- 1Department of Interventional Medicine, China-Japan Friendship Hospital, Beijing, China
- 2Department of Epidemiology and Health Statistics, School of Public Health, Capital Medical University, and Beijing Municipal Key Laboratory of Clinical Epidemiology, Beijing, China
- 3Pathology Department, China-Japan Friendship Hospital, Beijing, China
Introduction: Fine needle aspiration (FNA) biopsy is a widely accepted method for diagnosing thyroid nodules. However, the influence of maximum diameter (MD) of ACR TIRADS 5 (TR5) thyroid nodules on the FNA outcomes remains debated. This study examined the influence of MD on the FNA outcomes and investigated the optimal MD threshold for FNA in TR5 nodules.
Methods: We conducted a retrospective analysis of 280 TR5 thyroid nodules from 226 patients who underwent FNA from January to June 2022 in our department. Probably malignant (PM) group was defined as Bethesda V in cytopathology with confirmed BRAF V600E mutation or Bethesda VI, the other cytopathology outcomes were defined as probably benign (PB) group. We examined factors influencing malignant cytopathology outcomes and determined the optimal MD threshold for FNA in TR5 nodules using logistic regression and restricted cubic spline (RCS) analysis.
Results: Among these nodules, 58.2% (163/280) had PM outcomes. The PM group had a significantly larger MD than the PB group [6.5mm (range 5.0-8.4) vs. 5.3mm (range 4.0-7.0), p < 0.001]. In multivariate logistic regression fully adjusted for confounders, MD was significantly associated with PM outcomes [odds ratio 1.16, 95%CI 1.05-1.31; p = 0.042]. The highest quartile of MD had a greater likelihood of PM outcomes compared to the lowest quartile [odds ratio 4.71, 95% CI 1.97-11.69, p = 0.001]. The RCS analysis identified 6.2 mm as the optimal MD threshold for FNA in TR5 nodules.
Conclusion: MD significantly affects the probability of malignant outcomes in FNA of TR5 thyroid nodules. A MD threshold of ≥6.2mm is suggested for FNA in these nodules.
1 Introduction
Thyroid nodules is a worldwide prevalent diseases with the incidence ranging from 5 to 68% in the ultrasound examination or palpation depending on iodine supply, the age of the population studied (1–5). Moreover, subsequent examinations or treatments reveal that 5-15% of these nodules are malignant (6, 7). Consequently, the prompt differentiation of malignant thyroid nodules from benign ones is of paramount importance.
Fine needle aspiration (FNA) biopsy is recognized as a minimally invasive and highly accurate technique for the pathological diagnosis of thyroid nodules. This method is endorsed by almost guidelines concerning the management and treatment of thyroid nodules (2, 8, 9). Various factors, including age, sex, calcification, echogenicity, composition, as well as the maximum diameter (MD) and depth of the nodules, could influence the outcomes of FNA (10–15). Among these, the MD is an significant and important influencing factor (10, 15–18). Nevertheless, there is still absence of the universally recognized optimal MD threshold for FNA in suspicious thyroid nodules (9), which leads to variability in clinical management decisions. The American Thyroid Association recommended the FNA for high or intermediate suspicion thyroid nodules that are 1 cm or larger (2), aligning with recommendations from other guidelines (8, 9).
It merits emphasis that papillary thyroid microcarcinoma (PTMC), characterized as papillary thyroid carcinoma with a size under 1 cm, maintains a degree of invasiveness and a risk of lymph node metastasis. Prior research indicates that the incidence of lymph node metastasis in PTMC ranges between 13.5-37.0% (19–21). Thermal ablation emerges as an efficacious and minimally invasive option for treating thyroid nodules, and is recommended for the treatment of selected PTMC cases confirmed by preoperative FNA in various guidelines (22, 23). Given this context, it becomes crucial to comprehensively assess the efficacy of FNA in evaluating thyroid nodules smaller than 1 cm. Additionally, determining the optimal MD threshold for administering FNA on these suspicious nodules warrants thorough exploration.
