-8
26. Tiedje V, Fagin JA. Therapeutic breakthroughs for metastatic thyroid cancer. Nat Rev Endocrinol. (2020) 16:77–8. doi: 10.1038/s41574-019-0307-2
27. Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. (2018) 36:7–13. doi: 10.1200/JCO.2017.73.6785
28. Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. (2016) 375:1845–55. doi: 10.1056/NEJMoa1611299
Keywords: anaplastic thyroid cancer, distant metastasis, SEER, overall survival, nomogram
Citation: Wang L, Rao Y, Lai P and Lv Y (2024) Development of a novel dynamic nomogram for predicting overall survival in anaplastic thyroid cancer patients with distant metastasis: a population-based study based on the SEER database. Front. Endocrinol. 15:1375176. doi: 10.3389/fendo.2024.1375176
Received: 23 January 2024; Accepted: 19 June 2024;
Published: 04 July 2024.
Edited by:
Vincenzo Marotta, AOU S. Giovanni di Dio e Ruggi D’Aragona, ItalyReviewed by:
Barbara Maria Jarzab, Maria Skłodowska-Curie National Research Institute of Oncology, PolandJavier Ismael Altamirano García, National Institute of Cancerology (INCAN), Mexico
Daniele Barbaro, UO Endocrinologia ASL nord ovest Toscana, Italy
Copyright © 2024 Wang, Rao, Lai and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunxia Lv, ODMzOTQwNDVAcXEuY29t
†These authors have contributed equally to this work
 Liuhuan Wang
Liuhuan Wang Yanghua Rao†
Yanghua Rao† Yunxia Lv
Yunxia Lv