In the present study, clinical and imaging information of ACR TIRADS 5 (TR5) thyroid nodules underwent FNA was summarized, the influence factors of FNA were evaluated. Subsequently, the study delves into the relationship between the MD of these nodules and the results of FNA, aiming to identify the potential optimal MD threshold for FNA.
2 Methods
2.1 General information and patient enrollment
This retrospective, single-center study was approved by the ethics committee of China-Japan Friendship Hospital. Written informed consent was obtained from all participants. Written informed consent for the publication of data was waived because the examination results and radiological data of patients were published anonymously. Data of patients who underwent FNA for thyroid nodules between January 2022 and June 2022 were summarized.
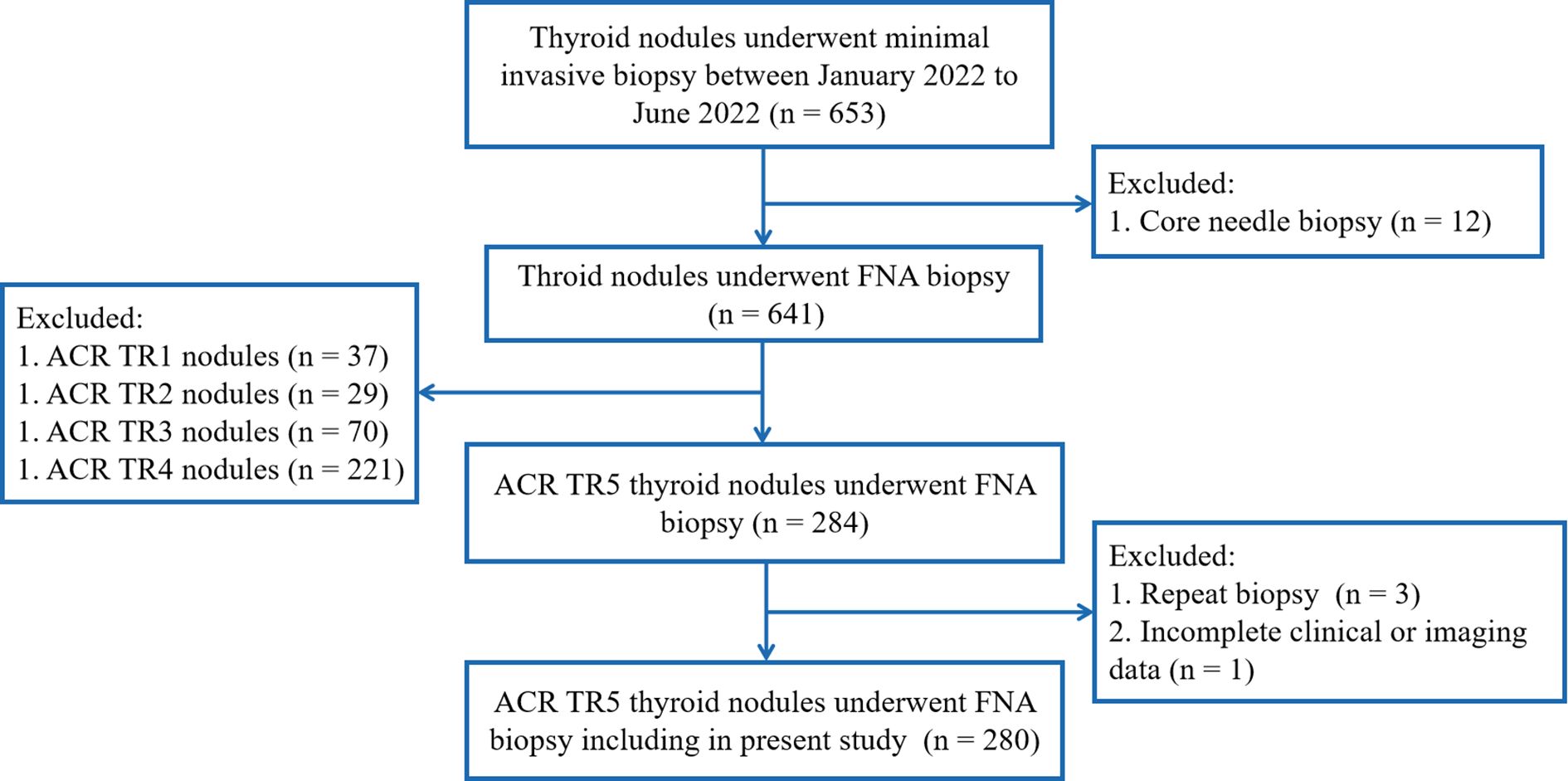
The inclusion criteria included: (1) the thyroid nodules underwent FNA biopsy, and (2) ACR TR5 thyroid nodules in ultrasound evaluation. Exclusion criteria were as follows: (1) repeat FNA on same thyroid nodules; (2) core needle biopsy of thyroid nodules, and (3) incomplete clinical or imaging data (Figure 1).
2.2 Preoperative evaluation and ultrasound-guided FNA
The ACR TIRADS was utilized to evaluate and classify the thyroid nodules in present study (9). Two doctors, each with over three years of experience in thyroid nodule ultrasound examination, conducted the evaluations. In cases of differing opinions, a senior doctor provided the final assessment. The ultrasound-guided FNA of thyroid nodules was performed by a doctor with a minimum of three years of clinical experience.
Prior to FNA, patients underwent laboratory tests including routine blood test, coagulation function tests, and calcitonin measurement. The preoperative evaluation and FNA were conducted using either the LOGIQ E9 scanner (GE Healthcare, Milwaukee, USA) or the Canon Aplio i900 (Canon Medical Systems, Otawara, Japan), both equipped with high-frequency linear array probes (9-18 MHz). Patients were positioned supine, and the anterior neck region was sterilized. Following local anesthesia, a 23G fine needle (Biopsy needles, Gallini S.R.L, Via S. Faustino, Modena, Italy) was precisely inserted into the center of the targeted thyroid nodules under ultrasound guidance. The needle core was withdrawn, and the needle sheath was then inserted into and retracted from the nodules several times without suction until the transparent needle hub showed a trace of blood (Figure 2) or the insertion was repeated more than 10 times. Post-procedure, the puncture site was compressed manually for 30 minutes to prevent hematoma formation.
Each targeted nodule was sampled thrice for FNA, followed by smear examination and liquid-based cytological testing. The eluate from the fine needles was analyzed for mutations in the B-type Raf kinase (BRAF) V600E gene.
2.3 Pathological outcomes
Pathological examinations and reporting were conducted by a senior pathologist specializing in thyroid cytopathology with three years of experience. The 2017 Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) guided the pathological reports for thyroid FNA specimens (6), which include: (I) non diagnostic or unsatisfactory; (II) benign; (III) atypia of undetermined significance or follicular lesion of undetermined significance; (IV) follicular neoplasm or suspicious for a follicular neoplasm; (V) suspicious for malignancy; (VI) malignant.
In our study, the thyroid nodules with Bethesda V in cytopathology and confirmed mutation of BRAF V600E were regarded as malignant nodules. These nodules, along with the nodules of Bethesda VI, were defined as malignant cytopathology outcomes and grouped into probably malignant (PM) group. Other thyroid nodules were grouped into probably benign (PB) group.
2.4 Study variables
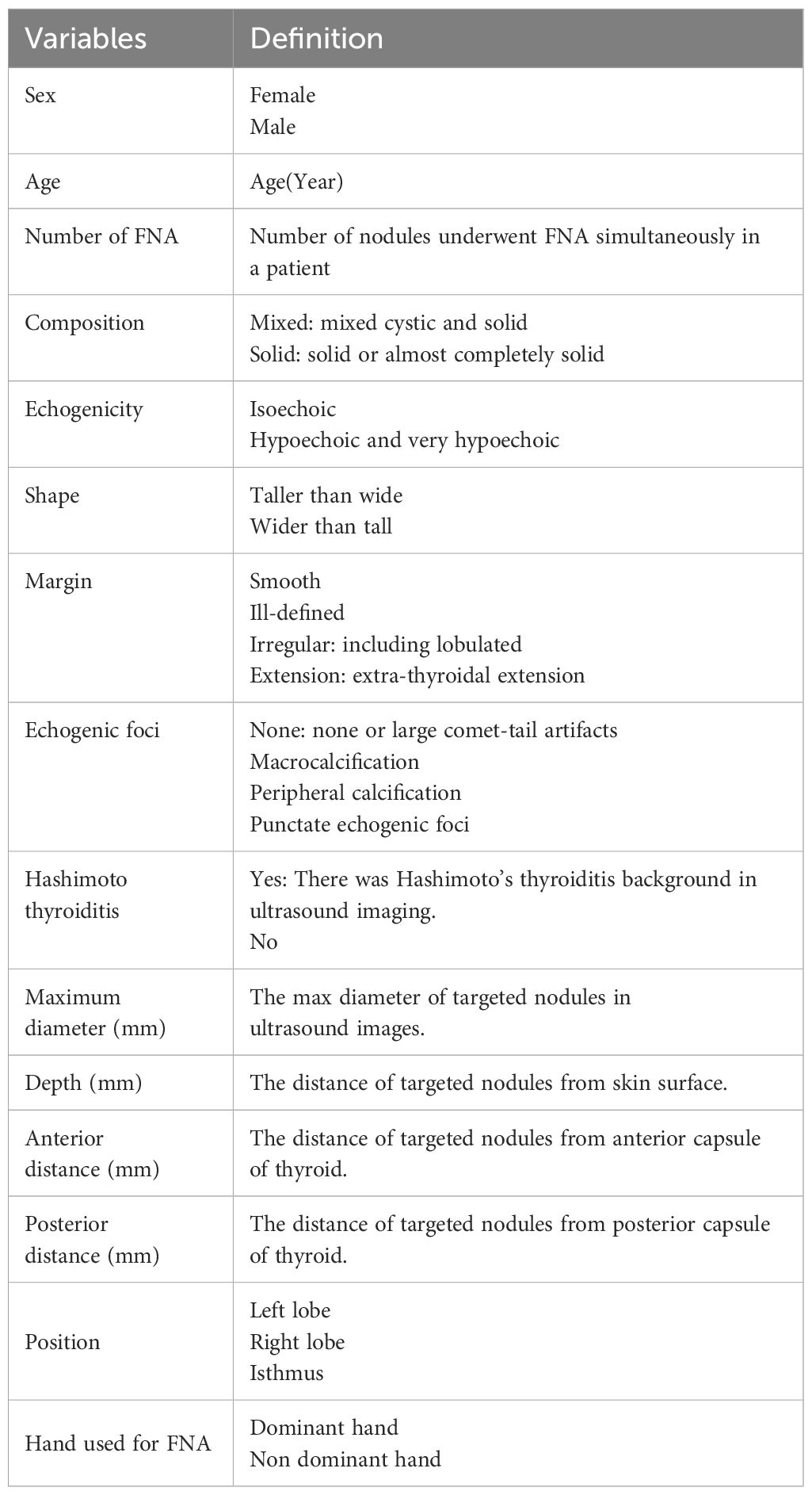
Demographic and clinical data of patients undergoing FNA were documented, including sex, age, and the number of nodules. Ultrasound images of the targeted thyroid nodules were reviewed, and characteristics such as composition, echogenicity, shape, margin, and echogenic foci were noted. Measurements including the MD, the vertical distance of nodules from the skin surface (depth), the distance of nodules from the anterior capsule of the thyroid (anterior distance), and the distance from the posterior capsule of the thyroid (posterior distance) (Figure 3) were taken and recorded. The presence of Hashimoto’s thyroiditis background in ultrasound imaging was noted due to its potential influence on nodule observation, characterized by heterogeneous echogenicity in the thyroid. Additionally, the dominance of the hand used for FNA was recorded. All variables and definitions used in the study are presented in Table 1.
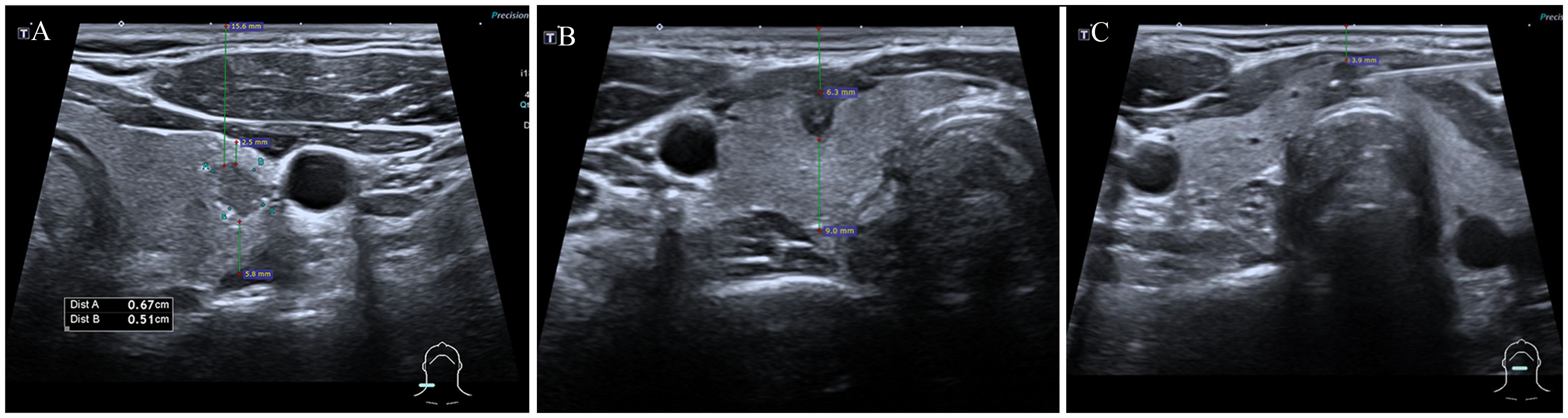
Figure 3 Measurements of thyroid nodules’ depth, anterior, and posterior distances. The depth of nodules refers to the vertical distance of nodules from the skin surface, anterior distance refers to the distance of nodules from the thyroid’s anterior capsule, posterior distance refers to the distance from the thyroid’s posterior capsuled. (A) depth: 15.6mm, anterior distance: 2.5mm, posterior distance: 5.8mm; (B) depth: 6.3mm, anterior distance: 0mm, posterior distance: 9.0mm; (C) depth: 3.9mm, anterior distance: 0mm, posterior distance: 0mm.
2.5 Statistical analysis
Continuous variables are presented as means and standard deviations (mean ± SD) for normally distributed data and as medians and interquartile ranges (IQR) for non-normally distributed data. Discrete variables are shown as frequencies and percentages. The Chi-square test was applied to compare discrete variables. For continuous variables, the Student’s t-test was used for normally distributed data, while the Mann-Whitney U test was employed for non-normally distributed data. Differences in baseline variables were analyzed using the CBCgrps package in R (24).
The present study explored the relationship between the MD of thyroid nodules and malignant outcomes in FNA using binary logistic regression. Model 1 did not adjust for any covariates; Model 2 was adjusted for age, gender, and ultrasound characteristics of the nodules; Model 3 included additional covariates that may influence FNA of thyroid nodules, such as position, hand used for FNA, depth, anterior distance, and posterior distance of the nodules. To investigate potential cutoff values for the MD of nodules for FNA, we performed a restricted cubic spline (RCS) analysis with three knots at the 10th, 50th, and 90th percentiles (25). Subsequently, MD was divided into two categories for further subgroup analysis. Statistical significance was set at p < 0.05. All statistical analyses were conducted using R software (version 4.3.1; R Statistical Software, R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Baseline information
In present study, 280 TR5 thyroid nodules from 226 patients underwent FNA. Of these, 143 nodules (41.1%, 143/280) were Bethesda VI in cytopathology, and 20 nodules (7.1%, 20/280) were Bethesda V with BRAF V600E mutation, these 163 nodules were categorized as PM group (58.2%, 163/280). The remaining 117 nodules were categorized as PB group (41.8%, 117/280), including 11 nodules with Bethesda I (3.9%, 11/280), 53 with Bethesda II (18.9%, 53/280), 25 with Bethesda III (8.9%, 25/280), 6 with Bethesda IV (2.1%, 6/280), and 22 with Bethesda V without BRAF V600E mutation (7.9%, 22/280).
Demographic, clinical, and imaging data for both groups are detailed in Table 2. The PM group exhibited a significantly larger MD of nodules compared with the PB group [Malignant group vs. benign cytopathology group, 6.5mm (5.0, 8.4) > 5.3mm (4.0, 7.0), p < 0.001]. Additionally, the PM group was significantly younger than PB group [Median age: 40 years (range 33-49) vs. 49 years (range 38-58), p < 0.001]. No statistical differences were observed in other variables between the two groups.
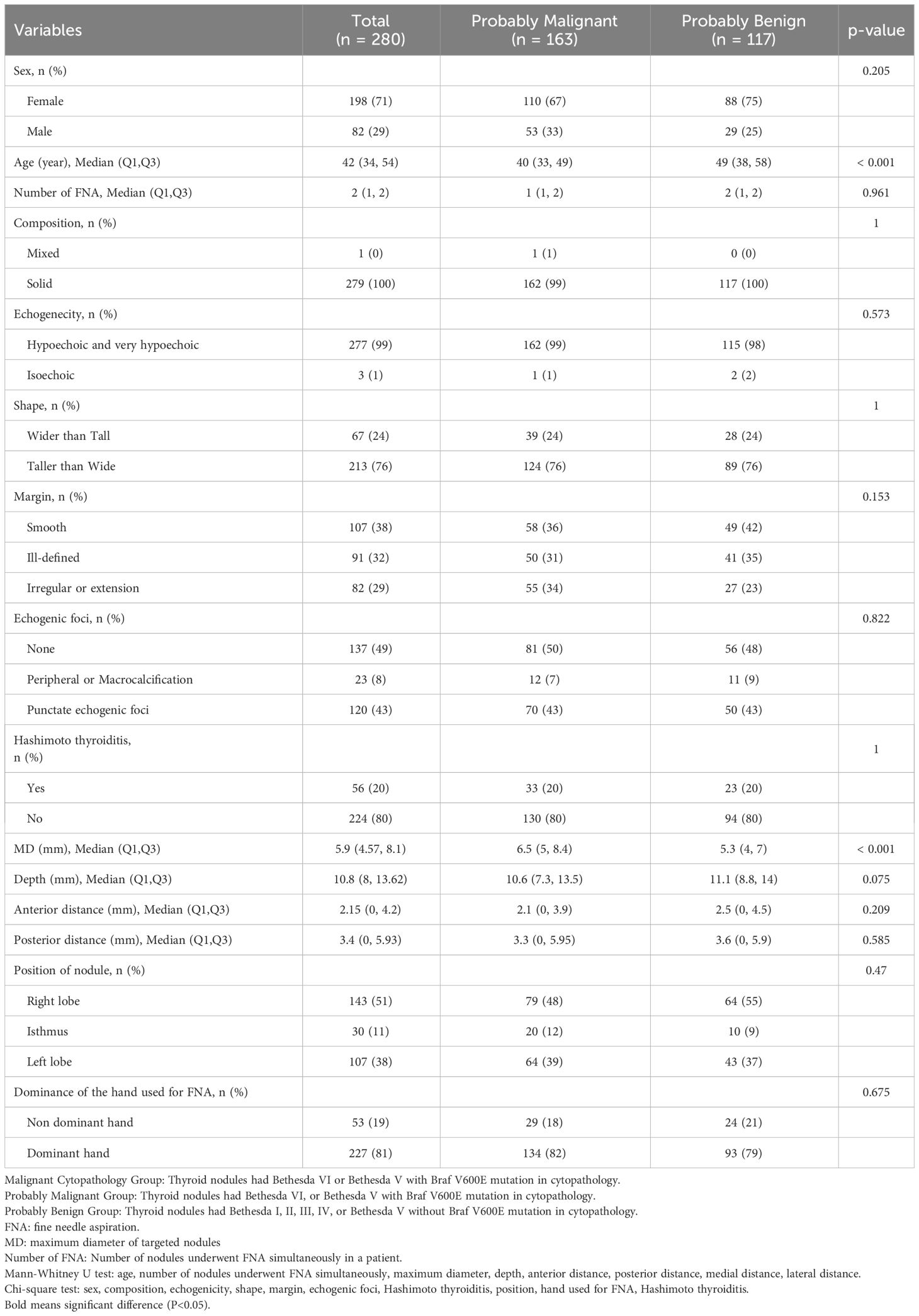
Table 2 Demographic, clinical and imaging information of probably malignant and probably benign groups.
3.2 Logistics regression
Three binary logistics regression models were constructed to investigated the relationship between MD of thyroid nodules and PM outcomes(Table 3). The MD was included in model as a continuous variables or a discontinuous variables [Quartiles 1 (Q1): 2.1-4.6mm; Q2: 4.6-5.9mm; Q3: 5.9-8.1mm; Q4:8.1-40.8mm] respectively.
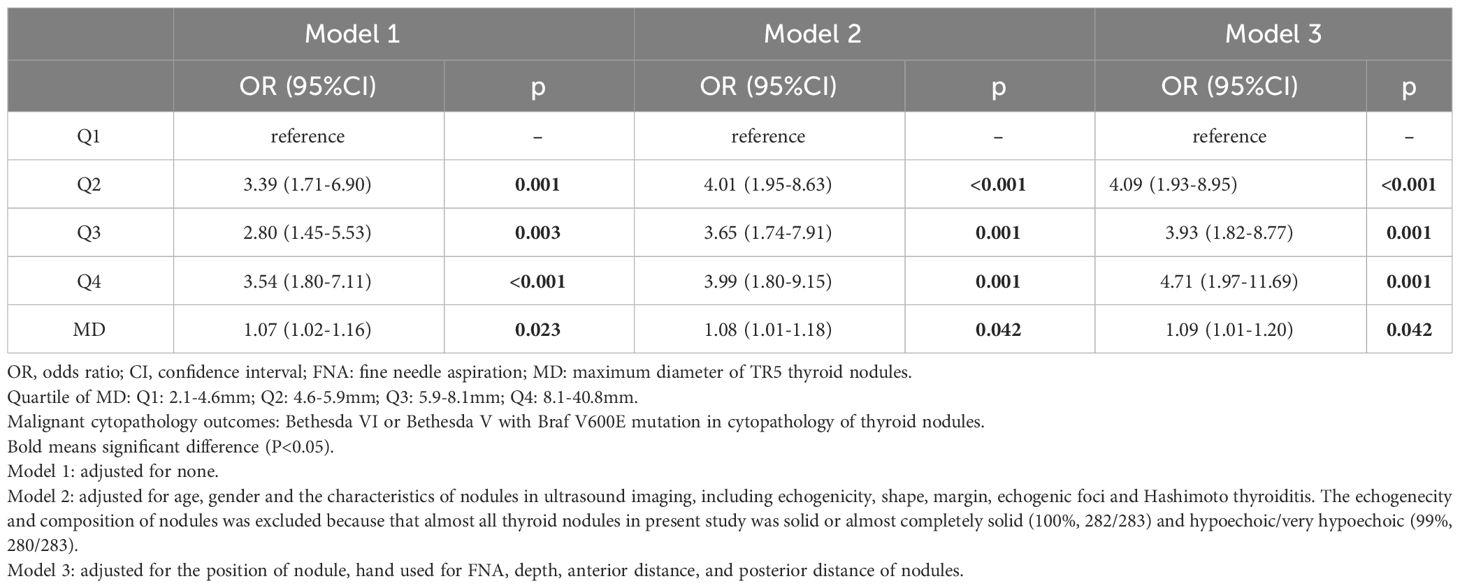
Table 3 Logistics regression model for association between MD and malignant cytopathologY outcomes in FNA of thyroid nodules.
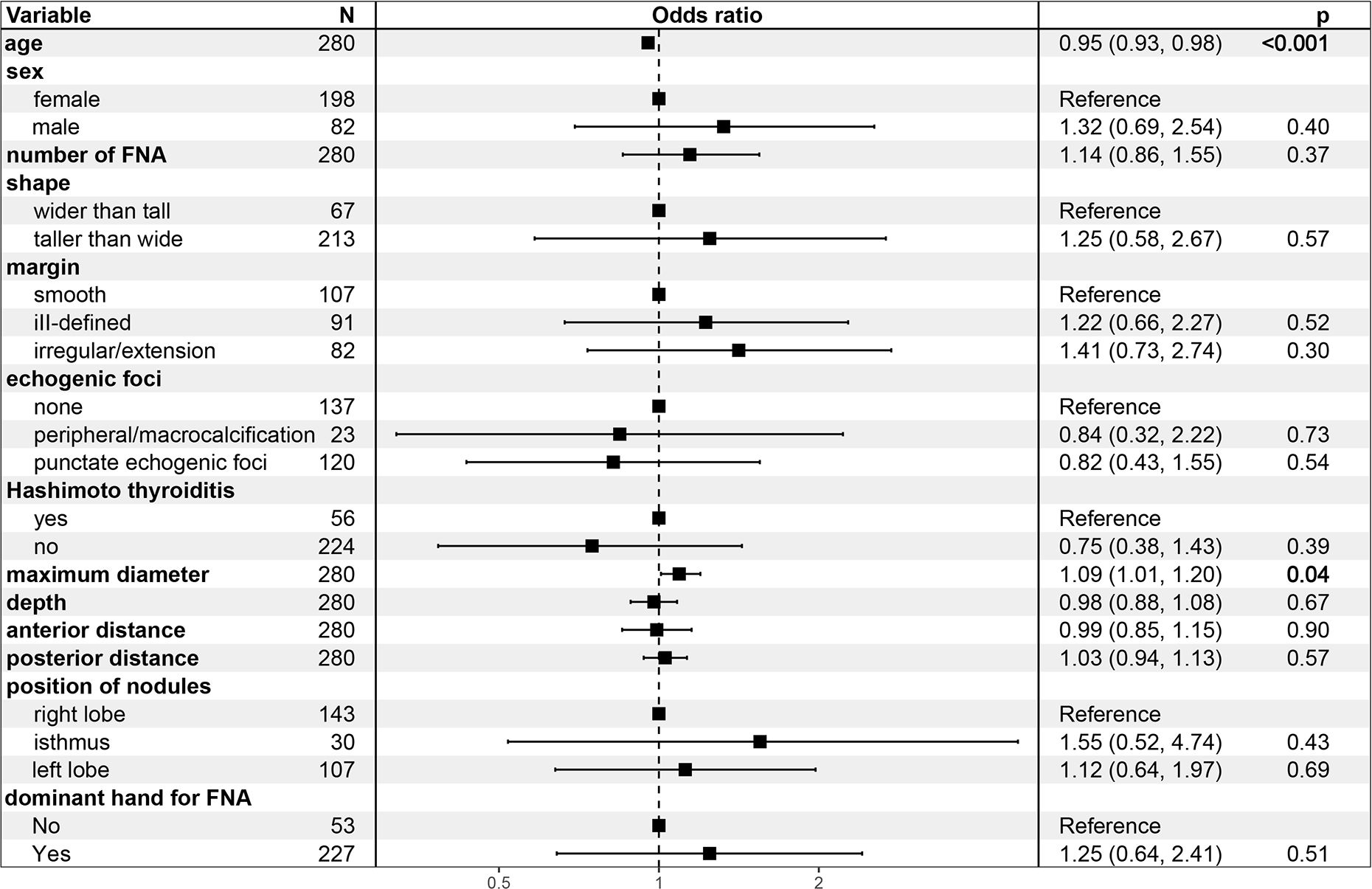
In Model 1, the odds ratio (OR) and 95% confidence interval (CI) of MD were 1.07 (1.02-1.16), p=0.023), suggesting an increased likelihood of PM outcomes in FNA with every unit increase in the MD of TR5 thyroid nodules. These relationship persisted in Model 2 [OR (95%CI): 1.08 (1.01-1.18), p=0.042] and Model 3 [OR (95%CI): 1.16 (1.05-1.31), p=0.007] after adjusted for potential influencing factors. The results of Model 3 indicated that age was a negative predictor of PM outcomes in FNA [OR (95%CI): 1.09 (1.01-1.20), p=0.042] (Figure 4). Furthermore, when MD was categorized into quartiles, this relationship remained significant. The Q2, Q3, and Q4 quartiles of MD showed a significantly higher probability of PM outcomes compared to the Q1 quartile in FNA of TR5 thyroid nodules (Table 3).
3.3 Restricted cubic spline
Using Model 3 as a basis, we constructed a RCS analysis to delve deeper into the relationship between MD and PM outcomes in FNA (Figure 5). A significant non-linear correlation was found between MD and PM outcomes (p=0.002). The inverted L-shaped RCS curve revealed an increasing probability of PM outcomes in TR5 thyroid nodules with higher MD. The RCS analysis for FNA in TR5 thyroid nodules identified 6.2mm as a potential MD cutoff value.
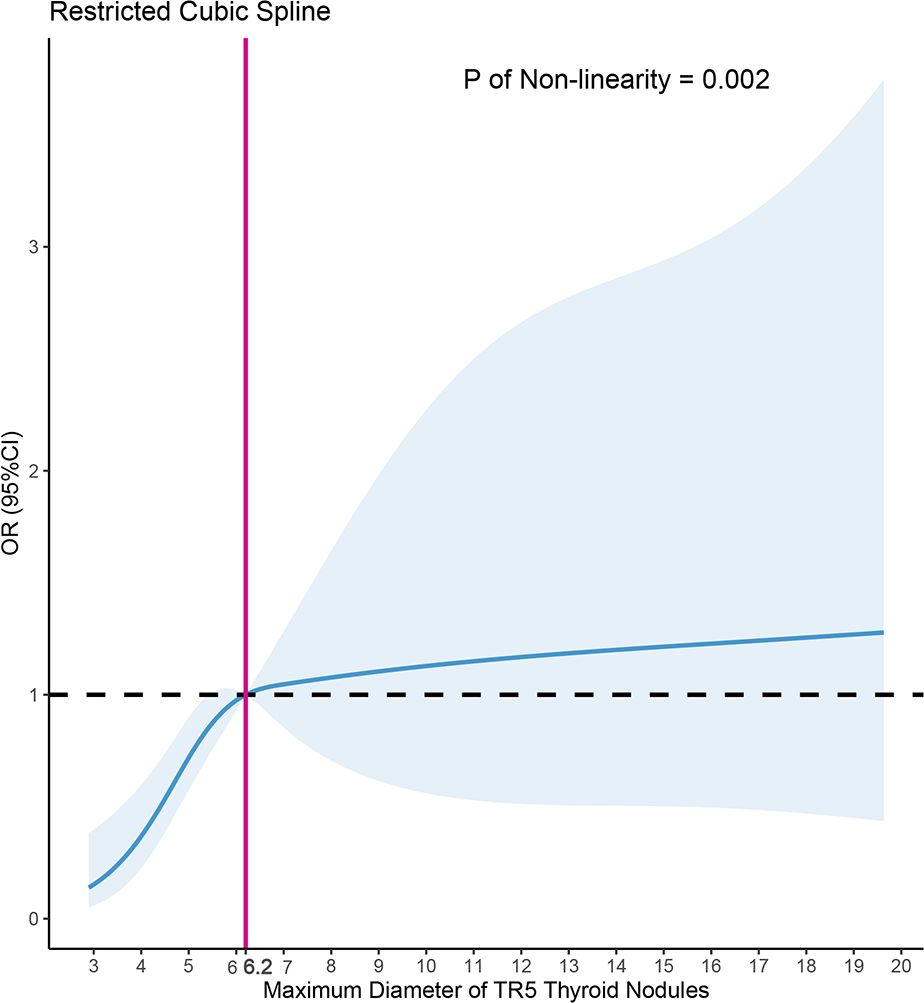
Figure 5 Nonlinear relationship of maximum diameter and malignant cytopathology outcomes in fine needle aspiration (FNA) of ACR TIRADS 5 thyroid nodules, the model was adjusted for age, gender, the shape, margin, echogenic foci of nodules, Hashimoto thyroiditis, position of nodules, dominance of the hand for FNA, depth, anterior distance, and posterior distance of nodules.
3.4 Subgroup logistics regression
Furthermore, TR5 thyroid nodules were categorized into two subgroups based on the results of RCS and the MD of the nodules: those with MD ≥ 6.2mm and those with MD < 6.2mm (Table 4). Logistic regression analyses were conducted separately for each subgroup. It was found that the MD of TR5 thyroid nodules was significantly positively correlated with PM outcomes in FNA for nodules with MD < 6.2mm [Odds Ratio (OR) 95% Confidence Interval (CI): 2.43 (1.60-3.86), p < 0.001]. However, this correlation was not significant in nodules with MD ≥ 6.2mm [OR (95% CI): 1.03 (0.94-1.15), p = 0.519].
4 Discussion
FNA is a widely recommended diagnostic tool for determining the nature of the thyroid nodules in most guidelines (2, 6, 9). However, the application of FNA for thyroid nodules smaller than 1cm is debated, with no established MD threshold for suspicious nodules identified (9). Additionally, due to the potential of lymph node metastasis in PTMC and the swift advancement of thermal ablation treatments for PTMC (19–21, 26, 27), FNA for thyroid nodules smaller than 1cm has become increasingly necessary in recent years. To address these issues, this study investigated the relationship between MD and malignant cytopathology outcomes in FNA cytopathology of thyroid nodules, and explored the appropriate MD threshold for TR5 thyroid nodules requiring FNA.
In present study, we summarized 280 TR5 nodules that underwent FNA, with 58.2% (163/280) exhibiting PM outcomes. The MD in the malignant group was 6.5mm (5.0, 8.4), notably larger than the 5.3mm (4.0, 7.0) in the PB group (p < 0.001). The multivariate logistic regression model revealed a positive correlation between MD and PM outcomes [OR (95%CI): 1.09 (1.01-1.20), p=0.042]. Further RCS analysis indicated that an MD of ≥ 6.2mm is a more suitable criterion for TR5 thyroid nodules to undergo FNA.
Remarkably, prior research has not specifically focused on the relationship between the MD of thyroid nodules and malignant outcomes in FNA. Although several studies have explored the relationship between the MD of thyroid nodules and FNA results, they predominantly concentrated on non-diagnostic rather than malignant cytopathology outcomes. Choi et al. suggested that the MD ≤ 5mm positively influenced non-diagnostic results in repeat FNA (OR = 1.742, p = 0.0331) (16). Similarly, studies by Leenhardt et al., Leung et al., Li et al., and Chen et al. observed a comparable relationship in FNA for thyroid nodules (10, 18, 28, 29). Conversely, other studies found no significant correlation between nodule MD and sample adequacy in FNA (12, 15, 30, 31). We believe that nodule MD does not influence sample size in FNA, as operators can obtain thyroid cells surrounding even very small nodules. Consequently, thyroid nodule size does not lead to inadequate sample sizes in FNA, but theoretically could increase the false negative rate. One study found that the sensitivity, specificity, accuracy, and predictive values for nodules ≤ 5mm were lower than those for nodules 6-10mm and >10mm, although the differences were not significant (31). However, another study found that thyroid nodules < 5mm had a significantly higher false negative rate (3.9%) compared to 5 - 10mm (0.9%) and > 10mm (0%) (17).
Furthermore, this study is the first to statistically analyze and identify a suitable MD for thyroid nodules undergoing FNA. We conducted RCS analysis to determine the potential MD threshold for TR5 thyroid nodules undergoing FNA. The inverted L-shaped RCS curve indicated that 6.2mm might be an appropriate MD threshold, a finding further corroborated by subgroup logistic regression analysis. These results suggest an increased likelihood of benign cytopathology outcomes in FNA of TR5 thyroid nodules smaller than 6.2mm, compared to those ≥ 6.2mm. Based on these findings, we propose that an MD ≥ 6.2mm could be a more indicative threshold for FNA. Additionally, for TR5 nodules smaller than 6.2mm with benign cytopathology FNA outcomes, patients should be informed about the elevated risk of false negatives and the need for more aggressive follow-up or treatment, particularly for nodules in high-risk locations, such as the paratracheal position.
Besides MD, numerous studies have identified various factors potentially influencing FNA outcomes in thyroid nodules (10, 12–16, 18, 28–30, 32, 33), including age (14), needle path (18), needle size (11), calcification (10, 12, 18), echogenicity (14, 16), composition (13), shape (12), the depth of nodule (29, 30), and Hashimoto thyroiditis (12) et al. However, these studies primarily focused on factors influencing non-diagnostic outcomes (Bethesda I or III), with few examining the potential factors impacting malignant outcomes in FNA of thyroid nodules.
In fact, the presumption prior to FNA in TR5 thyroid nodules is that the targeted nodules are malignant; therefore, malignant cytopathology outcomes from FNA could be a more appropriate outcome variable. In this study, Bethesda VI and Bethesda V with the BRAF V600E mutation were considered outcome variables, and logistic regression models were constructed. The MD of nodules was identified as the most significant factor influencing malignant cytopathology outcomes of FNA in TR5 thyroid nodules, showing a notably positive correlation in Models 1, 2, and 3. Other potential influencing factors, such as sex, shape, margin, echogenic foci, nodule depth, anterior distance, posterior distance, Hashimoto’s thyroiditis, and the hand used for FNA, were not statistically significant. Interestingly, age demonstrated a negative correlation with malignant cytopathology outcomes in FNA for TR5 thyroid nodules in Models 2 and 3. Similar findings were observed in previous studies (34, 35).
This study had several limitations, the first being its retrospective design, which inherently carries the risk of bias. Second, some potential influencing factors, like the blood supply of thyroid nodules, were not incorporated into our model due to the absence of relevant data, but could be considered in future, well-designed prospective studies. Third, the relatively small sample size and non-normal distribution resulted in wide confidence intervals in the latter part of the RCS analysis.
In conclusion, the MD of TR5 thyroid nodules significantly influences the likelihood of malignant outcomes in FNA, with 6.2mm emerging as a potential MD threshold. The TR5 thyroid nodules with MD of ≥ 6.2mm are thus more appropriately indicated for FNA. Significantly, this study is the first to investigate the potential MD threshold for TR5 thyroid nodules undergoing FNA based on the RCS analysis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Clinical Research Ethics Committee of China-Japan Friendship Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the examination results and radiological data of patients were published anonymously.
Author contributions
S-LC: Writing – original draft. W-YS: Methodology, Writing – review & editing. Y-RN: Validation, Writing – review & editing. Z-LZ: Resources, Writing – review & editing. YW: Supervision, Writing – review & editing. JW: Resources, Writing – review & editing. L-LP: Data curation, Writing – review & editing. YL: Data curation, Writing – review & editing. M-AY: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grant from National High Level Hospital Clinical Research Funding (Grant No. 2023-NHLHCRF-YYPP-TS-01), Beijing Research Ward Project (Grant No. 2022-YJXBF-04-04), National Natural Science Foundation of China (Grant No. 62176268) and National High Level Hospital Clinical Research Funding (Grant No. 2022-NHLHCRF-PY-07).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. (2009) 39:699–706. doi: 10.1111/j.1365-2362.2009.02162.x
2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 american thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
3. Tan GH, Gharib H. Thyroid incidentalomas: Management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. (1997) 126:226–31. doi: 10.7326/0003-4819-126-3-199702010-00009
4. Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. The spectrum of thyroid disease in a community: The Whickham survey. Clin Endocrinol (Oxf). (1977) 7:481–93. doi: 10.1111/j.1365-2265.1977.tb01340.x
5. Vander JB, Gaston EA, Dawber TR. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid Malignancy. Ann Intern Med. (1968) 69:537–40. doi: 10.7326/0003-4819-69-3-537
6. Cibas ES, Ali SZ. The 2017 bethesda system for reporting thyroid cytopathology. Thyroid: Off J Am Thyroid Assoc. (2017) 27:1341–6. doi: 10.1089/thy.2017.0500
7. Hegedus L. The thyroid nodule: Clinical practice. N Engl J Med. (2004) 351:1764–71. doi: 10.1056/NEJMcp031436
8. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and Imaging-Based management of thyroid nodules: Revised korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol. (2016) 17:370–95. doi: 10.3348/kjr.2016.17.3.370
9. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS committee. J Am Coll Radiol. (2017) 14:587–95. doi: 10.1016/j.jacr.2017.01.046
10. CHen Z, Qiu ZX, Guo DM, Wang JJ, Guo HT, Su HH, et al. Ultrasound features affecting the sample adequacy after fine-needle aspiration of thyroid nodules with different risk stratification. Clin Hemorheol Microcirc. (2023) 83:377–86. doi: 10.3233/CH-221659
11. Degirmenci B, Haktanir A, Albayrak R, Acar M, Sahin DA, Sahin O, et al. Sonographically guided fine-needle biopsy of thyroid nodules: The effects of nodule characteristics, sampling technique, and needle size on the adequacy of cytological material. Clin Radiol. (2007) 62:798–803. doi: 10.1016/j.crad.2007.01.024
12. Fu Y, Sun Y, Pei Q, Han X, Qin W, Mei F, et al. Factors influencing the sample adequacy of Ultrasound-Guided Fine-Needle aspiration from solid thyroid nodules for Liquid-Based cytology: A demographic, sonographic, and technical perspective. Medicina (Kaunas). (2022) 58:1639. doi: 10.3390/medicina58111639
13. Alexander EK, Heering JP, Benson CB, Frates MC, Doubilet PM, Cibas ES, et al. Assessment of nondiagnostic ultrasound-guided fine needle aspirations of thyroid nodules. J Clin Endocrinol Metab. (2002) 87:4924–7. doi: 10.1210/jc.2002-020865
14. Inci MF, Ozkan F, Yuksel M, Salk I, Sahin M. The effects of sonographic and demographic features and needle size on obtaining adequate cytological material in sonography-guided fine-needle aspiration biopsy of thyroid nodules. Endocrine. (2013) 43:424–9. doi: 10.1007/s12020-012-9784-y
15. Zargham R, Johnson H, Anderson S, Ciolino A. Conditions associated with the need for additional needle passes in ultrasound-guided thyroid fine-needle aspiration with rapid on-site pathology evaluation. Diagn Cytopathol. (2021) 49:105–8. doi: 10.1002/dc.24605
16. Choi YS, Hong SW, Kwak JY, Moon HJ, Kim EK. Clinical and ultrasonographic findings affecting nondiagnostic results upon the second fine needle aspiration for thyroid nodules. Ann Surg Oncol. (2012) 19:2304–9. doi: 10.1245/s10434-012-2288-4
17. Kim DW, Lee EJ, Kim SH, Kim TH, Lee SH, Kim DH, et al. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules: Comparison in efficacy according to nodule size. Thyroid. (2009) 19:27–31. doi: 10.1089/thy.2008.0106
18. Li Y, Yu JH, Du PJ, Xie Y, Das SK, Li B, et al. High-Score US-Suspicious subcentimeter thyroid nodules: What factors affect adequate sampling of US-Guided Fine-Needle aspiration biopsy? Int J Endocrinol. (2020) 2020:8464623. doi: 10.1155/2020/8464623
19. Varshney R, Pakdaman MN, Sands N, Hier MP, Rochon L, Black MJ, et al. Lymph node metastasis in thyroid papillary microcarcinoma: A study of 170 patients. J Laryngol Otol. (2014) 128:922–5. doi: 10.1017/S0022215114001704
20. Caliskan M, Park JH, Jeong JS, Lee CR, Park SK, Kang SW, et al. Role of prophylactic ipsilateral central compartment lymph node dissection in papillary thyroid microcarcinoma. Endocr J. (2012) 59:305–11. doi: 10.1507/endocrj.ej11-0366
21. So YK, Son YI, Hong SD, Seo MY, Baek CH, Jeong HS, et al. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: A study of 551 resections. Surgery. (2010) 148:526–31. doi: 10.1016/j.surg.2010.01.003
22. Orloff LA, Noel JE, Stack BJ, Russell MD, Angelos P, Baek JH, et al. Radiofrequency ablation and related ultrasound-guided ablation technologies for treatment of benign and Malignant thyroid disease: An international multidisciplinary consensus statement of the American Head and Neck Society Endocrine Surgery Section with the Asia Pacific Society of Thyroid Surgery, Associazione Medici Endocrinologi, British Association of Endocrine and Thyroid Surgeons, European Thyroid Association, Italian Society of Endocrine Surgery Units, Korean Society of Thyroid Radiology, Latin American Thyroid Society, and Thyroid Nodules Therapies Association. Head Neck. (2022) 44:633–60. doi: 10.1002/hed.26960
23. Kim JH, Baek JH, Lim HK, Ahn HS, Baek SM, Choi YJ, et al. 2017 thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. (2018) 19:632–55. doi: 10.3348/kjr.2018.19.4.632
24. Zhang Z, Gayle AA, Wang J, Zhang H, Cardinal-Fernandez P. Comparing baseline characteristics between groups: An introduction to the CBCgrps package. Ann Transl Med. (2017) 5:484. doi: 10.21037/atm.2017.09.39
25. Harrell FE. Regression modeling strategies: With applications to linear models, logistic and ordinal regression, and survival analysis. New York: Springer-Verlag (2015).
26. Papini E, Monpeyssen H, Frasoldati A, Hegedüs L. 2020 european thyroid association clinical practice guideline for the use of Image-Guided ablation in benign thyroid nodules. Eur Thyroid J. (2020) 9:172–85. doi: 10.1159/000508484
27. Wei Y, Niu WQ, Zhao ZL, Wu J, Peng LL, Li Y, et al. Microwave ablation versus surgical resection for solitary T1N0M0 papillary thyroid carcinoma. Radiology. (2022) 304:704–13. doi: 10.1148/radiol.212313
28. Leung VA, Kirpalani A, Mnatzakanian G, Colak E, Vlachou PA. Effect of a biopsy center on adequacy rates of thyroid nodule Fine-Needle aspiration. AJR Am J Roentgenol. (2017) 209:358–62. doi: 10.2214/AJR.16.17157
29. Leenhardt L, Hejblum G, Franc B, Fediaevsky LD, Delbot T, Le Guillouzic D, et al. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J Clin Endocrinol Metab. (1999) 84:24–8. doi: 10.1210/jcem.84.1.5418
30. Jiang C, Chang J, Chen X, Zhang H, Xu M. Independent risk factors and feasibility of ultrasound diagnosis of ultrasound-guided non-cytologically diagnostic thyroid nodules. Gland Surg. (2021) 10:2724–33. doi: 10.21037/gs-21-512
31. Zhong LC, Lu F, Ma F, Xu HX, Li DD, Guo LH, et al. Ultrasound-guided fine-needle aspiration of thyroid nodules: Does the size limit its efficiency? Int J Clin Exp Pathol. (2015) 8:3155–9.
32. Jack GA, Sternberg SB, Aronson MD, Mukamal KJ, Oshin A, Hennessey JV. Nondiagnostic Fine-Needle aspiration biopsy of thyroid nodules: Outcomes and determinants. Thyroid. (2020) 30:992–8. doi: 10.1089/thy.2019.0140
33. Karabacak U, Derebey M, Tarim IA, Polat AK. Factors affecting Malignancy in thyroid nodules with non-diagnostic fine needle aspiration biopsy result. Acta Endocrinol (Buchar). (2022) 18:187–93. doi: 10.4183/aeb.2022.187
34. Bessey LJ, Lai NB, Coorough NE, Chen H, Sippel RS. The incidence of thyroid cancer by fine needle aspiration varies by age and gender. J Surg Res. (2013) 184:761–5. doi: 10.1016/j.jss.2013.03.086
35. Rago T, Fiore E, Scutari M, Santini F, Di Coscio G, Romani R, et al. Male sex, single nodularity, and young age are associated with the risk of finding a papillary thyroid cancer on fine-needle aspiration cytology in a large series of patients with nodular thyroid disease. Eur J Endocrinol. (2010) 162:763–70. doi: 10.1530/EJE-09-0895
Keywords: fine needle aspiration, thyroid nodule, restricted cubic spline (RCS), diagnosis, ultrasound
Citation: Cao S-L, Shi W-Y, Niu Y-R, Zhao Z-L, Wei Y, Wu J, Peng L-L, Li Y and Yu M-A (2024) Influence of maximum diameter on fine-needle aspiration biopsy outcomes in ACR TI-RADS 5 thyroid nodules. Front. Endocrinol. 15:1374888. doi: 10.3389/fendo.2024.1374888
Received: 22 January 2024; Accepted: 22 April 2024;
Published: 14 May 2024.
Edited by:
Cristina Alina Silaghi, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaReviewed by:
Joachim Feldkamp, Bielefeld University, GermanyShahram Taeb, Gilan University of Medical Sciences, Iran
Copyright © 2024 Cao, Shi, Niu, Zhao, Wei, Wu, Peng, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-An Yu, eW1hMzAxQDE2My5jb20=
 Shi-Liang Cao
Shi-Liang Cao Wan-Ying Shi2
Wan-Ying Shi2